Abstract
The present study aimed to investigate the association between rate of torque development (RTD), rate of activation (RoA), and muscle structure [muscle cross-sectional area (CSA), intramuscular fat (IMAT) and high density lean muscle (HDL)] with the weight transfer phase (WTP) during a choice reaction step test (CST) in older adults. Fifteen healthy older adults (7 females) participated in this study. Stance leg hip adductors RTD at 100, 150, and 200 ms, showed a significant inverse correlation with WTP (r ≥ 0.658, P ≤ 0.010). There was a significant inverse relationship between WTP and adductor magnus and tensor fascia latae RoA at all time points (RoA0–50-RoA0–200; r ≥ 0.707, P ≤ 0.033). In contrast, the WTP was not significantly associated with the hip abductor RTD, gluteus medius RoA, or muscle structure (CSA, IMAT, and HDL). Swing leg showed no significant relationship between WTP and RTD, RoA or muscle structure of the hip abductor or adductor muscles. In conclusion, the present study showed that hip adductor torque-time capacity, as well as neuromuscular activation of the adductor magnus and tensor fascia latae of the stance leg during a maximal isometric test, is associated with the ability to transfer body weight before a step to the side occurs.
Keywords: Rapid torque, Neuromuscular activation, Muscle structure, Voluntary stepping, Older adults
1. Introduction
Falls are a global health concern resulting in injuries (Hausdorff et al., 2001; Phelan et al., 2015) which significantly increase with age (Kannus et al., 2018; Lord et al., 1993; Phelan et al., 2015). Fall avoidance requires fast movements that are generated by rapid muscle torque production to execute a protective step (Pijnappels et al., 2010). However, older adults have a decreased capacity to produce torque rapidly in the later stages of life (Izquierdo et al., 1999), possibly increasing their risk of falling. Older adults appear to be particularly vulnerable to falls when their balance is perturbed in the mediolateral direction (Mille et al., 2013). This may be due in part to the altered structure and composition of the hip abductors and adductors (i.e., increased fatty infiltration) (Addison et al., 2014; Inacio et al., 2018, 2019). Thus, understanding the muscle composition and performance capacity (e.g.; muscle contractile properties and activation) of the muscles that control mediolateral balance are important for quick stepping responses in older adults that are important for fall avoidance.
The choice reaction step test (CST; Table 1) has been frequently used in older adults to measure the capacity to respond to visual stimuli and perform a step. The time taken to initiate the step is a predictor of fall risk (Lord and Fitzpatrick, 2001; Pijnappels et al., 2010) and is associated with slower choice reaction times (Lord and Fitzpatrick, 2001), which might be related to physiological factors of the muscle such as decrease in muscle force and/or nerve conduction velocity (Melzer and Oddsson, 2004; Pijnappels et al., 2010). Additionally, the weight transfer phase (WTP; time to shift the weight from the stepping to the stance leg prior to initiating a protective step) preceding an induced step appears to be important for recovering balance (Inacio et al., 2019). Older adults take longer to initiate the WTP than young adults (Inacio et al., 2019), which may impact the ability to initiate a protective step (Orr et al., 2006). The WTP appears to be an important part of preparing for a protective step, and yet, little is known about its underlying mechanisms.
Table 1.
List of abbreviations.
