Abstract
Background
Whether individual cardiologist billings are associated with differences in ambulatory care management and clinical outcomes in patients with coronary artery disease (CAD) and heart failure (HF) remains poorly understood.
Methods
We conducted a population-based, retrospective cohort study of cardiologists who treat patients with CAD or HF using administrative claims data in Ontario, Canada. The primary exposure was cardiologist billing quintile. We then stratified median billing amounts into quintiles, from lowest (quintile 1) to highest billing physicians (quintile 5).
Results
The main outcomes of interest were cardiac diagnostic and therapeutic procedures that occurred within 365 days of the index visit. Our 2 cohorts respectively consisted of 170,959 patients with CAD seen by 1 of 423 cardiologists and 56,262 HF patients seen by 1 of 413 cardiologists. CAD patients of higher-billing cardiologists had higher rates of echocardiograms (adjusted odds ratio [aOR], 1.65; 95% confidence interval [CI], 1.39 to 1.94 for quintile 5 vs quintile 2) and stress tests (aOR, 1.50; 95% CI, 1.28-1.75) at 1 year, with a similar pattern for HF patients of echocardiogram (aOR, 1.40; 95% CI, 1.23-1.59; P < 0.001) and stress test (aOR, 1.32; 95% CI, 1.15-1.51) use. CAD patients of cardiologists in quintile 1 had a higher mortality rate (aOR, 1.16; 95% CI, 1.03-1.31), and HF patients of cardiologists in billing quintile 4 had a lower hospitalization rate at 1 year (OR, 0.94; 95% CI, 0.89-0.99; P = 0.02).
Conclusions
Cardiac patients seen by the highest-billing cardiologists received more noninvasive cardiac testing compared with lower-billing cardiologists.
Résumé
Introduction
On comprend mal que la facturation individuelle des cardiologues soit associée à des différences dans la prise en charge des soins ambulatoires et les résultats cliniques des patients atteints de coronaropathie et d’insuffisance cardiaque (IC).
Méthodes
Nous avons mené une étude de cohorte populationnelle rétrospective auprès de cardiologues, qui traitent les patients atteints de coronaropathie ou d’IC, à partir des données sur les réclamations administratives en Ontario, au Canada. La principale exposition était les quintiles de facturation des cardiologues. Nous avons donc stratifié les montants médians de la facturation en quintiles, soit des médecins qui facturaient le moins (quintile 1) aux médecins qui facturaient le plus (quintile 5).
Résultats
Les principaux critères d’intérêts étaient le diagnostic de cardiopathie et les interventions thérapeutiques qui survenaient dans les 365 jours de la consultation indicielle. Nos deux cohortes regroupaient respectivement 170 959 patients atteints d’une coronaropathie qui avaient été vus par un des 423 cardiologues et 56 262 patients atteints d'IC vus par un des 413 cardiologues. Les patients atteints d’une coronaropathie des cardiologues qui facturaient le plus avaient des taux plus élevés d’utilisation des échocardiogrammes (rapport de cotes ajusté [RCa], 1,65; intervalle de confiance [IC] à 95 %, 1,39-1,94 pour le quintile 5 vs le quintile 2) et des épreuves d’effort (RCa, 1,50; IC à 95 %, 1,28-1,75) après 1 an, et les patients atteints d’IC avaient un profil comparable d’utilisation des échocardiogrammes (RCa, 1,40; IC à 95 %, 1,23-1,59; P < 0,001) et des épreuves d’effort (RCa, 1,32; IC à 95 %, 1,15-1,51). Les patients atteints d’IC des cardiologues dans le quintile 1 avaient un taux de mortalité plus élevé (RCa, 1,16; IC à 95 %, 1,03-1,31), et les patients atteints d’IC des cardiologues dans le quintile de facturation 4 avaient un taux d’hospitalisation plus faible après 1 an (RC, 0,94; IC à 95 %, 0,89-0,99; P = 0,02).
Conclusions
Les patients cardiaques vus par les cardiologues qui facturaient le plus avaient plus d’examens non invasifs du cœur comparativement aux patients vus par les cardiologues qui facturaient le moins.
Low-value care is responsible for up to 30% of all health care spending in both Canada and the United States.1, 2, 3, 4, 5 Health system financing, particularly fee-for-service (FFS) payment models, has been identified as a driver of overuse of low value care.6
The impact of economic incentives on the use of low-value cardiac services holds interest for health service researchers. Previous research found significant variability in rates of stress testing in patients with stable coronary artery disease (CAD).7,8 Prior research found that ownership of stress testing equipment may influence use of cardiac services, but there is no research investigating the association between individual cardiologist billings in an FFS system and cardiac service use. It is not known whether billing differences between cardiologists affects practice variation or clinical outcomes.
We aimed to describe cardiologists’ billing patterns and determine if there was any association between individual physician billings, use of cardiac services, and clinical outcomes in patients with CAD and heart failure (HF). We hypothesized that cardiologists with higher annual billings would use more cardiac services per patient compared with cardiologists with lower billing.
Methods
Study design and data sources
We used administrative claims data from Ontario, Canada to conduct a population-based, retrospective cohort study of cardiologists who treat patients with CAD and HF. In Ontario, cardiac care is mainly delivered through physicians’ offices and not-for-profit hospitals.9 Databases used were: (1) Discharge Abstract Database, which includes information on hospital discharges; (2) National Ambulatory Care Reporting System, containing data on hospital- and community-based ambulatory care, including emergency department visits and same day surgeries; (3) Ontario Health Insurance Plan (OHIP) claims data, covering all billings made by physicians; (4) ICES Physician Database, detailing demographic information for physicians; and (5) CorHealth Cardiac Registry, containing specific entries on a variety of cardiac procedures. Baseline patient characteristics were identified through the Registered Persons Database, Postal Code Conversion File (PCCF+), and disease-specific registries. These datasets were linked using unique, encoded identifiers and analyzed at ICES.
