Abstract
The bone is previously considered as a dominant organ involved in the processes of locomotion. However, in the past two decades, a large number of studies have suggested that the skeletal system closely coordinated with the immune system so as to result in the emerging area of ‘osteoimmunology’. In the evolution of many kinds of bone destruction-related diseases, osteoclasts could differentiate from dendritic cells, which contributed to increased expression of osteoclast-related membrane receptors and relatively higher activity of bone destruction, inducing severe bone destruction under inflammatory conditions. Numerous factors could influence the interaction between osteoclasts and dendritic cells, contributing to the pathogenesis of several bone diseases in the context of inflammation, including both immunocytes and a large number of cytokines. In addition, the products of osteoclasts released from bone destruction area serve as important signals for the differentiation and activation of immature dendritic cells. Therefore, the border between the dendritic cell-related immune response and osteoclast-related bone destruction has gradually unravelled. Dendritic cells and osteoclasts cooperate with each other to mediate bone destruction and bone remodelling under inflammatory conditions. In this review, we will pay attention to the interactions between dendritic cells and osteoclasts in physiological and pathological conditions to further understand the skeletal system and identify potential new therapeutic targets for the future by summarizing their significant roles and molecular mechanisms in bone destruction.
Keywords: Bone destruction diseases, Dendritic cells, Osteoclasts, Osteoimmunology, Trans-differentiation
Introduction
In a previous study, the bone is known as the hardest tissue in the human body that not only makes effects on motion, protection, and calcium storage but also provides shelter for the haematopoietic system, including both myeloid and lymphoid progenitor cells.1 Bone is a highly dynamic tissue undergoing continuous reconstruction, including osteoclast (OCs)-dependent bone resorption and osteoblast-dependent bone formation, which maintains proper functions and homeostasis over the lifetime. There is a dynamic balance between bone resorption and bone formation, and multiple bone related diseases may occur when this balance is disrupted.
It has been well acknowledged that the skeletal system has a close connection and participates in deep crosstalk with the immune system since bone cells and immunocytes share a common micro-environment.2,3 It was shown that the emergence of terrestrial vertebrates with a strong body skeleton coincided with the development of the immune system from an evolutionary point of view. The reciprocal interactions of these two systems was are essential in maintaining the normal functions of the body and may mediate the progression of various diseases under pathological conditions, such as fracture or inflammation.4,5 Therefore, the new field ‘osteoimmunology’ was created to investigate the crosstalk between the skeletal system and immune system under physiological and pathological conditions. The possible relationships between these two systems especially the relationship between immunocytes and osteoclasts has been deeply explored because they are both involved in a multitude of inflammation-related bone destruction diseases. Dendritic cells (DCs), acting as sentinels of the immune system, which are specialized as antigen-presenting cells (APCs). Dendritic cells are named for the dendritic structures when these cells act as mature statement; dendritic structures serve as vital component during the recognition and presentation of antigens in the immune system, which can promote the adaptive immune response by interacting with T cells.6,7 Classic dendritic cells are differentiated from both lymphoid precursor cells derived from haematopoietic stem cells and precursor cells under stimulation with granulocyte macrophage colony stimulating factor (GM-CSF) and interleukin-4 (IL-4).8 According to the level of maturity, dendritic cells can be divided into mature dendritic cells and immature dendritic cells (iDCs). Compared with the former, immature dendritic cells mainly exist in nonlymphoid tissues, such as the blood vessel. Interestingly, the bone marrow not only has a rich blood supply but also acts as the source of haemopoietic stem cells. Therefore, the bone provides an imperative shelter for the migration and survival of dendritic cells. Some studies have shown that the numbers of immature dendritic cells in the bone marrow microcirculation increase under inflammatory pathological conditions, indicating the important role of dendritic cells in bone related diseases.3 Dendritic cells and osteoclasts obtain a large number of same characteristics because they are all differentiated from mononuclear progenitor cells and presented similar phagocytic functions. Osteoclasts, multinuclear giant cells derived from monocyte precursor cells, act as the only macrophages in the skeletal system that are involved in the destruction and absorption of the bone matrix.8 It has been shown that immature dendritic cells could trans-differentiate into osteoclasts under inflammatory conditions so as to mediate the process of bone destruction.9, 10, 11 In the joint synovium, the number of immature dendritic cells is increased at the resorption site during inflammatory osteolysis, which further induces osteoclast differentiation and results in bone matrix erosion. This process is activated and regulated by inflammatory factors and immune cells.12, 13, 14, 15 In this review, we will discuss the molecular mechanism of dendritic cell differentiation into osteoclasts under inflammatory conditions, which may be identified as possible therapeutic strategies for osteoclast-related osteolytic diseases.
Potential relationship between dendritic cells and osteoclasts
The osteoblasts in the skeletal system especially provide shelter for the survival of haematopoietic stem cells and regulate their differentiation, which is well known as the cell niche. The immunocytes that originate from haematopoietic stem cells in the bone marrow, sharing the common microenvironment with osteocytes, which indicate that there may exist many interactions among different cells from the skeletal system and those from the immune system. As the immune system is responsible for the recognition and make resistance to pathogens, it can also cooperate with the skeletal system to protect the body from large amount of bone related injuries.
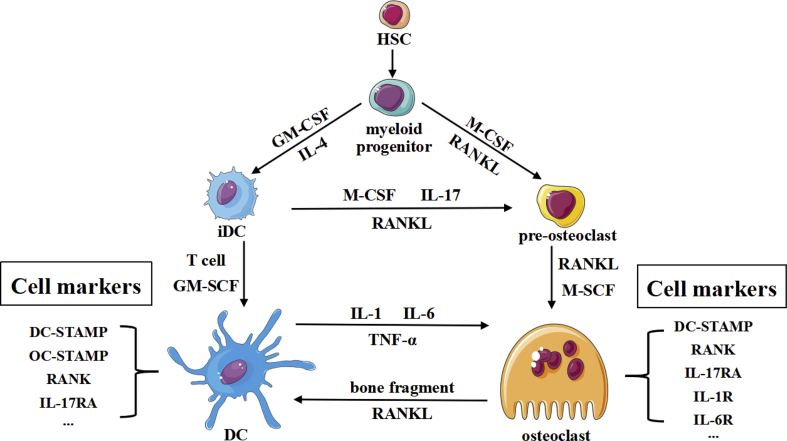
Prior to this, the critical interaction between macrophages and dendritic cells in inflammatory microenvironment has been reported.3 Osteoclasts are multinuclear giant cells derived from monocyte precursor cells that act as the only kind of macrophages in the skeletal system that perform phagocytosis.16 Osteoclasts are involved in bone remodelling and mediate bone destruction under inflammatory conditions. It is speculated that the increased number of immature dendritic cells in the microenvironment of the articular cavity under inflammatory conditions are coupled with osteoclasts. It is possible that dendritic cells and osteoclasts affect the functions of each other so as to adjust the balance of osteoimmunology and restrain the ‘inflammation/immunity-related bone destruction’ axis.12 Several lines of evidence support the possible link between dendritic cells and osteoclasts (Fig. 1). First, they are all originate from haemopoietic stem cells. Whole-genome analysis showed that they exhibited highly overlapping gene expression profiles and expressed the same surface markers, such as dendritic cell-specific transmembrane protein (DC-STAMP),17 suggesting that there is a certain genetic relationship between the two cell types, which may be considered a vertical differentiation related relationship. Second, the cells in the bone marrow include osteoblasts, osteoclasts, osteocytes, and dendritic cells. They share the same shelter in the bone microenvironment, which provide the possibility of the close relationship. In addition, dendritic cells and osteoclasts are both affected by multiple shared immune factors in the microenvironment.16,17 Many cytokines are vital to dendritic cells in the immune system and have been indicated to be equally important for osteoclasts differentiation in the skeletal system (Fig. 1). Several dendritic cell-related immune system disruptions also influence the balance of bone formation and destruction through affecting many signalling pathways. Both osteoclasts and dendritic cells signals through the receptor activator of NF-κB ligand (RANKL)-receptor activator of NF-κB (RANK) signalling pathway, which not only plays important roles in the development of the lymph nodes and dendritic cells in the immune system but also is significant in the differentiation of osteoclasts. RANKL-deficient mice show osteopetrosis due to an osteoclast deficiency and lack lymph node development as well.13 RANK exists in both dendritic cell and osteoclast surfaces,18 which activates developmental signalling pathways such as the NF-κB pathway. Moreover, Cathepsin K acts as an important functional molecule in osteoclasts for bone resorption, which plays a vital role in the intracellular toll like receptor 9 (TLR9) signalling pathway of dendritic cells.11 Many other important inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumour necrosis factor (TNF-α), are also important for both dendritic cells and osteoclasts. Overall, dendritic cells and osteoclasts share a multitude of molecular and signalling pathways, which further indicate that the close interaction among them is existed.
