Abstract
Purpose
The implantation of flow diverters requires administration of dual anti-platelet therapy, posing the potential for complications. The p48MW HPC (phenox, Bochüm, Germany) hydrophilic-coated flow diverting stent is designed to be anti-thrombotic, thus opening the potential for single anti-platelet therapy. We deploy a novel intravascular high-resolution imaging technique, high-frequency optical coherence tomography (HF-OCT), to study in an animal model the acute thrombus formation on coated p48MW devices versus uncoated control devices.
Methods
Three pigs were implanted with 4 flow diverters each, two test hydrophilic-coated devices, and two control uncoated devices (p48MW). Each pig was treated with a different anti-platelet regime: no anti-platelet therapy, aspirin only, aspirin and clopidogrel. Twenty minutes after the flow diverter was implanted, an HF-OCT data set was acquired. Acute clot formed on the flow diverter at each covered side branch was measured from the HF-OCT slices. Factors considered to be important were the device type (pHPC versus bare metal), aspirin, clopidogrel, and vessel location. A linear model was constructed from the significant factors.
Results
Both coating (p < 0.001) and aspirin (p = 0.003) were significantly related to reduction in clot burden, leading to an approximate 100-fold and 50-fold reduction in clot, respectively.
Conclusions
This study shows the power of HF-OCT not only in the detection of clot but also the quantification of clot burden. In an animal model, the pHPC-coated p48MW significantly reduced acute thrombus formation over jailed side branches as compared to the bare metal p48MW that was nearly eliminated when combined with aspirin administration.
Keywords: Stents, Optical coherence tomography, Thrombus, Flow diverter, Hydrophilic coating
Introduction
Over the past two decades, the use of stents in the neurovascular space has increased significantly [1–4]. Flow diverters and stent-assisted coiling allowed the treatment of aneurysms previously deemed inoperable to coiling alone. However, patients receiving intracranial endoluminal prosthesis require dual anti-platelet therapy (DAPT) administration to reduce thromboembolic complications [5]. A combination of aspirin with clopidogrel, ticagrelor, or prasugrel is often adopted [6]. Nevertheless, there are a number of disadvantages with this approach. DAPT should be used with caution in ruptured aneurysms [7], a broad variation in patient response is observed, constant monitoring and adjustment of the dosage is required, and patient non-compliance has been seen to be around 5% as early as 6 months, and reaches 10% by one year [8]. Although clopidogrel is often stopped after 6 months, aspirin is typically continued indefinitely [9]. Recent studies reported a high rate of thromboembolic complications, in approximately 10% of cases [5, 10, 11]. As such, the development of innovative stent designs, including new materials, surface modification, and coatings while maintaining its mechanical properties, could potentially reduce device thrombogenicity, lessen the need of DAPT use, and increase patient safety.
The use of coatings for stents has been previously explored. Coronary stents have been designed to elute antiproliferative drugs after implant [12–14], and these devices have shown a dramatic reduction of in-stent stenosis and rates of thromboembolic complications with the latest generation of devices [15, 16]. However, only recently have device surface modifications moved into the neurovascular space. The Pipeline Embolization Device with Shield technology has been recently introduced [17, 18], using a very thin (3 nm) phosphorylcholine surface modification. In preclinical settings, this coating has shown to reduce acute thrombus formation on the device [19, 20], while not impacting the aneurysm healing characteristics [21]. Testing of novel surface modifications and coatings is necessary for their successful translation to clinical practice.
In order to assess the risk of thromboembolic complication, high-resolution imaging, delivered by intravascular devices, would allow to visualize and quantify the burden of clot on the device. With the development of high frequency optical coherence tomography (HF-OCT) [22], in vivo assessment of neurovascular devices at a resolution approaching 10 μm is possible. A HF-OCT catheter prototype (Vis-M™, Gentuity LLC, Sudbury, MA) that can be delivered to locations accessible to an 0.017" inner diameter microcatheter was used in this study. By the means of this novel imaging technology, the effects of different types of flow-diverter design and anti-platelet regimes were tested in an animal model. Here, we study the hypothesis that a hydrophilic-coated flow diverter (p48MW-HPC) (phenox, Bochüm, Germany) may present a lower thrombus burden with respect to uncoated devices, similar to what was observed using in vitro models [23]. Furthermore, we investigated the effect of combining flow-diverter coating with aspirin to drastically reduce the formation of thrombus at the ostium of jailed side branches.
