Summary
Follicular T helper (Tfh) and regulatory (Tfr) cells are distinct subsets of CD4+ T lymphocytes, regulating humoral immune responses in the germinal center. It is widely accepted that dysregulated Tfh and Tfr cells are associated with autoimmunity. In this study, we evaluated the frequencies of circulating chemokine receptor (CXCR)5+ programmed cell death 1 (PD‐1+) Tfh (cTfh) and CXCR5+PD‐1+forkhead box protein 3 (FoxP3+) CD25+ Tfr (cTfr) cells, and their corresponding cytokines from the peripheral blood mononuclear cells of 28 patients with relapsing–remitting multiple sclerosis (MS) and 16 age‐ and sex‐matched healthy controls (HC). Subsets of cTfh cells by Th1‐ and Th17‐related surface markers (CXCR3 and CCR6) were also evaluated. We found that the frequency of cTfh cells was significantly higher in MS patients compared to that of HC. Conversely, the frequency of cTfr cells was lower in MS patients than that of HC. Interleukin (IL)‐21‐producing cTfh cells were significantly increased in MS patients, while IL‐10‐secreting cTfr cells were lower in MS patients compared to levels in HC. Among cTfh cells, cTfh17.1 cells were the major subtypes that were significantly increased in MS patients compared to HC, with the frequency of IL‐21‐secreting cells being the highest. These results suggest that an imbalanced distribution of cTfh and cTfr exist in MS patients, which contributes to the reciprocally altered IL‐21 and IL‐10 production.
Keywords: follicular regulatory T cells, follicular T helper cells, multiple sclerosis
This study describes an incresed frequency of circulating follicular helper T cells (cTfh) and reduced follicular regulatory T cells (cTfr) in patients with multiple sclerosis (MS). These imbalance distribution of cTfh and cTfr cells is associated with dyregulated IL‐21 and IL‐10 secretion in MS. Analysis of Tfh subsets revealed that cTfh17.1 cells were the major subtypes that were significantly increased in MS, with the frequency of IL‐21 secreting cells being the highest.
Introduction
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS), characterized by destruction of the myelin sheath and nerve axons [1]. CD4+ T cells are considered to be key players in MS; different subsets of CD4+ T cells orchestrate an inflammatory wave to initiate its pathogenesis [2, 3]. Additionally, B cells are known to play important roles in MS through antibody production or by influencing autoreactive T cell generation [4, 5, 6]. T follicular helper (Tfh) cells, a CD4+ T cell subset that resides in the germinal center (GC), are specialized for B cell help and antibody‐mediated immune responses [7]. Tfh cells possess unique phenotypical attributes and express chemokine receptor type 5 (CXCR5), inducible T cell co‐stimulator (ICOS) and programmed cell death 1 (PD‐1) at their surfaces, which are all controlled by a B cell lymphoma 6 transcription factor (Bcl‐6) [7, 8, 9, 10, 11]. Interleukin (IL)‐21, the signature cytokine produced by Tfh cells, is required for Tfh maintenance, B cell differentiation and antibody production [11, 12, 13]. Conversely, follicular regulatory T (Tfr) cells have been identified as a subtype of regulatory T cells (Treg) that share some common phenotypes with Tfh, together with conventional Treg markers such as forkhead box P3 (FoxP3) and CD25 [14, 15]. While Tfr cells secrete IL‐10 [16], as do the canonical Treg, their basic role is to regulate the functional activity of Tfh cells in the GC [17, 18].
Since their discovery, Tfh and Tfr cells have become crucial parts of antigen‐specific immune responses and self‐tolerance, which led to the exploration of their relevance in human health and disease [17, 19]. However, the characterization of bona fide Tfh and Tfr in humans has been poorly studied because of sampling difficulties. Instead, studies have focused upon their counterparts in peripheral blood, termed as circulating Tfh (cTfh) cells [20]. These cTfh cells are identified as a memory T cell subset, sharing common phenotypes with GC‐Tfh except for Bcl‐6 [21]. Like GC‐Tfh, cTfh cells are also capable of providing help to B cells [22]. Recently, cTfh cells have been reported to exert more potent cytokine‐mediated immune responses than GC‐Tfh [23]. Moreover, armed with memory‐like properties, cTfh cells may provide more rapid cellular immune responses than GC‐Tfh and undergo homeostatic proliferation. The presence of markers and cytokines specific to cTfh has been detected in the CNS of MS patients, implicating its potential CNS‐infiltrating mechanism and role in MS pathogenesis [24]. Morita et al. initially described the existence of T helper type 1 (Th1) (CXCR3) and Th17 [chemokine receptor (CCR6)]‐related surface markers on cTfh cells. cTfh cells were divided into cTfh1 (CXCR3+CCR6−), cTfh2 (CXCR3−CCR6−) and cTfh17 (CXCR3−CCR6+), each involved in producing IL‐21 and different sets of cytokines, such as interferon (IFN)‐γ, IL‐4 and IL‐17, respectively [25]. These diverse phenotypical markers and cytokines collectively reshape cTfh cells to provide more robust help to B cells and to induce both high‐affinity maturation of plasma cells as well as antibody production [20, 25].
Emerging evidence suggests that Tfh cells should be tightly regulated to maintain immune tolerance and avoid autoimmune responses. Uncontrolled activation of Tfh cells and insufficient frequency of Tfr cells can lead to the breakdown of self‐tolerance. An imbalance of Tfh and Tfr cells has been found in a number of autoimmune diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and myasthenia gravis (MG) [26, 27, 28, 29]. Moreover, impaired polarization of Tfh subsets has reportedly been involved in several autoimmune diseases, such as SLE, RA and immunoglobulin (Ig)G4‐related disease, which are associated with autoantibody production [30, 31, 32]. The relevance of Tfh and Tfr cells has been recognized in MS patients, suggesting their potential roles in MS pathogenesis [33, 34]. To address their impact upon MS, we investigated the frequency of cTfh (CD4+CXCR5+PD‐1+) cells, their subsets and cTfr (CD4+CXCR5+PD‐1+FoxP3+CD25+) cells in MS patients and compared them to those of healthy controls (HC). We also investigated the frequency of respective cytokines secreted by these cellular subtypes. We found that patients with MS display alterations to their cTfh and cTfr cells, both in terms of their frequencies and cytokine secretions.
Methods
Study participants
This study enrolled 28 relapsing–remitting MS (RRMS) patients from the Department of Neurology, National Cancer Center, South Korea. The diagnosis of MS was made according to the 2017 McDonald criteria [35]. Sixteen age‐ and sex‐matched healthy controls (HC) were also recruited. No patients had co‐existing CNS diseases or other autoimmune diseases. Among 28 MS patients, eight patients were treatment‐naive, 10 were treated with IFN‐β and the other 10 received glatiramer acetate (GA). None of the patients had received high‐dose steroids within the 2 months preceding blood sampling. Demographic and clinical characteristics of participants are summarized in Table 1. This study was approved by the Institutional Review Board and written informed consent was obtained from all participants. All procedures performed in this study were in accordance with the guidelines and regulations of Declaration of Helsinki.
Table 1.
Demographic and clinical features of participants
| Characteristics | Healthy control (n = 16) | MS patients (n = 28) | |
|---|---|---|---|
| Untreated (n = 8) | Treated (n = 20) | ||
| Age (years, mean ± s.d.) | 35.1 ± 6·9 | 30·9 ± 9·5 | 34·3 ± 9·9 |
| Women : men | 11 : 5 | 6 : 2 | 12 : 8 |
| Disease onset age (years, mean ± s.d.) | n.a. | 27·8 ± 9·0 | 29·3 ± 7·8 |
| Disease duration (years, mean ± s.d.) | n.a. | 3·1 ± 0·7 | 5·0 ± 4·3 |
| EDSS score (median, range) | n.a. | 1·0, 0~3·0 | 1·5, 0~3·0 |
| Treatment duration (years, mean ± s.d.) | n.a. | n.a. | 4·1 ± 3·97 |
EDSS = expanded disability status scale; s.d. = standard deviation; n.a. = not applicable.
