Abstract
Diabetes is an age-related disease, most of which is type 2 diabetes, and islet β cell dysfunction and insulin resistance are the main mechanisms of type 2 diabetes. Recent studies have revealed that autophagy plays an important role in maintaining the structure and function of islet beta cells and inhibiting insulin resistance and apoptosis induced by oxidative stress. In this review, we discussed the positive and negative effects of autophagy and its dysfunction on type 2 diabetes mellitus, which is the so-called double-edged sword, analysed its possible mechanism, and identified possible research hot spots.
Keywords: Apoptosis, Autophagic dysfunction, Insulin resistance, Islet beta cell dysfunction, Type 2 diabetes mellitus
Diabetic mellitus (DM) is the most common chronic disease worldwide.1 China ranked first in the world for the incidence of diabetes, and many studies have demonstrated that the prevalence of diabetes in the Chinese population has increased dramatically from 0.67% in 1980 to 11.6% in 2010, which continues to increase.1, 2, 3, 4 In addition, the prevalence of prediabetes in 2010 was 50.1%, suggesting that approximately 500 million Chinese adults may have prediabetes.4 This rapid growth in diabetic prevalence may be due to life-time dilation, high caloric diet and reduction of physical activity as economic growth and changes in lifestyle.1,5 Changes in these factors can cause diabetes in people with a gene that predisposes to the disease. Diabetes and its complications have become the most popular public health issue, which may impose a heavy social and economic burden due to its high morbidity and mortality.6 Therefore, elucidation of the mechanism involved in the pathogenesis of diabetes and the development of interventions based on its pathogenesis are urgently needed for the prevention of diabetes. As the most common diabetes in adults, type 2 diabetes (T2DM) is characterized by insulin resistance and islet β cell dysfunction.7 Although many risk factors have been reported to be related to T2DM, the pathogenesis remains unclear.
Autophagy is a cell-protective process that can maintain cellular homeostasis through the degradation and recycling of organelles and proteins.8 This process can respond to many conditions, such as starvation, growth factor deprivation, and endoplasmic reticulum (ER) stress.9 Autophagy can eliminate defective proteins and organelles, prevent the accumulation of abnormal proteins, and remove intracellular pathogens. Therefore, this important procedure helps maintain cellular homeostasis9 and affects the function and survival of cells. Extensive evidence has revealed the association between autophagic dysfunction and many diseases, such as tumours,10 inflammatory diseases,11 and neurodegenerative diseases.12 Autophagy can reallocate nutrients from unnecessary processes to pivotal processes required for survival,13 and it may play an important role in the pathogenesis of metabolic disorders, such as diabetes. Loss of function in autophagy has been observed under diabetic conditions according to several studies.14,15 Autophagy is a protective mechanism to eliminate toxic proteins or remove damaged organelles for survival. However, autophagy also leads to nonapoptotic cell death, which is the so-called type 2 programmed cell death that results in cell loss. Therefore, autophagy may have positive effects or negative effects on cellular survival and function. Whether autophagy results in positive or negative effects is unclear. In this article, we focused on the positive and negative effects of autophagic dysfunction on T2DM, analysed its possible mechanism, and suggested possible research hot spots for the future.
Autophagy signalling pathway
Autophagy, a term with a Greek origin, refers to a degradative pathway of proteins and organelles, by which cytoplasmic cargo can be delivered to the lysosomes.16 This process can be divided into three categories: macroautophagy, microautophagy, and chaperone-mediated autophagy. Macroautophagy is the major process of cellular metabolic regulation by which long-lived proteins and damaged organelles can be degraded. Herein, autophagy refers to macroautophagy if not noted otherwise. This process is initiated by forming phagophores via vesicle nucleation and vesicle elongation, followed by fusion of the edges of the phagophore to form double-membrane vesicles, the so-called autophagosomes. Autophagosomes can separate their cargos from the cytoplasm, and the captured material and inner membranes can be degraded and recycled when fused with lysosomes to form autolysosomes.17,18 Approximately 30 autophagy-related (Atg) genes encode proteins that are involved in the autophagic process.19 For example, the complex of Beclin 1/Atg6, Atg14L, UVRAG, Ambra1, vacuolar protein sorting 15, and Bif-1, the class III phosphatidylinositol 3-kinase (PI3K or Vps34), is important for the initiation of autophagy. Microtubule-associated protein 1 light chain 3 (LC3-I/Atg8) can combine with phosphatidylethanolamine to form LC3-II, which can be incorporated into the outer membrane of autophagosomes to maintain membrane-bound homeostasis.20 The Atg12-Atg5-Atg16 complex is essential for the formation of LC3-II and autophagosomes.21
A low level of autophagy has been suggested to maintain the normal metabolism of organelles and proteins in physiological environments, and this process is enhanced under some environments, such as starvation, infection, oxidative stress, ER stress, and overaccumulation of proteins in cells. In addition, many cellular signalling pathways have been reported to regulate the autophagic process. Mammalian target of rapamycin complex 1 (mTORC1) is one of the most important autophagic inhibitors. After activation by insulin, growth factors and other metabolic signals, mTORC1 can suppress autophagy by phosphorylating Atg1.22 AMPK, another energetic key protein, is known to activate autophagy by many mechanisms. For example, AMPK can compete directly with mTORC1 to activate unc-51 like autophagy activating kinase 1 and then induce autophagy.22 AMPK was reported to inhibit mTORC1 through phosphorylating tuberous sclerosis complex 2 and have an effect on regulatory associated protein of mTOR (Raptor) to activate autophagy. JNK123 and Beclin 124 can also be targeted to AMPK to activate the Vps34 complex and induce autophagy. In addition, some signalling pathways, such as PI3K/Akt and SIRT, have important effects on the autophagic process. Many studies have revealed that autophagy can be stimulated or inhibited pharmacologically in vivo or in vitro. For instance, inhibiting TOR kinase by rapamycin leads to the induction of autophagy.25 Furthermore, 3-methyladenine (3-MA) can suppress the formation of autophagosomes through PI3K, whereas bafilomycin A1 or chloroquine, which target lysosomal function, can inhibit autophagy by influencing the fusion of autophagosomes with lysosomes.25 As the regulation of these agents mentioned above is nonspecific for the autophagic pathway, inducing or inhibiting autophagy can be used to detect the role of autophagy in diseases, and these signalling factors may be favourable targets to prevent diabetes.