Participants
Cardiologists meeting the following criteria were included in the study: (1) cardiologists who billed for services between April 1, 2011 and March 31, 2016 documented in the OHIP database; (2) those with at least 80% of their billings during each year of practice as FFS payments; and (3) a billing period of 90 consecutive days or more during the 5-year period. Pediatric cardiologists were excluded. Other payments to physicians, including stipends and salary support for research or administrative activities, were not captured as part of this study.
Patients
Patients included in the CAD cohort were those seen by eligible cardiologists. We identified all OHIP claims dated between April 1, 2011 and March 31, 2016 for an outpatient visit to one of the study cardiologists. We then identified patient visits with a diagnosis of CAD, defined as meeting at least 1 of the following criteria within a 3-year lookback window: (1) at least 1 hospitalization or emergency department (ED) visit with International Classification of Disease–10th Revision (ICD-10) code I21 or I22 listed as the most responsible diagnosis10, 11, 12; (2) prior revascularization via coronary artery bypass and graft (CABG) or percutaneous coronary intervention10; and (3) documented angiographic findings of epicardial stenosis on cardiac catheterization. Patients were included in the HF cohort if they had a history of HF in the 3 years preceding their visit, defined as meeting at least 1 hospitalization or ED visit with an ICD-10 code of I5013, 14, 15 listed as the most responsible diagnosis. Supplemental Table S1 describes all OHIP fee codes used in the identification of both CAD and HF patients.16
We identified the earliest visit per unique CAD and HF patient and set the corresponding date as their index date. If patients had multiple visits on their index date, we excluded all visits that day if at least 2 of those visits were with different cardiologists. We excluded patients who were non-Ontario residents, < 18 or > 105 years old, in a long-term care home, ineligible for OHIP within 3 years before their visit, or with invalid or missing sociodemographic information (health card number, age, sex, or income quintile). Lastly, we revised the study population by excluding cardiologists who saw ≤ 50 CAD patients from the CAD cohort and cardiologists who saw < 25 HF patients from the HF cohort.
Covariates
The following physician characteristics were collected based on each physician’s first day of billing as a cardiologist during the study period: sex, years since medical school graduation, and international medical graduate status. We included a variable indicating whether the physician was an interventional cardiologist, defined by at least one percutaneous coronary intervention in the year before cohort entry.
Patient sociodemographic variables included age, sex, rurality, and neighborhood income quintile.17 The following medical complications within 3 years before the index visit were captured using ICD-10 and Canadian Classification of Health Interventions codes: myocardial infarction, coronary revascularization, renal dysfunction, stroke, and peripheral vascular disease. Evidence of chronic obstructive pulmonary disease, hyperlipidemia, diabetes mellitus, and hypertension any time before cohort entry were measured using validated algorithms.11,18, 19, 20
The primary exposure was cardiologists’ median billing amount per year for every year they billed for services during the accrual window, calculated using OHIP billing claims for services coded using professional fee codes. Median billing amounts were stratified into quintiles, from lowest (bottom 20%) to highest-billing physicians (top 20%).
Outcomes
The main outcomes of interest were cardiac diagnostic and therapeutic procedures. Claims for the following procedures were identified: transthoracic echocardiogram (TTE), stress testing, cardiac catheterization, and coronary revascularization, and, for the HF cohort, implantable cardiac defibrillator use. We independently observed the frequency of outpatient visits with a primary care physician or cardiologist. Clinical services were measured within 365 days of the index visit and remeasured annually for up to 7 years.
The following clinical outcomes were also captured: emergency department visits, hospitalizations, and death. Specifically, we measured nonelective all-cause hospitalization, ED visit or hospitalization for cardiovascular disease, HF hospitalization for the HF cohort, and hospitalization for myocardial infarction for the CAD cohort. Clinical outcomes were measured within 365 days of the index visit and remeasured annually thereafter until the end of the follow-up period for up to 7 years (death was measured once, 1 year from the index visit). Please see Supplemental Table S1 for relevant codes.
Statistical analysis
We compared distributions of baseline characteristics and unadjusted outcomes for the first year of follow-up among the quintiles of cardiologist billings using Kruskal-Wallis tests for continuous variables and χ2 tests for categorical variables. We then performed mixed effects logistic regression for each dichotomous outcome. Mixed effects linear regression was used for each count outcome, with values truncated at the 95th percentile and square-root transformed. The regression models adjusted for all baseline patient and physician characteristics listed previously and incorporated both physician- and patient-specific random effects. The former accounted for clustering of patients within physicians, whereas the latter accounted for repeated measurements per patient. The second physician income quintile was chosen as the reference category, as the distribution of the median annual income within the first quintile suggested members of this quintile were practicing part time, received income from other sources, and were likely to be systematically different from other physicians in the study population.
All analyses were performed in SAS 9.4 (SAS Institute) with statistical significance assessed via a 2-tailed P value ≤ 0.05.
Ethics approval
The use of data for this study was authorized under §45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board.
Results
Participant characteristics
We identified 423 eligible cardiologists for the CAD cohort and 413 cardiologists for the HF cohort after applying inclusion and exclusion criteria. Physician characteristics are detailed in Supplemental Table S2. Physicians in the highest billing quintiles of both cohorts were more likely male and saw more unique CAD and HF patients. There was no difference across quintiles in years since graduation from medical school, international medical graduate status, and whether the cardiologist billed for interventional cardiology procedures.
Figure 1 describes the selection of CAD and HF patients into the 2 cohorts. We identified 170,959 patients with CAD and 56,262 with HF. Baseline patient characteristics across billing quintiles for both cohorts are reported in Tables 1 and 2. Because of the large sample size, there were many statistically significant but not clinically significant differences across the quintiles.
Figure 1.
Patient cohort creation diagram. Asterisk indicates recent history of HF and CAD defined in Supplemental Table S1. Daggar indicates minimum number of HF or CAD patients for defining low-volume cardiologist was informed by distribution of patient volumes. CAD, coronary artery disease; HF, heart failure; OHIP, Ontario Health Insurance Plan.
Table 1.
