Abstract
IgA nephropathy is one of the leading causes of chronic kidney disease in Japan. Since the origin and mechanisms by which IgA nephropathy develops currently remain unclear, a confirmed disease diagnosis is currently only possible by highly invasive renal biopsy. With the background of the salivary microbiome as a rich source of biomarkers for systemic diseases, we herein primarily aimed to investigate the salivary microbiome as a tool for the non-invasive diagnosis of IgA nephropathy. In a comparison of salivary microbiome profiles using 16S rRNA amplicon sequencing, significant differences were observed in microbial diversity and richness between IgA nephropathy patients and healthy controls. Furthermore, recent studies reported that patients with IgA nephropathy are more likely to develop inflammatory bowel diseases and that chronic inflammation of the tonsils triggered the recurrence of IgA nephropathy. Therefore, we compared the salivary microbiome of IgA nephropathy patients with chronic tonsillitis and ulcerative colitis patients. By combining the genera selected by the random forest algorithm, we were able to distinguish IgA nephropathy from healthy controls with an area under the curve (AUC) of 0.90, from the ulcerative colitis group with AUC of 0.88, and from the chronic tonsillitis group with AUC of 0.70. Additionally, the genus Neisseria was common among the selected genera that facilitated the separation of the IgA nephropathy group from healthy controls and the chronic tonsillitis group. The present results indicate the potential of the salivary microbiome as a biomarker for the non-invasive diagnosis of IgA nephropathy.
Keywords: salivary microbiome, IgA nephropathy, oral microbiota, kidney disease, random forest algorithm
Chronic kidney disease (CKD) is a worldwide health issue that affects millions of individuals every year. According to the Japanese Society of Nephrology report in 2005, approximately 13.3 million people in Japan had CKD (Imai et al., 2009). Immunoglobulin A nephropathy (IgAN) is one of the leading causes of CKD in Japan (Tomino, 2016) and is also the most common form of primary glomerulonephritis reported globally (D’Amico, 1987; Schena, 1990). With 50% of IgAN patients progressing to end-stage renal disease (ESRD) (Moriyama et al., 2014), IgAN is a significant health burden, particularly in Japan, due to the rapidly aging population and annual increases in the number of patients undergoing dialysis (Masakane et al., 2018). Even after more than 50 years since its first classification, a confirmed diagnosis of IgAN is only achieved by kidney biopsy (Tomino, 2016), which is a highly invasive diagnostic tool and possesses greater risk of complications, particularly in the elderly. Therefore, the development of a non-invasive diagnostic tool for IgAN is urgently needed.
IgAN is an idiopathic disease, as indicated by the multi-hit hypothesis (Suzuki et al., 2011). The clinical diagnosis of IgAN includes the detection of differently glycosylated polymeric IgA1 (pIgA1) immune complex deposits in kidney glomeruli by histopathology, along with microscopic or macroscopic hematuria and proteinuria by a urinalysis (Sallustio et al., 2019). However, the trigger or maintenance of these immune reactions remains unclear. Genome-wide association studies (GWAS) on large IgAN cohorts discovered loci that only account for approximately 5% of the disease risk (Sallustio et al., 2019). Therefore, apart from genetics, other environmental factors (Sallustio et al., 2019) are considered to be associated with the progression and pathogenesis of IgAN.
Mucosal immunity is an essential factor in the pathogenesis of IgAN because the upper respiratory tract microflora has been implicated in recurrent macroscopic hematuria (Sallustio et al., 2019). The microbiota plays a vital role in the development of mucosal-associated lymphoid tissue (MALT), which, in turn, regulates the composition of the microbiota (Nakajima et al., 2018; Sallustio et al., 2019). IgA primarily originates from MALT, and GWAS of IgAN identified several risk loci involved in maintaining mucosal immunity (Sallustio et al., 2019). The oral mucosa is a gateway to the human body, and the salivary microbiome, which typically consists of more than 200 predominant species in the oral cavity (Krishnan et al., 2017), plays a crucial role in oral mucosal immunity (Moutsopoulos and Konkel, 2018). Salivary microbiome dysbiosis often reflects inflammatory responses and microbiome changes in the gut (Bajaj et al., 2015; Abe et al., 2018). Members of the salivary microbiome are potential diagnostic biomarkers for immunological diseases, such as rheumatoid arthritis (Chen et al., 2018), primary sclerosing cholangitis (Iwasawa et al., 2018), and pancreatic cancer (Torres et al., 2015; Coit et al., 2016). Furthermore, the collection and storage of saliva are non-invasive, and inexpensive (Hemadi et al., 2017). These factors may be advantageous for conducting salivary microbial profiling in IgAN patients to identify non-invasive microbial biomarkers for effective low-risk diagnostics of IgAN.
GWAS also revealed common risk loci between IgAN and IBD, such as Crohn’s disease (CD) and ulcerative colitis (UC) (Sallustio et al., 2019). A Swedish population-based study reported that patients with IgA nephropathy were more likely to develop inflammatory bowel diseases (Rehnberg et al., 2021). A transgenic murine model of IgAN highlighted the essential dependence of signals from the commensal microbiota for kidney IgA deposition in the pathogenesis of IgAN (McCarthy et al., 2011). Few studies have reported a change in the composition of the gut (De Angelis et al., 2014; Dong et al., 2020; Hu et al., 2020), salivary (Piccolo et al., 2015; Luan et al., 2019), periodontal (Cao et al., 2018), or tonsillar (Park et al., 2020) microbiota in Caucasian, Chinese, and Korean IgAN patients. However, none of these studies investigated microbiome changes in IgAN versus IBD.
In Japan, tonsillectomy monotherapy or tonsillectomy paired with steroid pulse therapy is one of the most effective therapeutic regimens for early-stage primary IgAN patients (Xie et al., 2003; Nihei et al., 2017). The efficacy of this treatment is attributed to the relationship between focal tonsillar infection and increases in pIgA-secreting plasma cells in the tonsils of IgAN patients (Meng et al., 2012). Previous studies detected pathogenic bacteria, commonly associated with chronic tonsillitis (CT) and periodontitis, in the tonsillar crypts of IgAN patients (Jensen et al., 2013; Nagasawa et al., 2014; Watanabe et al., 2017). Despite previous findings implicating periodontal pathogens in the pathogenesis of IgAN, no studies have examined the salivary microbiome of IgAN and compared it with that of chronic tonsillitis patients.
In the present study, we performed a 16S rRNA gene sequence-based analysis of the salivary microbiome of IgAN, UC, and CT patients and healthy subjects in a Japanese cohort. In our attempt to distinguish IgAN from other mucosal diseases (UC and CT), we identified a set of potential microbial biomarkers that may differentiate IgAN from other diseases and healthy individuals. According to the Ministry of Health, Labour and Welfare 2005 survey in Japan, male sex is one of the predictive factors for IgAN (Tomino, 2016). Therefore, we herein investigated the sex-specific association of the salivary microbiome in IgAN patients.
Materials and Methods
Sample collection and DNA extraction
We excluded patients with any history of antimicrobial usage within the past three months and gastrointestinal or hepatobiliary surgery from the present study. Saliva samples were collected preoperatively from IgAN and CT patients. All subjects were prohibited from eating or drinking for 2 h before sample collection. Signed consent was obtained from subjects before sampling. The present study followed all relevant guidelines and institutional policies, and was approved by the Azabu University Ethics Committee (029, 14 March 2013), Osaka University Ethics Committee (2413, 29 September 2014), and RIKEN Ethics Committee (H30-4, 29 August 2019). Following the self-collection of salivary samples in test tubes, samples were transported to the laboratory within 24 h of collection. At the laboratory, samples were immediately frozen using liquid nitrogen and stored at –80°C until further analyses. The extraction of bacterial DNA from these salivary samples was performed as described previously (Morita et al., 2007; Said et al., 2014). Briefly, 1 mL of saliva was centrifuged at 3,300×g at 4°C for 10 mins. The resulting bacterial cell pellets were then suspended in 10 mM Tris–HCl/10 mM EDTA buffer and incubated with 15 mg mL–1 lysozyme (Sigma-Aldrich) at 37°C for 1 h. Purified achromopeptidase (Wako Pure Chemical Industries) was added to the samples at a final concentration of 2,000 units mL–1 before they were incubated at 37°C for an additional 30 min. We treated the suspension with 1% (w/v) sodium dodecyl sulphate (SDS) and 1 mg mL–1 proteinase K (Merck Japan) and incubated samples at 55°C for 1 h. The resultant lysate was treated with phenol/chloroform/isoamyl alcohol (Life Technologies Japan) followed by centrifugation at 3,300×g at 4°C for 10 min. To precipitate DNA, a 1/10 volume of 3M sodium acetate (pH=4.5) and 2 volumes of ethanol (Wako Pure Chemical Industries) were added to the supernatant. DNA pellets, obtained by centrifugation at 3,300×g at 4°C for 15 min, were then rinsed with 75% ethanol, dried, and dissolved in 10 mM Tris–HCl/1 mM EDTA (TE) buffer. DNA samples were purified by a treatment with 1 mg mL–1 RNase A (Wako Pure Chemical Industries) at 37°C for 30 min and precipitated by adding equal volumes of 20% polyethylene glycol solution (PEG6000-2.5MNaCl). These samples were centrifuged at 8,060×g at 4°C and double-rinsed with 75% ethanol before pellets were dried and dissolved in TE buffer.
