Abstract
Background
Albumin is supposed to be associated with cancer risk. However, evidence on serum albumin and cancer risk among the Chinese population is sparse. This study was conducted to evaluate the association between pre‐diagnostic serum albumin and cancer risk among Chinese.
Methods
A total of 82,061 participants with baseline information on serum albumin concentration in the Kailuan cohort were recruited. Cox proportional hazards models and restricted cubic spline (RCS) analyses were used to evaluate the association between pre‐diagnostic serum albumin and cancer risk.
Results
Albumin levels were inversely associated with overall cancer risk (HR [95% CI]: Q2, Q3, Q4 vs. Q1: 0.91 [0.78–1.07], 0.80 [0.70–0.92], 0.73 [0.63–0.85]), and the risk of lung, colorectal, and liver cancer (HR [95% CI]: Q4 vs. Q1: lung: 0.70 [0.52–0.95], colorectal: 0.43 [0.26–0.72], liver: 0.59 [0.36–0.95]). After excluding new cancer cases within 2 years since enrollment, a more significant association was observed for liver cancer (HR [95% CI]: Q4 vs. Q1: 0.41 [0.21–0.78]), while associations converted to nonsignificant for lung and colorectal cancer. The RCS model suggested an inverse linear association between albumin and the risk of overall cancer (p‐overall < 0.0001, p‐nonlinear = 0.3716) and liver cancer (p‐overall = 0.0002, p‐nonlinear = 0.1807).
Conclusions
Our findings suggest that pre‐diagnostic serum albumin is inversely and linearly associated with cancer risk among the Chinese population. This study provides evidence that albumin may be valuable to the prediction and stratification of cancer risk in the general population. However, the biological mechanism and clinical significance remain to be elucidated. Population studies with longer follow‐up time as well as experimental studies are further required.
Keywords: associations, cancer, Chinese, prospective study, serum albumin
This is the first prospective cohort study to evaluate the association between pre‐diagnostic serum albumin and cancer risk. Inverse and linear associations were firstly reported between pre‐diagnostic serum albumin and the risks of overall cancer and liver cancer. These findings provide further evidence that albumin may be valuable to the prediction and stratification of cancer risk in the general population.

1. INTRODUCTION
With increasing incidence and mortality, cancer is now responsible for most global deaths in the 21st century. According to the report of the GLOBOCAN, an estimated 18.1 million new cancer cases and 9.6 million cancer deaths occurred worldwide in 2018, among which more than one fifth were from China. 1
As cancer becoming a major public health problem, several important risk factors have been identified in the literature, such as smoking, drinking, obesity, and hepatitis B virus (HBV) infection, 2 , 3 , 4 , 5 with some generic to all cancers and some specific for different cancer types. Albumin was proved to be closely associated with the advance and prognosis of cancer since 1989. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Hypotheses for decreased albumin concentration in cancer patients were varied, including malnutrition, increased albumin consumption due to the expression of cancer cells, and inhibition of albumin synthesis caused by systemic inflammation. 7 , 17 Furthermore, it was proposed that serum albumin was a kind of endogenous antioxidant, 18 which could reduce cancer risk through exerting anticarcinogenic properties. 19 A positive association between hypoalbuminemia and abnormal carcinoembryonic antigen were also reported. 20 Taken together, albumin was supposed to be associated with cancer risk.
Previous studies on serum albumin levels and cancer risk have yielded inconsistent results. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Results from a cohort study indicated that lower albumin levels were associated with an increased risk of new cancer diagnosis within a year. 21 Concerning each cancer type, inverse associations were confirmed by several studies between albumin levels and risks of colorectal 22 , 23 and breast cancer. 24 However, there were also studies reporting nonsignificant associations among colorectal, breast, lung, and prostate cancer, 25 , 26 , 27 , 28 , 29 and even positive associations among ovarian cancer 30 were reported.
Notably, among the above‐mentioned studies, only a few were prospective, and none was conducted among the Chinese population. Therefore, we conducted this study using data from the Kailuan cohort to confirm and further evaluate the association between pre‐diagnostic serum albumin and cancer risk among Chinese.
2. MATERIALS AND METHODS
2.1. Study population
The data were obtained from the Kailuan cohort study, which was a prospective study based on health examinations among employees of the Kailuan Company. The Kailuan Company is in Tangshan City, Hebei Province, and has developed a diversified economy, including coal mining, electric power, manufacturing, transportation, trading, and health care. Routine medical examinations of the employees were conducted every 2 years at 11 affiliated hospitals of the Kailuan Company. The Kailuan cohort study was initiated in May 2006. Four rounds of questionnaire interviews and health examinations were conducted in 2006–2007, 2008–2009, 2010–2011, and 2012–2013 to collect information on both risk factors and blood tests. New participants beyond the first round could enter the cohort at the second, third, and fourth round. Therefore, the Kailuan cohort was established as a population‐based dynamic cohort. In case of the potential survival bias, subjects with a previous diagnosis of any prevalent cancers before baseline recruitment were excluded. In total, 1,38,150 subjects were enrolled through four rounds of surveys and examinations, among which, participants without blood test results of serum albumin were further excluded for this analysis. Ultimately, 82,061 participants in the age range between 18 and 104 were enrolled in this study. The study was approved by the Medical Ethics Committee of Kailuan General Hospital and all participants provided written informed consent.
2.2. Laboratory tests
Standard protocols were applied for all measurements as described in previous studies. 31 , 32 , 33 Morning fasting venous blood samples were collected from the antecubital vein and transfused into vacuum tubes containing ethylenediaminetetraacetic acid (EDTA). Tubes were centrifuged at 3000 g for 10 min at room temperature and blood samples were processed into plasma, serum, and other samples. Serum samples were detected within 4 hours. As for serum albumin, it was measured using an autoanalyzer (Hitachi 747; Hitachi) according to the manufacturer's protocols at the central laboratory of the Kailuan General Hospital. Four categories of baseline serum albumin levels were classified according to quartiles of the study population (Q1: <44.6, Q2: 44.6–45.9, Q3: 46.0–47.9, Q4: ≥48.0 g/L).
