Abstract
Objective:
The aim of this study was to evaluate the mortality rates of 566,602 patients with coronavirus disease (COVID-19) based on sex, age, and the presence or absence of underlying diseases and determine whether the underlying disease provides prognostic information specifically related to death.
Methods:
The mortality rate was evaluated using conditional probability to identify the significant factors, and adjusted odds ratios (ORs) using a multivariable logistic regression analysis were estimated.
Results:
The mortality rate of patients with underlying health conditions was 12%, which was 4 times higher than that of patients without underlying health conditions. Furthermore, the mortality rates of women and men with underlying health conditions were 5.5 and 3.4 times higher than the mortality rates of patients without underlying health conditions, respectively. In a multivariable logistic regression analysis including sex, age, and underlying health conditions, male sex (OR: 1.83), age ≥ 41 y (ORs > 2.70), and underlying health conditions (OR: 2.20) were confirmed as risk factors for death.
Conclusions:
More attention should be paid to older patients with underlying diseases and male patients with underlying diseases as the probability of death in this population was significantly higher.
Keywords: COVID-19, mortality rate, underlying disease, health condition
Coronavirus disease 2019 (COVID-19) was first identified in December 2019 in Wuhan, China. In May 2020, the World Health Organization declared COVID-19 a pandemic. By February 3, 2021, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (the virus that causes the disease COVID-19) had spread to 223 countries with a mortality rate of 2.2%.1 Generally, COVID-19 is more severe in people aged >60 y or those with underlying diseases.2,3
Research on COVID-19 has been conducted from various perspectives, such as infection cases,4,5 clinical characteristics,6–8 and preventive measures.9 In addition, several studies have confirmed that COVID-19 is more severe in older people and those with underlying diseases, such as lung or heart disease, diabetes, and immunological diseases.10–12 The Mexican government provides information on several confirmed cases (566,602 patients), and studies on clinical significance and validation based on these data have been conducted.12–14
However, existing studies have targeted relatively few cases (less than 1000 cases),6,10,11,15,16 and comparative analysis of the association between the presence or absence of an underlying disease and the mortality rate based on age or sex remains inadequate. COVID-19 data from Mexico include many confirmed cases and 23 variables, including sex, age, date of symptom onset and death, and underlying disease status. Therefore, we can use these data to compare and analyze the mortality rates according to underlying diseases, age, and sex and obtain relevant information.
In this study, we evaluated the effect of COVID-19 on mortality rate in patients with underlying health conditions; moreover, we presented the probability values of mortality rate based on 9 underlying diseases, age, and sex. Furthermore, we determined the age-specific mortality rate and the mortality rate based on the presence or absence of underlying health conditions in each age group.
Methods
Data Source
We retrospectively analyzed the data provided by the Mexican government17 to evaluate the mortality rate of COVID-19 patients and identify important variables influencing mortality. The data of 566,602 patients included 23 parameters, such as sex, age, symptom onset date, death date, and 9 underlying health conditions, including diabetes, chronic obstructive pulmonary disease (COPD), asthma, immunosuppression, hypertension, chronic kidney disease (CKD), cardiovascular disease, obesity, and other diseases; these data were collected from January 1, 2020, to May 31, 2020.
Data Analysis
Conditional probability was calculated to ascertain the mortality rate for each factor. The chi-squared test was used to determine whether the presence or absence of an underlying disease had a significant effect on the mortality rate. Continuous variables are presented as mean (standard deviation). Welch’s t-test or Student’s t-test was used to describe the difference in time intervals depending on the presence or absence of the underlying health condition.18 In multivariate logistic regression analysis, the odds ratio (OR) was estimated for the mortality rate based on sex, age, and the underlying disease. All statistical analyses were performed using R (version 4.0.2) software, and values of P < 0.05 were considered statistically significant.
Results
Basic Characteristics of Confirmed Cases
Of the 566,602 patients, 287,112 (50.7%) were men, and 279,490 (49.3%) were women, and 36,176 (6.38%) patients had died. In addition, 212,910 (37.6%) had underlying diseases, whereas 353,692 (62.4%) had no underlying diseases; moreover, some patients had comorbidities (Table 1; Figure 1).
Table 1.
