Abstract
Objective:
To investigate the efficacy and safety of ICX72 or Sinus Buster, a proprietary homeopathic preparation of Capsicum annum and Eucalyptol, versus placebo administered continuously over 2 weeks in subjects with a significant component of nonallergic rhinitis (NAR).
Methods:
Forty-two consented subjects meeting inclusion/exclusion criteria were randomized to ICX72 (n = 20) or control (n = 22) administered twice daily over 2 weeks. The primary endpoint was change in total nasal symptom scores (TNSS) from baseline to end of study. Secondary endpoints included changes in individual symptom scores (ISS) over 2 weeks and average time to first relief. Mean TNSS and ISS were recorded after single dosing at different intervals over 60 minutes. Rhinitis quality-of-life, rescue medication, and safety endpoints were analyzed.
Results:
ICX72 versus placebo subjects exhibited significant differences in changes from baseline to end of study for TNSS and each ISS (P < .01), had an average time to first relief of 52.6 seconds (P < .01), and improvement in nasal congestion, sinus pain, sinus pressure, and headache at 5, 10, 15, and 30 minutes, persisting at 60 minutes for nasal congestion and sinus pain (P < .05). No difference between groups in adverse events or rescue medication was observed. ICX72 versus placebo subjects experienced no rebound congestion or impaired olfaction at the end of the study.
Conclusion:
This is the first controlled trial demonstrating intranasal capsaicin, when used continuously over 2 weeks, rapidly and safely improves symptoms in rhinitis subjects with a significant NAR component.
INTRODUCTION
Chronic rhinitis can be divided into inflammatory and noninflammatory rhinitis.1–3 Inflammatory rhinitis includes seasonal or perennial allergic rhinitis (AR), entopic (localized intranasal specific immunoglobulin E) rhinitis and nonallergic rhinitis eosinophilic syndrome, whereas noninflammatory rhinitis includes vasomotor rhinitis and other nonallergic rhinitis (NAR) conditions such as rhinitis medicamentosa and hormonally induced rhinitis.2,4 Because AR patients often exhibit significant symptoms in response to irritant triggers, clinicians have postulated the coexistence of AR and NA referred to as “mixed rhinitis” (MR).5 Cross-sectional que tionnaire survey studies have estimated that up to 75% chronic rhinitis patients have either NAR or MR.6
Currently, NAR conditions are diagnosed by exclusion, because their mechanism(s) are unknown, and there are no specific biomarkers or diagnostic tests.2,4,7,8 The most popular mechanism postulated for NAR is autonomic imbalance resulting in parasympathetic hyperactivity leading to nasal congestion and drainage.9,10 Recently, transient receptor protein (TRP) ion channel activation causing trigeminal nerve depolarization has been postulated to play an important role in NAR, because these ubiquitous receptors are activated by different temperatures, chemicals, osmolarity, and other physical stimuli that are well-recognized triggers of NAR symptoms.2,7,11
Only a few medications are approved for treatment of NAR. Although clinical studies have found that intranasal antihistamines, ipratropium bromide, and corticosteroids are relatively effective in relieving NAR symptoms, how they mechanistically exert their effect is unclear.12–16
Capsaicin, a pungent substance derived from red pepper extracts, is known to ablate sensory nerve fibers in NAR patients, leading investigators to postulate a role for afferent C-fibers in NAR.17 Early placebo-controlled studies in NAR patients found topical capsaicin significantly improved symptom scores compared with placebo, without changes in levels of mast cell mediators, including leukotrienes, prostaglandin D2, and tryptase, indicating that reduction in mast cell—mediated inflammation was not involved.17,18 Subsequently, capsaicin was found to activate the TRP vanilloid-1 ion channel, leading to desensitization of nasal sensory neural fibers and reduction in nasal hyperresponsiveness.11 Single intermittent short-term dosing studies with intranasal capsaicin in AR and NAR patients have confirmed that it reduces nasal congestion and discharge.17–21 However, the capsaicin concentration used was very irritating and required co-administration of a local anesthetic, making it impractical and intolerable to use on a daily basis.17–21 Therefore, the objective of this study was to assess the efficacy and safety of a newly formulated intranasal capsaicin (ICX72 or Sinus Buster) solution when used on a daily basis over 2 weeks in subjects with a significant NAR component.
METHODS
Study Design
A randomized, placebo-controlled, double-blind parallel study was conducted in subjects with a significant component of NAR.2,5 Because selecting a placebo with a transient burning sensation similar to what is often experienced with ICX72 was not possible, subjects were instructed not to discuss any sensations felt while taking the study medication, and written instructions were used to direct subjects throughout the study, thereby limiting unnecessary conversations that could bias the results.
Study Drug
ICX72 or Sinus Buster is a proprietary, homeopathic preparation of Capsicum annum and Eucalyptol. Eucalyptol was added to reduce the burning sensation of capsaicin without compromising its therapeutic effect.
Subject Participants
Subjects between 18 and 60 years of age were enrolled. All subjects signed an informed consent approved by Sterling IRB (Atlanta, Georgia) before screening. Subjects required a physician diagnosis of NAR with or without an allergic component (ie, MR) at least 6 months before enrollment. Nonallergic rhinitis was defined as having upper respiratory symptoms induced by chemical irritants, strong odors, weather, or temperature changes and negative skin prick testing or serum-specific immunoglobulin E to a panel of indigenous aeroallergens. Mixed rhinitis was defined as having 1 or more clinically relevant positive skin prick tests (wheal ≥ 3 mm in diameter with surrounding erythema compared with saline control in conjunction with a positive histamine control) to a panel of aeroallergens that correlated with clinical symptoms and significant upper respiratory symptoms induced by chemical irritants, strong odors, weather, or temperature changes.5 The relevance of each subject’s NAR component was confirmed by using an irritant index score previously demonstrated to correlate with a diagnosis of NAR or MR.2 To be enrolled, subjects were required to experience symptomatic relief using their currently prescribed rhinitis medications by medical history and to exhibit moderate to severe rhinitis symptoms off these medications at randomization. Symptom severity was defined as a morning or evening nasal congestion or postnasal drainage score of at least 5 on a 10-point scale on 3 separate symptom assessments during the 7-day prerandomization medication washout period, including within 24 hours before randomization.Subjects were required to comply with all study-related visits and procedures and to be in generally good health without uncontrolled chronic health problems.