2.3. Assessment of covariables
Face‐to‐face interviews were conducted by well‐trained staff using standardized questionnaires to collect information on demographics, socioeconomics, lifestyle factors, and personal medical history as potential risk factors. The status of coal mine dust exposure was obtained from the participants' work history recorded in the Kailuan Company. 34 , 35 In the questionnaire, family income per person was classified as <500 Chinese Yuan (CNY) per month, 500–1000 CNY per month, and ≥1,000 CNY per month, referred to the low‐income standard of Gross National Income (less than $1005) by the World Bank. Educational level was divided into four categories including illiterate or primary school, junior high school, senior high school, and college and above. Smoking status was classified as never, quit, occasional (<1 cigarette per day), and frequent smoking (≥1 cigarette per day). Alcohol drinking status was divided into ever drinkers or never drinkers. Coal dust exposure status was classified as non‐exposure or exposure. Bodyweight and height were measured with participants wearing light clothes and no shoes, and the body mass index (BMI) was calculated according to the equation that BMI = weight (kg)/height (m2). Enzyme‐linked immunosorbent assay was used to detect HBV infection status and the status was classified as HBsAg positive or negative. Serum C‐reactive protein (CRP) was measured using a commercial, high‐sensitivity nephelometry assay (Cias Latex CRP‐H, Kanto Chemical Co. Inc.).
2.4. Outcome ascertainment
Follow‐up of each participant began when baseline examinations were completed and ended at the first diagnosis of cancer, death, last documented follow‐up contact, or 31 December 2015, whichever came first. Incident cancer cases were identified mainly through biennial interviews. Medical records and death certificates were also used to add missing outcome information, which were attained from the medical insurance system of Tangshan city, the social security system of the Kailuan Group, and discharge summaries of affiliated hospitals. The diagnoses of incident cancers were confirmed by clinical experts according to comprehensive information of pathological, imaging diagnosis, and blood biochemical examination. Cancers were coded according to the International Classification of Diseases, Tenth Revision (ICD–10). Gastric, colorectal, liver, lung, and breast cancer were coded as C16, C18–19, C22, C34, and C50, respectively.
2.5. Statistical analyses
Baseline characteristics of the participants were described by percentages and compared using the Chi‐square test for categorical variables. For continuous variables, distributions were described by the mean (standard deviation) and compared using analysis of Variance. To evaluate the association between baseline serum albumin levels and incident cancer risk, cox proportional hazards regression models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Multivariable analyses were adjusted for potential confounders, including age (10‐year interval), gender (male, female), the education level (illiterate or primary school, junior high school, senior high school, and college and above), income status (<500 CNY, 500–1000 CNY, and ≥1000 CNY, per month), coal dust exposure status (non‐exposure or exposure), BMI (BMI < 18.5, 18.5 ≤ BMI < 25.0, 25.0 ≤ BMI < 30.0, BMI ≥ 30.0), the concentration of CRP (<0.5, 0.5–1.0, 1.1–2.5, >2.5, mg/L), smoking status (never, quit, <1 cigarette per day, ≥1 cigarette per day), alcohol drinking status (never, ever) and HBV infection status (HBsAg negative, HBsAg positive). Further subgroup analyses were conducted by subgroups of gender, BMI, and cigarette smoking status. For sensitivity analysis, we further excluded 563 participants who were diagnosed with cancers within the first 2 years after finishing the baseline examination of serum albumin. Moreover, the association between albumin concentrations and cancer risk were evaluated through restricted cubic spline (RCS) analysis based on multivariate Cox proportional hazard models. 36 Tests in this study were all two‐sided and p < 0.05 was considered statistically significant. All statistical analyses were conducted using the SAS statistical software, version 9.4 (SAS Institute Inc.).
3. RESULTS
3.1. Baseline characteristics of participants
Selected baseline characteristics of participants in the Kailuan cohort were listed in Table 1. This study consisted of 62,141 males and 19,920 females with a mean age of 50.32 ± 12.81 at baseline. More than half of the participants have been exposed to coal dust or were supposed to be overweight according to the WHO standard. The rate of current smoking and ever alcohol drinking were 32.04% and 40.58%, respectively, and the prevalence of HBV infection status was 2.96% in this study. Among the study population, mean serum concentrations of albumin were 46.47±7.90 g/L. Lower serum albumin levels were mainly observed among individuals above 60 years old (proportion of Q1: 35.30), individuals with education levels below primary school (proportion of Q1: 33.38) and those with income less than 500 CNY (proportion of Q1: 27.42).
TABLE 1.
Baseline characteristics of participants in the Kailuan cohort by serum albumin level
| Characteristics | Serum albumin (g/L) | χ 2 | p‐value | |||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Total | ||||
| Mean ± SD | No. (% a ) | No. (% a ) | No. (% a ) | No. (% a ) | No. (% b ) | |||
| Total | 46.47 ± 7.90 | <44.6 | 44.6–45.9 | 46.0–47.9 | ≥48.0 | |||
