Abstract
Head and neck cancers, especially in hypopharynx and oropharynx, are often detected at advanced stage with poor prognosis. Narrow band imaging enables detection of superficial cancers and transoral surgery is performed with curative intent. However, pathological evaluation and real‐world safety and clinical outcomes have not been clearly understood. The aim of this nationwide multicenter study was to investigate the safety and efficacy of transoral surgery for superficial head and neck cancer. We collected the patients with superficial head and neck squamous cell carcinoma who were treated by transoral surgery from 27 hospitals in Japan. Central pathology review was undertaken on all of the resected specimens. The primary objective was effectiveness of transoral surgery, and the secondary objective was safety including incidence and severity of adverse events. Among the 568 patients, a total of 662 lesions were primarily treated by 575 sessions of transoral surgery. The median tumor diameter was 12 mm (range 1–75) endoscopically. Among the lesions, 57.4% were diagnosed as squamous cell carcinoma in situ. The median procedure time was 48 minutes (range 2–357). Adverse events occurred in 12.7%. Life‐threatening complications occurred in 0.5%, but there were no treatment‐related deaths. During a median follow‐up period of 46.1 months (range 1–113), the 3‐year overall survival rate, relapse‐free survival rate, cause‐specific survival rate, and larynx‐preservation survival rate were 88.1%, 84.4%, 99.6%, and 87.5%, respectively. Transoral surgery for superficial head and neck cancer offers effective minimally invasive treatment.
Clinical trials registry number: UMIN000008276.
Keywords: head and neck cancer, larynx preservation, pharyngeal cancer, superficial cancer, transoral surgery
This is the first report on nationwide survival data and pathological criteria of superficial head and neck cancer. Transoral surgery for superficial head and neck cancer was safe and offered highly curative minimally invasive treatment preserving organs and their function. Subepithelial invasion was clinically useful predictor of recurrence after transoral surgery.
1. INTRODUCTION
Worldwide incidence and mortality of oropharyngeal cancer are reported as 92,887 and 51,005 and those of hypopharyngeal cancer are 80,608 and 34,984 in 2018. 1 The prognosis is still poor even with multimodal treatment because most patients have locally advanced disease with lymph node involvement at the time of diagnosis and have a propensity for developing distant metastasis. 2 , 3
The standard treatment for resectable oro‐ and hypopharyngeal cancer is laryngopharyngectomy with pharyngeal reconstruction, leading to a loss of natural speech and a difficulty of swallowing. 4 An alternative treatment is chemoradiotherapy, which can preserve organ and function. However, it often caused serious adverse effects, such as dysphagia, due to severe mucositis and xerostomia, negatively affecting patients’ quality of life. 5
The ideal approach to improve the patients’ survival and to preserve organ and function is early detection of cancer and applying minimally invasive treatment. 6 Tumor located within the epithelium and subepithelial layer was categorized as superficial cancer. 7 Muto et al. reported that narrow band imaging (NBI: Olympus Co., Ltd.) enabled virtual chromoendoscopy and early detection of superficial head and neck cancer. 8 Then, NBI is now widely used in clinical practice in many countries. 9 , 10 , 11 , 12 , 13 , 14 , 15
For superficial lesions, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have been indicated and showed effectiveness. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Recently, transoral video‐assisted surgery (TOVS) and endoscopic laryngopharyngeal surgery (ELPS) have also been indicated. 25 , 26 , 27 , 28 , 29 Together, EMR, ESD, TOVS, and ELPS are classified as transoral surgery (TOS). While TOS has been widely indicated for superficial head and neck cancer, their pathological evaluation is not standardized. Then, the clinical management after TOS is not also standardized. In addition, the real‐world effectiveness and safety of TOS for superficial head and neck cancer have not been well defined. We, therefore, conducted a national multi‐center survey of TOS in Japan.
2. MATERIALS AND METHODS
2.1. Participants
Patients who were primarily treated by TOS from April 2001 through July 2012 were retrospectively collected from 27 hospitals in Japan. The inclusion criteria were as follows: (a) tumors pathologically diagnosed as squamous cell carcinoma (SCC), (b) tumors invasion was pathologically limited within subepithelial layer, (c) no exposure of tumor cells to the vertical margin (negative vertical margin), (d) macroscopic tumor location in the oropharynx, hypopharynx, or supraglottis, (e) no regional lymph node metastasis on computed tomography, and (f) no other active advanced cancer in the head or neck region.
Written informed consent was obtained from all patients for the procedures in this study. Patients with concomitant primary cancer in any other organ were excluded. If cancers in other organs have been curatively treated when initial TOS was indicated, the patients were included. This study was approved by ethics committees in all participating hospitals and was registered in UMIN Clinical Trials Registry (UMIN000008276).
2.2. Transoral surgery (TOS)
TOS is defined as a procedure or an operation to perform mucosectomy for which a surgical device and visual guidance are inserted from mouth. Ablation procedure is not included in TOS. EMR and ESD were mainly performed by gastroenterologists. Others were mainly performed by head and neck surgeons.
2.3. Outcomes/Survey variables
The primary objective was effectiveness of TOS, and the secondary objective was safety including incidence and severity of adverse events. The survey variables were as follows: (a) the clinicopathological characteristics of patients with superficial SCC of head and neck, (b) adverse events associated with TOS, (c) incidences of local recurrence, regional lymph node recurrence, and distant recurrence after TOS and subsequent treatments, (d) incidence of and treatment regimen for metachronous cancer, and (e) the survival data on follow‐up duration (overall survival, relapse‐free survival, cause‐specific survival, and larynx‐preservation survival after TOS).