Subjects were excluded if they had a sinus infection in the past 30 days, nasal polyps or other nasal structural problems, treatment with oral corticosteroids in the past 7 days, were active smokers, or had passive smoke exposure at least 6 months before enrollment. Pregnant women were excluded, and child-bearing-aged women were required to use acceptable birth control throughout the study.
Before enrollment, the sponsor labeled each of 100 empty vials with a random permutation of numbers between 1 and 100. Next, 100 independent binary random digits (0, 1) were generated with equal probability (P[0] = 1/2 and P[1] = 1/2). After both sets of random numbers were generated, the digits 0 and 1 were randomly assigned to indicate ‘placebo’ or ‘ICX72.’ The numbered vials were labeled with a random digit in the order in which the digits were generated. A randomization grid was generated that linked the numbers 1 through 100 to the corresponding medication. The coordinator sequentially assigned a number from 1 to 100 (in ascending order) to each patient who consented to participate in the study. If the patient qualified to participate at visit 2 (baseline or predrug visit), that number was matched with the randomization grid. When a minimum sample size of 20 in both groups was reached, enrollment was stopped.
The research coordinator and principal investigator were blinded as to who received drug or placebo. The sponsor generated a randomization grid but had no knowledge of the number assigned to any patient. The biostatistician was the only one with access to the patient number assignment and the randomization grid and therefore was responsible for maintaining the blinding. The principal investigator had authority to unblind a patient’s assignment if a patient experienced a severe drug reaction.
Study Visits
Three visits and 1 interim phone call (Fig 1) were involved. During visit 1, informed consented subjects meeting all inclusion/exclusion criteria (except for symptom scores) were washed off all rhinitis medications for 7 days to determine whether they fulfilled symptom entry criteria. Diphenhydramine 25 mg, 1 to 2 pills every 4 to 6 hours, up to 200 mg/day, was permitted throughout the study as rescue medication. During visit 2, subjects fulfilling all inclusion/exclusion criteria including symptom score criteria were randomized to ICX72 or placebo (filtered water), 1 to 2 puffs each nostril, twice daily. During this visit, subjects were given their first dose of study drug or placebo. Using the stopwatch method,22 subjects clicked a stopwatch when they felt the first onset of symptom relief and immediately completed a symptom score card. Subjects also completed symptom score cards at baseline (defined as visit 2 predrug) and at 5-, 10-, 15-, 30-, and 60-minute intervals during this visit.
Figure 1.

Study time line.
An interim phone call was made to all subjects 7 days later to assess compliance with completing diaries, adherence to study medication, adverse events, and rescue medication requirements. The study ended at visit 3, 2 weeks after randomization.
During each visit, subjects had a directed medical history, nasal examination, and review of concomitant or rescue medications. During visits 1 and 3, all subjects underwent acoustic rhinometry (Ecco Vision, Hood Instruments, Pembroke, Massachusetts), and a subset of subjects underwent automated olfactometry (Osmic Enterprises, LLC, Cincinnati, Ohio) to assess nasal congestion and smell, respectively, because of concerns for rebound nasal congestion and anosmia previously reported for over-the-counter nasal preparations.23,24 Pregnancy tests were performed on all childbearing-aged women at visits 1 (screening) or 2 (randomization). Rescue medication and adverse events were assessed at visit 2, during the interim phone call, and visit 3. A Juniper rhinitis quality-oflife questionnaire (RQLQ) was administered during visits 2 (randomization) and 3 (end of study).25,26 Symptom scores and rescue medication requirements were recorded on home diaries over the 3-week study period (including during the 7-day washout period).
Primary Endpoint
The primary endpoint was a change in the 12-hour reflective total nasal symptom score (TNSS) recorded by the patient each evening in a daily diary, from baseline to end of the study (2 weeks). The TNSS was a composite score including nasal congestion, sinus pain, sinus pressure, headache, sneezing, rhinorrhea, and postnasal drainage. These symptoms were chosen based on the “Allergies in America survey,” which cited them as most commonly experienced by chronic rhinitis patients.27 Each symptom was rated on a 10-point Likert scale; the highest possible TNSS was 70; a higher score reflected a more intense symptom.
Secondary Endpoints
Secondary endpoints included changes in the 12-hour reflective individual symptom scores (ISS) of nasal congestion, sinus pain, sinus pressure, headache, sneezing, rhinorrhea, and postnasal drainage, recorded in patient daily diaries over a 2-week period and the time-to-first relief of symptoms recorded in the physician office at visit 2, after single dosing with ICX72 or placebo.
Additional Endpoints
These included changes in TNSS and ISS symptom scores after single dosing during visit 2 at different times over 1 hour, assessment of rescue medication requirements over 2 weeks, and the RQLQ, which compared ICX72 and placebo groups at baseline and completion of the study.
Safety Analyses
Adverse events, olfaction measured using an automated olfactometer (Osmic Enterprises, LLC), and nasal congestion using an acoustic rhinometer (Ecco Vision, Hood Instruments) to assess for increased nasal resistance were recorded before randomization while off all rhinitis medications and at the end of study.
Statistical Analysis
Sample size.
The sample sizes for ICX72 drug and placebo control groups were chosen based on feasibility and other intranasal capsaicin studies that reported clinically meaningful results.17 For sample sizes of 20 per group, the power was 80% to detect a difference of approximately 2 units (2.3 units) in TNSS mean scores from baseline (visit 2 predrug) to 14 days between drug and placebo groups, assuming a 2-tailed alpha = 0.05. The effect size (ratio of mean difference to TNSS standard deviation) was equal to 0.8.
