Abstract
p53 is a key tumor suppressor. As a transcription factor, p53 accumulates in cells in response to various stress signals and selectively transcribes its target genes to regulate a wide variety of cellular stress responses to exert its function in tumor suppression. In addition to tumor suppression, p53 is also involved in many other physiological and pathological processes, e.g. anti-infection, immune response, development, reproduction, neurodegeneration and aging. To maintain its proper function, p53 is under tight and delicate regulation through different mechanisms, particularly the posttranslational modifications. The tripartite motif (TRIM) family proteins are a large group of proteins characterized by the RING, B-Box and coiled-coil (RBCC) domains at the N-terminus. TRIM proteins play important roles in regulation of many fundamental biological processes, including cell proliferation and death, DNA repair, transcription, and immune response. Alterations of TRIM proteins have been linked to many diseases including cancer, infectious diseases, developmental disorders, and neurodegeneration. Interestingly, recent studies have revealed that many TRIM proteins are involved in the regulation of p53, and at the same time, many TRIM proteins are also regulated by p53. Here, we review the cross-talk between p53 and TRIM proteins, and its impact upon cellular biological processes as well as cancer and other diseases.
Keywords: Cancer, p53, Posttranslational modification, TRIM proteins, Tumor suppressor, Ubiquitination
Introduction
p53 plays a central role in tumor suppression, and disruption of p53 function leads to the initiation and/or progression of human cancers.1, 2, 3, 4, 5 The p53 gene is mutated in more than 50% of human cancers, and in almost every type of human cancers.1,3,5,6 It has been estimated that p53 function is impaired in majority of human cancers.7 Indeed, in addition to p53 mutations, the p53 signaling is often attenuated through different mechanisms in human cancers. For example, many DNA tumor viruses encode proteins that directly bind to p53 and inactivate it, such as the large T-antigen of simian virus 40 (SV40), the E6 oncoprotein of human papilloma virus (HPV) types 16 and 18, and the E1B-55 kDa protein of human adenovirus type 5.8,9 Furthermore, overexpression of many negative regulators of p53, such as MDM2, MDM4 (also known as MDMX), and PPM1D (also known as Wip1), can inactivate p53 in human cancers.1,10,11
As a transcription factor, p53 functions as a homotetramer and binds to the p53-responsive elements in p53 target genes to transcribe these genes in cells.12,13 The p53-responsive element is composed of two decamers separated by a spacer of 0–14 nucleotides: RRRCWWGYYY (spacer) RRRCWWGYYY, where R is a purine, W is A or T, and Y is a pyrimidine.9,12,13 So far, a large number of protein-coding genes have been identified to be direct p53 target genes. In addition, p53 also transcriptionally regulates non-coding genes, such as microRNAs (miRNAs) and long noncoding RNAs (LncRNAs), to exert its function in tumor suppression.14, 15, 16, 17 Through transcriptional regulation of select target genes in a highly context-dependent manner, p53 regulates a wide variety of important cellular processes, including cell cycle arrest, senescence, DNA repair, apoptosis, autophagy, ferroptosis, metabolism, and antioxidant defense, to maintain genomic integrity and tumor suppression2, 3, 4, 5,18,19 (Fig. 1). In addition to tumor suppression, studies have demonstrated that p53 is also involved in many other biological and pathological processes, including anti-infection, immune response, maternal reproduction, development, metabolic diseases, neurodegeneration, aging, etc.4,5,20, 21, 22, 23, 24, 25, 26
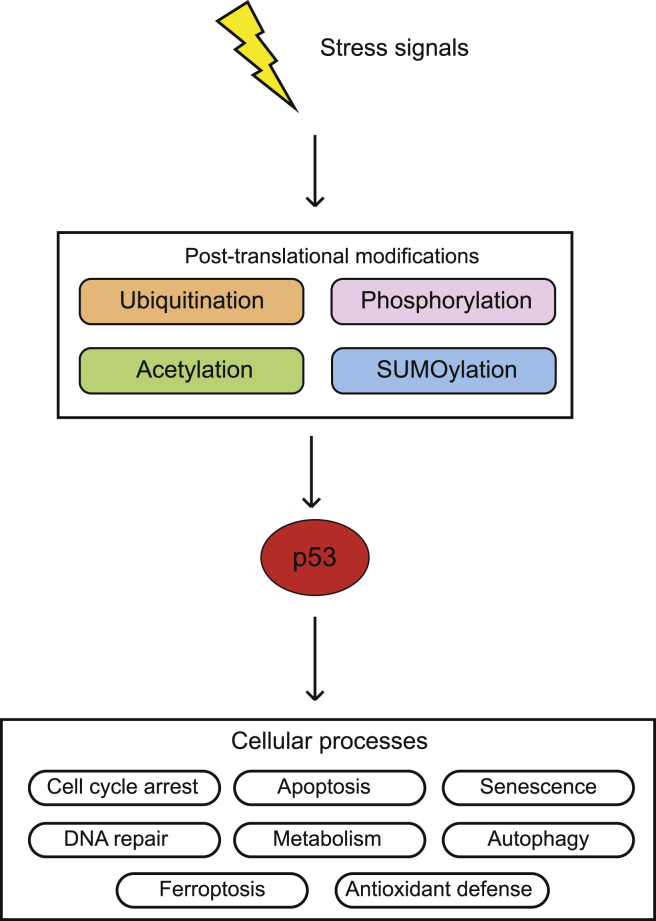
Figure 1.
The p53 signaling pathway. In response to stress signals, p53 protein is accumulated and activated to transcribe select target genes to regulate a wide variety of cellular processes, including cell cycle arrest, senescence, DNA repair, apoptosis, autophagy, ferroptosis, metabolism, and antioxidant defense. The posttranslational modifications of p53, including ubiquitination, phosphorylation, acetylation and SUMOylation, etc., play a critical role in regulation of p53 protein levels and activity in cells.
The tripartite motif (TRIM) proteins belong to a large protein family, which is characterized by the presence of a conserved N-terminal RBCC module consisting of a Really Interesting New Gene (RING) domain, one or two B-Boxes (B1/B2) and a coiled-coil (CC) domain.27, 28, 29 So far, more than 80 TRIM family members have been identified in humans. These TRIM proteins have been reported to be involved in a variety of important biological processes including transcription, signal transduction, cell proliferation, apoptosis, DNA repair, cell differentiation, stemness, antiviral infection and immune response.27, 28, 29, 30, 31 Alterations of functions and/or levels of TRIM proteins have been linked to different diseases including infectious diseases, developmental diseases, neuropsychiatric disorders, cardiovascular diseases, as well as cancers.27,28,31, 32, 33
Interestingly, recent studies have reported that many TRIM proteins regulate p53 levels and activity, and at the same time, p53 regulates expression of some TRIM proteins.34,35 The cross-talk between the p53 signaling pathway and TRIM family proteins plays an important role in regulating cellular biological processes and impacts cancer and other diseases. In this review, we summarize recent advances in studies on the cross-talk between the p53 signaling pathway and TRIM family proteins.
p53 and its regulation
Given the critical function of p53 in tumor suppression and many other fundamental biological processes, p53 protein levels and activity are under tight scrutiny and regulation in cells. Usually, under non-stressed conditions, p53 protein has a very short half-life and its levels are maintained at low in normal cells and tissues. In response to a wide variety of stress signals, including DNA damage, hypoxia, nutritional deprivation, impaired ribosome biogenesis, activation of oncogenes, etc., p53 protein half-life is increased and p53 protein accumulates in cells, leading to the activation of p53 transcriptional program to initiate various above-mentioned cellular responses.2,4,18 Many different mechanisms have been reported to regulate p53, among which the post-translational modifications are known to be the most critical and efficient mechanism to regulate the level, conformation, localization and activity of p53.4,36−39 These post-translational modifications include ubiquitination, phosphorylation, acetylation, SUMOylation, methylation, neddylation, etc.4,36, 37, 38, 39 Many proteins are involved in the regulation of p53, including E3 ubiquitin ligases, deubiquitinases, kinases, phosphatases, acetyltransferases, deacetylases, enzymes involved in SUMOylation and deSUMOylation, methylases, etc.4,36, 37, 38, 39 (Fig. 1).