Figure 1.
The origin and crosstalk of dendritic cells and osteoclasts.
It has been reported in previous studies that immature dendritic cells can trans-differentiate into osteoclasts under certain conditions (Fig. 1), for which there exist several points of support.19 First, it is suggested that there is a very similar genetic relationship between these two kinds of cells. RANK/RANKL-deficient mice not only show the bone loss caused by an osteoclast deficiency but also suffer developmental defects in B cells and T cells and even immune deficiency.20 At the genomic level, monocytes are the common precursors of dendritic cells and osteoclasts. From monocytes to osteoclasts, the expression of 3997 genes is downregulated or lost, while the expression of 3821 genes is upregulated.3 However, when immature dendritic cells differentiate into osteoclasts, only 2107 genes are downregulated and 1966 genes are upregulated.3 At the same time, the expression of RANK receptors, c-fms and TREM-2 receptors is increased on dendritic cells compared with osteoclasts, which suggests that dendritic cells and osteoclasts have closer connections than the relationship between monocytes and osteoclasts. In addition, since immature dendritic cells are immature cells that have a high differentiation potential, it has been suggested that the differentiation of dendritic cells into osteoclasts is faster and more efficient than that of monocytes into osteoclasts, which is also adapted to the requirement of increased bone destruction under inflammatory conditions.21 In addition, it is well known that the RANK-RANKL signalling pathway activates TRAF6 and downstream MAPK-JNK and NFATc1, a transcription factor. Among these molecules, NFATc1 is a master transcription factor for osteoclasts, which could regulate many osteoclast-related functional molecules such as matrix metalloenzyme 9 (MMP9), Cathepsin K and αvβ3.22,23 It is worth noting that dendritic cells have RANKL receptors on the surface and that RANK on the membrane can also recognize transmembrane RANKL or soluble RANKL produced on the surface membrane of other cells, especially osteoblasts and osteocytes, which are important sources of RANKL during the process of bone destruction.24 It has also been confirmed that dendritic cells may have the potential to produce osteoclast-related molecules and to differentiate into mature osteoclasts. In addition, many evidence have also demonstrated that monocytes can differentiate into immature dendritic cells under stimulation with GM-CSF and IL-4, further forming osteoclast-like cells after stimulation with M-CSF and RANKL.1 In addition to RANKL, the factors contributing to immature dendritic cell trans-differentiation include c-fms, the M-CSF receptor expressed on the surface of immature dendritic cells.25 It is found that if immature dendritic cells are stimulated with cytokines or components of microorganisms, the expression of c-fms is greatly downregulated, which means stimulation with M-CSF cannot induce the formation of osteoclast-like cells with bone destruction function. Some studies also showed that after blocking the c-fms receptor in vitro, dendritic cells could not transform into osteoclasts, further indicating the important role of c-fms in dendritic cell trans-differentiation.
The receptors co-expressed by dendritic cells and osteoclasts suggest that under inflammatory conditions, inflammatory mediators expressed at increased levels may act on both kinds of cells and induce their responses at the same time. However, there exists the question of whether the potential interaction between the two kinds of cells can influence the balance between the activation and termination of inflammation. Additionally, the questions of whether the bidirectional interaction between dendritic cells and osteoclasts is blocked by exogenous factors and whether the overactivated inflammatory reaction leading to bone destruction can be stopped remain. Therefore, elucidating the potential relationship between dendritic cells and osteoclasts will help us better understand the detailed mechanism underlying immunity-related diseases in the bone and may also provide a novel therapeutic target in immune-mediated bone destructive diseases. Both dendritic cells and osteoclasts originate from myeloid progenitor cells derived from haematopoietic stem cells. Myeloid progenitor cells differentiate into OCs in the presence of M-CSF and RANKL, while GM-CSF and IL-4 contribute to the differentiation of DCs. iDCs, which exist in the early stage of DC differentiation, are able to trans-differentiate into OCs under stimulation with RANKL and M-CSF or IL-17 during inflammation, which shows strong plasticity. At the same time, when DCs or OCs mature, they not only show affinity with each other by expressing similar receptors and signalling pathways but also exhibit functional crosstalk. The debris produced by OC bone resorption can be absorbed by DCs to promote their activation and maturation, while the inflammatory factors produced by DCs are essential for maintaining OCs and supporting OC functions.
The crosstalk between the dendritic cells and osteoclasts among molecular level
Since dendritic cells and osteoclasts both play important roles in the bone microenvironment under physiological and pathological conditions, it is important to determine how they interact with each other at the molecular level. As the dynamic balance between dendritic cells and osteoclasts is regulated by numerous factors, several kinds of skeletal diseases may occur once the balance is disturbed.
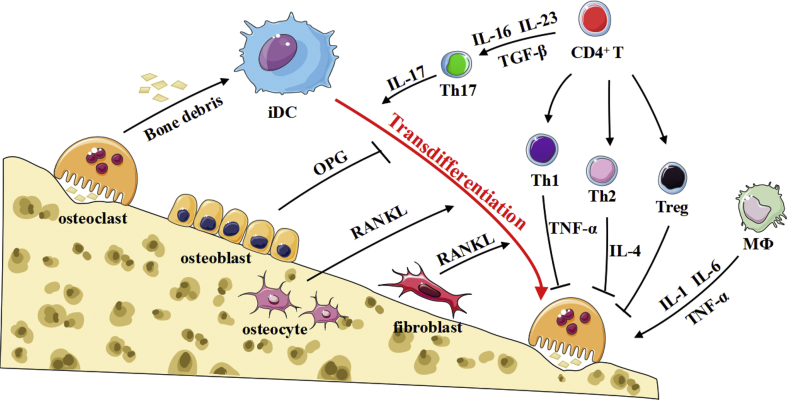
As mentioned above, immature dendritic cells (iDCs) can not only exist in the bone marrow microenvironment but also constantly migrate to the bone marrow microenvironment via activation by chemokines during the inflammatory stage.12,13 They, acting as the most changeable cells in the bone marrow, which can differentiate into osteoclasts with effective bone resorption activity under certain conditions, such as infection (Fig. 2). The inflammatory iDCs present under pathological conditions express increased levels of inflammatory factor receptors such as CD206 and F4/80 compared with classic antigen-presenting dendritic cells.1 Under infection or ‘inflammatory stress’ conditions, immature dendritic cells show increased chemotaxis to bone absorption sites and articular surfaces mediated by the stimulatory effect of inflammatory factors.2,14 The trans-differentiation of immature dendritic cells into osteoclasts has a higher efficiency than that of monocytes into osteoclasts, and immature dendritic cell-differentiated osteoclasts show increased bone resorption activity, which further promotes the bone destruction pathological process.20 It seems that this trans-differentiation could meet the need for bone destruction under inflammatory conditions, especially during bone destruction diseases such as multiple myeloma.26
Figure 2.