Statistical methods.
Baseline differences in demographic characteristics between subjects randomized to drug or placebo with respect to age, race, and sex were tested by unpaired t-tests and Fisher’s exact tests. Complete data were available for all analyses except olfaction, which included a subset of subjects, because this procedure was not available at the study onset. A repeated-measures analysis of variance (ANOVA) was performed in which mean changes between symptoms at baseline to end of study were compared between ICX72 and placebo groups. The TNSS (calculated as the average of all individual symptoms) and ISS were analyzed separately. For these analyses, baseline data collected at visit 2 after randomization before administration of drug were compared with the average of subsequent data, including 2 sets of 7-day diary scores, interim phone call data, and final office visit data. Additional analyses of TNSS and ISS were performed to evaluate group differences in mean changes from baseline to times of first relief. Because symptoms at first relief were not available for 4 placebo group patients who never indicated first relief, Tobit regression was used to account for the bias of changes calculated from reported data only.28 When a symptom at first relief was missing, the change (baseline minus first relief) was unknown. All unknown changes were conservatively assumed to be 1 or more units below the smallest known change for the same symptom, after adjustment for baseline. In the Tobit analyses, types of probabilities were combined to determine the join probability of the observed and unobserved changes for each symptom. Specifically, for each group (drug and placebo the individual probabilities that unknown changes were less than the minimum observable change were multiplied by individual probabilities of observed changes. The average change of each group was estimated by maximizing the log-transformed joint probability with respect to the group specific parameters. The probability of a difference betwee the mean change of drug and placebo groups was tested by t-statistic, using the SAS procedure PROC QLIM. Additional analyses of symptoms were performed to estimate means of TNSS and ISS after single dosing with ICX72 or placebo at specified intervals over 60 minutes. Trends of mean symptom scores over time were modeled as a quadratic (2nd degree polynomial function of time. Interactions between group and time were modeled to allow for possible differences between group means at specified time intervals. Contrast statements were written to estimate differences between mean symptom scores of ICX72 and placebo groups at 5, 10, 15, 30, and 60 minutes. Each of 7 domains in the Rhinitis Quality of Life Questionnaire, and combined domains, was analyzed by ANOVA to investigate group differences in changes from baseline to end of study. A similar ANOVA was performed for olfactory thresholds. For all analyses, a P-value < .05 was used to judge statistical significance unless stated otherwise. For primary and secondary endpoints, P-values adjusted for multiple comparisons were also calculated by the Bonferroni method. The analyses were performed using SAS for Windows, version 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
Subject Demographics
The subject population demographic characteristics are summarized in Table 1. No significant differences were found between ICX72 and placebo groups with respect to age, race, and sex. A total of 42 subjects were enrolled. Twenty subjects received ICX72, and 22 received placebo. By chance, 2 more subjects were randomized to the placebo group before the ICX72 group reached 20 subjects. Thirty-three participants had a diagnosis of MR, of which 16 received ICX72 and 17 placebo, whereas 9 had a diagnosis of NAR, of which 4 received ICX72 and 5 placebo.
Table 1.
Demographic Characteristics of Study Participants
| Capsaicin (N = 20) | Placebo (N = 22) | All (N = 42) | |
|---|---|---|---|
| Age, mean (min, max) | 46.9 (36, 59) | 45.2 (29, 58) | 46.0 (29, 59) |
| Race, N (%) | |||
| White | 17 (85%) | 17 (77%) | 34 (81%) |
| African American | 2 (10%) | 5 (23%) | 7 (17%) |
| Other | 1 (5%) | 0 (0%) | 1 (2%) |
| Sex, N (%) | |||
| Male | 4 (20%) | 3 (14%) | 7 (17%) |
| Female | 16 (80%) | 19 (86%) | 35 (83%) |
Note: No significant differences were seen between the ICX72 and placebo groups (P > .05).
Mean Changes in TNSS and Individual Symptom Scores from Baseline to End of Study for ICX72 and Placebo Groups
Table 2 shows mean differences (baseline minus end of study) for TNSS and ISS in the ICX72 and placebo groups. Mean symptom scores of the ICX72 and placebo groups were not significantly different at baseline (Table 2). A significantly greater reduction in mean symptom scores for the primary endpoint, TNSS, was observed for ICX72 compared with placebo. In addition, a significantly greater reduction for the mean ISS of nasal congestion, sinus pressure, sinus pain, and headache (secondary endpoints) was observed for ICX72 compared with placebo. Mean changes for sneezing, rhinorrhea, and postnasal drip were not significantly different between groups. Significance was maintained for TNSS and each ISS after Bonferroni adjustment (alpha level set at 0.05/8 = 0.006). Figure 2 illustrates the percent changes for TNSS and each ISS from baseline that were significantly greater for ICX72 compared with placebo, except for sneezing, rhinorrhea, and postnasal drainage.
Table 2.
Mean Change from Baseline to End of Study (Standard Error) of TNSS and Individual Symptom Scores for ICX72 and Placebo Groups
| Symptom | ICX72 (N = 20) Placebo (N = 22) |
|||||
|---|---|---|---|---|---|---|
| Baseline | Change: baseline–end | % Rel change | Baseline | Change: baseline–end | % Rel change | |
| TNSS | 23.7 (2.2) | 12.4 (1.1) | 52% | 26.0 (2.2) | 7.7 (1.2) | 30% |
| Nasal congestion | 5.1 (0.5) | 2.7 (0.3) | 53% | 5.3 (0.4) | 1.4 (0.3) | 27% |
| Sinus pressure | 5.4 (0.5) | 3.3 (0.3) | 62% | 3.7 (0.4) | 1.5 (0.3) | 29% |
| Sinus pain | 4.5 (0.5) | 2.8 (0.3) | 64% | 4.8 (0.5) | 1.6 (0.3) | 33% |
| Headache | 4.9 (0.5) | 3.2 (0.4) | 65% | 4.8 (0.5) | 1.8 (0.3) | 37% |
| Sneezing | 1.6 (0.4) | 0.9 (0.2) | 53% | 2.4 (0.4) | 0.8 (0.2) | 19% |
| Rhinorrhea | 2.6 (0.5) | 0.5 (0.3) | 19% | 2.6 (0.4) | 0.6 (0.3) | 24% |
| PND | 4.7 (0.5) | 2.2 (0.4) | 47% | 5.6 (0.5) | 1.7 (0.3) | 30% |
Abbreviation: N, number of patients.