2.4. Histopathological analysis
One certified pathologist (S.F.) performed centralized pathology review of registered patients and excluded the patients without SCC, those with SCC with muscularis propria invasion, and those with histologic cancer types other than SCC. As a second step, 10 certified pathologists developed a new set of diagnostic criteria to distinguish subepithelial invasive SCC from SCC in situ for this study. The criteria were as follows; at least one solitary nest of epithelial neoplastic cells is present in the stroma clearly separated from intraepithelial carcinoma or intraepithelial carcinoma with a thickness of 500 μm or greater. As a third step, we conducted a central pathological review board by three certified pathologists (M.F., T.N., and M.I.). This board defined the presence or absence of invasion blinded to the clinical findings according to the diagnostic criteria. Consensus decision making was used to make final pathological diagnosis.
2.5. Local, regional lymph node and distant recurrence
Local recurrence was defined as a development of tumor at the treatment site of TOS. Regional lymph node and distant recurrence were defined as an abnormal enlargement of lymph node and a new lesion in distant location detected on the computed tomography, respectively.
2.6. Metachronous cancer
Metachronous cancer was defined as cancer detected in the region after initial TOS that was clearly separate from the resection scar. Metachronous cancer in other organs was defined as cancer arising in organs other than the head and neck after initial TOS.
2.7. Statistical analysis
p‐values for categorical data were calculated by using Kruskal‐Wallis test for trends in the median procedure times and using Fisher's exact test for other variables related to the safety of transoral surgery, respectively. Overall survival rates, relapse‐free survival rates, and cause‐specific survival rates were estimated using the Kaplan–Meier method and tested by log‐rank tests. Cumulative incidence of metachronous head and neck cancers, metachronous cancers arising in other organs, and larynx‐preservation survival that events were laryngectomy and all death were estimated using the Kaplan–Meier method. We defined the time to the development of a metachronous cancer as the period from the day of TOS to the day of diagnosis of a metachronous cancer. All data were analyzed with SAS (version 9). All authors had access to the study data and have reviewed and approved the final manuscript.
3. RESULTS
3.1. Participants
A total of 599 patients with superficial head and neck cancer (700 lesions) were registered. We excluded 12 patients (14 lesions) with no available pathological specimens and 10 patients (11 lesions) with inadequate follow‐ups. The specimens of the remaining 577 patients (675 lesions) were carefully screened by one certified pathologist per protocol. This screening excluded 4 patients (4 lesions) with non‐cancerous lesions, 2 patients (2 lesions) with SCC invasion to muscular layer, 2 patients (5 lesions) with insufficient specimen to evaluate pathological findings, and 1 patient (2 lesions) with a tumor of a histologic type other than SCC (spindle cell carcinoma). Finally, a total of 568 patients (662 lesions) were included in the analysis. The total number of TOS sessions for 568 patients was 575 because 7 other sessions for synchronous lesions were performed on another day (Figure 1).
FIGURE 1.
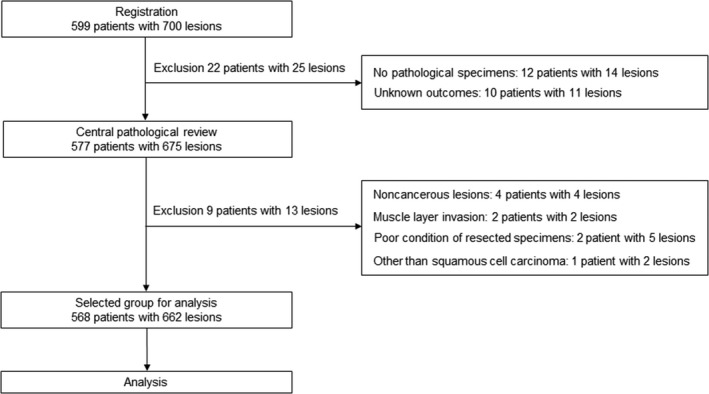
Flow chart of patients and lesions. *Seven other sessions for synchronous lesions were performed on another day
Table 1 shows the demographic characteristics of the study patients. The median age was 66 years (range 33 to 89), and 534 (94.0%) of the subjects were men. The performance status was 0 in 539 patients (94.9%). The most common reason for the detection of superficial head and neck cancer was endoscopic examination before or after the treatment of esophageal cancer (366 patients, 64.4%). The total number of patients with previous head and neck cancer and history of other cancer was 141 and 531 (includes overlapping patients), respectively. Among the 531 patients with previous cancer, 416 (78.3%) had history of esophageal cancer. Treatment for these cancers was summarized in Table 2.
TABLE 1.
Patient characteristics
| Total number of patients | 568 |
| Main factor leading to detection | |
| Before/after treatment of esophageal cancer | 366 (64.4%) |
| Before/after treatment of head and neck cancer | 83 (14.6%) |
| Medical checkups | 55 (9.7%) |
| Pharyngolaryngeal paresthesia | 49 (8.6%) |
| Before/after treatment of gastric cancer | 15 (2.6%) |
| Age, median (range) | 66 (33–89) |
| Sex (male) | 534 (94.0%) |
| Performance status (0/1/2/3/4) |
539 (94.9%) / 22 (3.9%) / 6 (1.1%) / 1 (0.2%) / 0 (0.0%) |
| History of head and neck cancer | |
| Total number of previous head and neck cancers a | 141 |
| Hypopharynx | 43 (30.5%) |
| Oral cavity | 38 (27.0%) |
| Larynx | 31 (22.0%) |
| Oropharynx | 25 (17.7%) |
| Primary unknown | 2 (1.4%) |
| Maxilla | 2 (1.4%) |
| History of cancer in other organ | |
| Total number of previous cancers a | 531 |
| Esophageal cancer | 416 (78.3%) |
| Gastric cancer | 74 (13.9%) |
| Colorectal cancer | 12 (2.3%) |
| Prostate cancer | 9 (1.7%) |
| Lung cancer | 5 (0.9%) |
| Liver cancer | 3 (0.6%) |
| Breast cancer | 2 (0.4%) |
| Skin cancer | 2 (0.4%) |
| Bladder cancer | 2 (0.4%) |
| Malignant lymphoma | 2 (0.4%) |
| Bile‐duct cancer | 1 (0.2%) |
| Thyroid cancer | 1 (0.2%) |
| Duodenal cancer | 1 (0.2%) |
| Anal canal cancer | 1 (0.2%) |
Including overlapping patients.
