Key Points
Question
Among patients with atherosclerotic cardiovascular disease (ASCVD), how are lipids managed in real-world clinical practice?
Findings
GOULD is a prospective observational registry study tracking lipid-lowering therapies for patients with ASCVD who had baseline low-density lipoprotein cholesterol level of 70 mg/dL or more or were taking a proprotein convertase subtilisin/kexin type 9 inhibitor. Over a 2-year period, only modest intensifications of lipid-lowering therapies were observed (17.1%), with only 1 in 3 patients achieving a low-density lipoprotein cholesterol level less than 70 mg/dL at 2 years.
Meaning
Further lipid-lowering therapy intensification efforts are needed to achieve optimal cholesterol management in patients with ASCVD.
This cohort study observed patients with low-density lipoprotein cholesterol levels greater than 70 mg/dL or proprotein convertase subtilisin/kexin type 9 inhibitor use for 2 years to determine treatment patterns.
Abstract
Importance
Guidelines for patients with atherosclerotic cardiovascular disease (ASCVD) recommend intensive statin therapy and adding nonstatin therapy if low-density lipoprotein cholesterol (LDL-C) levels are 70 mg/dL or more. Compliance with guidelines is often low.
Objective
To track LDL-C treatment patterns in the US over 2 years.
Design, Setting, and Participants
GOULD is a prospective observational registry study involving multiple centers. Patients with ASCVD receiving any lipid-lowering therapy (LLT) were eligible. Between December 2016 and July 2018, patients were enrolled in 1 of 3 cohorts: (1) those currently receiving proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) and 2 groups not receiving PCSK9i drugs, with (2) LDL-C levels of 100 mg/dL or more or (3) LDL-C levels of 70 to 99 mg/dL. Patients had medical record reviews and telephone interviews every 6 months. Analysis was done on data collected as of October 5, 2020.
Main Outcomes and Measures
The primary outcome was the change in LLT use in 2 years. Secondary outcomes included the number of LDL-C measurements, LDL-C levels, and responses to structured physician and patient questionnaires over 2 years.
Results
A total of 5006 patients were enrolled (mean [SD] age, 67.8 [9.9] years; 1985 women [39.7%]; 4312 White individuals [86.1%]). At 2 years, 885 (17.1%) had LLT intensification. In the cohorts with LDL-C levels of 100 mg/dL or more and 70 to 99 mg/dL, LLT intensification occurred in 403 (22.4%) and 383 (14.4%), respectively; statins were intensified in 115 (6.4%) and 168 (6.3%), ezetimibe added in 123 (6.8%) and 118 (4.5%), and PCSK9i added in 114 (6.3%) and 58 (2.2%), respectively. In the PCSK9i cohort, 508 of 554 (91.7%) were still taking PCSK9i at 2 years. Lipid panels were measured at least once over 2 years in 3768 patients (88.5%; PCSK9i cohort, 492 [96.1%]; LDL-C levels ≥100 mg/dL or more, 1294 [85.9%]; 70-99 mg/dL, 1982 [88.6%]). Levels of LDL-C fell from medians (interquartile ranges) of 120 (108-141) mg/dL to 95 (73-118) mg/dL in the cohort with LDL-C levels of 100 mg/dL or more, 82 (75-89) to 77 (65-90) mg/dL in the cohort with LDL-C levels of 70 to 99 mg/dL, and 67 (42-104) mg/dL to 67 (42-96) mg/dL in the PCSK9i cohort. Levels of LDL-C less than 70 mg/dL at 2 years were achieved by 308 patients (21.0%) and 758 patients (33.9%) in the cohorts with LDL-C levels of 100 mg/dL or more and 70 to 99 mg/dL, respectively, and 272 patients (52.4%) in the PCSK9i cohort. At 2 years, practice characteristics were associated with more LLT intensification (teaching vs nonteaching hospitals, 148 of 589 [25.1%] vs 600 of 3607 [16.6%]; lipid protocols or none, 359 of 1612 [22.3%] vs 389 of 2584 [15.1%]; cardiology, 452 of 2087 [21.7%] vs internal or family medicine, 204 of 1745 [11.7%] and other, 92 of 364 [25.3%]; all P < .001) and achievement of LDL-C less than 70 mg/dL (teaching vs nonteaching hospitals, 173 of 488 [35.5%] vs 823 of 2986 [27.6%]; lipid protocols vs none, 451 of 1411 [32.0%] vs 545 of 2063 [26.4%]; both P < .001; cardiology, 523 of 1686 [30.1%] vs internal or family medicine, 377 of 1472 [25.6%] and other, 96 of 316 [30.4%]; P = .003).
Conclusions and Relevance
Of patients with ASCVD, most with suboptimal LDL-C levels at baseline, only 17.1% had LLT intensification after 2 years, and two-thirds remained at an LDL-C level greater than 70 mg/dL. Further intensive efforts are needed to achieve optimal LDL-C management in patients with ASCVD.
Introduction
Lipid lowering is one of the most effective strategies to reduce cardiovascular morbidity and mortality.1 Clinical benefits of low-density lipoprotein cholesterol (LDL-C) level reduction have been seen in large randomized clinical trials with statins, ezetimibe, and most recently, proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i).1,2,3,4,5,6
In clinical practice, however, use of all classes of lipid-lowering therapies (LLTs) has not matched the guideline recommendations, whether in outpatients with atherosclerotic cardiovascular disease (ASCVD) or those hospitalized with a myocardial infarction.7,8,9,10,11,12 The American College of Cardiology/American Heart Association (ACC/AHA) Multisociety13 and European Society of Cardiology/European Atherosclerosis Society14 guidelines have both been updated to reflect new trial data, but limited data are available on the uptake of new therapies and LDL-C level attainment following release of these guidelines. The Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD) study is a prospective, multicenter, observational registry of patients with ASCVD. The primary study objective is to describe and track LDL-C treatment patterns over time in patients with clinical ASCVD in the US. In this report, we describe the primary 2-year results.