| CSA | Cross-sectional area |
| CST | Choice reaction step test |
| EMG | Electromyography |
| GM | Gluteus medius |
| HDL | High density lean muscle |
| IMAT | Intramuscular fat |
| LDL | Low density lean muscle |
| RoA | Rate of activation |
| RTD | Rate of torque development |
| TFL | Tensor fascia latae |
| WTP | Weight transfer phase |
Previous research indicates that kinetic (e.g., force), muscle structure (e.g., cross-sectional area), and neuromuscular responses (e.g., muscle activation) influence explosive force (isometrically in a single joint) (Folland et al., 2014; Maffiuletti et al., 2016). Yet, it remains uncertain which factors influence the ability to transfer weight quickly prior to initiating a rapid step. For instance, the ability of the muscle to produce torque [e.g., rate of torque development (RTD)] declines with age (Izquierdo et al., 1999; Schettino et al., 2014). More importantly, the rate of developing force is reduced in fallers compared to non-fallers (Morcelli et al., 2016). Generating torque rapidly may be more important to recover balance than muscle strength in older adults (Bento et al., 2010), especially in the hip joint (Addison et al., 2017; Inacio et al., 2018). For example, the time to achieve maximal force in older adults during an isometric task is around 500 ms (Klass et al., 2008). However, the phase prior to stepping (e.g., WTP) occurs is less than 200 ms (Inacio et al., 2019), well before the maximal force is reached. Thus, examining the WTP of the CST is important for understanding the factors that contribute to the capacity to initiate a step rapidly. Furthermore, the ability to produce force by activating the muscles in the first 200 ms may be more important for the WTP than the time it takes to produce the maximal contraction (~500 ms). Thus, investigating the neuromotor responses in the earlier phases of rapid muscle contractions [e.g., 0–50 up to 200 ms (Lanza et al., 2019a)] may be necessary to understand the performance of the WTP that happens prior to the initiation of the step. Moreover, the neural drive that would be needed to rapidly activate the muscles [e.g., rate of activation (RoA)] dictates how quickly torque would be developed (Aagaard et al., 2002; Folland et al., 2014), potentially contributing to faster reaction times (Inacio et al., 2019). Given the change in muscle properties with aging, such as a loss of Type 2 fibers, impact the motor unit recruitment and firing rate (Evans and Lexell, 1995), the contribution of RoA on the WTP during a rapid step in older adults is not clear. Although the advantage of a greater RoA would be a faster weight transfer and ultimately a quicker step initiation time (Inacio et al., 2019).
Declines in functional activities of daily living with age are associated with decreased muscle strength and muscle size (Janssen et al., 2002; Tieland et al., 2018). Muscle size, measured as cross-sectional area (CSA), is directly related to muscle power, (Minetti, 2002; Suchomel and Stone, 2017; Visser et al., 2005) with a decreased cross-sectional area associated with a risk of mobility loss (Visser et al., 2005). Muscle CSA includes fat content within the muscle [intramuscular fat (IMAT)], and high-density lean tissue (HDL; muscle without IMAT). Increased IMAT of the hip muscles that control medial-lateral balance, negatively impacts balance in older individuals (Addison et al., 2014, 2017). Those that had greater hip abductor muscle HDL were more likely to take a step in response to a lateral waist-pull perturbation that required a WTP (Addison et al., 2014, 2017). While intuitively, these same muscles would contribute to rapid voluntary stepping as observed during an induced stepping behaviour, it is unclear how the muscle composition measures (CSA, IMAT and/or HDL) contribute to rapid voluntary stepping. Therefore, the present study investigated the relationship between early phases of the rapid torque production (measure as RTD), early RoA, and muscle composition with the WTP of a CST in older adults. We hypothesized that a reduced earlier hip abductor and adductor muscle RTD, RoA, and CSA and HDL would be associated with a longer WTP, while greater IMAT would be associated with a longer WTP.
2. Methods
2.1. Subjects
Healthy community-dwelling older individuals were recruited for this study (Table 2). All participants provided written informed consent. This study was carried out in accordance with The Declaration of Helsinki and approved by the local Institutional Review Board. The following exclusion criteria were used: cognitive impairment (Folstein Mini-Mental Score Exam < 24); sedative use; use of an assistive device for ambulation; any clinically significant functional impairment related to musculoskeletal, neurological, cardiopulmonary, metabolic, or other general medical problem; depression (Centers for Epidemiological Studies Depression Survey >16); and BMI equal or greater than 35 kg/m2.
Table 2.
Participants demographics.