TABLE 2.
Treatment history of head and neck cancer and cancer in other organ
| Total number of previous cancers a | Surgery‐based therapy | Endoscopy‐based therapy | Chemoradiation –based therapy | Chemotherapy or hormonal therapy | Observation | TACE/RFA | Unknown | |
|---|---|---|---|---|---|---|---|---|
| Head and neck cancer | 141 | 75 | 0 | 60 | 5 | 1 | 0 | 0 |
| Esophageal cancer | 416 | 120 | 213 | 80 | 0 | 2 | 0 | 1 |
| Gastric cancer | 74 | 37 | 34 | 1 | 2 | 0 | 0 | 0 |
| Colorectal cancer | 12 | 9 | 2 | 0 | 1 | 0 | 0 | 0 |
| Prostate cancer | 9 | 4 | 0 | 1 | 4 | 0 | 0 | 0 |
| Lung cancer | 5 | 3 | 1 | 1 | 0 | 0 | 0 | 0 |
| Liver cancer | 3 | 1 | 0 | 0 | 0 | 0 | 2 | 0 |
| Bladder cancer | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Breast cancer | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Skin cancer | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Malignant lymphoma | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Bile‐duct cancer | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Duodenal cancer | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anal canal cancer | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Thyroid cancer | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 672 | 257 | 251 | 146 | 12 | 3 | 2 | 1 |
Abbreviations: RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization.
Including overlapping patients.
Table 3 shows the demographic characteristics of the treated lesions. Among the 662 lesions, 519 (78.4%) were located in the hypopharynx and 132 lesions (19.9%) were located in the oropharynx. The most common macroscopic types was flat (528 lesions, 79.8%). The procedures for TOS were EMR (307 lesions, 46.2%), ESD (264 lesions, 39.7%), ELPS (31 lesions, 4.7%), and TOVS (31 lesions, 4.7%). A total of 490 lesions (74.0%) underwent en bloc resection. The median tumor diameter was 12 mm (range 1–75) endoscopically and 14 mm (range 1–60) pathologically. The median diameters of the resected tumor specimens in EMR, ESD, ELPS, TOVS, and other procedures were 12, 15, 20, 16, and 13 mm, respectively (ranges: 1–45, 1–60, 2–58, 5–42, and 3–50 mm, respectively). Three hundred and eighty lesions (57.4%) were revealed to be intraepithelial SCC based on the central pathological review on the depth of invasion. The T categories were found to be Tis (380 lesions, 57.4%), T1 (181 lesions, 27.3%), T2 (89 lesions, 13.4%), T3 (11 lesions, 1.7%), and unknown (1 lesion, 0.2%). Subsequent treatment was performed immediately after initial TOS for 20 lesions (3.0%).
TABLE 3.
Lesion characteristics
| Total number of lesions | 662 |
| Tumor location | |
| Oropharynx | 132 (19.9%) |
| Anterior wall / Posterior wall / Lateral wall / Superior wall | 9/79/23/21 |
| Hypopharynx | 519 (78.4%) |
| Postcricoid / Pyriform sinus / Posterior wall | 33/404/82 |
| Larynx | 7 (1.1%) |
| Laryngeal epiglottis / Laryngeal arytenoid / Aryepiglottic folds | 4/2/1 |
| Oral cavity | 4 (0.6%) |
| Oral floor / Hard palate / Buccal mucosa | 1/1/2 |
| Macroscopic type | |
| Flat / Elevated / Unknown | 528 (79.8%) / 127 (19.2%) / 7 (1.1%) |
| Treatment methods | |
| Endoscopic mucosal resection (EMR) | 307 (46.2%) |
| Endoscopic submucosal dissection (ESD) | 264 (39.7%) |
| Endoscopic laryngopharyngeal surgery (ELPS) | 31 (4.7%) |
| Transoral videolaryngoscopic surgery (TOVS) | 31 (4.7%) |
| Laser microlaryngeal surgery | 17 (2.6%) |
| Direct mucosectomy | 12 (1.8%) |
| Number of resected specimens | |
| En bloc | 490 (74.0%) |
| Piecemeal | 172 (26.0%) |
| Number of segments obtained by piecemeal resection | |
| 2/3/4/5/6/7/8/9/10/11 | 85/39/13/11/10/7/3/1/2/1 |
| Tumor diameter on endoscopic images, median (range) a | 12 (1–75) |
| Tumor diameter of resected specimens, median (range) b | 14 (1–60) |
| EMR / ESD / ELPS / TOVS / Other procedures | 12 (1–45) / 15 (1–60) / 20 (2–58) / 16 (5–42) / 13 (3–50) |
| Endoscopic depth of invasion for resected lesions | |
| Intraepithelial / Subepithelial / Difficult to evaluate | 472 (71.0%) / 158 (23.8%) / 32 (4.8%) |
| Histopathological depth of invasion (central diagnosis) | |
| Intraepithelial / Subepithelial | 380 (57.4%) / 282 (42.6%) |
| T category | |
| Tis / T1 / T2 / T3 / Unknown | 380 (57.4%) / 181 (27.3%) / 89 (13.4%) / 11 (1.7%) / 1 (0.2%) |
| Lymphatic invasion | 19 (2.9%) |
| Venous invasion | 16 (2.4%) |
| Horizontal margin positive for cancer in the resected specimen | 309 (46.7%) |
| Subsequent treatment immediately after initial transoral surgery | 20 (3.0%) |
Missing data for 29 patients.
Missing data for 1 patient.