Role of autophagy in maintaining the normal structure and function of islets
A low level of autophagy is important for maintaining the physiological structure and function of islet β cells. Ebato et al found that autophagosomes were barely observed in the β cells of C57BL/6 mice fed a standard diet, and those identified as autophagosomes were small in size.14 To determine the physiological role of autophagy in islet β cells, the researchers constructed a mouse deficient in Atg7, an essential gene for autophagosome formation, specifically in islet β cells. In these Atg7-deficient mice, the LC3-I to LC3-II transition was suppressed, and p62 and polyubiquitin accumulated. In addition, the islets of Atg7-deficient mice contained multiple cyst-like structures measuring 15-20 mm in diameter. These cyst-like structures, occasionally associated with caspase-3-positive apoptotic cells, were not fat-laden adipocytes and could not be stained by oil red O. These results indicated that autophagy plays an important role in the maintenance of normal islet β cell structure.
Normal function of autophagy is important for the maintenance of islet function. However, the results focus on the relationship between autophagy and insulin secretion is still controversial. To further elucidate the role of autophagy in islet β cell function, Ebato et al conducted intraperitoneal glucose tolerance tests and insulin tolerance tests. Impaired insulin secretion was observed in Atg7-deficient mice, which confirmed the reduced glucose tolerance and insulin sensitivity in these mice compared with the control mice.14 However, another research suggested that inhibiting autophagy by Atg5/Atg7 knockdown in β cell increase the GSIS of mice,26 indicating that proinsulin accumulation is increased after autophagy inhibition, which may enhance insulin secretion after stimulation. The different results between these researches may be related to difference in cell states, viability and plasma glucose. Besides, the alternation of autophagy may also influence the insulin secretion of starved β cell. Goginashvili et al demonstrated that nutritional deprivation induce degradation of nascent secretory insulin granules by lysosomal recruitment in β cell, and inhibit autophagy by activating mTORC1 pathway, thus reducing the secretion of insulin.27 This phenomenon can provide the body against hypoglycemia caused by inappropriate insulin secretion. Recently, in vivo studies showed that LC3-I and LC3-II were obviously increased when INS-1 cells were treated with insulin at 100 nmol/L for 24 h compared with the control, which indicated that high levels of insulin upregulate autophagy.8 After treatment with 3-MA, an autophagy inhibitor, apoptosis was significantly increased compared to that in the INS-1 cells that were only treated with insulin. These results indicated that autophagy plays an important role in the maintenance of β cell function and survival.
Autophagy maintains the mass, structure and function of islet β cells; eliminates waste in the process of metabolism; and participates in the pathophysiological process of many diseases. The role of autophagic dysfunction in the aetiology of diabetes is not fully understood. Methods to improve the function of islet β cells through the regulation of autophagy may be a potential strategy for the treatment of diabetes.
Autophagy in β cells under diabetic conditions
As autophagy plays a role in maintaining the survival and function of islet β cells, an increasing number of studies have focused on its role in the pathogenesis of diabetes. One study of autophagy in type 2 diabetic patients demonstrated extensive accumulation of autophagic vacuoles in the islet β cells.28 Other studies of autophagy showed increased staining of p62-positive islet β cells in type 2 diabetic patients.29,30 Researchers also found that diabetic mice fed a high-fat (HF) diet have increased numbers of autophagosomes in islet β cells.14 There were more autophagosomes in many diabetic animal models, such as ob/ob mice,31 db/db mice14 and Zucker diabetic fatty rats, than the controls.32 These models are characterized as obesity due to leptin deficiency/resistance, causing diabetes by insulin resistance. Recently researches demonstrated that serum leptin is associated with the expression levels of autophagy genes,33 indicating the relationship between autophagy and leptin. It is noteworthy that regulation of autophagy by leptin seems to be highly cell/tissue specific.34 Even in same cell/tissue, effect of leptin on autophagy described in different studies are controversial. Gan et al suggested that leptin decreased the expression of autophagy genes in murine adipose and adipocytes34; However, another study indicated that leptin stimulate autophagy murine adipocyte cell-line in vitro.35 This may due to the differences between in vivo and in vitro experiments. Thus further study are needed to explore whether the autophagy dysfunction is associated with the leptin deficiency/resistance.
The increased number of autophagosomes in β cell of diabetes may be attributed to either the enhanced or inhibited autophagic flux. However, the accumulation of p62/SQSTM1, which is an adaptor protein interacting with LC3-II, was also observed in ob/ob mice and db/db mice.29,31 These results suggested that autophagic flux may not be sufficiently activated to address the increased demand for proteolysis in T2DM. Whether this process is a pathogenic or protective factor or even a consequence of diabetes is still unclear. A study indicated that HF diet-fed β cell-specific Atg7 knockout (Atg7f/f:RIP-Cre) mice had significantly higher nonfasting glucose levels and severely impaired glucose tolerance compared to the HF diet-fed Atg7f/f mice.16 β cell mass was reduced and apoptosis was significantly higher in the Atg7f/f:Cre mice than the HF-fed Atg7f/f mice. Similar results were observed in other diabetic models, such as ob/ob mice31 and Akita mice.36 This result indicated that inductive and smooth autophagy flux of β cells is truly an adaptive response and plays a protective role in T2DM.
The protection of islet β cells plays an important role in the progression of T2DM, and this process is a target in drug development for T2DM. Autophagy, as a defence mechanism of cells, has been recommended by an increasing number of studies. However, strategies to regulate autophagic flux at an appropriate time for protection of islet β cells require further exploration.
The effect of altered autophagy in islet β cells on T2DM
As mentioned above, there is a relationship between changes in autophagy and islet β cell function in diabetes, and several studies have attempted to investigate whether alterations in autophagy induce β cell injury or promote survival. Glucotoxicity, which means long-term exposure to hyperglycemia, may induce apoptosis and dysfunction of islet β cells. It is more obvious and more serious in hyperosmolar hyperglycemic syndrome, the most serious acute hyperglycemia emergency in T2DM. Chronic hyperglycemia may result in the defective insulin gene expression, diminished insulin content, and defective insulin secretion in β cell.37 Given the difficulty that studying human β cell in vivo, most of the knowledge was acquired from the postmortem islet from patients with T2DM or animal experiments. It is well accepted that patients with T2DM have failed insulin response to intravenous glucose.37 A recent publication reported that both expression of insulin gene and islet volume density of insulin were decreased in islet from T2DM patients.2 Besides this study also found that apoptosis in β cell was obviously increased in T2DM.38 Chen et al demonstrated that after incubation with high glucose (25 mmol/L) for 24 h, the viability of islet β cells was decreased compared with that of the control group.15 High glucose can induce autophagy, and MDC-labelled autophagosomes and LC3-II expression were increased compared with those of the control group. After inhibition of autophagy by 3-MA, the viability of islet β cells was decreased compared with that of the group treated only with high glucose. These results suggested that the induction of autophagy under high glucose conditions may protect islet β cells from death. High glucose increases the demand for insulin and then stimulates the synthesis of proinsulin by islet β cells. In this study, autophagy was induced to facilitate the delivery of protein. If autophagy is inhibited, misfolded proteins may accumulate in islet β cells to induce ER stress, which finally leads to cell death.