| n | 14 |
|---|---|
| Females | 7 |
| Age (years) | 71.1 ± 3.7 |
| Height (m) | 1.68 ± 0.05 |
| Weight (Kg) | 79.1 ± 12.4 |
| Body Mass Index (Kg/m2) | 27.9 ± 4.2 |
| Short Physical Performance Battery | |
| Balance Tests Score | (3.9/4) |
| Gait Speed Test Time | (3.2/4) |
| 5 Chair Stands | (3.5/4) |
| Total | 10.6 ± 0.5 |
| Four Square Step Test (s) | 8.0 ± 0.9 |
2.2. Testing and recording procedures
Participants performed standing unilateral hip abductor and adductor maximal voluntary isometric contractions (MVIC) on a BIODEX System 4 dynamometer (BIODEX, Shirley, NY). MVIC was performed at 30° of hip abduction (where 0° is the anatomical position). The tested limb was secured with a strap to the Biodex arm with the thigh pad proximal to the knee joint. Support was provided through the arms by a custom standing frame designed to facilitate postural stabilization during the contraction. A marker placed at the position of the great trochanter was aligned with the dynamometer axis of motion (Johnson et al., 2004). Each participant was asked to “push” (abduction) or “pull” (adduction) as fast and hard as possible for ~5 s and rested 90s between MVIC with ~5 min rest between the abductor and adductor MVICs. Three contractions were performed for abduction and adduction on each side (left and right). The testing order was consistent between all participants, left limb abduction, left limb adduction, right limb abduction, and right limb adduction. During the MVIC, surface electromyography (EMG) signals were recorded using a wireless EMG systemc at a frequency of 1500 Hz. Bipolar EMG electrodes (2 cm inter-electrode distance) were placed bilaterally over the TFL, GM, and adductor magnus (ADD) in accordance with SENIAM guidelines (Hermens et al., 2000). The EMG signals were collected in MyoResearch XP software (Noraxon, USA Inc.) and exported to Spike 2 software (CED, Cambridge, UK) for off-line analysis.
During off-line analysis, maximum voluntary torque (MVT) was identified as the highest instantaneous torque among the three contractions. Rate of torque development (RTD) was measured across 50, 100, 150, and 200 ms (RTD50, RTD100, RTD150, and RTD200 respectively) from torque onset [manually identified by visual identification by a trained investigator using a systematic approach (Tillin et al., 2013)] by dividing the torque at the time point by its respective time period (e.g., torque at 50 