PCR amplification of the V1-V2 hypervariable region of the 16S rRNA gene was performed using barcoded 27Fmod (5′-AGRGTTTGATYMTGGCTCAG-3′) and 338R (5′-TGCTGCCTCCCGTAGGAGT-3′) primers (Kim et al., 2013) according to a previously described protocol (Tsuda et al., 2015). Following purification using AMPure XP magnetic purification beads (Beckman Coulter) and quantification using the Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies Japan), equal amounts of PCR amplicons were pooled together and sequenced using the 454 GS FLX Titanium or 454 GS Junior system (Roche Applied Science) according to the manufacturer’s instructions (Said et al., 2014; Tsuda et al., 2015).
Data processing of 16S rRNA sequences
After sequencing, we used an analysis pipeline designed to process the 454 pyrosequencing data of the V1-V2 region of the 16S rRNA gene, as previously reported (Kim et al., 2013). Reads with an average quality score less than 25, lacking both universal primers and possible chimeric reads, accounting for 48–49% of all reads, were excluded from the analysis (Table S1). A total of 2,300 reads per sample were randomly chosen from high-quality reads for analysis in the present study. The selected reads were then sorted on the basis of the average quality score and grouped into OTUs using the UCLUST algorithm with a 96% identity threshold (Said et al., 2014). Taxonomic assignments for each OTU were appointed by similarity searching using the GLSEARCH program against the RDP, CORE, and NCBI genome databases. Sequence similarity thresholds of 70, 94, and 96%, respectively, were used for these assignments at the phylum, genus, and species levels.
Data availability
The high-quality 16S V1-V2 sequences used in the present study for a downstream analysis were deposited in the DDBJ/GenBank/EMBL database (accession no. DRA002611, DRA002617, and DRA002618 [Tsuda et al., 2015], DRA011285, and DRA011286).
Statistical analysis
Samples were sex-matched using the chi-squared test by comparing sex data across groups. Similarly, for age matching, the demographic data of samples were compared using a one-way ANOVA test. Between-sample diversity or beta diversity was assessed using the UniFrac distance metric (Lozupone et al., 2011) followed by a principal coordinate analysis (PCoA) to visualize any similarities or differences in the microbiome structure of the samples. We performed a permutational multivariate analysis of variance (PERMANOVA) to assess the significance of beta diversity between the groups, and the corresponding P values were adjusted for multiple testing using the Benjamin-Hochberg (BH) correction method. To evaluate the alpha diversity of the samples in terms of richness and diversity, we used the observed OTU and Chao1-estimated number, and the Shannon index, respectively. The significance of relative abundance at the taxonomic levels (phylum, genus, species, and OTU) was evaluated using the Wilcoxon test with BH corrections for multiple comparisons. A similar approach was used to clarify the sex-specific microbiome association. We grouped data into male and female samples and then assessed sex effects in UniFrac weighted and unweighted metrics using PERMANOVA. The groups that showed significance (P<0.05, the Wilcoxon test) were then investigated further with respect to alpha and beta diversities as well as the taxonomic profiles of microbiomes specific to male and female samples in the groups.
We used a Linear Discriminate Analysis (LDA) effect size (LEfSe) tool (http://huttenhower.sph.harvard.edu/lefse/) to identify differentially abundant taxa between the IgAN, healthy, and disease (CT and UC) groups at different taxa levels (genus and OTU levels). LefSe uses the non-parametric Kruskal-Wallis test and unpaired Wilcoxon rank sum test to identify differentially abundant taxa among groups of samples and the LDA method to estimate the effect size of each feature/taxa among the groups (Segata et al., 2011). In the present study, a LEfSe analysis was performed with the alpha value for statistical analyses set to 0.05 and the threshold on the logarithmic LDA score for discriminative features to 2.
We used the AUC-RF (version 1.1) (Calle et al., 2011) package to generate RF models, as described in the methods by Iwasawa et al. (2018), to identify the group of taxa among the selected biomarkers from the LefSe analysis that may be used to classify the IgAN group from other disease and HC groups. AUCRF package-based analyses were performed using the R version 3.6.1 within the RStudio environment (version 1.2.5019).
Results
Study subjects, age, and sex distribution
We recruited 43 IgAN, 20 CT, and 33 UC patients and 65 healthy volunteers without any disease symptoms. We collected salivary samples from 43 patients with IgAN (the IgAN group, median age 39 years), 20 with CT (the CT group, median age 34.5), 33 with UC (the UC group, median age 47), and 65 healthy controls (the HC group, median age 37). After age and sex matching, 11 UC and 15 HC samples were removed from the present study, which changed the median age of the UC group to 44.5 and the HC group to 37.5 (Table 1).
Table 1.
Subject demographics in four different groups—IgAN, CT, UC, and HC.
| Demographics | IgAN (n=43) |
CT (n=20) |
UC (n=22) |
HC (n=50) |
|---|---|---|---|---|
| Age, years, median (IQR) | 39 (20.5) | 34.5 (14.5) | 44.5 (7.75) | 37.5 (8) |
| Male | 20 | 13 | 11 | 36 |
| Female | 23 | 7 | 11 | 14 |
Median age in terms of years is shown for each group along with IQR in parentheses. Small IQR values represent data points that are spread closer to the median. IgAN, Immunoglobulin A nephropathy; CT, chronic tonsillitis; UC, ulcerative colitis; HC, healthy control; IQR, interquartile range.
The Ethics Committees of Azabu University, Osaka University, and RIKEN approved this study. Signed informed consent was collected from all subjects before sample collection.
Summary of 454 reads
We obtained 1,576,683 high-quality 16S reads from the four groups using the 454 GS FLX Titanium platform (Roche Applied Science) (for details see Materials and Methods, Table S1). After removing low quality and possibly chimeric reads, we obtained 809,607 reads from 135 samples. We randomly selected 2,300 reads per sample (310,500 reads from 135 samples), and further analyzed them using the pipeline for the 454 barcoded pyrosequencing of 16S PCR amplicons to minimize overestimations of species richness in clustering due to intrinsic sequencing errors (Kim et al., 2013). Good’s coverage index (Good, 1953; Singleton et al., 2001) of the 2,300 reads per sample was 0.996, indicating a high degree of coverage. Therefore, sequence data were sufficient for analyses in the present study.