Baseline CAD patient characteristics by billing quintile
| Characteristic∗ | Total N = 170,959 |
Physician billing quintile |
P value† | ||||
|---|---|---|---|---|---|---|---|
| Quintile 1 n = 18,215 | Quintile 2 n = 30,858 | Quintile 3 n = 30,029 | Quintile 4 n = 38,577 | Quintile 5 n = 53,280 | |||
| Age, median (Q1, Q3) | 67 (58,76) | 67 (58,76) | 67 (58,76) | 67 (58,76) | 67 (59,76) | 66 (58,75) | < 0.001 |
| Male sex, n (%) | 122,559 (71.7%) | 13,095 (71.9%) | 22,033 (71.4%) | 21,231 (70.7%) | 27,502 (71.3%) | 38,698 (72.6%) | < 0.001 |
| Income quintile, n (%) | < 0.001 | ||||||
| 1 (lowest) | 32,789 (19.2) | 3724 (20.4) | 5470 (17.7) | 5593 (18.6) | 7149 (18.5) | 10,853 (20.4) | — |
| 2 | 34,807 (20.4) | 3756 (20.6) | 6206 (20.1) | 5888 (19.6) | 7521 (19.5) | 11,436 (21.5) | — |
| 3 | 34,944 (20.4) | 3523 (19.3) | 6144 (19.9) | 6243 (20.8) | 7742 (20.1) | 11,292 (21.2) | — |
| 4 | 35,432 (20.7) | 3581 (19.7) | 6474 (21.0) | 6359 (21.2) | 8254 (21.4) | 10,764 (20.2) | — |
| 5 (highest) | 32,987 (19.3) | 3631 (19.9) | 6564 (21.3) | 5946 (19.8) | 7911 (20.5) | 8935 (16.8) | — |
| Rurality, n (%) | 22,243 (13.0) | 3119 (17.1) | 4871 (15.8) | 3616 (12.0) | 4689 (12.2) | 5948 (11.2) | < 0.001 |
| Prior myocardial infarction, n (%) | 62,887 (36.8) | 6927 (38.0) | 11,919 (38.6) | 10,524 (35.0) | 14,467 (37.5) | 19,050 (35.8) | < 0.001 |
| Prior coronary revascularization, n (%) | 87,473 (51.2) | 8770 (48.1) | 14,699 (47.6) | 13,967 (46.5) | 19,931 (51.7) | 30,106 (56.5) | < 0.001 |
| Renal dysfunction, n (%) | 20,857 (12.2) | 2277 (12.5) | 3487 (11.3) | 3513 (11.7) | 4784 (12.4) | 6873 (12.9) | < 0.001 |
| Previous stroke, n (%) | 11,071 (6.5) | 1298 (7.1) | 1880 (6.1) | 1799 (6.0) | 2427 (6.3) | 3667 (6.9) | < 0.001 |
| Peripheral vascular disease, n (%) | 13,803 (8.1) | 1612 (8.8) | 2379 (7.7) | 2325 (7.7) | 3129 (8.1) | 4358 (8.2) | < 0.001 |
| COPD | 22,948 (13.4) | 2587 (14.2) | 4091 (13.3) | 4068 (13.5) | 5249 (13.6) | 6953 (13.0) | 0.001 |
| Hyperlipidemia, n (%) | 136,460 (79.8) | 14,185 (77.9) | 23,557 (76.3) | 23,296 (77.6) | 30,822 (79.9) | 44,600 (83.7) | < 0.001 |
| Diabetes mellitus, n (%) | 71,590 (41.9) | 7479 (41.1) | 12,115 (39.3) | 12,060 (40.2) | 15,955 (41.4) | 23,981 (45.0) | < 0.001 |
| Hypertension, n (%) | 140,219 (82.0) | 14,797 (81.2) | 24,228 (78.5) | 24,273 (80.8) | 31,738 (82.3) | 45,183 (84.8) | < 0.001 |
COPD, chronic obstructive pulmonary disease; IQR, interquartile range; Q1, first quartile; Q3, third quartile.
Canadian Cardiovascular Society Angina score and left ventricle function not included due to high number of missing values.
P values for continuous variables calculated from Kruskal-Wallis tests, and P values for categorical variables calculated from χ2 tests of independence. A 2-tailed P value of < 0.05 was considered statistically significant.
Table 2.