Ebato et al attempted to demonstrate whether changes in autophagy are a protective or pathogenic factor in islet β cells under insulin resistance conditions.14 They found that HF-fed Atg7f/f:Cre mice had significantly higher nonfasting glucose levels and severely impaired glucose tolerance compared to the HF-fed Atg7f/f mice. Morphometric analysis found that after 12 weeks of HF diet feeding, the islet β cell mass was increased by 2-fold in the Atg7f/f mice but not in the Atg7f/f:Cre mice, which indicated the failure of compensatory hyperplasia and the increased apoptosis of islet β cells in the latter group. The degeneration of islets in the Atg7f/f:Cre mice was enhanced when these mice were fed a HF diet. The results revealed that inductive autophagy is an adaptive response of islet β cells in insulin resistance induced by HF diet feeding.
There are many studies on diabetic animal models, but the pathogenesis in human diabetes may not be similar to that in animals. For example, islet amyloid polypeptide (IAPP) accumulates in islet β cells in T2DM patients and is not found in diabetic animal models. Autophagy plays an important role in human diabetes because human IAPP is degraded by the autophagic system. Therefore, autophagic dysfunction may lead to human IAPP accumulation in islet β cells, which induces apoptosis of islet β cells for cytotoxicity. Transgenic mice expressing hIAPP are commonly used to mimic human diabetes. Only mice with mild hyperglycaemia were found to be positive for hIAPP, but they tended to develop diabetes when hybridized with RIP-Cre mice; Atg7F/F mice were used to generate hIAPP+Atg7Δβcell mice, in which autophagy is impaired in islet β cells.39,40 Apoptosis was increased in the hIAPP+Atg7Δβ cell mice compared with the hIAPP+Atg7 F/F or hIAPP−Atg7Δβ cell mice. Similar results were found in research using bafilomycin to inhibit autophagy in the hIAPP-expressing islets of monkeys.41 This result indicated that autophagy may play a protective role in hIAPP-induced diabetes. As mentioned above, autophagic dysfunction leads to a decrease in hIAPP degradation; therefore, oligomers of hIAPP accumulate and lead to apoptosis of islet β cells. Some researchers have attempted to determine whether inducing autophagy improves or aggregates islet β cell function in hIAPP-induced diabetes. Trehalose, an autophagy inducer, can improve the insulin secretion ability of islet β cells, decrease apoptosis, and ameliorate the accumulation of hIAPP in HF-fed hIAPP+ mice.41 This study revealed the similarity between T2DM and Alzheimer's disease, as well as other neurodegenerative diseases characterized by the accumulation of toxic amyloid protein. Autophagic dysfunction may play an important role in these diseases (see Fig. 1). Based on this study, we believe that preventing autophagic dysfunction may be an effective strategy for the treatment of β cell dysfunction, and this discovery may help to develop a new generation of treatment methods for T2DM.
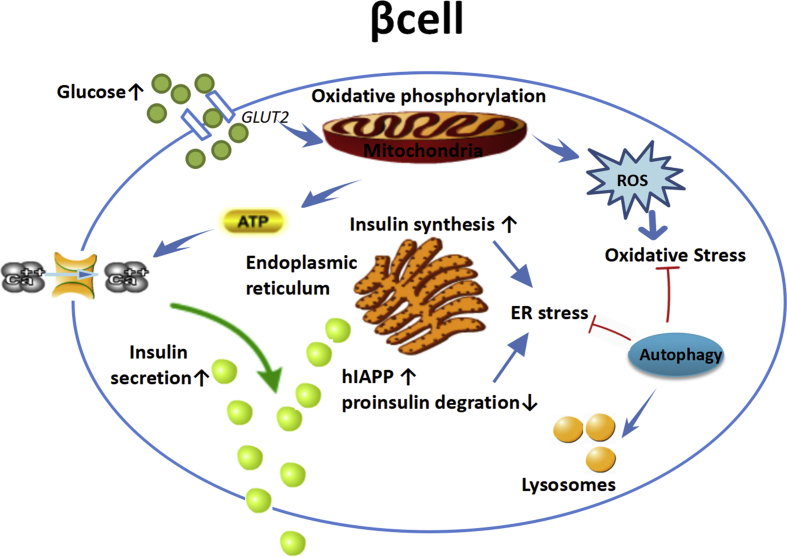
Figure 1.
β cell dysfunction and autophagy. Islet β cell regulates insulin secretion according to blood glucose level. Mitochondria metabolizes glucose and produces adenosine triphosphate (ATP) and reactive oxygen species (ROS), which are important signals for glucose to stimulate insulin secretion. Oxidative stress, endoplasmic reticulum (ER) stress, increased human islet amyloid polypeptide (hIAPP) and proinsulin degradation triggers the cell stress, which can be alleviated by autophagy. Hyperglycemia induces excessive ROS and leads to oxidative stress. Long term elevated insulin secretion leads to ER stress. Autophagy was up regulated by oxidative stress and ER stress to protect β cells from apoptosis.
Oxidative stress and autophagy in β cells
As insulin secretion relies on ATP generation during the process of oxidative metabolism, the normal function of mitochondria is essential for maintaining islet β cell function.42 Islet β cells are sensitive to oxidative injury, and excessive oxidative stress may lead to β cell dysfunction and diabetes. Mizukami et al found that the density of islet β cells was reduced in diabetic subjects compared with nondiabetic subjects,30 and the decrease in the density of islet β cells was associated with the increased expression of γH2AX, which is a marker of oxidative DNA injury. Another study indicated that mitochondrial oxidative stress was progressively increased in healthy subjects, prediabetic subjects, newly diagnosed diabetic patients and advanced diabetic patients.43 In prediabetic subjects, the increased expression of mitochondria-related markers was positively correlated with HOMA-β (the insulin secretion index), while the HOMA-β was decreased in the newly diagnosed diabetic group and the advanced diabetic group, especially the advanced diabetic group. These results revealed that oxidative stress increased with the elevation of glucose and may influence the function of β cells.