For each symptom, baseline differences between groups were not statistically different. Mean changes from baseline to end of study were significantly different between ICX72 and placebo for TNSS, nasal congestion, sinus pressure, sinus pain, and headache. Percent relative change of each symptom was larger for ICX72, compared with placebo, except rhinorrhea.
Figure 2.
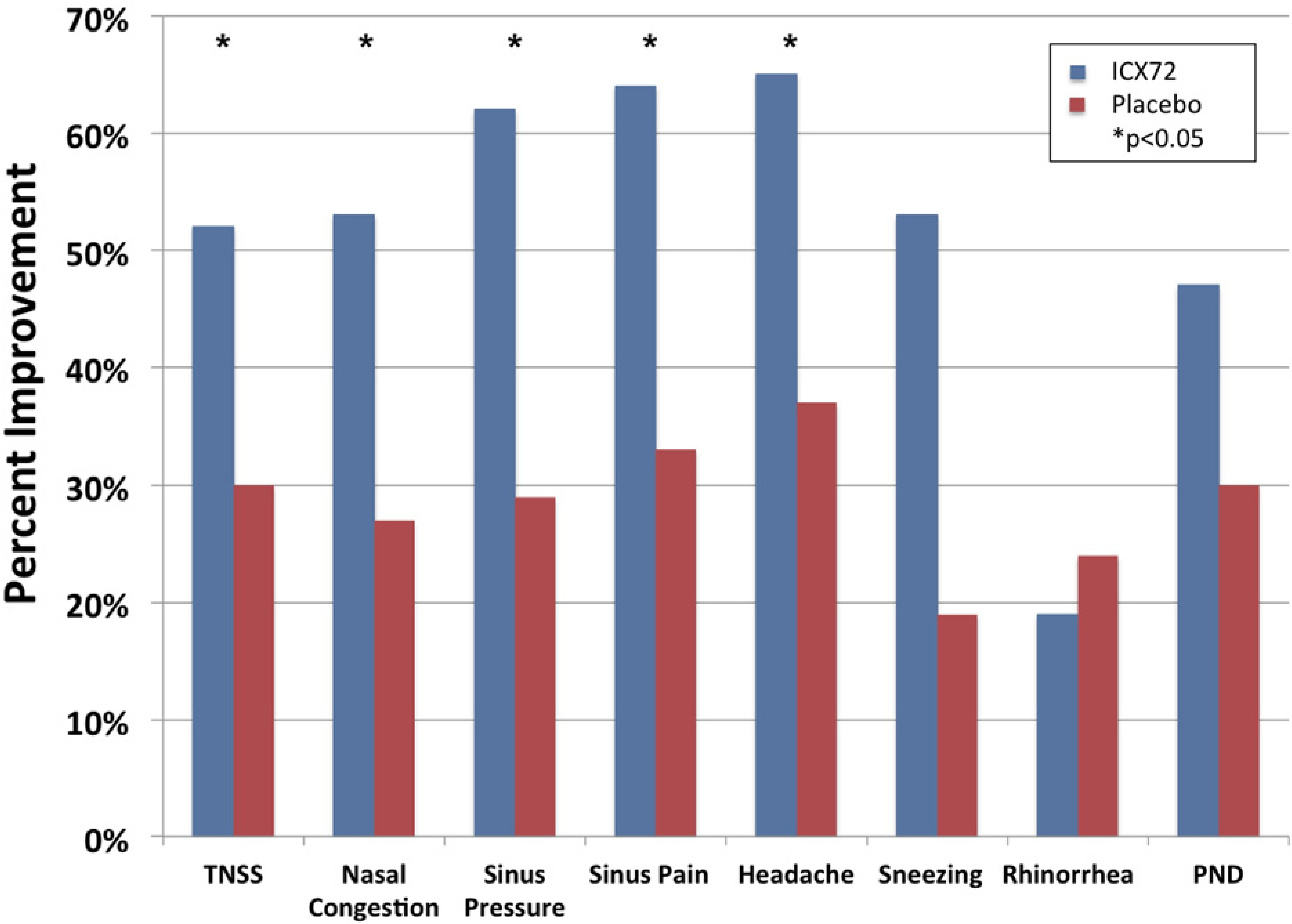
Percent improvement and symptom scores from baseline to end of study for ICX72 and placebo groups.
Time to First Relief
The average time to first relief for ICX72 was 52.6 seconds (0.8 minute) compared with placebo subjects, which was 184.32 seconds (P < .01) based on a censored log rank test because 4 subjects receiving placebo never experienced first relief. A significantly larger percentage of ICX72 (80%) compared with placebo subjects (45%) experienced first relief in less than 1 minute. Symptoms showing statistically significant improvement at time-to-first-relief were nasal congestion, sinus pressure, and headache (Table 3).
Table 3.