Methods
Patients
The design of GOULD has been previously reported (ClinicalTrials.gov identifier: NCT02993120).15 To be eligible for inclusion in the registry, patients had to be at least 18 years old and have established ASCVD, defined as having any 1 of the following clinical conditions: a history of myocardial infarction, coronary artery disease, coronary or other arterial revascularization, ischemic stroke or transient ischemic attack, carotid artery stenosis, or documented peripheral arterial disease secondary to atherosclerosis (an aortic aneurysm, an ankle-brachial index <0.9, imaging evidence of >50% stenosis in any peripheral artery, or intermittent claudication). Patients were enrolled into 1 of 3 cohorts: those (1) currently receiving a PCSK9i; (2) not receiving a PCSK9i with an LDL-C of 100 mg/dL or more (to convert to millimoles per liter, multiply by 0.0259); and (3) not receiving a PCSK9i with an LDL-C level of 70 to 99 mg/dL. All patients should have received some type of stable LLT for at least 4 weeks prior to enrollment, including those in the PCSK9i cohort, at the discretion of the treating physician. Each site obtained institutional review board approval; all patients provided informed consent. Patients were not eligible for inclusion in the registry if they were unable or unwilling to provide informed consent for reasons including cognitive or language barriers, or if they were currently participating or planning to participate in an interventional clinical study involving any investigational medical device or drug treatment at the time of enrollment; had a life expectancy of less than 12 months; or were currently pregnant, breastfeeding, or planning a pregnancy.
Study Procedures
Study sites screened potentially eligible patients between December 2016 and July 2018, and those who were eligible were asked to provide informed consent prior to enrollment. Data collection and medical record reviews were conducted by research personnel at the enrolling site at baseline and every 6 months for 2 years from the time of enrollment. The most recent values and medication use were recorded in the electronic case report forms at the follow-up points. Through biannual structured questionnaires (via telephone interviews), patients were asked about their perceptions and attitudes toward LLT. At each center, a physician was asked to fill out a structured questionnaire regarding their perceptions and patterns of use of LLT at the time of enrollment of the first patient and at 1 and 2 years following this date.
Outcome Variables
The primary outcome variable is the change in LLT over 2 years. This outcome includes initiation or discontinuation of statin therapy, ezetimibe, or PCSK9i; increasing or decreasing the dosage of a statin or PCSK9i; switching to a different type of statin or PCSK9i; changes in other LLTs (including fish oil/ω-3 preparations, bile acid sequestrants, mipomersen, lomitapide, apheresis, and any new LLT that entered the market after study initiation); and no changes in LLT. Intensive LLT was defined as use of one of the following: a high-intensity statin, any statin plus ezetimibe, or a PCSK9i. Secondary outcome variables assessed over 2 years include performance of a blood test measuring LDL-C and other lipid values, timing of lipid measurement, LDL-C and other lipid values, and responses to the physician and patient surveys.
Statistical Considerations
Descriptive statistics were used to describe management of lipids at the 2-year follow-up, including achieved LDL-C level and changes in LLT. For categorical variables, point estimates of outcomes were generated. Continuous variables were summarized via means (SDs) and medians (interquartile ranges). Comparisons between groups were made via χ2 test for categorical variables and via 2-sample t test for continuous variables. All analyses were performed using SAS version 9.4 (SAS Institute). Changes in LLT were defined as an increase in the dosage of a current medication or addition of another lipid-lowering agent. The last observation carried forward was used for follow-up LLT usage, and then the changes in LLT were rederived in patients without new data at a given visit point. Data in this report are based on a data set generated on October 5, 2020.
Results
Between December 2016 and July 2018, 5006 patients were enrolled at 119 participating centers evenly distributed across the US. As of October 5, 2020, 4257 patients (85.0%) had completed 2 years of follow-up: 3745 (84.1%) in the LDL-C cohorts and 512 (92.4%) in the PCSK9i cohort (eFigure 1 in Supplement 1).
At baseline, patients had a mean (SD) age of 67.8 (9.9) years; 3021 (60.3%) were men, and 1985 (39.7%) were women; 4312 (86.1%) were White; 4028 (80.5%) had coronary artery disease; and 1698 (33.9%) had type 2 diabetes (Table 1). The cohort with LDL-C levels of 100 mg/dL or more had higher percentage of Black/African American patients (PCSK9i, 33 of 554 [6.0%]; LDL-C ≥100 mg/dL, 261 of 1801 [14.5%]; LDL-C of 70-99 mg/dL, 208 of 2651 [7.8%]) and Hispanic patients (PCSK9i, 21 of 554 [3.8%]; LDL-C ≥100 mg/dL, 198 of 1801 [11.0%]; LDL-C of 70-99 mg/dL, 177 of 2651 [6.7%]) than the other cohorts. Baseline statin use was 1558 (86.5%) and 2525 (95.2%) in the cohort with LDL-C levels of 100 mg/dL or more (n = 1558) and the cohort with LDL-C levels of 70 to 99 mg/dL (n = 2525), respectively, although high-intensity statin therapy was used by only 717 patients (39.8%) in the cohort with LDL-levels of 100 mg/dL or more and 1225 (46.2%) in the cohort with LDL-C levels of 70 to 99 mg/dL. Ezetimibe was used by 194 (10.8%) and 228 (8.6%), respectively. Among those treated with a PCSK9i at enrollment, 193 (34.8%) were also receiving a statin and 114 (20.6%) were receiving ezetimibe at enrollment.