Ubiquitination is one of the most important posttranslational modifications for p53. Ubiquitination, which conjugates ubiquitin to the lysine (K) residues of substrate proteins, plays an important role in protein regulation, including protein degradation via the proteasome, regulation of the localization and activity of proteins, and modulating protein–protein interactions.40 Ubiquitin can be interlinked via any of its lysines (K6, K11, K27, K29, K33, K48 and K63). K48-linked ubiquitination usually induces proteasomal degradation of substrate proteins, whereas K63-linked polyubiquitin often affects the activity or localization of substrate proteins, or their abilities to interact with other proteins.40 Ubiquitination is a dynamic process catalyzed by E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin ligases that are responsible for recognition of substrate proteins.40 E3 ubiquitin ligase MDM2 is the most critical negative regulator of p53, which directly binds to and ubiquitinates p53 and leads to proteasomal degradation of p53.10,11 MDM2 is also a p53 target gene, which is transcriptionally induced by p53. Thus, MDM2 and p53 forms a negative feedback loop to keep p53 levels under fine-tuned control in cells.10,11 MDM2 knockout in mice results in embryonic lethality due to uncontrolled activation of p53 and p53-mediated apoptosis, which can be rescued by p53 knockout, demonstrating the vital role of MDM2 in negative regulation of p53.41,42 In addition to mediating proteasomal degradation of p53, MDM2 promotes p53 nuclear export to inhibit p53 transcriptional activation of its target genes.43,44 In addition to MDM2, MDM4 is a protein sharing structural homology with MDM2 and functions as another important negative regulator of p53.10,11 Although MDM4 has no detectable E3 ubiquitin ligase activity, it promotes MDM2-mediated ubiquitination and degradation of p53.10,11 Like MDM2, MDM4 knockout in mice results in the embryonic lethality, which can be rescued by p53 knockout, indicating its critical role in negative regulation of p53 in vivo.45 MDM2 and MDM4 are frequently amplified and/or overexpressed in different types of human cancers, and their overexpression is often mutually exclusive with p53 mutations in many human cancers, supporting the important roles of MDM2 and MDM4 in tumorigenesis through negative regulation of p53.1,10,11
In addition to MDM2, many other E3 ubiquitin ligases have been reported to ubiquitinate p53 for proteasomal degradation, such as Pirh2, Cop1, ARF-BP1, CHIP, UBE2T, RBCK1, and SYVN1.4,36, 37, 38 Like MDM2, some of these E3 ubiquitin ligases are also p53 target genes, such as Pirh2 and Cop1, which form negative feedback loops with p53. Moreover, some E3 ubiquitin ligases, including MDM2, WWP1 and MSL-2, can promote nuclear export of p53 through mono-ubiquitination of p53, removing p53 from the nucleus where p53 functions as a transcription factor to reduce its transcriptional activity.4,36, 37, 38 On the other hand, some deubiquitinases can bind to p53 and remove ubiquitin on p53. For instance, deubiquitinases USP7 (also called HAUSP), USP15 and USP49 deubiquitinate p53 to stabilize p53.36,46, 47, 48 UPS10 deubiquitinates p53 to stabilize p53 and also reverses MDM2-induced p53 nuclear export, leading to p53 activation.49 Interestingly, the deubiquitinase Otub1 abrogates p53 ubiquitination to stabilize p53 by inhibiting the MDM2 cognate ubiquitin-conjugating enzyme (E2) UbcH5 independently of its deubiquitinating enzyme activity.50 Furthermore, USP7 can either deubiquitinate p53 to stabilize it or deubiquitinate MDM2 to promote MDM2-mediated ubiquitination and degradation of p53 depending on the cellular context.51
In addition to ubiquitination, phosphorylation, acetylation and SUMOylation also play important roles in regulation of p53 levels and activity. In response to DNA damage, ATM and Chk1/2 kinases phosphorylate p53 at serine 15 and serine 20, respectively, and protect p53 from degradation by MDM2 to stabilize and activate p53.52,53 On the contrary, PPM1D54 and PP1 (protein phosphatase 1)55 inactivate p53 by dephosphorylating p53. PPM1D is frequently amplified and/or overexpressed in cancers, and its overexpression is often mutually exclusive with p53 mutations in cancers.1 The transcriptional coactivators CBP/p300 acetylate p53 at several lysines to activate p53 transcriptional activity.56,57 Some histone acetyltransferase (HAT) proteins, such as Tip60, MOF and MOZ, can promote p53 acetylation to increase p53 transcriptional activity.57, 58, 59, 60 In contrast, some histone deacetylases (HDACs), such as HDAC1 and HDAC8, deacetylate p53 and inhibit p53 transcriptional activity.61,62 p53 can also be modified by SUMOylation, the reversible modification of proteins by the small ubiquitin-like modifiers (SUMOs), which affects the stability, localization, activity and interaction of proteins.63 However, the biological consequence of p53 SUMOylation is still not clear, which might be affected by the SUMOylation site, cell and tissue types, stress signals, cellular microenvironment, and even experimental models and conditions.36,37,64 The SUMOylation modification of p53 was reported to enhance p53 transcriptional activity, or suppress p53 transcriptional activity and promote p53 nuclear export.36,37,64 Further studies are required to better understand the effect of the SUMOylation modification on p53.
p53 is also regulated by many non-coding genes, such as miRNAs and LncRNAs, which form a new layer of regulation for p53 in cells.14, 15, 16, 17 For instance, some miRNAs target p53 or its positive regulators to decrease p53 protein levels and activity.15,65, 66, 67, 68 In contrast, some miRNAs target negative regulators of p53, such as MDM2 and MDM4, to increase p53 protein levels and activity.15,17,65,68 Similarly, LncRNAs can exert inhibitory or activating effects on p53 through targeting p53 and its regulators.14,16 Thus, like these above-mentioned proteins, many miRNAs and LncRNAs function as negative or positive regulators for p53 and its signaling pathway.
TRIM family proteins and diseases
While most TRIM family proteins in humans possess the conserved N-terminal RBCC domains, TRIM proteins in humans can be subclassified into 11 subfamilies based on the differences in their C-terminal domain structure.27, 28, 29 The RING domain, a specialized type of zinc finger that binds a pair of zinc atoms and is involved in mediating protein–protein interactions, is present in many E3 ubiquitin ligases to confer E3 ligase activity.40 Through the RING domain, many TRIM proteins have the E3 ubiquitin ligase activity and are able to ubiquitinate substrate proteins to regulate their degradation, cellular locations, activities, and/or interactions with other proteins.27, 28, 29 In addition to the ubiquitination modification, some TRIM proteins can catalyze SUMOylation and ISGylation modifications of substrate proteins by ubiquitin-like modifiers SUMOs and ISG15 (Interferon-stimulated gene 15), respectively, to regulate the stability, localization, activity, or function of substrate proteins.27, 28, 29 The B-box domain of some TRIM proteins also contain zinc-binding motifs similar to the RING domain, which has been shown to modulate higher-order self-assembly, E3 ligase activity, and interaction with other proteins.69 For instance, TRIM16, which does not possess the RING domain, still has the E3 ubiquitin ligase activity due to a RING-like fold in its B-box domain.70 The coiled-coil domain can mediate oligomerization of TRIM proteins. Many TRIM proteins can form homodimers, and some TRIM proteins can form heterodimers (e.g. TRIM19-TRIM27, TRIM19-TRIM24, TRIM24-TRIM33 and TRIM24-TRIM28), and even heterotrimers (e.g. TRIM24-TRIM33-TRIM28).69,71 These dimerizations and higher-order assemblies of TRIM proteins may allow the TRIM proteins to perform different tasks and maintain the flexibility to adapt these structures to different intracellular and extracellular stimuli.69 The C-terminal region of TRIM proteins is responsible for the substrate recognition.72
Many TRIM proteins have been reported to play important roles in the antiviral host response.28,73 For example, TRIM28 inhibits integration of HIV-1 into the host genome via deacetylation of integrase.74 TRIM25 and TRIM65 induce K63-linked polyubiquitination of the viral RNA sensors RIG-I and MDA5, respectively, to facilitate the antiviral innate immune response.75,76 TRIM14 binds to cGAS and recruits USP14 to deubiqutinate and stabilize cGAS, enhancing the antiviral response against herpes simplex virus type 1 (HSV-1).77 Furthermore, TRIM56 and TRIM32 induce K63-linked polyubiquitination of STING to facilitate the induction of the antiviral response.78,79 In contrast, TRIM40 promotes K27- and K48-linked polyubiquitination and proteasomal degradation of both RIG-I and MAD5 to attenuate the antiviral immune responses.80 TRIM29 induces K48-linked ubiquitination of STING and promotes its degradation to repress innate immune responses.81
Some TRIM proteins have been linked to developmental diseases and neurodegenerative diseases.32 For instance, loss-of-function mutations in the TRIM18 gene (also known as MID1) are responsible for the X-linked form of Opitz G/BBB Syndrome (XLOS), a disorder characterized by defects in the development of embryonic midline structures.82 TRIM18 knockout mice display cerebellar development defects.83 TRIM32 ubiquitinates and degrades dysbindin and plays an important role in preventing two developmental diseases, limb-girdle muscular dystrophy and Bardet-Biedl syndrome.84 Mutations in the TRIM37 gene cause muscle-liver-brain-eye (mulibrey) nanism, a rare prenatal-onset autosomal recessive growth disorder. TRIM37 interacts with and mono-ubiquitinates peroxisomal targeting signal receptor PEX5 protein, whose deficiency causes fatal human peroxisomal biogenesis disorders, leading to stabilization of PEX5 and promoting peroxisomal targeting signal protein import, to prevent muscle-liver-brain-eye (mulibrey) nanism.85 TRIM2, which is highly expressed in the nervous system, binds to neurofilament light subunit (NF-L) and ubiquitinates it for degradation.86 TRIM2 deficiency in mice leads to NF-L accumulation in the nervous system and neurodegeneration.86 TRIM11 negatively regulates humanin, a neuroprotective peptide that specifically suppresses Alzheimer's disease-related neurotoxicity.87 Increased α-synuclein expression has been linked to Parkinson's disease. TRIM41 is an E3 ubiquitin ligase for ZSCAN21, a transcription factor that induces α-synuclein expression in neuronal cells.88 Furthermore, some rare genetic variants in ZSCAN21 and TRIM41 genes have been identified in patients with familial forms of Parkinson's disease, and their overexpression leads to the stabilization of the ZSCAN21 protein in neuronal cells.88
Interestingly, TRIM proteins are also involved in cardiovascular diseases.33 For instance, TRIM63 and TRIM54, also known as the muscle RING-finger protein-1 and 3 (MuRF1 and 3), respectively, act as E3 ubiquitin ligases and mediate ubiquitination and proteasomal degradation of beta/slow myosin heavy chain (beta/slow MHC) and MHCIIa.89 Mice deficient for TRIM63 or TRIM54 develop a skeletal muscle myopathy and hypertrophic cardiomyopathy characterized by subsarcolemmal MHC accumulation, myofiber fragmentation, and diminished muscle performance.89 Furthermore, TRIM54 degrades four-and-a-half LIM domain (FHL2) and γ-filamin, and TRIM54 deficient mice are more prone to cardiac rupture after acute myocardial infarction.90 Mutations in TRIM55 gene (also known as MuRF2) have been associated with familial hypertrophic cardiomyopathy. TRIM55 can protect against diabetic cardiomyopathy through mono-ubiquitination of transcription factors PPARα and PPARγ1 to stabilize them in the heart of mice, and TRIM55 knockout in mice results in the exaggerated diabetic cardiomyopathy.91
More and more studies have also shown that TRIM proteins are involved in cancer; some TRIM proteins suppress tumorigenesis, whereas some TRIM proteins promote tumorigenesis through different mechanisms. Altered expression of many TRIM proteins have been observed in different types of cancers.27,92,93 Furthermore, some TRIM proteins are also involved in chromosomal translocations and fused to other genes in cancers, resulting in the oncogenic gain-of-function.27,92, 93, 94 For example, TRIM19 is fused to retinoic acid receptor-α in the t (15; 17) translocation of acute promyelocytic leukemia; TRIM24 is fused to FGFR1 in the t (7; 8) translocation of 8p11 myeloproliferative syndrome and to BRAF in the t (7; 7) translocation of liver cancer; TRIM33 and TRIM27 are fused to RET in the t (1; 10) and t (6; 10) translocation of papillary thyroid carcinomas, respectively.27,92, 93, 94 Interestingly, a growing body of studies have reported that many TRIM proteins cross-talk with p53 and its signaling pathway to impact cancer and other diseases,34,35 which is summarized as follows.