3.2. Adverse events
Among the 575 treatment sessions, most of the procedure was underwent under general anesthesia (545 sessions, 94.8%). The median procedure time was 48 minutes (range 2–357). EMR was performed in a short time (32 minutes, p < 0.0001). Adverse events occurred in 12.7% (73/575). The main adverse events were laryngeal edema (33 sessions, 5.7%), subcutaneous emphysema (20 sessions, 3.5%), aspiration pneumonia (14 sessions, 2.4%), and bleeding (11 sessions, 1.9%). Subcutaneous emphysema frequently occurred in ESD (6.3%, p < 0.0178). Temporary tracheotomy was performed in 49 treatment sessions (8.5%). The main reasons for tracheotomy were development of laryngeal edema (22 sessions, 3.8%) and perioperative planned management (21 sessions, 3.7%). There were no treatment‐related deaths; however, 3 patients (0.5%) developed life‐threatening severe adverse events. Those were as follows: 1) one patient underwent an emergency tracheotomy because of suffocation caused by laryngeal edema after surgery, 2) one patient underwent an emergency tracheotomy because of arterial bleeding and hemostasis was achieved by ligation of the blood vessels, and 3) one patient had transient cardiopulmonary arrest caused by aspiration of food during a meal on the following day and recovered after removal of the foreign object through a tracheostoma (Table 4).
TABLE 4.
Variables related to the safety of transoral surgery
| Total | Endoscopic mucosal resection | Endoscopic submucosal dissection | Other procedures | p‐value | |
|---|---|---|---|---|---|
| Total number of treatment sessions | 575 | 263 | 222 | 90 | |
| Methods for anesthesia | 0.0353 | ||||
| General anesthesia | 545 (94.8%) | 242 (92.0%) | 214 (96.4%) | 89 (98.9%) | |
| Intravenous anesthesia | 29 (5.0%) | 20 (7.6%) | 8 (3.6%) | 1 (1.1%) | |
| None | 1 (0.2%) | 1 (0.4%) | 0 (0.0%) | 0 (0.0%) | |
| Procedure time, median (range), min a | 48 (2–357) | 32 (2–240) | 60 (15–357) | 71 (6–300) | <0.0001 |
| Adverse events | 73 (12.7%) | 28 (10.6%) | 36 (16.2%) | 9 (10.0%) | 0.1399 |
| Laryngeal edema | 33 (5.7%) | 18 (6.8%) | 13 (5.9%) | 2 (2.2%) | 0.2593 |
| Subcutaneous emphysema | 20 (3.5%) | 5 (1.9%) | 14 (6.3%) | 1 (1.1%) | 0.0178 |
| Aspiration pneumonia | 14 (2.4%) | 4 (1.5%) | 9 (4.1%) | 1 (1.1%) | 0.1704 |
| Bleeding | 11 (1.9%) | 5 (1.9%) | 5 (2.3%) | 1 (1.1%) | 0.923 |
| Stenosis | 3 (0.5%) | 1 (0.4%) | 2 (0.9%) | 0 (0.0%) | 0.7573 |
| Cerebral infarction | 2 (0.3%) | 1 (0.4%) | 1 (0.5%) | 0 (0.0%) | 1 |
| Dermatitis caused by iodine | 1 (0.2%) | 1 (0.4%) | 0 (0.0%) | 0 (0.0%) | 1 |
| Tooth injury | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 1 (1.1%) | 0.1565 |
| Mediastinitis | 1 (0.2%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 0.5426 |
| Temporary tracheotomy | 49 (8.5%) | 22 (8.4%) | 22 (9.9%) | 5 (5.6%) | 0.4964 |
| Reason for tracheotomy | |||||
| Development of laryngeal edema b | 22 (3.8%) | 17 (6.5%) b | 4 (1.8%) | 1 (1.1%) | 0.0113 |
| Perioperative planned management b | 21 (3.7%) | 6 (2.3%) b | 12 (5.4%) | 3 (3.3%) | 0.1869 |
| Difficulty for intraoperative bleeding management | 2 (0.3%) | 0 (0.0%) | 1 (0.5%) | 1 (1.1%) | 0.1453 |
| Unknown | 5 (0.9%) | 0 (0.0%) | 5 (2.3%) | 0 (0.0%) | 0.0243 |
| Life‐threatening severe adverse event | 3 (0.5%) | 1 (0.4%) | 2 (0.9%) | 0 (0.0%) | 0.7573 |
| Treatment‐related death | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | — |
Missing data for 21 patients.
One overlapping patient.
3.3. Local, regional lymph node and distant recurrence
Median follow‐up period was 46.1 months (range 1–113). Recurrence data and their treatment were summarized in Table 5. Among 662 lesions treated by TOS, 53 lesions (8.0%) developed local recurrence. Local recurrence rates of EMR, ESD, and other procedures were 11.7% (35/298), 2.7% (7/258), and 10.4% (11/106), respectively (p < 0.0001). The median diameters of the resected tumor specimens which developed local recurrence and specimens that did not develop local recurrence were 16 (range: 3–45 mm) and 14 mm (range: 1–60 mm), respectively. There was no relation between tumor size and local recurrence (p = 0.13). Thirty‐nine lesions (73.6%) were treated by re‐TOS. Traditional open surgery with and without laryngectomy were performed in 3 lesions (5.7%) and 2 lesions (3.8%), respectively. Remaining 9 lesions (17.0%) were treated with non‐surgical treatment. Regional lymph node recurrence developed in 26 patients (4.6%). Among them, 20 patients (76.9%) developed on the same side of the neck. Radical neck dissection was performed in 15 patients (57.7%), and 8 patients (30.8%) received neck dissection plus postoperative chemotherapy and/or radiotherapy. Three patients (11.5%) received definitive chemoradiotherapy. Three patients (0.5%) had distant recurrence; two had lung metastasis and remaining one had lung and liver metastasis. Two patients (66.7%) were followed up without any treatment, and 1 patient (33.3%) received chemotherapy.
TABLE 5.