Table 1. Baseline Characteristicsa.
| Characteristic | Patient cohort, No. (%) | |||
|---|---|---|---|---|
| PCSK9i (n = 554) | LDL-C | Total (N = 5006) | ||
| ≥100 mg/dL (n = 1801) | 70-99 mg/dL (n = 2651) | |||
| Age, mean (SD), y | 65.9 (9.7) | 66.6 (10.3) | 69.0 (9.6) | 67.8 (9.9) |
| Male | 310 (56.0) | 959 (53.2) | 1752 (66.1) | 3021 (60.3) |
| Female | 244 (44.0) | 842 (46.8) | 899 (33.9) | 1985 (39.7) |
| Race/ethnicity | ||||
| White | 505 (91.2) | 1463 (81.2) | 2344 (88.4) | 4312 (86.1) |
| Black or African American | 33 (6.0) | 261 (14.5) | 208 (7.8) | 502 (10.0) |
| Asian | 6 (1.1) | 31 (1.7) | 56 (2.1) | 93 (1.9) |
| Other or multiple | 9 (1.6) | 39 (2.2) | 38 (1.4) | 86 (1.7) |
| Hispanic or Latino | 21 (3.8) | 198 (11.0) | 177 (6.7) | 396 (7.9) |
| Geographic US region | ||||
| Northeast | 115 (20.8) | 236 (13.1) | 406 (15.3) | 757 (15.1) |
| Northwest | 182 (32.9) | 337 (18.7) | 507 (19.1) | 1026 (20.5) |
| South | 179 (32.3) | 944 (52.4) | 1255 (47.3) | 2378 (47.5) |
| West | 78 (14.1) | 284 (15.8) | 483 (18.2) | 845 (16.9) |
| BMI, mean (SD) | 30.2 (5.3) | 30.8 (6.4) | 30.5 (6.0) | 30.6 (6.1) |
| Cardiovascular disease | ||||
| Coronary artery disease | 489 (88.3) | 1361 (75.6) | 2178 (82.2) | 4028 (80.5) |
| Cerebrovascular accident | 47 (8.5) | 214 (11.9) | 252 (9.5) | 513 (10.2) |
| Peripheral arterial disease | 73 (13.2) | 256 (14.2) | 347 (13.1) | 676 (13.5) |
| Myocardial infarction | 156 (28.2) | 572 (31.8) | 857 (32.3) | 1585 (31.7) |
| Type 2 diabetes | 143 (25.8) | 655 (36.4) | 900 (33.9) | 1698 (33.9) |
| Lipids, median (IQR), mg/dL | ||||
| LDL-C | 67 (42-104) | 120 (108-141) | 82 (75-89) | 91 (78-113) |
| HDL-C | 49 (41-60) | 47 (39-58) | 47 (39-57) | 47 (39-58) |
| Triglycerides | 128 (92-178) | 137 (97-193) | 115 (84-159) | 124 (89-173) |
| Total cholesterol | 146 (119-188) | 200 (182-224) | 156 (144-168) | 168 (149-195) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor.
SI conversion factor: To convert HDL-C, LDL-C, and total cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.
Data are as of October 5, 2020.
Over 2 years after enrollment, 3768 patients (88.5%) had their LDL-C remeasured at least once (eFigure 2 in Supplement 1). Lipid testing occurred more frequently among patients in the PCSK9i cohort. The median (interquartile range) time from preenrollment LDL-C value to the first LDL-C measurement was 189 (139-276) days. Levels of LDL-C were measured twice in 1033 patients (24.3%) and 3 times or more in 1855 patients (43.6%) by year 2. The mean (SD) time from medical record review to next lipid profile was 210 (186) days.
Changes in LLT Over Time
By the 2-year follow-up, only 855 of 5006 patients (17.1%) had some type of LLT intensification (Table 2), with 4191 (83.7%) of these receiving a statin (with 2181 [43.6%] receiving a high-intensity statin), 717 (14.3%) receiving ezetimibe, and 678 (13.5%) receiving a PCSK9i (Figure 1A). In the cohorts with LDL-C levels of 100 mg/dL or more and 70 to 99 mg/dL, LLT intensification was carried out in 403 patients (22.4%) and 383 patients (14.4%), respectively: statin dosage was intensified in 115 patients (6.4%) and 168 patients (6.3%), a statin was initiated in 85 (4.7%) and 54 (2.0%), ezetimibe was added in 123 (6.8%) and 118 (4.5%), and a PCSK9i was added in 114 (6.3%) and 58 (2.2%), respectively (Table 2). Figure 1B, C, and D describes LLT used in patients at baseline and the 2-year point in each patient group. Of note, 950 patients (52.7%) and 1451 patients (54.7%) in the cohorts with LDL-C levels of 100 mg/dL or more and LDL-C levels of 70 to 99 mg/dL, respectively, were receiving intensive LLT at 2 years, compared with 787 (43.7%) and 1310 (49.4%) at baseline.
Table 2. Changes in Lipid-Lowering Therapy Use in Patients With Follow-up at 2 Yearsa.
| Parameter | Patients, No. (%) | |||
|---|---|---|---|---|
| PCSK9i (n = 554)b | Low-density lipoprotein cholesterol | Total (N = 5006) | ||
| ≥100 mg/dL (n = 1801) | 70-99 mg/dL (n = 2651) | |||
| Any change in lipid-lowering therapy | 232 (41.9) | 643 (35.7) | 714 (26.9) | 1589 (31.7) |
| Lipid treatment intensification | 69 (12.5) | 403 (22.4) | 383 (14.4) | 855 (17.1) |
| Statin intensifiedc | 7 (1.3) | 115 (6.4) | 168 (6.3) | 290 (5.8) |
| Statin added | 20 (3.6) | 85 (4.7) | 54 (2.0) | 159 (3.2) |
| Ezetimibe added | 22 (4.0) | 123 (6.8) | 118 (4.5) | 263 (5.3) |
| PCSK9i added | 10 (1.8)d | 114 (6.3) | 58 (2.2) | 182 (3.6) |
| Lipid treatment deescalation | 103 (18.6) | 153 (8.5) | 214 (8.1) | 470 (9.4) |
| Statin downtitrated | 6 (1.1) | 33 (1.8) | 58 (2.2) | 97 (1.9) |
| Statin discontinued | 28 (5.1) | 108 (6.0) | 108 (4.1) | 244 (4.9) |
| Ezetimibe discontinued | 22 (4.0) | 38 (2.1) | 22 (0.8) | 82 (1.6) |
| PCSK9i discontinued | 46 (8.3)e | 2 (0.1)f | 0 | 48 (1.0) |
Abbreviation: PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor.
Data are as of October 5, 2020.
Receiving PCSK9i at baseline.