TRIM proteins regulate p53 and its function
TRIM proteins that bind to and regulate p53
Studies have shown that quite a few TRIM proteins with the RING domain can bind to p53, such as TRIM24, TRIM39, TRIM32, TRIM59, TRIM31, TRIM71, TRIM69 and TRIM23, leading to ubiquitination and degradation of p53 (Fig. 2A). TRIM24, also known as transcription intermediary factor 1α (TIF1α), was the first reported TRIM protein that directly targetes p53.95 TRIM24 binds to p53 and promotes its ubiquitination, leading to decreased p53 levels and transcriptional activity in a RING-dependent manner.95 Furthermore, TRIM24 can be phosphorylated at serine 768 by ATM in response to DNA damage, leading to the degradation of TRIM24 and the subsequent stabilization and activation of p53.96 Increased expression of TRIM24 was observed in many types of tumors, including breast cancer, prostate cancer and glioblastoma, which may contribute to p53 dysfunction in these tumors.97, 98, 99 TRIM39 (also known as RNF23) can also bind to p53 and ubiquitinate p53 for degradation in a RING-dependent manner.100 Interestingly, TRIM39 binds to p21, an important p53 target that mediates p53 function in inducing cell cycle arrest, and blocks p21 ubiquitination and degradation mediated by E3 ubiquitin ligase CRL4Cdt2, which in turn contributes to the role of TRIM39 in tumor suppression.101 TRIM32, TRIM59 and TRIM31 have also been reported to interact with p53 and ubiquitinate p53, leading to the proteasomal degradation of p53.102, 103, 104 TRIM32 negatively regulates p53 levels to reduce the p53-dependent apoptosis, cell cycle arrest and senescence.102 TRIM32 overexpression has been observed in different types of human cancers, including colorectal, lung, liver, and breast cancers, which promotes proliferation and chemoresistance of cancer cells and is associated with poor prognosis of cancer patients.102,105, 106, 107, 108 TRIM59 was reported to be overexpressed in human gastric tumors, leading to the decreased p53 levels and enhanced cell proliferation and metastasis.103 TRIM31, which is up-regulated in the anchorage-deprived hepatocellular carcinoma (HCC) cells, enhances K48-linked poly-ubiquitination and degradation of p53, which in turn promotes resistance of HCC cells to anoikis, a form of programmed cell death that occurs in anchorage-dependent cells when they detach from the surrounding extracellular matrix.104 TRIM71 (also known as LIN41) binds to p53 and degrades p53 through ubiquitination to antagonize p53-dependent pro-apoptosis and pro-differentiation during stem cell differentiation.109 TRIM69 interacts with p53 and induces its ubiquitination and degradation in human lens epithelial cells, and inhibition of TRIM69 expression leads to p53 activation and cataract formation.110 TRIM69 expression is up-regulated in pregnancy-associated breast cancer, suggesting its potential role in this type of cancer through inhibition of p53 function.111 Additionally, TRIM23, which is overexpressed in colorectal cancer and associated with poor prognosis of colorectal cancer patients, interacts with p53 and results in the ubiquitination and degradation of p53, thereby promoting cancer cell proliferation.112
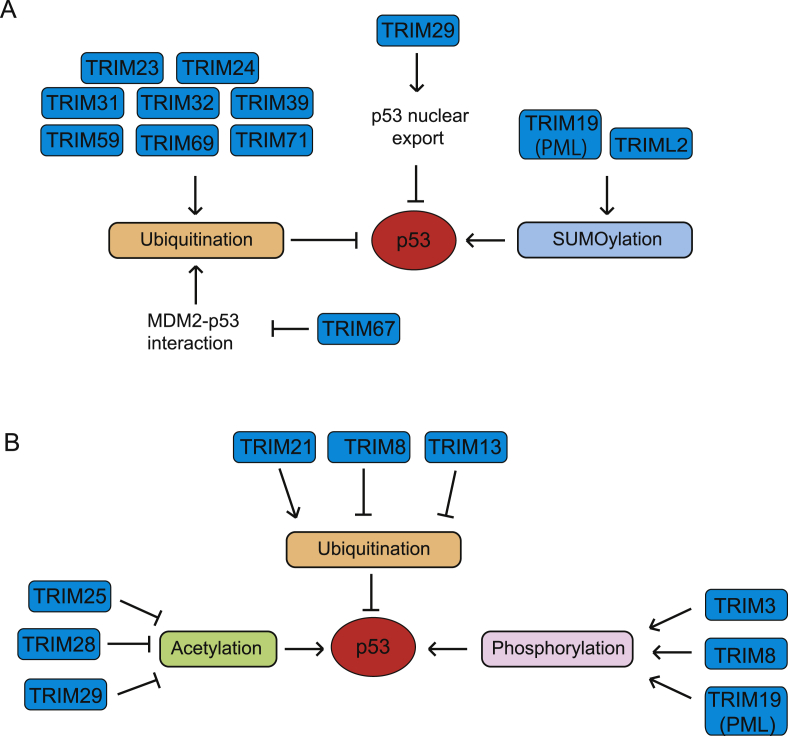
Figure 2.
TRIM proteins regulate p53. (A) TRIM proteins that interact with p53 and directly regulate p53. TRIM23, TRIM24, TRIM31, TRIM32, TRIM39, TRIM59, TRIM69, and TRIM71 negatively regulate p53 through binding to and ubiquitinating p53, whereas TRIM19 (PML), TRIM67 and TRIML2 bind to and positively regulate p53. In addition, TRIM29 binds to and sequesters p53 in the cytoplasm to negatively regulate p53. (B) TRIM proteins that indirectly regulate p53 through regulating upstream p53 regulators. Through modulating the regulators for p53, TRIM3, TRIM8, TRIM13, and TRIM19 positively regulate p53, whereas TRIM21, TRIM25, TRIM28, and TRIM29 negatively regulate p53.
In addition to ubiquitination, TRIM proteins also modify p53 with SUMO. TRIM19, also known as promyelocytic leukemia protein (PML), is essential for the formation of the subnuclear structure known as PML nuclear bodies (PML-NBs), which are thought to be sites of transcription, DNA repair and viral replication. TRIM19 directly binds to p53 and induces its SUMOylation at lysine 386, leading to stabilization and enhanced transcriptional activity of p53.113,114 Similarly, TRIML2 was reported to bind to p53, promoting the SUMOylation of p53 and leading to transactivation of pro-apoptotic p53 target genes.115 Interestingly, TRIML2 was reported to be overexpressed in oral squamous cell carcinoma and triple-negative breast cancer,116,117 suggesting that the positive regulation of p53 by TRIML2 may be cancer type specific.