ms/0.05 s) as previously used (Lanza et al., 2019a). RTD was normalized by MVT in each time period for each contraction, and a mean value was calculated by averaging the trials. EMG signals were recorded with a bandpass filter (20–500 Hz bandwidth) and filtered using a fourth-order Butterworth digital bandpass filter. The EMG signal was separated in epochs, 0–50 (RoA0–50), 0–100 (RoA0–100), 0–150 (RoA0–150), and 0–200 (RoA0–200) from EMG onset, as previously performed (Hannah et al., 2014; Lanza et al., 2019a) and the EMG signal was calculated as the root mean square across each epoch. Next, each average value was divided by the respective time period (e.g., EMG from 0 to 50 ms/0.05 s) to generate the RoA (Fig. 2). The EMG signal was normalized in each epoch by the EMG at the MVIC, which was calculated over a 500 ms epoch, 250 ms either side, from peak torque.
Fig. 2.
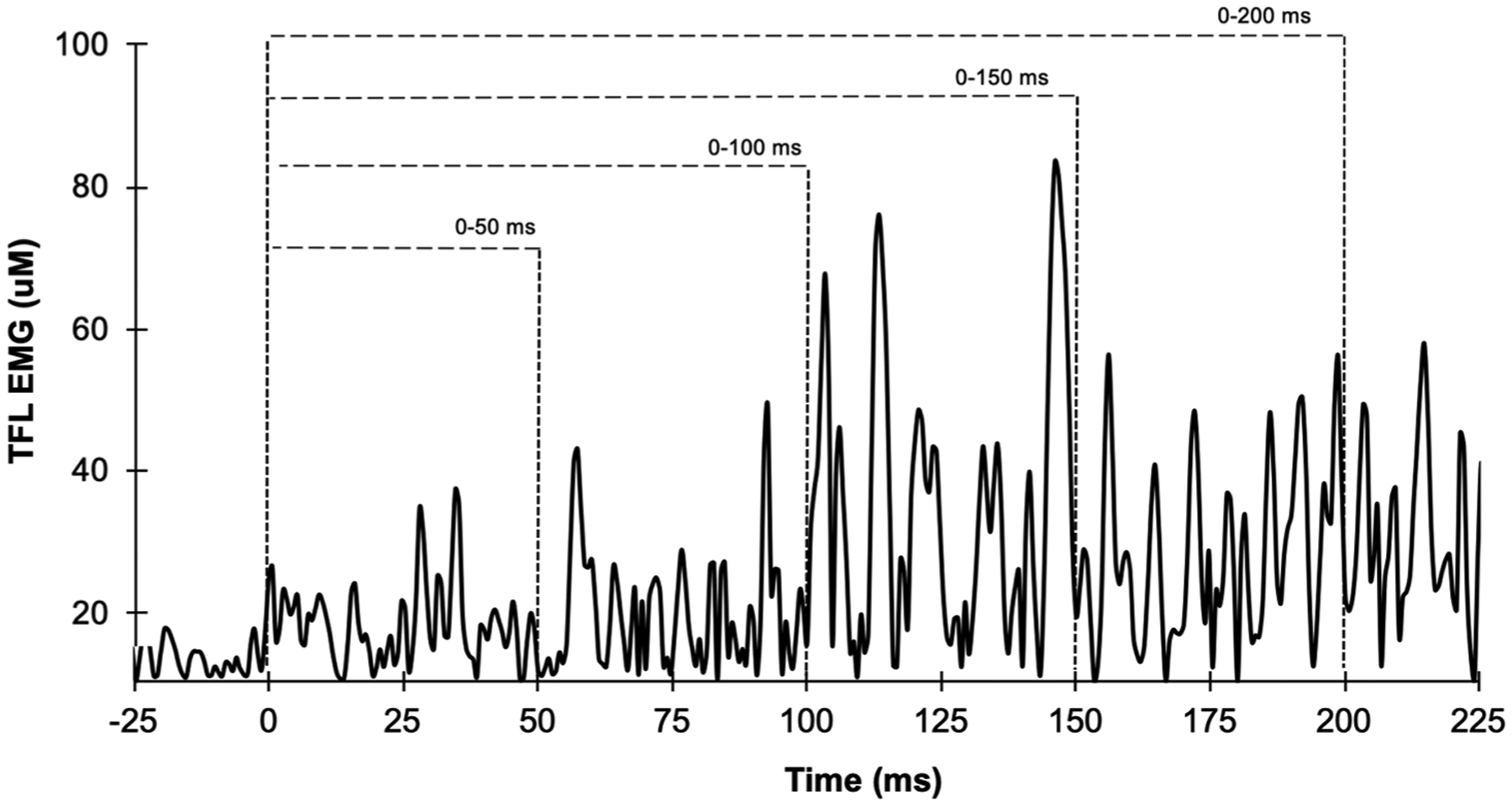
Example of sample recording of electromyography amplitude (filtered and root mean squared processed) from EMG onset (0 ms) during hip abduction maximum voluntary isometric contraction from the tensor fascia latae (TFL) of one participant.
Clinical measures of balance and mobility were assessed to characterize the balance function. Each participant performed the Short Physical Performance Battery that assesses gait speed, static balance, and the time to complete 5 repeated chair rises (lower extremity strength). Each task is scored between 0 and 4, with a total score of 12 (Guralnik et al., 1994). Also, participants completed the timed Four Square Step Test to assess the ability to step quickly over an object forward, sideways, and backward (Dite and Temple, 2002).
After the MVIC, participants stood on two adjacent force platforms (AMTI, Watertown, MA) and performed ten voluntary lateral choice reaction steps. Participants were instructed to take a lateral step as fast as possible following a visual “go” cue” without prior knowledge of the stepping leg (CST, 5 trials × 2 sides). The “go” cue was preceded by a warning signal of an inter-stimulus delay of 100 ms. The direction of the step was randomly assigned. The force platform data were collected at a frequency of 600 Hz. The data analyses for the CST targeted the WTP (Fig. 3) by focusing on the interval from the onset of lateral weight transfer to the instant of foot lift-off, as previously described (Inacio et al., 2018).
Fig. 3.