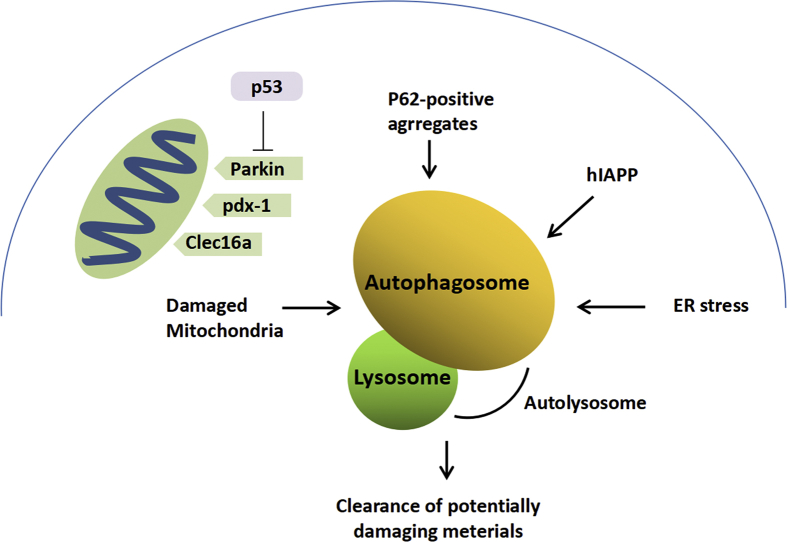
Mitophagy is a special kind of autophagy in which dysfunctional mitochondria can be selectively degraded. Thus, mitophagy is important for maintaining the normal function of β cells by maintaining the normal quality and quantity of mitochondria.44 The process of mitophagy is regulated by several genes, such as PTEN-induced putative kinase 1 (PINK1), PARKIN, LC3 and lysosome-associated membrane protein-2 (LAMP-2), as well as the mitophagic receptors NIP3 like protein X (NIX) and mitofusin2.45 Injured mitochondria can be identified by NIX and PINK1, followed by induction of the formation of autophagosomes. Then, these bodies are fused with lysosomes mediated by LAMP-2 and degraded. Under physiological conditions, low levels of ROS can activate many defensive mechanisms to protect β cells against oxidative injury.46 Chronic elevated glucose, also known as glucotoxicity, may lead to the production of excessive ROS and abnormal mitochondria in β cells.47 In this condition, the inhibition of PARKIN translocation leads to disruption of the mitophagic pathway.48 Therefore, glucotoxicity may result in oxidative imbalance. Researchers have reported that patients with T2DM exhibit inhibition of the mitophagic pathway.43 In addition, the mutation of several mitophagy-related genes, such as PINK1, PARKIN, CLEC16A, and PDX1, was shown to be correlated with both T1DM and T2DM.49, 50, 51 In animal models, pancreas-specific knockout of clec16a or PDX1 resulted in a deficiency in insulin secretion52 (see Fig. 2). Above all, these findings suggested that disruption of mitophagy may aggravate the function of β cells, accelerating the progression of diabetes. The normal function of mitophagy may be a target for the prevention and treatment of diabetes in the future.
Figure 2.
Macroautophagy and β cell dysfunction. Macroautophagy is involved in the removal of damaged organelles, including mitochondria, and promotes the degradation of misfolded proteins and protein aggregates, which is the heavy burden of special secretion machines found in β cells.
Crinophagy and autophagy in β cells
Insulin is produced and secreted by islet β cells. Insulin is stored in β cells as large dense-core secretory granules, which are called β-granules. Normally, the insulin content in β cells is kept in an appropriate range to maintain glucose homeostasis. After stimulation, insulin can be released by exocytosis, and proinsulin is upregulated to replenish the insulin content in β cells, while β-granules that do not undergo exocytosis may be degraded by autophagy or crinophagy exocytosis.52,53 In crinophagy, the membrane of β-granules can be directly fused with secretary vesicles in β cells and then delivered to lysosomes for degradation.54 Recently, some Atgs have been shown to participate in this process.55, 56, 57, 58
Both the decrease in β cell mass an insulin resistance in T2DM lead to the dysfunction of insulin secretion in β cells,59,60 resulting in an imbalance of insulin production and secretion. Rab3A is an essential molecule for transporting insulin granules to the cell surface for exocytosis in β cells. The defects in β-granule transfer in Rab3A (−/−) mice have been suggested to result in dysfunctional insulin secretion.61 Another study used electron microscopy tomography for 3D analysis of β cell organelles62 and found reduced insulin secretion without affecting insulin production,63 suggesting a relatively increased number of insulin-containing granules in β cells. However, Marsh et al indicated that in Rab3A (−/−) mice, the number of insulin granules is maintained in the normal range by increasing crinophagy and insulin degradation.62 In addition, in Rab3A (−/−) mice, crinophagy was further activated by treatment with diazoxide, an agent that inhibits insulin secretion.64 These results suggested that crinophagy maintains the quantity and quality of insulin-containing granules in β cells. However, the molecular mechanism of crinophagy has not been fully elucidated. Considering that crinophagy involves the direct fusion of lysosomes with hormone-containing granules, the mechanism regulating crinophagy is likely different from that of macroautophagy. In addition, because of the lack of methods to activate or inhibit crinophagy, the physiological role of crinophagy in islet β cells is still unknown (see Fig. 3).
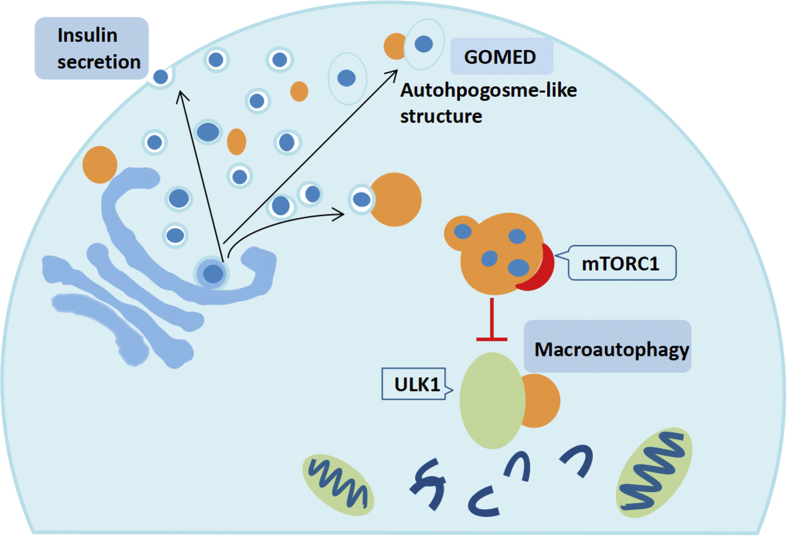
Figure 3.