Baseline HF patient characteristics by billing quintile
| Characteristic∗ | Total N = 56,262 |
Physician billing quintile |
P value† | ||||
|---|---|---|---|---|---|---|---|
| Quintile 1 n = 7529 | Quintile 2 n = 8645 | Quintile 3 n = 9795 | Quintile 4 n = 14,121 | Quintile 5 n = 16,172 | |||
| Age, median (Q1, Q3) | 76 (67,84) | 75 (64,83) | 76 (67,84) | 77 (67,84) | 77 (67,84) | 76 (67,83) | < 0.001 |
| Male sex, n (%) | 31,555 (56.1) | 4376 (58.1) | 4939 (57.1) | 5374 (54.9) | 7880 (55.8) | 8986 (55.6) | < 0.001 |
| Income quintile, n (%) | < 0.001 | ||||||
| 1 (lowest) | 12,484 (22.2) | 1757 (23.3) | ,858 (21.5) | 2116 (21.6) | 3055 (21.6) | 3698 (22.9) | |
| 2 | 12,185 (21.7) | 1613 (21.4) | 1893 (21.9) | 2101 (21.4) | 2952 (20.9) | 3626 (22.4) | |
| 3 | 11,138 (19.8) | 1383 (18.4) | 1629 (18.8) | 1914 (19.5) | 2777 (19.7) | 3435 (21.2) | |
| 4 | 10,856 (19.3) | 1343 (17.8) | 1671 (19.3) | 1952 (19.9) | 2869 (20.3) | 3021 (18.7) | |
| 5 (highest) | 9598 (17.1) | 1433 (19.0) | 1594 (18.4) | 1712 (17.5) | 2468 (17.5) | 2392 (14.8) | |
| Rurality, n (%) | 6250 (11.1) | 1097 (14.6) | 894 (10.3) | 1065 (10.9) | 1646 (11.7) | 1548 (9.6) | < 0.001 |
| Prior myocardial infarction, n (%) | 9085 (16.1) | 1233 (16.4) | 1517 (17.5) | 1583 (16.2) | 2226 (15.8) | 2526 (15.6) | 0.001 |
| Prior coronary revascularization, n (%) | 7585 (13.5) | 991 (13.2) | 1316 (15.2) | 1242 (12.7) | 1784 (12.6) | 2252 (13.9) | < 0.001 |
| Renal dysfunction, n (%) | 13,426 (23.9) | 1932 (25.7) | 2132 (24.7) | 2275 (23.2) | 3239 (22.9) | 3848 (23.8) | < 0.001 |
| Previous stroke, n (%) | 3566 (6.3) | 508 (6.7) | 608 (7.0) | 621 (6.3) | 850 (6.0) | 979 (6.1) | 0.008 |
| Peripheral vascular disease, n (%) | 4867 (8.7) | 700 (9.3) | 831 (9.6) | 815 (8.3) | 1188 (8.4) | 1333 (8.2) | < 0.001 |
| COPD, n (%) | 15,415 (27.4) | 2010 (26.7) | 2378 (27.5) | 2713 (27.7) | 3,921 (27.8) | 4393 (27.2) | 0.44 |
| Hyperlipidemia, n (%) | 39,594 (70.4) | 5257 (69.8) | 6205 (71.8) | 6809 (69.5) | 9763 (69.1) | 11,560 (71.5) | < 0.001 |
| Diabetes mellitus, n (%) | 30,247 (53.8) | 3896 (51.7) | 4598 (53.2) | 5125 (52.3) | 7539 (53.4) | 9089 (56.2% | < 0.001 |
| Hypertension, n (%) | 50,767 (90.2) | 6606 (87.7) | 7810 (90.3) | 8753 (89.4) | 12,750 (90.3) | 14,848 (91.8) | < 0.001 |
COPD, chronic obstructive pulmonary disease; IQR, interquartile range; Q1, first quartile; Q3, third quartile.
Canadian Cardiovascular Society Angina score and left ventricle function not included due to high number of missing values.
P values for continuous variables calculated from Kruskal-Wallis tests, and P values for categorical variables calculated from χ2 tests of independence. A 2-tailed P value < 0.05 was considered statistically significant.
Use of cardiac services
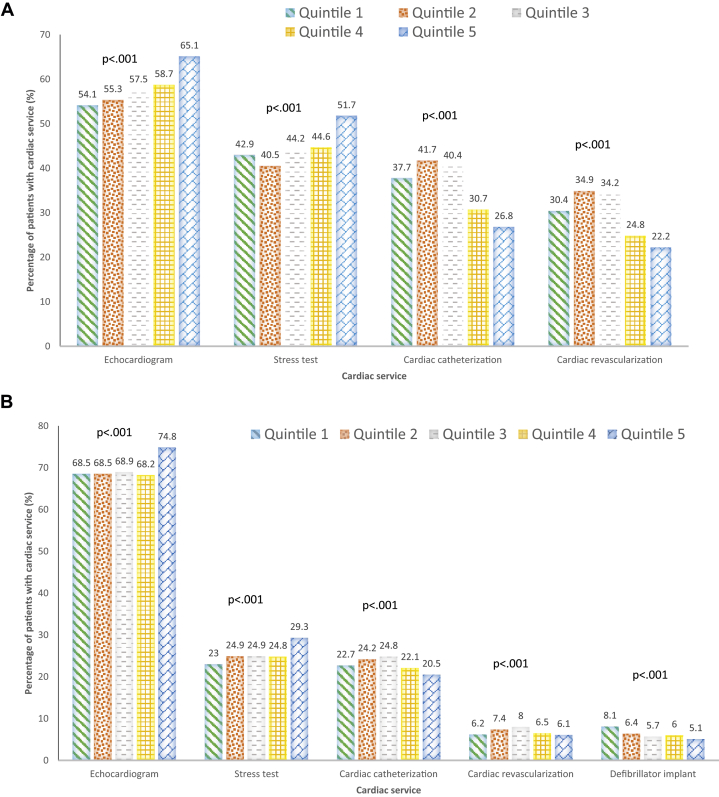
Figure 2 shows unadjusted use of cardiac services for both CAD and HF patients within 1 year. The proportion of CAD patients who had a TTE was highest in patients seen by the highest billing cardiologists (65.1% in quintile 5 vs 55.3% in quintile 2; P < 0.001), as was stress testing (51.7% vs 40.5%; P < 0.001). Conversely, the portion of CAD patients who had a cardiac catheterization were lowest in the highest billing quintile (26.8% in quintile 5 vs 41.7% in quintile 2; P < 0.001) as was coronary revascularization (22.2% vs 34.9%; P < 0.001).
Figure 2.
(A-D) Unadjusted cardiac service use and clinical outcomes at 1 year. CAD, coronary artery disease; ED, emergency department; HF, heart failure; MI, myocardial infarction.
The proportion of HF patients who had a TTE within 1 year was highest in the highest billing quintile (74.8% in quintile 5 vs 68.5% in quintile 2; P < 0.001), as was stress test use (29.3% vs 24.9%; P < 0.001). The proportion of HF patients with a cardiac catheterization were lowest in the highest billing quintile (20.5% in quintile 5 vs 24.2% in quintile 2; P < 0.001), as was coronary revascularization (6.1% vs 7.4%; P < 0.001). The proportion of HF patients with an implantable cardiac defibrillator placement was lowest in the high billing quintile (5.1% in quintile 5 vs 6.4% in quintile 2; P < 0.001).
CAD patients in the highest billing quintile had more unadjusted office visits per year than the lower billing quintile (1.86 ± 2.57 in quintile 5 vs 1.65 ± 2.02 in quintile 2; P < 0.001). HF patients of cardiologists in the highest billing quintile had fewer cardiology office visits per year than lower-billing cardiologists (2.57 ± 3.27 in quintile 5 vs 2.61 ± 3.06 in quintile 2; P < 0.001).