Possible pathways of osteoclasts formation and activation in inflammatory pathology.
DC-OC trans-differentiation is regulated by several kinds of signalling pathways (Fig. 2). At the cellular level, T cells, especially Th17 cells, are thought to play an important role in the trans-differentiation of dendritic cells, as T cells can produce cytokines that promote the differentiation of dendritic cells.4 However, it is possible that T cells may serve as a balanced lever in the process of DC-OC trans-differentiation, working as a switch between the osteoclast formation state and the resting stage.3,14,27, 28, 29 T cells can not only activate the process of dendritic cell trans-differentiation but also inhibit this process by secreting other cytokines. Under normal physiological circumstances, there are relatively few functional T cells accompanied by an increased frequency of initial non-functional T cells in the resting stage, and these cells may not produce enough cytokines to induce the differentiation of dendritic cells, such as IL-17 that is derived from Th17 cell subsets.26,30 In vitro experiments confirmed that T cell-derived IFN-γ could inhibit RANKL signalling by blocking TRAF6 to inhibit osteoclast maturation and activation, forming an antagonistic equilibrium relationship between IFN-γ and RANKL.30 Treg cells also make inhibitory effects on osteoclast differentiation. Moreover, activated T cells not only produce RANKL but also secrete TRAIL, an osteoclast inhibitor.2 However, the number of Th17 cells and percentage of IL-17 are obviously increased in inflammatory pathology, which may be involved in the induction of dendritic cell differentiation and antagonize negative signals such as TRAIL, IFN-γ and Treg cells.31 Overall, T cells play dual roles in dendritic cell trans-differentiation. The balancing function of T cells needs to be confirmed by subsequent studies.
IL-17 is considered to be an important participant in trans-differentiation. The specific molecular signalling pathway of IL-17 produced by activated Th17 cells is not clear. It is suggested that dendritic cells have the specific receptor IL-17RA, and IL-17/IL-17RA interaction activates the downstream TRAF6-NF-κB or CEBP-β pathway, which transmits intracellular signals and ultimately activates Ca2+/NFATc1, thus upregulating the expression of osteoclast-related bone-absorbing functional proteins.32 Additionally, it has been noted that IL-17 can also be used as a stimulator to promote the secretion of RANKL by synovial fibroblasts or osteoblasts.16 Therefore, we believe that IL-17 utilizes both direct and indirect mechanisms to exert its functions, not only by acting on IL-17 receptors but also by stimulating synovial fibroblast production of RANKL, which promotes the trans-differentiation from dendritic cells into osteoclasts. Additionally, IL-17 also has other functions in osteoclast formation, such as stimulating osteoblasts and osteocytes to produce M-CSF and promoting the expression of RANK receptor. IL-17 can also be detected in the dendritic cell environment of patients with Langerhans cell hyperplasia (LCH) or periodontitis.33 The infiltration of a large amount of Th17 cells can be found in intestinal mucous membrane biopsies from patients with inflammatory bowel disease (IBD).34,35 These diseases are accompanied by severe bone destruction. Inhibition of the IL-17 receptor with everolimus, a drug used for immunologic suppression, can effectively inhibit the conformational change in the CREB molecule, which further changes the fate of dendritic cell differentiation into osteoclasts. Taken together, the evidence suggests positive effects of IL-17 on the growth and development of osteoclasts. Because of the increase in the immature dendritic cell frequency in inflammatory microenvironments, IL-17 may lead to dendritic cell trans-differentiation during inflammation. However, the specific full mechanism of IL-17-mediated dendritic cell trans-differentiation is not clear at present.
Another molecular factor that is worth considering is RANKL, which is produced by several kinds of cells such as CD4+ T cells. In the course of development, it has been confirmed that osteocytes and the bone matrix are relatively heterogeneous compared with other tissues of the body, which means that they may be recognized as non-autologous by the immune system under inflammatory conditions. As the bone marrow is also the source of all immune cells, it may be the close origin relationship between the skeletal and immune systems that promotes tolerance of the matrix in the immune response during development. There is a protective layer of osteoblasts on the surface of the bone matrix, isolating the matrix and osteocytes from the external microenvironment.36 However, with the bone destruction that occurs during inflammation, osteoblasts may undergo damage and apoptosis, which may expose the bone matrix to the immune microenvironment, attracting osteoclasts and precursor dendritic cells that can detect this minimal damage to the bone and be recruited to related bone destruction areas, where dendritic cells can identify the bone matrix particles in the inflammatory environment and present an inflammatory signal to resting CD4+ T cells to induce activation. This process results in the expression of membrane RANKL anchored on T cells, which further activates osteoclast polarization, adhesion and bone destruction activity. Moreover, RANK is also expressed on the surface of dendritic cells, and CD4+ T cell-derived RANKL binds to dendritic cells, which leads to NFATc1-mediated osteoclast-related gene transcription in the dendritic cells and induces DC differentiation into neonatal osteoclasts. CD4+ T cells can also release M-CSF, acting as an important first signal for dendritic cell differentiation into osteoclasts. Thus, RANKL-associated signalling plays vital roles in both the formation of DC-derived OCs and bone resorption activity of OCs.
The activation and differentiation of osteoclasts require not only RANK/RANKL as the necessary first signal but also costimulatory signal as the second signal, such as osteoclast-related receptor (OSCAR), which contains numerous signal receptors ITAM (a kind of tyrosine-activated motif) used to activate the downstream FcR-γ or DAP12-PLCγ pathway, leading to the release of calcium ions and activation of the Ca2+/NFATc1 self-amplification pathway, which may upregulate OC-relevant gene expression.37 It has been confirmed that type Ⅰ and Ⅱ collagens, which are the main components of the bone matrix, can enhance the ability of dendritic cells to release inflammatory factors by activating OSCAR on the dendritic cell surface and stimulate dendritic cell maturation.37 Increasing the expression of dendritic cell receptors can enhance the ability to activate T cells for specific immunity. Activation of OSCAR on the osteoclast surface by type Ⅰ and Ⅱ collagens can also promote the differentiation of osteoclasts, which confirms the special significance of matrix collagen. Therefore, we speculate that dendritic cells may also have this double signal activation mode. IL-17R may be a costimulatory receptor of dendritic cells, and it passes signals through downstream Ca2+/CEBP-β, playing an important role in the isomerization of CEBP-β and participating in crosstalk with the classic first signal activation pathway. These costimulatory signals may be important signals mediating the nuclear fusion of dendritic cells or osteoclasts. It was also found that dendritic cell-specific transmembrane protein (DC-STAMP), a co-stimulatory receptor, and osteoclast-specific transmembrane protein (OC-STAMP) interact with each other in both dendritic cells and osteoclasts. DC-STAMP is also involved in the induction of OC-STAMP mRNA expression and works as a costimulatory signal complementing RANK.37,38 Moreover, it is suggested that the RANK signal and costimulatory signals, such as DC-STAMP, comprise a cross-linked system and influence each other.38 When the differentiation of DCs into OCs is increasing, the phenomenon of nuclear fusion is more obvious under inflammatory conditions than physiological conditions. Overall, these costimulatory molecules may be overexpressed under inflammatory conditions, which provides a precondition for the design of drug targets.