Peripheral blood mononuclear cell (PBMC) isolation
PBMCs were isolated from fresh blood by density gradient centrifugation over Ficoll‐PaqueTM PLUS (GE Healthcare, Danderyd, Sweden), according to the manufacturer’s protocols.
Surface staining and intracellular staining
A total of 1·5 × 106 and 2 × 106 PBMCs were used for Tfh and Tfr analysis, respectively. PBMCs were stained with a fixable viability dye 700‐alexa fluorophore at 4°C for 15 min in the dark (for dead cell discrimination) prior to surface and intracellular staining. PBMCs were subsequently stained with surface markers at 4°C for 30 min in the dark with the following monoclonal antibodies: anti‐CD3‐phycoerythrin‐cyanin 7 (PE‐cy7), anti‐CD4‐Brilliant Violet (BV)496, anti‐CD8‐fluorescein isothiocyanate (FITC), anti‐CXCR5‐peridinin chlorophyll (PerCp‐cy)‐5·5, anti‐PD‐1‐BV786, anti‐CXCR3‐PE anti‐CCR6‐BV711 and anti‐CD25‐allophycocyanin (APC)‐cy‐7. All monoclonal antibodies were purchased from BD Bioscience (San Jose, CA, USA). For intracellular cytokine staining (ICS), PBMCs were stimulated for 5 h with phorbol 12‐myristate 13‐acetate (PMA) (50 ng/ml), ionomycin (500 ng/ml) (Sigma‐Aldrich, St Louis, MO, USA) and GolgiStop (monensin; 0·6 µl/ml) (BD Bioscience). Following stimulation, PBMCs were fixed and permeabilized with cytofix/cytoperm cell permeabilization buffer (BD Bioscience). ICS was then performed using monoclonal antibodies against IFN‐γ‐APC, IL‐17‐BV650, IL‐21‐BV421, IL‐10‐PE‐CF594 (BD Bioscience) and IL‐4‐BV605 (Biolegend, San Diego, CA, USA) following incubation under the same conditions described above for surface staining. Simultaneously, FoxP3 staining was performed for 30 min in the dark at 4°C using anti‐FoxP3‐PE antibody, according to the manufacturer’s instructions (eBioscience, San Diego, CA, USA).
Flow cytometry
After surface and intracellular staining, PBMCs were acquired for analysis using an LSRFortessaTM flow cytometer (BD Bioscience), followed by analysis using FlowJo software (TreeStar, Inc., Ashland, OR, USA). At least 500 000 live events were acquired for the lymphocyte and monocyte populations. Fine singlets were constructed from PBMCs by removing doublets and cell clumps (Fig. 1a).
Fig. 1.
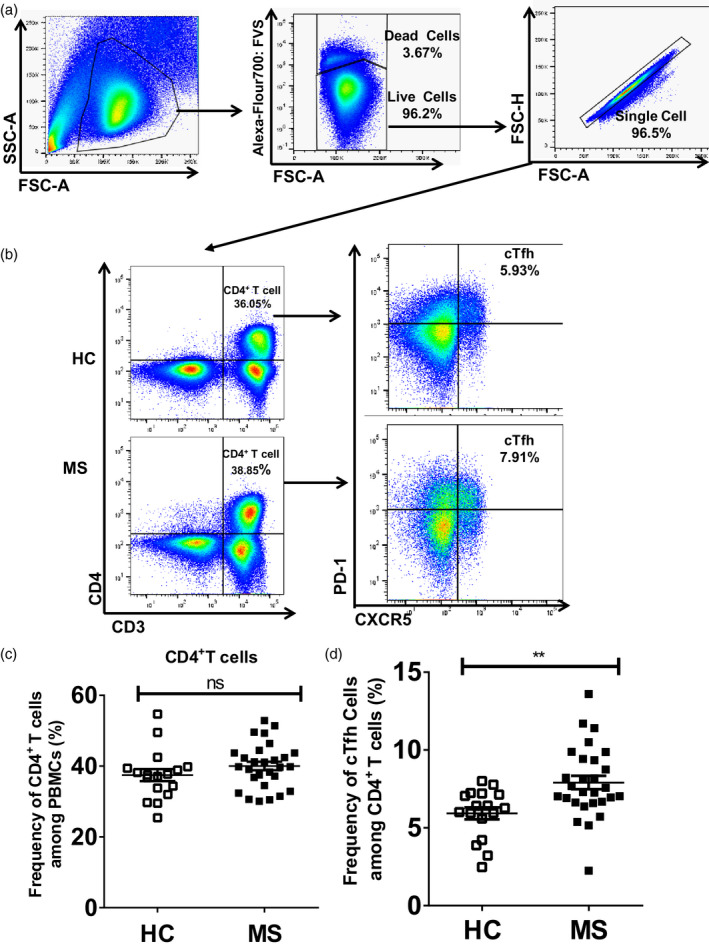
Increased frequency of circulating T follicular helper (cTfh) cells in multiple sclerosis (MS) patients than in healthy controls (HC). Fresh peripheral blood mononuclear cells (PBMCs) from HC and MS patients were surface‐stained with anti‐CD3, anti‐CD4, anti‐chemokine receptor type 5 (CXCR5) and anti‐programmed cell death 1 (PD‐1) antibodies. (a) Construction of fine singlets; representative example of flow cytometry gating of total live PBMCs by exclusion of debris, doublets and dead cells; (b) gating strategy: CD3+CD4+ T cells (left) were defined by gating them on PBMCs after constructing fine singlets, CXCR5+PD‐1+ cells (right) defined as cTfh cells which were selected by gating from CD3+CD4+ T cells (left); (c) percentages of CD4+ T cells in MS and HC were calculated and compared; (d) percentage of cTfh cells in MS patients was calculated and compared to that of HC, **P = 0·003. Each data point represents each individual.
Statistical analysis
Data were statistically analyzed using GraphPad Prism version 5 (GraphPad Software Inc., San Diego, CA, USA). All mean values are expressed as mean ± standard error of the mean (s.e.m.). The non‐parametric Mann–Whitney U‐test was performed to compare between healthy controls and MS patients descriptively. A probability value of less than 0·05 (P < 0·05) was considered statistically significant.
Ethics approval
The Institutional Review Board of NCC approved the present study, and written informed consent was obtained from all participants.
Results
Increased frequency of cTfh cells in MS patients
Circulating Tfh cells were analyzed by gating CXCR5+PD‐1+ cells from CD3+CD4+ T cells. As shown in Fig. 1b (right) and 1d, the average frequency of cTfh cells in MS patients was significantly higher than that observed in HC (7·91 ± 0·43 versus 5·93 ± 0·39%, P = 0·003). The percentage of CD4+ T cells in the PBMC of HC versus MS patients was not significantly different [Fig. 1b (left) and 1c].