Differences in alpha and beta diversities of the salivary microbiota in IgAN, CT, UC, and HC groups
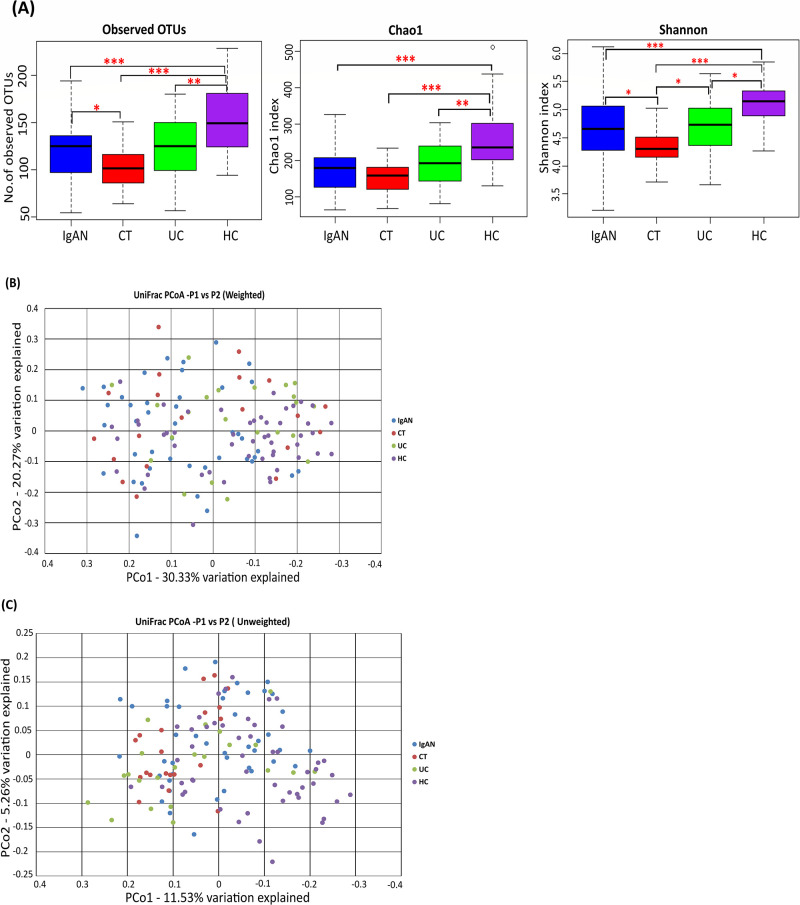
The observed OTU number in the IgAN group was significantly lower than that in the HC group (P=5.31E-05) and was significantly higher than that in the CT group (P=0.04407) (Fig. 1A). No significant difference was observed between OTU numbers in the IgAN and UC groups (Fig. 1A). Chao1-estimated OTU numbers were significantly higher in the HC group than in the IgAN, CT, and UC groups (P=1.40E-06, 6.79E-07, and 0.00459, respectively) (Fig. 1A). Within-sample diversity or alpha diversity, indicated by the Shannon index, showed a significant difference between all groups, except for between the IgAN and UC groups (Fig. 1A). The HC group had a significantly higher alpha diversity than the IgAN, CT, and UC groups (P=0.00048, 4.34E-08, and 0.01055, respectively), while the CT group had a significantly lower alpha diversity than the IgAN and UC groups (P=0.02099 and 0.01763, respectively) (Fig. 1A).
Fig. 1.
Alpha and beta diversities in IgAN, CT, UC, and HC subjects. Samples from 43 IgAN (blue), 20 CT (red), 22 UC (green), and 50 HC (purple) subjects are shown. (A) Observed and Chao1-estimated OTU numbers, and the Shannon index of the salivary microbiome from the four groups. * P<0.05; ** P<0.01; *** P<0.001 based on the Wilcoxon test with the Benjamin-Hochberg correction. (B) Weighted UniFrac–PCoA and (C) unweighted UniFrac–PCoA of the salivary microbiome from the four groups. OTU, operational taxonomic unit; PCoA, principal coordinate analysis; IgAN, immunoglobulin A nephropathy; CT, chronic tonsillitis; UC, ulcerative colitis; HC, healthy controls.
PCoA based on the unweighted UniFrac distance metric showed that many of the IgAN, CT, and UC samples were segregated from HC samples (Fig. 1C). PERMANOVA revealed a significant difference between the IgAN and CT groups in the unweighted UniFrac metric (P<0.01), but not in the weighted UniFrac metric (Table 2). Additionally, PERMANOVA showed that the IgAN group was significantly different from both the HC and UC groups for unweighted (P<0.01) and weighted (P<0.01) UniFrac metrics (Table 2). According to unweighted UniFrac metrics, in comparisons with the HC group, the IgAN group had lower dysbiosis than the CT and UC groups, as shown by the respective R2 values from PERMANOVA (R2 value=0.03 [IgAN vs HC]; 0.06 [CT vs HC]; 0.05 [UC vs HC]; Table 2).
Table 2.
Permutational multivariate analysis of variance (PERMANOVA) in salivary microbiome samples among four groups—IgAN, CT, UC, and HC.
| Category | No. of Subjects | Weighted UniFrac | Unweighted UniFrac | |||
|---|---|---|---|---|---|---|
| R2 | Adjusted P value | R2 | Adjusted P value | |||
| CT vs HC | CT: 20 HC: 50 |
0.06 | 0.006 | 0.06 | 0.001 | |
| IgAN vs CT | IgAN: 43 CT: 20 |
0.02 | 0.217 | 0.03 | 0.003 | |
| IgAN vs HC | IgAN: 43 HC: 50 |
0.07 | 0.003 | 0.03 | 0.001 | |
| CT vs UC | CT: 20 UC: 22 |
0.04 | 0.179 | 0.04 | 0.002 | |
| UC vs HC | UC: 22 HC: 50 |
0.04 | 0.043 | 0.05 | 0.001 | |
| IgAN vs UC | IgAN: 43 UC: 22 |
0.06 | 0.003 | 0.04 | 0.001 | |
P values were adjusted for multiple testing by the Benjamin-Hochberg method. P values <0.05 are in bold.
Variations in salivary microbiome taxonomic profiles between all groups
We taxonomically assigned OTUs according to phylotypes in public microbial 16S rRNA gene databases. Phyla with a relative mean abundance of more than 0.1% across all groups (IgAN, CT, UC, and HC) and, thus, accounting for 99.8% of total abundance were Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacteria, Candidatus Saccharibacteria (TM7), and Streptophyta. The relative mean abundance of Bacteroidetes was significantly lower (P=0.0014), whereas that of Proteobacteria was significantly higher (P=0.0021) in the IgAN group than in HC group. The relative mean abundance of Actinobacteria was significantly lower in the IgAN group than in the UC group (P=0.0256). Candidatus Saccharibacteria (TM7) was significantly more abundant in the IgAN group than in the CT group (P=0.0074). The ratio of the relative mean abundance of Firmicutes/Bacteroidetes was higher in the disease groups (IgAN, CT, and UC groups) than in the HC group. On the other hand, the Firmicutes/Proteobacteria ratio was lower in the disease groups than in the HC group.
The taxonomic assignment at the genus level identified 270 bacterial genera, 51 of which (Table S2) had a relative mean abundance of more than 0.1%, accounting for 97.8% of the total abundance. Neisseria was significantly more abundant in the IgAN group than in the HC and UC groups (P=0.0005 and 0.0032, respectively), whereas Prevotella, Megasphaera, and Solobacterium were significantly less abundant in the IgAN group than in the HC and UC groups (P=0.0019, 0.0335, 0.0241, 0.0261, 0.0417, and 0.0391, respectively). On the other hand, Stomatobaculum was significantly more abundant in the IgAN group than in the CT and UC groups (P=0.0385 and 0.005, respectively), but was significantly less abundant than in the HC group (P=0.0008). The abundance of Peptostreptococcus was also significantly lower in the IgAN group than in the HC group (P=0.0044), but higher than in the CT group (P=0.0431). Peptococcus was significantly more abundant in the IgAN group than in the CT group (P=0.0361). However, Schaalia was significantly less abundant in the IgAN group than in the CT and UC groups (P=0.0147 and 0.0017, respectively). Actinomyces and Selenomonas were significantly less abundant, whereas Gemella was significantly more abundant in the IgAN group than in the UC group (P=0.0020, 0.0006, and 0.0098, respectively).
Similar patterns were observed in genus abundance in comparisons of the disease groups with the HC group. The abundance of Stomatobaculum, Staphylococcus, Cutibacterium, and Peptostreptococcus was significantly lower in all disease groups (IgAN, UC, and CT) than in the HC group (P<0.05, Table S2). Similarly, the abundance of the genera Veillonella, Solobacterium, and Corynebacterium was significantly lower in the IgAN and CT groups than in the HC group (P<0.05, Table S2). The mean relative abundance of the genus Enhydrobacter was significantly lower in the IgAN and UC groups than in the HC group (P=0.0021 and 0.0457, respectively).