Methods and Materials
Experimental Procedures
Under a protocol approved by the Institutional Animal Care and Use Committee (IACUC), castrated male Yorkshire pigs with weight between 31 and 35 kg were used in this study (n = 3). Each animal received a different anti-platelet therapy regime, verified by aspirin reaction unit (ARU) and P2Y12 reaction unit (PRU) testing (Table 1), starting 5 days prior to implantation.1 Femoral arterial access was gained via the modified Seldinger technique, followed by the placement of a 6F hemostatic sheath. Each pig was also given 100 IU/kg heparin following arterial access, and then maintained on 50 IU/kg heparin each hour until the femoral sheath was removed. Each pig received multiple flow diverter devices (n = 4). The internal maxillary (IMAX) and ascending pharyngeal (APA) arteries were targeted for implant due to size, stability, and existence of side branch arteries. Two different length devices were implanted, for the IMAX a 3 × 18 mm device was used, for the APA a 3 × 15 mm device was used, and test and control devices were the same size. Uncoated p48MW flow diverters were implanted on left side arteries as control devices (n = 2 per animal) in the APA and IMAX, respectively; similarly, hydrophilic polymer-coated devices, p48MW-HPC, were implanted on the right side (n = 2 per animal). Figure 1 shows a representative digital subtraction angiography (DSA) image before implant. Both a high-resolution single shot and DSA were acquired after implant. Twenty minutes after flow diverter implantation, HF-OCT data sets were acquired using the Vis-M intravascular imaging device prototype. The Vis-M was delivered to the arterial segment of interest by the means of a 0.017" microcatheter (Excelsior SL-10, Stryker, Freemont CA). In order to clear arterial blood, a contrast agent (Omnipaque-240, GE Healthcare, Cork, Ireland) was injected via power injector at a rate of 4 mL/s for the IMAX, and 2 mL/s for the APA, for a duration of 4 s through a 6F Navien catheter (Medtronic, Irvine, CA). Finally, the femoral sheath was removed, the artery was ligated, and the pig recovered.
Table 1.
Anti-platelet therapy per animal and associated aspirin reaction units (ARU)/P2Y12 reaction units (PRU). An ARU < 550 is considered therapeutic, while a PRU < 240 is considered therapeutic
| Animal: | Anti-platelet therapy | ARU | PRU |
|---|---|---|---|
| 1 | No anti-platelet therapy | 586 | 360 |
| 2 | Aspirin (81 mg) Only | 493 | 284 |
| 3 | Aspirin (81 mg) and clopidogrel (75 mg) | 450 | 229 |
Fig. 1.