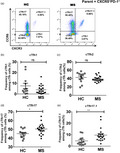
Tfh cells have been categorized into three different subsets based on Th1 (CXCR3)‐ and Th17 (CCR6)‐related surface markers [25]. Previous work has shown that CXCR3+ Th1 and CCR6+ Th17 cells play essential roles in MS [36]. Here, we explored the expression of CXCR3 and CCR6 markers in Tfh cells to classify and evaluate the frequency of different subsets in MS patients. Our data demonstrate that the frequency of cTfh17 (CXCR3−CCR6+ Tfh) cells was significantly increased in MS patients compared to that of HC (50·45 ± 1·36 versus 45·18 ± 1·44%, P = 0·02) (Fig. 2a,d). CXCR3+CCR6+ T cells have been defined as T helper 1‐like Th 17 (Th17.1) cells [37, 38]. We also observed CXCR3+CCR6+ cells among cTfh cells and recognized them as cTfh17.1 cells. Interestingly, the frequency of cTfh17.1 (CXCR3+CCR6+ Tfh) cells was significantly higher in MS patients than that in HC (9·66 ± 1·32 versus 4·58 ± 1·14%, P = 0·005) (Fig. 2a,e). In contrast, there were no significant differences in the frequencies of both cTfh1 (CXCR3+CCR6− Tfh) and cTfh2 (CXCR3−CCR6− Tfh) in comparisons between HC and MS patients (Fig. 2a–c).
Fig. 2.
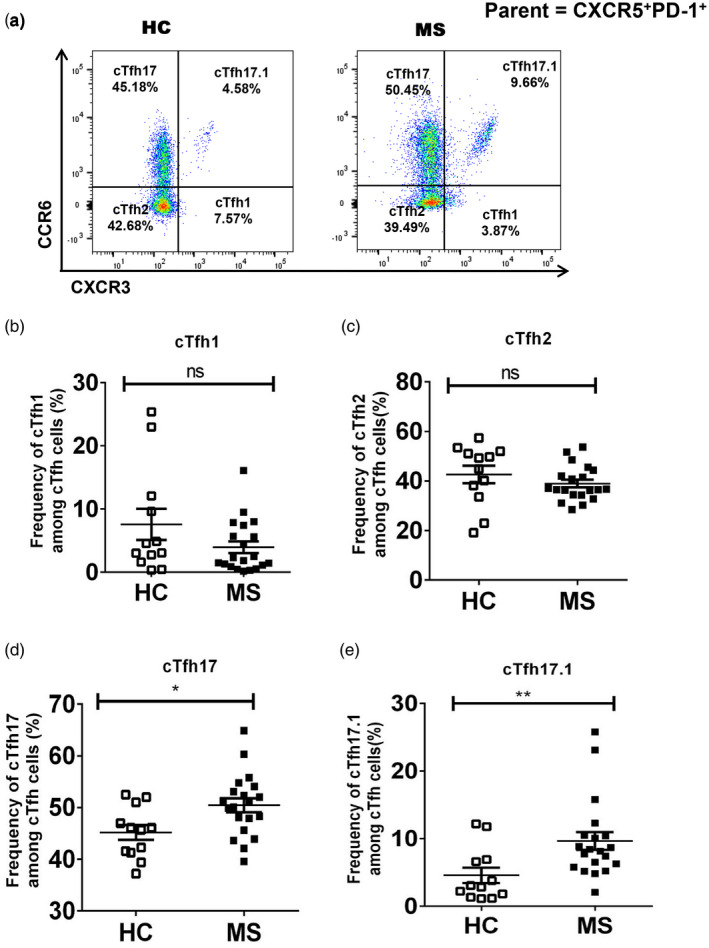
Circulating T follicular helper (cTfh)17 and cTfh17.1 cells were the major subtypes of cTfh cells with increased frequencies in multiple sclerosis (MS) patients. Fresh peripheral blood mononuclear cells (PBMCs) from healthy controls (HC) and MS patients were surface‐stained with anti‐CD3, anti‐CD4, anti‐chemokine receptor (CXCR)5 anti‐programmed cell death 1 (PD‐1), anti‐CXCR3, anti‐CCR6 antibodies (a) gating strategy; CXCR3 and CCR6 were gated from CXCR5+PD‐1+ cells to delineate cTfh subtypes, where CXCR3+CCR6− defined as cTfh1, CXCR3−CCR6− as cTfh2, CXCR3−CCR6+ as cTfh17 and CXCR3+CCR6+ as cTfh17.1 cell; (b–e) percentages of different subtypes of cTfh cells were calculated in MS patients and compared to those of HC, *P = 0·02 and **P = 0·005, respectively.
IL‐21‐secreting cTfh cells were higher in MS patients
Aberrant secretion of inflammatory cytokines plays an important role in autoimmune diseases. We evaluated some signature cytokines, such as IFN‐γ, IL‐4, IL‐17 and IL‐21. Our data indicate that the frequency of IL‐21‐secreting PBMCs was significantly higher in MS patients than that in HC (2·57 ± 0·29 versus 0·95 ± 0·20%, P < 0·0001) (Fig. 3a,c), whereas no significant differences were observed for comparisons of IFN‐γ, IL‐4 and IL‐17 production between MS patients and HC (Supporting information, Fig. S1). The frequency of IL‐21‐secreting CD3+ T cells was higher in MS patients than that in HC (2·02 ± 0·19 versus 0·84 ± 0·28%, P = 0·0009) (Fig. 3c), whereas no significant differences in the frequencies of IL‐21‐producing B cells and monocytes were observed between MS patients and HC (data not shown). Comparative analyses of IL‐21 secretion by CD4+ T cells and CD8+ T cells showed that a significantly higher percentage of CD4+ T cells secreted IL‐21 in MS patients compared to that in HC (2·27 ± 0·19 versus 1·11 ± 0·26%, P = 0·001), while IL‐21‐producing CD8+ T cells showed no significant difference between HC and MS patients (Fig. 3b,c).
Fig. 3.
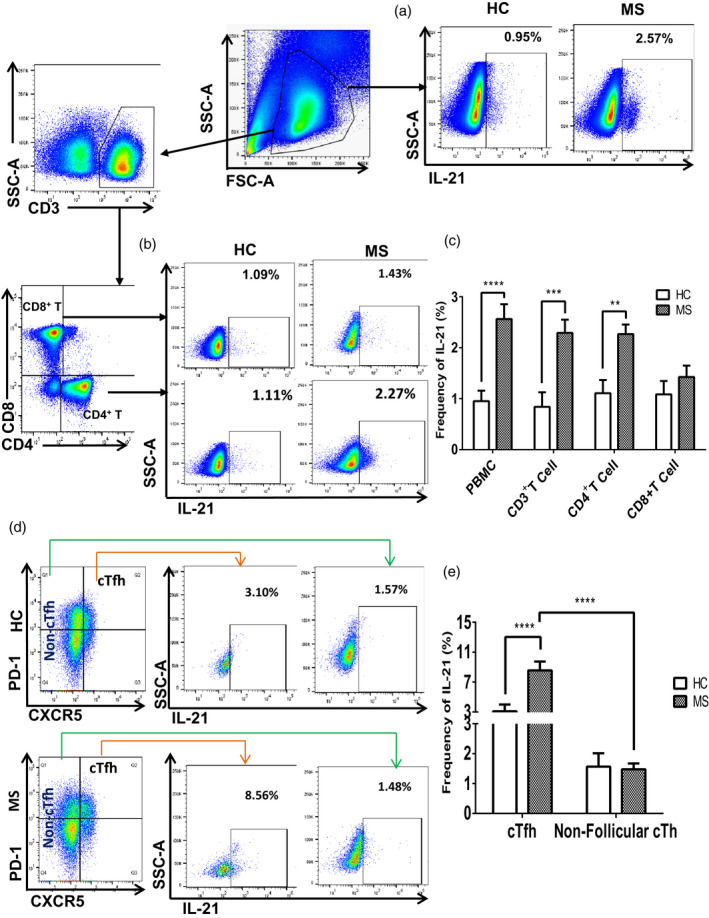
Frequency of interleukin (IL)‐21‐secreting circulating T follicular helper (cTfh) cells were higher in multiple sclerosis (MS) patients than in healthy controls (HC). Fresh peripheral blood mononuclear cells (PBMCs) from HC and MS patients were surface‐stained with monoclonal antibodies against CD3, CD4, CD8, chemokine receptor type 5 (CXCR5) and programmed cell death 1 (PD‐1). Intracellular cytokine staining was performed using anti‐IL‐21 antibody after stimulating for 5 h with phorbol myristate acetate (PMA), ionomycin and GolgiStop (a,b) gating strategy; IL‐21‐positive population was selected from PBMCs, CD4+ T cells and CD8+ T cells, c) percentages of IL‐21 secretion in PBMCs, CD3+ T, CD4+ T and CD8+ T cells from MS patients were calculated and compared to those of HC, ****P < 0·0001, ***P = 0·0004 and **P = 0·001; (d) gating strategy: IL‐21‐positive cells were selected from CXCR5+PD‐1+ Tfh cells and CD4+CXCR5− non‐follicular cTh cells; (e) average frequencies of IL‐21‐producing cTfh and non‐follicular circulating T cells (cTh) cells were calculated and compared between HC and MS; ****P < 0·0001 on the left indicates the difference of IL‐21‐secreting cTfh cells between MS patients and HC; ****P < 0·0001 on the right represents the statistical analysis of IL‐21‐producing circulating T follicular helper (cTfh) versus non‐follicular cTh cells in MS patients.