| Gender | ||||||||
| Female | 45.88 ± 8.07 | 5822 (29.23) | 3998 (20.07) | 6079 (30.52) | 4021 (20.19) | 19920 (24.27) | 904.9050 | <0.0001 |
| Male | 46.66 ± 7.84 | 14296 (23.01) | 10473 (16.85) | 18412 (29.63) | 18960 (30.51) | 62141 (75.73) | ||
| Age (year) | ||||||||
| <40 | 47.54 ± 7.30 | 2289 (14.73) | 1959 (12.60) | 4587 (29.51) | 6709 (43.16) | 15544 (18.94) | 3721.2277 | <0.0001 |
| 40–49 | 46.53 ± 6.94 | 4732 (22.52) | 3800 (18.08) | 6541 (31.13) | 5939 (28.26) | 21012 (25.61) | ||
| 50–59 | 46.34 ± 7.62 | 6970 (24.76) | 5410 (19.22) | 8640 (30.28) | 7127 (25.32) | 28147 (34.30) | ||
| ≥60 | 45.66 ± 9.66 | 6127 (35.30) | 3302 (19.02) | 4723 (27.21) | 3206 (18.47) | 17358 (21.15) | ||
| Income (yuan/month) | ||||||||
| <500 | 46.09 ± 6.01 | 4448 (27.42) | 2924 (18.02) | 4870 (30.02) | 3980 (24.53) | 16222 (20.80) | 825.3477 | <0.0001 |
| 500–1000 | 46.43 ± 8.91 | 10448 (24.20) | 8225 (19.05) | 13252 (30.70) | 11246 (26.05) | 43171 (55.36) | ||
| ≥1000 | 46.95 ± 7.01 | 4259 (22.90) | 2665 (14.33) | 5027 (27.03) | 6644 (35.73) | 18595 (23.84) | ||
| Education | ||||||||
| Illiterate or primary school | 45.80 ± 7.72 | 2251 (33.38) | 1194 (17.70) | 1823 (27.03) | 1476 (21.89) | 6744 (8.54) | 824.3316 | <0.0001 |
| Junior high school | 46.44 ± 7.64 | 12590 (24.06) | 9860 (18.84) | 15887 (30.35) | 14001 (26.75) | 52338 (66.31) | ||
| Senior high school | 46.97 ± 6.37 | 2663 (22.86) | 1687 (14.48) | 3208 (27.53) | 4093 (35.13) | 11651 (14.76) | ||
| College and above | 46.72 ± 11.93 | 1881 (22.94) | 1185 (14.45) | 2480 (30.24) | 2655 (32.37) | 8201 (10.39) | ||
| Smoking status | ||||||||
| Never | 46.38 ± 8.49 | 12263 (25.57) | 8817 (18.38) | 14205 (29.62) | 12679 (26.43) | 47964 (64.91) | 273.0513 | <0.0001 |
| Quit | 46.48 ± 9.11 | 549 (24.34) | 361 (16.00) | 699 (30.98) | 647 (28.68) | 2256 (3.05) | ||
| Occasional (<1 cigarette per day) | 47.26 ± 10.33 | 330 (19.86) | 264 (15.88) | 467 (28.10) | 601 (36.16) | 1662 (2.25) | ||
| Frequent (≥1 cigarette per day) | 46.68 ± 6.58 | 5016 (22.79) | 3559 (16.17) | 6583 (29.90) | 6856 (31.14) | 22014 (29.79) | ||
| Alcohol drinking status | ||||||||
| Never drinker | 46.35 ± 8.34 | 12170 (25.87) | 8767 (18.64) | 13867 (29.48) | 12235 (26.01) | 47039 (59.42) | 339.0104 | <0.0001 |
| Ever drinker | 46.70 ± 7.56 | 7278 (22.65) | 5196 (16.17) | 9591 (29.85) | 10066 (31.33) | 32131 (40.58) | ||
| Coal dust exposure status | ||||||||
| Non‐exposure | 46.46 ± 9.28 | 8650 (25.84) | 5813 (17.36) | 9726 (29.05) | 9288 (27.74) | 33477 (42.46) | 50.9545 | <0.0001 |
| Exposure | 46.51 ± 6.99 | 10720 (23.63) | 8102 (17.86) | 13650 (30.09) | 12890 (28.42) | 45362 (57.54) | ||
| BMI (kg/m2) | ||||||||
| BMI < 18.5 | 46.48 ± 3.09 | 490 (23.91) | 324 (15.81) | 595 (29.04) | 640 (31.23) | 2049 (2.51) | 78.7321 | <0.0001 |
| 18.5 ≤ BMI < 25.0 | 46.38 ± 8.49 | 9810 (25.41) | 6948 (18.00) | 11456 (29.68) | 10388 (26.91) | 38602 (47.31) | ||
| 25.0 ≤ BMI < 30.0 | 46.55 ± 7.39 | 7481 (23.83) | 5458 (17.39) | 9442 (30.08) | 9010 (28.70) | 31391 (38.47) | ||
| BMI ≥ 30.0 | 46.62 ± 7.92 | 2180 (22.82) | 1676 (17.55) | 2859 (29.93) | 2837 (29.70) | 9552 (11.71) | ||
| HBsAg status | ||||||||
| Negative | 46.50 ± 7.95 | 18205 (24.30) | 13293 (17.75) | 22345 (29.83) | 21062 (28.12) | 74905 (97.04) | 39.7021 | <0.0001 |
| Positive | 46.06 ± 3.45 | 678 (29.72) | 346 (15.17) | 623 (27.31) | 634 (27.79) | 2281 (2.96) | ||
| Crp | N.A. | 2.95 ± 9.21 | 2.63 ± 6.31 | 2.48 ± 5.21 | 2.55 ± 6.05 | 2.64 ± 6.79 | 108.6861 | <0.0001 |
For Crp, mean ± SD were calculated in each quartile, and t value and relevant p value were provided.
Abbreviation: BMI, body mass index; Crp, C‐reactive protein.
Row percentage.
Column percentage.
3.2. Risk associations
Results of Cox regression analyses on serum albumin levels and cancer risk were shown in Table 2. Till 31 December 2015, a total of 1482 new cancer cases occurred with a median follow‐up duration of 4.69 years. After multivariable adjustment for potential confounders (age, gender, education level, income, coal dust exposure, concentration of CRP, BMI, frequency of smoking, frequency of alcohol drinking, and HBsAg infection status), participants with higher serum albumin levels were at decreased cancer risk of 9%–27%, compared with those whose serum albumin concentrations were below 44.6 g/L (Q2 vs. Q1: HR 0.91, 95% CI 0.78–1.07; Q3 vs. Q1: HR 0.80, 95% CI 0.70–0.92; Q4 vs. Q1: HR 0.73, 95% CI 0.63–0.85). With respect to cancers of specific types, albumin levels were significantly inversely associated with risks of lung cancer (Q2 vs. Q1: HR 0.82, 95% CI 0.60–1.12; Q3 vs. Q1: HR 0.68, 95% CI 0.51–0.90; Q4 vs. Q1: HR 0.70, 95% CI 0.52–0.95), colorectal cancer (Q2 vs. Q1: HR 0.87, 95% CI 0.57–1.34; Q3 vs. Q1: HR 0.95, 95% CI 0.65–1.38; Q4 vs. Q1: HR 0.43, 95% CI 0.26–0.72), and liver cancer (Q2 vs. Q1: HR 0.55, 95% CI 0.31–0.95; Q3 vs. Q1: HR 0.44, 95% CI 0.26–0.73; Q4 vs. Q1: HR 0.59, 95% CI 0.36–0.95). In the sensitivity analysis, higher albumin levels remained to be associated with decreased overall cancer risks (Q2 vs. Q1: HR 0.93, 95% CI 0.76–1.13; Q3 vs. Q1: HR 0.83, 95% CI 0.69–0.99; Q4 vs. Q1: HR 0.75, 95% CI 0.61–0.91), and significantly lower risk of liver cancer was found related to higher albumin levels (Q2 vs. Q1: HR 0.49, 95% CI 0.25–0.97; Q3 vs. Q1: HR 0.40, 95% CI 0.22–0.74; Q4 vs. Q1: HR 0.41, 95% CI 0.21–0.78). However, no significant associations between albumin levels and risks of lung and colorectal cancer were observed after excluding participants who had new cancer diagnoses within 2 years since enrollment.
TABLE 2.