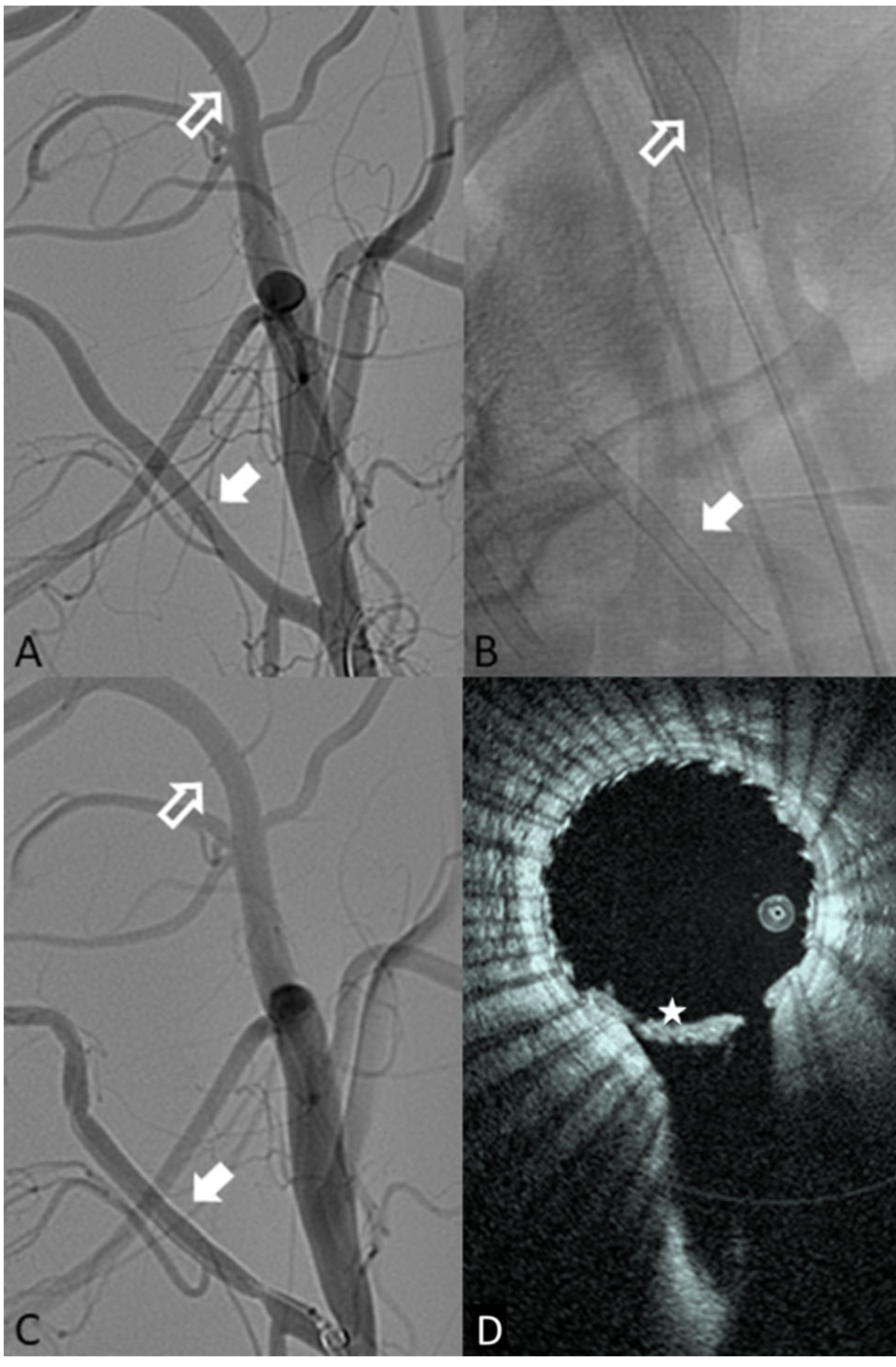
A Pre-implant DSA showing the internal maxillary target location (hollow white arrow) and the ascending pharyngeal artery target zone (solid white arrow). B Single-shot fluoroscopic image of both devices once deployed (IMAX—hollow arrow; APA—solid arrow). C Post-implant example DSA of both locations deployed (IMAX—hollow arrow; APA—solid arrow). D HF-OCT slice from the APA side branch, the star (*) shows a large amount of thrombus seen on HF-OCT but not DSA
Image Analysis
Following interventional procedures, HF-OCT cross-sectional images were analyzed using ImageJ (National Institutes of Health, USA), first selecting for slices that contained side branch origins. For each slice, the area of clots covering the ostium of the side branches was manually traced by a reviewer with experience in endovascular image analysis blinded to both device type and anti-platelet therapy. In order to determine the volume of thrombus, the area of thrombus on each slice was combined using a Riemann sum. Each branch was considered as an independent observation. APA arteries showed a median of 2 (range 1–2) side branches, while the IMAX showed median of 5 (range 3–5) branches. Following clot analysis, each branch opening ostium area was measured to normalize the result. Statistical testing was used to interrogate the significance of the four test factors, namely: device type (p48MW-HPC vs p48MW), location (APA vs IMAX), aspirin administration, and clopidogrel administration. Once the significant factors were identified using a significance threshold equal to α ≤ 0.05, a linear model was constructed to determine their relative impact on the thrombus formation in covered side branch origins.
