Abstract
Cancer is one of the diseases with high morbidity and mortality on a global scale. Chemotherapy remains the primary treatment option for most cancer patients, including patients with progressive, metastatic, and recurrent diseases. To date, hundreds of chemotherapy drugs are used to treat various cancers, however, the anti-cancer efficacy and outcomes are largely hampered by chemotherapy-associated toxicity and acquired therapeutic resistance. The natural product (NP) oridonin has been extensively studied for its anti-cancer efficacy. More recently, oridonin has been shown to overcome drug resistance through multiple mechanisms, with yet-to-be-defined bona fide targets. Hundreds of oridonin derivative analogs (oridonalogs) have been synthesized and screened for improved potency, bioavailability, and other drug properties. Particularly, many of these oridonalogs have been tested against oridonin for tumor growth inhibition, potential for overcoming therapeutic resistance, and immunity modulation. This concise review seeks to summarize the advances in this field in light of identifying clinical-trial level drug candidates with the promise for treating progressive cancers and reversing chemoresistance.
Keywords: Cancer therapy, Chemoresistance, Derivatives, Drug resistance, Oridonalogs, Oridonin
Introduction
Cancer has been a major health threat to human beings worldwide. According to the International Agency for Research on Cancer (IARC), there were 17 million new cancer cases diagnosed and 9.5 million cancer-associated deaths reported in 2018. Unfortunately, with a growing, aging population in most countries, the cancer incidence and mortality have been sustainably increasing over the past decades and are predicted to remain high in future years. To date, surgery, chemotherapy, and radiotherapy are among the major therapeutic options for cancer patients, with addition of the more recent targeted therapy and immunotherapy, which may benefit selective patient subpopulations. Chemotherapy has always been the mainstream treatment for the majority of cancer patients with the exception of some early-staged patients. With the prevalent use of chemotherapies, the drug-associated toxicities and adverse side effects became serious clinical concerns, particularly with acquired drug resistance, or chemoresistance, developed during and after chemotherapy administration, as the most significant contributor to such challenges. Chemoresistance seriously hampers the application of cancer drugs, in general, to achieve optimal and satisfactory anti-cancer efficacy in patients. Efforts to reduce toxicity, overcome resistance of chemotherapy, and enhance the efficiency of anti-cancer drugs are the vigorous focus of research via elucidation of the underlying mechanisms and development of agents capable of overcoming chemoresistance.
Natural products (NPs) have provided a rich source in developing a plethora of cancer drugs in use, and the NPs possessing low toxicity can be ideal candidates in the identification of initial chemical entities in the development of cancer drugs capable of overcoming chemoresistance. Design based on the structure of those NP entities and their structural modulation can target individual or multiple pathways critical for developing chemoresistance. In this review, we focus on one of these NPs, oridonin,1 and its derivative compounds developed in recently years by our team and other laboratories. While oridonin possesses moderate to potent anti-cancer efficacy, its potential clinical use has been limited due to its low aqueous solubility and bioavailability. To improve its overall drug-like properties, many oridonin analogs (oridonalogs) have been developed by modifying the A, B, and D rings of oridonin to achieve higher anti-cancer efficacy, lower toxicity, and the ability to overcome de novo and acquired chemoresistance.2,3 In fact, many of such oridonalogs have shown favorable capacity in suppression of tumor growth and progression, overcome of therapeutic resistance, and blockade of metastasis and recurrence.
Oridonin and its use for treating human diseases
Oridonin (C20H28O6), a kaurene-type diterpenoid isolated from Rabdosia rubescens, is called “Donglingcao” in Chinese or “Hara” in Japanese.4,5 Its chemical structure and antitumor effect were characterized by Fujita E. et al in 1976 (Table 1).1 Since its first report as a potential anticancer agent, accumulating evidence demonstrates that oridonin can modulate multiple biological functions by acting as anti-inflammatory,6 antibacterial,7 anti-fibrotic,8,9 auto-immune-regulatory, and neuro-regulatory agent in diverse diseases.10
Table 1.
Approaches in optimizing oridonin.
| Modified Structure/Name of Compounds | Chemical Structures | Targeting Pathways | Models Tested | Ref |
|---|---|---|---|---|
| Oridonin | 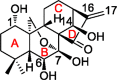 |
1 | ||
| 1-O- and 14-O-derivatives | 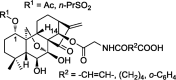 |
– | BGC-7901, SW-480, HL-60, BEL-7402, A549, B16 | 50 |
| Ent-6,7-seco-oridonin derivatives | – | A549, Bel-7402, K562, MGC-803, CaEs-17 | 52 | |
| 13p |  |
Mitochondrial pathway | MCF-7 | 53 |
| DS2 |  |
MMP, ROS | EC9706, EC109, HEECs, HL-7702 | 54 |
| Geridonin | 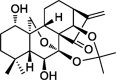 |
ROS-mediated PTEN, PI3K/Akt pathway | MGC 803 | 55,56 |
| Furoxan-based nitric oxide-releasing derivative | 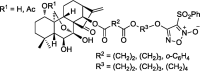 |
– | Bel-7402, K562, MGC-803, CaEs-17 | 57 |
| Spirolactone-type diterpenoid derivatives | – | Bel-7402, K562, MGC-803, CaEs-17 | 58 | |
| Enmein-type diterpenoid analogs |  |
Mitochondria-related caspase-dependent pathway | Bel-7402, K562, MGC-803, CaEs-17 | 59 |
| H2S releasing ent-kaurane diterpenoid oridonin derivatives | 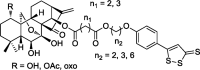 |
Extrinsic and intrinsic apoptosis pathways | HepG2, MCF-7, HCT-116, B16, K562, L-02, PBMC |
60 |
| Nitric oxide (NO)-releasing oridonin derivatives |  |
Apoptosis and cell cycle arrest at S phase | Bel-7402 | 63 |
| Seven-membered C-ring-expanded 6,7-seco-ent-kaurenes | 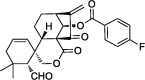 |
Apoptosis and cell cycle arrest | MCF-7 | 64 |
| Enmein-type diterpenoid amino acid ester derivatives | 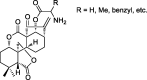 |
Intrinsic apoptosis pathway | Bel-7402, SGC-7901, HL-60, PC-3, A549, K562, L-02 | 66 |
| Ent-kaurane and spirolactone-type 6,7-seco-ent-kaurane derivatives | 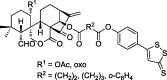 |
Intrinsic apoptosis pathway | K562, Bel-7402, SGC-7901, A549, L-02, PBMC |
67 |
| A-ring nitrogen-enriched modifications |  |
Mitochondria-dependent pathway; antifibrosis through NF-κB pathway; STAT3 |
MDA-MB-232, MCF-7, MCF-7/ADR, HMEC, LX-2, HSC-T6, A2780, OVCAR3, OVCAR8, SKOV3 |
68-70, 78, 79 |
| CYD-6-17 (CYD0617) |  |
Wnt/β-catenin pathway | T24-P, UMUC3 | 71 |
| AKT/PDPK1 | 786-0 KD, HK-2 KD resistant to mTOR and tyrosine kinase inhibitors | 72 | ||
| D-ring modifications |  |
NRF-2/RHOA/ROCK signaling pathway | MDA-MB-231, GI101, GILM2, GILM3 | 74 |
| Oridonin A-ring-based diverse constructions of enone functionality | 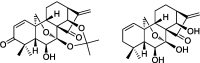 |
p53-dependent apoptosis | LX-2 cells | 73, 76 |
| α-formylenone in the A-ring and introduction of an acetonide moiety to 7,14-dihydroxyl |  |
S-phase cell cycle arrest, apoptosis | LX-2 cells | 77 |
| HAO472, prodrug with an amino acid residue | 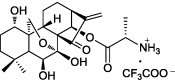 |
Suppressing NF-κB pathway | mouse colitis model | 5 |
| Benzene analogues at C17 position | 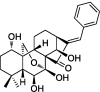 |
– | AGS, MGC803, Bel7402, HCT116, A549, HeLa cells | 81 |
| 14-substituted oridonin analogs | 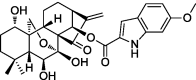 |
p53-MDM2 pathway | HCT116, BEL7402, MCF-7 | 82 |
As a traditional Chinese herb, Rabdosia rubescens was firstly used as anti-inflammation herb for sore throat and tonsillitis and has been included in the Chinese Pharmacopoeia since 1977.11,12 The leaves of Rabdosia rubescens are still used for tea in China for their antibacterial function.13 It was reported that the anti-inflammatory effect of oridonin is primarily associated with suppressing the nuclear factor-kappa B (NF-κB) signaling pathway, reducing secretion of serum cytokines including interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), and inhibiting expression and function of toll-like receptors 4 (TLR4) as well as the p38-mitogen activated protein kinase (p38-MAPK) in endometritis,14 diabetic nephropathy,15 vascular inflammation,16 acute lung injury,17 liver injury,18 inflammatory bowel disease,5 and sepsis.19 Recently, oridonin was reported to inhibit NLRP3 which is a key component in the NLPR3 inflammasome by targeting the Cys279 residue of NLRP3 in the NACHT domain.6
Oridonin was also found to suppress Th1/Th17 cells and the proliferation of CD4+ T cells by inhibiting the NF-κB signaling pathway in a mouse Crohn's disease model.20 There is increasing evidence that oridonin modulates the immune system by yet undefined mechanisms. Oridonin was reported to increase the weights of spleen and bursa, as well as the number of proliferating peripheral blood T and B lymphocytes and serum cytokines, such as IL-2, IL-4, and TNF-α in broiler chickens.21 Furthermore, in broiler chickens, oridonin improves immune functions against infection of Salmonella pullorum, by decreasing splenic inflammatory cytokines, through down-regulating the B lymphocyte stimulator (BLyS) which is a TNF family member. BLyS can irritate B cell proliferation and survival in vitro and adjust B cell homeostasis in vivo to regulate the TH1/Th2 balance.22 Oridonin also promotes CD4+/CD5+ regulatory T cell differentiation, and modulates the TH1/Th2 balance, by inducing HO-1, an anti-inflammatory target.23 In an asthma mouse model, oridonin was used to treat asthma by regulating the Th1/Th2 balance.24 Oridonin inhibits the proliferation of fibroblast-like synoviocytes and induces mitochondria-dependent apoptosis in rheumatoid arthritis, a chronic inflammatory autoimmune disease.25 In vitro and in vivo, oridonin modulates immunity via down-regulation of B-cell activating factor and B-cell maturation and differentiation in systemic lupus erythematosus.26 The aforementioned research provides evidence that oridonin modulates immunity and may therefore impact its own ability to suppress cancer growth and progression, and may also facilitate other immunity-targeting drugs in additive or synergistic manner.