Includes uptitration of the dosage of a previous statin or adding or switching to a more potent statin.
Ten individuals in the PCSK9i cohort started taking a PCSK9i on or after the date of enrollment and thus were considered to have added a PCSK9i after the baseline period.
Discontinuation of a PCSK9i was defined as a patient receiving a PCSK9i drug at baseline (prior to the enrollment date) and not receiving a PCSK9i drug at 24 months.
Seven individuals in the cohort with low-density lipoprotein cholesterol levels of 100 mg/dL or more were receiving a PCSK9i at baseline; 2 discontinued taking a PCSK9i during 2 years of follow-up.
Figure 1. Use of Lipid-Lowering Therapies (LLT) at Baseline and 2 Years Among Patients Enrolled in the GOULD Registry.
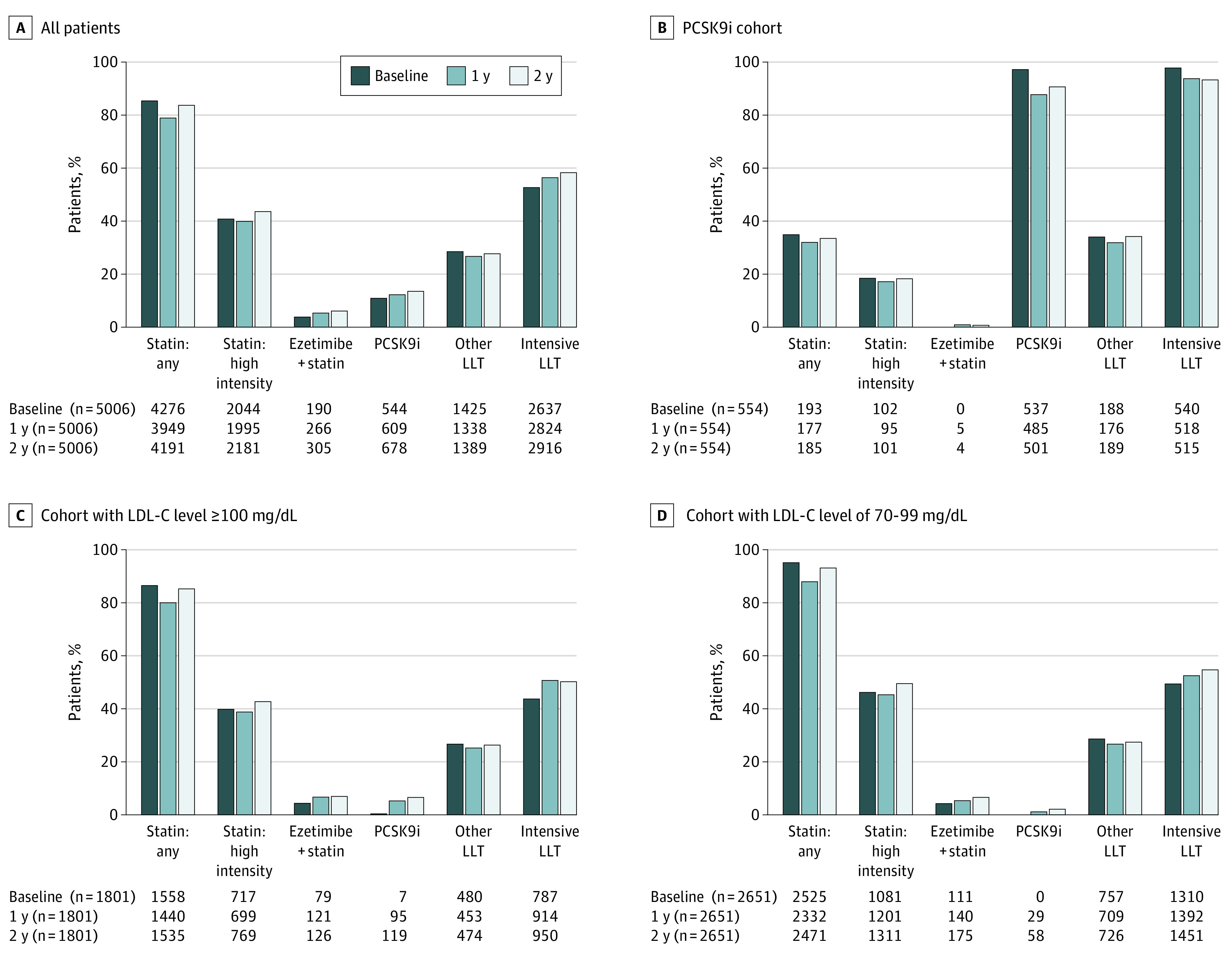
GOULD indicates Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management; LDL-C, low-density lipoprotein cholesterol; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor. To convert LDL-C levels to millimoles per liter, multiply by 0.0259.
In the PCSK9i cohort, 69 patients (12.5%) had LLT intensification in the 2 years since enrollment (Table 2), with 185 (33.4%) receiving a statin (with 101 [18.2%] receiving a high-intensity statin), 114 (20.6%) receiving ezetimibe, and 501 (90.4%) continuing to take a PCSK9i (Figure 1B). Of the 501 patients who continued to take a PCSK9i, 180 (35.9%) were receiving PCSK9i monotherapy and 321 (64.1%) were receiving a PCSK9i plus another LLT (data not shown). Since baseline, 7 (1.3%) uptitrated their statin, 20 (3.6%) added a statin, and 22 (4.0%) added ezetimibe. Furthermore, 28 (5.1%) discontinued and 6 (1.1%) downtitrated their baseline statin (Table 2). Of the 250 patients receiving PCSK9i (45.1%) who had LDL-C levels greater than 70 mg/dL at baseline, 41 (16.4%) had LLT intensification at 2 years; 5 (2.0%) uptitrated their statin, 12 (4.8%) added ezetimibe, 8 (3.2%) increased the dosage of the PCSK9i, and 13 (5.2%) added another type of LLT (data not shown).
eFigure 3 in Supplement 1 shows changes in the use of different types of LLT over time, including before and after publication of the 2018 ACC/AHA cholesterol guidelines. Little change was noted over time in the PCSK9i cohort. In the LDL-C cohorts, there was an absolute increase of 4.0% (from 42.3% to 46.3%) in the use of high-intensity statin therapy the year before the guideline published, but only 2.5% (from 46.3% to 48.8%) after the guideline was published. For ezetimibe and PCSK9i before and after the guideline published, annual increases of approximately 3% for ezetimibe (2018, 9.8%; 2019, 13.1%; 2020, 16.1%) and 2% for PSCK9i (2018, 0.9%; 2019, 2.9%; 2020, 4.6%) were noted in the LDL-C cohorts.