TRIM29, also known as ataxia telangiectasia group D-complementing (ATDC), is a TRIM protein without the RING domain. TRIM29 interacts with p53 and sequesters p53 in the cytoplasm, which in turn inhibits p53 transcriptional activity in colorectal cancer cells.118 Interestingly, TRIM29 was reported to be overexpressed in multiple types of human cancers, including colorectal cancer and gastric cancer,119, 120, 121 but downregulated in HCC and head and neck squamous cell carcinomas,122,123 suggesting the tissue and tumor type specific function of TRIM29. In addition, TRIM67, which is frequently downregulated in colorectal cancer, binds to p53 to inhibit MDM2-mediated p53 ubiquitination and degradation. Knockout of TRIM67 accelerates colorectal tumorigenesis in ApcMin/+ mice and chemical carcinogen azoxymethane-induced colorectal cancer in mice.124
TRIM proteins regulating p53 indirectly
In addition to binding to p53 to regulate p53 directly, some TRIM proteins also indirectly regulate p53 levels and activity (Fig. 2B). Several TRIM proteins have been reported to regulate p53 through modulating MDM2. TRIM13, also known as ret finger protein 2 (RFP2) or LEU5 (leukemia associated gene 5), forms a complex with MDM2, leading to ubiquitination and degradation of MDM2 in a RING-dependent manner, which in turn stabilizes p53 and induces apoptosis.125 TRIM13 is induced by γ-irradiation, which may contribute to the accumulation of p53 protein in cells and p53-mediated apoptosis in response to γ-irradiation.125 TRIM13 is deleted in some B-cell chronic lymphocytic leukemia, and its expression is decreased in breast cancer and non-small-cell lung carcinoma, suggesting that loss of TRIM13 may contribute to p53 dysfunction in these cancers.126, 127, 128 TRIM8 stabilizes p53 by degradation of MDM2 in a RING-dependent manner, leading to cell cycle arrest and inhibition of cell proliferation.129 The expression of TRIM8 is decreased in clear cell renal cell carcinomas and gliomas.130,131 Further, the downregulation of TRIM8 expression in gliomas is associated with poor prognosis of cancer patients.131
Some TRIM proteins have been reported to regulate p53 activity through modulating the phosphorylation and acetylation modifications of p53. PML (TRIM19) recruits p53 into the PML-NBs, mediating the p53 phosphorylation by HIPK2 at serine 46 and thus facilitating p53 acetylation by CBP at lysine 382, which enhances p53 transcriptional activity and induces growth arrest and apoptosis of cells.132 PML also recruits Chk2 and CK1 kinases into the PML-NBs, enhancing p53 phosphorylation at serine 20 and threonine 18, respectively, in response to DNA damage, which in turn inhibits MDM2-mediated ubiquitination and degradation of p53.133,134 Furthermore, PML mediates p53 acetylation at lysine 120 and lysine 382 by recruiting MOZ/KAT6A, enhancing p53 transcription activity and p53-mediated senescence.135
TRIM8 can enhance p53 phosphorylation at serines 15 and 20 through an unknown mechanism, leading to the transcriptional activation of p53 targets involved in cell cycle arrest and apoptosis.129 In cervical cancer cells, TRIM3 activates p53 through regulating p38 MAPK signaling, which is known to phosphorylate p53 at serine 33.136,137 The downregulation of TRIM3 expression has been reported in liver cancer and colorectal cancer and is associated with poor prognosis in cancer patients.138,139 TRIM28, also known as KRAB-associated protein 1 (KAP1) or transcriptional intermediary factor 1 β (TIF-1β), binds to MDM2 and stimulates the formation of p53-HDAC1 complex and inhibits p53 acetylation, promoting p53 ubiquitination and degradation to inhibit cellular apoptosis.140 In line with its role as a negative regulator of p53, overexpression of TRIM28 has been observed in lung, breast and cervical cancers.141, 142, 143 TRIM29 (ATDC) binds to the acetyltransferase Tip60 and promotes its degradation to inhibit the acetylation of p53 at lysine 120 by Tip60, which in turn promotes cell proliferation and enhances transforming ability of cells in soft agar.144 TRIM25 interferes with the formation of p53-MDM2-p300 complex, suppressing the acetylation of p53 to inhibit p53-dependent cell death and DNA damage response.145 In addition, TRIM25 interacts with G3BP2 and promotes the G3BP2/RanBP2-mediated SUMOylation and nuclear export of p53 to inhibit p53 transcriptional activity, which in turn promotes cell proliferation and inhibits chemotherapeutic agent docetaxel-induced apoptosis of prostate cancer cells.146 TRIM21 (also known as Ro52) regulates p53 via a mechanism involving USP7 and the guanosine 5′-monophosphate synthase (GMPS).147 Under non-stress conditions, TRIM21 binds to and ubiquitinates GMPS and sequesters GMPS in the cytoplasm. Under stress conditions, GMPS is released from its interaction with TRIM21 and translocates to the nucleus, where it transfers p53 from MDM2 to a GMPS-USP7 deubiquitination complex, leading to p53 stabilization and activation.147
Other TRIM proteins regulating p53
In addition to the above-mentioned TRIM proteins, some TRIM proteins have also been reported to regulate p53 with unclear mechanisms. For instance, TRIM66 and TRIM11 negatively regulate p53 protein levels.148,149 TRIM66, also known as transcription intermediate factor 1δ (TIF1 δ), is a TRIM protein without the RING domain. TRIM66 is overexpressed in osteosarcomas.148 TRIM66 suppresses the apoptosis of osteosarcoma cells by down-regulating protein levels of p53, and caspases 7 and 9.148 TRIM11 is highly expressed in breast cancer, lung cancer, liver cancer and ovarian cancer.149, 150, 151, 152 TRIM11 negatively regulates p53 protein levels to promote proliferation, migration, and invasion of HCC cells.149 However, the mechanisms by which TRIM66 and TRIM11 negatively regulate p53 protein levels remain unclear. In addition, TRIM52 was reported to promote the cell cycle progression of specific glioblastoma cell lines in a p53-dependent manner, suggesting that TRIM52 negatively regulates p53.153 Currently, the precise effect of TRIM52 on p53 and its underlying mechanism are unclear, which need to be addressed by future studies.
TRIM proteins regulated by p53
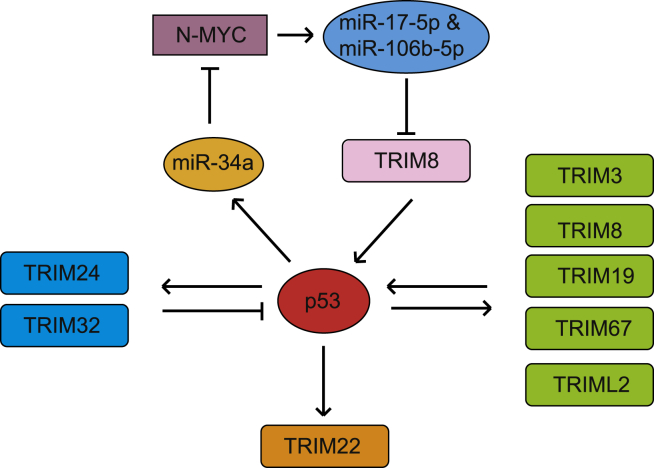
While many TRIM proteins regulate p53 as summarized above, many TRIM proteins are also regulated by p53. Studies have shown that genes encoding some TRIM proteins are direct p53 target genes, and some TRIM proteins can be indirectly regulated by p53 through different mechanisms (Fig. 3). For example, TRIM24 and TRIM32, two TRIM proteins that ubiquitinate p53, are direct transcriptional targets of p53.96,102 p53 binds to the p53-responsive elements in TRIM24 and TRIM32 genes and induces their expression in response to stress. At the same time, TRIM24 and TRIM32 can bind to p53 and ubiquitinate and degrade p53. Thus, like MDM2, TRIM24 and TRIM32 form negative feedback loops with p53 to regulate p53 levels and activity.96,102 In addition to these two p53 negative regulators, TRIM19 (PML), TRIM3, TRIM8 and TRIM67, which positively regulate p53 levels and activity, are also direct p53 target genes.124,129,154,155 Therefore, TRIM19 (PML), TRIM3 (BERP), TRIM 8, and TRIM67 form positive feedback loops with p53, which may amplify p53 signaling in response to stress signals in cells. As a p53 target, TRIM3 regulates the intracellular trafficking of GABA(A) receptors to the postsynaptic membrane, and TRIM3 deficient mice exhibit increased resistance to pentylenetetrazol-induced seizures, suggesting a potential role for p53 in the central nervous system.154 In addition to the direct transcriptional regulation by p53, TRIM8 can also be regulated by p53 indirectly. N-MYC promotes expression of miR-17-5p and miR-106b-5p to negatively regulate TRIM8 in clear cell renal cell carcinoma and colorectal cancer.156 It is known that N-MYC protein levels is downregulated by the miR-34a,157 which is a transcription target of p53.158 Thus, p53 indirectly induces TRIM8 expression via the miR-34a/N-MYC/miR-17-5p & miR-106b-5p pathway, and thereby forms a positive feedback loop with TRIM8 to regulate cell proliferation and therapeutic responsiveness.156 TRIML2 is a direct target of p53, and interestingly, its expression is preferentially induced by a p53 variant with arginine at codon 72.115 Since TRIML2 stabilizes p53 by SUMOylation, TRIML2 forms a positive feedback loop to activate p53 particularly in cells with the p53 variant with arginine at codon 72.115 In addition, TRIM22 (also known as Staf50) is also a p53 target gene; p53 induces TRIM22 expression.159 Ectopic expression of TRIM22 reduces clonogenic growth of leukemic cells, suggesting that TRIM22 contributes to the tumor suppressive function of p53.159 The downregulation of TRIM22 expression has been reported in breast cancer and Wilms tumors,160,161 which may contribute to the dysfunction of p53 in these tumors.
Figure 3.
TRIM proteins regulated by p53. p53 transcriptionally regulates TRIM3, TRIM8, TRIM19, TRIM22, TRIM24, TRIM32, TRIM67, and TRIML2. At the same time, TRIM24 and TRIM32 negatively regulate p53 and form negative feedback loops with p53; TRIM3, TRIM8, TRIM19, TRIM67 and TRIML2 positively regulate p53 and form positive feedback loops with p53. In addition, TRIM8 is regulated by p53 through p53 target miR-34a, which forms a positive feedback loop with p53.