The association between serum albumin and cancer risk, Kailuan cohort
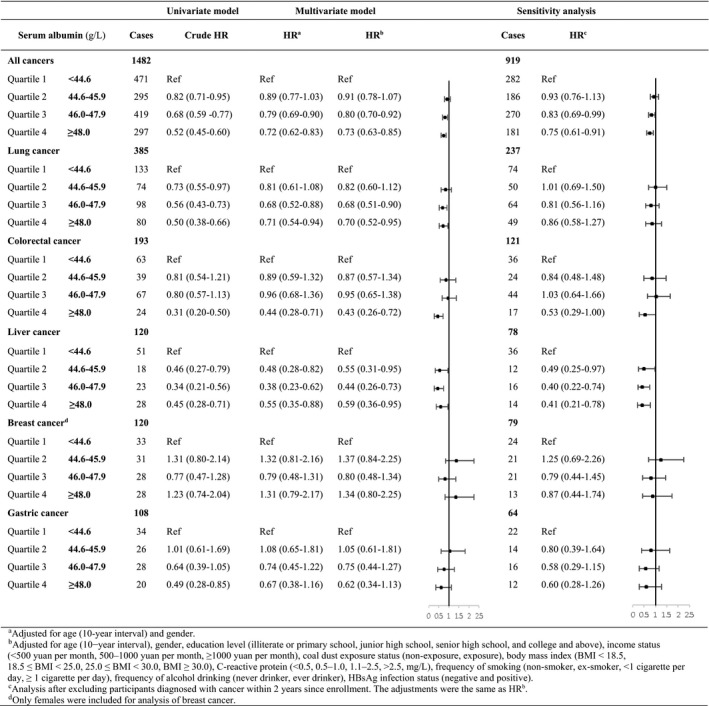
Subgroup analyses by gender, BMI, and smoking status were displayed in Table 3. The associations between albumin levels and cancer risks were not significant among females, while for males, inverse associations remained. In general, individuals with higher albumin levels were at decreased overall cancer risk among both the normal‐weight and overweight population. However, for lung cancer risk, a significant inverse association was only observed among normal weight individuals, while for risk of colorectal cancer, the inverse association was only significant among the overweight. Moreover, high levels of albumin were found to be associated with lung, colorectal, and liver cancer especially among non‐smokers.
TABLE 3.
Hazard ratios (HRs) and 95% confidence intervals (CIs) of cancer risk in relationship to serum albumin by gender, body mass index (BMI), and smoking status, Kailuan cohort
| Serum albumin (g/L) | Gender | BMI | Smoking status | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | Normal weight (BMI < 25 kg/m2) | Overweight (BMI ≥ 25 kg/m2) | Non‐smoker | Ever smoker | ||
| All cancers | |||||||
| Quartile 1 | <44.6 | Ref | Ref | Ref | Ref | Ref | Ref |
| Quartile 2 | 44.6–45.9 | 0.86 (0.72–1.03) | 1.08 (0.81–1.44) | 0.89 (0.72–1.11) | 0.94 (0.76–1.17) | 0.93 (0.77–1.12) | 0.89 (0.69–1.16) |
| Quartile 3 | 46.0–47.9 | 0.82 (0.70–0.97) | 0.80 (0.61–1.06) | 0.83 (0.68–1.01) | 0.78 (0.64–0.96) | 0.77 (0.65–0.92) | 0.86 (0.69–1.08) |
| Quartile 4 | ≥48.0 | 0.73 (0.61–0.87) | 0.85 (0.61 −1.18) | 0.74 (0.59–0.92) | 0.73 (0.58–0.91) | 0.75 (0.61–0.91) | 0.73 (0.56–0.93) |
| Lung cancer | |||||||
| Quartile 1 | <44.6 | Ref | Ref | Ref | Ref | Ref | Ref |
| Quartile 2 | 44.6–45.9 | 0.76 (0.54–1.07) | 1.18 (0.56–2.48) | 0.72 (0.47–1.09) | 0.97 (0.62–1.52) | 0.82 (0.55–1.24) | 0.81 (0.51–1.29) |
| Quartile 3 | 46.0–47.9 | 0.70 (0.52–0.95) | 0.52 (0.22–1.24) | 0.63 (0.43–0.93) | 0.75 (0.49–1.14) | 0.51 (0.34–0.77) | 0.89 (0.60–1.32) |
| Quartile 4 | ≥48.0 | 0.69 (0.50–0.96) | 0.85 (0.34–2.11) | 0.60 (0.39–0.92) | 0.84 (0.54–1.31) | 0.71 (0.47–1.08) | 0.71 (0.45–1.12) |
| Colorectal cancer | |||||||
| Quartile 1 | <44.6 | Ref | Ref | Ref | Ref | Ref | Ref |
| Quartile 2 | 44.6–45.9 | 0.74 (0.45–1.21) | 1.61 (0.62–4.18) | 1.16 (0.62–2.18) | 0.67 (0.37–1.23) | 0.78 (0.45–1.33) | 1.07 (0.52–2.21) |
| Quartile 3 | 46.0–47.9 | 0.86 (0.57–1.31) | 1.36 (0.55–3.35) | 1.28 (0.73–2.23) | 0.73 (0.44–1.22) | 0.94 (0.59–1.48) | 0.97 (0.50–1.86) |
| Quartile 4 | ≥48.0 | 0.40 (0.23–0.69) | 0.61 (0.16–2.32) | 0.78 (0.39–1.53) | 0.22 (0.10–0.51) | 0.39 (0.20–0.76) | 0.53 (0.23–1.21) |
| Breast cancer a | |||||||
| Quartile 1 | <44.6 | N.A. | Ref | Ref | Ref | Ref | N.A. |
| Quartile 2 | 44.6–45.9 | N.A. | 1.37 (0.84–2.25) | 2.04 (0.88–4.71) | 1.14 (0.60–2.15) | 1.42 (0.86–2.33) | N.A. |
| Quartile 3 | 46.0–47.9 | N.A. | 0.80 (0.48–1.34) | 1.34 (0.58–3.07) | 0.57 (0.28–1.16) | 0.83 (0.49–1.39) | N.A. |
| Quartile 4 | ≥48.0 | N.A. | 1.34 (0.80–2.25) | 1.91 (0.81–4.52) | 1.13 (0.57–2.22) | 1.39 (0.82–2.34) | N.A. |
| Liver cancer | |||||||
| Quartile 1 | <44.6 | Ref | Ref | Ref | Ref | Ref | Ref |
| Quartile 2 | 44.6–45.9 | 0.47 (0.26–0.87) | 1.71 (0.34–8.60) | 0.45 (0.18–1.11) | 0.63 (0.31–1.27) | 0.64 (0.33–1.27) | 0.40 (0.15–1.06) |
| Quartile 3 | 46.0–47.9 | 0.44 (0.26–0.75) | 0.34 (0.04–3.25) | 0.44 (0.20–0.97) | 0.45 (0.23–0.87) | 0.36 (0.18–0.75) | 0.55 (0.27–1.13) |
| Quartile 4 | ≥48.0 | 0.58 (0.35–0.95) | 0.73 (0.07–7.15) | 0.53 (0.24–1.15) | 0.63 (0.33–1.17) | 0.52 (0.26–1.02) | 0.68 (0.34–1.37) |
| Gastric cancer b | |||||||
| Quartile 1 | <44.6 | Ref | N.A. | Ref | Ref | Ref | Ref |
| Quartile 2 | 44.6–45.9 | 1.20 (0.66–2.18) | N.A. | 1.06 (0.50–2.23) | 1.04 (0.47–2.30) | 1.22 (0.62–2.40) | 0.80 (0.32–2.01) |
| Quartile 3 | 46.0–47.9 | 0.91 (0.52–1.62) | N.A. | 0.85 (0.42–1.71) | 0.65 (0.29–1.44) | 0.62 (0.30–1.29) | 0.94 (0.44–2.02) |
| Quartile 4 | ≥48.0 | 0.78 (0.42–1.47) | N.A. | 0.57 (0.24–1.34) | 0.67 (0.29–1.58) | 0.76 (0.35–1.63) | 0.47 (0.18–1.24) |
Adjusted for age (10‐year interval), gender, education level (illiterate or primary school, junior high school, senior high school, and college and above), income status (<500 yuan per month, 500–1000 yuan per month, ≥1000 yuan per month), coal dust exposure status (non‐exposure, exposure), BMI (BMI <18.5, 18.5 ≤ BMI <25.0, 25.0 ≤ BMI <30.0, BMI ≥30.0), C‐reactive protein (<0.5, 0.5–1.0, 1.1–2.5, >2.5, mg/L), frequency of smoking (non‐smoker, ex‐smoker, <1 cigarette per day, ≥1 cigarette per day), frequency of alcohol drinking (never drinker, ever drinker), HBsAg infection status (negative and positive). Grouping variables were excluded for each subgroup.