Next, we analyzed the secretion of cytokines by cTfh cells and compared their frequencies between MS patients and HC. The frequency of IL‐21‐secreting cTfh cells was markedly higher in MS patients compared to that in HC (8·56 ± 1·18 versus 3·10 ± 0·89%, P < 0·0001) (Fig. 3d,e), whereas no significant differences were observed in IFN‐γ, IL‐4 and IL‐17‐secreting cTfh cells between MS patients and HC (Supporting information, Fig. S2). Comparative analysis of IL‐21 production by follicular versus non‐follicular Th cells revealed that a higher percentage of cTfh cells secreted IL‐21 compared to their counterpart, non‐follicular Th cells, in both HC (3·10 ± 0·89 versus 1·57 ± 0·45%, P = 0·08) and MS patients (8·56 ± 1·18 versus 1·48 ± 0·19%, P < 0·0001). Interestingly, the frequency of IL‐21‐secreting cTfh cells was significantly higher in MS patients compared to that in HC (Fig. 3d,e). Both higher frequencies of IL‐21‐secreting Tfh cells in MS patients than in HC and cTfh cells participating in IL‐21 secretion than non‐follicular Th cells suggest that the elevated IL‐21 secretions in MS patients may be attributable to up‐regulations of cTfh cells.
cTfh17.1 cells were the major subtype for IL‐21 production in MS patients
As shown in Fig. 3d,e, the level of IL‐21‐secreting cTfh cells was significantly higher in MS patients than in HC. We then examined the secretion of IL‐21 by different subsets of cTfh cells and compared them between HC and MS patients. Although all four subtypes of cTfh cells produced IL‐21, cTfh17.1 cells were associated with the greatest production of IL‐21 in both HC and MS patients (Fig. 4a,b). Interestingly, the frequency of IL‐21‐secreting cTfh17.1 cells was significantly higher in MS patients (52·52 ± 4·86 versus 24·81 ± 5·34%, P = 0·002) than that in HC (Fig. 4a,b). However, other subsets of IL‐21‐secreting cTfh cells did not differ significantly between HC and MS. We also analyzed and compared IL‐17‐secreting cTfh17 cells in HC and MS patients, followed by a comparative analysis of IL‐17 production by cTfh17 versus non‐follicular Th17 cells. We found no noticeable difference in IL‐17‐secreting cTfh17 cells between HC and MS patients (Fig. 4c and Supporting information, Fig. S3a). Moreover, there was no significant difference in the frequencies of IL‐17‐secreting cTfh17 versus non‐follicular Th17 cells between HC and MS patients (Fig. 4d and Supporting information, Fig. S3a,b,e,f). However, a significant increase of CXCR3+CCR6+ cTfh17.1 cells in MS patients led us to investigate them further based on the cytokine types, especially IFN‐γ and IL‐17. Together with IL‐21, cTfh17.1 cells also produced IFN‐γ and IL‐17 with different frequencies (Supporting information, Fig. S3c,d and Fig. S4e,f), although no significant difference was observed between HC and MS patients regarding IFN‐γ‐ and IL‐17‐secreting cTfh17.1 cells. Importantly, as IL‐21 was the major cytokine secreted by the cTfh17.1 subtype over others, it implies that the elevated frequency of cTh17.1 cells is largely responsible for increased IL‐21 levels in MS patients.
Fig. 4.
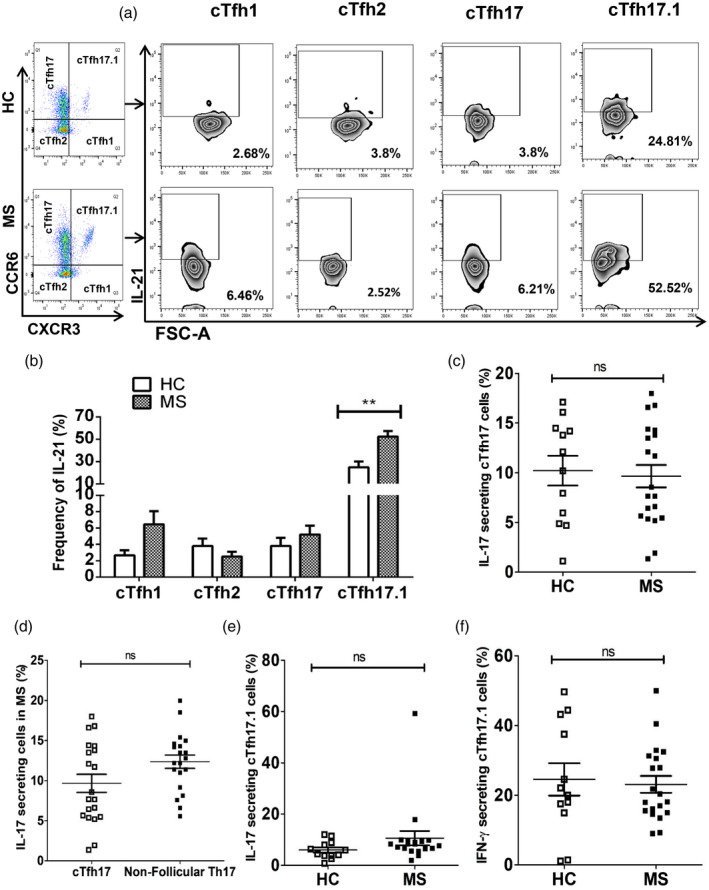
T follicular helper (Tfh) 17.1 cell was the major subtype for interleukin (IL)‐21 production in multiple sclerosis (MS) patients. Fresh peripheral blood mononuclear cells (PBMCs) from healthy controls (HC) and MS patients were surface‐stained with antibodies as described in Fig. 2. Intracellular cytokine staining was additionally performed using anti‐interferon (IFN)‐γ, anti‐IL‐17, anti‐IL‐4 and anti‐IL‐21 antibodies after stimulating for 5 h with phorbol myristate acetate (PMA), ionomycin and GolgiStop (a) gating strategy; IL‐21 population was positively selected from different subsets of cTfh cells; (b) frequencies of IL‐21 production by different subtypes of circulating T follicular helper (cTfh) cells were calculated in MS and compared to those of HC, **P = 0·002; (c) proportion of IL‐17‐producing cTfh17 cells in MS and HC were calculated and compared; (d) percentages of IL‐17 production by follicular and non‐follicular cTh17 cells of MS patients were calculated and compared against each other; (e) percentage of IL‐17‐producing cTfh17.1 cells in MS patients was calculated and compared to that of HC; (f) frequency of IFN‐γ‐producing cTfh17.1 cells was calculated in MS patients and compared to that of HC. Each data point represents each individual.