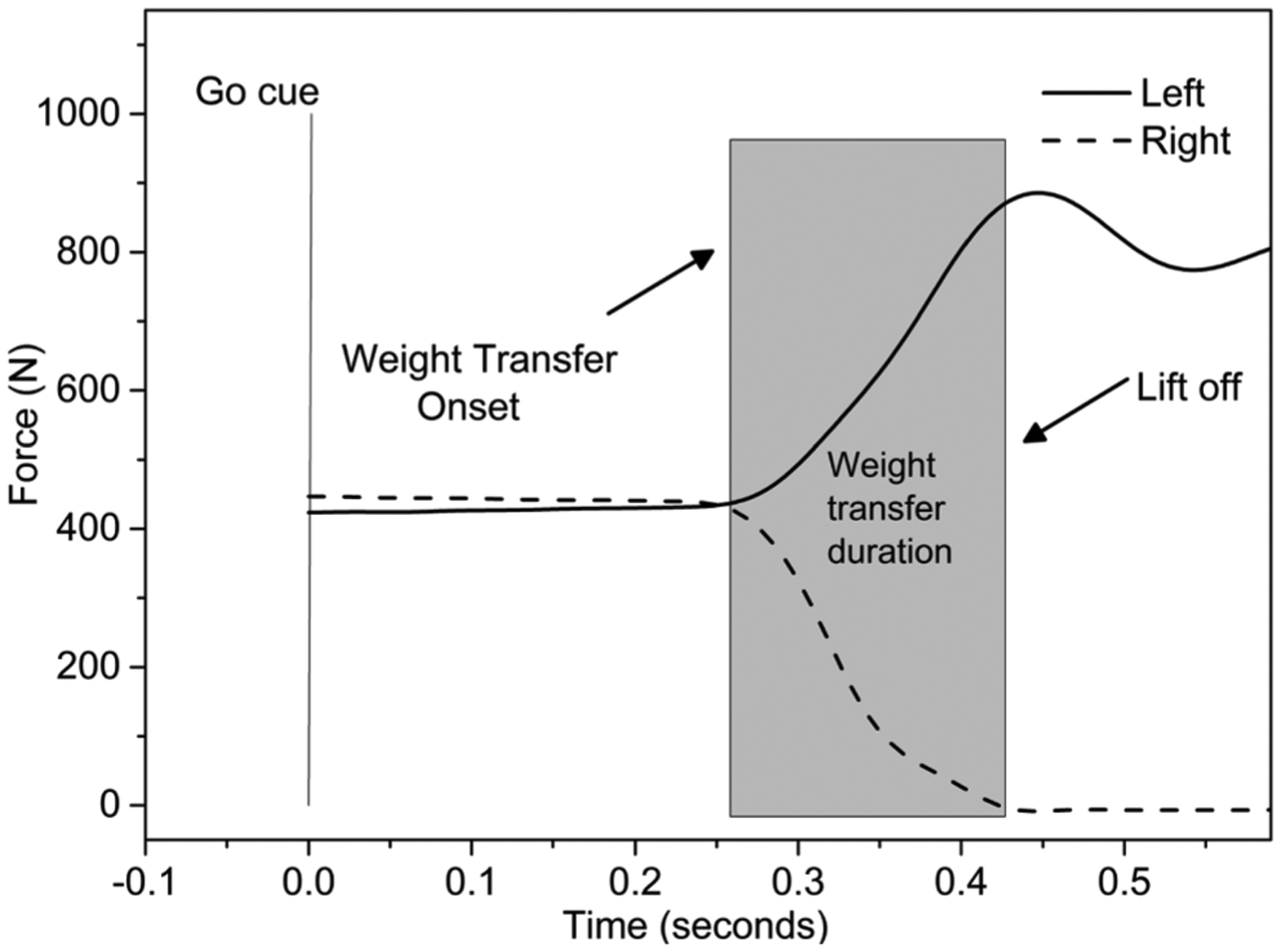
Example of sample recording of force data during a right step choice reaction test (CST) of one participant.
Muscle composition was determined with a computed tomography scan using methods previously reported in detail (Inacio et al., 2014). A computerized spiral tomography scan was performed starting at L2-L3 and ending at the patella using a Siemens Somatom Sensation 64 Scanner. The gluteus medius (GM), tensor fascia latae (TFL), and adductor longus/magnus (ADDLM) were assessed (Addison et al., 2014). In brief, CSA, HDL, low-density lean tissue (LDL), and adipose tissue content (fat) within each muscle were determined using Medical Image Processing, Analysis and Visualization (MIPAV, v. 7.0 NIH) software (Fig. 1). Previous work used both LDL and fat as a measure of IMAT; hence both were included in this study (Addison et al., 2014). Computed tomography data for each muscle was expressed as CSA of tissue (cm2) using Hounsfield units (HU) for HDL between 30–100, LDL, 0–29, and FAT −30- to 0–190 (Addison et al., 2014). Muscle composition was normalized for the respective muscle size by calculating the percent of each measure relative to the total muscle CSA (Bouche et al., 2011).