Only females were included for analysis of breast cancer. For female ever smokers, the analysis was infeasible due to the limitation of subjects’ number.
The analysis of gastric cancer risk among female was infeasible due to the limitation of cancer cases.
The RCS model showed a significant linear association between serum albumin concentrations and overall cancer risk among the subjects (p‐overall < 0.0001, p‐nonlinear = 0.3716). As the 50th quintile of albumin concentrations (46 g/L) was chosen to be the reference, the HRs of overall cancer risk related to albumin levels rise sharply when albumin levels were below 46 g/L. Concerning each cancer type, there was a linear association between albumin and liver cancer risk (p‐overall = 0.0002, p‐nonlinear = 0.1807), and a slightly significant association was also observed for lung cancer (p‐overall = 0.0456, p‐nonlinear = 0.2113) (Figure 1).
FIGURE 1.
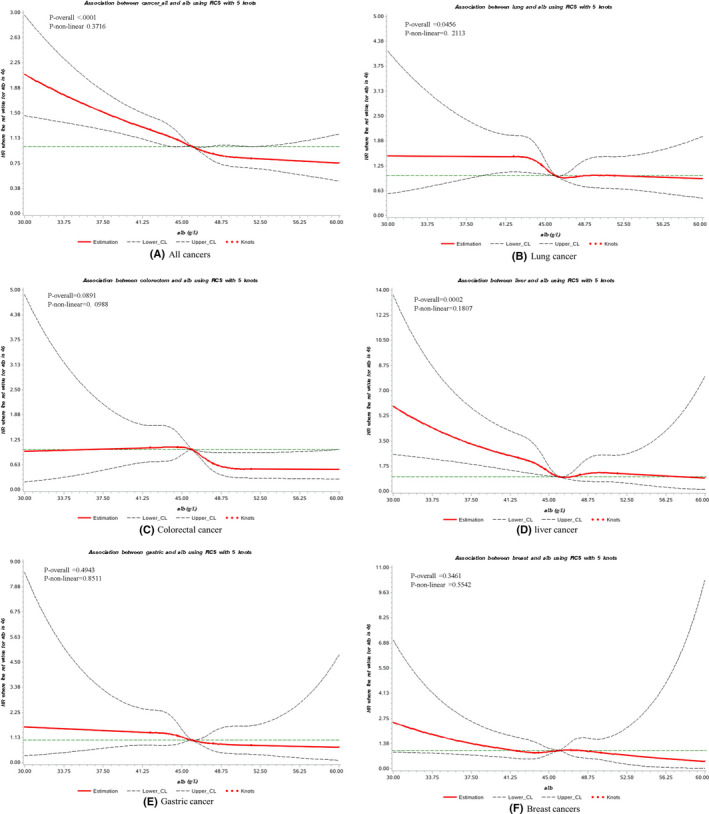
Cubic spline graph of the adjusted HR (represented by solid line) and 95% CI (represented by the dotted lines) for the association between serum albumin and risk of primary cancer in Kailuan cohort, 2006–2013. (A) All cancers, (B) lung cancer, (C) colorectal cancer, (D) liver cancer, (E) gastric cancer, (F) breast cancer. Knots: 42, 44.6,46, 48 and 51.5 (95th) of the distribution of serum albumin concentration (g/L); Reference: 46 g/L, 50th of the distribution of serum albumin concentration. Analyses are adjusted for age 10−yearinterval, gender, education level (illiterate or primary school, junior high school, senior high school, and college and above), income status (<500 yuan per month, 500–1000 yuan per month, ≥1000 yuan per month), coal dust exposure status (non‐exposure, exposure), BMI (BMI <18.5, 18.5 ≤ BMI <25.0, 25.0 ≤ BMI <30.0, BMI ≥30.0), C‐reactive protein (<0.5, 0.5–1.0, 1.1–2.5, >2.5, mg/L), smoking status (never, quit, <1 cigarette per day, ≥1 cigarette per day), alcohol drinking status (never, ever) and HBV infection status (HBsAg negative, HBsAg positive). BMI, body mass index; HBV, hepatitis B virus
4. DISCUSSION
Based on a large prospective cohort study, this analysis displayed the associations between pre‐diagnostic serum albumin and cancer risks. After controlling for pertinent risk factors, results suggested that serum albumin was inversely associated with overall cancer risk with a significant linear association. When analyzed by cancer type, the association between albumin and overall cancer risk was mainly attributed to lung, colorectal and especially, liver cancer. Moreover, a significant inverse linear relationship was observed between serum albumin concentration and liver cancer risk.