Table 3 lists the odds ratios (OR) of cardiac services for both cohorts. Compared with CAD patients of physicians in billing quintile 2, patients of physicians in higher billing quintiles were more likely to receive an echocardiogram (OR, 1.65; 95% confidence interval [CI], 1.39 to 1.94 for quintile 5) and stress test (OR, 1.50; 95% CI, 1.28-1.75 for quintile 5) at 1 year. Conversely, patients of physicians in the higher billing quintiles had lower adjusted rates of cardiac catheterization (OR, 0.86; 95% CI, 0.77-0.95 for quintile 5), whereas revascularization rates were not significantly different (OR, 0.93; 95% CI, 0.86-1.01 for quintile 5).
Table 3.
Mixed effects logistic regression results for diagnostic and therapeutic procedures over 7 years
| Outcome∗ | Median billing quintile (ref: quintile 2) | CAD |
HF |
||
|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P value† | Adjusted OR (95% CI) | P value† | ||
| At least 1 echocardiogram in 1 year | 1 | 0.96 (0.83-1.12) | 0.63 | 0.94 (0.84-1.06) | 0.31 |
| 3 | 1.04 (0.89-1.20) | 0.64 | 1.01 (0.90-1.13) | 0.90 | |
| 4 | 1.20 (1.03-1.40) | 0.02 | 1.12 (0.99-1.26) | 0.07 | |
| 5 | 1.65 (1.39-1.94) | < 0.001 | 1.40 (1.23-1.59) | < 0.001 | |
| At least 1 stress test in 1 year | 1 | 1.13 (0.98-1.31) | 0.09 | 1.00 (0.88-1.14) | 0.98 |
| 3 | 1.27 (1.10-1.46) | 0.001 | 1.12 (0.99-1.26) | 0.08 | |
| 4 | 1.30 (1.12-1.51) | 0.001 | 1.13 (1.00-1.29) | 0.06 | |
| 5 | 1.50 (1.28-1.75) | < 0.001 | 1.32 (1.15-1.51) | < 0.001 | |
| At least 1 revascularization in 1 year | 1 | 1.09 (1.00-1.18) | 0.05 | 0.89 (0.75-1.06) | 0.19 |
| 3 | 0.98 (0.91-1.05) | 0.55 | 1.06 (0.90-1.24) | 0.51 | |
| 4 | 0.92 (0.86-0.99) | 0.03 | 1.02 (0.86-1.21) | 0.80 | |
| 5 | 0.93 (0.86-1.01) | 0.09 | 0.95 (0.80-1.14) | 0.58 | |
| At least 1 catheterization in 1 year | 1 | 1.13 (1.02-1.25) | 0.02 | 1.01 (0.88-1.16) | 0.91 |
| 3 | 0.97 (0.88-1.07) | 0.52 | 1.03 (0.90-1.18) | 0.66 | |
| 4 | 0.88 (0.79-0.97) | 0.01 | 1.01 (0.87-1.16) | 0.93 | |
| 5 | 0.86 (0.77-0.95) | 0.005 | 0.97 (0.84-1.13) | 0.73 | |
| At least 1 defibrillator implant in 1 year‡ | 1 | 1.09 (0.93-1.26) | 0.28 | ||
| 3 | 1.00 (0.86-1.16) | 0.99 | |||
| 4 | 1.01 (0.87-1.18) | 0.88 | |||
| 5 | 0.91 (0.77-1.06) | 0.22 | |||
CAD, coronary artery disease; CI, confidence interval; HF, heart failure; OR, odds ratio.
A 2-tailed P value of < 0.05 was considered statistically significant.
Analyses are adjusted for year, patient, and physician characteristics.
Analysis of defibrillator implant only included for HF cohort.
HF patients of physicians in higher billing quintiles were more likely to receive at least 1 echocardiogram within 1 year compared with those in quintile 2 (OR 1.40; 95% CI, 1.23-1.59) and stress test (OR 1.32; 95% CI, 1.15-1.51). There was no difference in adjusted rates of cardiac catheterization, revascularization, or cardiac defibrillator implantation.
The adjusted rates of physician visits for both cohorts are shown in Supplemental Table S3. There were no differences in the number of physician visits in 1 year across billing quintiles for both cohorts.
Clinical outcomes
Figure 2 shows unadjusted clinical outcomes at 1 year for both CAD and HF cohorts. CAD patients seen by cardiologists in the highest billing quintile had a lower rate of death (4.3% in quintile 5 vs 4.9% in quintile 2; P < 0.001), hospitalization (28.2% in quintile 5 vs 30.5% in quintile 2; P < 0.001) and ED visits or hospitalizations for CAD (24.3% in quintile 5 vs 27.4% in quintile 2; P < 0.001). Similarly, HF patients seen by higher-billing cardiologists had a lower rate of death (17.0% in quintile 5 vs 17.7% in quintile 2; P = 0.026), all-cause hospitalization (48.6% in quintile 5 vs 50.5% in quintile 2; P < 0.001), and HF hospitalization (19.2% in quintile 5 vs 20.3% in quintile 2; P = 0.035).
Table 4 shows adjusted clinical outcomes for both the CAD and HF cohorts. After adjustment, there were no significant differences in 1-year clinical outcomes across billing quintiles for CAD patients, except that all-cause mortality was higher in the lowest billing quintile (OR, 1.16; 95%; CI, 1.03-1.31). In the HF cohort, all adjusted results were similar, except patients seen by cardiologists in billing quintile 4 had a slightly lower hospitalization rate (OR, 0.94; 95% CI, 0.89-0.99).
Table 4.