Reduced frequency of cTfr cells in MS patients
Next, we evaluated the frequency of cTfr cells, which was determined by selecting FoxP3+CD25+ cells from cTfh cells (Fig. 1). The frequency of cTfr cells was significantly lower in MS patients compared to that in HC (1·37 ± 0·12 versus 3·30 ± 0·41%, P < 0·0001) [Fig. 5a (right) and 5b]. The frequency of non‐follicular cTreg cells also displayed a similar trend to that of cTfr between MS and HC (2·11 ± 0·28 versus 3·21 ± 0·30%, P = 0·01) (Fig. 5c and Supporting information, Fig. S4a). Accordingly, the frequency of cTreg (CD4+FoxP3+CD25+) cells among CD4+ T cells was significantly lower in MS patients compared to that in HC (1·65 ± 1·17 versus 3·31 ± 0·33%, P < 0·0001) [Supporting information, Fig. S4b (left) and c]. We also examined the expression of CXCR5+PD‐1+ markers in cTreg cells. Interestingly, the percentage of CXCR5+PD‐1+ cells among cTreg cells was also significantly reduced in MS patients compared to that observed in HC (4·53 ± 0·51 versus 8·81 ± 1·67%, P = 0·0005) (Supporting information, Fig. S4b, right). Taken together, these data suggest that cTfr cells were down‐regulated in MS patients.
Fig. 5.
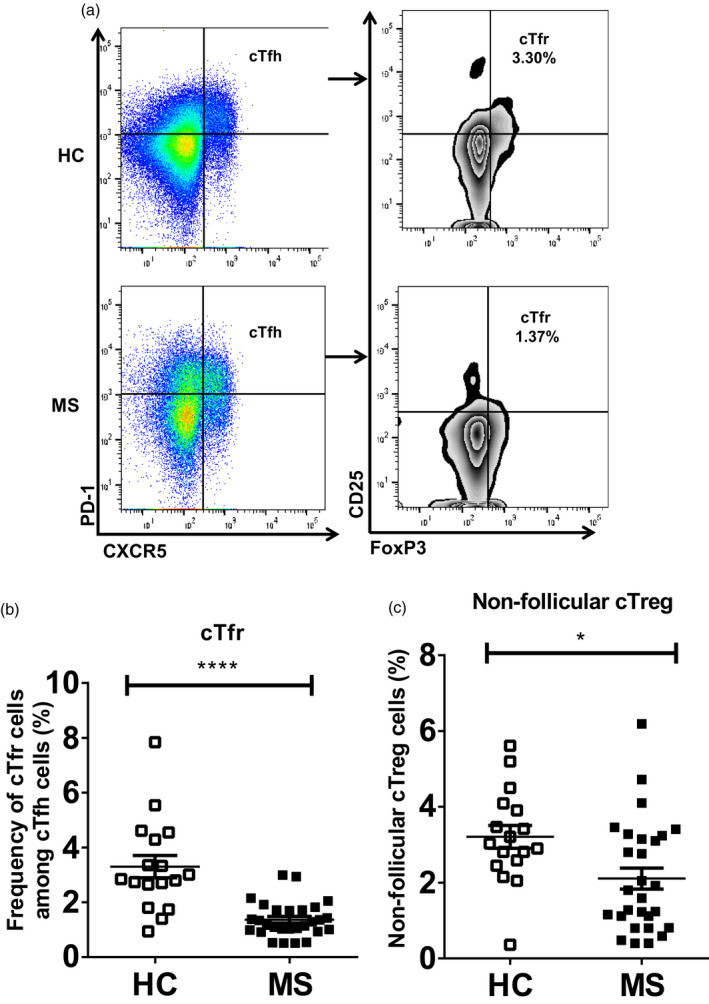
Significantly lower frequency of circulating regulatory T cells (cTfr) cells in multiple sclerosis (MS) patients than in healthy controls (HC). Fresh peripheral blood mononuclear cells (PBMCs) from both HC and MS were surface‐stained with anti‐CD3, anti‐CD4, anti‐chemokine receptor type 5 (CXCR5), anti‐programmed cell death 1 (PD‐1) and anti‐CD25 antibodies. Forkhead box protein 3 (FoxP3) staining with anti‐FoxP3 antibody was performed according to the manufacturer’s protocol (eBioscience); (a) gating strategy: cTfr cells (right) were selected by gating FoxP3+CD25+ cells on CXCR5+PD‐1+circulating T follicular helper (cTfh) cells (left); (b) percentage of cTfr cells was calculated in MS patients and compared to that of HC, ****P < 0·0001, (c) percentage of non‐follicular circulating regulatory T cells (cTreg) cells was calculated in MS patients and compared to that of HC, *P = 0·01. Each data point indicates each individual.
IL‐10‐producing cTfr cells were lower in MS patients
Being the most common anti‐inflammatory cytokine, we analyzed and compared the secretion of IL‐10 between HC and MS patients. In PBMCs, the frequency of IL‐10‐secreting cells was significantly lower in MS patients compared to that in HC (0·67 ± 0·10 versus 1·69 ± 0·32%, P = 0·01) (Fig. 6a,d). Next, we investigated the production of IL‐10 by follicular and non‐follicular cTreg cells, followed by a comparative observation between HC and MS patients. The results showed that compared to non‐follicular cTreg cells, higher percentages of cTfr cells secreted IL‐10 in both HC (21·50 ± 2·30 versus 3·73 ± 0·71%, P < 0·0001) and MS patients (17·78 ± 3·44 versus 2·24 ± 0·22%, P < 0·0001), respectively (Fig. 6b,c,e). Similar to the trend of lower frequency of cTfr cells in MS, the frequency of IL‐10‐producing cTfr cells was significantly lower in MS patients compared to that in HC (17·78 ± 3·44 versus 21·50 ± 2·30%, P = 0·009) (Fig. 6b,c,e). The percentage of IL‐10‐producing non‐follicular cTreg cells was also reduced in MS patients compared to that in HC (Fig. 6c,e).
Fig. 6.