Base characteristics of confirmed cases
| Confirmed (%) | Deaths (%) | |
|---|---|---|
| Overall | 566,602(100) | 36,176(6.38) |
| Sex | ||
| Female | 279,490(49.3) | 12,696(2.24) |
| Male | 287,112(50.7) | 23480(4.14) |
| Age | ||
| Mean (SD) | 42.6(±16.7) years | 60.8(±15.4) years |
| 0-20 | 34,709(6.1) | 455(0.08) |
| 21-30 | 103,492(18.3) | 690(0.12) |
| 31-40 | 136,498(24.1) | 2085(0.37) |
| 41-50 | 122,707(21.7) | 5149(0.91) |
| 51-60 | 87,476(15.4) | 8690(1.53) |
| 61-70 | 46,940(8.3) | 9234(1.63) |
| 71-80 | 23,640(4.2) | 6623(1.17) |
| 81+ | 11,140(2.0) | 3250(0.57) |
| Underlying health condition | ||
| No | 353,692(62.4) | 10,694(1.89) |
| Yes | 212,910(37.6) | 25482(4.50) |
| Diabetes | 70,831(12.5) | 13251(2.34) |
| COPD | 9,013(1.6) | 2033(0.36) |
| Asthma | 18,026(3.2) | 737(0.13) |
| Immunosuppression | 8,978(1.6) | 1364(0.24) |
| Hypertension | 92,508(16.4) | 15136(2.67) |
| CKD | 11,250(2.0) | 2786(0.49) |
| Cardiovascular disease | 12,775(2.3) | 2219(0.39) |
| Obesity | 92,272(16.3) | 8444(1.49) |
| Other disease | 17,084(3.0) | 2265(0.40) |
Note: Underlying health condition = Yes indicates patients with diabetes, COPD, asthma, immunosuppression, hypertension, CKD, cardiovascular disease, obesity, or other diseases. Some patients have comorbidities.
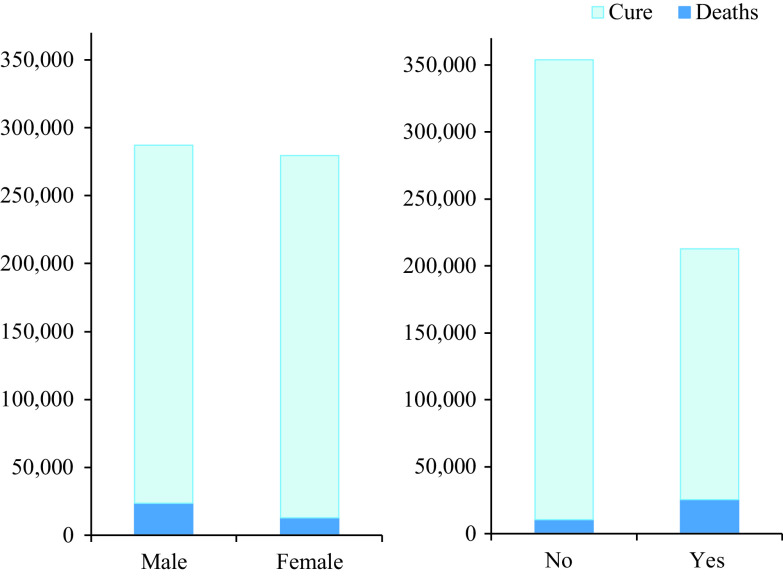
Figure 1.
Mortality rates based on sex and the presence or absence of underlying health conditions. Left, The mortality rate for men higher than for women. Right, Contrast between the number of deaths of patients with and without underlying diseases.
Mortality Rate Based on Sex, Age, and Underlying Health Conditions
Figure 1 shows the mortality rate based on sex and the presence or absence of underlying health conditions. The proportion of COVID-19 patients without underlying health conditions was higher than that of patients with underlying health conditions; however, the mortality rate was higher in COVID-19 patients with underlying health conditions. In other words, the probability of death for patients with underlying health conditions, P(Deaths|Yes), was 0.12, which was 4 times higher than that of patients with no underlying health conditions, P(Deaths|No) = 0.03. Specifically, the proportion of COVID-19 patients with and without underlying health conditions was 37.6% and 62.4%, respectively; however, 70.4% of the total number of deaths occurred in patients with underlying health conditions, and only 29.6% of deaths occurred in patients without an underlying disease (PDeaths|Yes] = 0.704, P[Deaths|No] = 0.296). The mortality rate was 1.8 times as high in men (8.18%) than in women (4.54%). The proportions of men (50.7%) and women (49.3%) among the study participants were similar; however, 64.9% and 35.1% of deaths occurred in men and women, respectively (P[Male|Deaths] = 0.649, P[Female|Deaths] = 0.351).