Fig. 1.
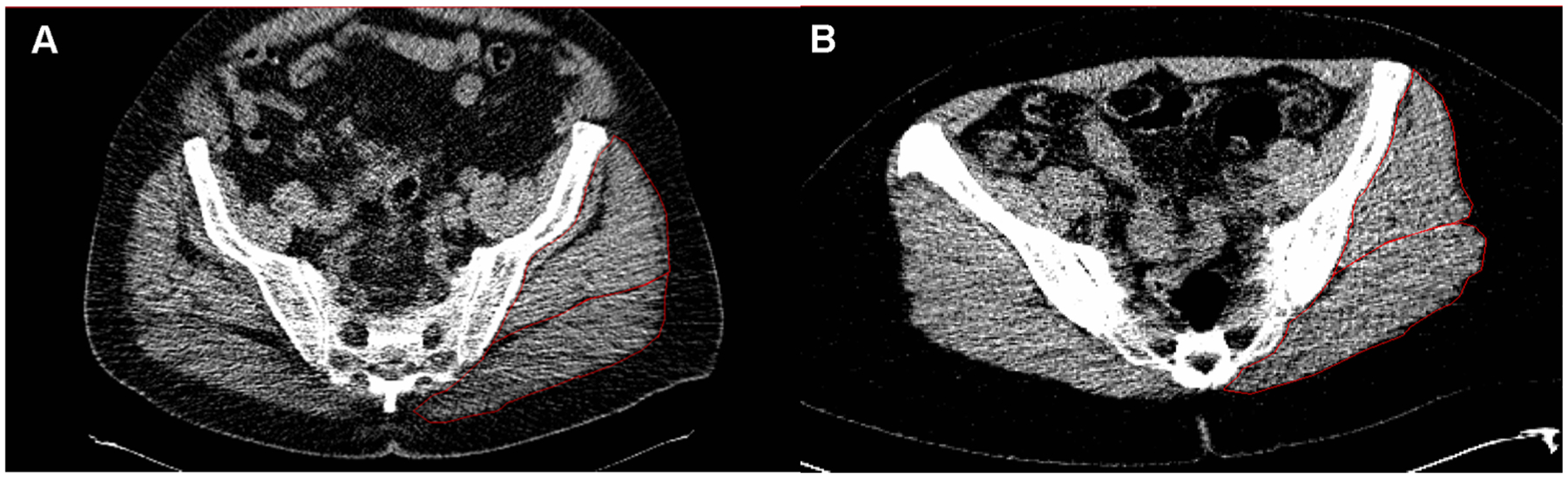
CT scans of two individuals one with differing amoutns of lean tissue and IMAT in the hip abductors. A. demonstrates low attenuation and high levels of IMAT in the gluteus medius/minimus (outlined in red). B. demonstrates a high attenuation and low levels of IMAT in the gluteal muscles.
2.3. Statistical analysis
Shapiro-Wilk test assessed the normality of the data. SPSS version 23 (IBM Corporation, Armonk, New York, USA) was used to calculate Pearson’s product-moment bivariate correlations between WTP and the stance leg variables (RTD, RoA, FAT, LDL, HDL, and CSA) and stepping leg variables that were contralateral to WTP. The stance leg is defined as the leg that supports the body weight while the stepping leg moves to perform the step. Considering the clinical relevance and importance of the WTP, correlations were performed for the weaker leg (Table 3). To identify differences between the epochs of RTD and RoA a repeated measure one-way ANOVA was performed, and post-hoc comparisons were conducted using Bonferroni tests. Data are presented as mean and standard deviations. The significance level was set at P ≤ 0.05.
Table 3.