Mean Change from Baseline to First Relief (Standard Error) of TNSS and Individual Symptom Scores for ICX72 and Placebo Groups
| Symptom | ICX72 (N = 20) |
Placebo (N = 22)* |
||||
|---|---|---|---|---|---|---|
| Baseline | Change: baseline –first relief | % Rel change | Baseline | Change: baseline –first Relief | % Rel change | |
| TNSS | 23.7 (2.2) | 3.4 (1.5) | 14% | 26.0 (2.2) | 2.3 (7.7) | 9% |
| Nasal congestion | 5.1 (0.5) | 1.3 (0.4) | 25% | 5.3 (0.4) | 0 (2.2) | 0 |
| Sinus pressure | 5.4 (0.5) | 1.7 (0.5) | 32% | 3.7 (0.4) | −0.1 (1.9) | −1% |
| Sinus pain | 4.5 (0.5) | 1.2 (0.6) | 27% | 4.8 (0.5) | −0.2 (2.7) | −5% |
| Headache | 4.9 (0.5) | 2.2 (0.6) | 44% | 4.8 (0.5) | −0.1 (2.0) | −2% |
| Sneezing | 1.6 (0.4) | 1.2 (0.3) | 75% | 2.4 (0.4) | 0.5 (1.8) | 19% |
| Rhinorrhea | 2.6 (0.5) | −2.3 (0.6) | −89% | 2.6 (0.4) | −1.9 (4.3) | −74% |
| PND | 4.7 (0.5) | 0.4 (0.7) | 7% | 5.6 (0.5) | −0.6 (5.4) | −10% |
Abbreviation: N, number of patients.
For each symptom, baseline differences between groups were not statistically different. Mean changes from baseline to first relief were significantly different between ICX72 and placebo for nasal congestion, sinus pressure, and headache (P < .05). Percent relative change of each symptom was larger for ICX72, compared with placebo, except rhinorrhea.
Four of 22 placebo patients had missing data at first relief for each symptom. When a symptom at first relief was missing, less relief was assumed than was determined from all reported symptom changes at first relief, after adjusting for baseline. The minimum values at first relief of missing data were symptom-specific. Mean symptom changes in the placebo group were obtained from Tobit analysis, which accounts for the upward bias of changes calculated from observed data only. The probability of unreported symptoms reflecting less relief than the minimum relief of all other patients was combined with the probabilities of the observed symptom values at first relief, to estimate mean changes at first relief of each symptom for the placebo group.
Improvement in TNSS and Symptoms Scores for Intranasal Capsaicin Compared with Placebo at Fixed Time Periods
This additional analysis required subjects to complete symptom score questionnaires at fixed time periods beginning at 5 minutes up to 60 minutes after the first dose (Fig 3). Differences were statistically significant for nasal congestion, sinus pressure, sinus pain, and headache at 5, 10, 15, and 30 minutes, which persisted for nasal congestion, sinus pain, and sinus pressure up to 60 minutes. Differences were statistically significant for TNSS at 10, 15, 30, and 60 minutes. Also of note, statistically significant improvement occurred in postnasal drainage at 30 minutes and for sneezing at 5 and 10 minutes.
Figure 3.
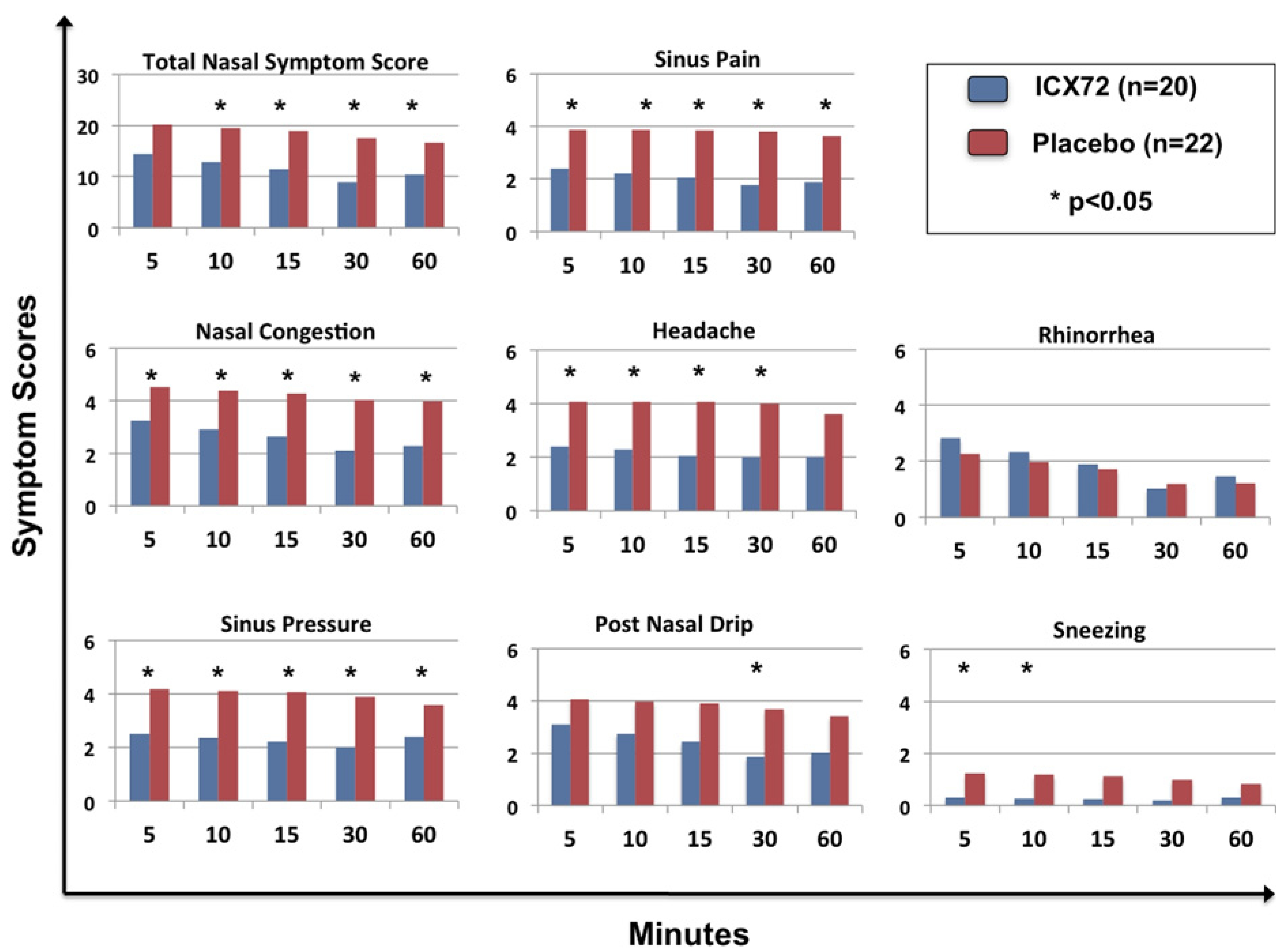
Mean scores of TNSS and individual symptoms after single dosing with ICX72 over 60 minutes vs placebo.