Macroautophagy independent lysosomal degradation of insulin granules and Golgi. The fate of secretory granules is controlled by the accumulation induced starvation-induced nascent granule degradation (SINGD) in the megaphagocytic active cells and the Golgi membrane associated degradation (GOMED) in the macroautophagy dysfunctional cells. SINGD pathway promotes autophagic lysosomal degradation of newborn granules, which leads to lysosomal recruitment and mTORC1 activation, thus inhibiting macroautophagy.
The mechanism of crinophagy as well as its role in diabetes needs further exploration. The role of microautophagy-mediated autophagy remains to be explored in islet β cells. Identification of the molecular mechanisms controlling these processes may provide new opportunities for drug development in diabetes.
Treatment targeting autophagy in T2DM
T2DM is caused by insulin resistance and deficiency of the secretion function of islet β cells. Mitochondrial damage plays an important role in insulin resistance and islet β cell damage. Research has suggested that autophagy, as a cell self-protective mechanism, plays an important role in maintaining the structure and function of mitochondria and improving insulin resistance. As mentioned above, autophagic dysfunction is closely related to the pathogenesis of T2DM; thus, targeting autophagic function has become a hot topic for the prevention and treatment of diabetes. Calorie restriction effectively reduces the level of blood glucose in patients with T2DM in the clinic. Cheng et al discovered that a fasting-mimicking diet can promote β cell regeneration in a T2DM model.65 This effect can be mimicked by suppression of autophagic inhibitors such as mTORC1 or PKA, suggesting that normal autophagy is important to maintain the function of islet β cells and insulin production. Researchers have demonstrated that intermittent fasting can promote β cell neogenesis, accompanied by inducing autophagy.65, 66, 67 However this phenomenon was abrogated when autophagy is suppressed,66 indicating autophagy may play an important role in intermittent fasting induced-β cell neogenesis. It is revealed that autophagy induced by in intermittent fasting can inhibit the Notch1 pathway, thus boosting the expression of neurogenin-3 to increase the β-cell mass.66 The liver is an important organ for glucose and lipid metabolism in the body. Autophagy can maintain cell metabolism and normal function of the liver, and these effects can help to maintain healthy metabolism and T2DM.
Several antidiabetic drugs used in the clinic, such as metformin, rosiglitazone, GLP-1 agonist, SGLT2 inhibitor and others, were investigated to determine their effects on autophagic dysfunction in β cells. The results suggested that metformin and rosiglitazone can prevent lipotoxicity induced-apoptosis of β cells by inducing autophagy in vitro via the AMPK pathway.68,69 Similar effects were found with GLP-1 agonists, including GLP-1 receptor agonists and DPP-IV inhibitors.70 AMPK has been known as a central regulator of glucose metabolism. It is revealed that it is closely related to glucose uptake and glycogen synthesis. AMPK can be activated by nutritious stress. As is mentioned above, AMPK is an autophagy activator by inhibiting mTORC1 pathway or activate the Vps34 complex by regulating JNK1 and Beclin 1.68 Therefore, many antidiabetic drugs may protect islet β cells at least partially through mediating the function of autophagy. The results also indicated that the pathogenesis of diabetes is related to autophagic dysfunction. SGLT2 inhibition reduced the risk and severity of kidney and cardiac injury in T2DM patients and animal models.71 Whether SGLT2 inhibitors directly or indirectly acted by improving glycolipid metabolic repair in autophagic dysfunction to reduce apoptosis of islet β cells requires further study. AMPK is also a central signal molecule associated with various diseases, such as tumours, cardiovascular disease, and others. Whether regulation of autophagic dysfunction can affect the occurrence and development of diabetic cardiovascular complications or tumours needs further exploration.
Par-4 induces autophagic dysfunction and leads to apoptosis in tumour cells, but few studies have been conducted on the islet β cells
Prostate apoptosis response protein 4 (Par-4) was recently identified as a proapoptotic factor located on human chromosome 12q21, and it may participate in the regulation of cell apoptosis at the transcriptional level. Par-4 is widely expressed in many kinds of cells. Telomeres are DNA-protein structures, and the human telomere sequence is a repeat of 5′-TTAGGG-3'. This sequence cannot encode proteins but has the following biological functions: maintaining the stability of chromosomes, preventing the end fusion of chromosomes, protecting chromosome structure, and determining the lifespan of cells. Zhou Ping and his colleagues found that Par-4 interacts with TERT, and further study showed that the inhibition of TERT reduces the tumour size in nude mice and increases the nuclear expression of Par-4. Our study also found that Par-4 binds and interacts with TERT and participates in the regulation of islet β cell apoptosis. Diabetes increases the expression of Par-4 in the nucleus, induces Par-4 binding to TERT in the cytoplasm, decreases the expression of TERT, increases the expression of Par-4 in the nucleus and activates NF-κB.