LDL-C Levels
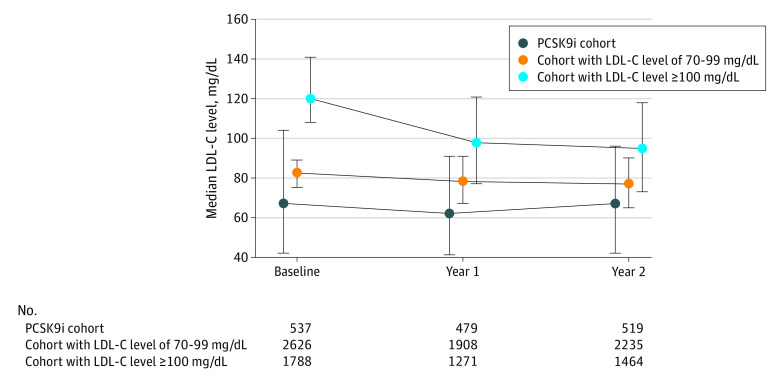
The levels of LDL-C fell from a median (interquartile range) of 120 (108-141) mg/dL to 95 (73-118) mg/dL in the cohort with LDL-C levels of 100 mg/dL or more (P < .001), from 82 (75-89) mg/dL to 77 (65-90) mg/dL in the cohort with LDL-C levels of 70 to 99 mg/dL (P < .001), and was stable at 68 (43-104) mg/dL at baseline and 67 (42-96) mg/dL at 2 years in the PCSK9i cohort (P = .77) (Figure 2). Across all patients, 281 (6.7%) had LDL-C levels less than 70 mg/dL at baseline (1 [0.1%] in the cohort with LDL-C levels of ≥100 mg/dL, 19 [0.9%] in the cohort with LDL-C of 70-99 mg/dL, and 261 [51.6%] in the PCSK9i cohort), and this increased to 1338 (31.7%) at 2 years, indicating that approximately 1057 patients (25.1%) lowered their LDL-C level below the current threshold at 2 years. Levels of LDL-C less than 70 mg/dL at 2 years were achieved by 308 patients (21.0%), 758 patients (33.9%), and 272 patients (52.4%) of patients in the cohort with LDL-C levels of 100 mg/dL or more, the cohort with LDL-C levels of 70 to 99 mg/dL, and the PCSK9i cohort, respectively (Figure 3A). Among patients in the PCSK9i cohort receiving monotherapy or a PCSK9i plus another LLT, 78 (46%) and 128 (64%) achieved an LDL-C level less than 70 mg/dL, respectively (Figure 3B).
Figure 2. Median Low-Density Lipoprotein Cholesterol (LDL-C) Over 2 Years of Follow-up in the GOULD Registry.
GOULD indicates Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor. To convert LDL-C levels convert to millimoles per liter, multiply by 0.0259.
Figure 3. Follow-up at 2 Years.
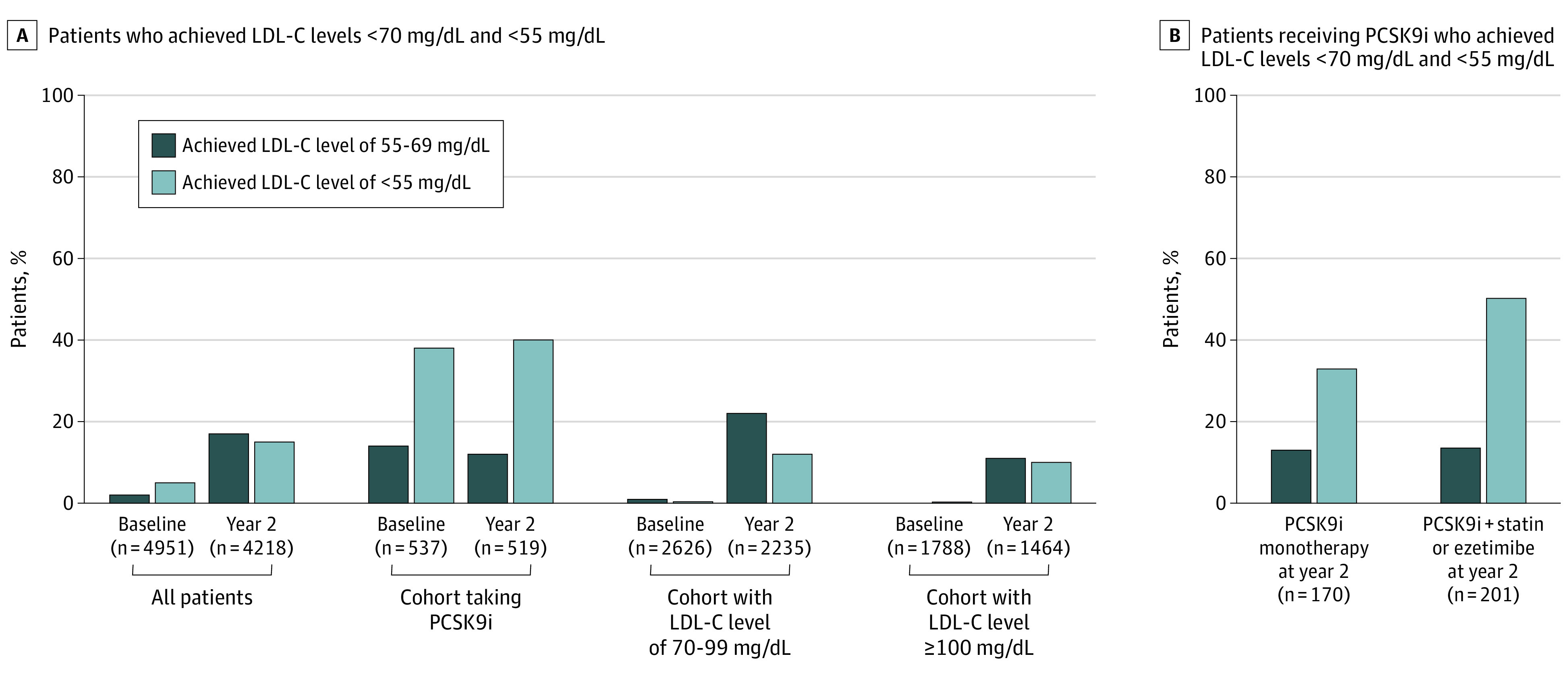
A, Percentage of patients who achieved low-density lipoprotein cholesterol (LDL-C) levels less than 70 mg/dL and less than 55 mg/dL (to convert to millimoles per liter, multiply by 0.0259) at baseline and 2 years of follow-up in the Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD) registry. B, Percentage of patients who achieved LDL-C levels less than 70 mg/dL and less than 55 mg/dL at 2 years among users of proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) drugs as monotherapy and in combination with a statin drug or ezetimibe.