The average ages of discharged and deceased patients with COVID-19 were 41.4 (±16.0) y and 60.8 (±15.4) y, respectively. Expressly, patients with COVID-19 who died were approximately 20 y older than those who survived. Therefore, age was an important determining factor of the mortality rate. Hence, we evaluated the mortality rates of the following age groups: 0-20, 21-30, 31-40, 41-50, 51-60, 61-70, 71-80, 81+ y.
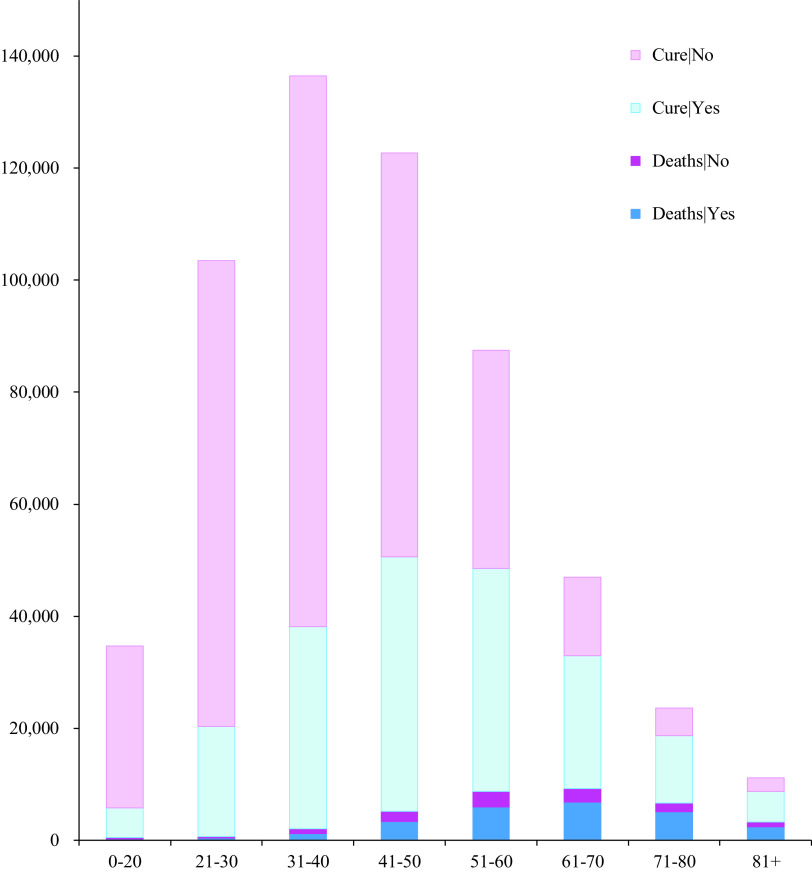
Figure 2 shows the mortality rates by age group and the presence or absence of underlying disease. Although there were more patients with COVID-19 in the age groups 21-30, 31-40, and 41-50, more deaths occurred in the age groups 51-60, 61-70, 71-80, and 81+.
Figure 2.
Mortality rates of age groups based on the presence or absence of underlying health conditions.
Regarding only the mortality rate (P[Deaths|No] + P[Deaths|Yes]), it was higher in people aged over 51 y; however, the trend of rates varied by age group as well as the presence or absence of disease. Similarly, the proportion of discharged patients (cured), 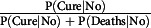 or
or 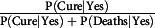 differ based on the age group and the presence or absence of an underlying disease.
differ based on the age group and the presence or absence of an underlying disease.