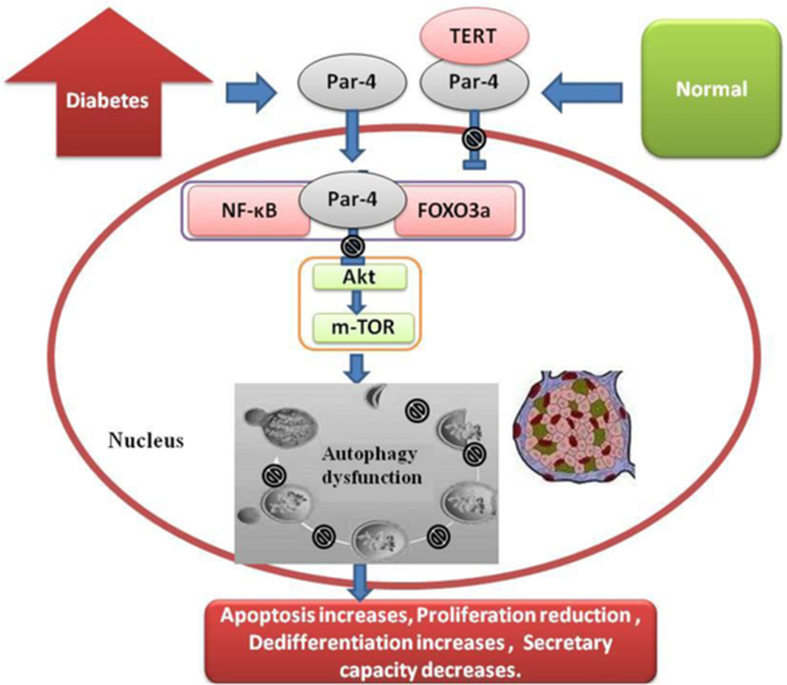
In recent studies, Par-4 was shown to induce cell death through autophagic dysfunction: 1) various stresses increase Par-4 expression, autophagic marker accumulation, autophagic obstruction and autophagic dysfunction, leading to cell death72, 73, 74; 2) upregulation of Par-4 inhibits the binding of Bcl-2 with the autophagic gene Becn1, resulting in autophagic obstruction, autophagic dysfunction and cell death.75 This finding indicates that Par-4 may induce cell death and dysfunction through autophagic dysfunction.76 Previous studies suggested that Par-4 expression can induce the death of islet β cells. Some studies have shown that the transcription factors FOXO3a and NF-κB, which are regulated by Par-4, are involved in the development of islet β cell autophagy.30,77 Therefore, Par-4 may participate in the death of islet β cells through autophagy. Our study found that Par-4 interacts with TERT and participates in the regulation of apoptosis.78 Recent studies have shown that TERT is also a key factor in the development of autophagic disorders. Overexpression of TERT inhibited the mTOR pathway, increased the expression of LC-3II, upregulated the level of autophagy, and reduced apoptosis.79,80 The autophagic inducer rapamycin upregulated the expression of TERT in the mTOR pathway.81 However, after knockout of TERT in mouse renal tubular epithelial cells, autophagy was impaired and the p62 protein accumulated, and rapamycin only partially restored the autophagy and ischaemia/reperfusion injury. These findings suggested that TERT may be an important factor in mTOR-mediated autophagy. In addition, the activity of TERT is closely related to the Akt pathway. After inhibition of Akt phosphorylation, the expression of TERT was also decreased.82 One of the important effects of Par-4 activation is the inhibition of Akt expression. Therefore, the inhibition of Akt by Par-4 activation may also be an important factor in autophagic dysfunction.83,84 Akt is a key signalling pathway regulating autophagy (see Fig. 4). Whether the cells undergo apoptosis or continue to survive after autophagy depends predominantly on the activation of the Akt signalling pathway.85 Whether autophagic dysfunction results in positive or negative effects is still unclear.
Figure 4.
Par-4 -TERT/ Akt and autophagy dysfunction of islet β cells.
Conclusion
Autophagy, a special process for clearing abnormal proteins and organelles in cells, is essential for maintaining the normal structure and function of cells. Researchers have reported that autophagy plays a vital role in the regulation of β cell mass and function. In diabetes, autophagosomes in β cells are increased, with an increase in P62 and SQTSM1 both in vitro and in vivo. Inhibition of autophagy by a genetic approach or chemical methods in diabetic animals may lead to aggravated loss of β cell mass and function. Insulin secretion relies on the normal function of mitochondria. Mitophagy inhibition in diabetic conditions leads to an imbalance of oxidative stress and accelerates cellular injury. Hence, regulating the autophagic pathway has recently become a hot spot in research. Some autophagic activators, such as resveratrol and rapamycin, can improve islet β cell function in diabetic animals. However, these activators are nonspecific, and until now, there has been a lack of evidence to demonstrate whether these drugs can be used in clinical practice. In addition, some antidiabetic drugs, such as metformin, rosiglitazone, GLP-1 agonists, and SGLT2 inhibitors, have also been found to improve autophagy and the function of β cells in diabetic models, but the exact mechanism remains unknown. Further studies are needed to reveal the role of autophagic dysfunction in diabetes. In addition, autophagy is related to tumours, ageing and cardiovascular diseases. Although new mechanisms are constantly being reported, there are still many issues to be clarified regarding the function and mechanism of autophagy-related genes. Further research on autophagic mechanisms is not only of profound theoretical significance but also of great practical value.
Conflict of Interests
The authors declare no conflicts of interest.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant number 81370885), Research Institute performance incentive and guidance project of Chongqing Science and Technology Bureau (grant number cstc2019jxjl130006), General program of Natural Science Foundation of Chongqing Science and Technology Bureau (grant number cstc2019jcyj-msxm0827), 2019 Merck Diabetes Research Fund (grant number g-x-2019-056).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Saeedi P., Petersohn I., Salpea P. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.National Diabetes Research Group A mass survey of diabetes mellitus in a population of 300,000 in 14 provinces and municipalities in China. Zhonghua Nei Ke Za Zhi. 1981;20(11):678–683. [PubMed] [Google Scholar]
- 3.Pan X.R., Yang W.Y., Li G.W., Liu J. National diabetes prevention and control cooperative group. Prevalence of diabetes and its risk factors in China,1994. Diabetes Care. 1997;20(11):1664–1669. doi: 10.2337/diacare.20.11.1664. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y., Wang L., He J. 2010 China noncommunicable disease surveillance group. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 5.Yin J., Kong P.S., Chan C.N. Prevention and care programs addressing the growing prevalence of diabetes in China. Curr Diabetes Rep. 2016;16(12) doi: 10.1007/s11892-016-0821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan J.C., Lau E.S., Luk A.O. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am J Med. 2014;127(7):616–624. doi: 10.1016/j.amjmed.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Watada H., Fujitani Y. Minireview: autophagy in pancreatic β-cells and its implication in diabetes. Mol Endocrinol. 2015;29(3):338–348. doi: 10.1210/me.2014-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Li Y.B., Yin J.J. Autophagy regulates inflammation following oxidative injury in diabetes. Autophagy. 2013;9(3):272–277. doi: 10.4161/auto.23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Bailo B., El-Sohemy A., Haddad P.S. Vitamins D, C, and E in the prevention of type 2 diabetes mellitus: modulation of inflammation and oxidative stress. Biologics. 2011;5:7–19. doi: 10.2147/BTT.S14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine B. Cell biology: autophagy and cancer. Nature. 2007;446(7137):745–747. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa I., Amano A., Mizushima N. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306(5698):1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- 12.Zare-shahabadi A., Masliah E., Johnson Gail V.W. Autophagy in Alzheimer's disease. Rev Neurosci. 2015;26(4):385–395. doi: 10.1515/revneuro-2014-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuma A., Hatano M., Matsui M. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 14.Ebato C., Uchida T., Arakawa M. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metabol. 2008;8(4):325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z.F., Li Y.B., Han J.Y. Liraglutide prevents high glucose level induced insulinoma cells apoptosis by targeting autophagy. Chin Med J. 2013;126(5):937–941. [PubMed] [Google Scholar]