Looking at the lower European Society of Cardiology goal, 618 (14.7%) of all enrolled patients, 146 patients (10%) in the cohort with LDL-C levels of 100 mg/dL or more, 265 patients (11.9%) in the cohort with LDL-C levels of 70 to 99 mg/dL, and 207 (39.9%) in the PCSK9i cohort achieved an LDL-C level of less than 55 mg/dL at 2 years (Figure 3A). Among patients in the PCSK9i cohort receiving monotherapy and PCSK9i plus another LLT, 56 (33%) and 101 (50%) achieved an LDL-C level less than 55 mg/dL, respectively (Figure 3B). Conversely, 1057 patients (25.1%) still had LDL-C levels greater than 100 mg/dL at the 2-year follow-up.
Subgroups
eTable 1 in Supplement 1 shows subgroups of patients and their rates of LLT intensification and of achievement of LDL-C less than 70 mg/dL at 2 years. Those with baseline LDL-C levels of 100 mg/dL or more had more intensification of therapy but achieved guideline-recommended LDL-C levels less often. Intensification of LLT was seen more often in those who were married (unmarried, 233 of 1474 [15.8%] vs married, 513 of 2652 [19.3%]; P = .005), had a high household income (<$75 000, 496 of 3075 [16.1%]; ≥$75 000, 245 of 1034 [23.7%]; P < .001), and had insurance (private, Medicare, and Medicaid; insured, 692 of 3808 [18.2%] vs uninsured, 94 of 644 [14.6%];P = .03). We observed that patients of non-White race and Hispanic ethnicity both had significantly lower rates of LLT intensification (White, 697 of 3807 [18.3%] vs non-White, 89 of 645 [13.8%]; P = .005; Hispanic, 46 of 375 [12.3%] vs non-Hispanic, 736 of 4056 [18.1%]; P = .004) and LDL-C goal achievement (White, 946 of 3191 [29.6%] vs non-White, 120 of 508 [23.6%]; P = .005; Hispanic, 70 of 314 [22.3%] vs non-Hispanic, 991 of 3367 [29.4%]; P = .008) at 2 years. Achievement of LDL-C levels less than 70 mg/dL occurred more frequently among patients who had diabetes (without diabetes, 645 of 2350 [27.4%] vs with diabetes, 421 of 1349 [31.2%]; P = .02) and those with baseline LDL-C levels of 70 to 99 mg/dL (70-99 mg/dL, 758 of 2235 [33.9%] vs ≥100 mg/dL, 308 of 1464 [21.0%]; P < .001). These patients were also more often male (women, 333 of 1435 [23.2%] vs men, 733 of 2264 [32.4%]; P < .001), married (unmarried, 322 of 1201 [26.8%] vs married, 693 of 2253 [30.8%]; P = .02), college educated (no, 616 of 2227 [27.7%] vs yes, 398 of 1213 [32.8%]; P = .002), and with an annual household income of $75 000 or more (no, 712 of 2548 [27.9%] vs yes, 302 of 893 [33.8%]; P < .001).
eTable 2 in Supplement 1 examines some characteristics of the practices that associated with LLT intensification and achievement of LDL-C levels less than 70 mg/dL: both were more common at those sites affiliated with teaching hospitals (LLT intensification: teaching hospitals, 148 of 589 [25.1%] vs nonteaching hospitals, 600 of 3607 [16.6%]; P < .001; LDL-C levels <70 mg/dL: teaching hospitals, 173 of 488 [35.5%] vs nonteaching hospitals, 823 of 2986 [27.6%]; P < .001), those where lipid protocols were in place (LLT intensification: lipid protocols, 359 of 1612 [22.3%] vs no lipid protocols, 389 of 2584 [15.1%]; P < .001; LDL-C levels <70 mg/dL: lipid protocols, 451 of 1411 [32.0%] vs no lipid protocols, 545 of 2063 [26.4%]; P < .001), and at cardiology sites (LLT intensification: cardiology, 452 of 2087 [21.7%] vs internal or family medicine, 204 of 1745 [11.7%] and other, 92 of 364 [25.3%]; P < .001; LDL-C levels <70 mg/dL: cardiology, 523 of 1686 [30.1%] vs internal or family medicine, 377 of 1472 [25.6%] and other, 96 of 316 [30.4%]; P = .003). Some regional differences existed, with the highest rates of LLT intensification in the Northwest (178 of 798 patients [22.3%]). On the physician survey, if the lead physician noted that the ideal goal for patients with ASCVD was an LDL-C level of less than 70 mg/dL or reported prescribing an nonstatin LLT if an LDL-C level remains high despite statin use, patients at those practices more often had intensification of LLT prescribed (described as a goal LDL-C level <50 mg/dL: 25 of 187 [13.4%] vs <70 mg/dL, 578 of 2995 [19.3%]; P < .001; prescribed a nonstatin if the LDL-C level remained high, 558 of 2922 [19.1%] vs did not prescribe, 187 of 1255 [14.9%]; P = .001) and achieved an LDL-C less than 70 mg/dL (described as a goal LDL-C level <50 mg/dL: 34 of 163 [20.9%] vs <70 mg/dL, 767 of 2487 [30.8%]; P < .001; prescribed a nonstatin if the LDL-C level remained high, 718 of 2405 [29.9%] vs did not prescribe, 274 of 1055 [26.0%]; P = .02).