Our findings suggested that pre‐diagnostic albumin levels were inversely associated with overall cancer risk. This inverse association was in line with a recent study based on a UK database of adult primary care patients. 21 Additionally, we observed a significant linear relationship between serum albumin concentrations and cancer risk. Referred to the median of albumin concentrations, HRs of overall cancer risk increased sharply when albumin concentrations were below 46 g/L.
For decades, it has been confirmed that albumin was associated with the overall mortality, 6 , 8 and especially the prognosis of cancer, 7 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 whereas only a few studies were conducted concerning cancer risk. Hypotheses for decreased albumin concentration with increased cancer risk raised by the previous study were varied from malnutrition, increased albumin consumption due to the expression of cancer cells, to antioxidant properties and inhibition of albumin synthesis caused by systemic inflammation. First, albumin is one of the various inflammation‐related markers. In previous studies, pooled analyses of inflammation‐related markers including the ratio of albumin to globulin, 37 C‐reactive protein to albumin, 38 albumin‐to‐alkaline phosphatase, 39 and albumin to fibrinogen 40 were conducted concerning cancer prognosis. For these indexes, the prognostic value of clinical significance has already been proved. Considering that serum albumin is a negative acute‐phase protein, inflammation could suppress albumin synthesis and lead to low albumin concentrations. A longitudinal study has observed decreased albumin in women with early‐stage ovarian cancer from a mean of 51.3–40.9 g/L (p < 0.001). 41 Though few studies explained the mechanism of the influence of serum albumin on cancer risk and mortality, a previous study conducted among dialysis patients suggested that the influence of serum albumin on mortality was partly explained by the inflammatory pathway. 42 Besides, albumin is supposed to be an important extracellular antioxidant. 18 In animal experiments, serum albumin was shown to be protective through reducing arterial reactivity in endotoxemia as an antioxidant and improving the anti‐inflammatory effect. However, no further research explored the mechanism of albumin in relation to cancer in the human body. Two previous studies have explored the association between cancer risk, mortality, and metabolic markers including albumin, bilirubin, and uric acid, which were all considered as non‐nutrient antioxidants of physiologic importance. A nested case‐control study showed that the risk of colon cancer increased from the highest to the lowest quartiles of serum albumin (p trend = 0.05) whereas no statistically significant dose‐response trend was observed for bilirubin or uric acid. 22 Another cohort study also reported that albumin and uric acid levels were associated with breast cancer risk. The theory of extracellular antioxidants was still insufficient and required further exploration. Moreover, cancer is recognized as a kind of consumptive disease with clinical symptoms including weight loss in the late stages. It was supposed that a lower level of serum albumin associated with higher cancer risk may indicate the influence on nutrition intake before cancer diagnoses. However, there was no prior hypothesis concerning nutrition intake and cancer risk and this just remains as a supposition.
With respect to liver cancer risk, we observed an inverse association with albumin levels, and after excluding participants who were diagnosed with cancer within 2 years since enrollment, a more significant association was observed. Significant linear associations were also suggested by the RCS model for liver cancer. Though there was no previous research exploring the association between albumin and liver cancer risk, it has been confirmed that albumin was produced by hepatocytes and abnormal albumin levels were in relation to hepatic dysfunction. 43 , 44
For colorectal cancer, we observed an inverse association between pre‐diagnostic albumin levels and colorectal cancer risk, which was consistent with the results of several previous studies. 22 , 23 , 45 Nevertheless, there were also two studies reporting nonsignificant associations between albumin and colon cancer risk. 25 , 26 Albumin was suggested to play a role in the resistance of the gastrointestinal mucosal membrane to lipid peroxidation. 46 Therefore, it is believed that albumin is closely related to the development of gastrointestinal cancer and many studies focused on its association with risks of gastrointestinal cancer, especially colon cancer. 22 , 23 , 25 , 26 Furthermore, a previous study showed that hypoalbuminemia in patients with colorectal cancer reflects both increased nutritional risk and greater systemic inflammatory response. 47
For lung cancer, the inverse association was observed between lung cancer risk and albumin concentrations. However, the inverse association converted to nonsignificant after excluding participants who had cancer diagnoses within 2 years since recruitment. Besides, there was also a cohort study reporting nonsignificant results between albumin and lung cancer. 29 Though the physiological and functional properties of the lung have little to do with albumin, the association between albumin and lung cancer risk may reflect systemic inflammation. However, the inverse association between lung cancer risk and serum albumin was novel and should be interpreted cautiously.
In our analysis, nonsignificant associations were observed for cancer subtypes including gastric and breast cancer, which might be related to the limited number of cancer cases and low proportion of females in the study population. The lack of association between breast cancer risk and albumin in our study was consistent with findings of the AMORIS study, 27 whereas a recent cohort study reported a weak inverse relationship, 24 with the HRs being 0.71 (95% CI 0.51–0.99) for participants in the highest albumin quartile. However, there was no previous study reporting the association related to gastric cancer.
Taken together, the positive results in our analysis could only indicate the association between serum albumin and cancer risk. Lack of research on the basic biological mechanism, these results should be interpreted with caution and further evidence on biological mechanism was required.
Since gender, smoking status, and BMI were suggested to be closely associated with cancer risk, 2 we further conducted subgroup analyses by these factors. Significant inverse associations were observed especially for the non‐smokers and males. Concerning lung cancer risk, an inverse association was observed among normal‐weight individuals, whereas for colorectal cancer, a significant association was observed among the overweight. As suggested in studies on BMI and cancer risk, obesity was suggested as a risk factor of colorectal cancer, 48 whereas a protective effect was indicated for lung cancer. 5 Taken together, our findings were consistent with the significant association between obesity and cancer risks. As the established risk factors played a leading role in the development of cancer, the effect of albumin was still significant.
The present study had several limitations. First, the follow‐up time in the cohort was 4.69 years, which was relatively short to identify new cancer cases. An evaluation of weaker associations, for example, between albumin levels and gastric cancer risk was precluded due to low case numbers. Besides, analyses by cancer types and subgroups were also restricted. Second, it is known that blood biochemistry markers would undergo successive changes individually over time. However, with limited follow‐up duration, the albumin concentration used in this analysis was at one single time point, and changes of cancer risk along with albumin concentrations were not analyzed. Third, most participants enrolled in this study were baseline healthy subjects, and the albumin concentrations mainly ranged between 35 and 50 g/L. Hypoproteinemia was unable to be analyzed separately with only 0.20% (167/82061) of albumin concentrations below 35 g/L. Fourth, since the proportion of females in this study was less than 25%, these results were limited when interpreting associations for females. Last but not the least, though our study added to evidence that albumin was significantly associated with total cancer risk as well as risks of lung, colorectal, and liver cancer, the biological plausibility was still moderate and the clinical significance remains to be elucidated. The positive results with serum albumin were novel and should be interpreted cautiously.