As mentioned above, immature dendritic cell-derived functional multinucleated osteoclasts show a relatively high level of bone absorption function, which perpetuates the need for a large amount of bone remodeling under inflammatory conditions. The expression of tartrate-resistant acid phosphatase (TRAP) and Cathepsin K, along with MMP9, is significantly higher in bone-absorbing osteoclasts under inflammatory conditions than under normal physiological conditions during bone remodelling.3 Excessive Cathepsin K also acts as a chemokine to induce more haematopoietic stem cell migration and differentiation. The remodelling process of the bone is divided into four stages, one of which is called the inversion stage, in which osteoclasts absorb the bone matrix and release bone debris under physiological conditions. The debris released by osteoclasts is then removed by macrophage-like cells, so we speculate that dendritic cell uptake and presentation may occur in the inversion stage of bone remodelling and that bone debris is recognized and phagocytosed by immature dendritic cells. The damaged bone structure under inflammatory conditions also releases several inflammatory factors, producing a cascade amplification effect and chemotactically recruiting more immature dendritic cells to the destruction site. It is worth mentioning that while osteocytes and osteoblasts are destroyed, their membrane-anchored and soluble RANKL molecules are also released, further resulting in dendritic cell or osteoclast activation. In general, the resorption caused by osteoclasts creates conditions for the migration and differentiation of dendritic cells that present small antigen molecules at same times. The role of immature dendritic cells can be divided into two aspects because they not only transform into osteoclasts and join osteoclast-related bone destruction directly but also play vital roles in producing osteoclast differentiation-related molecules. For example, under inflammatory conditions, dendritic cells can produce IL-1β, IL-6, TNF-α and other factors to stimulate monocyte-derived osteoclast activation. The continuous activation of immature dendritic cells may induce osteoclastogenesis and further result in pathological osteolysis.
Several kinds of cell populations are involved in DC-OC trans-differentiation, including T cells, B cells, innate immune cells, synovial fibroblasts, osteocytes and osteoclasts. In the first phase, chemotactic immature dendritic cells are recruited to the site of microinjury. At the same time, multinuclear osteoclasts absorb bone matrix and collagen molecules, which are also presented to CD4+ T cells. Then, through the cross-linking of T cells with dendritic cells, the expression of RANKL by T cells promotes the transformation of dendritic cells into osteoclast-like cells with absorptive function. In addition, IL-17 produced by activated Th17 cells stimulates the production of soluble RANKL and binds to IL-17A receptor on the surface of immature dendritic cells to activate NF-κB signalling and costimulatory signalling, which mediates the differentiation and nuclear fusion of dendritic cells. In addition, cytokines produced by macrophages in the microenvironment also promote the differentiation and maturation of osteoclasts. Overall, T cells in the site may serve to establish balance, such as the balance between RANKL and IFN-γ or the balance between Th17 and Treg cells.
DC-OC trans-differentiation under pathologic conditions
Inflammatory bone disease
Inflammatory bone destruction is a systemic bone destruction disease caused by infection, autoimmunity or other related pathogenic processes. The pathological feature is the excessive activation of osteoclasts, which leads to bone resorption and formation imbalance. The clinical manifestations of rheumatoid arthritis (RA) are symmetrical and widespread destruction of multiple small joints with the main pathological changes of synovitis and vasculitis (Table 1). Immune disorder is the main pathogenesis of RA.39 A large amount of IL-17, along with activated CD4+ T cells and antigen-presenting cells, can be found in the synovial fluid of patients.25 Small injuries may lead to the loss of the osteoblast collar above the bone matrix, which exposes the bone matrix to the inflammatory microenvironment. In patients with RA, the matrix is more heterogeneous after citrullination of vital amino acid residues, which makes it possible to recruit immature dendritic cells for antigen recognition and presentation and then activate CD4+ T cells to express or release soluble RANKL.40 Then, RANKL acts on dendritic cells, inducing them to differentiate into osteoclasts, which corresponds with RA inflammatory conditions.41, 42, 43 Newly formed osteoclasts absorb residual bone debris and form a DC-OC loop by inducing chemotaxis of immature dendritic cells, resulting in constant severe bone destruction. At the same time, fibroblasts mediate the migration of Th17 cells to bone destruction sites, and activated Th17 cells release a large amount of IL-17 to stimulate the secretion of RANKL by bone stromal cells, which may also promote the nuclear fusion of immature dendritic cells induced by IL-17A receptors.31,36 In vitro experiments have shown that IL-17 can promote the differentiation of dendritic cells into osteoclasts and mediate bone damage. In addition, dendritic cells can release IL-1β, IL-6, TNF-α and other inflammatory factors to promote the production of RANKL along with the differentiation and maturation of osteoclasts, so their initiating and assistive roles can be fully observed. IL-17 also has a self-magnifying feed-forward cascade reaction mediated by stimulating synovial cells to release IL-32, which can in turn act on Th17 cells to promote increased expression of IL-17.7
Table 1.
The DC-OC trans-differentiation contributes to severe kinds of diseases.