Discussion
In this prospective, national registry of patients with ASCVD across a wide spectrum of practices in the US, we observed that only a small percentage of patients (17.1%) who started with an LDL-C level above the threshold of 70 mg/dL had their therapy intensified over the next 2 years. With practice tracked over time, these modest changes (absolute increases between 2% and 4% annually) in the use of high-intensity statin therapy, ezetimibe, and PCSK9i (eFigure 3 in Supplement 1) showed no apparent change in the rate of increase after publication of the 2018 ACC/AHA guidelines.13 While LDL-C levels did improve, especially in those with initial LDL-C levels greater than 100 mg/dL, even after 2 years, two-thirds of patients overall still had LDL-C levels greater than 70 mg/dL. In those treated with PCSK9i drugs, LDL-C levels had a mean of less than 70 mg/dL, and use of these agents had high persistence. However, with two-thirds of the patients still at or above the recommended LDL-C threshold of 70 mg/dL at 2 years, further intensive efforts are needed to achieve optimal LDL-C management in patients with ASCVD.
There was variability in those who underwent LLT intensification, with variation noted among different patient groups and by physician practice type and approach to LLT at baseline. As noted in many prior studies, we too saw that at baseline, high-intensity statin therapy and ezetimibe are underused in these patients with ASCVD in real-world practice in the US.7,8,9,15 When looking at trends over time, there are only modest changes in the use of high-intensity statin therapy, ezetimibe, and PCSK9i drugs, despite new large cardiovascular outcomes trials being published in 2017,3 2018,4 and 201916 with both PCSK9i monoclonal antibodies and ezetimibe, as well as the 2018 ACC/AHA Multisociety cholesterol guidelines.13 This illustrates how slowly clinical practice changes, even with the publication of new evidence and guidelines. Cost hurdles and administrative burdens in prescribing also have been factors for newer therapies, such as PCSK9i drugs. Despite announcements of 60% cost reductions in late 2018 and early 2019, we saw no apparent change in PCSK9i use relative to the modest steady increases already occurring.
Furthermore, we observed significantly lower rates of LLT intensification in patients of non-White race and Hispanic ethnicity. Our data highlight the need for new approaches to improve implementations of the guidelines into clinical practice, especially across diverse populations. Our finding that physician attitude on the ideal goal for LDL-C level was one of the factors associated with more intensification and LDL-C level achievement suggests that more intensive education programs may help improve guideline implementation and adherence. Interestingly, even simple monitoring of lipids (in patients who were not at an ideal LDL-C level and thus should have had repeated lipid measurements taken) was underused, with 89% of patients having follow-up lipid measurement over 2 years of follow-up. Among these patients, approximately 1 in 3 achieved an LDL-C level of less than 70 mg/dL, which implies that only 20% of patients in the total cohort lowered their LDL-C level below the currently recommended threshold for consideration of additional LLT.
The 2019 European Society of Cardiology/European Atherosclerosis Society guidelines14 have recommended an LDL-C level goal of less than 55 mg/dL and at least 50% reduction in LDL-C level from baseline for patients at very high risk, such as those enrolled in this registry, on the basis of recent trials.2,3,4 We found that by 2 years, in those with LDL-C values measured, about 15% achieved LDL-C levels less than 55 mg/dL. Interestingly, very few patients who were in the LDL-C cohorts achieved this, but among those taking a PCSK9i, 40% had their LDL-C values below this level. Thus, as guidelines adopt lower goals, the need for more potent agents, such as PCSK9i drugs, will be greater.
Our data at baseline reinforce the findings in the Patient and Provider Assessment of Lipid Management (PALM) registry.17 This registry enrolled 7938 patients with ASCVD or risk factors from 140 practices in the US.18 They observed that only 47.3% were receiving the recommended statin intensity. They also found that patients managed by cardiologists were more often prescribed high-intensity statin therapy. The GOULD registry now adds an understanding how treatment patterns evolved the following 2 years. While some improvement was seen, it was disappointing to see that most patients still did not lower their LDL-C level below the current threshold of 70 mg/dL.
In addition to physician clinical inertia and guideline updates, patient adherence to statins and high-intensity statin therapy is suboptimal. Among patients who fill a high-intensity statin prescription following discharge for a myocardial infarction, adherence declines to 58.9% at 6 months and 41.6% at 2 years.8 Low rates of persistence and adherence to statin therapy in patients with coronary heart disease is more frequent in those who are newly using high-intensity statins and Black and Hispanic patients than White patients.8 Efforts to initiate moderate-intensity statin therapy in patients who discontinue high-intensity statin therapy after a myocardial infarction may improve persistence19 and reduce recurrence and mortality.20
Limitations
Our study has several limitations. This registry is not a population-based study and is being conducted solely in the US, which has implications for generalizability outside the US. Practices participating in the registry may provide better quality of care than the national average. Also, the population enrolled may not represent all demographics and underrepresented groups. However, we enrolled patients from a wide range of centers across all regions of the US and different practice settings (eTable 2 in Supplement 1) to make the registry as representative as possible. We should also note that this is a selective cohort of patients who agreed to volunteer and provide consent. While prescribed medications are documented in the medical records, we do not have information on patient adherence. We did not collect a pretreatment LDL-C level, so we could not calculate the percentage change in LDL-C level, another metric cited in the guidelines. There was incomplete follow-up in some patients, and there are some differences in the demographics of those who completed the follow-up (eTable 3 in Supplement 1). Interestingly, those with prior myocardial infarction or percutaneous coronary intervention tended to have better follow-up than those without. Finally, there could be unmeasured factors not assessed that might explain differences (or lack thereof) in prescribing LLT.