Recurrence, metachronous head and neck cancer, and their treatment after transoral surgery
| Local recurrence (n = 662 lesions) | 53 (8.0%) |
| Treatment for recurrent lesions | |
| Transoral surgery | 39 (73.6%) |
| Traditional open surgery | 5 (9.4%) |
| With laryngectomy | 3 (5.7%) |
| Without laryngectomy | 2 (3.8%) |
| Observation | 3 (5.7%) |
| Definitive chemoradiotherapy | 2 (3.8%) |
| Radiotherapy | 2 (3.8%) |
| Argon plasma coagulation | 1 (1.9%) |
| Laser ablation | 1 (1.9%) |
| Regional lymph node recurrence (n = 568 patients) | 26 (4.6%) |
| Location of recurrent lesions | |
| Only same side | 20 (76.9%) |
| Only opposite side | 2 (7.7%) |
| Both sides | 2 (7.7%) |
| Unknown | 2 (7.7%) |
| Treatment for recurrent lesions | |
| Neck dissection | 15 (57.7%) |
| Neck dissection + postoperative chemotherapy | 3 (11.5%) |
| Neck dissection + postoperative radiotherapy | 3 (11.5%) |
| Neck dissection + postoperative chemoradiotherapy | 2 (7.7%) |
| Definitive chemoradiotherapy | 3 (11.5%) |
| Distant recurrence (n = 568 patients) | 3 (0.5%) |
| Location of recurrent lesions | |
| Lung | 2 (66.7%) |
| Lung + Liver | 1 (33.3%) |
| Treatment for recurrent lesions | |
| Chemotherapy | 1 (33.3%) |
| Observation | 2 (66.7%) |
| Metachronous head and neck cancer (n = 568 patients) | 132 (23.2%) with 234 lesions |
| Treatment for metachronous lesions | |
| Transoral surgery | 207 (88.5%) |
| Traditional open surgery | 5 (2.1%) |
| With laryngectomy | 1 (0.4%) |
| Without laryngectomy | 4 (1.7%) |
| Argon plasma coagulation | 9 (3.8%) |
| Radiotherapy | 6 (2.6%) |
| Observation | 3 (1.3%) |
| Definitive chemoradiotherapy | 1 (0.4%) |
| Chemotherapy | 1 (0.4%) |
| Unknown | 2 (0.9%) |
3.4. Metachronous cancer
A total of 234 metachronous head and neck cancers were diagnosed in 132 patients (23.2%) during the follow‐up period. The 3‐year cumulative incidence rate of metachronous head and neck cancers after TOS was 16.7% (95% confidence interval, 13.7% to 20.2%) (Figure 2A). Among 234 lesions, 207 (88.5%) were again treated by TOS. Traditional open surgery with and without laryngectomy were performed in 1 lesion (0.4%) and 4 lesions (1.7%), respectively. Other 20 lesions (8.5%) were treated with non‐surgical treatment and the treatment details of 2 lesions (0.9%) were not available (Table 5).
FIGURE 2.
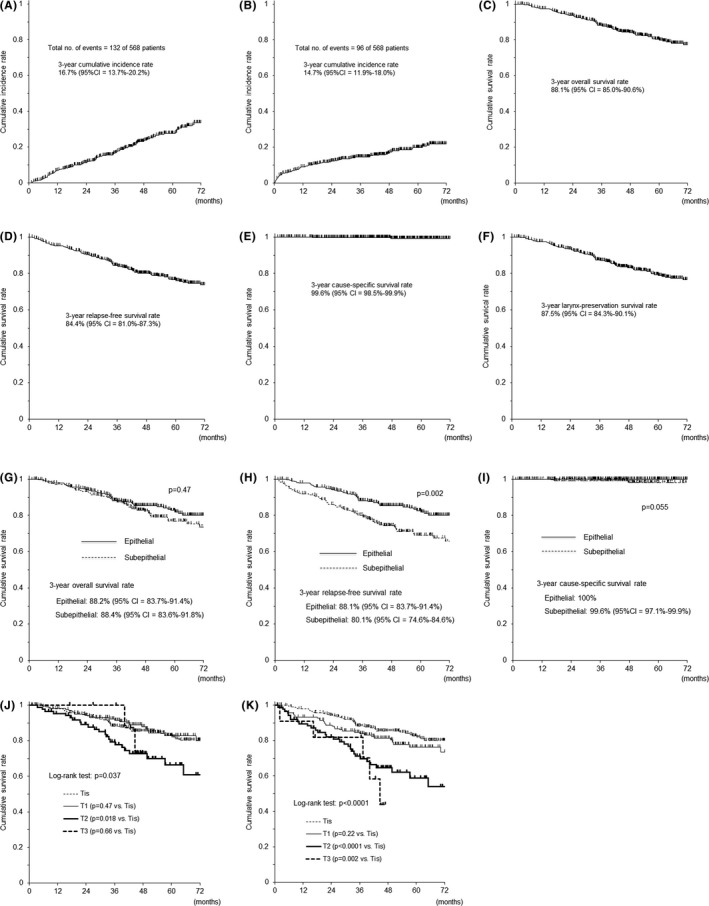
Cumulative incidence of metachronous cancers and survival rate. A, Cumulative incidence rate of metachronous head and neck cancers. B, Cumulative incidence rate of metachronous cancers arising in other organs. C, Overall survival rate. D, Relapse‐free survival rate. E, Cause‐specific survival rate. F, Larynx‐preservation survival rate. G, Overall survival rates according to the histopathological depth of invasion. H, Relapse‐free survival rates according to the histopathological depth of invasion. I, Cause‐specific survival rates according to the histopathological depth of invasion. J, Overall survival rates according to the T category. K, Relapse‐free survival rates according to the T category
A total of 131 metachronous cancers arising in other organ were diagnosed in 96 patients (16.9%) during the follow‐up period. The 3‐year cumulative incidence rate of metachronous cancers arising in other organs after TOS was 14.7% (95% CI, 11.9% to 18.0%) (Figure 2B). And treatment for these cancers were summarized in Table 6. Esophagus was the main sites of metachronous cancer arising in other organ. Among 90 metachronous esophageal cancers, endoscopic resection was performed in 74 lesions (82.2%), surgery‐based treatment in 11 lesions (12.2%), and chemoradiation‐based treatment in 5 lesions (5.6%).