Conclusion and perspective
As summarized above, p53 plays a pivotal role in cell fate decision in response to various exogenous and endogenous stress signals. A wide variety of proteins and many different mechanisms have been reported to regulate p53 in a highly dynamic and context-dependent manner to maintain proper p53 levels and activity for accurately exerting its function in cell fate decision. Interestingly, recent studies have revealed that many TRIM proteins are negative or positive regulators for p53, and at the same time, many TRIM proteins are regulated by p53 to mediate p53 functions in cellular stress response and tumor suppression. This cross-talk between p53 and TRIM proteins adds a new layer of regulation to this already very complex p53 signaling pathway. While these findings bring TRIM proteins as exciting new players in the p53 signaling pathway, they also raise some important questions which need to be addressed. For example, why do cells need so many TRIM proteins to regulate p53 when there are already so many other proteins, especially many other E3 ubiquitin ligases, involved in the p53 regulation? How is the cross-talk between p53 and TRIM proteins precisely regulated in different physiological and pathological processes? Can p53 be regulated by these TRIM proteins in cell, tissue and organ type-specific, stress signal-specific, and developmental stage-specific manners? While many studies on the cross-talk between p53 and TRIM proteins are derived from in vitro cell culture systems, it is unclear whether all of these regulations can be observed in vivo in animal models. These are vital and urgent questions to be solved by future studies, particularly studies with different TRIM transgenic animal models. In addition to tumor suppression, p53 has been known to regulate many other physiological and pathological processes, including anti-viral infection, metabolism, reproduction, neurodegeneration, and aging. While many studies on TRIM proteins have focused on the role of p53 in tumor suppression, future studies are needed to determine the contribution of TRIM proteins to the roles of p53 in the above-mentioned biological processes and diseases. For example, both p53 and TRIM proteins have been shown to play a critical role in anti-viral infection and immune response. However, it remains unclear how p53 and TRIM proteins cooperate to efficiently modulate the immune system to exert the anti-viral infection function. Similarly, while both p53 and TRIM proteins have been shown to be involved in neurodegenerative diseases, the impact of their cross-talk upon neurodegenerative diseases is largely unknown. Answering these questions will help us further understand the cross-talk between p53 and TRIM family proteins, and its impact upon biological and pathological processes, which will benefit the clinical application of targeting p53 signaling pathway and TRIM family proteins for therapies in cancer and other diseases including infection diseases and neurodegenerative diseases.
Conflict of Interests
These authors declare no conflicts of interest.
Funding
This work was supported in part by grants from NIH (grant number R01CA214746) and DOD (grant number BC171968) to Z.F., and by grants from NIH (grant number R01CA203965) and DOD (grant number W81XWH-16-1-0358) to W.H.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Wenwei Hu, Email: wh221@cinj.rutgers.edu.
Zhaohui Feng, Email: fengzh@cinj.rutgers.edu.
References
- 1.Donehower L.A., Soussi T., Korkut A. Integrated analysis of TP53 gene and pathway alterations in the cancer genome atlas. Cell Rep. 2019;28(5):1370–1384. doi: 10.1016/j.celrep.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vousden K.H., Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137(3):413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 3.Muller P.A., Vousden K.H. p53 mutations in cancer. Nat Cell Biol. 2013;15(1):2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 4.Levine A.J. The many faces of p53: something for everyone. J Mol Cell Biol. 2019;11(7):524–530. doi: 10.1093/jmcb/mjz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vousden K.H., Lane D.P. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8(4):275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 6.Yue X., Zhao Y., Xu Y., Zheng M., Feng Z., Hu W. Mutant p53 in cancer: accumulation, gain-of-function, and therapy. J Mol Biol. 2017;429(11):1595–1606. doi: 10.1016/j.jmb.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polager S., Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9(10):738–748. doi: 10.1038/nrc2718. [DOI] [PubMed] [Google Scholar]
- 8.Levine A.J. The common mechanisms of transformation by the small DNA tumor viruses: the inactivation of tumor suppressor gene products: p53. Virology. 2009;384(2):285–293. doi: 10.1016/j.virol.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 9.Freed-Pastor W.A., Prives C. Mutant p53: one name, many proteins. Genes Develop. 2012;26(12):1268–1286. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karni-Schmidt O., Lokshin M., Prives C. The roles of MDM2 and MDMX in cancer. Annu Rev Pathol. 2016;11:617–644. doi: 10.1146/annurev-pathol-012414-040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y., Yu H., Hu W. The regulation of MDM2 oncogene and its impact on human cancers. Acta Biochim et Biophys Sin. 2014;46(3):180–189. doi: 10.1093/abbs/gmt147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.el-Deiry W.S., Kern S.E., Pietenpol J.A., Kinzler K.W., Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992;1(1):45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- 13.Feng Z., Levine A.J. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 2010;20(7):427–434. doi: 10.1016/j.tcb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dangelmaier E., Lazar S.B., Lal A. Long noncoding RNAs: p53's secret weapon in the fight against cancer? PLoS Biol. 2019;17(2) doi: 10.1371/journal.pbio.3000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., Zhang C., Zhao Y., Feng Z. MicroRNA control of p53. J Cell Biochem. 2017;118(1):7–14. doi: 10.1002/jcb.25609. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhary R., Lal A. Long noncoding RNAs in the p53 network. Wiley Interdiscip Rev RNA. 2017;8(3) doi: 10.1002/wrna.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Z., Cui R., Tili E., Croce C. Friend or foe: MicroRNAs in the p53 network. Cancer Lett. 2018;419:96–102. doi: 10.1016/j.canlet.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Levine A.J., Hu W., Feng Z. The P53 pathway: what questions remain to be explored? Cell Death Differ. 2006;13(6):1027–1036. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- 19.Liu J., Zhang C., Hu W., Feng Z. Tumor suppressor p53 and metabolism. J Mol Cell Biol. 2019;11(4):284–292. doi: 10.1093/jmcb/mjy070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W. The role of p53 gene family in reproduction. Cold Spring Harb Perspect Biol. 2009;1(6) doi: 10.1101/cshperspect.a001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu W., Feng Z., Teresky A.K., Levine A.J. p53 regulates maternal reproduction through LIF. Nature. 2007;450(7170):721–724. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- 22.Chang J.R., Ghafouri M., Mukerjee R., Bagashev A., Chabrashvili T., Sawaya B.E. Role of p53 in neurodegenerative diseases. Neurodegener Dis. 2012;9(2):68–80. doi: 10.1159/000329999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Checler F., Alves da Costa C. p53 in neurodegenerative diseases and brain cancers. Pharmacol Ther. 2014;142(1):99–113. doi: 10.1016/j.pharmthera.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Kung C.P., Murphy M.E. The role of the p53 tumor suppressor in metabolism and diabetes. J Endocrinol. 2016;231(2):R61–R75. doi: 10.1530/JOE-16-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y., Wu L., Yue X. A polymorphism in the tumor suppressor p53 affects aging and longevity in mouse models. Elife. 2018;7 doi: 10.7554/eLife.34701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agupitan A.D., Neeson P., Williams S., Howitt J., Haupt S., Haupt Y. P53: a guardian of immunity becomes its saboteur through mutation. Int J Mol Sci. 2020;21(10) doi: 10.3390/ijms21103452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11(11):792–804. doi: 10.1038/nrc3139. [DOI] [PubMed] [Google Scholar]
- 28.van Gent M., Sparrer K.M.J., Gack M.U. TRIM proteins and their roles in antiviral host defenses. Annu Rev Virol. 2018;5(1):385–405. doi: 10.1146/annurev-virology-092917-043323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meroni G. Genomics and evolution of the TRIM gene family. Adv Exp Med Biol. 2012;770:1–9. doi: 10.1007/978-1-4614-5398-7_1. [DOI] [PubMed] [Google Scholar]
- 30.Granata M., Panigada D., Galati E. To trim or not to trim: progression and control of DSB end resection. Cell Cycle. 2013;12(12):1848–1860. doi: 10.4161/cc.25042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaworska A.M., Wlodarczyk N.A., Mackiewicz A., Czerwinska P. The role of TRIM family proteins in the regulation of cancer stem cell self-renewal. Stem Cell. 2020;38(2):165–173. doi: 10.1002/stem.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meroni G. TRIM E3 ubiquitin ligases in rare genetic disorders. Adv Exp Med Biol. 2020;1233:311–325. doi: 10.1007/978-3-030-38266-7_14. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J.R., Li X.X., Hu W.N., Li C.Y. Emerging role of TRIM family proteins in cardiovascular disease. Cardiology. 2020;145(6):390–400. doi: 10.1159/000506150. [DOI] [PubMed] [Google Scholar]