Table 2 shows the mortality rate and the average time interval owing to the underlying disease, separated by sex and age. The average mortality rate was 6.38%, and the average time interval from symptom onset to death was 10.3 (±6.29) d. The time intervals from symptom onset to death of patients with and without underlying health conditions were 10.1 (±6.23) and 10.7 (±6.42) d, respectively. The P-value of Welch’s t-test was < 0.0001, and the time intervals of the 2 groups were statistically significantly different.
Table 2.
Mortality rate of patients based on sex, age group, and underlying health condition, and the average time interval between initial symptom onset and death
| Underlying health condition = Yes or No | |||||||
|---|---|---|---|---|---|---|---|
| Mortality rate (%) | Time intervals (days) | ||||||
| P(Deaths|Yes) | P(Deaths|No) | P-Value | Yes (mean ± SD) | No (mean ± SD) | P-Value | ||
| Overall | 6.38 | 11.97 | 3.02 | < .0001 | 10.1 ± 6.23 | 10.7 ± 6.42 | < .0001 |
| Sex | |||||||
| Female | 4.54 | 9.27 | 1.68 | < .0001 | 9.89 ± 6.17 | 10.1 ± 6.50 | .0770 |
| Male | 8.18 | 14.61 | 4.32 | < .0001 | 10.3 ± 6.26 | 10.9 ± 6.37 | < .0001 |
| Age group (y) | |||||||
| 0-20 | 1.31 | 3.98 | 0.81 | .4146 | 7.6 ± 6.83 | 7.07 ± 6.20 | .8051 |
| 21-30 | 0.67 | 2.03 | 0.34 | .5594 | 8.83 ± 6.15 | 9.61 ± 6.53 | .0548 |
| 31-40 | 1.53 | 3.28 | 0.87 | .0088 | 9.82 ± 6.10 | 10.5 ± 6.19 | .0037 |
| 41-50 | 4.20 | 6.87 | 2.44 | < .0001 | 10.4 ± 6.22 | 11.2 ± 6.53 | < .0001 |
| 51-60 | 9.93 | 13.06 | 6.50 | < .0001 | 10.5 ± 6.28 | 11.2 ± 6.41 | < .0001 |
| 61-70 | 19.67 | 22.25 | 14.88 | < .0001 | 10.5 ± 6.30 | 10.9 ± 6.42 | .0031 |
| 71-80 | 28.02 | 29.66 | 23.72 | < .0001 | 9.86 ± 6.05 | 10.4 ± 6.36 | .0009 |
| 81+ | 29.17 | 30.85 | 25.08 | < .0001 | 9.07 ± 6.02 | 9.22 ± 5.95 | .2790 |
Note: Underlying health condition = Yes indicates patients with diabetes, COPD, asthma, immunosuppression, hypertension, CKD, cardiovascular disease, obesity, or other diseases. Some patients have comorbidities.
Sex
The mortality rates of women and men with COVID-19 were 4.54% and 8.18%, respectively; therefore, mortality rate was sex-related. Women with underlying diseases had a mortality rate of 9.27%, which was 5.5 times higher than that of women without underlying diseases (1.68%). The mortality rate of men with underlying diseases was 14.61%, which was 3.4 times higher than that of men without underlying diseases (4.32%).
Age
The 21-30 age group had the lowest mortality rate (0.67%); furthermore, the mortality rates for the 51-60, 61-70, 71-80, and 81+ age groups were 9.93%, 19.67%, 28.02%, and 29.17%, respectively. Furthermore, in all age groups, the mortality rate of patients with underlying health conditions was higher than that of those without underlying health conditions. The mortality rate of patients aged ≤ 60 y with underlying health conditions was at least twice as high as that of patients without underlying health conditions. However, patients aged ≥ 71 y were less affected by underlying health conditions than other age groups.
Underlying Health Conditions
The mortality rate of diabetic patients was 18.71%, which was 4 times higher than that of patients without diabetes (4.59%). Additionally, the mortality rate of patients with underlying diseases was 2.5-4 times higher than that of patients without underlying diseases, excluding asthma. Moreover, the mortality rates of obese patients with and without underlying diseases were 9.15% and 5.81%, respectively.