Furthermore, oridonin was reported to possess anti-osteoporotic ability, by inhibiting the NF-κB pathway and p65 nuclear translocation in degenerative diseases.27 Through inhibiting NF-κB signaling, oridonin was used for treating Alzheimer's disease to prevent synaptic loss, by modulating β-amyloid in vitro and in vivo.28, 29, 30, 31 Oridonin was reported to be potentially useful in cardiovascular disease and liver fibrosis. Also, oridonin induces mitochondria-dependent apoptosis of pulmonary artery smooth muscle cells (PASMC) to reduce pulmonary artery pressure, and inhibits pulmonary artery structural remodeling in pulmonary arterial hypertension (PAH).32 It was reported that oridonin induces apoptosis of hepatic stellate cells (HSCs), which promotes liver fibrosis, through decreasing intracellular glutathione (GSH) and the production of the reactive oxygen species (ROS) in rats.8 Other than inducing apoptosis, oridonin inhibits the proliferation of HSCs time- and dose-dependently, arrests S-phase cell cycle progression, and reduces expression of extracellular matrix (ECM) proteins.9 Collectively, these research findings provide rich evidence that oridonin can treat inflammatory and immune diseases by targeting a number of pathways and genes, such as BLyS, HO-1, and NF-κB, that are important in inflammation and immune system function. This may be achieved through maintaining Th cell balance and cytokine secretion.
Oridonin as cancer therapy
Despite the evidence supporting oridonin's therapeutic efficacy with many human diseases, a major focus for its potential clinical use is for cancer therapy. Using the term “oridonin”, we searched the PubMed.gov database and found 468 research articles published as of January, 2020, while searching “oridonin AND cancer” generated 341 articles. There were limited studies of oridonin for cancer therapy in the 1990s, only the cytostatic effects of oridonin in leukemia L1210 cells and gastric cancer cells were reported.33,34 Since 2004, there has been an increasing number of publications demonstrating oridonin's anti-proliferative effects via induction of apoptosis in lung cancer cells,35 HeLa cells,36 and melanoma cells,37 via modulation of apoptotic signaling including the caspase family, Bcl-2/Bax family, and the mitogen-activated protein kinase (MAPK) pathway.37, 38, 39 In addition, oridonin stimulates phagocytosis of apoptotic tumor cells through regulation of macrophage functioning that involves TNFα and IL-1β.40 Furthermore, oridonin induces apoptosis of breast cancer cells by enhancing autophagy.41 Oridonin was also shown to arrest G2/M cell cycle progression in cancer cells leading to apoptosis and inhibition of cancer progression.42 ROS is the initial and pivotal step in inducing apoptosis or autophagy with oridonin treatment, involving p53 and MAPK pathways,43 SIRT1,44 and NF-κB.45 A recent study showed that oridonin suppresses epithelial-mesenchymal transition (EMT) through TGF-β1/Smad2/3 signaling pathway in osteosarcoma 143B cells,46 while in pancreatic cancer SW1990 cells this effect is achieved through Wnt/β-catenin pathway.47 Additionally, oridonin induces autophagy in colorectal cancer cells via inhibition of glucose metabolism.48 These studies demonstrate that oridonin processes impressive anti-cancer activity in many cancer types through modulation of cell cycle progression and induction of phagocytosis and autophagy, eventually leading to apoptosis.
It is believed that its intrinsic chemical structure endows oridonin's multiple efficacy against inflammation and cancers, by inhibition of cellular proliferation and induction of apoptosis. Although Rabdosia rubescens has been used as a natural anti-bacterial/inflammatory therapy for a long time in China and Asia, its major active component, oridonin, has a low oral bioavailability, merely less than 5%, due to its low water-solubility.49 This property adversely impacted its potential clinical implications. To date, oridonin is only used as an over-the-counter herbal medicine for anti-inflammation in China.12 There are no drugs based on oridonin approved by U.S. Food and Drug Administration (FDA) for clinical use for cancer medication or other diseases.
Oridonin derivative compounds as potential cancer therapies
Optimization of oridonin and new oridonin-derived oridonalogs
With its discovered and verified anti-tumor effects, a number of laboratories attempted to modify the structure of oridonin in order to achieve improved efficacy and drug properties, such as better bioavailability, higher potency, and less toxicity, as summarized in Table 1. Xu J. et al in 2008, synthesized 1-O- and 14-O-derivative compounds from oridonin and found that these compounds were more effective than oridonin itself in inhibiting BGC-7901, SW-480, HL-60, BEL-7402, A549, and B16 cancer cell lines.50 In 2011, Liu H. et al reported three oridonin derivative compounds complexing with β-cyclodextrin (βCD), a commonly used drug delivery compound, to increase drug solubility51; however no evaluation of their biological activity was reported. Later, Ent-6,7-seco-oridonin derivatives were synthesized in 2012 to enhance the activity of oridonin.52 It was reported that 13-p, an A-ring-modified analogue, showed 200-fold higher efficacy than oridonin, in breast cancer cell line MCF-7, by targeting the mitochondrial pathway.53 Another oridonin analog, DS2, was reported to induce apoptosis in human esophageal cancer cells, through modulating the mitochondrial membrane potential (MMP) and ROS.54 More recently, geridonin, an oridonin-based analog was reported to inhibit gastric cancer cells, alone or in combination with paclitaxel, via up-regulating PTEN and down-regulating the PI3K/Akt signaling pathway.55,56 Li D. et al used oridonin as a nitric oxide donor and designed furoxan-based nitric oxide-releasing (NO-releasing) derivatives, and also designed spirolactone-type and enmein-type diterpenoid derivative compounds. These derivatives demonstrated more powerful anti-cancer effects than oridonin.57, 58, 59 Hydrogensulfide (H2S) was also used to improve the bioavailability of oridonin, instead of the NO-releasing gasotransmitter. Recently, H2S-releasing ent-kaurane diterpenoid oridonin derivatives were designed.60 Most of these derivatives were reported to induce apoptosis through a mitochondrial-dependent pathway in cancer cells, a same mechanism of action in antibacterial effect.13,53,61,62 Other oridonin-based derivatives were reported in which adiazen-1-ium-1,2-diolate nitric oxide donor moiety was added and were demonstrated to arrest S phase cell cycle progression in hepatoma Bel-7402 cells.63 In 2017, spiro-lactone-type ent-kaurene derivatives were reported to be able to induce apoptosis and arrest cell cycle progression in MCF-7 human breast cancer cells.64 Xu S. et al also reported a modification with the trans-cinnamic acid moiety on the oridonin structure, which rendered the derivatives stronger anti-mycobacterial activity.65 Recently, a series of enmein-type diterpenoid amino acid ester derivatives were developed and tested in multiple human cancer cell lines. Among them, the compound 19 was found to act against proliferation by inducing apoptosis and cell cycle arrest through a mitochondria-dependent pathway.66 Similarly, another two ent-kaurane derivatives, linking different H2S donors, also induced mitochondria-dependent apoptosis.67 These studies demonstrate that there are aroused interest in the synthesis and identification of potent oridonalogs as potential cancer therapies (Table 1).