- 34.Elabd S., Meroni G., Blattner C. TRIMming p53's anticancer activity. Oncogene. 2016;35(43):5577–5584. doi: 10.1038/onc.2016.33. [DOI] [PubMed] [Google Scholar]
- 35.Valletti A., Marzano F., Pesole G., Sbisa E., Tullo A. Targeting chemoresistant tumors: could TRIM proteins-p53 Axis Be a possible answer? Int J Mol Sci. 2019;20(7) doi: 10.3390/ijms20071776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y., Tavana O., Gu W. p53 modifications: exquisite decorations of the powerful guardian. J Mol Cell Biol. 2019;11(7):564–577. doi: 10.1093/jmcb/mjz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hock A.K., Vousden K.H. The role of ubiquitin modification in the regulation of p53. Biochim Biophys Acta. 2014;1843(1):137–149. doi: 10.1016/j.bbamcr.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Hafner A., Bulyk M.L., Jambhekar A., Lahav G. The multiple mechanisms that regulate p53 activity and cell fate. Nat Rev Mol Cell Biol. 2019;20(4):199–210. doi: 10.1038/s41580-019-0110-x. [DOI] [PubMed] [Google Scholar]
- 39.Meek D.W. Regulation of the p53 response and its relationship to cancer. Biochem J. 2015;469(3):325–346. doi: 10.1042/BJ20150517. [DOI] [PubMed] [Google Scholar]
- 40.Yau R., Rape M. The increasing complexity of the ubiquitin code. Nat Cell Biol. 2016;18(6):579–586. doi: 10.1038/ncb3358. [DOI] [PubMed] [Google Scholar]
- 41.Jones S.N., Roe A.E., Donehower L.A., Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378(6553):206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 42.Montes de Oca Luna R., Wagner D.S., Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378(6553):203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 43.Li M., Brooks C.L., Wu-Baer F., Chen D., Baer R., Gu W. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science. 2003;302(5652):1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- 44.Tao W., Levine A.J. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc Natl Acad Sci U S A. 1999;96(6):3077–3080. doi: 10.1073/pnas.96.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parant J., Chavez-Reyes A., Little N.A. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat Genet. 2001;29(1):92–95. doi: 10.1038/ng714. [DOI] [PubMed] [Google Scholar]
- 46.Li M., Chen D., Shiloh A. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416(6881):648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- 47.Liu W.T., Huang K.Y., Lu M.C. TGF-beta upregulates the translation of USP15 via the PI3K/AKT pathway to promote p53 stability. Oncogene. 2017;36(19):2715–2723. doi: 10.1038/onc.2016.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tu R., Kang W., Yang X. USP49 participates in the DNA damage response by forming a positive feedback loop with p53. Cell Death Dis. 2018;9(5) doi: 10.1038/s41419-018-0475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan J., Luo K., Zhang L., Cheville J.C., Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell. 2010;140(3):384–396. doi: 10.1016/j.cell.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun X.X., Challagundla K.B., Dai M.S. Positive regulation of p53 stability and activity by the deubiquitinating enzyme Otubain 1. EMBO J. 2012;31(3):576–592. doi: 10.1038/emboj.2011.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tavana O., Gu W. Modulation of the p53/MDM2 interplay by HAUSP inhibitors. J Mol Cell Biol. 2017;9(1):45–52. doi: 10.1093/jmcb/mjw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shieh S.Y., Ikeda M., Taya Y., Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91(3):325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 53.Shieh S.Y., Ahn J., Tamai K., Taya Y., Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Develop. 2000;14(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- 54.Lu X., Nannenga B., Donehower L.A. PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes & development. 2005;19(10):1162–1174. doi: 10.1101/gad.1291305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li D.W., Liu J.P., Schmid P.C. Protein serine/threonine phosphatase-1 dephosphorylates p53 at Ser-15 and Ser-37 to modulate its transcriptional and apoptotic activities. Oncogene. 2006;25(21):3006–3022. doi: 10.1038/sj.onc.1209334. [DOI] [PubMed] [Google Scholar]
- 56.Gu W., Roeder R.G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90(4):595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 57.Reed S.M., Quelle D.E. p53 acetylation: regulation and consequences. Cancers (Basel) 2014;7(1):30–69. doi: 10.3390/cancers7010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sykes S.M., Mellert H.S., Holbert M.A. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol Cell. 2006;24(6):841–851. doi: 10.1016/j.molcel.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang Y., Luo J., Zhang W., Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol Cell. 2006;24(6):827–839. doi: 10.1016/j.molcel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 60.Rokudai S., Aikawa Y., Tagata Y., Tsuchida N., Taya Y., Kitabayashi I. Monocytic leukemia zinc finger (MOZ) interacts with p53 to induce p21 expression and cell-cycle arrest. J Biol Chem. 2009;284(1):237–244. doi: 10.1074/jbc.M805101200. [DOI] [PubMed] [Google Scholar]
- 61.Luo J., Su F., Chen D., Shiloh A., Gu W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature. 2000;408(6810):377–381. doi: 10.1038/35042612. [DOI] [PubMed] [Google Scholar]
- 62.Qi J., Singh S., Hua W.K. HDAC8 inhibition specifically targets inv(16) acute myeloid leukemic stem cells by restoring p53 acetylation. Cell Stem Cell. 2015;17(5):597–610. doi: 10.1016/j.stem.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seeler J.S., Dejean A. SUMO and the robustness of cancer. Nat Rev Cancer. 2017;17(3):184–197. doi: 10.1038/nrc.2016.143. [DOI] [PubMed] [Google Scholar]
- 64.Wu S.Y., Chiang C.M. p53 sumoylation: mechanistic insights from reconstitution studies. Epigenetics. 2009;4(7):445–451. doi: 10.4161/epi.4.7.10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoffman Y., Pilpel Y., Oren M. microRNAs and Alu elements in the p53-Mdm2-Mdm4 regulatory network. J Mol Cell Biol. 2014;6(3):192–197. doi: 10.1093/jmcb/mju020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu W., Chan C.S., Wu R. Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol Cell. 2010;38(5):689–699. doi: 10.1016/j.molcel.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le M.T., Teh C., Shyh-Chang N. MicroRNA-125b is a novel negative regulator of p53. Genes Develop. 2009;23(7):862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hunten S., Siemens H., Kaller M., Hermeking H. The p53/microRNA network in cancer: experimental and bioinformatics approaches. Adv Exp Med Biol. 2013;774:77–101. doi: 10.1007/978-94-007-5590-1_5. [DOI] [PubMed] [Google Scholar]
- 69.Napolitano L.M., Meroni G. TRIM family: pleiotropy and diversification through homomultimer and heteromultimer formation. IUBMB Life. 2012;64(1):64–71. doi: 10.1002/iub.580. [DOI] [PubMed] [Google Scholar]
- 70.Bell J.L., Malyukova A., Holien J.K. TRIM16 acts as an E3 ubiquitin ligase and can heterodimerize with other TRIM family members. PloS One. 2012;7(5) doi: 10.1371/journal.pone.0037470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herquel B., Ouararhni K., Khetchoumian K. Transcription cofactors TRIM24, TRIM28, and TRIM33 associate to form regulatory complexes that suppress murine hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2011;108(20):8212–8217. doi: 10.1073/pnas.1101544108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Micale L., Chaignat E., Fusco C., Reymond A., Merla G. The tripartite motif: structure and function. Adv Exp Med Biol. 2012;770:11–25. [PubMed] [Google Scholar]
- 73.Khan R., Khan A., Ali A., Idrees M. The interplay between viruses and TRIM family proteins. Rev Med Virol. 2019;29(2) doi: 10.1002/rmv.2028. [DOI] [PubMed] [Google Scholar]
- 74.Allouch A., Di Primio C., Alpi E. The TRIM family protein KAP1 inhibits HIV-1 integration. Cell Host Microbe. 2011;9(6):484–495. doi: 10.1016/j.chom.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Gack M.U., Shin Y.C., Joo C.H. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446(7138):916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 76.Lang X., Tang T., Jin T., Ding C., Zhou R., Jiang W. TRIM65-catalized ubiquitination is essential for MDA5-mediated antiviral innate immunity. J Exp Med. 2017;214(2):459–473. doi: 10.1084/jem.20160592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen M., Meng Q., Qin Y. TRIM14 inhibits cGAS degradation mediated by selective autophagy receptor p62 to promote innate immune responses. Mol Cell. 2016;64(1):105–119. doi: 10.1016/j.molcel.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J., Hu M.M., Wang Y.Y., Shu H.B. TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. J Biol Chem. 2012;287(34):28646–28655. doi: 10.1074/jbc.M112.362608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsuchida T., Zou J., Saitoh T. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity. 2010;33(5):765–776. doi: 10.1016/j.immuni.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 80.Zhao C., Jia M., Song H. The E3 ubiquitin ligase TRIM40 attenuates antiviral immune responses by targeting MDA5 and RIG-I. Cell Rep. 2017;21(6):1613–1623. doi: 10.1016/j.celrep.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 81.Xing J., Zhang A., Zhang H. TRIM29 promotes DNA virus infections by inhibiting innate immune response. Nat Commun. 