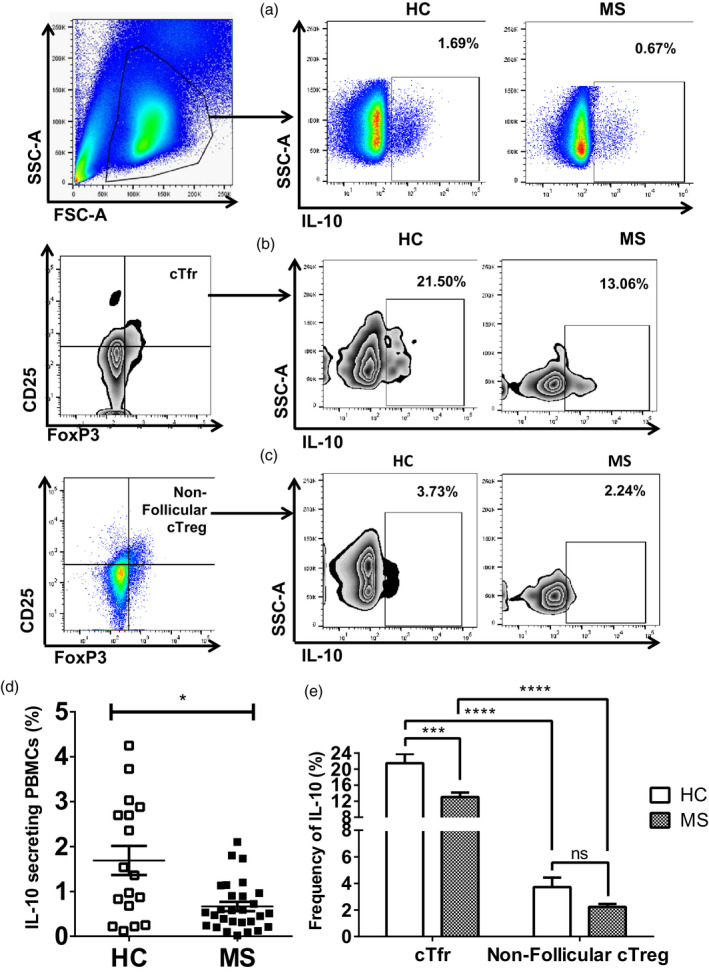
Frequency of interleukin (IL)‐10‐producing circulating regulatory T cells was lower in multiple sclerosis (MS) patients than in healthy controls (HC). Fresh peripheral blood mononuclear cells (PBMCs) from 12 HC and 20 MS patients were surface‐stained with monoclonal antibodies as described in Fig. 5. Intracellular cytokine staining was additionally performed using anti‐IL‐10 antibody after stimulating PBMCs with phorbol myristate acetate (PMA), ionomycin and GolgiStop for 5 h; (a–c) gating strategy: IL‐10‐positive cells were gated on PBMCs (top); cTfr cells (middle) and non‐follicular circulating regulatory T cells (cTreg) cells (bottom), respectively; (d) percentage of IL‐10 secretion in the PBMC of MS patients was calculated and compared to that of HC, *P = 0·01; (e) average frequencies of IL‐10‐producing cTfr cells and non‐follicular cTreg cells were calculated in MS patients and compared to those of HC, ***P = 0·001 represents the comparative analysis of IL‐10 secretion by cTfr cells between HC and MS, whereas *P = 0·05 indicates the statistical difference of IL‐10 production by non‐follicular cTreg cells in HC versus MS, and finally ****P < 0·0001 demonstrates the statistical difference of IL‐10 secretion by follicular versus non‐follicular cTreg cells in HC and MS patients, respectively. Each data point indicates each individual.
Comparison of treated and untreated MS patients
To attribute our findings to the characteristics of the disease and not the effects of treatment, we conducted statistical comparisons among MS patients who received IFN‐β, GA or no treatment. Due to the scarcity of fresh PBMCs from untreated MS patients, frozen PBMCs of untreated MS patients were inevitably used for comparison. We validated the usage of frozen PBMCs with our previous analysis, where there was no significant difference between fresh and frozen PBMCs in their distributions of T cell subsets and cytokine‐producing cells (data not shown). However, we discovered inconsistencies in their Th1/Th2/Th17/Th17.1 subsets that could not be attributed to disease or treatment effect, which consequently led us to exclude them from this comparison.
No significant differences were observed between IFN‐β‐ and GA‐treated patients (data not shown). More importantly, untreated MS patients maintained the same imbalance of cTfh and cTfr frequencies as seen in treated MS patients, in addition to their similar IL‐21 and IL‐10 secretion profiles (Supporting information, Fig. S6a–d). The only major difference resided in the IL‐17 distribution profiles, where treated MS patients exhibited a significantly lower frequency of IL‐17‐secreting T cells than that of untreated MS patients, akin to that of HC (Supporting information, Fig. S6e). These comparisons thus support the idea that our findings regarding the imbalance in follicular T cell populations and irregular IL‐21 and IL‐10 secretion profiles are characteristic of MS.
Discussion
Tfh and Tfr cells are CD4+ T cells that constitute a composite immune regulatory system, where disturbance of this system can cause autoimmune diseases [26, 29, 30, 31]. Despite ongoing research, little is known about the role of Tfh and Tfr cells in MS pathogenesis.
In the present study, we showed an increased frequency of circulating CXCR5+PD‐1+ Tfh cells in MS patients compared to HC. Together with higher frequency of cTfh cells, IL‐21 secretion was also elevated significantly in MS patients. Subsequent analysis of IL‐21 production by follicular and non‐follicular Th cells revealed that cTfh cells were engaged with the highest production of IL‐21, where its frequency was remarkably increased in MS patients. The role of Tfh cells in autoimmune disease was initially described in murine models of Roquinsan/san mice, where the mice are characterized by their excessive Tfh numbers and GC developments in the absence of foreign antigens [39]. However, recent reports with human data in RA, SLE, MG and autoimmune thyroid disease have revealed that the frequency of Tfh cells and their cytokine, IL‐21, were elevated, associating them with disease activity as well as severity by the amplification of autoreactive B cells and pathogenic autoantibody production [28, 29, 30, 40]. Our data are consistent with those results and, more importantly for MS, we connect cTfh cells to high levels of IL‐21 production, suggesting that these cells may play a vital role in the immunopathogenesis of MS. Our results are also supported by previous studies in MS, where both the frequency of memory Tfh and IL‐21 levels in the plasma and CSF were higher in MS patients [33, 41, 42]. Nicolas et al. reported that elevated cTfh cells can be reverted in patients with neuromyelitis optica after treatment with rituximab [43], suggesting exploring new therapeutic solutions for MS.
Proportional bias among different subtypes of Tfh cells has been reported to be associated with several autoimmune diseases, such as SLE, MG, RA and IgG4‐related disease [29, 30, 31, 32]. cTfh17 cells have been reported to be enriched in primary progressive MS compared to those in HC and RRMS [41]. The exact role of cTfh17 cells in MS is not yet clear; while Morita et al. have reported that cTfh17 cells help naive and memory B cells to produce Igs (IgG, IgA and IgM), the process needs to be elucidated in MS [25]. Cunill et al. have shown an increased percentage of cTfh17.1 cells in untreated RRMS compared to that in HC, but no significant difference was observed in cTfh17 frequency [44]. In our study, the frequencies of both cTfh17 and cTfh17.1 cells were highly elevated in MS patients compared to those in HC. However, the increased frequency of cTfh17.1 cells, but not of cTfh17 cells, was positively associated with a significantly higher production of IL‐21 in MS patients. Additionally, among the four subtypes, only cTfh17.1 cells were involved in producing substantial amounts of various cytokines, including IFN‐γ, IL‐17 and IL‐21, where the level of IL‐21 secretion was the greatest for MS patients. These observations collectively suggest that cTfh17.1 cells may be one of the key players in the disease pathogenesis. However, an extensive study with transcriptional profiling and evaluation of a broader range of cytokines may provide more insight into the role of cTfh17.1 cells in MS pathogenesis.
The frequency of IL‐17‐producing Th17 cells was reported to be increased in untreated MS patients compared to that in HC [44, 45, 46] and to be reduced in MS patients after treatment with IFN‐S or dimethyl fumarate [47, 48]. In this study, there was no significant difference in the frequency of Th17 cells and IL‐17 secretion profiles between HC and MS patients. This discrepancy may be attributed to the fact that most of the enrolled patients were continuously undergoing disease‐modifying treatments. Indeed, comparisons between the IL‐17 secretions of untreated and IFN‐r‐ or GA‐treated MS patients showed a significant difference, in line with prior reports, suggesting that first‐line treatments such as IFN‐β or GA influence IL‐17, but not IL‐21.