In 2013, our team systematically modified the A-ring of oridonin and developed a series of new oridonalogs as potential therapies for cancer and other human diseases. We incorporated a nitrogen moiety into the core scaffold of oridonin to improve solubility. Most of the nitrogen-enriched oridonalogs not only showed improved aqueous solubility, but also enhanced ability of inducing apoptosis in the triple-negative breast cancer cell line, MDA-MB-231, and other cancer cell lines, as well as tumors in mice. An important discovery was that our compounds, such as CYD0618, were able to overcome resistance in drug-resistant MCF-7 cells,68, 69, 70 consistent with the reported anti-chemoresistance effect of oridonin. Another of our compounds, CYD0617, also showed inhibition on transition cell carcinoma (TCC) cells by targeting the Wnt/β-catenin pathway in vitro and in vivo.71 CYD0617 also regulates AKT by targeting 3-phosphoinositide-dependent protein kinase 1 (PDPK1) in drug-resistant renal cell carcinoma in vitro and in vivo.72 Using an additional α, β-unsaturated ketone system to modify the A-ring structure, we developed a new generation of oridonin-based compounds, which could more efficiently induce apoptosis in highly aggressive breast cancer cells.73 Another D-ring modification oridonalog, YD0514, was discovered through aziridination and mediated covalent warheads, was shown to inhibit breast cancer metastasis to the lungs by targeting the nuclear factor erythroid 2-related factor 2 (Nrf2)/RHOA/ROCK signaling pathway.74,75 Oridonalog, CYD0682, was developed through oridonin ring A-based diverse constructions of enone functionality, which acquired a more anti-fibrogenic effect than oridonin in the LX-2 human hepatic stellate cell line.76 The anti-fibrogenic effects have also been observed in oridonalog CYD0692, which has an additional α-formylenone in the A-ring and an acetonide moiety attached to 7,14-dihydroxyl.77 Recently, CYD0618 was reported to inhibit fibrosis in hepatic stellate cells through suppression of the NF-κB pathway.78 CYD0618 was also reported to target STAT3 signaling in ovarian cancer.79 A water-soluble oridonin prodrug, HAO472, was recently reported in a Phase I clinical trial for treating acute myelogenous leukemia, as reviewed.3 HAO472 has been demonstrated to modulate inflammation by suppressing NF-κB signaling in a mouse colitis model at 5.0–7.5 mg/kg doses.5 An oridonin phosphate was recently reported to be able to induce apoptosis by up-regulating autophagy in breast cancer cells.80 Shen Q. et al synthesized benzene analogs with modifications at the C17 position of the oridonin structure and the derivatives showed inhibition of proliferating cancer cells.81 Substitution on the 14-OH position of oridonin structure also rendered a new derivative compound to induce apoptosis and arrest cell cycle progression by suppressing p53-MDM2 signaling pathway.82 Together, vigorous efforts have been made in this field in the modification of different ring structures of oridonin to generate new compounds for better efficacy and drug properties (Table 1). Some of those have already shown promising profiles for further development into potential drug candidates for treatment of cancer and other diseases.
Oridonin and its derivative compounds for treating cancers
To date, hundreds of oridonin derivatives have been developed to improve solubility and enhance bioavailability of oridonin. The majority has shown improved efficacy against proliferation and fibrosis compared to oridonin. Targeting the intrinsic apoptosis pathway primarily contributes to the anti-cancer effect of oridonin and its derivative compounds. Here, we summarized the major modification strategies used in optimizing oridonin, chemical structures of oridonin and its derivative compounds, targeting pathways, and the model systems for testing the compounds (Table 1).
Oridonin and its derivative compounds tested in combination with other chemotherapy drugs
Chemotherapy has been the most important therapeutic option for treating cancers, particularly, for hematologic malignancies and certain cancer types reaching unresectable or advanced stage of disease. No matter how the chemotherapeutic drugs are used as adjuvant, cytotoxic, or palliative therapies, adverse side effects are unavoidable in chemotherapy, and drug resistance develops during and/or after regimens, becoming a serious barrier to achieve expected therapeutic outcomes and long-term survival. Thus, combination therapy with natural or non-/low-toxic herbs to enhance cytotoxicity or reverse drug resistance arouses continuous interest in this regard. There is a plethora of reports showing oridonin's anti-tumor activities via promotion of ROS, induction of apoptosis and autophagy, inhibition of EMT, and modulation of cell cycle progression in various cancer cells. Oridonin has been investigated in the experimental settings for treatment in combination with other chemotherapeutic agents for synergistic anticancer activity and overcoming drug resistance or chemoresistance.
As summarized and reviewed above, oridonin plays an important role in inducing apoptosis, which would be a significant contribution in combination therapy. Either the classical apoptosis pathway, such as the caspase-depended pathway or the mitogen-activated protein kinase (MAPK) pathway, which includes ERK, c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) and p38 kinases,83,84 is involved in inducing cell death by oridonin. Oridonin was shown to enhance the effects of gemcitabine in pancreatic cancer via mitochondrial caspase-dependent signaling pathway.85 Oridonin synergizes with JQ1, a bromodomain and extra-terminal domain (BET) inhibitor, to enhance the sensitivity of JQ1 in hepatocellular carcinoma (HCC) cells by inducing apoptosis involving NF-κB, Bcl-2, and other pro-apoptotic factors.86 Synergizing with oridonin and other chemotherapeutic agents has been widely tested in experimental hematological malignancies. Intriguingly, in a recent study with the combination of oridonin and valproic acid (VPA) in treating HL-60 leukemia cells, caspase-dependent apoptosis pathways contributed to cell death.87 Homoharringtonine (HHT) induces apoptosis through targeting c-KIT protein which is a member of type III tyrosine kinase subclass in t (8; 21) acute myeloid leukemia (AML), one of the most common AML subtypes.88 The combination of oridonin with HHT, showed synergistic inhibition in the same cell line through inducing MMP loss and enhancing HHT-induced c-KIT downregulation. Interestingly, HHT also increased intracellular oridonin level and enhanced its influx by inhibiting ATP-binding cassette (ABC)-mediated efflux of oridonin. In vivo, the combination therapy prolonged survival of mice bearing t (8; 21) AML.89 RUNX1-ETO (RE) fusion protein is also a plausible target for t (8; 21) AML in association with ERK2 (MAPK1) kinase. Oridonin and ERK2 inhibitors synergistically induced apoptosis in RE-positive cells, but not RE-negative cells,90 and the synergistic effect of oridonin depends on RE expression. In other hematological malignancies like non-germinal center B cell-like subtype of diffuse large B cell lymphoma (non-GCB DLBCL), oridonin and NVP-BEZ235, a PI3K/mTOR inhibitor, also showed strong inhibition and less toxicity than in their individual use. The synergy promoted AKT/mTOR-, NF-κB-, and ROS-mediated DNA damage.91 Additionally, LYN/mTOR, Raf/MEK/ERK and SATA5 signaling pathways were reported to be involved in combining oridonin and imatinib for treating Ph+ acute lymphoblastic leukemia cells.92 These studies suggest that oridonin and oridonalogs, whether in single or combination use, have huge potential for treating hematological malignancies.
Other than hematological malignancies, oridonin has been shown to trigger ROS generation in solid tumor cells. ROS generated by aerobic metabolism induces oxidative stress.93 Unfortunately, cancer cells especially those in advanced disease stages have higher tolerance to exogenous stress. Cancer cells with redox adaptation survive better than other cancer cells and are thought to be associated with chemoresistance.94 Arsenic trioxide (As2O3) induces ROS-dependent apoptosis in liver cancer cells and was adopted as the first-line treatment for acute promyelocytic leukemia (APL).95,96 HCC cell lines including Bel7402, HepaG2, and SMMC7721 became more sensitive to As2O3 after administration of oridonin. Synergistic elevation of cellular ROS level is related to low GSH level. Interestingly, low dose oridonin promotes ROS generation, but not induce cell death in Bel7402 cells,97 with undefined mechanisms. Nevertheless, oridonin can activate Nrf2 and attenuate arsenic-induced toxicity, through the Nrf2 signaling pathway at a low dose in vitro, whereas, at a high dose it induces apoptosis and inhibits the Nrf2-dependent survival pathway.98 A recent study reported that the combination of oridonin with cetuximab, a mouse-human chimeric anti-EGFR monoclonal antibody of epidermal growth factor receptor (EGFR), synergistically activates the ROS–mediated JNK pathway and downregulates p-EGFR in laryngeal squamous cell carcinoma cell lines, HEp-2 and Tu212.99 Thus, oridonin enhances chemotherapeutic efficacy, and may simultaneously alleviate the adverse side effects of chemotherapy.
Radiotherapy has been an increasingly important modality among cancer therapies, especially for radiation-sensitive cancers. However, there are concerns and side effects, before and after radiotherapy, and radiation resistance compromises the clinical efficacy. Recently, oridonin was reported to enhance irradiation-induced cell death in non-small cell lung cancer (NSCLC) cells by promoting DNA damage. NSCLC H460 cells were treated with oridonin (1 and 2.5 μM) before exposure to different doses of gamma rays. A 2.5 μM oridonin dose with 4 Gy gamma rays showed strong inhibition of H460 cell viability. This was achieved by inducing ROS, which leads to DNA damage and apoptosis, together with consistent in vivo inhibition of tumor growth and enhanced sensitivity towards radiotherapy.100 This study suggests that oridonin and its derivatives may be used as radiosensitizer to enhance radiotherapy.
There is no doubt that a combination of multiple chemo-therapeutic agents, not only potentially reduces the dose of oridonin needed, but also reduces the doses of combining chemotherapeutic agents, and hence diminishes adverse side effects as well. Oridonin was shown to sensitize cells to lentinan, a medicinal polysaccharide isolated from the Shiitake mushroom. Combination of both NPs showed more effective anticancer activity, compared to treatment with oridonin, lentinan, or cisplatin alone in a human hepatoblastoma cell line, HepG2. The combination increased caspase-3, caspase-9, p53, and p21 levels, and inhibited NF-κB signaling which contributes to enhanced apoptosis.101,102 Other groups reported γ-tocotrienol as a nontoxic natural phytochemical in the vitamin E family,103 and combination of oridonin and γ-tocotrienol inhibits malignant + SA mammary epithelial cells, but displayed nearly no toxicity with normal CL-S1 mammary epithelial cell.104 These studies, as summarized in Fig. 1, Table 2, and Table S1, suggest that oridonin in combination with other anti-cancer agents may significantly increase the efficacy of those agents and also reduce side-effects in many occasions, thus providing windows and venues for further investigation. Oridonin was also tested in metastatic conditions. A recent study reported that oridonin in combination with nutlin-3 promotes the nutlin-3-mediated anticancer effect in osteoscarcoma (OS) cells within wild-type p53, but no impact on p53-deficient OS cells was observed.105 This study suggests that p53 plays a key mechanistic role in mediating oridonin's anti-cancer effect.