Statistical Analysis
All data are presented as mean ± standard deviation. An ANOVA test was used for data that were found to be normally distributed, followed by a Tukey post hoc test. In case of factors not following a normal distribution, a nonparametric Mann–Whitney U with Bonferroni correction was used for multiple comparisons. Finally, for each of the significant factors, a linear model was constructed assuming a binary yes/no determination. All statistical tests were performed using software R 3.5.2 (The R Foundation).
Results
In this study, we identified a total of 514 HF-OCT slices that included side branches (n = 35 side branches), over a total of 12 implanted arteries, with a slice thickness of 53 μm for the APA and 66 μm for the IMAX. Of these 514 slices, 234 were from the p48MW-HPC group, while the remaining 280 were from the uncoated p48MW device. Since two of the three animals were on aspirin, 344 of the slices included came from animals treated with at least single anti-platelet therapy. Overall, study results indicated that both device coating with HPC and aspirin use were significantly associated with reduction of clot burden (p < 0.001 and p = 0.003, respectively). Table 2 reports the average and standard deviation for the four factors tested, along with the associated p value. The effect of p48MW-HPC coating was seen to have a greater than 100-fold reduction in the clot burden, while aspirin usage was seen to reduce clot burden by 50-fold. The combined effect approached a 1000-fold reduction. Figure 2 shows a representative HF-OCT slice from each of the three pigs, from each side. It is notable that the pig not receiving any anti-platelet therapy showed a large clot burden on the control devices, but almost none on the coated devices. The linear regression model comparing the relative effect of device and aspirin usage showed that both had a reductive effect, and that either factor reduced the clot burden near to zero. The mean clot burden for control devices without anti-platelet was 0.224 mm3/mm2, with pHPC coating reducing the clot burden by 0.223 mm3/mm2 and aspirin reducing the clot burden by 0.216 mm3/mm2. The addition of clopidogrel did not have any significant impact on the mean clot burden (p = 0.43); however, this factor had a very small sample size.
Table 2.
Factor level analysis for clot burden
| Factor | Group | Mean clot burden (μm3/μm2) ± SD | p value | Significant |
|---|---|---|---|---|
| Device | p48MW-HPC (test) | 0.33 ± 0.79 | < 0.001 | Yes |