Conclusions
In this national registry of patients with ASCVD, most who had elevated LDL-C levels at baseline showed only modest LLT intensification and improvement in LDL-C levels over 2 years, with no apparent change in the rate of increase after publication of the 2018 ACC/AHA guidelines. Among the patients who had an LDL-C level remeasured during 2 years of follow-up, approximately 1 in 3 achieved an LDL-C level less than 70 mg/dL, and 1 in 10 achieved an LDL-C level less than 55 mg/dL. New efforts to facilitate more intensification of LLT are necessary to optimize lipids in this ASCVD population.
eTable 1. LLT Intensification and Achievement of LDL-C <70 mg/dL at 2 Years by Subgroup in the LDL-C Cohorts
eTable 2. LLT Intensification and Achievement of LDL- C <70 mg/dL at 2 Years by Site Characteristics and Physician Survey Responses
eTable 3. Subject Characteristics at Baseline - Comparing Patients Completing Follow-up vs. Patients Dropping out [not due to death]
eFigure 1. Consort diagram
eFigure 2. Frequency of lipid panels observed in clinical care over 2 years of follow-up in the GOULD registry
eFigure 3. Use of lipid-lowering therapies over time among patients enrolled in the GOULD registry
eAppendix. List of Committees and Investigators
Nonauthor Collaborators.
References
- 1.Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon CP, Blazing MA, Giugliano RP, et al. ; IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 3.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GG, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Committees and Investigators . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi: 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
- 5.Cannon CP, Braunwald E, McCabe CH, et al. ; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495-1504. doi: 10.1056/NEJMoa040583 [DOI] [PubMed] [Google Scholar]
- 6.LaRosa JC, Grundy SM, Waters DD, et al. ; Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425-1435. doi: 10.1056/NEJMoa050461 [DOI] [PubMed] [Google Scholar]
- 7.Cannon CP, Khan I, Klimchak AC, Reynolds MR, Sanchez RJ, Sasiela WJ. Simulation of lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2(9):959-966. doi: 10.1001/jamacardio.2017.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colantonio LD, Huang L, Monda KL, et al. Adherence to high-intensity statins following a myocardial infarction hospitalization among Medicare beneficiaries. JAMA Cardiol. 2017;2(8):890-895. doi: 10.1001/jamacardio.2017.0911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenson RS, Farkouh ME, Mefford M, et al. Trends in use of high-intensity statin therapy after myocardial infarction, 2011 to 2014. J Am Coll Cardiol. 2017;69(22):2696-2706. doi: 10.1016/j.jacc.2017.03.585 [DOI] [PubMed] [Google Scholar]
- 10.Colantonio LD, Rosenson RS, Deng L, et al. Adherence to statin therapy among US adults between 2007 and 2014. J Am Heart Assoc. 2019;8(1):e010376. doi: 10.1161/JAHA.118.010376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maddox TM, Song Y, Allen J, et al. Trends in U.S. ambulatory cardiovascular care 2013 to 2017: JACC review topic of the week. J Am Coll Cardiol. 2020;75(1):93-112. doi: 10.1016/j.jacc.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 12.Rosenson RS, Kent ST, Brown TM, et al. Underutilization of high-intensity statin therapy after hospitalization for coronary heart disease. J Am Coll Cardiol. 2015;65(3):270-277. doi: 10.1016/j.jacc.2014.09.088 [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285-e350. doi: 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 14.Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 15.Cannon CP, de Lemos JA, Rosenson RS, et al. ; GOULD Investigators . Getting to an Improved Understanding of Low-Density Lipoprotein-Cholesterol and Dyslipidemia Management (GOULD): methods and baseline data of a registry of high cardiovascular risk patients in the United States. Am Heart J. 2020;219:70-77. doi: 10.1016/j.ahj.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 16.Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140(12):992-1003. doi: 10.1161/CIRCULATIONAHA.118.039415 [DOI] [PubMed] [Google Scholar]
- 17.Navar AM, Wang TY, Goldberg AC, et al. Design and rationale for the Patient and Provider Assessment of Lipid Management (PALM) registry. Am Heart J. 2015;170(5):865-871. doi: 10.1016/j.ahj.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 18.Navar AM, Wang TY, Li S, et al. Lipid management in contemporary community practice: results from the Provider Assessment of Lipid Management (PALM) Registry. Am Heart J. 2017;193:84-92. doi: 10.1016/j.ahj.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth JN III, Colantonio LD, Chen L, et al. Statin discontinuation, reinitiation, and persistence patterns among Medicare beneficiaries after myocardial infarction: a cohort study. Circ Cardiovasc Qual Outcomes. 2017;10(10):e003626. doi: 10.1161/CIRCOUTCOMES.117.003626 [DOI] [PubMed] [Google Scholar]
- 20.Hickson RP, Robinson JG, Annis IE, Killeya-Jones LA, Fang G. It’s not too late to improve statin adherence: association between changes in statin adherence from before to after acute myocardial infarction and all-cause mortality. J Am Heart Assoc. 2019;8(7):e011378. doi: 10.1161/JAHA.118.011378 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. LLT Intensification and Achievement of LDL-C <70 mg/dL at 2 Years by Subgroup in the LDL-C Cohorts
eTable 2. LLT Intensification and Achievement of LDL- C <70 mg/dL at 2 Years by Site Characteristics and Physician Survey Responses
eTable 3. Subject Characteristics at Baseline - Comparing Patients Completing Follow-up vs. Patients Dropping out [not due to death]
eFigure 1. Consort diagram
eFigure 2. Frequency of lipid panels observed in clinical care over 2 years of follow-up in the GOULD registry
eFigure 3. Use of lipid-lowering therapies over time among patients enrolled in the GOULD registry
eAppendix. List of Committees and Investigators
Nonauthor Collaborators.