TABLE 6.
Treatment of metachronous cancer arising in other organ
| Total number of metachronous cancers a | Endoscopic resection | Surgery‐based therapy | Chemoradiation‐based therapy | Chemotherapy or hormonal therapy | Observation | TACE/RFA b | |
|---|---|---|---|---|---|---|---|
| Esophageal cancer | 90 | 74 | 11 | 5 | 0 | 0 | 0 |
| Gastric cancer | 16 | 12 | 4 | 0 | 0 | 0 | 0 |
| Lung cancer | 10 | 1 | 6 | 2 | 1 | 0 | 0 |
| Colorectal cancer | 5 | 1 | 3 | 0 | 0 | 1 | 0 |
| Bile‐duct cancer | 4 | 0 | 3 | 0 | 1 | 0 | 0 |
| Liver cancer | 2 | 0 | 1 | 0 | 0 | 0 | 1 |
| Duodenal cancer | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Prostate cancer | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Urinary tract cancer | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Thyroid cancer | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Total | 131 | 88 | 30 | 7 | 3 | 2 | 1 |
Including overlapping patients.
TACE: transcatheter arterial chemoembolization, RFA: radiofrequency ablation.
3.5. Survival
During a median follow‐up period of 46.1 months (range 1–113), 3 patients died of superficial head and neck cancer because of 2 distant metastasis and 1 local lymph node metastasis and 25 patients died of metachronous cancer arising in other organ. Specific sites of cancer among those 25 patients were esophageal cancer in 8 patients, lung cancer in 6 patients, colorectal cancer in 3 patients, gastric cancer in 2 patients, liver cancer in 2 patients, bile duct cancer in 2 patients, duodenal cancer in 1 patient, and ureteral cancer in 1 patient.
The 3‐year overall survival rate (Figure 2C) was 88.1% (95% CI, 85.0% to 90.6%), the 3‐year relapse‐free survival rate (Figure 2D) was 84.4% (95% CI, 81.0% to 87.3%), the 3‐year cause‐specific survival rate (Figure 2E) was 99.6% (95% CI, 98.5% to 99.9%), and the 3‐year larynx‐preservation survival rate (Figure 2F) was 87.5% (95% CI, 84.3% to 90.1%).
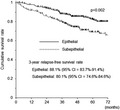
Survival was analyzed based on the depth of invasion (carcinoma in situ vs. cancer with subepithelial invasion) and results were compared. The 3‐year overall survival rates (Figure 2G), the 3‐year relapse‐free survival rates (Figure 2H), and the 3‐year cause‐specific survival rates (Figure 2I) were 88.2% (95% CI, 83.7% to 91.4%) vs. 88.4% (95% CI, 83.6% to 91.8%) (p = 0.47), 88.1% (95% CI, 83.7% to 91.4%) vs. 80.1% (95% CI, 74.6% to 84.6%) (p = 0.002), and 100% vs. 99.6% (95% CI, 97.1% to 99.9%) (p = 0.055), respectively.
Survival based on the T category was analyzed. The 3‐year overall survival rates of Tis, T1, T2, and T3 tumors (Figure 2J) were 88.2% (95% CI, 83.7% to 91.4%), 92.2% (95% CI, 86.7% to 95.5%), 79.3% (95% CI, 68.4% to 86.8%), and 100% (p = 0.037), respectively. The 3‐year relapse‐free survival rates of Tis, T1, T2, and T3 tumors (Figure 2K) were 88.1% (95% CI, 83.7% to 91.4%), 84.7% (95% CI, 78.0% to 89.5%), 71.2% (95% CI, 59.8% to 79.9%), and 81.8% (95% CI, 44.7% to 95.1%) (p < 0.0001), respectively. The 3‐year cause‐specific survival rates of Tis, T1, T2, and T3 tumors were 100%, 98.7% (95% CI, 94.9% to 99.7%), 100%, and 100% (p = 0.068), respectively.
4. DISCUSSION
This is the first report of national multi‐center survey of TOS for superficial head and neck cancer based on the standardized pathological evaluation. During a median follow‐up period of 46.1 months (range 1–113), the 3‐year overall survival rate and the 3‐year cause‐specific survival rate was 88.1% (95% CI. 85.0% to 90.6%) and 99.6% (95% CI, 98.5% to 99.9%), respectively. There was no treatment‐related death.
The most important clinical benefit of TOS was that it could preserve organ and function sparing patients from potentially devastating adverse events of radial surgery or chemoradiation. In this study, a total of 53 local recurrence (8.0%) developed after completion of TOS. However, 39 recurrent lesions (73.6%) were treated by re‐TOS. As for regional lymph node recurrence, most of the patients were treated by radical neck dissection or radical neck dissection plus chemotherapy and/or radiotherapy. Only 4 patients (0.7%) underwent laryngectomy (3 for local recurrence and 1 for metachronous head and neck cancer). Therefore, 99.3% (564/568) of the patients overall enjoyed preservation of organ and function. Calculated 3‐year larynx‐preservation survival rate was very high at 87.5%.
The local recurrence rate was significantly lower in the en bloc resection (5.3%) than in the piecemeal resection (15.7%, p < 0.0001). Previous studies reported that the size of tumors that can be resected en bloc by EMR is limited and that EMR tends to have a higher rate of local recurrence. 30 , 31 In this study, en bloc resection rate of EMR was 55.4% for median tumor size of 14 mm. Because the average size of en bloc resected specimens by EMR is 10.3 ± 6.1 mm, EMR may be suitable for small lesions if en bloc resection can be performed.