| Diseases | Factors | Effect | Reference |
|---|---|---|---|
| Multiple myeloma | RANKL, IL-17 | MM raises iDC through SDF-1 | 8, 9, 10 |
| CD47, SDF-1 | MM cell produces IL-17 and RANKL make DC differentiation | 18,21 | |
| Rheumatoid arthritis | IL-17, RANKL | Bone cells release RANKL | 1,4 |
| IL-1, IL-6 | Th-17 joint infiltration mediates trans-differentiation. | 14,15,17 | |
| TNF-α, IL-1β | |||
| periodontitis | IL-17 | Th-17 cell invade the parodontium | 27,35 |
| RANKL | And induce the trans-differentiation | ||
| Langerhans cell hyperplasia | IL-1, TNFα, IL-6, RANKL, M-CSF, IL-17 | DC chemotaxis to superficial dermis and secrete IL-17 | 24 |
| Increased IL-17 in microenvironment |
Bone infectious diseases often result in serious bone destruction, such as periodontitis (Table 1), which is a chronic infectious disease of the bone.39, 40, 41, 42 In vitro, it was found that coculturing immature dendritic cells and T cells with bacterial products such as lipopolysaccharide (LPS) leads to increased expression levels of osteoclast-relevant molecules, such as TRAP, Cathepsin K, and MMP9. In contrast, neither coculture of immature dendritic cells with bacterial products nor culture of T cells with LPS could produce detectable osteoclast-like multinucleated giant cells and their specific markers in nutrient medium, which implies the possibility of interactions among T cells, DCs and LPS.1 In particular, clinical research has increasingly pointed out that the gram-negative bacteria infection rate is increasing.42 Gram-negative bacterium-derived LPS is also able to stimulate bone stromal cells to release RANKL.44 This indicates that there may exist a process in which dendritic cells present bacterial antigens to activate T cells and then stimulate DC-OC trans-differentiation to mediate bone destruction pathogenesis.45
Inflammatory bowel disease (IBD) is a chronic recurrent intestinal inflammatory disease caused by abnormal immune system activity (Table 1). Clinical follow-up showed that 40% of patients with IBD exhibited bone destruction.1 In recent years, it has been found by pathological biopsies that Th17 cells are present in the superficial layer of the mucous membrane of IBD patients.35,46,47 Metastatic tumour cells, such as prostate or breast carcinoma cells, in the bone often result in bone loss, apart from the cause of carcinoma cell biology and the abundant blood supply of the bone. These tumour cells often migrate to the vertebrae, which also have a plentiful blood supply. Therefore, we speculated that inflammatory conditions caused by tumours can recruit Th17 cells, causing Th17 cells and their product IL-17 to travel through the blood to the bone, ultimately meditating serious bone destruction and contributing to metastasis. Taken together, these findings indicate that during the pathology of these inflammatory bone diseases, dendritic cell-derived osteoclasts may also contribute to inflammation-related bone destruction. Th17 cells and IL-17 mediate RANKL production-induced immature dendritic cell trans-differentiation and nuclear fusion after they arrive at the corresponding bone site.
Abnormal hyperplastic disease of bone marrow
Multiple myeloma (MM) is a myelodysplastic malignant tumour derived from plasma cells. Multiple myeloma cells can release the soluble chemokine SDF-1 (CXCL-12) to induce immature dendritic cell chemotaxis via the corresponding receptor CXCR4 on the iDC surface,2,16 and then multiple myeloma cells and dendritic cells interact with each other to transmit signals (Table 1). Multiple myeloma expresses TACI receptor anchored on the surface of dendritic cells by TRAIL (an immature dendritic cell marker), and then dendritic cells increase pro-proliferative signalling to multiple myeloma cells. The secretion of soluble RANKL by multiple myeloma cells promotes the activation and differentiation of immature dendritic cells into osteoclast-like cells with bone resorption function. Multiple myeloma cells not only produce RANKL themselves but also stimulate OBs and stromal cells to produce RANKL.48 Although osteoclast proliferation in patients with multiple myeloma can destroy multiple myeloma cells, it leads to bone destruction as an adverse effect, which may be a prerequisite for the invasion of multiple myeloma and produce a large enough volume for the expansion and erosion of tumour cells in the later stage of disease. In addition, it has been confirmed that IL-17 plays vital roles in the occurrence and development of multiple myeloma.32,49 The level of IL-17 in the synovial fluid of patients with multiple myeloma is increased, probably because multiple myeloma cells act as tumour cells and can activate immune cells, especially Th17 cells, to produce IL-17.
Langerhans cell hyperplasia (LCH) is a group of diseases with dendritic cell monoclonal hyperplasia as the pathological change, which is often accompanied by serious bone destruction (Table 1). It was found that the connection between Langerhans cells and differentiated osteoclast-like cells is relatively close, suggesting that abnormal presentation of antigens may lead to the pathological activation and differentiation of dendritic cells.50 Additionally, the expression levels of IL-1, IL-6, TNF-α, RANKL, and M-CSF in the local microenvironment are increased so osteoclasts are activated directly through these mediators and the dendritic cell trans-differentiation pathway, playing important roles in bone destruction in patients with LCH. In summary, a good understanding of osteoclast differentiation and maturation may lead to a new therapeutic strategy for treatment of diseases with abnormal hyperplasia of the bone marrow.
Metabolic bone diseases
A multitude of metabolic diseases are often accompanied by bone destruction, which suggests the endocrine role of the skeletal system (Table 1). Hyperlipidaemia is a group of metabolic abnormalities characterized by hyperlipidaemia, especially an increase in the low-density lipoprotein (LDL) concentration. Epidemiological studies have found that coronary atherosclerosis (AS) caused by hyperlipidaemia is positively correlated with the incidence of osteoarthritis (OA). It is suggested that the electron pairs of oxidized low-density lipoprotein (OX-LDL) can induce the production of oxygen free radicals, which attack and destroy osteoblasts and expose the bone matrix, inducing immune inflammation. Due to the lack of oestrogen and the weakening of the vascular protective effect of oestrogen in postmenopausal osteoporosis (POP), women with POP are prone to produce OX-LDL, which can damage endothelial cells in bone vessels, resulting in the formation of vascular plaques, which block and affect the blood supply. Therefore, ischaemia in related blood vessels leads to small-segment osteonecrosis and the formation of neovasculature induced by hypoxic activation of the HIF-VEGF pathway.51 However, because the permeability of the neovasculature does not reach the normal capillary level, it further aggravates lipid penetration. However, the bone is similar to a container with a constant volume that cannot contain too much fat unless more effective space is released by bone destruction. The protective effect of oestrogen on the bone is weakened, and the scavenging of free radicals is also weakened, which ultimately leads to bone damage. The remaining part of the bone is replaced with lipids so that the bone becomes fatty and vacuolated, which affects the growth and differentiation of normal sinuses and HSCs, contributing to an increased level of HSC differentiation into adipocytes.52 The high levels of triglycerides and cholesterols in the microenvironment are a kind of inflammatory stress, which may induce the production of a large number of proinflammatory factors. Osteoclast activation and bone destruction are mediated by free radicals, and macrophages and osteoclasts, the only macrophages of the skeletal system, have the function of scavenging free radicals.53 Osteoclasts can significantly proliferate and become activated, further causing serious bone loss, under the condition of a high fat concentration, and the lost cavity will be replaced with lipids to form a positive feedback loop. Furthermore, a study also showed that the lipid content was closely related to the activity of osteoclasts. Cholesterol efflux from the osteoclast intracellular space can induce apoptosis in osteoclasts, suggesting that lipids are vital in the survival and function of osteoclasts.54 Treatment of hyperlipidaemia with drugs can reverse osteoporosis, which has been effectively confirmed in the clinic. Adipocytes are closely related to the bone and originate from shared precursors. Mesenchymal stem cells can differentiate into two kinds of cells at the same time, and leptin produced by adipocytes also has the ability to regulate bone mass.55, 56, 57 Under normal circumstances, there is a balance in the activation of osteoclasts. It is possible that the overactivation of osteoclasts may be related to dendritic cells. The exposure of the bone matrix provides an ideal condition for the function of dendritic cells. The inflammatory microenvironment not only contains inflammatory factors inducing those that induce chemotaxis and the transformation of immature dendritic cells into osteoclasts but also provides necessary conditions for the survival of osteoclasts. In addition, necrotic bone also produces soluble RANKL to activate the dendritic cell differentiation signalling pathway. However, the exact mechanism by which adipocytes can transform dendritic cells into osteoclasts and the origins of RANKL and M-CSF need to be further studied. In addition, how CD4+ T cells are involved in inflammatory microenvironments with a high fat concentration also remains to be explored. The role of DC-OC trans-differentiation in bone metabolic diseases remains to be further discussed.