2017;8(1) doi: 10.1038/s41467-017-00101-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li B., Zhou T., Zou Y. Mid1/Mid2 expression in craniofacial development and a literature review of X-linked opitz syndrome. Mol Genet Genomic Med. 2016;4(1):95–105. doi: 10.1002/mgg3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lancioni A., Pizzo M., Fontanella B. Lack of Mid1, the mouse ortholog of the Opitz syndrome gene, causes abnormal development of the anterior cerebellar vermis. J Neurosci. 2010;30(8):2880–2887. doi: 10.1523/JNEUROSCI.4196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Locke M., Tinsley C.L., Benson M.A., Blake D.J. TRIM32 is an E3 ubiquitin ligase for dysbindin. Hum Mol Genet. 2009;18(13):2344–2358. doi: 10.1093/hmg/ddp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang W., Xia Z.J., Farre J.C., Subramani S. TRIM37, a novel E3 ligase for PEX5-mediated peroxisomal matrix protein import. J Cell Biol. 2017;216(9):2843–2858. doi: 10.1083/jcb.201611170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balastik M., Ferraguti F., Pires-da Silva A. Deficiency in ubiquitin ligase TRIM2 causes accumulation of neurofilament light chain and neurodegeneration. Proc Natl Acad Sci U S A. 2008;105(33):12016–12021. doi: 10.1073/pnas.0802261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Niikura T., Hashimoto Y., Tajima H. A tripartite motif protein TRIM11 binds and destabilizes Humanin, a neuroprotective peptide against Alzheimer's disease-relevant insults. Eur J Neurosci. 2003;17(6):1150–1158. doi: 10.1046/j.1460-9568.2003.02553.x. [DOI] [PubMed] [Google Scholar]
- 88.Lassot I., Mora S., Lesage S. The E3 ubiquitin ligases TRIM17 and TRIM41 modulate alpha-synuclein expression by regulating ZSCAN21. Cell Rep. 2018;25(9):2484–2496. doi: 10.1016/j.celrep.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Fielitz J., Kim M.S., Shelton J.M. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J Clin Invest. 2007;117(9):2486–2495. doi: 10.1172/JCI32827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fielitz J., van Rooij E., Spencer J.A. Loss of muscle-specific RING-finger 3 predisposes the heart to cardiac rupture after myocardial infarction. Proc Natl Acad Sci U S A. 2007;104(11):4377–4382. doi: 10.1073/pnas.0611726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He J., Quintana M.T., Sullivan J. MuRF2 regulates PPARgamma1 activity to protect against diabetic cardiomyopathy and enhance weight gain induced by a high fat diet. Cardiovasc Diabetol. 2015;14 doi: 10.1186/s12933-015-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cambiaghi V., Giuliani V., Lombardi S., Marinelli C., Toffalorio F., Pelicci P.G. TRIM proteins in cancer. Adv Exp Med Biol. 2012;770:77–91. doi: 10.1007/978-1-4614-5398-7_6. [DOI] [PubMed] [Google Scholar]
- 93.Mandell M.A., Saha B., Thompson T.A. The tripartite nexus: autophagy, cancer, and tripartite motif-containing protein family members. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crawford L.J., Johnston C.K., Irvine A.E. TRIM proteins in blood cancers. J Cell Commun Signal. 2018;12(1):21–29. doi: 10.1007/s12079-017-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allton K., Jain A.K., Herz H.M. Trim24 targets endogenous p53 for degradation. Proc Natl Acad Sci U S A. 2009;106(28):11612–11616. doi: 10.1073/pnas.0813177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jain A.K., Allton K., Duncan A.D., Barton M.C. TRIM24 is a p53-induced E3-ubiquitin ligase that undergoes ATM-mediated phosphorylation and autodegradation during DNA damage. Mol Cell Biol. 2014;34(14):2695–2709. doi: 10.1128/MCB.01705-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Groner A.C., Cato L., de Tribolet-Hardy J. TRIM24 is an oncogenic transcriptional activator in prostate cancer. Cancer Cell. 2016;29(6):846–858. doi: 10.1016/j.ccell.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsai W.W., Wang Z., Yiu T.T. TRIM24 links a non-canonical histone signature to breast cancer. Nature. 2010;468(7326):927–932. doi: 10.1038/nature09542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lv D., Li Y., Zhang W. TRIM24 is an oncogenic transcriptional co-activator of STAT3 in glioblastoma. Nat Commun. 2017;8(1) doi: 10.1038/s41467-017-01731-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang L., Huang N.J., Chen C., Tang W., Kornbluth S. Ubiquitylation of p53 by the APC/C inhibitor Trim39. Proc Natl Acad Sci U S A. 2012;109(51):20931–20936. doi: 10.1073/pnas.1212047110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang L., Mei Y., Fu N.Y. TRIM39 regulates cell cycle progression and DNA damage responses via stabilizing p21. Proc Natl Acad Sci U S A. 2012;109(51):20937–20942. doi: 10.1073/pnas.1214156110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu J., Zhang C., Wang X.L. E3 ubiquitin ligase TRIM32 negatively regulates tumor suppressor p53 to promote tumorigenesis. Cell Death Differ. 2014;21(11):1792–1804. doi: 10.1038/cdd.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou Z., Ji Z., Wang Y. TRIM59 is up-regulated in gastric tumors, promoting ubiquitination and degradation of p53. Gastroenterology. 2014;147(5):1043–1054. doi: 10.1053/j.gastro.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 104.Guo P., Qiu Y., Ma X. Tripartite motif 31 promotes resistance to anoikis of hepatocarcinoma cells through regulation of p53-AMPK axis. Exp Cell Res. 2018;368(1):59–66. doi: 10.1016/j.yexcr.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 105.Ito M., Migita K., Matsumoto S. Overexpression of E3 ubiquitin ligase tripartite motif 32 correlates with a poor prognosis in patients with gastric cancer. Oncol Letters. 2017;13(5):3131–3138. doi: 10.3892/ol.2017.5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao T.T., Jin F., Li J.G. TRIM32 promotes proliferation and confers chemoresistance to breast cancer cells through activation of the NF-kappaB pathway. J Cancer. 2018;9(8):1349–1356. doi: 10.7150/jca.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Du Y., Zhang W., Du B. TRIM32 overexpression improves chemoresistance through regulation of mitochondrial function in non-small-cell lung cancers. Onco Targets Ther. 2018;11:7841–7852. doi: 10.2147/OTT.S176689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu J., Zhu Y., Hu W., Feng Z. TRIM32 is a novel negative regulator of p53. Mol Cell Oncol. 2015;2(2) doi: 10.4161/23723548.2014.970951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nguyen D.T.T., Richter D., Michel G. The ubiquitin ligase LIN41/TRIM71 targets p53 to antagonize cell death and differentiation pathways during stem cell differentiation. Cell Death Differ. 2017;24(6):1063–1078. doi: 10.1038/cdd.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rong X., Rao J., Li D., Jing Q., Lu Y., Ji Y. TRIM69 inhibits cataractogenesis by negatively regulating p53. Redox Biol. 2019;22 doi: 10.1016/j.redox.2019.101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang J., Zhou Y.J., Yu Z.H. Identification of core genes and clinical roles in pregnancy-associated breast cancer based on integrated analysis of different microarray profile datasets. Biosci Rep. 2019;39(6) doi: 10.1042/BSR20190019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Han Y., Tan Y., Zhao Y. TRIM23 overexpression is a poor prognostic factor and contributes to carcinogenesis in colorectal cancer. J Cell Mol Med. 2020;24(10):5491–5500. doi: 10.1111/jcmm.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chu Y., Yang X. SUMO E3 ligase activity of TRIM proteins. Oncogene. 2011;30(9):1108–1116. doi: 10.1038/onc.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ivanschitz L., Takahashi Y., Jollivet F., Ayrault O., Le Bras M., de The H. PML IV/ARF interaction enhances p53 SUMO-1 conjugation, activation, and senescence. Proc Natl Acad Sci U S A. 2015;112(46):14278–14283. doi: 10.1073/pnas.1507540112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kung C.P., Khaku S., Jennis M., Zhou Y., Murphy M.E. Identification of TRIML2, a novel p53 target, that enhances p53 SUMOylation and regulates the transactivation of proapoptotic genes. Mol Canc Res : MCR. 2015;13(2):250–262. doi: 10.1158/1541-7786.MCR-14-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Song X., Zhang C., Liu Z., Liu Q., He K., Yu Z. Characterization of ceRNA network to reveal potential prognostic biomarkers in triple-negative breast cancer. PeerJ. 2019;7 doi: 10.7717/peerj.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayashi F., Kasamatsu A., Endo-Sakamoto Y. Increased expression of tripartite motif (TRIM) like 2 promotes tumoral growth in human oral cancer. Biochem Biophy Res Commun. 2019;508(4):1133–1138. doi: 10.1016/j.bbrc.2018.12.060. [DOI] [PubMed] [Google Scholar]
- 118.Yuan Z., Villagra A., Peng L. The ATDC (TRIM29) protein binds p53 and antagonizes p53-mediated functions. Mol Cell Biol. 2010;30(12):3004–3015. doi: 10.1128/MCB.01023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun J., Zhang T., Cheng M. TRIM29 facilitates the epithelial-to-mesenchymal transition and the progression of colorectal cancer via the activation of the Wnt/beta-catenin signaling pathway. J Exp Clin Cancer Res. 2019;38(1) doi: 10.1186/s13046-019-1098-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Jiang T., Tang H.M., Lu S., Yan D.W., Yang Y.X., Peng Z.H. Up-regulation of tripartite motif-containing 29 promotes cancer cell proliferation and predicts poor survival in colorectal cancer. Med Oncol. 2013;30(4) doi: 10.1007/s12032-013-0715-4. [DOI] [PubMed] [Google Scholar]