Figure 1.
Targeted pathways of oridonin in combination therapy.
Table 2.
Targeting therapeutic resistance with oridonin and its derivatives in vitro.
| aCell Lines Tested | Potential Mechanisms | Ref |
|---|---|---|
| Ovarian cancer A278/DDP and SKOV3/DDP cell lines | Induction of apoptosis, increase of cells in G0/G1 phase, downregulation of Bcl-2, upregulation of Bax, and decrease of MMP-2 and MMP-9 | 114 |
| Leukemia K562/ADR cell line | Upregulation of BIM-S by diminishing miRNA-7 and miRNA-20a | 118 |
| AML MV4-11/DDP and MOLM-13/DDP cell lines | Induction of apoptosis, inhibition of MMP-2 and MMP-9 | 115 |
| Renal cell carcinoma 786-O cell line | Induction of necrosis by depleting GSH and enhancing ROS. | 120 |
| Colorectal cancer HCT-15 and HCT-15/5FU-R cell line | Upregulation of ROS/JNK/c-Jun axis | 121 |
| Gastric cancer SGC7901/DDP cell line | Downregulation of P-gp, MRP1 and cyclin D1 | 110 |
| Pancreatic cancer PANC-1 (PANC-1/Gem) gemcitabine-resistant cell line | Inhibition of GST pi and LRP1/ERK/JNK signaling | 122 |
| NSCLC H1975-gefitinib-resistant cell line | Suppression of EGFR/ERK/MMP-12 and CIP2A/PP2A/Akt signaling pathways | 123 |
| Leukemia Ph+ (K562, KU812 and SUP-B15) cell lines | Depletion of BCR-ABL through activating HSF-1 for chaperone-mediated degradation | 126 |
| Leukemia imatinib-sensitive (K562–S) and imatinib-resistant (K562-R) cell lines | Downregulation of p-Lyn and inhibition of mTOR and Bcl-2 | 127 |
Oridonin and oridonalogs as potential therapies to sensitize cancer cells and overcome therapeutic resistance
Chemoresistance is one of the major challenges in cancer chemotherapy, as intrinsic or acquired chemoresistance is related to poor prognosis in cancer patients. Intrinsic drug resistance occurs in initial chemotherapy while acquired chemoresistance relates to patients that may be initially sensitive to chemotherapy, but later become resistant to the same or similar drugs.106 Furthermore, multi-drug resistance (MDR) is the resistance to other chemotherapeutic drugs, after exerting resistance to one drug, dependent on similar or different mechanisms. It was reported that the adenosine triphosphate-binding cassette (ABC) transporter family members play a key role in acquired chemoresistance.107 Other factors such as drug properties and cancer stem cells also contribute to chemoresistance.108 Current literature supports the notion that oridonin, used alone or in combination with other chemotherapeutic agents, primarily exhibits anti-chemoresistance activity via induction of apoptosis, potentially by regulating drug resistance-associated molecules such as P-glyconprotein (P-gp) and MDR-associated protein 1 (MRP1).109,110
Nearly all chemotherapeutic drugs are able to induce apoptosis of cancer cells.111 However, after cancer cells develop chemoresistance, they become resistant to apoptosis, which eventually lead to the escape from immune surveillance.112 Oridonin has been shown to induce apoptosis of many cancer cell types with potent efficacy. For example, platinum-based chemotherapeutic agents such as cisplatin (DDP), carboplatin, and nedaplatin are widely used in ovarian cancer chemotherapy. However, these drugs did not achieve satisfactory clinical outcomes to prolong survival, either due to chemoresistance or severe side effects.113 Oridonin in combination with DDP decreases the IC50 of DDP in A278/DDP and SKOV3/DDP cells via induction of apoptosis and arrest of cells in G0/G1 phase.114 This was also evident in cisplatin-resistant AML cells via apoptosis induction.115 Zhao Y. et al reported that oridonin enhances the sensitivity of A2780CP and SKOV3/DDP cells to cisplatin via cisplatin-mediated autophagy.116 Intriguingly, the paclitaxel-resistant cell line PTX10 showed higher sensitivity to oridonin compared to the chemo-sensitive cell line A2780.117 Leukemia K562/ADR cells with resistance to cytarabine (Ara-C) and etoposide (VP-16) showed high sensitivity to oridonin. This was achieved through upregulating BIM-S, which belongs to the BCL-2 family but acts as an apoptotic activator, via diminishing the expression of miRNA-17 and miRNA-20a, whereas, neither Ara-C nor VP-16 had any impact on BIM-S-mediated mitochondria-dependent apoptosis via miRNA-17 or miRNA-20a.118 In summary, oridonin demonstrated strong activity against chemoresistance by modulating multiple apoptotic pathways to induce apoptosis of cancer cells, and miRNA may play a major role in developing and maintaining chemoresistance in cancer cells.
Recent literature shows evidence that oridonin also induces other forms of programmed cell death such as necroptosis, which can enhance chemotherapy or overcome apoptotic resistance.111 Necroptosis is a complex cellular process involving multiple factors such as receptor-interacting protein kinases-1 and -3 (RIPK1 and RIPK3), mixed-lineage kinase domain-like protein (MLKL), and other factors.119 It was shown that 5-FU alone only induces apoptosis, but in combination with oridonin can induce apoptosis and necroptosis in 786-O renal carcinoma cells.120 Necroptosis induced by oridonin was accomplished through depleting GSH and enhancing ROS generation via regulation of the ROS/JNK/c-Jun axis in colorectal cancer cells.121 Thus, ROS production may be a rate-limiting factor in cells in order for oridonin to induce apoptosis, necrosis, or both. An additional benefit of combining oridonin with 5-FU or other chemotherapeutic agents is to reduce doses of chemo-drugs, leading to less toxicity and side effects. These studies demonstrate that induction of programmed cell death is one of its major mechanisms of action for oridonin.
MDR-associated gene overexpression and/or activation essentially contribute to chemoresistance in many cancer types. Human DDP-resistant cell line, SGC7901/DDP, showed upregulated P-gp, MRP1, and cyclin D1. Administration of oridonin at a 10 μM concentration effectively reduced the expression of those proteins by at least 1.25-fold, and combination of oridonin and DDP showed a synergistic effect in decreasing the IC50 of DDP, achieved via suppression of the CIP2A/PP2A/Akt signaling pathway.110 Other than modulating P-gp and MRP1, oridonin overcomes gemcitabine resistance by regulating GST, which is involved in MDR and LRP/1 ERK/JNK signaling in PANC-1/Gem cells.122 In addition, oridonin is also effective against gefitinib-resistant NSCLC cells by modulating the CIP2A//PP2A/Akt signaling pathway.123 By using silico-based technology to validate potential targets of oridonin in various drug-resistant tumor cells, one study showed that oridonin interacts with signaling molecules of the Akt/EGFR pathway. Accumulating evidence and our unpublished data suggest that Akt and STAT3 signaling are critically involved in oridonin's effect against chemoresistance.124
The BCR-ABL gene encodes tyrosine kinase which is a hot therapeutic target for leukemia, and gefitinib and imatinib are frequently used tyrosine kinase inhibitors (TKIs). It was reported that BCR-ABL gene amplification or point mutations in the kinase domain leads to imatinib-associated resistance.125 Oridonin was reported to target BCR-ABL by binding to its cysteine-153 residue in the HSF1 domain, which regulates the expression of HSP70 and ubiquitin proteins, UBB and UBC, to downregulate BCR-ABL in leukemia cells.126 Further, oridonin was shown to inhibit K562 cells with imatinib-resistance via downregulation of Bcl-2 and p-Lyn, which is a member of Src family of protein tyrosine kinases thought to be involved in developing resistance to imatinib via the mTOR pathway.127 These reports suggest that oridonin reverses resistance raised from targeted therapies including BCR-ABL, and this effect may be non-specific towards any tyrosine kinase inhibitors.
Oridonin was reported to dose-dependently decrease the levels of MMP-2 and MMP-9, which are related to invasion and metastasis.114,115 Oridonin also inhibits migration, invasion, and adhesion of gefitinib-resistant NSCLC cell line H1975 in vitro and in vivo through EGFR/ERK/MMP-12 pathway.123 Thus, oridonin may suppress cancer metastasis and serve as a therapy for late-stage/metastatic cancers.
Targets of oridonin and its derivative compounds
As mentioned earlier, oridonin and its various derivative compounds can suppress cancer cell proliferation in vitro and tumor growth in vivo. Oridonin combined with other chemical agents enhances the anticancer effect and decreases adverse side effects. In terms of potency and toxicity, many oridonin derivatives demonstrated improved efficacy, higher bioavailability, and less toxicity, justifying the previous and ongoing efforts to overcome the noticeable disadvantages of oridonin. Accumulating evidence suggests that oridonin and its analogs, alone or combination with other drugs, trigger apoptosis, autophagy, cell cycle arrest, and EMT through differential molecular pathways and mechanisms. However, deconvolution of targets for oridonin and its derivatives has been a significant challenge. Here we summarized the reported signaling pathways involved in the mechanism of action of oridonin in an attempt to facilitate future endeavors to identify targets directly and/or indirectly interacting with oridonin associated with chemoresistance or other disease settings (Fig. 2 and Table S2).
Figure 2.
Potential targets and signaling pathways of oridonin and its derivative compounds.