On the other hand, this study had strengths over previous studies. This analysis was based on a population‐based cohort with well‐organized follow‐up. Adjustment for potential confounders was comprehensively performed including adjustment for C‐reactive protein, which helped when exploring weak associations. It is noteworthy that this study first conducted RCS analysis to analyze the nonlinear relationship between pre‐diagnostic serum albumin and cancer risk.
In conclusion, our findings suggest that pre‐diagnostic serum albumin is inversely and linearly associated with cancer risk among the Chinese population. And the significant association between albumin and overall cancer risk is mainly attributed to lung, colorectal and especially, liver cancer. This study provides evidence that albumin may be valuable to the prediction and stratification of cancer risk in the general population. Since albumin concentration is widely used in physical examination, this marker is easy to obtain and well accepted by the population. When combined with other detection indexes, it becomes an indicator of both feasibility and considerable economic value before the invasive examination. Nevertheless, the biological mechanism and clinical significance remain to be elucidated. Population studies with longer follow‐up time as well as experimental studies are further required.
AUTHOR CONTRIBUTIONS
Ni Li, Min Dai, Jie He, and Shouling Wu were responsible for the study concepts and design. Zhuoyu Yang, Yadi Zheng, Zheng Wu, Yan Wen, Gang Wang, and Shuohua Chen contributed to data acquisition. Fengwei Tan and Jiang Li were involved in quality control and data interpretation. Zhuoyu Yang, Yadi Zheng, Zheng Wu, and Yan Wen were responsible for paper preparation. Statistical analyses were performed by Zhuoyu Yang, Yadi Zheng, Zheng Wu, and Yan Wen. Ni Li and Jie He contributed to manuscript editing. All authors approved the final version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The studies involving human participants were reviewed and approved by the ethics committee of the Kailuan General Hospital. The patients/participants provided their written informed consent to participate in this study. This study was performed in accordance with the Declaration of Helsinki.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGMENTS
The authors thank all the participants in the study and the members of the survey team from the Kailuan Cohort Study for their painstaking efforts to conduct the baseline survey and follow‐up.
Zhuoyu Yang and Yadi Zheng should be considered joint first author.
Funding information
This study was funded by grants from the National Key Research and Development Program of China (no. 2018YFC1315000), Non‐profit Central Research Institute Fund of Chinese Academy of Medical Sciences (no. 2019PT320027, 2020PT330001, 2019PT320023, 2018RC330002), Training Program Foundation for the Talents in Beijing City (no. 2017000021223TD05), National Natural Science Foundation of China (no. 81673265).
Contributor Information
Ni Li, Email: nli@cicams.ac.cn.
Jie He, Email: hejie@cicams.ac.cn.
DATA AVAILABILITY STATEMENT
The datasets for this manuscript are not publicly available under the regulation of both the National Cancer Center of China and the Kailuan Group. However, data are available from the authors upon reasonable request. Requests to access the datasets should be directed to JH, hejie@cicams.ac.cn and SLW, drwusl@163.com.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General's report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179(4):403‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site‐specific cancer risk: a comprehensive dose‐response meta‐analysis. Br J Cancer. 2015;112(3):580‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song CI, Lv J, Liu Y, et al. Associations between hepatitis B virus infection and risk of all cancer types. JAMA Netw Open. 2019;2(6):e195718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body‐mass index and incidence of cancer: a systematic review and meta‐analysis of prospective observational studies. Lancet (London, England). 2008;371(9612):569‐578. [DOI] [PubMed] [Google Scholar]
- 6. Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet (London, England). 1989;2(8677):1434‐1436. [DOI] [PubMed] [Google Scholar]
- 7. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klonoff‐Cohen H, Barrett‐Connor EL, Edelstein SL. Albumin levels as a predictor of mortality in the healthy elderly. J Clin Epidemiol. 1992;45(3):207‐212. [DOI] [PubMed] [Google Scholar]
- 9. Ataseven B, du Bois A, Reinthaller A, et al. Pre‐operative serum albumin is associated with post‐operative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol. 2015;138(3):560‐565. [DOI] [PubMed] [Google Scholar]
- 10. Chandrasinghe PC, Ediriweera DS, Kumarage SK, Deen KI. Pre‐operative hypoalbuminaemia predicts poor overall survival in rectal cancer: a retrospective cohort analysis. BMC Clin Pathol. 2013;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lis CG, Grutsch JF, Vashi PG, Lammersfeld CA. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr. 2003;27(1):10‐15. [DOI] [PubMed] [Google Scholar]
- 12. Ouyang X, Dang Y, Zhang F, Huang Q. Low serum albumin correlates with poor survival in gastric cancer patients. Clin Lab. 2018;64(3):239‐245. [DOI] [PubMed] [Google Scholar]
- 13. Parker D, Bradley C, Bogle SM, et al. Serum albumin and CA125 are powerful predictors of survival in epithelial ovarian cancer. Br J Obstet Gynaecol. 1994;101(10):888‐893. [DOI] [PubMed] [Google Scholar]
- 14. Shen Q, Liu WU, Quan HU, et al. Prealbumin and lymphocyte‐based prognostic score, a new tool for predicting long‐term survival after curative resection of stage II/III gastric cancer. Br J Nutr. 2018;120(12):1359‐1369. [DOI] [PubMed] [Google Scholar]
- 15. Xu S‐S, Li S, Xu H‐X, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol. 2020;26(8):828‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamashita K, Ushiku H, Katada N, et al. Reduced preoperative serum albumin and absence of peritoneal dissemination may be predictive factors for long‐term survival with advanced gastric cancer with positive cytology test. Eur J Surg Oncol. 2015;41(10):1324‐1332. [DOI] [PubMed] [Google Scholar]
- 17. Stehle G, Sinn H, Wunder A, et al. Plasma protein (albumin) catabolism by the tumor itself–implications for tumor metabolism and the genesis of cachexia. Crit Rev Oncol/Hematol. 1997;26(2):77‐100. [DOI] [PubMed] [Google Scholar]