- 121.Wang C., Zhou Y., Chen B., Yuan W., Huang J. Prognostic value of tripartite motif containing 29 expression in patients with gastric cancer following surgical resection. Oncol Letters. 2018;15(4):5792–5798. doi: 10.3892/ol.2018.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu M., Hu J., Zhou B., Zhong Y., Lin N., Xu R. TRIM29 prevents hepatocellular carcinoma progression by inhibiting Wnt/beta-catenin signaling pathway. Acta Biochim et Biophys Sin. 2019;51(1):68–77. doi: 10.1093/abbs/gmy151. [DOI] [PubMed] [Google Scholar]
- 123.Yanagi T., Watanabe M., Hata H. Loss of TRIM29 alters keratin distribution to promote cell invasion in squamous cell carcinoma. Cancer Res. 2018;78(24):6795–6806. doi: 10.1158/0008-5472.CAN-18-1495. [DOI] [PubMed] [Google Scholar]
- 124.Wang S., Zhang Y., Huang J. TRIM67 activates p53 to suppress colorectal cancer initiation and progression. Cancer Res. 2019;79(16):4086–4098. doi: 10.1158/0008-5472.CAN-18-3614. [DOI] [PubMed] [Google Scholar]
- 125.Joo H.M., Kim J.Y., Jeong J.B. Ret finger protein 2 enhances ionizing radiation-induced apoptosis via degradation of AKT and MDM2. Eur J Cell Biol. 2011;90(5):420–431. doi: 10.1016/j.ejcb.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 126.Kapanadze B., Kashuba V., Baranova A. A cosmid and cDNA fine physical map of a human chromosome 13q14 region frequently lost in B-cell chronic lymphocytic leukemia and identification of a new putative tumor suppressor gene, Leu5. FEBS Lett. 1998;426(2):266–270. doi: 10.1016/s0014-5793(98)00357-3. [DOI] [PubMed] [Google Scholar]
- 127.Chen W.X., Cheng L., Xu L.Y., Qian Q., Zhu Y.L. Bioinformatics analysis of prognostic value of TRIM13 gene in breast cancer. Biosci Rep. 2019;39(3) doi: 10.1042/BSR20190285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu L., Wu Q., Zhou X., Wu Q., Fang M. TRIM13 inhibited cell proliferation and induced cell apoptosis by regulating NF-kappaB pathway in non-small-cell lung carcinoma cells. Gene. 2019;715 doi: 10.1016/j.gene.2019.144015. [DOI] [PubMed] [Google Scholar]
- 129.Caratozzolo M.F., Micale L., Turturo M.G. TRIM8 modulates p53 activity to dictate cell cycle arrest. Cell Cycle. 2012;11(3):511–523. doi: 10.4161/cc.11.3.19008. [DOI] [PubMed] [Google Scholar]
- 130.Caratozzolo M.F., Valletti A., Gigante M. TRIM8 anti-proliferative action against chemo-resistant renal cell carcinoma. Oncotarget. 2014;5(17):7446–7457. doi: 10.18632/oncotarget.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Micale L., Fusco C., Fontana A. TRIM8 downregulation in glioma affects cell proliferation and it is associated with patients survival. BMC Canc. 2015;15 doi: 10.1186/s12885-015-1449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hofmann T.G., Moller A., Sirma H. Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nat Cell Biol. 2002;4(1):1–10. doi: 10.1038/ncb715. [DOI] [PubMed] [Google Scholar]
- 133.Louria-Hayon I., Grossman T., Sionov R.V., Alsheich O., Pandolfi P.P., Haupt Y. The promyelocytic leukemia protein protects p53 from Mdm2-mediated inhibition and degradation. J Biol Chem. 2003;278(35):33134–33141. doi: 10.1074/jbc.M301264200. [DOI] [PubMed] [Google Scholar]
- 134.Alsheich-Bartok O., Haupt S., Alkalay-Snir I., Saito S., Appella E., Haupt Y. PML enhances the regulation of p53 by CK1 in response to DNA damage. Oncogene. 2008;27(26):3653–3661. doi: 10.1038/sj.onc.1211036. [DOI] [PubMed] [Google Scholar]
- 135.Rokudai S., Laptenko O., Arnal S.M., Taya Y., Kitabayashi I., Prives C. MOZ increases p53 acetylation and premature senescence through its complex formation with PML. Proc Natl Acad Sci U S A. 2013;110(10):3895–3900. doi: 10.1073/pnas.1300490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Song Y., Guo Q., Gao S., Hua K. Tripartite motif-containing protein 3 plays a role of tumor inhibitor in cervical cancer. Biochem Biophy Res Commun. 2018;498(3):686–692. doi: 10.1016/j.bbrc.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 137.Sanchez-Prieto R., Rojas J.M., Taya Y., Gutkind J.S. A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Canc Res. 2000;60(9):2464–2472. [PubMed] [Google Scholar]
- 138.Piao M.Y., Cao H.L., He N.N. Potential role of TRIM3 as a novel tumour suppressor in colorectal cancer (CRC) development. Scand J Gastroenterol. 2016;51(5):572–582. doi: 10.3109/00365521.2015.1124285. [DOI] [PubMed] [Google Scholar]
- 139.Chao J., Zhang X.F., Pan Q.Z. Decreased expression of TRIM3 is associated with poor prognosis in patients with primary hepatocellular carcinoma. Med Oncol. 2014;31(8) doi: 10.1007/s12032-014-0102-9. [DOI] [PubMed] [Google Scholar]
- 140.Wang C., Ivanov A., Chen L. MDM2 interaction with nuclear corepressor KAP1 contributes to p53 inactivation. EMBO J. 2005;24(18):3279–3290. doi: 10.1038/sj.emboj.7600791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu L., Zhao E., Li C. TRIM28, a new molecular marker predicting metastasis and survival in early-stage non-small cell lung cancer. Cancer Epidemiol. 2013;37(1):71–78. doi: 10.1016/j.canep.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 142.Li F., Wang Z., Lu G. TRIM28 promotes cervical cancer growth through the mTOR signaling pathway. Oncol Rep. 2018;39(4):1860–1866. doi: 10.3892/or.2018.6235. [DOI] [PubMed] [Google Scholar]
- 143.Addison J.B., Koontz C., Fugett J.H. KAP1 promotes proliferation and metastatic progression of breast cancer cells. Canc Res. 2015;75(2):344–355. doi: 10.1158/0008-5472.CAN-14-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sho T., Tsukiyama T., Sato T. TRIM29 negatively regulates p53 via inhibition of Tip60. Biochim Biophys Acta. 2011;1813(6):1245–1253. doi: 10.1016/j.bbamcr.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 145.Zhang P., Elabd S., Hammer S. TRIM25 has a dual function in the p53/Mdm2 circuit. Oncogene. 2015;34(46):5729–5738. doi: 10.1038/onc.2015.21. [DOI] [PubMed] [Google Scholar]
- 146.Takayama K.I., Suzuki T., Tanaka T. TRIM25 enhances cell growth and cell survival by modulating p53 signals via interaction with G3BP2 in prostate cancer. Oncogene. 2018;37(16):2165–2180. doi: 10.1038/s41388-017-0095-x. [DOI] [PubMed] [Google Scholar]
- 147.Reddy B.A., van der Knaap J.A., Bot A.G. Nucleotide biosynthetic enzyme GMP synthase is a TRIM21-controlled relay of p53 stabilization. Mol Cell. 2014;53(3):458–470. doi: 10.1016/j.molcel.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 148.Chen Y., Guo Y., Yang H. TRIM66 overexpresssion contributes to osteosarcoma carcinogenesis and indicates poor survival outcome. Oncotarget. 2015;6(27):23708–23719. doi: 10.18632/oncotarget.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu J., Rao J., Lou X., Zhai J., Ni Z., Wang X. Upregulated TRIM11 exerts its oncogenic effects in hepatocellular carcinoma through inhibition of P53. Cell Physiol Biochem : Int J Exp Cell Physiol Biochem Pharmacol. 2017;44(1):255–266. doi: 10.1159/000484678. [DOI] [PubMed] [Google Scholar]
- 150.Chen Y., Sun J., Ma J. Proliferation and invasion of ovarian cancer cells are suppressed by knockdown of TRIM11. Oncol Letters. 2017;14(2):2125–2130. doi: 10.3892/ol.2017.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dai X., Geng F., Li M., Liu M. Tripartite motifcontaining 11 regulates the proliferation and apoptosis of breast cancer cells. Oncol Rep. 2019;41(4):2567–2574. doi: 10.3892/or.2019.7015. [DOI] [PubMed] [Google Scholar]
- 152.Wang X., Shi W., Shi H. TRIM11 overexpression promotes proliferation, migration and invasion of lung cancer cells. J Exp Clin Cancer Res. 2016;35(1) doi: 10.1186/s13046-016-0379-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 153.Benke S., Agerer B., Haas L. Human tripartite motif protein 52 is required for cell context-dependent proliferation. Oncotarget. 2018;9(17):13565–13581. doi: 10.18632/oncotarget.24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cheung C.C., Yang C., Berger T. Identification of BERP (brain-expressed RING finger protein) as a p53 target gene that modulates seizure susceptibility through interacting with GABA(A) receptors. Proc Natl Acad Sci U S A. 2010;107(26):11883–11888. doi: 10.1073/pnas.1006529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.de Stanchina E., Querido E., Narita M. PML is a direct p53 target that modulates p53 effector functions. Mol Cell. 2004;13(4):523–535. doi: 10.1016/s1097-2765(04)00062-0. [DOI] [PubMed] [Google Scholar]
- 156.Mastropasqua F., Marzano F., Valletti A. TRIM8 restores p53 tumour suppressor function by blunting N-MYC activity in chemo-resistant tumours. Mol Cancer. 2017;16(1) doi: 10.1186/s12943-017-0634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wei J.S., Song Y.K., Durinck S. The MYCN oncogene is a direct target of miR-34a. Oncogene. 2008;27(39):5204–5213. doi: 10.1038/onc.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Raver-Shapira N., Marciano E., Meiri E. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26(5):731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 159.Obad S., Brunnstrom H., Vallon-Christersson J., Borg A., Drott K., Gullberg U. Staf50 is a novel p53 target gene conferring reduced clonogenic growth of leukemic U-937 cells. Oncogene. 2004;23(23):4050–4059. doi: 10.1038/sj.onc.1207524. [DOI] [PubMed] [Google Scholar]
- 160.Sun Y., Ho G.H., Koong H.N. Down-regulation of tripartite-motif containing 22 expression in breast cancer is associated with a lack of p53-mediated induction. Biochem Biophy Res Commun. 2013;441(3):600–606. doi: 10.1016/j.bbrc.2013.10.110. [DOI] [PubMed] [Google Scholar]
- 161.Zirn B., Hartmann O., Samans B. Expression profiling of Wilms tumors reveals new candidate genes for different clinical parameters. Int J Cancer. 2006;118(8):1954–1962. doi: 10.1002/ijc.21564. [DOI] [PubMed] [Google Scholar]