Conclusions and perspectives
In recent years, the concept of osteoimmunology has been recognized by more people thanks to the development of osteoimmunology based on the contributions of other scholars.58 Osteoimmunology has demonstrated a possible relationship between the skeletal and immune systems because they not only have similar origins but also share common microenvironment.59 They are closely related to each other and have shared similar receptors and molecular pathways, such as RANK/RANKL, which are vital to both the differentiation of mature osteoclasts and the development of immune organs. RANK/RANKL knockout mice show bone sclerosis, which is accompanied by deficiencies in secondary immune organs, such as spleen and lymph node developmental loss.60
The regulation of bone mass under physiological conditions is undertaken by the differentiation and nuclear fusion of monocytes, the microphage system. However, under inflammatory conditions, osteoclasts can be formed by the trans-differentiation of immature dendritic cells.1,61,62 The antigen-presenting ability of immature dendritic cells, which are called ‘inflammatory dendritic cells’, is weak, but their chemotactic ability is obviously strong.63 Under inflammatory conditions, immature dendritic cell chemotaxis plays an essential role in the inflamed site, and these cells differentiate into bone-destructive cells under stress. Therefore, dendritic cell-derived osteoclasts have increased expression of osteoclast-related molecules and show relatively strong bone resorption activity. At the same time, the bone matrix and debris are presented to CD4+ T cells to further amplify the inflammatory cascade in response to the need for bone destruction in the context of acute or chronic inflammation.64 In addition, dendritic cells also produce a variety of inflammatory factors to support the activation and maturation of the original osteoclasts. Therefore, in the relationship between dendritic cells and osteoclasts, dendritic cells are the source of differentiating osteoclasts and play an assistive and auxiliary role in osteoclast activation and maturation. Additionally, osteoclasts are not only the executor of bone resorption but also helpers, which can transfer resorption products to dendritic cells for presentation. The cavity that is produced by osteoclast resorption is vital in tumour cell growth during cancer metastasis.65 During bone destruction, chips and small molecules are released to induce immature dendritic cell recruitment and trans-differentiation, which in turn contributes to further bone destruction and construct a closed loop of bone matrix damage. It has been discovered that monocytes play important roles in DC-OC differentiation by releasing several kinds of inflammatory factors. Monocytes, which are well known as the precursors of osteoclasts, can produce inflammatory mediators to promote osteoclast maturation. In addition, B cells are also important components of the immune system, which are needed to be further clarified in bone destruction and DC-OC-related diseases.
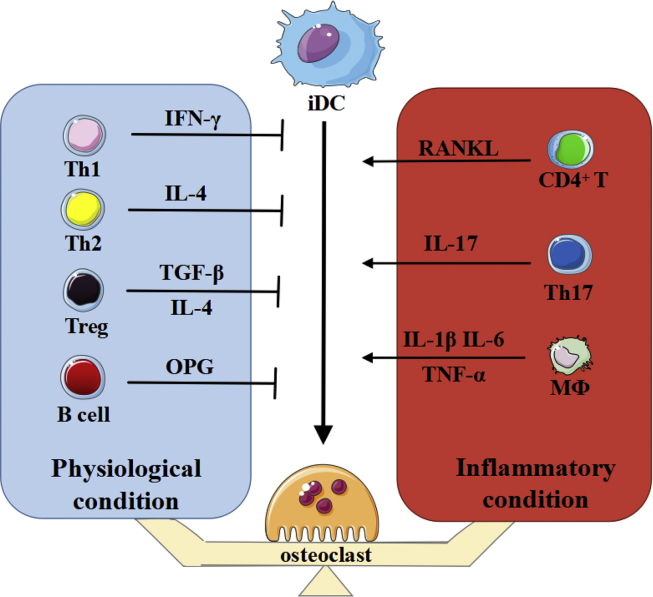
In recent years, the effect of CD4+ T cells on DC-OC trans-differentiation has become a heated topic. CD4+ T cells may be used as a switch to determine the balance between inflammation intensification and resolution (Fig. 3).30 Activated CD4+ T cells can not only produce RANKL to stimulate dendritic cell differentiation but also exert inhibitory effects by secreting TRAIL, a suppressive signal antagonistic to RANKL. In addition, IFN-γ produced by T cells can inhibit the RANK/RANKL pathway by blocking TRAF6, further indicating the balancing effect of T cells. However, under pathological conditions, the balancing effect of T cells breaks down, resulting in increased RANKL production and leading to the occurrence and development of bone destruction diseases (Fig. 3). In addition, by analysing and summarizing the signalling pathway of IL-17, we have found that IL-17 can promote dendritic cell differentiation by participating in not only the canonical NF-κB pathway but also the TRAF6-CEBP-β costimulatory pathway, indicating its dual actions during dendritic cell trans-differentiation. CEBP-β is an important costimulatory signalling molecule in osteoclasts that mediates the nucleation of osteoclasts. The co-coupling of dendritic cells and osteoclasts has become a hot issue because it reveals the mechanism underlying the interaction and transformation between dendritic cells and osteoclasts, which will provide a new therapeutic target for a variety of osteoclast-related bone destruction diseases. For instance, in multiple myeloma, there are a multitude of tumour markers that are presented by dendritic cells and activate dendritic cell trans-differentiation into osteoclasts to exert a bone-destructive effect. At the same time, in a hyperlipidaemia model, a high-fat inflammatory microenvironment may also induce immature dendritic cell chemotaxis and promote their activation and trans-differentiation into functional osteoclasts.
Figure 3.
The role of immunocytes in the process of trans-differentiation from immature dendritic cells to osteoclasts.
Immunocytes are involved in iDC trans-differentiation into OCs and are essential in mediating pathological bone mass loss. In the physiological state, a large amount of inhibitory signalling molecules are produced to prevent abnormal formation and activation of OCs. In contrast, promotive signals from immunocytes have been increased dramatically during inflammation, contributing to increased trans-differentiation of iDCs into OC and osteolysis. Therefore, immunocytes play essential roles in the formation of OC from iDCs. Several kinds of bone destruction diseases may occur when the equilibrium breaks down.
The differentiation of DCs into OCs reveals a new pathway for osteoclast formation, providing a novel target for clinical application. Previous researchers tried to inhibit the activation of osteoclasts by inhibiting the NF-κB pathway, but the side effects were obvious and the activated osteoclasts were difficult to control. Recently, it has been suggested that using RANKL antagonists during inflammation may inhibit the activation of osteoclasts, which is being tested in a clinical trial stage.65,66 Dendritic cell migration plays an important role in bone destruction under inflammatory conditions. It is possible to inhibit the migration and differentiation of dendritic cells in the treatment of RA and other diseases.67 Blocking the cross-linking between dendritic cells and multiple myeloma cells may inhibit the proliferation of multiple myeloma cells. We also speculate that IL-17A antagonists may have an efficient clinical effect due to its essential effect. In addition, immunosuppressive agents may exert an effect by inhibiting T cells' activity since these essential roles in the DC-OC trans-differentiation. More drug targets need to be supported by further basic experiments and clinical research.
DC-OC trans-differentiation has become an accepted mechanism of osteoclast formation. It is strongly believed that more mechanisms of DC-OC trans-differentiation will be clarified and more drug targets for related diseases in this area will be detected in further studies.