The most frequent adverse event was laryngeal edema. Temporary tracheostomy was indicated in 49 (8.5%) of 575 treatment sessions. Among them, 22 procedures (44.9%, 22/49) directly attributed to laryngeal edema and 2 procedures (0.3%) were due to difficulty for intraoperative bleeding management. In contrast, 21 procedures (42.9%, 21/49) were indicated for the planned tracheostomy to avoid airway obstruction potentially caused by laryngeal edema, bleeding, or aspiration after TOS even in the cases with absence of intraoperative adverse events. However, the indication for planned tracheostomy was not clear because all such adverse events did not cause airway obstruction. Then, we have to clear the definite indication of planned tracheostomy to introduce the TOS as a minimally invasive treatment.
The rate of postoperative stenosis in the present study was only 0.5% (3/575). In the three cases who developed stenosis, the pathological tumor diameters were 15, 16, and 45 mm, respectively. And, all lesion located in the pyriform sinus. The possible reason developed stenosis might be associated with the tumor lesion regardless of the tumor size because the pyriform sinus is directly connected to the cervical esophagus which is physiological stenotic part.
Indication for TOS has not been clearly determined. In this study, pathological criteria for intraepithelial SCC and subepithelial SCC have been clearly defined. Using this criteria, relapse‐free survival rates were significantly different between two groups, while overall survival was similar. Cause‐specific survival rate was not statistically different between the two groups because both groups had nearly 100% cause‐specific survival. These results indicated that our pathological criteria for subepithelial invasion is clinically useful to stratify the risk for recurrence but not survival after TOS.
Early detection of head and neck cancer continues to be difficult worldwide. Screening of cancer in the head and neck is not a common practice. However, early detection is important because advanced head and neck cancer has poor prognosis and conventional treatments adversely affect the patients’ quality of life. Image enhanced endoscopy such as NBI is revealed to be useful for early detection of head and neck cancer. 12 However, it is not routinely used in Western countries, while they were high incidence area for head and neck cancer. We would like to emphasize the benefit of image‐enhanced endoscopy and hope it will be used in routine clinical practice especially in countries with known high incidence of head and neck cancer.
Our study has several limitations. This is a retrospective study and the duration of follow‐up was relatively short. Although this national multi‐center survey showed real‐world outcomes and benefit of TOS for superficial head and neck cancer and we have shown clinically meaningful pathological criteria of subepithelial invasion, a prospective study would provide a better assessment of individual management of TOS.
In conclusion, TOS for superficial head and neck cancer appears to be an excellent organ preserving minimally invasive treatment that results in excellent cause‐specific survival.
CONFLICT OF INTEREST
Professor Manabu Muto received a joint research fund between Kyoto University and Olympus Corporation out of this study. Dr. Ryuichi Hayashi received a grant from the Japan Agency for Medical Research and Development (Grant No. 19ck0106510h0001). Other authors have not declared a specific grant for this study from any funding agency in the public, commercial, or not‐for‐profit sectors.
AUTHOR CONTRIBUTIONS
CK, MM, SF, TEY, YAS, HW, TS, AO, TAK, and RH contributed to conception and design. CK, KOK, YAS, and MI contributed to collection and assembly of data. CK, MM, SF, TEY, YAS, and RH contributed to data analysis and interpretation. CK, MM, SF, and TEY wrote the manuscript. RH provided financial support. CK, KOK, YAS, MI, MM, and RH contributed to administrative support. TOY, AW, TI, SY, IT, HM, YUS, AT, TOK, NH, MA, AS, KOK, YA, TM, HU, KO, KG, SH, YO, ST, YN, KT, KEK, MA, NS, and AA provided study materials or patients. All authors provided final approval of manuscript.
ETHICAL CONSIDERATION
This study was approved by the institutional review board at Kitasato University School of Medicine (Approval ID; B10‐134).
ACKNOWLEDGMENTS
Professor Norio Fukami (Mayo Clinic in Scottsdale) and Kenneth K. Wang (Mayo Clinic in Rochester) are acknowledged for their support of the writing and editing of this article.
Funding information
This study was supported by a grant from the Japan Agency for Medical Research and Development 19ck0106510h0001.
DATA AVAILABILITY STATEMENT
The data and other items supporting the results of the study will be made available upon reasonable request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Hoffman HT, Karnell LH, Shah JP, et al. Hypopharyngeal cancer patient care evaluation. Laryngoscope. 1997;107:1005‐1017. [DOI] [PubMed] [Google Scholar]
- 3. Seiwert TY, Cohen EE. State‐of‐the‐art management of locally advanced head and neck cancer. Br J Cancer. 2005;92:1341‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bova R, Goh R, Poulson M, Coman WB. Total pharyngolaryngectomy for squamous cell carcinoma of the hypopharynx: a review. Laryngoscope. 2005;115:864‐869. [DOI] [PubMed] [Google Scholar]
- 5. Chao KS, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head‐and‐neck cancers receiving intensity‐modulated or three‐dimensional radiation therapy: initial results. Int J Radiat Oncol Biol Phys. 2001;49:907‐916. [DOI] [PubMed] [Google Scholar]