| p48MW (control) | 87 ± 150 | |||
| Aspirin | 81 mg | 4.1 ± 18 | 0.003 | Yes |
| None | 160 ± 170 | |||
| Clopidogrel | 75 mg | 8.3 ± 29 | 0.43 | No |
| None | 68 ± 140 | |||
| Location | APA | 12 ± 29 | 0.95 | No |
| IMAX | 59 ± 130 | |||
Fig. 2.
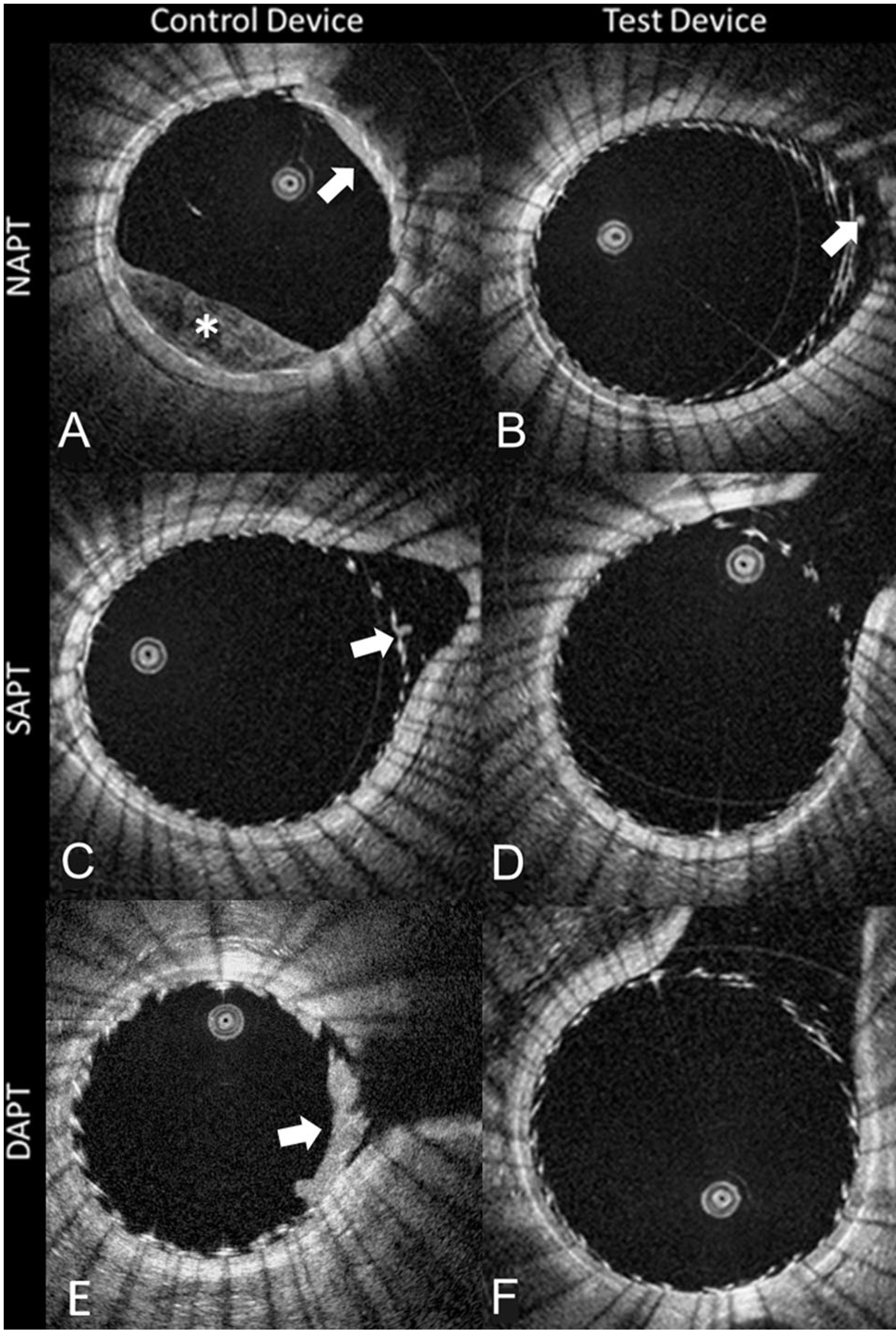
A single OCT slice derived from each of the 6 testing conditions. The first column (A, C, E) shows the control device (p-48MW), while the second column (B, D, F) shows the test device (p48MW-HPC). Along the rows shows the different anti-platelet therapy groups. Of note, only conditions A, B, C, and E show an appreciable burden of clot (white arrows), confirming that the combination of the test device and aspirin to remove almost all the clot. The (*) in panel A shows incomplete blood clearance in the lumen (NAPT; no anti-platelet therapy, SAPT: single anti-platelet therapy, DAPT; dual anti-platelet therapy)
Discussion
The use of flow diversion and new generation intrasaccular devices for the treatment of aneurysms significantly increased in the last decade. However, a major complicating factor remains, the need of DAPT in almost all patients. With the recent development of HF-OCT, it will be possible to image these devices at the time of the implant, generating a new array of treatment metrics. Also, this technology could be used to follow the formation of neointima and determine the optimal timing for cessation of DAPT. Here, in an acute imaging study, we investigated the clot burden over side branches jailed by a flow diverter. In a swine animal model, HF-OCT enabled us to evaluate the effect of a new surface coating (HPC), characterizing its interaction with different anti-platelet therapies.
Previous works investigated the formation of clots on the surface of flow diversion devices, with and without surface modification either using a single or dual anti-platelet therapy [19, 20]. However, the major limitation of these studies was the use of an OCT system that was designed for cardiac imaging, not neuroimaging. Furthermore, in the current study, HF-OCT data sets were used to accurately measure and quantify the true size of the clot burden, instead of a binary identification of absence or present of clots. Interestingly, we observed a similar trend, showing that both anti-platelet therapy and hydrophilic device coating resulted in a reduced clot burden, and we characterized the contribution of each one of those factors and their combination.