Rescue Medications
Fewer subjects in the ICX72 group (n = 6; 30% of subjects) versus the placebo group (n = 10; 45% of subjects) reported rescue medication usage during the first week of randomization. Similarly, fewer subjects in the ICX72 group (n = 4; 20% of subjects) versus the placebo group (n = 10; 45%) reported rescue medication use during the second week of randomization.
Rhinitis Quality of Life Questionnaire
Table 4 shows mean differences (baseline minus end of study) of RQLQ domains for each group. Lower RQLQ scores indicate better quality of life. Baseline means of each domain except sleep were significantly lower in the ICX72, compared with placebo groups (P < .05). The mean difference between groups with respect to change from baseline to end of study was not significant between groups for each domain. However, percent relative changes for each domain were qualitatively assessed and observed to be larger for ICX72 subjects, compared with placebo.
Table 4.
Mean Change from Baseline to End of Study (Standard Error) of RQLQ Scores for ICX72 and Placebo Groups
| Domain of RQLQ scores | ICX72 (N = 20) |
Placebo (N = 22) |
||||
|---|---|---|---|---|---|---|
| Baseline | Change: baseline-end | % Rel change | Baseline | Change: baseline-end | % Rel change | |
| Activities | 3.1 (0.3) | 1.3 (0.4) | 41% | 4.1 (0.2) | 1.0 (0.4) | 21% |
| Sleep | 2.4 (0.3) | 1.0 (0.3) | 43% | 3.2 (0.3) | 0.6 (0.3) | 18% |
| Non-nasal/eye symptoms | 2.4 (0.3) | 1.1 (0.3) | 45% | 3.3 (0.2) | 0.7 (0.3) | 21% |
| Practical problems | 2.3 (0.3) | 1.0 (0.3) | 43% | 3.4 (0.4) | 0.4 (0.4) | 13% |
| Nasal symptoms | 2.4 (0.2) | 0.8 (0.3) | 32% | 3.2 (0.2) | 0.5 (0.2) | 15% |
| Eye symptoms | 1.8 (0.2) | 0.6 (0.2) | 31% | 2.6 (0.3) | 0.7 (0.3) | 25% |
| Emotional factors | 1.9 (0.3) | 0.9 (0.3) | 47% | 3.0 (0.2) | 0.8 (0.2) | 27% |
| Combined domains | 2.3 (0.2) | 0.9 (0.2) | 41% | 3.2 (0.2) | 0.7 (0.2) | 20% |
Abbreviation: N, number of patients.
Lower RQLQ scores indicate improvement in quality of life.
For each domain, baseline differences between groups were significantly different, except for the Sleep domain. Mean changes from baseline to end of study of each domain were not significantly different. Percent relative change of each domain was larger for ICX72, compared with placebo.
Safety Analyses
Olfactometry.
Table 5 shows the mean difference (baseline minus end of study) of olfactory threshold in the ICX72 and placebo groups. Only 14 subjects receiving ICX72 and 17 receiving placebo underwent this testing, because it was introduced as an additional safety analysis after enrollment had begun. Baseline means and mean change from baseline to end of study were not significantly different between groups. A negative relative change, however, was observed among ICX72 subjects (−14.6), and a positive change (+1.2%) was observed for placebo subjects. End of study means were significantly different for ICX72 vs placebo, suggesting tha the ICX72 group had improved olfaction.
Table 5.
Mean Change from Baseline to End of Study (Standard Error) of Olfactory Thresholds for ICX72 and Placebo Groups
| ICX72 (N = 14) |
Placebo (N = 17) |
|||
|---|---|---|---|---|
| Baseline | Change: baseline–end | Baseline | Change: baseline–end | |
| Olfactory Threshold | 6.7 (0.4) | −1.0 (0.6) | 6.1 (0.6) | +0.1 (0.5) |
Abbreviation: N, number of patients.
A negative change (higher value at end of study) indicates improved olfaction.
Baseline (defined as data collected during visit 2 predrug) differences between groups were not significantly different (P > .05). Mean changes from baseline to end of study were not significantly different between ICX72 and placebo groups (P > .05). End of study means were significantly different between ICX72 (7.7) and placebo (6.0) (P < .05).
Acoustic rhinometry.
No significant difference was seen in changes of nasal mean cross-sectional areas at baseline when subjects were off rhinitis medications and end of study between ICX72 and placebo subjects, suggesting that rebound nasal congestion was not a side effect after using ICX72 continuously over 2 weeks.
Adverse events.
No significant difference was seen between ICX72 and placebo in reported adverse events, which included increased nasal congestion, headaches, postnasal drainage, rhinorrhea, transient stinging/burning lasting only a few seconds, blood-tinged mucus, and fatigue. Ten percent of subjects on ICX72 and 27% on placebo, respectively, reported adverse events during the first week after randomization, whereas 45% of subjects on ICX72 and 32% on placebo reported adverse events during the second week after randomization.