- 18. Halliwell B. Albumin—an important extracellular antioxidant? Biochem Pharmacol. 1988;37(4):569‐571. [DOI] [PubMed] [Google Scholar]
- 19. Ching S, Ingram D, Hahnel R, Beilby J, Rossi E. Serum levels of micronutrients, antioxidants and total antioxidant status predict risk of breast cancer in a case control study. J Nutr. 2002;132(2):303‐306. [DOI] [PubMed] [Google Scholar]
- 20. Lai C‐C, You J‐F, Yeh C‐Y, et al. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis. 2011;26(4):473‐481. [DOI] [PubMed] [Google Scholar]
- 21. Merriel SW, Carroll R, Hamilton F, Hamilton W. Association between unexplained hypoalbuminaemia and new cancer diagnoses in UK primary care patients. Fam Pract. 2016;33(5):449‐452. [DOI] [PubMed] [Google Scholar]
- 22. Ko WF, Helzlsouer KJ, Comstock GW. Serum albumin, bilirubin, and uric acid and the anatomic site‐specific incidence of colon cancer. J Natl Cancer Inst. 1994;86(24):1874‐1875. [DOI] [PubMed] [Google Scholar]
- 23. Ghuman S, Van Hemelrijck M, Garmo H, et al. Serum inflammatory markers and colorectal cancer risk and survival. Br J Cancer. 2017;116(10):1358‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kühn T, Sookthai D, Graf ME, et al. Albumin, bilirubin, uric acid and cancer risk: results from a prospective population‐based study. Br J Cancer. 2017;117(10):1572‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knekt P, Hakulinen T, Leino A, Heliövaara M, Reunanen A, Stevens R. Serum albumin and colorectal cancer risk. Eur J Clin Nutr. 2000;54(6):460‐462. [DOI] [PubMed] [Google Scholar]
- 26. Prizment AE, Anderson KE, Visvanathan K, Folsom AR. Association of inflammatory markers with colorectal cancer incidence in the atherosclerosis risk in communities study. Cancer Epidemiol Biomark Prev. 2011;20(2):297‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wulaningsih W, Holmberg L, Garmo H, et al. Prediagnostic serum inflammatory markers in relation to breast cancer risk, severity at diagnosis and survival in breast cancer patients. Carcinogenesis. 2015;36(10):1121‐1128. [DOI] [PubMed] [Google Scholar]
- 28. Van Hemelrijck M, Jungner I, Walldius G, et al. Risk of prostate cancer is not associated with levels of C‐reactive protein and other commonly used markers of inflammation. Int J Cancer. 2011;129(6):1485‐1492. [DOI] [PubMed] [Google Scholar]
- 29. Sprague BL, Trentham‐Dietz A, Klein BE, et al. Physical activity, white blood cell count, and lung cancer risk in a prospective cohort study. Cancer Epidemiol Biomark Prev. 2008;17(10):2714‐2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwartz GG, Tretli S, Vos L, Robsahm TE. Prediagnostic serum calcium and albumin and ovarian cancer: a nested case‐control study in the Norwegian Janus Serum Bank Cohort. Cancer Epidemiol. 2017;49:225‐230. [DOI] [PubMed] [Google Scholar]
- 31. Wang F, Wu S, Song Y, et al. Waist circumference, body mass index and waist to hip ratio for prediction of the metabolic syndrome in Chinese. Nutr Metab Cardiovasc Dis. 2009;19(8):542‐547. [DOI] [PubMed] [Google Scholar]
- 32. Wu S, Li Y, Jin C, et al. Intra‐individual variability of high‐sensitivity C‐reactive protein in Chinese general population. Int J Cardiol. 2012;157(1):75‐79. [DOI] [PubMed] [Google Scholar]
- 33. Wu S, Huang Z, Yang X, et al. Prevalence of ideal cardiovascular health and its relationship with the 4‐year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes. 2012;5(4):487‐493. [DOI] [PubMed] [Google Scholar]
- 34. Feng X, Wang G, Li NI, et al. The association between fasting blood glucose and the risk of primary liver cancer in Chinese males: a population‐based prospective study. Br J Cancer. 2017;117(9):1405‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lyu Z, Li NI, Wang G, et al. Independent and joint associations of blood lipids and lipoproteins with lung cancer risk in Chinese males: a prospective cohort study. Int J Cancer. 2019;144(12):2972‐2984. [DOI] [PubMed] [Google Scholar]
- 36. Desquilbet L, Mariotti F. Dose‐response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037‐1057. [DOI] [PubMed] [Google Scholar]
- 37. Li J, Wang Y, Wu Y, Li J, Che G. Prognostic value of pretreatment albumin to globulin ratio in lung cancer: a meta‐analysis. Nutr Cancer. 2021;73(1):75‐82. [DOI] [PubMed] [Google Scholar]
- 38. Zang Y, Fan Y, Gao Z. Pretreatment C‐reactive protein/albumin ratio for predicting overall survival in pancreatic cancer: a meta‐analysis. Medicine. 2020;99(23):e20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xie H, Wei L, Tang S, Gan J. Prognostic value of pretreatment albumin‐to‐alkaline phosphatase ratio in cancer: a meta‐analysis. Biomed Res Int. 2020;2020:6661097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun DW, An L, Lv GY. Albumin‐fibrinogen ratio and fibrinogen‐prealbumin ratio as promising prognostic markers for cancers: an updated meta‐analysis. World J Surg Oncol. 2020;18(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwartz GG, Tretli S, Klug MG, Robsahm TE. Women who develop ovarian cancer show an increase in serum calcium and a decrease in serum albumin. A longitudinal study in the Janus Serum Bank Cohort. Gynecol Oncol. 2020;159(1):264‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr. 2009;19(2):127‐135. [DOI] [PubMed] [Google Scholar]
- 43. Spinella R, Sawhney R, Jalan R. Albumin in chronic liver disease: structure, functions and therapeutic implications. Hep Intl. 2016;10(1):124‐132. [DOI] [PubMed] [Google Scholar]
- 44. Garcia‐Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology (Baltimore, MD). 2013;58(5):1836‐1846. [DOI] [PubMed] [Google Scholar]
- 45. Stevens RG, Jones DY, Micozzi MS, Taylor PR. Body iron stores and the risk of cancer. N Engl J Med. 1988;319(16):1047‐1052. [DOI] [PubMed] [Google Scholar]
- 46. Balasubramanian KA, Nalini S, Cheeseman KH, Slater TF. Nonesterified fatty acids inhibit iron‐dependent lipid peroxidation. Biochem Biophys Acta. 1989;1003(3):232‐237. [DOI] [PubMed] [Google Scholar]
- 47. Almasaudi AS, Dolan RD, Edwards CA, McMillan DC. Hypoalbuminemia reflects nutritional risk, body composition and systemic inflammation and is independently associated with survival in patients with colorectal cancer. Cancers. 2020;12(7):1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8(1):e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets for this manuscript are not publicly available under the regulation of both the National Cancer Center of China and the Kailuan Group. However, data are available from the authors upon reasonable request. Requests to access the datasets should be directed to JH, hejie@cicams.ac.cn and SLW, drwusl@163.com.