Alternatively, it is known that the pathogenesis of MS is linked to the failure of regulatory activities by Treg cells [15, 49, 50]. In this study, one of our main focuses was to investigate whether or not the proportion of CXCR5+PD‐1+FoxP3+CD25+ cTfr cells is altered in MS patients. The data revealed that the frequency of cTfr cells was significantly lower in MS patients compared to that in HC. Additionally, the reduced CXCR5+PD‐1+ phenotypes among cTreg cells in MS patients compared to those in HC indicate the unique behavior of cTfr cells in MS. Unlike GC‐Tfr, cTfr does not express Bcl‐6 and ICOS, although they share the same functional property to control excessive humoral‐mediated immunity [21]. In‐vitro co‐culture assays of GC‐Tfh and cTfr cells, each with responder T cells, have shown that both cTfr and GC‐Tfh cells provide equal suppressive activities over the responder cells [34]. The frequency of cTfr cells was found to be reduced in different autoimmune diseases [26, 29, 34]. These findings are consistent with our results, and therefore suggest that impaired cTfr cells may participate in uncontrolled immunoregulation in MS.
The mechanism underlying the suppressive activity of Tfr cells over other effector T cells still remains elusive. It is not clear whether Tfr cells directly inhibit Tfh cells by blocking their transcriptional activations or if they use the IL‐10 signaling pathway to set up their inhibitory milieus. Our data has shown that the frequency of IL‐10‐producing cTfr cells was significantly higher than those of their non‐follicular cTreg counterpart in both HC and MS patients, suggesting that cTfr cells may contribute towards significant portions of reduced IL‐10 production in MS. Interestingly, we observed a lower frequency of cTfr cells associated with a parallel reduction of IL‐10 in MS patients compared to that in HC. Taken together, these data demonstrate that the lower frequency of cTfr is probably linked to the reduced IL‐10 secretion in MS patients. However, the exact role of IL‐10 in MS remains elusive. Impaired IL‐10 secretion was reported to increase the severity of MS [51, 52]. One possible hypothesis is that IL‐10 exerts a beneficial effect in MS by regulating other cytokines, resulting in less tissue damage [53]. Failure of cytokine regulation by defective IL‐10 has been reported in progressive MS [54]. Our findings, together with previous reports, suggest that a lower level of IL‐10 secretion by cTfr cells may be involved in MS pathogenesis, serving as a potential therapeutic target to treat MS.
Conclusions
While our study has some limitations, including the small sample size, a particularly low number of treatment‐naive patients and the lack of confirmative studies using animal models, we discovered a correlation between the increased frequency of cTfh cells and cytokine production, particularly cTfh17.1 cells and the enhanced production of IL‐21, in MS patients. Conversely, cTfr cells and their signature cytokine, IL‐10, were significantly impaired in MS patients. Taken together, our study demonstrates an alteration of cTfh and cTfr cells, which may contribute to dysregulated cytokine productions in MS. An additional study with a larger cohort is warranted to validate our observations regarding the role of Tfh cells, their subsets and Tfr cells in MS. Functional analysis through in‐vitro co‐culture of purified cTfh, cTfr and B cells may provide a clearer understanding of MS and other autoimmune diseases that could potentially guide us to new therapeutic interventions.
Disclosures
H. R., K. Y., P. K., J. H., K. S. Y. and L. H. report no conflicts of interest. K. H. J. received a grant from the National Research Foundation of Korea; received consultancy/speaker fees from Alexion, Aprilbio, Biogen, Celltrion, Eisai, GC Pharma, HanAll BioPharma, MDimune, Merck Serono, Novartis, Sanofi Genzyme, Teva‐Handok and Viela Bio; serves on a steering committee for MedImmune/Viela Bio; is a co‐editor for the Multiple Sclerosis Journal and an associated editor for the Journal of Clinical Neurology.
Author contributions
Conceptualization of study and design of experiments: R. H., H. J. K.; data acquisition, experimentation, analysis and investigation: R. H., Y. K., K. P., H J., S. Y. K., H. L., H. J. K.; study supervision and funding acquisition: H. J. K.; writing original manuscript: R. H., H. J. K.: writing revised manuscript: K. P., H. J. K.; all authors read and approved the final manuscript.
Supporting information
Fig. S1. No significant difference in the levels of IL‐17, IL‐4, and IFN‐γ secretions between HC and MS.
Fig. S2. Levels of IFN‐γ, IL‐17, and IL‐4 secretion by cTfh cells in HC and MS patients.
Fig. S3. Levels of IL‐17 and IFN‐γ secretion by cTfh17, non‐follicular Th17, and cTfh17.1 cells in HC and MS patients.
Fig. S4. Frequency of cTreg cells was lower in MS patients than in HC.
Fig. S5. Frequency of IL‐10 positive cTreg cells was lower in MS patients than in HC.
Fig. S6. Comparison of treated and untreated MS patients.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF‐2018R1A5A2023127) and International Cooperation and Education Program (NCCRI.NCCI 52210‐52211, 2019) of the National Cancer Center, South Korea.
Data Availability Statement
All data generated or analyzed during this study are included this published article.
References
- 1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med 2018; 378:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chitnis T. The role of CD4 T cells in the pathogenesis of multiple sclerosis. Int Rev Neurobiol 2007; 79:43–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaskow BJ, Baecher‐Allan C. Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med 2018; 8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol 2008; 7:852–8. [DOI] [PubMed] [Google Scholar]
- 5. Li R, Patterson KR, Bar‐Or A. Reassessing B cell contributions in multiple sclerosis. Nat Immunol 2018; 19:696–707. [DOI] [PubMed] [Google Scholar]
- 6. Jelcic I, Al Nimer F, Wang J et al. Memory B cells activate brain‐homing, autoreactive CD4+ T cells in multiple sclerosis. Cell 2018; 175:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crotty S. Follicular helper CD4 T cells (TFH). Ann Rev Immunol 2011; 29:621–63. [DOI] [PubMed] [Google Scholar]
- 8. Yu DI, Rao S, Tsai LM et al. The transcriptional repressor Bcl‐6 directs T follicular helper cell lineage commitment. Immunity 2009; 31:457–68. [DOI] [PubMed] [Google Scholar]
- 9. Bossaller L, Burger J, Draeger R et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol 2006; 177:4927–32. [DOI] [PubMed] [Google Scholar]
- 10. Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H. PD‐1 controls follicular T helper cell positioning and function. Immunity 2018; 49:264–74.e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chtanova T, Tangye SG, Newton R et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non‐Th1/Th2 effector cells that provide help for B cells. J Immunol 2004; 173:68–78. [DOI] [PubMed] [Google Scholar]
- 12. Nurieva RI, Chung Y, Hwang D et al. Generation of T follicular helper cells is mediated by interleukin‐21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008; 29:138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bryant VL, Ma CS, Avery DT et al. Cytokine‐mediated regulation of human B cell differentiation into Ig‐secreting cells: predominant role of IL‐21 produced by CXCR5+ T follicular helper cells. J Immunol 2007; 179:8180–90. [DOI] [PubMed] [Google Scholar]
- 14. Chung Y, Tanaka S, Chu F et al. Follicular regulatory T cells expressing Foxp3 and Bcl‐6 suppress germinal center reactions. Nat Med 2011; 17:983–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Venken K, Hellings N, Thewissen M et al. Compromised CD4+ CD25(high) regulatory T‐cell function in patients with relapsing‐remitting multiple sclerosis is correlated with a reduced frequency of FOXP3‐positive cells and reduced FOXP3 expression at the single‐cell level. Immunology 2008; 123:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laidlaw BJ, Lu Y, Amezquita RA et al. Interleukin‐10 from CD4(+) follicular regulatory T cells promotes the germinal center response. Sci Immunol 2017; 2:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wollenberg I, Agua‐Doce A, Hernandez A et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol 2011; 187:4553–60. [DOI] [PubMed] [Google Scholar]