DISCUSSION
This study demonstrated that continuous treatment with ICX7 (Sinus Buster) over 2 weeks significantly improved TNSS, nasal congestion, sinus pressure, sinus pain, and headache compared with placebo. Furthermore, ICX72 has a quick onset of action, as the average time to first relief was less than 1 minute (52.6 seconds). The time to first relief by subjects receiving placebo was likely much longer than the 184.32 seconds indicated by the censored data analyzed, because 4 subjects never reported first relief. However, the rapid improvement observed for nasal congestion, sinus pressure, and headache among subjects receiving ICX72 at first relief compared with placebo is consistent with the significantly faster time of onset observed for subjects receiving ICX72. This therapeutic response continued over time, as nasal congestion, sinus pressure, sinus pain, and headache significantly improved for ICX72 vs placebo at 5, 10, 15, and 30 minutes and persisted for nasal congestion and sinus pain at 60 minutes.
ICX72 was safe to use daily over 2 weeks, as there was no evidence of increased nasal congestion or impaired olfaction at the end of study. In fact, ICX72 subjects had improved olfactory threshold levels vs placebo at the end of the study. Other than a transient stinging sensation, ICX72 was well tolerated.
Several important points regarding this study should be emphasized. This is the first study to demonstrate that daily treatment with a low concentration of intranasal capsaicin combined with eucalyptol over a 2-week period is safe and effective at improving symptoms in MR and NAR subjects. Second, because subjects were required to have a significant component of NAR at enrollment, the beneficial effect of ICX72 supports an important mechanistic role for TRPV1 ion channels in MR and NAR. Although this effect of intranasal capsaicin in NAR has been previously reported, it has only recently been possible to attribute its effect through a specific pathway.29 Finally, the observation that subjects dosed with ICX72 also experienced less sinus pain, sinus pressure, nasal congestion, and headache is remarkable but not entirely surprising when one considers the critical role TRP receptors play in chronic pain and headache.30 The rapid onset of action of ICX72 in improving rhinitis symptoms and headaches suggest overlapping neurogenic mechanistic pathways for MR, NAR, and headaches that requires further investigation.31
Several potential limitations of this study warrant discussion. First, similar to other intranasal medications,12 formulating a placebo that caused mild transient stinging similar to ICX72 was not possible. However, to ensure that this study remained blinded, common written instructions were used to instruct subjects, thereby limiting unnecessary conversations that could bias the results. Second, subjects receiving placebo in some instances experienced modest improvement in TNSS and ISS compared with baseline, a frequently observed effect in clinical trials.32 However, the finding that compared with placebo, ICX72 demonstrated improvement in TNSS as well as in each individual symptom score at the end of the study after adjusting for multiple comparisons, supports its robust therapeutic effect. Third, despite significant baseline differences in RQLQ domain scores, each domain score with the exception of eye symptoms significantly improved at the end of the study to a greater extent in subjects on ICX72 compared with placebo. The failure to observe a significant change in RQLQ from baseline to end of study is not surprising because the study was not powered to detect this effect. Fourth, although enrolled subjects were required to have a history of response to conventional rhinitis medications, a biased response to ICX72 is unlikely, because this was a placebo-controlled parallel-group study. Finally, the eucalyptol that was incorporated into the ICX72 formulation to reduce the burning sensation associated with capsaicin may have had a therapeutic effect in our subjects, as aromatherapy agents such as menthol and eucalyptus activate TRP ion channels.9,33
In summary, this is the first controlled clinical trial to demonstrate that an intranasal capsaicin spray, ICX72 (Sinus Buster), provides a rapid onset of relief for MR and NAR subjects, while also providing safe, sustained symptom relief over a 2-week treatment period.
Funding Sources:
This study was funded by Dynova Laboratories.
Footnotes
Disclosures: The corresponding author and lead investigator is a consultant for Dynova Laboratories. Jennifer Cooper is the Vice President of Research and Development for Dynova Laboratories. Benjamin Davis is an Allergy fellow; Jillian Picard, a clinical research coordinator; and Shu Zheng and Linda Levin, statisticians without conflicts of interests.