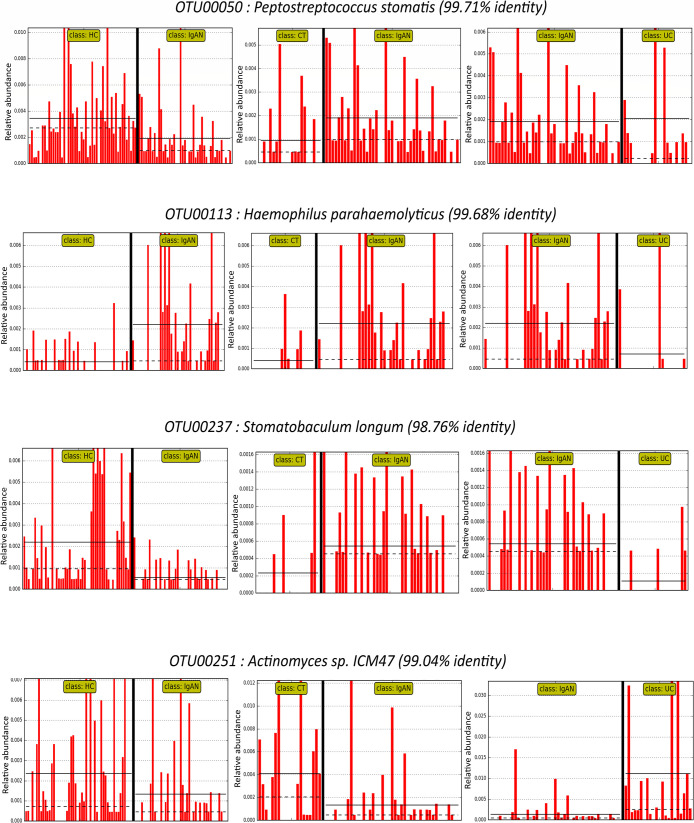
To further evaluate salivary microbiome differences among IgAN, CT, and UC patients and healthy subjects, the LDA LefSe method was used to identify significant discriminative features between the groups (with a logarithmic LDA score threshold >2). At the OTU level, we identified 19, 33, and 36 differential taxa between the IgAN and CT, IgAN and UC, and IgAN and HC groups, respectively (Fig. S1). Four of these taxa, namely, OTU00113 (99.68% similarity with Haemophilus parahaemolyticus), OTU00237 (98.76% similarity with Stomatobaculum longum), OTU00251 (99.04% similarity with Actinomyces sp. ICM47), and OTU00050 (99.71% similarity with Peptostreptococcus stomatis) were common differential features between the IgAN group and the other three groups (CT, UC, and HC) (Fig. 2).
Fig. 2.
Common differential features between IgAN and three other groups (CT, UC, and HC) from a LefSe analysis. With a threshold LDA score >2, differentially abundant taxa were identified between the IgAN vs CT, IgAN vs UC, and IgAN vs HC groups. The four common taxa at the OTU level are shown. Individual red bars represent the relative abundance of the taxa in a sample. LefSe, Linear discriminant analysis (LDA) effect size; IgAN, immunoglobulin A nephropathy; CT, chronic tonsillitis; UC, ulcerative colitis; HC, healthy controls.
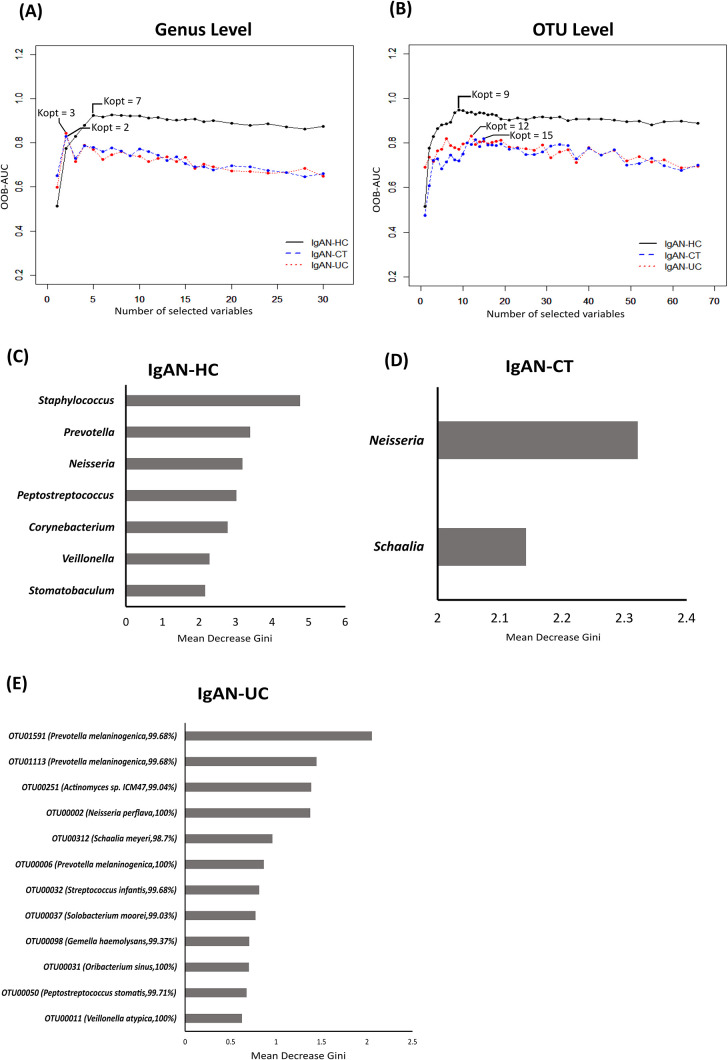
To assess the potential value of the identified microbial biomarkers for clinically differentiating the IgAN group from the disease (CT and UC) and HC groups, we generated random forest (RF) models using the AUC-RF package. We generated models for two taxa levels (genus and OTU) (Table 3). We used the area under the curve (AUC) of the receiver operating curve (ROC), that was, in turn, based on the out-of-bag (OOB) error rate, to identify the combination of multiple taxa from the 30 differential genera selected by the LefSe tool that contributes to discriminating the IgAN group from the other groups. The models with the best AUC values at the genus level were observed for 7, 2, and 3 genera between the IgAN and HC, IgAN and CT, and IgAN and UC groups, respectively (Fig. 3A). Among the selected genera, Neisseria (Fig. S2C) was a common contributor for distinguishing the IgAN group from the HC, CT, and UC groups, and Schaalia for distinguishing the IgAN group from the CT and UC groups. The abundance of Neisseria was significantly higher in the IgAN group than in the HC and UC groups (P=0.0005 and 0.0032, respectively), whereas no significant differences were observed between the IgAN and CT groups. The abundance of Schaalia was significantly lower in the IgAN group than in the CT (Fig. S2B) and UC groups (P=0.01 and 0.002, respectively). Six out of the 7 genera (Staphylococcus, Prevotella, Peptostreptococcus, Corynebacterium, Veillonella, and Stomatobaculum) contributed to distinguishing the IgAN group from the HC group (Fig. S2). Among 3 genera, the genus Selenomonas contributed to distinguishing the IgAN group from the UC group. We confirmed these results using a 10-fold cross-validation of AUC-RF models repeated 20 times to obtain mean AUCs, which were 0.90 between the IgAN and HC groups, 0.707 between the IgAN and CT groups, and 0.851 between the IgAN and UC groups (Table 3).
Table 3.
Mean area under curve for salivary microbiome samples among IgAN, CT, UC, and HC groups.
| Category | cvAUC | |
|---|---|---|
| OTU Level | Genus Level | |
| IgAN vs HC | 0.88 | 0.90 |
| IgAN vs CT | 0.62 | 0.707 |
| IgAN vs UC | 0.88 | 0.851 |
The mean AUCs (cvAUC) of the 10-fold cross validation process repeated 20 times using the best RF model in the AUC-RF package are shown here. cvAUC, mean area under curve from 20 repetitive 10-fold cross validations of the random forest model; RF, Random Forest.
Fig. 3.
Random Forest (RF) analysis of the salivary microbiota of four groups using the AUC-RF package at the OTU level. Best RF models (comparisons based on a combination of the best mean area under the curve [AUC] value) was obtained at the (a) genus and (b) OTU levels. The selected features of the best RF models for (c) IgAN-HC, (d) IgAN-CT, and (e) IgAN-UC comparisons are shown. Kopt=optimal number of features to distinguish between two groups under comparison; IgAN, immunoglobulin A nephropathy; CT, chronic tonsillitis; UC, ulcerative colitis; HC, healthy controls.
At the OTU level, we used 66 differential OTUs selected by the LefSe tool to build the RF model. The models with the best AUC values had 9, 15, and 12 OTUs between the IgAN and HC, IgAN and CT, and IgAN and UC groups, respectively (Fig. 3B). Out of these OTUs, OTU00002 (100% similarity with Neisseria perflava) and OTU00050 (99.71% identity with P. stomatis) were common contributors to distinguishing the IgAN group from the HC, CT, and UC groups. The UC and HC groups had a significantly higher abundance, whereas the CT group had a significantly lower abundance of the OTU, OTU00050 (99.71% identity with P. stomatis) than the IgAN group (P=0.02, 0.01, and 0.03, respectively). OTU00002 (100% similarity with N. perflava) was significantly higher in abundance in the IgAN group than in the HC and UC groups (P=0.0002 and 0.0060, respectively); however, the difference between its abundance in the IgAN and CT groups was not significant (P>0.05). Four OTUS, OTU00312 (Schaalia meyeri, 98.7%), OTU00251 (Actinomyces sp. ICM47, 99.04%), OTU00011 (Veillonella atypica, 100%), and OTU00031 (Oribacterium sinus, 100%) were common contributors to distinguishing the IgAN group from the other two disease groups. OTU00312 (S. meyeri, 98.7% identity) was significantly lower in abundance in the IgAN group than in the CT and UC groups (P=0.005 and 0.005, respectively). OTU00251 (Actinomyces sp. ICM47, 99.04% identity) had significantly lower abundance, whereas OTU00031 (O. sinus, 100%) had significantly higher abundance in the IgAN group than in the UC group (P=0.0005 and 0.04, respectively). No significant differences were observed in the abundance of OTU00011 (V. atypica, 100%) between the IgAN group and the other two disease groups. The mean AUC of the 10-fold cross-validation repeated 20 times was 0.88 between the IgAN and HC groups, 0.62 between the IgAN and CT groups, and 0.88 between the IgAN and UC groups (Table 3).