Extrinsic and intrinsic apoptotic pathways have been demonstrated as the primary mechanisms of action involved in oridonin and its derivatives' anti-cancer activities. The caspase family, Bcl-2 family, p53, MDM2, NF-κB, PI3K/Akt/mTOR, MAPK-p38, and JAK/STAT have been shown to contribute to oridonin and its derivatives’ effects. Among them, the NF-κB signaling pathway plays a key-role in anti-tumor, anti-fibrosis, anti-inflammatory, and immune regulation. As shown in Fig. 2 and Table S2, multiple pathways and potential targets of oridonin were reported, however, direct targets are not fully determined. Previous studies suggest that two oridonin derivative compounds developed by our team, CYD0617 and CYD0618, may target β-catenin and PDPK1, and STAT3 signaling, respectively.71,72,79 Huang H. et al found that c-Myc, a helix-loop-helix leucine zipper transcription factor, is one of the oridonin targets in the K562 cell model, through comparing the miRNA expression with oridonin exposure.128 Oridonin was shown to directly bind to the cysteine-153 residue of HSF1 to activate HSF1 and enhance the expression of HSP70, eventually inducing BCR-ABL degradation in leukemia.126 Involvement of miRNA such as miRNA-7 and miRNA-20a may help deconvolute downstream targets of oridonin and its derivative compounds. These studies suggest that oridonin and its derivative compounds directly and indirectly target multiple pathways and molecules to exert their various anti-cancer efficacy. Further research is therefore warranted to identify the bona fide and the key indirect targets of oridonin and oridonalogs for better understanding of their mechanisms of action.
Perspectives and conclusions
Oridonin as a natural product with a relatively safe profile has displayed potent pharmacological activities, such as anti-tumor, anti-inflammatory, and immune regulation. It targets many genes or proteins important in various signaling pathways involved in human diseases including cancers. In the past decade, oridonin has been vastly investigated, alone or in combination, for synergistic effects with other therapeutic agents. With its moderate efficacy and relatively low bioavailability, over one hundred oridonin-based structurally diversified derivative compounds were synthesized and evaluated in various in vitro and in vivo systems. Combination therapy with oridonin, not only enhances chemotherapy, but also reduces commonly seen adverse side effects. Extended implication for oridonin and its oridonalogs is to overcome therapeutic resistance developed during or after cancer treatment. Beyond induction of apoptosis as the primary mechanism of action, oridonin also has yet-to-elucidate functions in inflammation and immunity regulation. Further investigation of the putative targets for oridonin and its novel analogs is therefore warranted in order to obtain more supporting evidence. With improved overall drug-like properties including aqueous solubility and bioavailability, oridonin-based new scaffolds may provide promising drug candidates with potential to be advanced into future human clinical trials.
Authors contribution
X. Liu, J. Zhou and Q. Shen conceived the concept. X. Liu conducted literature search and summarization. X. Liu wrote the manuscript and J. Xu contributed in part to Table 1. X. Liu, J. Xu, J. Zhou and Q. Shen revised the manuscript. All authors reviewed manuscript and granted approval for submission.
Conflict of Interests
The authors declare that they have no conflict of interest.
Acknowledgements
Q. Shen and J. Zhou were supported in part by the National Institutes of Health (NIH)/National Cancer Institute (NCI) grants (grant numbers R01CA231150, R01CA226001). J. Zhou was also supported in part by the John D. Stobo, M.D. Distinguished Chair Endowment Fund, University of Texas Medical Branch. We thank Doerte Raphi Fricke, M.S., and Susan Theodosiou, Ph.D., Stanley S. Scott Cancer Center, School of Medicine, Louisiana State University Health Sciences Center, for their editing of the manuscript.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2020.06.010.
Contributor Information
Jia Zhou, Email: jizhou@utmb.edu.
Qiang Shen, Email: qshen@lsuhsc.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Combination therapy with oridonin in different in vitro models
Targets and signaling pathways of oridonin and its derivative compounds
References
- 1.Fujita E., Nagao Y., Node M., Kaneko K., Nakazawa S., Kuroda H. Antitumor activity of the Isodon diterpenoids: structural requirements for the activity. Experientia. 1976;32(2):203–206. doi: 10.1007/BF01937766. [DOI] [PubMed] [Google Scholar]
- 2.Xu J., Wold E.A., Ding Y., Shen Q., Zhou J. Therapeutic potential of oridonin and its analogs: from anticancer and antiinflammation to neuroprotection. Molecules. 2018;23(2) doi: 10.3390/molecules23020474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding Y., Ding C., Ye N. Discovery and development of natural product oridonin-inspired anticancer agents. Eur J Med Chem. 2016;122:102–117. doi: 10.1016/j.ejmech.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Q.B., Li M.L., Li S.H., Mou Y.K., Lin Z.W., Sun H.D. Ent-kaurane diterpenoids from isodon rubescens var. lushanensis. Chem Pharm Bull (Tokyo) 2003;51(7):790–793. doi: 10.1248/cpb.51.790. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q.Q., Wang H.L., Chen K. Oridonin derivative ameliorates experimental colitis by inhibiting activated T-cells and translocation of nuclear factor-kappa B. J Dig Dis. 2016;17(2):104–112. doi: 10.1111/1751-2980.12314. [DOI] [PubMed] [Google Scholar]
- 6.He H., Jiang H., Chen Y. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018;9(1) doi: 10.1038/s41467-018-04947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadota S., Basnet P., Ishii E., Tamura T., Namba T. Antibacterial activity of trichorabdal a from Rabdosia trichocarpa against Helicobacter pylori. Zentralbl Bakteriol. 1997;286(1):63–67. doi: 10.1016/s0934-8840(97)80076-x. [DOI] [PubMed] [Google Scholar]
- 8.Kuo L.M., Kuo C.Y., Lin C.Y., Hung M.F., Shen J.J., Hwang T.L. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules. 2014;19(3):3327–3344. doi: 10.3390/molecules19033327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohanon F.J., Wang X., Ding C. Oridonin inhibits hepatic stellate cell proliferation and fibrogenesis. J Surg Res. 2014;190(1):55–63. doi: 10.1016/j.jss.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y., Xue Y., Wang Y., Feng D., Lin S., Xu L. Multiple-modulation effects of Oridonin on the production of proinflammatory cytokines and neurotrophic factors in LPS-activated microglia. Int Immunopharm. 2009;9(3):360–365. doi: 10.1016/j.intimp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Tan W., Lu J., Huang M. Anti-cancer natural products isolated from Chinese medicinal herbs. Chin Med. 2011;6(1) doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Z., Hu C., Zhang Y. Therapeutic effect of Rabdosia rubescens aqueous extract on chronic pharyngitis and its safety. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36(2):170–173. doi: 10.3969/j.issn.1672-7347.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Li D., Han T., Xu S. Antitumor and antibacterial derivatives of oridonin: a main composition of Dong-Ling-cao. Molecules. 2016;21(5):e575. doi: 10.3390/molecules21050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou M., Yi Y., Hong L. Oridonin ameliorates lipopolysaccharide-induced endometritis in mice via inhibition of the TLR-4/NF-kappaB pathway. Inflammation. 2019;42(1):81–90. doi: 10.1007/s10753-018-0874-8. [DOI] [PubMed] [Google Scholar]
- 15.Li J., Bao L., Zha D. Oridonin protects against the inflammatory response in diabetic nephropathy by inhibiting the TLR4/p38-MAPK and TLR4/NF-kappaB signaling pathways. Int Immunopharm. 2018;55:9–19. doi: 10.1016/j.intimp.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Huang W., Huang M., Ouyang H., Peng J., Liang J. Oridonin inhibits vascular inflammation by blocking NF-kappaB and MAPK activation. Eur J Pharmacol. 2018;826:133–139. doi: 10.1016/j.ejphar.2018.02.044. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G., Zhang T., Ma X. Oridonin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-induced RAW264.7 cells and acute lung injury. Oncotarget. 2017;8(40):68153–68164. doi: 10.18632/oncotarget.19249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng Y., Chen C., Yu H. Oridonin ameliorates lipopolysaccharide/D-galactosamine-induced acute liver injury in mice via inhibition of apoptosis. Am J Transl Res. 2017;9(9):4271–4279. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y.J., Lv H., Xu P.B. Protective effects of oridonin on the sepsis in mice. Kaohsiung J Med Sci. 2016;32(9):452–457. doi: 10.1016/j.kjms.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S., Zhang Y., Saas P. Oridonin’s therapeutic effect: suppressing Th1/Th17 simultaneously in a mouse model of Crohn’s disease. J Gastroenterol Hepatol. 2015;30(3):504–512. doi: 10.1111/jgh.12710. [DOI] [PubMed] [Google Scholar]