One of the hypotheses investigated in this study was if aspirin, when combined with an pHPC-coated implant, can reduce the amount of thrombus on the flow diverting stent surface and eliminate the need of DAPT. The results of this preliminary study seem to support that conclusion, with a near complete elimination of acute thrombus with the combination of the pHPC and aspirin. Previous research into the effects of aspirin and clopidogrel has shown mixed results, with some studies showing no difference in the addition of clopidogrel over aspirin alone [24]; however, others have shown an effect in the reduction of myocardial infarction and stroke [25]. In either case, the effect of the clopidogrel is small, and based on the results of our study, we hypothesize that pHPC-coated flow diverters are potentially able to replace the use of DAPT.
Although this study is limited by the number of test animals (n = 3), the ability to image multiple side branches per implant, and the ability to deploy both test and control devices on each swine, reduced the inter-animal variability and the number of required samples. Secondarily, only acute results were evaluated and given the absence of follow-up time points, complications from this larger clot burden were not evaluated. Independent studies have shown the short-term safety of the p48MW-HPC device, and that the pHPC did not have any adverse inflammatory or cell loss effects [26]. Future studies would be needed to characterize what clot size and clot properties are safe when observed over the ostium of side branches, and what characteristics could lead to complications.
Supplementary Material
Funding
This study was funded by phenox and the Massachusetts Life Sciences Center Bits-to-Bytes program.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00270-020-02482-w) contains supplementary material, which is available to authorized users.
The anesthesiological protocol can be found in the online supplement of the article.
Compliance with Ethical Standards
Conflict of interest Robert M. King, Erin T. Langan, Chris M. Raskett declare that they have no conflicts of interest. Giovanni J. Ughi declares he is an employee of Gentuity LLC and holds stock. Hans Henkes declares he is a founder of phenox GmbH. Ajit S. Puri declares he has been a consultant for Stryker, Cerenovus, Medtronic, Cerevasc, and Microvention; holds stock in InNeuroCo; and has received research support from National Institutes of Health (NIH). Matthew J. Gounis declares he has been a consultant on a fee-per-hour basis for Cerenovus, Imperative Care, phenox, Medtronic Neurovascular, Route 92 Medical, Stryker Neurovascular; holds stock in Imperative Care and Neurogami; and has received research support from the National Institutes of Health (NIH), the United States – Israel Binational Science Foundation, Anaconda, ApicBio, Axovant, Cerenovus, Cook Medical, Gentuity, Imperative Care, InNeuroCo, Magneto, MicroVention, Medtronic Neurovascular, MIVI Neurosciences, Neuravi, Neurogami, Philips Healthcare, Rapid Medical, Route 92 Medical, Stryker Neurovascular, Syntheon, and the Wyss Institute.
Ethical Approval All procedures performed in this study were in accordance with ethical standards of the Institutional Animal Care and Use Committee (IACUC).