Sex-specific microbiome association
To establish whether there is any underlying sex-specific association of the salivary microbiome, we performed PERMANOVA on samples grouped according to sex. The results obtained revealed a significant difference between the male and female groups with respect to weighted and unweighted UniFrac metrics irrespective of the disease state grouping (Table S3). Upon further analyses of the dataset group-wise, a significant difference was observed between male and female samples in the IgAN group for the weighted UniFrac metric and in the HC group for the unweighted UniFrac metric only (Table S3). Observed OTU numbers were significantly lower in the IgAN male and IgAN female groups than in the HC male and HC female groups, respectively (P=0.0010 and 0.04, respectively) (Fig. S3A). Similarly, chao1-estimated OTU numbers were significantly lower in the IgAN male and IgAN female groups than in the HC male and HC female groups, respectively (P=0.0002 and 0.003, respectively) (Fig. S3A). Alpha diversity, indicated by the Shannon index, showed a significant difference between the IgAN female and HC female groups (P=0.005), but not between the IgAN male and HC male groups (Fig. S3A). PCoA based on the unweighted UniFrac distance metric segregated IgAN male and IgAN female samples from HC male and HC female samples, respectively (Fig. S3B). Similar sample segregation was observed between IgAN male and HC male samples and between IgAN female and HC female samples in PCoA plots based on weighted UniFrac distance metrics (Fig. S3B). A significant difference was also noted between the IgAN male and HC male groups for both weighted and unweighted UniFrac metrics (Table S3). Similar results were obtained in PERMANOVA between the IgAN female and HC female groups (Table S4).
Regarding the taxonomic composition, no phylogenetic demarcation was detected between taxa that were differentially abundant in two sex-based analyses. At the phylum level, Bacteroidetes was significantly less abundant in the IgAN male group than in the HC male group, while Candidatus Saccharibacteria (TM7) was significantly less abundant in the IgAN female group than in the HC female group. However, the abundance of the phylum Proteobacteria was significantly higher in the IgAN male and IgAN female groups than in the HC male and HC female groups, respectively. At the genus level, the abundance of 12 genera (Neisseria, Rothia, Oribacterium, Turicibacter, Campylobacter, Peptostreptococcus, Romboutsia, Stomatobaculum, Enhydrobacter, Peptococcus, Acinetobacter, and Dialister) with a mean relative abundance of more than 0.1% was significantly higher in the IgAN male group than in the HC male group. The abundance of 3 genera (Atopobium, Corynebacterium, and Micrococcus), with a mean relative abundance of more than 0.1%, was significantly lower, while that of the genus Streptococcus was significantly higher in the IgAN female group than in the HC female group (Fig. S4).
Discussion
The human microbiome is closely associated with mucosal immunity, and previous findings implicated the gut, tonsil, periodontal, and salivary microbiota in IgAN (De Angelis et al., 2014; Nagasawa et al., 2014; Piccolo et al., 2015; Watanabe et al., 2017; Cao et al., 2018; Luan et al., 2019; Dong et al., 2020; Hu et al., 2020; Park et al., 2020). However, despite Japan having the second highest incidence and frequency of IgAN (Schena and Nistor, 2018), few studies have been conducted on the IgAN-associated microbiota in a Japanese cohort. To the best of our best knowledge, the present study is the first to characterize the salivary microbiome of patients with IgAN and compare it with those of CT and UC patients and healthy subjects.
The present results revealed that the salivary microbial composition in IgAN patients was different from that in healthy subjects, as indicated by lower species richness and microbial diversity in the salivary microbiota of IgAN patients. Similar results were obtained on the salivary microbiome composition in CT and UC patients. Similarly, salivary microbial richness and diversity were previously reported to be lower (P>0.05) in IgAN than in HC in a Caucasian population (Piccolo et al., 2015), whereas a Chinese population-based study of the IgAN salivary microbiota did not report any significant differences (Luan et al., 2019). In fecal microbiome studies associated with IgAN, few studies reported lower microbial richness (De Angelis et al., 2014; Hu et al., 2020) and diversity (De Angelis et al., 2014) in IgAN than in HC, whereas another study did not find any significant difference (Dong et al., 2020). These differences may be attributed to variability in the study design, such as the use of different hypervariable regions for 16S rRNA sequencing as well as different sequencing platforms, sample sizes, and ethnicities. A statistical analysis of phylogeny-based weighted and unweighted UniFrac metrics showed that the observed dysbiosis was associated with differences in the presence or absence of the microbial taxa as well as their abundance in the population. Similar to the findings of Caucasian (De Angelis et al., 2014; Piccolo et al., 2015) and Chinese (Luan et al., 2019; Dong et al., 2020) population studies, microbial dysbiosis was detected in IgAN patients in the present study. In PERMANOVA of unweighted UniFrac distance metrics, the IgAN and CT groups significantly differed in terms of the microbial taxa composition only. In contrast, the composition and abundance of the microbial taxa contributed to a significant difference between the IgAN and UC groups. These results indicate that the overall microbial population was similar in the CT and IgAN groups. However, since this is the first study to compare the salivary microbiome of IgAN, CT, and UC patients, a more detailed and larger population-based study investigating the relationships among these diseases in terms of the microbiome is required.
Differences were observed at the taxonomic level between the IgAN group and the other groups. The phylum Firmicutes dominated all samples and was the most abundant in the IgAN group, which is consistent with the findings of fecal and salivary microbiota studies on IgAN patients (De Angelis et al., 2014; Luan et al., 2019; Hu et al., 2020). The Firmicutes/Proteobacteria ratio was lower in the IgAN group than in the HC group, which was similar to that reported in a salivary microbiota study on a Caucasian population (Piccolo et al., 2015) and in a periodontal microbiome study on an Asian population (Cao et al., 2018), but differed from that in a salivary microbiota study on a Chinese cohort (Luan et al., 2019).
The present results revealed that the Firmicutes/Bacteroides ratio was higher in the UC group than in the HC group, which is similar to the findings of other gut microbiome studies (Kabeerdoss et al., 2015; Xun et al., 2018). Furthermore, the abundance of the phylum Actinobacteria was significantly higher in the UC group than in the IgAN and HC groups, which is consistent with a meta-analysis of the gut microbiota composition in IBD (Walters et al., 2014) and salivary microbiome ecotypes associated with UC (Xun et al., 2018). Based on these findings, an abnormal physiological state is associated with microbial dysbiosis in gut inflammatory diseases (Xun et al., 2018).
The significant differences observed between the IgAN group and the three other groups (CT, UC, and HC) in terms of the microbial taxa prompted us to investigate the capacity of these selected biomarkers to discriminate the IgAN group from the CT, UC, and HC groups. In the present study, a combination of salivary taxa separated IgAN from healthy individuals with AUC of 0.88 and 0.90 at the OTU and genus levels, respectively. A Chinese cohort study reported the separation of IgAN from HC with a predictive accuracy of up to 80% by using salivary microbial OTUs in combination with biochemical characteristics (Luan et al., 2019). These findings indicate that salivary microbiome-derived biomarkers are applicable for a predictive diagnosis of IgAN from healthy population.
Neisseria was a common contributor for distinguishing IgAN from healthy individuals and CT. The abundance of this genus was higher in the IgAN group than in the CT, UC, and HC groups, which is consistent with the findings of a previous study (Piccolo et al., 2015). Furthermore, at the OTU level, some OTUs belonging to the genus Neisseria were among the selected taxa for differentiating the IgAN group from the UC group and were significantly enriched in IgAN patients. Salivary microbiome studies previously reported the co-occurrence of Neisseria and Haemophilus in the salivary ecosystem (De Angelis et al., 2014; Takeshita et al., 2016). A salivary community type comprising Neisseria, Haemophilus, Gemella, Porphyromonas, and Streptococcus mitis in combination with reduced phylogenetic diversity was associated with better periodontal health (Takeshita et al., 2016). Furthermore, a salivary microbiome study on IBD patients revealed a correlation between elevated salivary IgA levels and a lower abundance of Neisseria, Haemophilus, Gemella, and Streptococcus (Said et al., 2014). Salivary IgA levels were previously shown to be elevated in IgAN patients (Yamabe et al., 1987). In the present study, the abundance of the genera Neisseria, Haemophilus, Gemella, and Streptococcus increased in IgAN patients, and, thus, was not directly associated with salivary IgA responses in the pathogenesis of IgAN. This may be attributed to differences in the disease pathogeneses of IgAN and IBD. However, since this is the first study to compare the salivary microbiome in IgAN and UC and data on salivary IgA levels in IgAN patients were not collected, further studies are needed to elucidate the relationship between salivary IgA levels and the microbiome in these two diseases.