- 21.Wu Q.J., Zheng X.C., Wang T., Zhang T.Y. Effects of dietary supplementation with oridonin on the growth performance, relative organ weight, lymphocyte proliferation, and cytokine concentration in broiler chickens. BMC Vet Res. 2018;14(1):34. doi: 10.1186/s12917-018-1359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q.J., Zheng X.C., Wang T., Zhang T.Y. Effects of oridonin on immune cells, Th1/Th2 balance and the expression of BLys in the spleens of broiler chickens challenged with Salmonella pullorum. Res Vet Sci. 2018;119:262–267. doi: 10.1016/j.rvsc.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Hu A.P., Du J.M., Li J.Y., Liu J.W. Oridonin promotes CD4+/CD25+ Treg differentiation, modulates Th1/Th2 balance and induces HO-1 in rat splenic lymphocytes. Inflamm Res. 2008;57(4):163–170. doi: 10.1007/s00011-007-7193-0. [DOI] [PubMed] [Google Scholar]
- 24.Wang J., Li F., Ding J. Investigation of the antiasthmatic activity of Oridonin on a mouse model of asthma. Mol Med Rep. 2016;14(3):2000–2006. doi: 10.3892/mmr.2016.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shang C.H., Zhang Q.Q., Zhou J.H. Oridonin inhibits cell proliferation and induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 2016;39(2):873–880. doi: 10.1007/s10753-016-0318-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhou L., Sun L., Wu H. Oridonin ameliorates lupus-like symptoms of MRL(lpr/lpr) mice by inhibition of B-cell activating factor (BAFF) Eur J Pharmacol. 2013;715(1–3):230–237. doi: 10.1016/j.ejphar.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Xie Z., Yu H., Sun X. A novel diterpenoid suppresses osteoclastogenesis and promotes osteogenesis by inhibiting Ifrd1-mediated and IkappaBalpha-mediated p65 nuclear translocation. J Bone Miner Res. 2018;33(4):667–678. doi: 10.1002/jbmr.3334. [DOI] [PubMed] [Google Scholar]
- 28.Seo E.J., Fischer N., Efferth T. Phytochemicals as inhibitors of NF-kappaB for treatment of Alzheimer’s disease. Pharmacol Res. 2018;129:262–273. doi: 10.1016/j.phrs.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Wang S., Yu L., Yang H. Oridonin attenuates synaptic loss and cognitive deficits in an abeta1-42-induced mouse model of alzheimer’s disease. PloS One. 2016;11(3) doi: 10.1371/journal.pone.0151397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z.Y., Daniels R., Schluesener H.J. Oridonin ameliorates neuropathological changes and behavioural deficits in a mouse model of cerebral amyloidosis. J Cell Mol Med. 2013;17(12):1566–1576. doi: 10.1111/jcmm.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S., Yang H., Yu L. Oridonin attenuates Abeta1-42-induced neuroinflammation and inhibits NF-kappaB pathway. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0104745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L.X., Sun Y., Chen C. Effects and mechanism of oridonin on pulmonary hypertension induced by chronic hypoxia-hypercapnia in rats. Chin Med J. 2009;122(12):1380–1387. [PubMed] [Google Scholar]
- 33.Wang M.Y., Lin C., Zhang T.M. [Cytokinetic effects of oridonin on leukemia L1210 cells] Zhongguo Yaoli Xuebao. 1985;6(3):195–198. [PubMed] [Google Scholar]
- 34.Li X.T., Lin C., Li P.Y. [Characteristics of the cytostatic effects of oridonin in vitro] Zhongguo Yaoli Xuebao. 1986;7(4):361–363. [PubMed] [Google Scholar]
- 35.Liu J.J., Huang R.W., Lin D.J. Anti-proliferative effects of oridonin on SPC-A-1 cells and its mechanism of action. J Int Med Res. 2004;32(6):617–625. doi: 10.1177/147323000403200606. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C.L., Wu L.J., Tashiro S., Onodera S., Ikejima T. Oridonin induced A375-S2 cell apoptosis via bax-regulated caspase pathway activation, dependent on the cytochrome c/caspase-9 apoptosome. J Asian Nat Prod Res. 2004;6(2):127–138. doi: 10.1080/1028602031000147375. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C.L., Wu L.J., Zuo H.J., Tashiro S., Onodera S., Ikejima T. Cytochrome c release from oridonin-treated apoptotic A375-S2 cells is dependent on p53 and extracellular signal-regulated kinase activation. J Pharmacol Sci. 2004;96(2):155–163. doi: 10.1254/jphs.fpj04008x. [DOI] [PubMed] [Google Scholar]
- 38.Huang J., Wu L., Tashiro S., Onodera S., Ikejima T. Bcl-2 up-regulation and P-p53 down-regulation account for the low sensitivity of murine L929 fibrosarcoma cells to oridonin-induced apoptosis. Biol Pharm Bull. 2005;28(11):2068–2074. doi: 10.1248/bpb.28.2068. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J.F., Liu J.J., Liu P.Q., Lin D.J., Li X.D., Chen G.H. Oridonin inhibits cell growth by induction of apoptosis on human hepatocelluar carcinoma BEL-7402 cells. Hepatol Res. 2006;35(2):104–110. doi: 10.1016/j.hepres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y.Q., You S., Zhang C.L., Tashiro S., Onodera S., Ikejima T. Oridonin enhances phagocytosis of UV-irradiated apoptotic U937 cells. Biol Pharm Bull. 2005;28(3):461–467. doi: 10.1248/bpb.28.461. [DOI] [PubMed] [Google Scholar]
- 41.Cui Q., Tashiro S., Onodera S., Minami M., Ikejima T. Autophagy preceded apoptosis in oridonin-treated human breast cancer MCF-7 cells. Biol Pharm Bull. 2007;30(5):859–864. doi: 10.1248/bpb.30.859. [DOI] [PubMed] [Google Scholar]
- 42.Qi X., Zhang D., Xu X. Oridonin nanosuspension was more effective than free oridonin on G2/M cell cycle arrest and apoptosis in the human pancreatic cancer PANC-1 cell line. Int J Nanomed. 2012;7:1793–1804. doi: 10.2147/IJN.S29483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang J., Wu L., Tashiro S., Onodera S., Ikejima T. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J Pharmacol Sci. 2008;107(4):370–379. doi: 10.1254/jphs.08044fp. [DOI] [PubMed] [Google Scholar]
- 44.Zeng R., Chen Y., Zhao S., Cui G.H. Autophagy counteracts apoptosis in human multiple myeloma cells exposed to oridonin in vitro via regulating intracellular ROS and SIRT1. Acta Pharmacol Sin. 2012;33(1):91–100. doi: 10.1038/aps.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao Y., Wei W., Zhang N. Oridonin stabilizes retinoic acid receptor alpha through ROS-activated NF-kappaB signaling. BMC Canc. 2015;15 doi: 10.1186/s12885-015-1219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y., Jiang X., Lu Y. Oridonin prevents epithelial-mesenchymal transition and TGF-beta1-induced epithelial-mesenchymal transition by inhibiting TGF-beta1/Smad2/3 in osteosarcoma. Chem Biol Interact. 2018;296:57–64. doi: 10.1016/j.cbi.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Liu Q.Q., Chen K., Ye Q., Jiang X.H., Sun Y.W. Oridonin inhibits pancreatic cancer cell migration and epithelial-mesenchymal transition by suppressing Wnt/beta-catenin signaling pathway. Canc Cell Int. 2016;16 doi: 10.1186/s12935-016-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao Z., Xie F., Li M. Oridonin induces autophagy via inhibition of glucose metabolism in p53-mutated colorectal cancer cells. Cell Death Dis. 2017;8(2) doi: 10.1038/cddis.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu W., Sun J., Zhang T.T. Pharmacokinetic behaviors and oral bioavailability of oridonin in rat plasma. Acta Pharmacol Sin. 2006;27(12):1642–1646. doi: 10.1111/j.1745-7254.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- 50.Xu J., Yang J., Ran Q. Synthesis and biological evaluation of novel 1-O- and 14-O-derivatives of oridonin as potential anticancer drug candidates. Bioorg Med Chem Lett. 2008;18(16):4741–4744. doi: 10.1016/j.bmcl.2008.06.097. [DOI] [PubMed] [Google Scholar]
- 51.Liu W., Zhao B., Li Y.C., Liu H.M. NMR spectra and structures of oridonin derivatives complexes with beta-cyclodextrin. Magn Reson Chem : MRC. 2011;49(9):611–615. doi: 10.1002/mrc.2770. [DOI] [PubMed] [Google Scholar]
- 52.Wang L., Li D., Xu S. The conversion of oridonin to spirolactone-type or enmein-type diterpenoid: synthesis and biological evaluation of ent-6,7-seco-oridonin derivatives as novel potential anticancer agents. Eur J Med Chem. 2012;52:242–250. doi: 10.1016/j.ejmech.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 53.Xu S., Yao H., Luo S. A novel potent anticancer compound optimized from a natural oridonin scaffold induces apoptosis and cell cycle arrest through the mitochondrial pathway. J Med Chem. 2017;60(4):1449–1468. doi: 10.1021/acs.jmedchem.6b01652. [DOI] [PubMed] [Google Scholar]
- 54.Ma Y.C., Ke Y., Zi X. Induction of the mitochondria-mediated apoptosis in human esophageal cancer cells by DS2, a newly synthetic diterpenoid analog, is regulated by Bax and caused by generation of reactive oxygen species. Oncotarget. 2016;7(52):86211–86224. doi: 10.18632/oncotarget.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S.Q., Wang C., Chang L.M. Geridonin and paclitaxel act synergistically to inhibit the proliferation of gastric cancer cells through ROS-mediated regulation of the PTEN/PI3K/Akt pathway. Oncotarget. 2016;7(45):72990–73002. doi: 10.18632/oncotarget.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S.Q., Wang C., Wang J.W. Geridonin, a novel derivative of oridonin, inhibits proliferation of MGC 803 cells both in vitro and in vivo through elevating the intracellular ROS. J Pharm Pharmacol. 2017;69(2):213–221. doi: 10.1111/jphp.12678. [DOI] [PubMed] [Google Scholar]