- 16.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizushima N., Levine B., Cuervo A.M. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroemer G., Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9(12):1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi S., Liang Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochim Biophys Acta. 2015;1852(2):252–261. doi: 10.1016/j.bbadis.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Kabeya Y., Mizushima N., Yamamoto A. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117(Pt 13):2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Kim J., Kundu M., Viollet B. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He C., Zhu H., Li H. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62(4):1270–1281. doi: 10.2337/db12-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J., Kim Y.C., Fang C. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152(1–2):290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinsztein D.C., Gestwicki J.E., Murphy L.O. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6(4):304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 26.Riahi Y., Wikstrom J.D., Bachar-Wikstrom E. Autophagy is a major regulator of beta cell insulin homeostasis. Diabetologia. 2016;59(7):1480–1491. doi: 10.1007/s00125-016-3868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goginashvili A., Zhang Z., Erbs E. Insulin granules. Insulin secretory granules control autophagy in pancreatic β cells. Science. 2015;347(6224):878–882. doi: 10.1126/science.aaa2628. [DOI] [PubMed] [Google Scholar]
- 28.Masini M., Bugliani M., Lupi R. Autophagy in human type 2 diabetes pancreatic β cells. Diabetologia. 2009;52(6):1083–1086. doi: 10.1007/s00125-009-1347-2. [DOI] [PubMed] [Google Scholar]
- 29.Abe H., Uchida T., Hara A. Exendin-4 improves β-cell function in autophagy- deficient β-cells. Endocrinology. 2013;154(12):4512–4524. doi: 10.1210/en.2013-1578. [DOI] [PubMed] [Google Scholar]
- 30.Mizukami H., Takahashi K., Inaba W. Involvement of oxidative stress-induced DNA damage, endoplasmic reticulum stress, and autophagy deficits in the decline of β-cell mass in Japanese type 2 diabetic patients. Diabetes Care. 2014;37(7):1966–1974. doi: 10.2337/dc13-2018. [DOI] [PubMed] [Google Scholar]
- 31.Quan W., Hur K.Y., Lim Y. Autophagy deficiency in β cells leads to compromised unfolded protein response and progression from obesity to diabetes in mouse. Diabetologia. 2012;55(2):392–403. doi: 10.1007/s00125-011-2350-y. [DOI] [PubMed] [Google Scholar]
- 32.Li X., Zhang L., Meshinchi S. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes. 2006;55(11):2965–2973. doi: 10.2337/db06-0733. [DOI] [PubMed] [Google Scholar]
- 33.Slutsky N., Vatarescu M., Haim Y. Decreased adiponectin links elevated adipose tissue autophagy with adipocyte endocrine dysfunction in obesity. Int J Obes. 2016;40(6):912–920. doi: 10.1038/ijo.2016.5. [DOI] [PubMed] [Google Scholar]
- 34.Gan L., Liu Z., Luo D. Reduced endoplasmic reticulum stress-mediated autophagy is required for leptin alleviating inflammation in adipose tissue. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.01507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein N., Haim Y., Mattar P. Leptin stimulates autophagy/lysosome-related degradation of long-lived proteins in adipocytes. Adipocyte. 2019;8(1):51–60. doi: 10.1080/21623945.2019.1569447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachar-Wikstrom E., Wikstrom J.D., Ariav Y. Stimulation of autophagy improves endoplasmic reticulum stress-induced diabetes. Diabetes. 2013;62(4):1227–1237. doi: 10.2337/db12-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson R.P., Harmon J.S. Diabetes, glucose toxicity, and oxidative stress: a case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med. 2006;41(2):177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 38.Butler A.E., Janson J., Bonner-Weir S. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 39.Kim K.H., Lee M.S. Autophagy-a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014;10(6):322–337. doi: 10.1038/nrendo.2014.35. [DOI] [PubMed] [Google Scholar]
- 40.Rivera J.F., Costes S., Gurlo T., Glabe C.G., Butler P.C. Autophagy defends pancreatic β cells from human islet amyloid polypeptide-induced toxicity. J Clin Invest. 2014;124(8):3489–3500. doi: 10.1172/JCI71981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y.S., Silwal P., Kim S.Y. Autophagy-activating strategies to promote innate defense against mycobacteria. Exp Mol Med. 2019;51(12) doi: 10.1038/s12276-019-0290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maechler P., Wollheim C.B. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001;414(6865):807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- 43.Bhansali S., Bhansali A., Walia R. Alterations in mitochondrial oxidative stress and mitophagy in subjects with prediabetes and Type 2 diabetes mellitus. Front Endocrinol. 2017;8 doi: 10.3389/fendo.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Twig G., Elorza A., Molina A.J. Fission and selective fusion govern mitochondria segregation and elimination by autophagy. EMBO (Eur Mol Biol Organ) J. 2008;27(2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding W.X., Yin X.M. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem. 2012;393(7):547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shanbhag R., Shi G., Rujiviphat J. The emerging role of proteolysis in mitochondrial quality control and the etiology of Parkinson's disease. Parkinsons Dis. 2012;2012 doi: 10.1155/2012/382175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ScherzShouval R., Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17(9):422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Hoshino A., Ariyoshi M., Okawa Y. Inhibition of p53 preserves Parkin-mediated mitophagy and pancreatic b-cell function in diabetes. Proc Natl Acad Sci USA. 2014;111(8):3116–3121. doi: 10.1073/pnas.1318951111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin H.S., Kim J., Lee S.J. The PARK2 gene is involved in the maintenance of pancreatic beta-cell functions related to insulin production and secretion. Mol Cell Endocrinol. 2014;382(1):178–189. doi: 10.1016/j.mce.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 50.Soleimanpour S.A., Ferrari A.M., Raum J.C. Diabetes susceptibility genes Pdx1 and Clec16a function in a pathway regulating mitophagy in beta-cells. Diabetes. 2015;64(10):3475–3484. doi: 10.2337/db15-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qu Y., Sun L., Yang Z. Variation in the PTEN-induced putative kinase 1 gene associated with the increase risk of type 2 diabetes in northern Chinese. J Genet. 2011;90(1):125–128. doi: 10.1007/s12041-011-0020-y. [DOI] [PubMed] [Google Scholar]
- 52.Halban P.A., Wollheim C.B. Intracellular degradation of insulin stores by rat pancreatic islets in vitro. An alternative pathway for homeostasis of pancreatic insulin content. J Biol Chem. 1980;255(13):6003–6006. [PubMed] [Google Scholar]