Time Intervals
Time intervals were defined as the disease durations from initial symptom onset to death. The means and standard deviations of the time interval based on sex, age group, and the presence or absence of underlying health conditions are shown in Table 2 and Table 3. The t-test was performed to evaluate the time intervals for each factor. We observed significant differences in time intervals for the following factors: diabetes, COPD, immunosuppression, hypertension, CKD, cardiovascular disease, other diseases, the 31-40, 41-50, 51-60, 61-70, and 71-80 age groups, and male sex. However, no significant differences in time intervals were observed between the 0-20, 21-30, and 81+ age groups and the female sex (P > 0.05).
Table 3.
Mortality rate of patients based on the presence or absence of underlying disease, and the average time interval between initial symptom onset and death
| Underlying health condition | Mortality rate (%) | Time intervals (days) | ||||
|---|---|---|---|---|---|---|
| P(Deaths|Yes) | P(Deaths|No) | P-Value | Yes (mean ± SD) | No (mean ± SD) | P-Value | |
| Diabetes | 18.71 | 4.59 | < .0001 | 9.89 ± 6.12 | 10.6 ± 6.36 | < .0001 |
| COPD | 22.27 | 6.10 | < .0001 | 9.21 ± 6.05 | 10.4 ± 6.29 | < .0001 |
| Asthma | 4.09 | 6.43 | < .0001 | 10.5 ± 6.26 | 10.3 ± 6.28 | .8390 |
| Immunosuppression | 15.19 | 6.22 | < .0001 | 9.14 ± 6.36 | 10.4 ± 6.28 | < .0001 |
| Hypertension | 16.36 | 4.40 | < .0001 | 10 ± 6.15 | 10.5 ± 6.37 | < .0001 |
| CKD | 24.76 | 5.99 | < .0001 | 8.81 ± 5.98 | 10.4 ± 6.29 | < .0001 |
| Cardiovascular disease | 17.37 | 6.10 | < .0001 | 9.3 ± 6.28 | 10.4 ± 6.28 | < .0001 |
| Obesity | 9.15 | 5.81 | < .0001 | 10.4 ± 6.13 | 10.3 ± 6.33 | .1451 |
| Other diseases | 13.26 | 6.13 | < .0001 | 9.62 ± 6.44 | 10.4 ± 6.27 | < .0001 |
Note: Some patients have comorbidities.
Multivariable Logistic Regression
A multivariable logistic regression analysis that included sex, age, and underlying health conditions showed that men had a 1.83 higher OR (95% confidence interval (CI): 1.79-1.88) for mortality rate than women (Table 4). The OR positively correlated with age, indicating that the patient’s age was significantly related to the high COVID-19 mortality rate; ORs > 2.70 in ≥ 41 y group. The OR for patients with underlying diseases was 2.20 (95% CI: 2.15-2.26), suggesting that the mortality rate of patients with underlying disease increases by at least 2.15 times.
Table 4.
Univariate and multivariate analyses for mortality rate: adjusted ORs and their 95% CIs based on sex, age, and underlying health condition
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | P-Value | Adjusted OR (95% CI) | P-Value | |
| Sex | ||||
| Female | Reference | |||
| Male | 1.87 (1.83, 1.91) | < .0001 | 1.83 (1.79, 1.88) | < .0001 |
| Age | ||||
| 0-20 | Reference | |||
| 21-30 | 0.51 (0.45, 0.57) | < .0001 | 0.50 (0.44, 0.56) | < .0001 |
| 31-40 | 1.17 (1.05, 1.29) | .0029 | 1.06 (0.96, 1.7) | .268 |
| 41-50 | 3.30 (2.99, 3.63) | < .0001 | 2.70 (2.45, 2.98) | < .0001 |
| 51-60 | 8.30 (7.55, 9.13) | < .0001 | 6.16 (5.60, 6.78) | < .0001 |
| 61-70 | 18.44 (16.76, 20.28) | < .0001 | 12.46 (11.33, 13.73) | < .0001 |
| 71-80 | 29.30 (26.60, 32.28) | < .0001 | 19.08 (17.32, 21.06) | < .0001 |
| 81+ | 31.01 (28.03, 34.31) | < .0001 | 20.98 (18.96, 23.27) | < .0001 |
| Underlying health condition | ||||
| Yes | 4.36 (4.26, 4.46) | < .0001 | 2.20 (2.15, 2.26) | < .0001 |
| No | reference | |||
Note: Underlying health condition = Yes indicates patients with diabetes, COPD, asthma, immunosuppression, hypertension, CKD, cardiovascular disease, obesity, or other diseases. Some patients have comorbidities.