- 18. Linterman MA, Pierson W, Lee SK et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med 2011; 17:975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Linterman MA, Vinuesa CG. T follicular helper cells during immunity and tolerance. Prog Mol Biol Transl Sci 2010; 92:207–48. [DOI] [PubMed] [Google Scholar]
- 20. Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol 2014; 35:436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sage PT, Alvarez D, Godec J, von Andrian UH, Sharpe AH. Circulating T follicular regulatory and helper cells have memory‐like properties. J Clin Invest 2014; 124:5191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simpson N, Gatenby PA, Wilson A et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum 2010; 62:234–44. [DOI] [PubMed] [Google Scholar]
- 23. Asrir A, Aloulou M, Gador M, Pérals C, Fazilleau N. Interconnected subsets of memory follicular helper T cells have different effector functions. Nat Commun 2017; 8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hale JS, Ahmed R. Memory T follicular helper CD4 T cells. Front Immunol 2015; 6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morita R, Schmitt N, Bentebibel S‐E et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu B, Wang S, Zhou M et al. The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus. Clin Immunol 2017; 183:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu C, Wang D, Lu S et al. Increased circulating follicular Treg cells are associated with lower levels of autoantibodies in patients with rheumatoid arthritis in stable remission. Arthritis Rheumatol 2018; 70:711–21. [DOI] [PubMed] [Google Scholar]
- 28. Liu R, Wu Q, Su D et al. A regulatory effect of IL‐21 on T follicular helper‐like cell and B cell in rheumatoid arthritis. Arthritis Res Ther 2012; 14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang C‐J, Gong YE, Zhu W et al. Augmentation of circulating follicular helper T cells and their impact on autoreactive B cells in myasthenia gravis. J Immunol 2016; 197:2610–7. [DOI] [PubMed] [Google Scholar]
- 30. Le Coz C, Joublin A, Pasquali JL, Korganow AS, Dumortier H, Monneaux F. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLOS ONE 2013; 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arroyo‐Villa I, Bautista‐Caro M‐B, Balsa A et al. Constitutively altered frequencies of circulating follicullar helper T cell counterparts and their subsets in rheumatoid arthritis. Arthritis Res Ther 2014; 16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akiyama M, Suzuki K, Yamaoka K et al. Number of circulating follicular helper 2 T cells correlates with IgG4 and interleukin‐4 levels and plasmablast numbers in IgG4‐related disease. Arthritis Rheumatol 2015; 67:2476–81. [DOI] [PubMed] [Google Scholar]
- 33. Fan X, Jin T, Zhao S et al. Circulating CCR7+ICOS+ memory T follicular helper cells in patients with multiple sclerosis. PLOS ONE 2015; 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhaeze T, Peelen E, Hombrouck A et al. Circulating follicular regulatory T cells are defective in multiple sclerosis. J Immunol 2015; 195:832–40. [DOI] [PubMed] [Google Scholar]
- 35. Thompson AJ, Banwell BL, Barkhof F et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17:162–73. [DOI] [PubMed] [Google Scholar]
- 36. Arellano G, Acuña E, Reyes LI et al. Th1 and Th17 cells and associated cytokines discriminate among clinically isolated syndrome and multiple sclerosis phenotypes. Front Immunol 2017; 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Langelaar J, van der Vuurst de Vries RM, Janssen M et al. T helper 17.1 cells associate with multiple sclerosis disease activity: perspectives for early intervention. Brain 2018; 141:1334–49. [DOI] [PubMed] [Google Scholar]
- 38. Ramstein J, Broos CE, Simpson LJ et al. IFN‐gamma‐producing T‐helper 17.1 cells are increased in sarcoidosis and are more prevalent than T‐helper type 1 cells. Am J Respir Crit Care Med 2016; 193:1281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Linterman MA, Rigby RJ, Wong RK et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med 2009; 206:561–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu C, Ma J, Liu Y et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab 2012; 97:943–50. [DOI] [PubMed] [Google Scholar]
- 41. Christensen JR, Börnsen L, Ratzer R et al. Systemic inflammation in progressive multiple sclerosis involves follicular T‐helper, Th17‐and activated B‐cells and correlates with progression. PLOS ONE 2013; 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tzartos JS, Craner MJ, Friese MA et al. IL‐21 and IL‐21 receptor expression in lymphocytes and neurons in multiple sclerosis brain. Am J Pathol 2011; 178:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nicolas P, Ruiz A, Cobo‐Calvo A et al. The balance in T follicular helper cell subsets is altered in neuromyelitis optica spectrum disorder patients and restored by rituximab. Front Immunol 2019; 10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cunill V, Massot M, Clemente A et al. Relapsing–remitting multiple sclerosis is characterized by a T follicular cell pro‐inflammatory shift. reverted by dimethyl fumarate treatment. Front immunol 2018; 9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Babaloo Z, Aliparasti MR, Babaiea F, Almasi S, Baradaran B, Farhoudi M. The role of Th17 cells in patients with relapsing–remitting multiple sclerosis: Interleukin‐17A and interleukin‐17F serum levels. Immunol Lett 2015; 164:76–80. [DOI] [PubMed] [Google Scholar]
- 46. Matusevicius D, Kivisäkk P, He B et al. Interleukin‐17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 1999; 5:101–4. [DOI] [PubMed] [Google Scholar]
- 47. Zhang X, Markovic‐Plese S. Interferon beta inhibits the Th17 cell‐mediated autoimmune response in patients with relapsing–remitting multiple sclerosis. Clin Neurol Neurosurg 2010; 112:641–5. [DOI] [PubMed] [Google Scholar]
- 48. Wu Q, Wang Q, Mao GA‐O, Dowling CA, Lundy SK, Mao‐Draayer Y. Dimethyl fumarate selectively reduces memory T Cells and shifts the balance between Th1/Th17 and Th2 in multiple sclerosis patients. J Immunol 2017; 198:3069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Viglietta V, Baecher‐Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 2004; 199:971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mexhitaj I, Nyirenda MH, Li R et al. Abnormal effector and regulatory T cell subsets in paediatric‐onset multiple sclerosis. Brain 2019; 142:617–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van Boxel‐Dezaire AH, Hoff SC, van Oosten BW et al. Lower interleukin‐10 and increased interleukin‐12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann Neurol 1999; 45:695–703. [DOI] [PubMed] [Google Scholar]
- 52. Waubant E, Gee L, Bacchetti P et al. Relationship between serum levels of IL‐10, MRI activity and interferon beta‐1a therapy in patients with relapsing remitting MS. J Neuroimmunol 2001; 112:139–45. [DOI] [PubMed] [Google Scholar]
- 53. Petereit HF, Pukrop R, Fazekas F et al. Low interleukin‐10 production is associated with higher disability and MRI lesion load in secondary progressive multiple sclerosis. J Neurol Sci 2003; 206:209–14. [DOI] [PubMed] [Google Scholar]
- 54. Balashov KE, Comabella M, Ohashi T et al. Defective regulation of IFN‐γ and IL‐12 by endogenous IL‐10 in progressive MS. Neurology 2000; 55:192–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. No significant difference in the levels of IL‐17, IL‐4, and IFN‐γ secretions between HC and MS.
Fig. S2. Levels of IFN‐γ, IL‐17, and IL‐4 secretion by cTfh cells in HC and MS patients.
Fig. S3. Levels of IL‐17 and IFN‐γ secretion by cTfh17, non‐follicular Th17, and cTfh17.1 cells in HC and MS patients.
Fig. S4. Frequency of cTreg cells was lower in MS patients than in HC.
Fig. S5. Frequency of IL‐10 positive cTreg cells was lower in MS patients than in HC.
Fig. S6. Comparison of treated and untreated MS patients.
Data Availability Statement
All data generated or analyzed during this study are included this published article.