Mixed effects logistic regression results for clinical outcomes over 7 years
| Outcome∗ | Median billing quintile (ref: quintile 2) | CAD |
HF |
||
|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P value† | Adjusted OR (95% CI) | P value† | ||
| All-cause mortality in one year‡ | 1 | 1.16 (1.03-1.31) | 0.01 | 1.09 (0.99-1.21) | 0.08 |
| 3 | 1.05 (0.94-1.18) | 0.35 | 1.00 (0.92-1.10) | 0.93 | |
| 4 | 1.04 (0.93-1.16) | 0.49 | 0.98 (0.90-1.08) | 0.72 | |
| 5 | 1.01 (0.90-1.14) | 0.85 | 0.92 (0.83-1.01) | 0.08 | |
| At least 1 non-elective admission in one year | 1 | 1.05 (0.98-1.11) | 0.14 | 0.99 (0.94-1.05) | 0.86 |
| 3 | 1.00 (0.94-1.05) | 0.87 | 1.02 (0.97-1.08) | 0.37 | |
| 4 | 0.98 (0.93-1.04) | 0.54 | 0.94 (0.89-0.99) | 0.02 | |
| 5 | 0.99 (0.94-1.06) | 0.84 | 0.98 (0.92-1.04) | 0.43 | |
| At least 1 acute MI admission in one year | 1 | 0.96 (0.85-1.08) | 0.49 | 1.05 (0.97-1.14) | 0.25 |
| 3 | 0.97 (0.87-1.09) | 0.61 | 1.01 (0.94-1.09) | 0.75 | |
| 4 | 0.97 (0.86-1.09) | 0.57 | 0.95 (0.88-1.03) | 0.19 | |
| 5 | 0.97 (0.86-1.10) | 0.67 | 0.99 (0.91-1.08) | 0.85 | |
| At least 1 ED visit or admission due to CVD in one year | 1 | 1.00 (0.95-1.06) | 0.90 | 0.96 (0.91-1.02) | 0.21 |
| 3 | 1.00 (0.95-1.06) | 0.86 | 1.04 (0.98-1.10) | 0.16 | |
| 4 | 0.97 (0.92-1.03) | 0.29 | 0.96 (0.91-1.02) | 0.17 | |
| 5 | 1.00 (0.94-1.06) | 0.90 | 1.00 (0.95-1.06) | 0.92 | |
CAD, coronary artery disease; CI, confidence interval; CVD, cardiovascular disease; ED, emergency department; HF, heart failure; MI, myocardial infarction; OR, odds ratio.
Analyses are adjusted for year, patient, and physician characteristics.
A 2-tailed P value of < 0.05 was considered statistically significant.
All-cause mortality outcome measured 1 year from index date only.
Discussion
In this large, retrospective cohort study of CAD and HF patients, we observed significant outpatient practice variation by cardiologists associated with annual physician billings. Across both cohorts, high-billing cardiologists saw more patients and ordered a significantly higher number of TTEs and stress tests per patient than lower-billing cardiologists. Importantly, there were no significant differences in 1-year mortality, hospitalizations, and ED visits across billing groups, except for CAD patients in the lowest billing group who had higher 1-year mortality. These results suggest that cardiac patients seen by higher billing cardiologists receive more noninvasive cardiac testing with questionable impact on outcomes.
Prior research suggests that economic incentives, including ownership of imaging equipment and self-referral, impact imaging rates.21, 22, 23 A study by Shah et al.24 found that stress testing after coronary revascularization was more commonly ordered by cardiologists who billed for the cardiac services than those who did not. Primary care physicians who were paid using an FFS reimbursement model had more patient visits, specialty referrals, and diagnostic services than capitated physicians.25 These studies only assessed the relationship of billing status to clinical activity, whereas our study assesses the relationship between total billing amounts, clinical activity, and outcomes. Our study also has the advantage of taking a population-based approach, including more than 220,000 patients seen across a geographically and demographically diverse jurisdiction and adjusting for many patient and physician covariates.
Concerns regarding increasing overuse of low-value medical care has led to the creation of Choosing Wisely campaigns in over 20 countries worldwide.26,27 In addition, increased use of cardiac imaging has sparked the American College of Cardiology to create Appropriate Use Criteria in an attempt to provide clinical guidance around the rational use of cardiac testing.28, 29, 30 One proposed barrier to appropriate use of cardiac imaging is FFS reimbursement models, although there is little evidence to draw conclusions as to the impact of payments on ordering behaviours.31,32 High-billing cardiologists ordered more noninvasive cardiac testing per patient with a lower proportion of patients undergoing invasive testing. This finding suggests that either lower billing cardiologists see sicker patients with a higher burden of disease, or higher billing cardiologists have a more liberal noninvasive testing strategy leading to a lower diagnostic yield, which may be in part due to economic incentives.
CAD patients seen by cardiologists in the lowest billing quintile did have higher mortality than those in other quintiles, a finding that does provide some pause. It is possible that cardiologists in the lowest billing quintile are academic cardiologists, and patients are seen in academic centres where there may be higher medical complexity, particularly unmeasured confounders, which could include complex coronary anatomy, although we do not have practice data to confirm this. We should interpret these results with caution and consider further research that includes data from angiograms, echocardiograms, and other diagnostic testing.
The results of this study have substantial health policy implications that are broadly applicable. Our results reinforce the idea that reimbursement models affect physician behaviour. In this instance, although higher-billing cardiologists saw more patients, they also ordered more noninvasive tests per patient. This pattern exists presumably because cardiologists can bill for the noninvasive tests in their office or clinic; prior research has found that the presence of cardiac testing facilities is associated with higher rates of testing.33 Second, reimbursement considerations should be considered when designing interventions to reduce low-value cardiac testing. The impact of interventions, such as audit and feedback, may be blunted when juxtaposed with financial considerations.31,34 Finally, these results reinforce the idea that physician reimbursement should be aligned with health system goals, particularly with value-based payments being tested in multiple jurisdictions.35, 36, 37
Our results need to be interpreted within the context of some important limitations. Administrative data lack the clinical granularity to determine appropriateness of testing.38 The lack of clinical, laboratory, and cardiac testing data may mean that we have underestimated the severity of illness. We do not include any income derived from on-call, administrative, or academic stipends, underestimating the physician funding envelope. We do not have data on physician overhead, so we cannot calculate physician income. We cannot determine whether cardiac testing was ordered by the billing cardiologist or another physician, limiting our ability to comment on self-referral. During the study period, there were fee schedule changes that affected cardiac testing, and we are unable to determine if those changes affected physician behaviour. Finally, we only examined a portion of cardiology practice, selecting a clearly defined population of CAD and HF patients to more easily allow for comparability between cardiologists, but this cohort does not represent most cardiology patients.