- 53.Schnell A.H., Swenne I., Borg L.A. Lysosomes and pancreatic islet function. A quantitative estimation of crinophagy in the mouse pancreatic B-cell. Cell Tissue Res. 1988;252(1):9–15. doi: 10.1007/BF00213820. [DOI] [PubMed] [Google Scholar]
- 54.Orci L., Ravazzola M., Amherdt M. Insulin, not Cpeptide (proinsulin), is present in crinophagic bodies of the pancreatic B-cell. J Cell Biol. 1984;98(1):222–228. doi: 10.1083/jcb.98.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levine B., Klionsky D.J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004 Apr;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 56.Shintani T., Klionsky D.J. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):910–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishino I., Fu J., Tanji K. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406(6798):906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 58.Eskelinen E.L., Illert A.L., Tanaka Y. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol Biol Cell. 2002;13(9):3355–3368. doi: 10.1091/mbc.E02-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhodes C.J. Type-2 diabetes-a matter of b-cell life and death? Science. 2005;307(5708):380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 60.Kahn S.E. Clinical review 135: the importance of betacell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86(9):4047–4058. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- 61.Marsh B.J., Soden C. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Mol Endocrinol. 2007 Sep;21(9):2255–2269. doi: 10.1210/me.2007-0077. [DOI] [PubMed] [Google Scholar]
- 62.Marsh B.J., Mastronarde D.N., Howell K.E. 3D structure studies in pancreatic beta cells by high resolution cellular tomography. Microsc Microanal. 2003;9(Suppl. 2):1156–1159. [Google Scholar]
- 63.Yaekura K., Julyan R., Wicksteed B.L. Insulin secretory deficiency and glucose intolerance in Rab3A null mouse. J Biol Chem. 2003;278(11):9715–9721. doi: 10.1074/jbc.M211352200. [DOI] [PubMed] [Google Scholar]
- 64.Marsh B.J., Soden C., Alarcón C. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine-cells. Mol Endocrinol. 2007;21(9):2255–2269. doi: 10.1210/me.2007-0077. [DOI] [PubMed] [Google Scholar]
- 65.Cheng C.W., Villani V., Buono R. Fasting-mimicking diet promotes Ngn3-driven b-cell regeneration to reverse diabetes. Cell. 2017;168(5):775–788. doi: 10.1016/j.cell.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu H., Javaheri A., Godar R.J. Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagylysosome pathway. Autophagy. 2017;13(11):1952–1968. doi: 10.1080/15548627.2017.1368596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei S., Han R., Zhao J. Intermittent administration of a fastingmimicking diet intervenes in diabetes progression, restores β cells and reconstructs gut microbiota in mice. Nutr Metab. 2018;15 doi: 10.1186/s12986-018-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang Y., Huang W., Wang J. Metformin plays a dual role in MIN6 pancreatic b cell function through AMPK-dependent autophagy. Int J Biol Sci. 2014;10(3):268–277. doi: 10.7150/ijbs.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu J., Wu J.J., Yang L.J. Rosiglitazone protects against palmitate-induced pancreatic beta-cell death by activation of autophagy via 50-AMP-activated protein kinase modulation. Endocrine. 2013;44(1):87–98. doi: 10.1007/s12020-012-9826-5. [DOI] [PubMed] [Google Scholar]
- 70.Janzen K.M., Steuber T.D., Nisly S.A. GLP-1 agonists in type 1 diabetes mellitus. Ann Pharmacother. 2016;50(8):656–665. doi: 10.1177/1060028016651279. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka S., Sugiura Y., Saito H. Sodium-glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int. 2018;94(5):912–925. doi: 10.1016/j.kint.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 72.Wang L.J., Chen P.R., Hsu L.P. Concomitant induction of apoptosis and autophagy by prostate apoptosis response-4 in hypopharyngeal carcinoma cells. Am J Pathol. 2014;184(2):418–430. doi: 10.1016/j.ajpath.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 73.Thayyullathil F., Rahman A., Pallichankandy S. ROS-dependent prostate apoptosis response-4 (Par-4) up-regulation and ceramide generation are the prime signaling events associated with curcumin-induced autophagic cell death in human malignant glioma. FEBS Open Bio. 2014;4:763–776. doi: 10.1016/j.fob.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Notaro A., Sabella S., Pellerito O. Involvement of PAR-4 in cannabinoid-dependent sensitization of osteosarcoma cells to TRAIL-induced apoptosis. Int J Biol Sci. 2014;10(5):466–478. doi: 10.7150/ijbs.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rah B., Rasool R., Nayak D. PAWR-mediated suppression of BCL2 promotes switching of 3-azido withaferin A (3-AWA)-induced autophagy to apoptosis in prostate cancer cells. Autophagy. 2015;11(2):314–331. doi: 10.1080/15548627.2015.1017182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma S., Kaul D., Arora M. Oncogenic nature of a novel mutant AATF and its interactome existing within human cancer cells. Cell Biol Int. 2015;39(3):326–333. doi: 10.1002/cbin.10379. [DOI] [PubMed] [Google Scholar]
- 77.Shin H.J., Kim H., Oh S. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature. 2016;534(7608):553–557. doi: 10.1038/nature18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu C., Wu Q.N., Lei X.T. TERT and Akt are involved in the Par-4-dependent apoptosis of islet ß cells in type 2 diabetes. J Diabetes Res. 2018;2018 doi: 10.1155/2018/7653904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ali M., Devkota S., Roh J.I. Telomerase reverse transcriptase induces basal and amino acid starvation-induced autophagy through mTORC1. Biochem Biophys Res Commun. 2016;478(3):1198–1204. doi: 10.1016/j.bbrc.2016.08.094. [DOI] [PubMed] [Google Scholar]
- 80.Liu Q., Sun Y., Lv Y. TERT alleviates irradiation-induced late rectal injury by reducing hypoxia-induced ROS levels through the activation of NF-κB and autophagy. Int J Mol Med. 2016;38(3):785–793. doi: 10.3892/ijmm.2016.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pospelova T.V., Bykova T.V., Zubova S.G. Rapamycin induces pluripotent genes associated with avoidance of replicative senescence. Cell Cycle. 2013;12(24):3841–3851. doi: 10.4161/cc.27396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng H., Fan X., Lawson W.E. Telomerase deficiency delays renal recovery in mice after ischemia-reperfusion injury by impairing autophagy. Kidney Int. 2015;88(1):85–94. doi: 10.1038/ki.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao T., Hu F., Liu X. Blockade of telomerase reverse transcriptase enhances chemosensitivity in head and neck cancers through inhibition of AKT/ERK signaling pathways. Oncotarget. 2015;6(34):35908–35921. doi: 10.18632/oncotarget.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song B.Q., Chi Y., Li X. Inhibition of Notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell Physiol Biochem. 2015;36(5):1991–2002. doi: 10.1159/000430167. [DOI] [PubMed] [Google Scholar]
- 85.Dall'Armi C., Devereaux K.A., Di Paolo G. The role of lipids in the control of autophagy. Curr Biol. 2013;23(1):R33–R45. doi: 10.1016/j.cub.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]