Some of the OTUs belonging to the genus Gemella differentiated the IgAN group from the UC group (Fig. S1B). In contrast to previous findings (Piccolo et al., 2015), this taxa was more abundant in the IgAN group than in the UC and HC groups. These differences in the IgAN salivary microbiome across populations supports the heterogeneity of the IgAN pathology across different ethnicities (Yeo et al., 2018).
Prevotella is one of the selected genera that contributes to differentiating IgAN patients from healthy controls. Consistent with previous findings (Piccolo et al., 2015; Cao et al., 2018; Luan et al., 2019), the abundance of Prevotella was significantly higher in the HC group than in the IgAN group. A Chinese fecal microbiome study comparing IgAN patients, membranous nephropathy patients, and healthy controls noted a positive correlation between Prevotella and a higher serum albumin level. Serum albumin plays an essential role in reducing oxidative stress in mesangial cells by attenuating the production of reactive oxygen species, such as hydrogen peroxide, thereby reducing the risk of IgAN progression towards ESRD (Kawai et al., 2018). Therefore, the genus Prevotella appears to play a protective role in the salivary and gut ecosystems, and a decline in its abundance may facilitate systemic disease progression, such as IgAN.
Although the OTU clustering methods performed in the present study were not sufficient for species level assignment, we identified several OTUs assigned to Prevotella melaninogenica species, with more than 99% identity, which were among the selected taxa for distinguishing IgAN patients from UC patients, with a significantly higher abundance in UC patients than in IgAN patients. Even though P. melaninogenica has been identified as a member of the Japanese salivary core microbiome (Takeshita et al., 2016), previous studies implicated it in the development of oral and systemic diseases (Brook, 1995; Sibley et al., 2011; Kondo et al., 2018). The expression of TLR2 was previously shown to be up-regulated in the blood mononuclear cells of IgAN patients (Chang and Li, 2020; Saito et al., 2016) and in the dendritic cells of IBD patients (CD and UC) (Hug et al., 2018). Since P. melaninogenica reportedly stimulates cytokine responses via TLR2 pathways (Kondo et al., 2018), it has the potential to contribute to the progression of inflammatory disorders, such as UC and IgAN. On the other hand, the depletion of CD4+ T cells correlated with an increase in the abundance of P. melaninogenica in the salivary microbiota of HIV patients (Lewy et al., 2019). Therefore, the lower abundance of P. melaninogenica in IgAN patients in the present study implied the autoimmune nature of IgAN, and further studies are needed to clarify the role of this genus in the T helper cell imbalance in IgAN.
Streptococcus is the predominant genus in the phylum Firmicutes, and was the most abundant in the CT group, followed by the IgAN group. In previous studies, Streptococcus was enriched in the subgingival microbiome of chronic periodontitis patients with IgAN (Cao et al., 2018) and was also identified as one of the core members of the tonsillar crypt microbiome (Jensen et al., 2013). 16S rDNA reads of the Streptococcaeae family were found to be elevated in the fecal microbiome of IgAN patients with persistent proteinuria (De Angelis et al., 2014). Another study showed that the level of the cell surface collagen-binding Cnm protein of S. mutans was higher in tonsillar specimens from IgAN patients than in those from CT patients (Ito et al., 2019). In the present study, the mean relative abundance of this species was higher in the IgAN group than in the CT group (P>0.05). Therefore, some bacterial causal agents of focal tonsillar infection, such as Streptococcus, may play a role in the pathogenesis of IgAN because immunological assays of renal tissues from IgAN patients detected streptococcal proteins (Schmitt et al., 2010).
Several OTUs belonging to the genus Haemophilus varied significantly between the IgAN and UC groups only. Some members of the genus Hemophilus, such as H. parahaemolyticus, are commensal microflora in the upper respiratory tract, and may be opportunistic pathogens that cause invasive and severe diseases (Le Floch et al., 2013). H. parahaemolyticus belongs to the H. parainfluenzae group and is one of the few species in the genus Haemophilus, including H. influenzae, producing IgA1 protease, which is responsible for its pathogenicity in its human host (Norskov-Lauritsen, 2014). Piccolo et al. reported an increase in H. parainfluenzae in the salivary microbiota of IgAN patients with the lowest grade of proteinuria (Piccolo et al., 2015). A previous study that recommended the use of H. influenzae-derived IgA protease to treat IgAN reported that the lack of mesangial IgA1 specificity by the H. influenzae-derived IgA protease limits its immediate application to IgAN therapy (Eitner and Floege, 2008). These findings suggest that some members of the genus Hemophilus contribute to the early pathogenic stages of IgAN. Further studies based on these associative members may contribute to a more detailed understanding of the progression of IgAN and, thus, expedite therapeutic applications.
In the present study, the microbial diversity and richness of the salivary microbiome were significantly lower in UC patients than in HC. This was in contrast to previous studies that reported no significant difference in terms of richness or diversity between these two groups (Said et al., 2014; Xun et al., 2018). Furthermore, unlike previous findings (Xun et al., 2018), the Firmicutes/Bacteroidetes ratio was lower in the UC group than in the HC group. The abundance of Oribacterium was significantly lower in UC patients than in HC, which is consistent with previous studies (Said et al., 2014; Xun et al., 2018). While the present study and another study (Xun et al., 2018) reported the enrichment of Streptococcus in the salivary microbiome of UC patients, Said et al. (2014) showed that it was depleted in UC patients. Moreover, in contrast to previous findings (Said et al., 2014), Veillonella was less abundant in the UC group than in the HC group in the present study. We also observed a decrease in the abundance of Prevotella in the UC group (though P>0.05). This was similar to the findings of the Chinese cohort study (Xun et al., 2018) that showed the significant depletion of Prevotella in the UC group. However, Said et al. (2014) reported an increase in the abundance of this genus in UC group. These discrepancies may be attributed to multiple factors, such as study design, sample size, and the genetic history of the study subjects.
While some epidemiological studies observed a male predominance among North American and Western European populations (Barratt and Feehally, 2014; Sukcharoen et al., 2020), others showed an equal distribution of the incidence of IgAN between the sexes in an Asian population (Lee et al., 2012; Cheng et al., 2013; Feehally and Barratt, 2015). A single-center study in Japan reported that primary glomerulonephritis was more prevalent in men than in women (Moriyama et al., 2010). In microbiome studies, sex may have acted as a confounding factor because some showed a significant difference between the male and female microbiomes (Raju et al., 2019; Minty et al., 2020). Therefore, upon investigating the existence of a sex-associated microbiota in our cohort, we found that the abundance of some genera significant differed between HC and IgAN males (Fig. S4A), whereas others differed between HC and IgAN females (Fig. S4B). These results suggest that the microbiota plays a role in the sex-associated risk of IgAN disease progression and, thus, further research in this field is needed.
There were some limitations that need to be addressed. The present study included a small and non-uniform sample size across the groups. The imbalance in the dataset was not significant and did not affect the microbiome analysis. Disease predictive modeling is affected by an imbalanced dataset; however, the RF classifier is relatively robust when dealing with an imbalanced dataset (Dittman et al., 2015) and the AUC-RF package uses a RF classifier for modeling. Furthermore, data associated with the stage of disease for IgAN patients was not available for a clinical metadata correlation analysis. However, since tonsillectomy is recommended and effective in the early stages of IgAN (Hotta et al., 2001) and IgAN patients in the present study provided saliva samples before tonsillectomy, these patients were in the early stage of the disease. Moreover, our study design is limited by the lack of clinical data reporting serum or salivary IgA levels in IgAN patients. Since secretory IgA plays an important role in the pathogenesis of IgAN, a future microbiome study that investigates the relationship between secretory IgA levels and the predominant microbiota associated with IgAN is warranted.
In conclusion, the results of the present study, which examined variations in the salivary microbiome profiles of IgAN patients, UC patients, CT patients, and HC, indicate the potential of the salivary microbiome as a biomarker source for the development of a non-invasive diagnostic tool for IgAN. However, the biological role of the microbial biomarkers identified in this study in the pathogenesis and disease progression of IgAN is a scope for further research.