Conclusions
In this population-based retrospective cohort study, cardiac patients seen by high-billing cardiologists were more likely to receive noninvasive testing compared with patients seen by lower-billing cardiologists.
Acknowledgements
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Parts of this material are based on data and information compiled and provided by MOHLTC and the Canadian Institute for Health Information. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. The authors acknowledge that the clinical registry data used in this publication is from participating hospitals through CorHealth Ontario, which serves as an advisory body to the MOHLTC is funded by the MOHLTC, and is dedicated to improving the quality, efficiency, access and equity in the delivery of the continuum of adult cardiac, vascular, and stroke services in Ontario, Canada. We would also like to thank IMS Brogan Inc for use of their Drug Information Database.
Funding Sources
R.S.B. is supported by a Clinician Investigator Award from the Heart and Stroke Foundation of Canada and the F.M. Hill Chair in Health Systems Solutions at Women’s College Hospital. P.C.A. and D.T.K. are supported by a Mid-Career Investigator award from the Heart and Stroke Foundation of Canada. S.G.G. is supported by the University of Toronto Heart and Stroke Foundation of Ontario (Polo) Chair. Drs R.S.B., D.T.K., P.D., H.R., and S.G.G. are cardiologists who bill the Ontario Health Insurance Plan (OHIP) for clinical services.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
See page 766 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.02.002.
Supplementary Material
References
- 1.Berwick D.M., Hackbarth A.D. Eliminating waste in US health care. JAMA. 2012;307:1513–1516. doi: 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]
- 2.Bouck Z., Pendrith C., Chen X., et al. Measuring the frequency and variation of unnecessary care across Canada. BMC Health Serv Res. 2019;19:446. doi: 10.1186/s12913-019-4277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brett J., Maust D.T., Bouck Z., et al. Benzodiazepine use in older adults in the United States, Ontario, and Australia from 2010 to 2016. J Am Geriatr Soc. 2018;66:1180–1185. doi: 10.1111/jgs.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pendrith C., Hawker G.A., Jaglal S.B., et al. Frequency of and variation in low-value care in primary care: a retrospective cohort study. CMAJ Open. 2017;5:E45–E51. doi: 10.9778/cmajo.20160095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkham K.R., Wijeysundera D.N., Pendrith C., Ng R. Preoperative testing before low-risk surgical procedures. CMAJ. 2015;187:349–358. doi: 10.1503/cmaj.150174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saini V., Brownlee S., Elshaug A.G., Glasziou P., Heath I. Addressing overuse and underuse around the world. Lancet. 2017;390:105–107. doi: 10.1016/S0140-6736(16)32573-9. [DOI] [PubMed] [Google Scholar]
- 7.Luca S.R., Koh M., Qiu F., et al. Stress testing after percutaneous coronary interventions: a population-based study. CMAJ Open. 2017;5:E417–E423. doi: 10.9778/cmajo.20160159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin G.A., Dudley R.A., Lucas F., Malenka D., Vittinghoff E., Redberg R. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA. 2008;300:1765–1773. doi: 10.1001/jama.300.15.1765. [DOI] [PubMed] [Google Scholar]
- 9.Ivers N., Brown A.D., Detsky A.S. Lessons from the Canadian experience with single-payer health insurance just comfortable enough with the status quo. JAMA Intern Med. 2018;178:1250–1255. doi: 10.1001/jamainternmed.2018.3568. [DOI] [PubMed] [Google Scholar]
- 10.Tran C., Wijeysundera H.C., Qui F., Tu J.V., Bhatia R.S. Comparing the ambulatory care and outcomes for rural and urban patients with chronic ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2014;7:835–843. doi: 10.1161/CIRCOUTCOMES.114.001076. [DOI] [PubMed] [Google Scholar]
- 11.Tu J.V., Chu A., Donovan L.R., et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8:204–212. doi: 10.1161/CIRCOUTCOMES.114.001416. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia R.S., Bouck Z., Ivers N.M., et al. Electrocardiograms in low-risk patients undergoing an annual health examination. JAMA Intern Med. 2017;177:1326–1333. doi: 10.1001/jamainternmed.2017.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colla C.H., Morden N.E., Sequist T.D., Schpero W.L., Rosenthal M.B. Choosing Wisely : Prevalence and correlates of low-value health care services in the United States. J Gen Intern Med. 2011;30:221–228. doi: 10.1007/s11606-014-3070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz A.L., Landon B.E., Elshaug A.G., Chernew M.E., McWilliams J.M. Measuring low-value care in Medicare. JAMA Intern Med. 2014;174:1067–1076. doi: 10.1001/jamainternmed.2014.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colla C.H., Sequist T., Rosenthal M.B., et al. Use of non-indicated cardiac testing in low-risk patients: choosing Wisely. BMJ Qual Saf. 2015;24:149–153. doi: 10.1136/bmjqs-2014-003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministry of Health and Long Term Care. Ontario Health Insurance Plan (OHIP) Schedule of Benefits and Fees. 2019. http://www.health.gov.on.ca/en/pro/programs/ohip/sob/ Available at:
- 17.Statistics Canada Statistics Canada Postal Code Conversion File Plus (PCCF+) reference guide. 2017. http://data.library.utoronto.ca/datapub/codebooks/cstdli/pccf_health/pccf6a1/82-F0086-XDB-2014v6a-eng.pdf Available at:
- 18.Tu K., Campbell N.R., Chen Z.L., Cauch-Dudek K.J., McAlister F.A. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1:e18–26. [PMC free article] [PubMed] [Google Scholar]