- 57.Li D., Wang L., Cai H., Zhang Y., Xu J. Synthesis and biological evaluation of novel furozan-based nitric oxide-releasing derivatives of oridonin as potential anti-tumor agents. Molecules. 2012;17(6):7556–7568. doi: 10.3390/molecules17067556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li D., Cai H., Jiang B. Synthesis of spirolactone-type diterpenoid derivatives from kaurene-type oridonin with improved antiproliferative effects and their apoptosis-inducing activity in human hepatoma Bel-7402 cells. Eur J Med Chem. 2013;59:322–328. doi: 10.1016/j.ejmech.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Li D., Xu S., Cai H. Enmein-type diterpenoid analogs from natural kaurene-type oridonin: synthesis and their antitumor biological evaluation. Eur J Med Chem. 2013;64:215–221. doi: 10.1016/j.ejmech.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Li H., Mu J., Sun J. Hydrogen sulfide releasing oridonin derivatives induce apoptosis through extrinsic and intrinsic pathways. Eur J Med Chem. 2020;187 doi: 10.1016/j.ejmech.2019.111978. [DOI] [PubMed] [Google Scholar]
- 61.Li D., Han T., Tian K. Novel nitric oxide-releasing spirolactone-type diterpenoid derivatives with in vitro synergistic anticancer activity as apoptosis inducer. Bioorg Med Chem Lett. 2016;26(17):4191–4196. doi: 10.1016/j.bmcl.2016.07.059. [DOI] [PubMed] [Google Scholar]
- 62.Li D., Hu X., Han T. NO-releasing enmein-type diterpenoid derivatives with selective antiproliferative activity and effects on apoptosis-related proteins. Molecules. 2016;21(9) doi: 10.3390/molecules21091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu S., Wang G., Lin Y. Novel anticancer oridonin derivatives possessing a diazen-1-ium-1,2-diolate nitric oxide donor moiety: design, synthesis, biological evaluation and nitric oxide release studies. Bioorg Med Chem Lett. 2016;26(12):2795–2800. doi: 10.1016/j.bmcl.2016.04.068. [DOI] [PubMed] [Google Scholar]
- 64.Xu S., Yao H., Hu M. 6,7-Seco-ent-Kauranoids derived from oridonin as potential anticancer agents. J Nat Prod. 2017;80(9):2391–2398. doi: 10.1021/acs.jnatprod.7b00057. [DOI] [PubMed] [Google Scholar]
- 65.Xu S., Li D., Pei L. Design, synthesis and antimycobacterial activity evaluation of natural oridonin derivatives. Bioorg Med Chem Lett. 2014;24(13):2811–2814. doi: 10.1016/j.bmcl.2014.04.119. [DOI] [PubMed] [Google Scholar]
- 66.Hu X., Bai Z., Qiao J. Effective enmein-type mimics of clinical candidate HAO472: design, synthesis and biological evaluation. Eur J Med Chem. 2019;171:169–179. doi: 10.1016/j.ejmech.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 67.Li H., Gao X., Huang X. Hydrogen sulfide donating ent-kaurane and spirolactone-type 6,7-seco-ent-kaurane derivatives: design, synthesis and antiproliferative properties. Eur J Med Chem. 2019;178:446–457. doi: 10.1016/j.ejmech.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 68.Ding C., Zhang Y., Chen H. Novel nitrogen-enriched oridonin analogues with thiazole-fused A-ring: protecting group-free synthesis, enhanced anticancer profile, and improved aqueous solubility. J Med Chem. 2013;56(12):5048–5058. doi: 10.1021/jm400367n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding C., Wang L., Chen H. ent-Kaurane-based regio- and stereoselective inverse electron demand hetero-Diels-Alder reactions: synthesis of dihydropyran-fused diterpenoids. Org Biomol Chem. 2014;12(42):8442–8452. doi: 10.1039/c4ob01040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding C., Zhang Y., Chen H. Overcoming synthetic challenges of oridonin A-ring structural diversification: regio- and stereoselective installation of azides and 1,2,3-triazoles at the C-1, C-2, or C-3 position. Org Lett. 2013;15(14):3718–3721. doi: 10.1021/ol4015865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen W., Zhou J., Wu K. Targeting XBP1-mediated β-catenin expression associated with bladder cancer with newly synthetic Oridonin analogues. Oncotarget. 2016;7(35):56842–56854. doi: 10.18632/oncotarget.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou J., Yun E.-J., Chen W. Targeting 3-phosphoinositide-dependent protein kinase 1 associated with drug-resistant renal cell carcinoma using new oridonin analogs. Cell Death Dis. 2017;8(3) doi: 10.1038/cddis.2017.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding C., Zhang Y., Chen H. Oridonin ring A-based diverse constructions of enone functionality: identification of novel dienone analogues effective for highly aggressive breast cancer by inducing apoptosis. J Med Chem. 2013;56(21):8814–8825. doi: 10.1021/jm401248x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li D., Wang H., Ding Y. Targeting the NRF-2/RHOA/ROCK signaling pathway with a novel aziridonin, YD0514, to suppress breast cancer progression and lung metastasis. Canc Lett. 2018;424:97–108. doi: 10.1016/j.canlet.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 75.Ding Y., Li D., Ding C. Regio- and stereospecific synthesis of oridonin D-ring aziridinated analogues for the treatment of triple-negative breast cancer via mediated irreversible covalent warheads. J Med Chem. 2018;61(7):2737–2752. doi: 10.1021/acs.jmedchem.7b01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bohanon F.J., Wang X., Graham B.M. Enhanced effects of novel oridonin analog CYD0682 for hepatic fibrosis. J Surg Res. 2015;199(2):441–449. doi: 10.1016/j.jss.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bohanon F.J., Wang X., Graham B.M. Enhanced anti-fibrogenic effects of novel oridonin derivative CYD0692 in hepatic stellate cells. Mol Cell Biochem. 2015;410(1–2):293–300. doi: 10.1007/s11010-015-2562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cummins C.B., Wang X., Xu J. Antifibrosis effect of novel oridonin analog CYD0618 via suppression of the NF-kappaB pathway. J Surg Res. 2018;232:283–292. doi: 10.1016/j.jss.2018.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shen X., Zhao L., Chen P. A thiazole-derived oridonin analogue exhibits antitumor activity by directly and allosterically inhibiting STAT3. J Biol Chem. 2019;294(46):17471–17486. doi: 10.1074/jbc.RA119.009801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y., Wang Y., Wang S., Gao Y., Zhang X., Lu C. Oridonin phosphate-induced autophagy effectively enhances cell apoptosis of human breast cancer cells. Med Oncol. 2015;32(1) doi: 10.1007/s12032-014-0365-1. [DOI] [PubMed] [Google Scholar]
- 81.Shen Q.-K., Chen Z.-A., Zhang H.-J. Design and synthesis of novel oridonin analogues as potent anticancer agents. J Enzym Inhib Med Chem. 2018;33(1):324–333. doi: 10.1080/14756366.2017.1419219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen Q.-K., Deng H., Wang S.-B., Tian Y.-S., Quan Z.-S. Synthesis, and evaluation of in vitro and in vivo anticancer activity of 14-substituted oridonin analogs: a novel and potent cell cycle arrest and apoptosis inducer through the p53-MDM2 pathway. Eur J Med Chem. 2019;173:15–31. doi: 10.1016/j.ejmech.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 83.Zhang W., Liu H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 84.Bu H.Q., Luo J., Chen H. Oridonin enhances antitumor activity of gemcitabine in pancreatic cancer through MAPK-p38 signaling pathway. Int J Oncol. 2012;41(3):949–958. doi: 10.3892/ijo.2012.1519. [DOI] [PubMed] [Google Scholar]
- 85.Liu D.-L., Bu H.-Q., Jin H.-M., Zhao J.-F., Li Y., Huang H. Enhancement of the effects of gemcitabine against pancreatic cancer by oridonin via the mitochondrial caspase-dependent signaling pathway. Mol Med Rep. 2014;10(6):3027–3034. doi: 10.3892/mmr.2014.2584. [DOI] [PubMed] [Google Scholar]
- 86.Zhang H.-P., Li G.-Q., Guo W.-Z. Oridonin synergistically enhances JQ1-triggered apoptosis in hepatocellular cancer cells through mitochondrial pathway. Oncotarget. 2017;8(63):106833–106843. doi: 10.18632/oncotarget.21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi M., Ren X., Wang X. A novel combination of oridonin and valproic acid in enhancement of apoptosis induction of HL-60 leukemia cells. Int J Oncol. 2016;48(2):734–746. doi: 10.3892/ijo.2015.3294. [DOI] [PubMed] [Google Scholar]
- 88.Lennartsson J., Ronnstrand L. The stem cell factor receptor/c-Kit as a drug target in cancer. Curr Cancer Drug Targets. 2006;6(1):65–75. doi: 10.2174/156800906775471725. [DOI] [PubMed] [Google Scholar]
- 89.Zhang W., Lu Y., Zhen T. Homoharringtonine synergy with oridonin in treatment of t(8; 21) acute myeloid leukemia. Front Med. 2019;13(3):388–397. doi: 10.1007/s11684-018-0624-1. [DOI] [PubMed] [Google Scholar]