Publisher's Disclaimer: Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lylyk P, et al. Endovascular reconstruction of intracranial arteries by stent placement and combined techniques. J Neurosurg. 2002;97(6):1306–13. [DOI] [PubMed] [Google Scholar]
- 2.Piotin M, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke. 2010;41(1):110–5. [DOI] [PubMed] [Google Scholar]
- 3.Wakhloo AK, et al. Closed-cell stent for coil embolization of intracranial aneurysms: clinical and angiographic results. AJNR Am J Neuroradiol. 2012;33(9):1651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becske T, et al. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery. 2017;80(1):40–8. [DOI] [PubMed] [Google Scholar]
- 5.Adeeb N, et al. Use of platelet function testing before pipeline embolization device placement: a multicenter cohort study. Stroke. 2017;48(5):1322–30. [DOI] [PubMed] [Google Scholar]
- 6.Podlasek A, et al. Outcome of intracranial flow diversion according to the antiplatelet regimen used: a systematic review and meta-analysis. J Neurointerv Surg. 2020;12(2):148–55. [DOI] [PubMed] [Google Scholar]
- 7.Kan P, et al. Expanding indications for flow diverters: ruptured aneurysms, blister aneurysms, and dissecting aneurysms. Neurosurgery. 2020;86(Supplement_1):S96–S103. [DOI] [PubMed] [Google Scholar]
- 8.Cutlip DE, et al. Thrombotic complications associated with early and late nonadherence to dual antiplatelet therapy. JACC Cardiovasc Interv. 2015;8(3):404–10. [DOI] [PubMed] [Google Scholar]
- 9.Rajah G, Narayanan S, Rangel-Castilla L. Update on flow diverters for the endovascular management of cerebral aneurysms. Neurosurg Focus. 2017;42(6):E2. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro M, et al. Stent-supported aneurysm coiling: a literature survey of treatment and follow-up. AJNR Am J Neuroradiol. 2012;33(1):159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daou B, et al. P2Y12 reaction units: effect on hemorrhagic and thromboembolic complications in patients with cerebral aneurysms treated with the pipeline embolization device. Neurosurgery. 2016;78(1):27–33. [DOI] [PubMed] [Google Scholar]
- 12.Sousa JE, et al. Sirolimus-eluting stent for the treatment of in-stent restenosis: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2003;107(1):24–7. [DOI] [PubMed] [Google Scholar]
- 13.Sousa JE, et al. Two-year angiographic and intravascular ultrasound follow-up after implantation of sirolimus-eluting stents in human coronary arteries. Circulation. 2003;107(3):381–3. [DOI] [PubMed] [Google Scholar]
- 14.Sousa JE, et al. Use of rapamycin-impregnated stents in coronary arteries. Transplant Proc. 2003;35(3 Suppl):165S–70S. [DOI] [PubMed] [Google Scholar]
- 15.Dola J, et al. Results of PCI with drug-eluting stents in an all-comer population depending on vessel diameter. J Clin Med. 2020;9(2):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youn YJ, et al. Randomized comparison of everolimus- and zotarolimus-eluting coronary stents with biolimus-eluting stents in all-comer patients. Circ Cardiovasc Interv. 2020;13(3): e008525. [DOI] [PubMed] [Google Scholar]
- 17.Pasarikovski CR, et al. Pipeline embolisation device with shield technology for the treatment of ruptured intracranial aneurysm. Neuroradiol J. 2019;32(3):189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trivelato FP, et al. Safety and effectiveness of the pipeline flex embolization device with shield technology for the treatment of intracranial aneurysms: midterm results from a multicenter study. Neurosurgery. 2019. 10.1093/neuros/nyz356. [DOI] [PubMed]
- 19.Marosfoi M, et al. Acute thrombus formation on phosphorilcholine surface modified flow diverters. J Neurointerv Surg. 2018;10(4):406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda Y, et al. Preliminary outcomes of single antiplatelet therapy for surface-modified flow diverters in an animal model: analysis of neointimal development and thrombus formation using OCT. J Neurointerv Surg. 2019;11(1):74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King RM, et al. Communicating malapposition of flow diverters assessed with optical coherence tomography correlates with delayed aneurysm occlusion. J Neurointerv Surg. 2018;10(7): 693–7. [DOI] [PubMed] [Google Scholar]
- 22.Gounis MJ, et al. Intravascular optical coherence tomography for neurointerventional surgery. Stroke, 2018: p. STROKE AHA118022315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenz-Habijan T, et al. Hydrophilic stent coating inhibits platelet adhesion on stent surfaces: initial results in vitro. Cardiovasc Intervent Radiol. 2018;41(11):1779–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatt DL, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–17. [DOI] [PubMed] [Google Scholar]
- 25.Donadini MP, Bellesini M, Squizzato A. Aspirin plus clopidogrel vs aspirin alone for preventing cardiovascular events among patients at high risk for cardiovascular events. JAMA. 2018;320(6):593–4. [DOI] [PubMed] [Google Scholar]
- 26.Martinez Moreno R, et al. In vivo canine study of three different coatings applied to p64 flow-diverter stents: initial biocompatibility study. Eur Radiol Exp. 2019;3(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