- 19.Gershon A.S., Wang C., Guan J., Vasilevska-Ristovska J., Cicutto L., To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD J Chronic Obstr Pulm Dis. 2009;6:388–394. doi: 10.1080/15412550903140865. [DOI] [PubMed] [Google Scholar]
- 20.Lipscombe L.L., Hwee J., Webster L., Shah B.R., Booth G.L., Tu K. Identifying diabetes cases from administrative data: a population-based validation study. BMC Health Serv Res. 2018;18:316. doi: 10.1186/s12913-018-3148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes D.R., Bhargavan M., Sunshine J.H. Imaging self-referral associated with higher costs and limited impact on duration of illness. Health Aff. 2010;29:2244–2251. doi: 10.1377/hlthaff.2010.0413. [DOI] [PubMed] [Google Scholar]
- 22.Gazelle S.G., Halpern E.F., Ryan H.S., Tramontano A.C. Utilization of diagnostic medical imaging: comparison of radiologist referral versus same-specialty referral. Radiology. 2007;245:517–522. doi: 10.1148/radiol.2452070193. [DOI] [PubMed] [Google Scholar]
- 23.Hillman B.J., Joseph C.A., Mabry M.R., Sunshine J.H., Kennedy S.D., Noether M. Frequency and costs of diagnostic imaging in office practice- a comparison of self-referring and radiologist-referring physicians. N Engl J Med. 1990;323:1604–1608. doi: 10.1056/NEJM199012063232306. [DOI] [PubMed] [Google Scholar]
- 24.Shah B.R., Cowper P.A., O’Brien S.M., et al. Association between physician billing and cardiac stress testing patterns following coronary revascularization. JAMA. 2011;306:1993–2000. doi: 10.1001/jama.2011.1604. [DOI] [PubMed] [Google Scholar]
- 25.Gosden T., Forland F., Kristiansen I., et al. Capitation, salary, fee-for-service and mixed systems of payment: effects on the behaviour of primary care physicians. Cochrane Database Syst Rev. 2000:CD002215. doi: 10.1002/14651858.CD002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerr E.A., Kullgren J.T., Saini S.D. Choosing wisely: how to fulfill the promise in the next 5 years. Health Aff (Millwood) 2017;36:2012–2018. doi: 10.1377/hlthaff.2017.0953. [DOI] [PubMed] [Google Scholar]
- 27.Levinson W., Kallewaard M., Bhatia R.S., Wolfson D., Shortt S., Kerr E.A. ‘ Choosing Wisely ’ : a growing international campaign. BMJ Qual Saf. 2015;24:167–174. doi: 10.1136/bmjqs-2014-003821. [DOI] [PubMed] [Google Scholar]
- 28.Douglas P.S., Garcia M.J., Haines D.E., et al. American College of Cardiology Foundation/American Society of Echocardiography/American Heart Association/American Society of Nuclear Cardiology/Heart Failure Society of America/Heart Rhythm Society/Society for Cardiovascular Angiography/Society of Critical Care Medicine/Society of Cardiovascular Computed Tomography/Society for Cardiovascular Magnetic Resonance Imaging. 2011 appropriate use criteria for echocardiography: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011;57:1126–1166. doi: 10.1016/j.jacc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Hendel R.C., Berman D.S., Di Carli M.F., et al. American College of Cardiology Foundation/American Society Nuclear Cardiology/American College of Radiology/American Heart Association/American Society of Echocardiography/Society of Cardiovascular Computed Tomography/Society for Cardiovascular Magnetic Resonance Imaging/Society of Nuclear Medicine 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119:e561–e587. doi: 10.1161/CIRCULATIONAHA.109.192519. [DOI] [PubMed] [Google Scholar]
- 30.Hendel R.C., Patel M.R., Kramer C.M., et al. American College of Cardiology Foundation/American College of Radiology/Society for Cardiovascular Computed Tomography/Society for Cardiovascular Magnetic Resonance /American Society of Nuclear Cardiology/North American Society for Cardiac Imaging/Society for Cardiovascular Angiography and Interventions/Society of Interventional Radiology 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia R.S., Alabousi M., Dudzinski D.M., Weiner R.B. Appropriate use criteria: a review of need, development and applications. Expert Rev Cardiovasc Ther. 2016;14:281–290. doi: 10.1586/14779072.2016.1131125. [DOI] [PubMed] [Google Scholar]
- 32.Shaw L.J., Marwick T.H., Zoghbi W.A., et al. Why all the focus on cardiac imaging? JACC Cardiovasc Imaging. 2010;3:789–794. doi: 10.1016/j.jcmg.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Wennberg D., Dickens J., Soule D., et al. The relationship between the supply of cardiac catheterization laboratories, cardiologists and the use of invasive cardiac procedures in northern New England. J Health Serv Res Policy. 1997;2:75–80. doi: 10.1177/135581969700200204. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia R.S., Ivers N.M., Yin C.X., et al. Improving the appropriate use of transthoracic echocardiography. J Am Coll Cardiol. 2017;70:1135–1144. doi: 10.1016/j.jacc.2017.06.065. [DOI] [PubMed] [Google Scholar]
- 35.McWilliams J.M., Chernew M.E., Landon B.E., Schwartz A.L. Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372:1927–1936. doi: 10.1056/NEJMsa1414929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colla C.H., Lewis V., Shortell S.M., Fisher E. First national survey of ACOs finds that physicians are playing strong leadership and ownership roles. Health Aff (Millwood) 2014;33:964–971. doi: 10.1377/hlthaff.2013.1463. [DOI] [PubMed] [Google Scholar]
- 37.Shortell S.M., Waters T.M., Clarke K.W.B. Physicians as double agents: maintaining trust in an era of multiple accountabilities. JAMA. 1998;280:1102–1108. doi: 10.1001/jama.280.12.1102. [DOI] [PubMed] [Google Scholar]
- 38.Wadhera R.K., Sukul D., Secemsky E.A., et al. Temporal trends in unstable angina diagnosis codes for outpatient percutaneous coronary interventions. JAMA Intern Med. 2019;179:259–261. doi: 10.1001/jamainternmed.2018.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.