- 6. Gogarty DS, Shuman A, O’Sullivan EM, et al. Conceiving a national head and neck cancer screening programme. J Laryngol Otol. 2016;130:8‐14. [DOI] [PubMed] [Google Scholar]
- 7. Japan Society for Head and Neck Cancer . General rules for clinical studies on head and neck cancer. Tokyo: Kanehara Co., Ltd.; 2018. (in Japanese). [Google Scholar]
- 8. Muto M, Nakane M, Katada C, et al. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375‐1381. [DOI] [PubMed] [Google Scholar]
- 9. Katada C, Tanabe S, Koizumi W, et al. Narrow band imaging for detecting superficial squamous cell carcinoma of the head and neck in patients with esophageal squamous cell carcinoma. Endoscopy. 2010;42:185‐190. [DOI] [PubMed] [Google Scholar]
- 10. Katada C, Muto M, Nakayama M, et al. Risk of superficial squamous cell carcinoma developing in the head and neck region in patients with esophageal squamous cell carcinoma. Laryngoscope. 2012;122:1291‐1296. [DOI] [PubMed] [Google Scholar]
- 11. Katada C, Yokoyama T, Yano T, et al. Drinking alcohol, multiple dysplastic lesions and the risk of field cancerization of squamous cell carcinoma in the esophagus and head and neck region. Gastroenterology. 2016;151:860‐869. [DOI] [PubMed] [Google Scholar]
- 12. Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566‐1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goda K, Dobashi A, Yoshimura N, et al. Dual‐focus versus conventional magnification endoscopy for the diagnosis of superficial squamous neoplasms in the pharynx and esophagus: a randomized trial. Endoscopy. 2016;48:321‐329. [DOI] [PubMed] [Google Scholar]
- 14. Tateya I, Morita S, Muto M, et al. Magnifying endoscope with NBI to predict the depth of invasion in laryngo‐pharyngeal cancer. Laryngoscope. 2015;125:1124‐1129. [DOI] [PubMed] [Google Scholar]
- 15. Kikuchi D, Iizuka T, Yamada A, et al. Utility of magnifying endoscopy with narrow band imaging in determining the invasion depth of superficial pharyngeal cancer. Head Neck. 2015;37:846‐850. [DOI] [PubMed] [Google Scholar]
- 16. Shimizu Y, Yamamoto J, Kato M, et al. Endoscopic submucosal dissection for treatment of early stage hypopharyngeal carcinoma. Gastrointest Endosc. 2006;64:255‐259. [DOI] [PubMed] [Google Scholar]
- 17. Iizuka T, Kikuchi D, Hoteya S, Yahagi N, Takeda H. Endoscopic submucosal dissection for treatment of mesopharyngeal and hypopharyngeal carcinomas. Endoscopy. 2009;41:113‐117. [DOI] [PubMed] [Google Scholar]
- 18. Muto M, Satake H, Yano T, et al. Long‐term outcome of transoral organ‐preserving pharyngeal endoscopic resection for superficial pharyngeal cancer. Gastrointest Endosc. 2011;74:477‐484. [DOI] [PubMed] [Google Scholar]
- 19. Okada K, Tsuchida T, Ishiyama A, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for en bloc resection of superficial pharyngeal carcinomas. Endoscopy. 2012;44:556‐564. [DOI] [PubMed] [Google Scholar]
- 20. Hanaoka N, Ishihara R, Takeuchi Y, et al. Clinical outcomes of endoscopic mucosal resection and endoscopic submucosal dissection as a transoral treatment for superficial pharyngeal cancer. Head Neck. 2013;35:1248‐1254. [DOI] [PubMed] [Google Scholar]
- 21. Imai K, Tanaka M, Hasuike N, et al. Feasibility of a "resect and watch" strategy with endoscopic resection for superficial pharyngeal cancer. Gastrointest Endosc. 2013;78:22‐29. [DOI] [PubMed] [Google Scholar]
- 22. Satake H, Yano T, Muto M, et al. Clinical outcome after endoscopic resection for superficial pharyngeal squamous cell carcinoma invading the subepithelial layer. Endoscopy. 2015;47:11‐18. [DOI] [PubMed] [Google Scholar]
- 23. Hanaoka N, Ishihara R, Takeuchi Y, et al. Endoscopic submucosal dissection as minimally invasive treatment for superficial pharyngeal cancer: a phase II study. Gastrointest Endosc. 2015;82:1002‐1008. [DOI] [PubMed] [Google Scholar]
- 24. Yoshio T, Tsuchida T, Ishiyama A, et al. Efficacy of double‐scope endoscopic submucosal dissection and long‐term outcomes of endoscopic resection for superficial pharyngeal cancer. Dig Endosc. 2017;29:152‐159. [DOI] [PubMed] [Google Scholar]
- 25. Shiotani A, Tomifuji M, Araki K, Yamashita T, Saito K. Videolaryngoscopic transoral en bloc resection of supraglottic and hypopharyngeal cancers using laparoscopic surgical instruments. Ann Otol Rhinol Laryngol. 2010;119:225‐232. [DOI] [PubMed] [Google Scholar]
- 26. Okami K, Ebisumoto K, Sakai A, et al. Transoral en bloc resection of superficial laryngeal and pharyngeal cancers. Head Neck. 2013;35:1162‐1167. [DOI] [PubMed] [Google Scholar]
- 27. Nakayama M, Katada C, Mikami T, et al. A clinical study of transoral pharyngectomies to treat superficial hypopharyngeal cancers. Jpn J Clin Oncol. 2013;43:782‐787. [DOI] [PubMed] [Google Scholar]
- 28. Tateya I, Muto M, Morita S, et al. Endoscopic laryngo‐pharyngeal surgery for superficial laryngo‐pharyngeal cancer. Surg Endosc. 2016;30:323‐329. [DOI] [PubMed] [Google Scholar]
- 29. Watanabe A, Taniguchi M, Kimura Y, et al. Synopsis of transoral endoscopic laryngopharyngeal surgery for superficial pharyngeal cancers. Head Neck. 2017;39:1779‐1787. [DOI] [PubMed] [Google Scholar]
- 30. Ishihara R, Iishi H, Takeuchi Y, et al. Local recurrence of large squamous‐cell carcinoma of the esophagus after endoscopic resection. Gastrointest Endosc. 2008;67:799‐804. [DOI] [PubMed] [Google Scholar]
- 31. Furue Y, Katada C, Tanabe S, et al. Effectiveness and safety of endoscopic aspiration mucosectomy and endoscopic submucosal dissection in patients with superficial esophageal squamous‐cell carcinoma. Surg Endosc. 2019;33:1433‐1440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and other items supporting the results of the study will be made available upon reasonable request.