Limitations
A limitation to this study is that the results cannot be generalized because it was based on a retrospective analysis of COVID-19 patients in Mexico. Because our research focused on underlying health conditions rather than factors, such as ethnicity, socioeconomic deprivation, and culture, a careful approach and interpretation of the study results is required.
Discussion
To date, COVID-19 has spread to 223 countries with a mortality rate of 2.2%. Although many studies have been conducted on this virus, only a few focused on the correlation between underlying health conditions and mortality rate. Among the study participants, 37.6% had underlying health conditions, with a 4 times higher risk of death than patients without underlying diseases. Although the incidence of COVID-19 was similar between men and women, we observed an approximately 2-fold mortality rate in men (64.9%) compared with women (35.1%). This suggests that COVID-19 could be more severe in men, and this is consistent with the findings of Clark et al.2 and McCullough et al.15 Furthermore, the mortality rates of women and men with underlying diseases were 5.5 and 3.4 times higher than the mortality rates of women and men without underlying diseases, respectively. In a multivariable logistic regression that included sex, age, and underlying health conditions, we similarly confirmed that the male sex (OR 1.83) and underlying health conditions (OR 2.20) were risk factors for death.
In this study, we used the data of 566,602 patients with COVID-19, which were considerably large compared with those of Cortés-Álvarez et al.,8 Kang,11 McCullough et al.,15 and Zhang et al.,16 who studied the correlation between underlying disease and COVID-19; thus, the reliability of our study results is somewhat adequate. Patients with underlying health conditions could be at increased risk of COVID-19, and this risk varies considerably with age. The mortality rate of patients with underlying health conditions increased rapidly in the 41-50, 51-60, and 61-70 age groups, whereas patients aged ≥ 71 y were less likely to be affected by underlying conditions. In addition, the mortality rates of patients with diabetes, COPD, immunosuppression, hypertension, CKD, cardiovascular disease, and other diseases were approximately 2.5-4 times higher than those without underlying conditions. However, in cases of asthma and obesity, no significant differences were observed in mortality rates. Except for those over 71 y of age, the mortality rate of patients with underlying health conditions was more than 2 times higher than that for patients without underlying conditions. However, patients over 71 y of age were less affected by their underlying health conditions, suggesting that age is a more important factor than the underlying disease for patients over 71 y.
The time from initial symptom onset to death (time interval) was reduced by the following underlying factors: diabetes, COPD, immunosuppression, hypertension, CKD, cardiovascular disease, and other diseases. This was probably because of the deteriorating state of health due to the disease. Thus, COVID-19 patients with underlying diseases are recommended to undergo a comprehensive assessment at early diagnosis due to their vulnerability.
Conclusions
We observed that COVID-19 patients with underlying diseases, male patients, and those above 41 y have an increased risk of death. Particularly, age was confirmed as the most important factor positively correlated with mortality rather than the presence or absence of underlying diseases in those aged above 71 y. Hence, more attention should be paid to older and male patients, because the probability of death can be significantly high. In addition, COVID-19 patients with no underlying diseases could have a decreased risk of death. Although our estimates include uncertainty, they provide relevant information regarding the group of patients to place on further observation.
Funding statement
This work was supported by the research fund of Hanyang University(HY-2019).
References
- 1. World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed May 7, 2021.