Citation
Khasnobish, A., Takayasu, L., Watanabe, K., Nguyen, T. T. T., Arakawa, K., Hotta, O., et al. (2021) Dysbiosis in the Salivary Microbiome Associated with IgA Nephropathy—A Japanese Cohort Study. Microbes Environ 36: ME21006.
https://doi.org/10.1264/jsme2.ME21006
Supplementary Material
Acknowledgements
The first author was supported by the Japanese Government through a MONBUKAGAKUSHO (MEXT) Scholarship during her study at Okayama University. The authors are very grateful to Chie Shindo for her technical assistance. All the subjects involved in this study gave informed consent. The authors have no conflicts of interest related to this work.
References
- Abe, K., Takahashi, A., Fujita, M., Imaizumi, H., Hayashi, M., Okai, K., and Ohira, H. (2018) Dysbiosis of oral microbiota and its association with salivary immunological biomarkers in autoimmune liver disease. PLoS One 13: e0198757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj, J.S., Betrapally, N.S., Hylemon, P.B., Heuman, D.M., Daita, K., White, M.B., et al. (2015) Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 62: 1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt, J., and Feehally, J. (2014) Immunoglobulin A nephropathy and related disorders. In National Kidney Foundation Primer on Kidney Diseases, 6th edn. Gilbert, S.J., and Weiner, D.E. (eds). Philadelphia, PA: W.B. Saunders, pp. 185–192. [Google Scholar]
- Brook, I. (1995) Prevotella and Porphyromonas infections in children. J Med Microbiol 42: 340–347. [DOI] [PubMed] [Google Scholar]
- Calle, M.L., Urrea, V., Boulesteix, A.L., and Malats, N. (2011) AUC-RF: a new strategy for genomic profiling with random forest. Hum Hered 72: 121–132. [DOI] [PubMed] [Google Scholar]
- Cao, Y., Qiao, M., Tian, Z., Yu, Y., Xu, B., Lao, W., et al. (2018) Comparative analyses of subgingival microbiome in chronic periodontitis patients with and without IgA nephropathy by high throughput 16S rRNA sequencing. Cell Physiol Biochem 47: 774–783. [DOI] [PubMed] [Google Scholar]
- Chang, S., and Li, X.-K. (2020) The role of immune modulation in pathogenesis of IgA nephropathy. Front Med (Lausanne) 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B., Zhao, Y., Li, S., Yang, L., Wang, H., Wang, T., et al. (2018) Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci Rep 8: 17126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, G.Y., Liu, D.W., Zhang, N., Tang, L., Zhao, Z.Z., and Liu, Z.S. (2013) Clinical and prognostic implications of serum uric acid levels on IgA nephropathy: a cohort study of 348 cases with a mean 5-year follow-up. Clin Nephrol 80: 40–46. [DOI] [PubMed] [Google Scholar]
- Coit, P., Mumcu, G., Ture-Ozdemir, F., Unal, A.U., Alpar, U., Bostanci, N., et al. (2016) Sequencing of 16S rRNA reveals a distinct salivary microbiome signature in Behçet’s disease. Clin Immunol (Amsterdam, Neth) 169: 28–35. [DOI] [PubMed] [Google Scholar]
- D’Amico, G. (1987) The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 64: 709–727. [PubMed] [Google Scholar]
- De Angelis, M., Montemurno, E., Piccolo, M., Vannini, L., Lauriero, G., Maranzano, V., et al. (2014) Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS One 9: e99006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman, D.J., Khoshgoftaar, T.M., and Napolitano, A. (2015) The effect of data sampling when using random forest on imbalanced bioinformatics data. In Proceedings of the 2015 IEEE International Conference on Information Reuse and Integration. Washington, DC: Institute of Electrical and Electronics Engineers (IEEE) Computer Society, pp. 457–463. [Google Scholar]
- Dong, R., Bai, M., Zhao, J., Wang, D., Ning, X., and Sun, S. (2020) A comparative study of the gut microbiota associated with immunoglobulin a nephropathy and membranous nephropathy. Front Cell Infect Microbiol 10: 557368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitner, F., and Floege, J. (2008) Bacterial protease for the treatment of IgA nephropathy. Nephrol, Dial, Transplant 23: 2173–2175. [DOI] [PubMed] [Google Scholar]
- Feehally, J., and Barratt, J. (2015) The genetics of IgA nephropathy: An overview from Western Countries. Kidney Dis (Basel) 1: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good, I.J. (1953) The population frequencies of species and the estimation of population parameters. Biometrika 40: 237–264. [Google Scholar]
- Hemadi, A.S., Huang, R., Zhou, Y., and Zou, J. (2017) Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int J Oral Sci 9: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta, O., Miyazaki, M., Furuta, T., Tomioka, S., Chiba, S., Horigome, I., et al. (2001) Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis 38: 736–743. [DOI] [PubMed] [Google Scholar]
- Hu, X., Du, J., Xie, Y., Huang, Q., Xiao, Y., Chen, J., et al. (2020) Fecal microbiota characteristics of Chinese patients with primary IgA nephropathy: A cross-sectional study. BMC Nephrol 21: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug, H., Mohajeri, M.H., and La Fata, G. (2018) Toll-like receptors: Regulators of the immune response in the human gut. Nutrients 10: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, E., Horio, M., Watanabe, T., Iseki, K., Yamagata, K., Hara, S., et al. (2009) Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol 13: 621–630. [DOI] [PubMed] [Google Scholar]
- Ito, S., Misaki, T., Naka, S., Wato, K., Nagasawa, Y., Nomura, R., et al. (2019) Specific strains of Streptococcus mutans, a pathogen of dental caries, in the tonsils, are associated with IgA nephropathy. Sci Rep 9: 20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasawa, K., Suda, W., Tsunoda, T., Oikawa-Kawamoto, M., Umetsu, S., Takayasu, L., et al. (2018) Dysbiosis of the salivary microbiota in pediatric-onset primary sclerosing cholangitis and its potential as a biomarker. Sci Rep 8: 5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, A., Fagö-Olsen, H., Sørensen, C.H., and Kilian, M. (2013) Molecular mapping to species level of the tonsillar crypt microbiota associated with health and recurrent tonsillitis. PLoS One 8: e56418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeerdoss, J., Jayakanthan, P., Pugazhendhi, S., and Ramakrishna, B.S. (2015) Alterations of mucosal microbiota in the colon of patients with inflammatory bowel disease revealed by real time polymerase chain reaction amplification of 16S ribosomal ribonucleic acid. Indian J Med Res 142: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, Y., Masutani, K., Torisu, K., Katafuchi, R., Tanaka, S., Tsuchimoto, A., et al. (2018) Association between serum albumin level and incidence of end-stage renal disease in patients with Immunoglobulin A nephropathy: A possible role of albumin as an antioxidant agent. PLoS One 13: e0196655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.W., Suda, W., Kim, S., Oshima, K., Fukuda, S., Ohno, H., et al. (2013) Robustness of gut microbiota of healthy adults in response to probiotic intervention revealed by high-throughput pyrosequencing. DNA Res 20: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo, Y., Sato, K., Nagano, K., Nishiguchi, M., Hoshino, T., Fujiwara, T., and Nakayama, K. (2018) Involvement of PorK, a component of the type IX secretion system, in Prevotella melaninogenica pathogenicity. Microbiol Immunol 62: 554–566. [DOI] [PubMed] [Google Scholar]
- Krishnan, K., Chen, T., and Paster, B. (2017) A practical guide to the oral microbiome and its relation to health and disease. Oral Dis 23: 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floch, A.-S., Cassir, N., Hraiech, S., Guervilly, C., Papazian, L., and Rolain, J.-M. (2013) Haemophilus parahaemolyticus septic shock after aspiration pneumonia, France. Emerging Infect Dis 19: 1694–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H., Kim, D.K., Oh, K.-H., Joo, K.W., Kim, Y.S., Chae, D.-W., et al. (2012) Mortality of IgA nephropathy patients: A single center experience over 30 years. PLoS One 7: e51225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy, T., Hong, B.Y., Weiser, B., Burger, H., Tremain, A., Weinstock, G., et al. (2019) Oral microbiome in HIV-infected women: Shifts in the abundance of pathogenic and beneficial bacteria are associated with aging, HIV load, CD4 count, and antiretroviral therapy. AIDS Res Hum Retroviruses 35: 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone, C., Lladser, M.E., Knights, D., Stombaugh, J., and Knight, R. (2011) UniFrac: An effective distance metric for microbial community comparison. ISME J 5: 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan, S., Zhang, S., Zhong, H., Zhang, Y., Wei, X., Lin, R., et al. (2019) Salivary microbial analysis of Chinese patients with immunoglobulin A nephropathy. Mol Med Rep 20: 2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masakane, I., Taniguchi, M., Nakai, S., Tsuchida, K., Wada, A., Ogata, S., et al. (2018) Annual Dialysis Data Report 2016, JSDT Renal Data Registry. Ren Replace Ther 4: 45. [Google Scholar]