Conflict of Interests
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by grants from the Key Program of Nature Science Foundation of China (grant number 81930067), the Medical Science and Technology Youth Cultivation Project of PLA China (grant number 20QNPY022), Medical innovation capability upgrading Plan of Southwest Hospital China (grant number SWH2018LJ-03) and Medical innovation of graduate students in Chongqing China (grant number CYS19360).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Yueqi Chen, Email: chenyueqi1012@sina.com.
Shiwu Dong, Email: dongshiwu@tmmu.edu.cn.
Abbreviations
- DC
dendritic cells
- iDC
immature dendritic cells
- GM-CSF
granulocyte macrophage colony stimulating factor
- M-CSF
macrophage colony stimulating factor
- IL-4
Interleukin-4
- BMP
bone morphogenetic protein
- TGF-β
transforming growth factor-beta
- RANKL
receptor activator of nuclear factor κB ligand
- Th0
naive T cell
- Th1
type 1 T helper (cell)
- Th2
type 2 T helper (cell)
- Th17
type 17 T helper (cell)
- Treg
regulatory T (cell)
References
- 1.Olivier L., Claudine B.W., Jerome G. Dendritic-cell-derived osteoclasts: a new game changer in boneresorption-associated diseases. Drug Discov Today. 2016;21(9):1345–1354. doi: 10.1016/j.drudis.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L., Dong Y., Wang Y., Hu W., Dong S., Chen Y. Sphingosine-1-phosphate (S1P) receptors: promising drug targets for treating bone-related diseases. J Cell Mol Med. 2020;24(8):4389–4401. doi: 10.1111/jcmm.15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kazuo O., Tomoki N., Masahiro S. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. 2017;97(4):1295–1349. doi: 10.1152/physrev.00036.2016. [DOI] [PubMed] [Google Scholar]
- 4.Ono T., Takayanagi H. Osteoimmunology in bone fracture healing. Curr Osteoporos Rep. 2017;15(4):367–375. doi: 10.1007/s11914-017-0381-0. [DOI] [PubMed] [Google Scholar]
- 5.Rauner M., Buttgereit F., Distler J. Osteoimmunologie – IMMUNOBONE. Zeitschrift Für. Rheumatologie. 2018;77(S1):12–15. doi: 10.1007/s00393-018-0455-0. [DOI] [PubMed] [Google Scholar]
- 6.Ono Y., Terashima K., Liu A. Follicular dendritic cell sarcoma with microtubuloreticular structure and virus-like particle production in vitro. Pathol Int. 2009;59(5):332–344. doi: 10.1111/j.1440-1827.2009.02375.x. [DOI] [PubMed] [Google Scholar]
- 7.Herter S., Osterloh P., Hilf N. Dendritic cell aggresome-like-induced structure formation and delayed antigen presentation coincide in influenza virus-infected dendritic cells. J Immunol. 2005;175(2):891–898. doi: 10.4049/jimmunol.175.2.891. [DOI] [PubMed] [Google Scholar]
- 8.Ono T., Nakashima T. Recent advances in osteoclasts biology. Histochem Cell Biol. 2018;149(4):325–341. doi: 10.1007/s00418-018-1636-2. [DOI] [PubMed] [Google Scholar]
- 9.Gallois A., Lachuer J., Yvert G. Genome-wide expression analyses establish dendritic cells as a new osteoclasts precursor able to generate bone-resorbing cells more efficiently than monocytes. J Bone Miner Res. 2010;25(3):661–672. doi: 10.1359/jbmr.090829. [DOI] [PubMed] [Google Scholar]
- 10.Alnaeeli M., Penninger J.M., Teng Y.T. Immune interactions with CD4+ T cells promote the development of functional osteoclasts from murine CD11c+ dendritic cells. J Immunol. 2006;177(5):3314–3326. doi: 10.4049/jimmunol.177.5.3314. [DOI] [PubMed] [Google Scholar]
- 11.Maitra R., Follenzi A., Yaghoobian A. Dendritic cell-mediated in vivo bone resorption. J Immunol. 2010;185(3):1485–1491. doi: 10.4049/jimmunol.0903560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schett G., Lories R.J., D'Agostino M.A. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol. 2017;13(7):31–41. doi: 10.1038/nrrheum.2017.188. [DOI] [PubMed] [Google Scholar]
- 13.Klein-Hessling S., Muhammad K., Klein M. NFATc1 controls the cytotoxicity of CD8+ T cells. Nat Commun. 2017;8(1) doi: 10.1038/s41467-017-00612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava R.K., Dar H.Y., Mishra P.K. Immunoporosis: immunology of osteoporosis-role of T cells. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudo O., Fujikawa Y., Itonaga I. Proinflammatory cytokine (TNFalpha/IL-1alpha) induction of human osteoclast formation. J Pathol. 2002;198(2):220–227. doi: 10.1002/path.1190. [DOI] [PubMed] [Google Scholar]
- 16.Hirbe A.C., Morgan E.A., Weilbaecher K.N. The CXCR4/SDF-1 chemokine axis: a potential therapeutic target for bone metastases? Curr Pharmaceut Des. 2010;16(11):1284–1290. doi: 10.2174/138161210791034012. [DOI] [PubMed] [Google Scholar]
- 17.Azab A.K., Runnels J.M., Pitsillides C. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tucci M., Stucci S., Strippoli S. Dendritic cells and malignant plasma cells: an alliance in multiple myeloma tumor progression? Oncol. 2011;16(7):1040–1048. doi: 10.1634/theoncologist.2010-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evangelos T., Ioannis N.S., Meletios A.D. Myeloma bone disease: from biology findings to treatment approaches. Blood Spotlight. 2019;133(14):1–11. doi: 10.1182/blood-2018-11-852459. [DOI] [PubMed] [Google Scholar]
- 20.Alnaeeli M., Park J., Mahamed D. Dendritic cells at the osteoimmune interface: implications for inflammation induced bone loss. J Bone Miner Res. 2007;22(6):775–780. doi: 10.1359/jbmr.070314. [DOI] [PubMed] [Google Scholar]
- 21.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7(4):292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Dou C., Yi J. Inhibitory effect of vanillin on RANKL-induced osteoclast formation and function through activating mitochondrial-dependent apoptosis signaling pathway. Life Sci. 2018;208:305–314. doi: 10.1016/j.lfs.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima T., Hayashi M., Fukunaga T. Evidence for osteocytes regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 24.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2013;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 25.Fabienne C., Olivier P., Irma M.G. Osteoimmunology of bone loss in inflammatory rheumatic diseases. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kavita M., Dhodapkar S.B., Phillip M. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112(7):2878–2885. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J., Paul W.E. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):57–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato K., Suematsu A., Okamoto K. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):73–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monasterio G., Castillo F., Ibarra J.P. Alveolar bone resorption and Th1/Th17-associated immune response triggered during Aggregatibacter actinomycetemcomitans-induced experimental periodontitis are serotype-dependent. J Periodontol. 2018;89(10):49–61. doi: 10.1002/JPER.17-0563. [DOI] [PubMed] [Google Scholar]
- 30.Takayanagi H. T cell-mediated regulation of osteoclastogenesis by signalling crosstalk between RANKL and IFN-γ. Nature. 2000;408(6812):600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 31.Adamopoulos I.E., Chao C., Geissler R. Interleukin-17A upregulates receptor activator of NF-κB on osteoclasts precursors. Arthritis Res Ther. 2010;12(1) doi: 10.1186/ar2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ying F., Cunjing Z., Zongpu Z. IL-17A neutralizing antibody attenuates eosinophilic meningitis caused by Angiostrongylus cantonensis by involving IL-17RA/Traf6/NF-κB signaling. Exp Cell Res. 2019;384(1) doi: 10.1016/j.yexcr.2019.111554. [DOI] [PubMed] [Google Scholar]