- 90.Spirin P., Lebedev T., Orlova N. Synergistic suppression of t(8;21)-positive leukemia cell growth by combining oridonin and MAPK1/ERK2 inhibitors. Oncotarget. 2017;8(34):56991–57002. doi: 10.18632/oncotarget.18503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qing K., Jin Z., Fu W. Synergistic effect of oridonin and a PI3K/mTOR inhibitor on the non-germinal center B cell-like subtype of diffuse large B cell lymphoma. J Hematol Oncol. 2016;9(1) doi: 10.1186/s13045-016-0303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo Y., Shan Q., Gong Y., Lin J., Yang X., Zhou R. Oridonin in combination with imatinib exerts synergetic anti-leukemia effect in Ph+ acute lymphoblastic leukemia cells in vitro by inhibiting activation of LYN/mTOR signaling pathway. Canc Biol Ther. 2012;13(13):1244–1254. doi: 10.4161/cbt.21460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 95.Sadaf N., Kumar N., Ali M., Ali V., Bimal S., Haque R. Arsenic trioxide induces apoptosis and inhibits the growth of human liver cancer cells. Life Sci. 2018;205:9–17. doi: 10.1016/j.lfs.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 96.Li J.X., Shen Y.Q., Cai B.Z. Arsenic trioxide induces the apoptosis in vascular smooth muscle cells via increasing intracellular calcium and ROS formation. Mol Biol Rep. 2010;37(3):1569–1576. doi: 10.1007/s11033-009-9561-z. [DOI] [PubMed] [Google Scholar]
- 97.Chen G., Wang K., Yang B.Y., Tang B., Chen J.X., Hua Z.C. Synergistic antitumor activity of oridonin and arsenic trioxide on hepatocellular carcinoma cells. Int J Oncol. 2012;40(1):139–147. doi: 10.3892/ijo.2011.1210. [DOI] [PubMed] [Google Scholar]
- 98.Du Y., Villeneuve N.F., Wang X.J. Oridonin confers protection against arsenic-induced toxicity through activation of the Nrf2-mediated defensive response. Environ Health Perspect. 2008;116(9):1154–1161. doi: 10.1289/ehp.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cao S., Xia M., Mao Y. Combined oridonin with cetuximab treatment shows synergistic anticancer effects on laryngeal squamous cell carcinoma: involvement of inhibition of EGFR and activation of reactive oxygen species-mediated JNK pathway. Int J Oncol. 2016;49(5):2075–2087. doi: 10.3892/ijo.2016.3696. [DOI] [PubMed] [Google Scholar]
- 100.Park H., Jeong Y.J., Han N.K., Kim J.S., Lee H.J. Oridonin enhances radiation-induced cell death by promoting DNA damage in non-small cell lung cancer cells. Int J Mol Sci. 2018;19(8) doi: 10.3390/ijms19082378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun Z., Han Q., Duan L., Yuan Q., Wang H. Oridonin increases anticancer effects of lentinan in HepG2 human hepatoblastoma cells. Oncol Lett. 2018;15(2):1999–2005. doi: 10.3892/ol.2017.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dong X., Liu F., Li M. Inhibition of nuclear factor κB transcription activity drives a synergistic effect of cisplatin and oridonin on HepG2 human hepatocellular carcinoma cells. Anti Canc Drugs. 2016;27(4):286–299. doi: 10.1097/CAD.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 103.Tiwari R.V., Parajuli P., Sylvester P.W. gamma-Tocotrienol-induced autophagy in malignant mammary cancer cells. Exp Biol Med. 2014;239(1):33–44. doi: 10.1177/1535370213511022. [DOI] [PubMed] [Google Scholar]
- 104.Tiwari R.V., Parajuli P., Sylvester P.W. Synergistic anticancer effects of combined gamma-tocotrienol and oridonin treatment is associated with the induction of autophagy. Mol Cell Biochem. 2015;408(1–2):123–137. doi: 10.1007/s11010-015-2488-x. [DOI] [PubMed] [Google Scholar]
- 105.Wang X.H., Zhang S.F., Bao J.T., Liu F.Y. Oridonin synergizes with Nutlin-3 in osteosarcoma cells by modulating the levels of multiple Bcl-2 family proteins. Tumour Biol. 2017;39(6) doi: 10.1177/1010428317701638. 1010428317701638. [DOI] [PubMed] [Google Scholar]
- 106.Wang Z., Li Y., Ahmad A. Targeting miRNAs involved in cancer stem cell and EMT regulation: an emerging concept in overcoming drug resistance. Drug Resist Updates. 2010;13(4–5):109–118. doi: 10.1016/j.drup.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu Q., Yang Z., Nie Y., Shi Y., Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Canc Lett. 2014;347(2):159–166. doi: 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 108.Pan S.T., Li Z.L., He Z.X., Qiu J.X., Zhou S.F. Molecular mechanisms for tumour resistance to chemotherapy. Clin Exp Pharmacol Physiol. 2016;43(8):723–737. doi: 10.1111/1440-1681.12581. [DOI] [PubMed] [Google Scholar]
- 109.Efferth T., Davey M., Olbrich A., Rucker G., Gebhart E., Davey R. Activity of drugs from traditional Chinese medicine toward sensitive and MDR1- or MRP1-overexpressing multidrug-resistant human CCRF-CEM leukemia cells. Blood Cells Mol Dis. 2002;28(2):160–168. doi: 10.1006/bcmd.2002.0492. [DOI] [PubMed] [Google Scholar]
- 110.He Z., Xiao X., Li S. Oridonin induces apoptosis and reverses drug resistance in cisplatin resistant human gastric cancer cells. Oncol Lett. 2017;14(2):2499–2504. doi: 10.3892/ol.2017.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yuan R., Hou Y., Sun W. Natural products to prevent drug resistance in cancer chemotherapy: a review. Ann N Y Acad Sci. 2017;1401(1):19–27. doi: 10.1111/nyas.13387. [DOI] [PubMed] [Google Scholar]
- 112.Igney F.H., Krammer P.H. Death and anti-death: tumour resistance to apoptosis. Nat Rev Canc. 2002;2(4):277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 113.Damia G., Broggini M. Platinum resistance in ovarian cancer: role of DNA repair. Cancers. 2019;11(1) doi: 10.3390/cancers11010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ma S., Tan W., Du B. Oridonin effectively reverses cisplatin drug resistance in human ovarian cancer cells via induction of cell apoptosis and inhibition of matrix metalloproteinase expression. Mol Med Rep. 2016;13(4):3342–3348. doi: 10.3892/mmr.2016.4897. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Y., Wang L., Zi Y., Zhang L., Guo Y., Huang Y. Oridonin effectively reverses the drug resistance of cisplatin involving induction of cell apoptosis and inhibition of MMP expression in human acute myeloid leukemia cells. Saudi J Biol Sci. 2017;24(3):678–686. doi: 10.1016/j.sjbs.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao Y., Xia H. Oridonin elevates sensitivity of ovarian carcinoma cells to cisplatin via suppressing cisplatin-mediated autophagy. Life Sci. 2019;233 doi: 10.1016/j.lfs.2019.116709. [DOI] [PubMed] [Google Scholar]
- 117.Chen S., Cooper M., Jones M. Combined activity of oridonin and wogonin in advanced-stage ovarian cancer cells: sensitivity of ovarian cancer cells to phyto-active chemicals. Cell Biol Toxicol. 2011;27(2):133–147. doi: 10.1007/s10565-010-9176-0. [DOI] [PubMed] [Google Scholar]
- 118.Weng H., Huang H., Dong B., Zhao P., Zhou H., Qu L. Inhibition of miR-17 and miR-20a by oridonin triggers apoptosis and reverses chemoresistance by derepressing BIM-S. Canc Res. 2014;74(16):4409–4419. doi: 10.1158/0008-5472.CAN-13-1748. [DOI] [PubMed] [Google Scholar]
- 119.Geserick P., Wang J., Schilling R. Absence of RIPK3 predicts necroptosis resistance in malignant melanoma. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng W., Zhou C.Y., Zhu X.Q. Oridonin enhances the cytotoxicity of 5-FU in renal carcinoma cells by inducting necroptotic death. Biomed Pharmacother. 2018;106:175–182. doi: 10.1016/j.biopha.2018.06.111. [DOI] [PubMed] [Google Scholar]
- 121.Zhang D., Zhou Q., Huang D. ROS/JNK/c-Jun axis is involved in oridonin-induced caspase-dependent apoptosis in human colorectal cancer cells. Biochem Biophys Res Commun. 2019;513(3):594–601. doi: 10.1016/j.bbrc.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 122.Wang B., Shen C., Li Y. Oridonin overcomes the gemcitabine resistant PANC-1/Gem cells by regulating GST pi and LRP/1 ERK/JNK signalling. OncoTargets Ther. 2019;12:5751–5765. doi: 10.2147/OTT.S208924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xiao X., He Z., Cao W. Oridonin inhibits gefitinib-resistant lung cancer cells by suppressing EGFR/ERK/MMP-12 and CIP2A/Akt signaling pathways. Int J Oncol. 2016;48(6):2608–2618. doi: 10.3892/ijo.2016.3488. [DOI] [PubMed] [Google Scholar]
- 124.Kadioglu O., Saeed M., Kuete V., Greten H.J., Efferth T. Oridonin targets multiple drug-resistant tumor cells as determined by in silico and in vitro analyses. Front Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shah N.P., Nicoll J.M., Nagar B. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Canc Cell. 2002;2(2):117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 126.Huang H., Weng H., Dong B., Zhao P., Zhou H., Qu L. Oridonin triggers chaperon-mediated proteasomal degradation of BCR-ABL in leukemia. Sci Rep. 2017;7 doi: 10.1038/srep41525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shan Q.Q., Guo Y., Gong Y.P., Lin J., Wang Y.S. Anti-Leukemia effect and mechanism of oridonin on imatinib-sensitive and imatinib-resistant K562 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25(5):1378–1383. doi: 10.7534/j.issn.1009-2137.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 128.Huang H.-L., Weng H.-Y., Wang L.-Q. Triggering Fbw7-mediated proteasomal degradation of c-Myc by oridonin induces cell growth inhibition and apoptosis. Mol Canc Therapeut. 2012;11(5):1155–1165. doi: 10.1158/1535-7163.MCT-12-0066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Combination therapy with oridonin in different in vitro models
Targets and signaling pathways of oridonin and its derivative compounds