- McCarthy, D.D., Kujawa, J., Wilson, C., Papandile, A., Poreci, U., Porfilio, E.A., et al. (2011) Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 121: 3991–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, H., Ohtake, H., Ishida, A., Ohta, N., Kakehata, S., and Yamakawa, M. (2012) IgA production and tonsillar focal infection in IgA nephropathy. J Clin Exp Hematop 52: 161–170. [DOI] [PubMed] [Google Scholar]
- Minty, M., Loubières, P., Canceill, T., Azalbert, V., Burcelin, R., Tercé, F., and Blasco-Baque, V. (2020) Gender-associated differences in oral microbiota and salivary biochemical parameters in response to feeding. J Physiol Biochem doi: 10.1007/s13105-020-00757-x [DOI] [PubMed] [Google Scholar]
- Morita, H., Kuwahara, T., Ohshima, K., Sasamoto, H., Itoh, K., Hattori, M., et al. (2007) An improved DNA isolation method for metagenomic analysis of the microbial flora of the human intestine. Microbes Environ 22: 214–222. [Google Scholar]
- Moriyama, T., Suzuki, K., Sugiura, H., Itabashi, M., Tsukada, M., Takei, T., et al. (2010) Frequency of renal disease in Japan: an analysis of 2,404 renal biopsies at a single center. Nephron Clin Pract 115: c227–236. [DOI] [PubMed] [Google Scholar]
- Moriyama, T., Tanaka, K., Iwasaki, C., Oshima, Y., Ochi, A., Kataoka, H., et al. (2014) Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One 9: e91756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos, N.M., and Konkel, J.E. (2018) Tissue-specific immunity at the oral mucosal barrier. Trends Immunol 39: 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa, Y., Iio, K., Fukuda, S., Date, Y., Iwatani, H., Yamamoto, R., et al. (2014) Periodontal disease bacteria specific to tonsil in IgA nephropathy patients predicts the remission by the treatment. PLoS One 9: e81636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, A., Vogelzang, A., Maruya, M., Miyajima, M., Murata, M., Son, A., et al. (2018) IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J Exp Med 215: 2019–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei, H., Sakai, K., Shishido, S., Sibuya, K., Edamatsu, H., and Aikawa, A. (2017) Efficacy of tonsillectomy for the treatment of immunoglobulin A nephropathy recurrence after kidney transplantation. Ren Replace Ther 3: 10. [Google Scholar]
- Norskov-Lauritsen, N. (2014) Classification, identification, and clinical significance of Haemophilus and Aggregatibacter species with host specificity for humans. Clin Microbiol Rev 27: 214–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.I., Kim, T.Y., Oh, B., Cho, H., Kim, J.E., Yoo, S.H., et al. (2020) Comparative analysis of the tonsillar microbiota in IgA nephropathy and other glomerular diseases. Sci Rep 10: 16206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo, M., De Angelis, M., Lauriero, G., Montemurno, E., Di Cagno, R., Gesualdo, L., and Gobbetti, M. (2015) Salivary microbiota associated with immunoglobulin A nephropathy. Microb Ecol 70: 557–565. [DOI] [PubMed] [Google Scholar]
- Raju, S.C., Lagström, S., Ellonen, P., de Vos, W.M., Eriksson, J.G., Weiderpass, E., and Rounge, T.B. (2019) Gender-specific associations between saliva microbiota and body size. Front Microbiol 10: 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehnberg, J., Symreng, A., Ludvigsson, J.F., and Emilsson, L. (2021) Inflammatory bowel disease is more common in patients with IgA nephropathy and predicts progression of ESKD: A Swedish population-based cohort study. J Am Soc Nephrol 32: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said, H.S., Suda, W., Nakagome, S., Chinen, H., Oshima, K., Kim, S., et al. (2014) Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res 21: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, A., Komatsuda, A., Kaga, H., Sato, R., Togashi, M., Okuyama, S., et al. (2016) Different expression patterns of toll-like receptor mRNAs in blood mononuclear cells of IgA nephropathy and IgA vasculitis with nephritis. Tohoku J Exp Med 240: 199–208. [DOI] [PubMed] [Google Scholar]
- Sallustio, F., Curci, C., Di Leo, V., Gallone, A., Pesce, F., and Gesualdo, L. (2019) A new vision of IgA nephropathy: The missing link. Int J Mol Sci 21: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena, F.P. (1990) A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am J Med 89: 209–215. [DOI] [PubMed] [Google Scholar]
- Schena, F.P., and Nistor, I. (2018) Epidemiology of IgA nephropathy: A global perspective. Semin Nephrol 38: 435–442. [DOI] [PubMed] [Google Scholar]
- Schmitt, R., Carlsson, F., Mörgelin, M., Tati, R., Lindahl, G., and Karpman, D. (2010) Tissue deposits of IgA-binding streptococcal M proteins in IgA nephropathy and Henoch-Schonlein purpura. Am J Pathol 176: 608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W.S., and Huttenhower, C. (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12: R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley, C.D., Grinwis, M.E., Field, T.R., Eshaghurshan, C.S., Faria, M.M., Dowd, S.E., et al. (2011) Culture enriched molecular profiling of the cystic fibrosis airway microbiome. PLoS One 6: e22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton, D.R., Furlong, M.A., Rathbun, S.L., and Whitman, W.B. (2001) Quantitative comparisons of 16S rRNA gene sequence libraries from environmental samples. Appl Environ Microbiol 67: 4374–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukcharoen, K., Sharp, S.A., Thomas, N.J., Kimmitt, R.A., Harrison, J., Bingham, C., et al. (2020) IgA nephropathy genetic risk score to estimate the prevalence of IgA nephropathy in UK Biobank. Kidney Int Rep 5: 1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, H., Kiryluk, K., Novak, J., Moldoveanu, Z., Herr, A.B., Renfrow, M.B., et al. (2011) The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita, T., Kageyama, S., Furuta, M., Tsuboi, H., Takeuchi, K., Shibata, Y., et al. (2016) Bacterial diversity in saliva and oral health-related conditions: The Hisayama Study. Sci Rep 6: 22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomino, Y. (2016) Diagnosis and treatment of patients with IgA nephropathy in Japan. Kidney Res Clin Pract 35: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, P.J., Fletcher, E.M., Gibbons, S.M., Bouvet, M., Doran, K.S., and Kelley, S.T. (2015) Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ 3: e1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda, A., Suda, W., Morita, H., Takanashi, K., Takagi, A., Koga, Y., and Hattori, M. (2015) Influence of proton-pump inhibitors on the luminal microbiota in the gastrointestinal tract. Clin Transl Gastroenterol 6: e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, W.A., Xu, Z., and Knight, R. (2014) Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett 588: 4223–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, H., Goto, S., Mori, H., Higashi, K., Hosomichi, K., Aizawa, N., et al. (2017) Comprehensive microbiome analysis of tonsillar crypts in IgA nephropathy. Nephrol, Dial, Transplant 32: 2072–2079. [DOI] [PubMed] [Google Scholar]
- Xie, Y., Nishi, S., Ueno, M., Imai, N., Sakatsume, M., Narita, I., et al. (2003) The efficacy of tonsillectomy on long-term renal survival in patients with IgA nephropathy. Kidney Int 63: 1861–1867. [DOI] [PubMed] [Google Scholar]
- Xun, Z., Zhang, Q., Xu, T., Chen, N., and Chen, F. (2018) Dysbiosis and ecotypes of the salivary microbiome associated with inflammatory bowel diseases and the assistance in diagnosis of diseases using oral bacterial profiles. Front Microbiol 9: 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamabe, H., Ozawa, K., Fukushi, K., Ohsawa, H., Chiba, N., and Onodera, K. (1987) Elevated salivary IgA in patients with IgA nephropathy. Nephron 45: 176. [DOI] [PubMed] [Google Scholar]
- Yeo, S.C., Cheung, C.K., and Barratt, J. (2018) New insights into the pathogenesis of IgA nephropathy. Pediatr Nephrol (Berlin) 33: 763–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The high-quality 16S V1-V2 sequences used in the present study for a downstream analysis were deposited in the DDBJ/GenBank/EMBL database (accession no. DRA002611, DRA002617, and DRA002618 [Tsuda et al., 2015], DRA011285, and DRA011286).