- 33.Coury F., Annels N., Rivollier A. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cells fusion. Nat Med. 2008;14(1):81–87. doi: 10.1038/nm1694. [DOI] [PubMed] [Google Scholar]
- 34.Uta S., Britta S. Bone marrow Th17 TNF-α cells induce osteoclasts differentiation and link bone destruction to IBD. Gut. 2015;64(7):1072–1081. doi: 10.1136/gutjnl-2014-306947. [DOI] [PubMed] [Google Scholar]
- 35.Shuvra R., Carlo D.S., Theresa T.P. Central role of IL-17/Th17 immune responses and the gut microbiota in the pathogenesis of intestinal fibrosis. Curr Opin Gastroenterol. 2014;30(6):531–538. doi: 10.1097/MOG.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X., Zhi X., Wang J. RANKL signaling in bone marrow mesenchymal stem cells negatively regulates osteoblastic bone formation. Bone Res. 2018;6 doi: 10.1038/s41413-018-0035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nitze L.M., Zeuthen L.H., Keller P. Collagen induces maturation of human monocyte-derived dendritic cells by signaling through osteoclast-associated receptor. J Immunol. 2015;194(7):3169–3179. doi: 10.4049/jimmunol.1402800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeshi M. The dendritic cells-specific transmembrane protein DC-STAMP is essential for osteoclasts fusion and osteoclasts bone-resorbing activity. Mod Rheumatol. 2006;16(6):341–342. doi: 10.1007/s10165-006-0524-0. [DOI] [PubMed] [Google Scholar]
- 39.Genovese M.C., Kalunian K., Gottenberg J.E. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA. 2019;322(4):315–325. doi: 10.1001/jama.2019.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akilan K.A., Jimmy Y., Meng S. Citrullination control dendritic cell trans-differentiation into osteoclasts. J Immunol. 2019;202(11):3143–3150. doi: 10.4049/jimmunol.1800534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato K., Takayanagi H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr Opin Rheumatol. 2006;18(4):419–426. doi: 10.1097/01.bor.0000231912.24740.a5. [DOI] [PubMed] [Google Scholar]
- 42.Zhou L., Li L., Wang Y. Effects of RANKL on the proliferation and apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis through regulating the NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(21):9215–9221. doi: 10.26355/eurrev_201911_19413. [DOI] [PubMed] [Google Scholar]
- 43.Popova V., Vazhev Z., Geneva-Popova M. Comparison of RANKL expression, inflammatory markers, and cardiovascular risk in patients with acute coronary syndrome with and without rheumatoid arthritis. Rheumatol Int. 2019;39(10):67–73. doi: 10.1007/s00296-019-04367-9. [DOI] [PubMed] [Google Scholar]
- 44.Corneliu S., Ana V., Michael G. Macrophage immunomodulation in chronic osteolytic diseases—the case of periodontitis. J Leukoc Biol. 2018;105(3):1–15. doi: 10.1002/JLB.1RU0818-310R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexios D., Iliadis M.R. Paediatric bone and joint infection. EFORT Open Reviews. 2017;2(1):7–12. doi: 10.1302/2058-5241.2.160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali T., Lam D., Bronze M.S. Osteoporosis in inflammatory bowel disease. Am J Med. 2009;122:599–604. doi: 10.1016/j.amjmed.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carrie N., Arnold G., Jennifer E.T. A role for IL-12 in IBD after all? Immunity. 2019;51(2):209–211. doi: 10.1016/j.immuni.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Sezer O., Heider U., Jakob C. Human bone marrow myeloma cells express RANKL. J Clin Oncol. 2002;20(1):353–354. doi: 10.1200/JCO.2002.20.1.353. [DOI] [PubMed] [Google Scholar]
- 49.Noonan K., Marchionni L., Anderson J. A novel role of IL-17 producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood. 2010;116(18):3554–3563. doi: 10.1182/blood-2010-05-283895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Da Costa C.E., Annels N.E., Faaij C.M. Presence of osteoclasts-like multinucleated giant cells in the bone and nonostotic lesions of Langerhans cell histiocytosis. J Exp Med. 2005;201(5):687–693. doi: 10.1084/jem.20041785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semenza G.L. Hypoxia-Inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawrence R.C., Felson D.T., Helmick C.G. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part Ⅱ. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y., Sun J., Dou C. Alliin attenuated RANKL-induced osteoclastogenesis by scavenging reactive oxygen species through inhibiting Nox1. Int J Mol Sci. 2016;17(9) doi: 10.3390/ijms17091516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luegmayr E., Glantschnig H., Wesolowski G.A. Osteoclast formation, survival and morphology are highly dependent on exogenous cholesterol/lipoproteins. Cell Death Differ. 2004;11(Suppl 1):S108–S118. doi: 10.1038/sj.cdd.4401399. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang H., Zhang X., Zhu C. Molecular mechanisms of PPAR-γ governing MSC osteogenic and adipogenic differentiation. Curr Stem Cell Res Ther. 2016;11(3):255–264. doi: 10.2174/1574888x10666150531173309. [DOI] [PubMed] [Google Scholar]
- 56.Chen X.X., Yang T. Roles of leptin in bone metabolism and bone diseases. J Bone Miner Metabol. 2015;33(5):474–485. doi: 10.1007/s00774-014-0569-7. [DOI] [PubMed] [Google Scholar]
- 57.Liu K., Liu P., Liu R. Relationship between serum leptin levels and bone mineral density: a systematic review and meta-analysis. Clin Chim Acta. 2015;444:260–263. doi: 10.1016/j.cca.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 58.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5(12):667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 59.Calvi L.M., Adams G.B., Weibrecht K.W. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J., Niu C., Ye L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 61.Xiao Y., Palomero J., Grabowska J. Macrophages and osteoclasts stem from a bipotent progenitor downstream of a macrophage/osteoclast/dendritic cell progenitor. Blood Adv. 2017;1(23):69–77. doi: 10.1182/bloodadvances.2017008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiu Y.H., Schwarz E., Li D. Dendritic cell-specific transmembrane protein (DC-STAMP) regulates osteoclast differentiation via the Ca/NFATc1 Axis. J Cell Physiol. 2017;232(9):2538–2549. doi: 10.1002/jcp.25638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arai F., Hirao A., Ohmura M. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Xing L., Schwarz E.M., Boyce B.F. Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev. 2005;208(1):19–29. doi: 10.1111/j.0105-2896.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 65.Wittrant Y., Théoleyre S., Chipoy C. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta. 2004;1704(2):49–57. doi: 10.1016/j.bbcan.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Hameed A., Brady J.J., Dowling P. Bone disease in multiple myeloma: pathophysiology and management. Canc Growth Metastasis. 2014;7:33–42. doi: 10.4137/CGM.S16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venkataraman A., Almas K. Rheumatoid arthritis and periodontal disease: an update. N Y State Dent J. 2015;81(5):30–36. [PubMed] [Google Scholar]