REFERENCES
- 1.Settipane RA, Lieberman P. Update on nonallergic rhinitis. Ann Allergy Asthma Immunol. 2001;86:494–507; quiz 8. [DOI] [PubMed] [Google Scholar]
- 2.Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1–84. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein JA. Characteristics of non-allergic rhinitis. World Allergy Journal. 2009;2:102–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaliner M. Classification of nonallergic rhinitis syndromes with a focus on vasomotor rhinitis, proposed to be known henceforth as nonallergic rhinopathy. World Allergy Journal. 2009;2:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein JA, Rezvani M. Mixed rhinitis: a new subclass of chronic rhinitis. In: Kaliner M, ed. Current Review of Rhinitis, 2nd ed. Philadelphia, PA, Current Medicine, LLC: 2006:69–78. [Google Scholar]
- 6.Settipane RA. Demographics and epidemiology of allergic and nonallergic rhinitis. Allergy Asthma Proc. 2001;22:185–189. [PubMed] [Google Scholar]
- 7.Brandt D, Bernstein JA. Questionnaire evaluation and risk factor identification for nonallergic vasomotor rhinitis. Ann Allergy Asthma Immunol. 2006;96:526–532. [DOI] [PubMed] [Google Scholar]
- 8.Garay R. Mechanisms of vasomotor rhinitis. Allergy. 2004;59 Suppl 76:4–9; discussion 10. [DOI] [PubMed] [Google Scholar]
- 9.Baraniuk JN. Sensory, parasympathetic, and sympathetic neural influences in the nasal mucosa. J Allergy Clin Immunol. 1992;90:1045–1050. [DOI] [PubMed] [Google Scholar]
- 10.Jones AS. Autonomic reflexes and non-allergic rhinitis. Allergy. 1997;52: 14–19. [DOI] [PubMed] [Google Scholar]
- 11.Seki N, Shirasaki H, Kikuchi M, Sakamoto T, Watanabe N, Himi T. Expression and localization of TRPV1 in human nasal mucosa. Rhinology. 2006;44:128–134. [PubMed] [Google Scholar]
- 12.Banov CH, Lieberman P. Efficacy of azelastine nasal spray in the treatment of vasomotor (perennial nonallergic) rhinitis. Ann Allergy Asthma Immunol. 2001;86:28–35. [DOI] [PubMed] [Google Scholar]
- 13.Georgitis JW, Banov C, Boggs PB, Dockhorn R, Grossman J, Tinkelman D, et al. Ipratropium bromide nasal spray in non-allergic rhinitis: efficacy, nasal cytological response and patient evaluation on quality of life. Clin Exp Allergy. 1994;24:1049–1055. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman PL, Settipane RA. Azelastine nasal spray: a review of pharmacology and clinical efficacy in allergic and nonallergic rhinitis. Allergy Asthma Proc. 2003;24:95–105. [PubMed] [Google Scholar]
- 15.Scadding GK, Lund VJ, Jacques LA, Richards DH. A placebo-controlled study of fluticasone propionate aqueous nasal spray and beclomethasone dipropionate in perennial rhinitis: efficacy in allergic and non-allergic perennial rhinitis. Clin Exp Allergy. 1995;25:737–743. [DOI] [PubMed] [Google Scholar]
- 16.Ciprandi G. Treatment of nonallergic perennial rhinitis. Allergy. 2004; 59(Suppl 76):16–22; discussion 3. [DOI] [PubMed] [Google Scholar]
- 17.Blom HM, Van Rijswijk JB, Garrelds IM, Mulder PG, Timmermans T, Gerth van Wijk R. Intranasal capsaicin is efficacious in non-allergic, non-infectious perennial rhinitis: a placebo-controlled study. Clin Exp Allergy. 1997;27:796–801. [DOI] [PubMed] [Google Scholar]
- 18.Blom HM, Severijnen LA, Van Rijswijk JB, Mulder PG, Van Wijk RG, Fokkens WJ. The long-term effects of capsaicin aqueous spray on the nasal mucosa. Clin Exp Allergy. 1998;28:1351–1358. [DOI] [PubMed] [Google Scholar]
- 19.Lacroix JS, Buvelot JM, Polla BS, Lundberg JM. Improvement of symptoms of non-allergic chronic rhinitis by local treatment with capsaicin. Clin Exp Allergy. 1991;21:595–600. [DOI] [PubMed] [Google Scholar]
- 20.Sanico AM, Philip G, Proud D, Naclerio RM, Togias A. Comparison of nasal mucosal responsiveness to neuronal stimulation in non-allergic and allergic rhinitis: effects of capsaicin nasal challenge. Clin Exp Allergy. 1998;28:92–100. [DOI] [PubMed] [Google Scholar]
- 21.Van Rijswijk JB, Boeke EL, Keizer JM, Mulder PG, Blom HM, Fokkens WJ. Intranasal capsaicin reduces nasal hyperreactivity in idiopathic rhinitis: a double-blind randomized application regimen study. Allergy 2003;58:754–761. [DOI] [PubMed] [Google Scholar]
- 22.Black P, Max MB, Desjardins P, Norwood T, Ardia A, Pallotta T. A randomized, double-blind, placebo-controlled comparison of the analgesic efficacy, onset of action, and tolerability of ibuprofen arginate and ibuprofen in postoperative dental pain. Clin Ther. 2002;24:1072–1089. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein JA, Bernstein IL. A novel case of mealworm-induced occupational rhinitis in a school teacher. Allergy Asthma Proc. 2002;23:41–44. [PubMed] [Google Scholar]
- 24.Rezvani M, Brandt D, Bernstein JA, Hastings L, Willwerth J. Investigation of olfactory threshold responses in chronic rhinitis subtypes. Ann Allergy Asthma Immunol. 2007;99:571–572. [DOI] [PubMed] [Google Scholar]
- 25.Juniper EF, Guyatt GH, Griffith LE, Ferrie PJ. Interpretation of rhinoconjunctivitis quality of life questionnaire data. J Allergy Clin Immunol. 1996;98:843–845. [DOI] [PubMed] [Google Scholar]
- 26.Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J Allergy Clin Immunol. 1999;104:364–369. [DOI] [PubMed] [Google Scholar]
- 27.Blaiss MS, Meltzer EO, Derebery MJ, Boyle JM. Patient and healthcare-provider perspectives on the burden of allergic rhinitis. Allergy Asthma Proc. 2007;28(Suppl 1):S4–10. [DOI] [PubMed] [Google Scholar]
- 28.Amemiya T. Tobit models: a survey. Journal of Econometrics 1984;24: 3–61. [Google Scholar]
- 29.Marabini S, Ciabatti PG, Polli G, Fusco BM, Geppetti P. Beneficial effects of intranasal applications of capsaicin in patients with vasomotor rhinitis. Eur Arch Otorhinolaryngol. 1991;248:191–194. [DOI] [PubMed] [Google Scholar]
- 30.Belmonte C, Viana F. Molecular and cellular limits to somatosensory specificity. Mol Pain. 2008;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rapoport AM, Bigal ME, Tepper SJ, Sheftell FD. Intranasal medications for the treatment of migraine and cluster headache. CNS Drugs. 2004;18:671–685. [DOI] [PubMed] [Google Scholar]
- 32.Spector SL. The placebo effect is nothing to sneeze at. J Allergy Clin Immunol. 1992;90:1042–1043. [DOI] [PubMed] [Google Scholar]
- 33.Anand P. Capsaicin and menthol in the treatment of itch and pain: recently cloned receptors provide the key. Gut. 2003;52:1233–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]