- 2. Clark A, Jit M, Warren-Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003-e1017. doi: 10.1016/S2214-109X(20)30264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertsimas D, Lukin G, Mingardi L, et al. COVID-19 mortality risk assessment: an international multi-center study. PLoS One. 2020;15(12). doi: 10.1371/journal.pone.0243262 [DOI] [PMC free article] [PubMed]
- 4. Kang Y-J. Lessons learned from cases of COVID-19 infection in South Korea. Disaster Med Public Health Prep. 2020;14(6):818-825. doi: 10.1017/dmp.2020.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giangreco G. Case fatality rate analysis of Italian COVID-19 outbreak. J Med Virol. 2020;92(7):919-923. doi: 10.1002/jmv.25894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun YJ, Feng YJ, Chen J, et al. Clinical features of fatalities in patients with COVID-19. Disaster Med Public Health Prep. 2020:1-3. doi: 10.1017/dmp.2020.235 [DOI] [PMC free article] [PubMed]
- 7. Jamshidi B, Bekrizadeh H, Zargaran SJ, et al. Modeling propagation of COVID-19 in the UK. Disaster Med Public Health Prep. 2020:1-2. doi: 10.1017/dmp.2020.383 [DOI] [PMC free article] [PubMed]
- 8. Cortés-Álvarez NY, Piñeiro-Lamas R, Vuelvas-Olmos CR. Psychological effects and associated factors of COVID-19 in a Mexican sample. Disaster Med Public Health Prep. 2020;14(3):413-424. doi: 10.1017/dmp.2020.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramage-Morin P, Polsky JY. Health-related concerns and precautions during the COVID-19 pandemic: a comparison of Canadians with and without underlying health conditions. Health Rep. 2020;31(5):3-8. doi: 10.25318/82-003-x202000500001-eng [DOI] [PubMed] [Google Scholar]
- 10. Chen Y, Li T, Ye Y, et al. Impact of fundamental diseases on patients with COVID-19. Disaster Med Public Health Prep. 2020;14(6):776-781. doi: 10.1017/dmp.2020.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang Y-J. Mortality rate of infection with COVID-19 in Korea from the perspective of underlying disease. Disaster Med Public Health Prep. 2020;14(3):384-386. doi: 10.1017/dmp.2020.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105(8):dgaa346. doi: 10.1210/clinem/dgaa346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martos-Benítez FD, Soler-Morejón CD, García-Del Barco D. Chronic comorbidities and clinical outcomes in patients with and without COVID-19: a large population-based study using national administrative healthcare open data of Mexico. Intern Emerg Med. 2021:1-11. doi: 10.1007/s11739-020-02597-5 [DOI] [PMC free article] [PubMed]
- 14. Mancilla-Galindo J, Vera-Zertuche JM, Navarro-Cruz AR, et al. Development and validation of the patient history COVID-19 (PH-Covid19) scoring system: a multivariable prediction model of death in Mexican patients with COVID-19. Epidemiol Infect. 2020;148:e286. doi: 10.1017/S0950268820002903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCullough SA, Goyal P, Krishnan U, et al. Electrocardiographic findings in coronavirus disease-19: insights on mortality and underlying myocardial processes. J Card Fail. 2020;26(7):626-632. doi: 10.1016/j.cardfail.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Lu S, Wang X, et al. Do underlying cardiovascular diseases have any impact on hospitalised patients with COVID-19? Heart. 2020;106(15):1148-1153. doi: 10.1136/heartjnl-2020-316909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaggle. COVID-19 patient pre-condition datset. https://www.kaggle.com/tanmoyx/covid19-patient-precondition-dataset. Accessed May 7, 2021.
- 18. Welch BL. The generalization of student’s problem when several different population variances are involved. Biometrika. 1947;34(1-2):28-35. doi: 10.1093/biomet/34.1-2.28 [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Wang J, Tan N, et al. Risk factors in patients with diabetes hospitalized for COVID-19: findings from a multicenter retrospective study. J Diabetes Res. 2021;2021:3170190. doi: 10.1155/2021/3170190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ioannidis JPA, Axfors C, Contopoulos-Ioannidis DG. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ Res. 2020;188:109890. doi: 10.1016/j.envres.2020.109890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu Y, Deng H, Huang L, et al. Analysis of characteristics in death patients with COVID-19 pneumonia without underlying diseases. Acad Radiol. 2020;27(5):752. doi: 10.1016/j.acra.2020.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peters SAE, MacMahon S, Woodward M. Obesity as a risk factor for COVID-19 mortality in women and men in the UK biobank: comparisons with influenza/pneumonia and coronary heart disease. Diabetes Obes Metab. 2021;23(1):258-262. doi: 10.1111/dom.14199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lunski MJ, Burton J, Tawagi K, et al. Multivariate mortality analyses in COVID-19: comparing patients with cancer and patients without cancer in Louisiana. Cancer. 2021;127(2):266-274. doi: 10.1002/cncr.33243 [DOI] [PubMed] [Google Scholar]