Mean values and standard deviations of rate of torque development (RTD), MVT, rate of activation (RoA), muscle cross-sectional area (CSA), fat percentage relative to CSA (FAT%), low density lean tissue relative to CSA (LDL%), high-density lean tissue relative to CSA (HDL%) and weight transfer phase (WTP) (n = 14).
| RTD50 (MVT.s-1) | RTD100 (MVT.s-1) | RTD150 (MVT.s-1) | RTD200 (MVT.s-1) | MVT (N.m) | |
|---|---|---|---|---|---|
| Hip Abduction | 3.93 ± 2.26 | 4.44 ± 1.87 | 3.71 ± 1.29 | 2.95 ± 0.75* | 33 ± 15 |
| Hip Adduction | 0.53 ± 0.29‡ | 1.15 ± 0.78‡ | 1.59 ± 1.06‡ | 1.90 ± 1.08‡ | 87 ± 20 |
| RoA0–50 (% EMGMVT) | RoA0–100 (% EMGMVT) | RoA0–150 (% EMGMVT) | RoA0–200 (% EMGMVT) | ||
| Gluteus Medius | 13 ± 6 | 7 ± 3‡ | 14 ± 6 | 15 ± 6 | |
| Tensor Fascia Latae | 15 ± 6 | 9 ± 4‡ | 17 ± 9 | 16 ± 8 | |
| Adductor Magno | 11 ± 6 | 6 ± 3‡ | 13 ± 7 | 12 ± 6 | |
| CSA (cm2) | FAT (% CSA) | LDL (% CSA) | HDL (% CSA) | ||
| Gluteus Medius + Minimus | 42 ± 11 | 10 ± 6 | 24 ± 3 | 50 ± 10 | |
| Tensor Fascia Latae | 6 ± 2 | 13 ± 11 | 26 ± 8 | 39 ± 18 | |
| Adductor Magno + Longus | 26 ± 10 | 3 ± 4 | 26 ± 8 | 57 ± 19 | |
| WTP (s) | |||||
| Choice Reaction Step Test (Weaker leg) | 0.219 ± 0.052 | ||||
| Choice Reaction Step Test (Stronger leg) | 0.207 ± 0.035 |
Symbols indicates:
lower than RTD100 and RTD150;
different from the others.
3. Results
The participant characteristics are presented in Table 2. The mean time on the Four Square Step Test was 8.0 ± 0.9 s, and the mean score was 10.6 ± 0.5 on the Short Physical Performance Battery. During MVIC, hip abductor and adductor RTD and RoA (all muscles) were different among the time points (P < 0.001; Table 3). For hip abduction, RTD200 was smaller than RTD100 and RTD150 (P ≤ 0.015). For hip adduction, all the time points were different from each other (P ≤ 0.004), and the largest RTD was at RTD200. All the muscles showed a reduced activation at RoA0–100 compared to the other time points (P ≤ 0.001).
3.1. Stance leg
There was a significant negative correlation between WTP and hip adductor peak MVT and torque production at RTD100, RTD150, and RTD200 (Table 4). No significant correlation was found in the early epoch of RTD50 (Fig. 4). There was a significant inverse relationship between the WTP and ADD RoA and TFL RoA at all time points (RoA0–50-RoA0–200) (Table 5). The WTP was not significantly correlated with the hip abductor RTD, hip abductor MVT (Table 4), or GM RoA (Table 5). There was no significant relationship between muscle structure (CSA, IMAT, and HDL; Table 6) and WTP.
Table 4.
Pearson correlations between weight transfer phase (WTP) of the stance leg during the lateral choice reaction step and rate of torque development (RTD) at 50, 100, 150, 200 ms and maximal voluntary torque (MVT) during maximal isometric voluntary contraction of the hip abductor and adductor muscles.
| WTP | ||
|---|---|---|
| Hip Abduction | ||
| RTD50 | −0.257 | r |
| 0.374 | P | |
| RTD100 | −0.317 | r |
| 0.274 | P | |
| RTD150 | −0.405 | r |
| 0.151 | P | |
| RTD200 | −0.510 | r |
| 0.090 | P | |
| MVT | −0.490 | r |
| 0.075 | P | |
| Hip Adduction | ||
| RTD50 | −0.388 | r |
| 0.171 | P | |
| RTD100 | −0.697 | r |
| 0.006* | P | |
| RTD150 | −0.658 | r |
| 0.01* | P | |
| RTD200 | −0.665 | r |
| 0.009* | P | |
| MVT | −0.570 | r |
| 0.033* | P |
Symbol (*) indicates the Pearson correlation (r) was statistically significant.
Fig. 4.
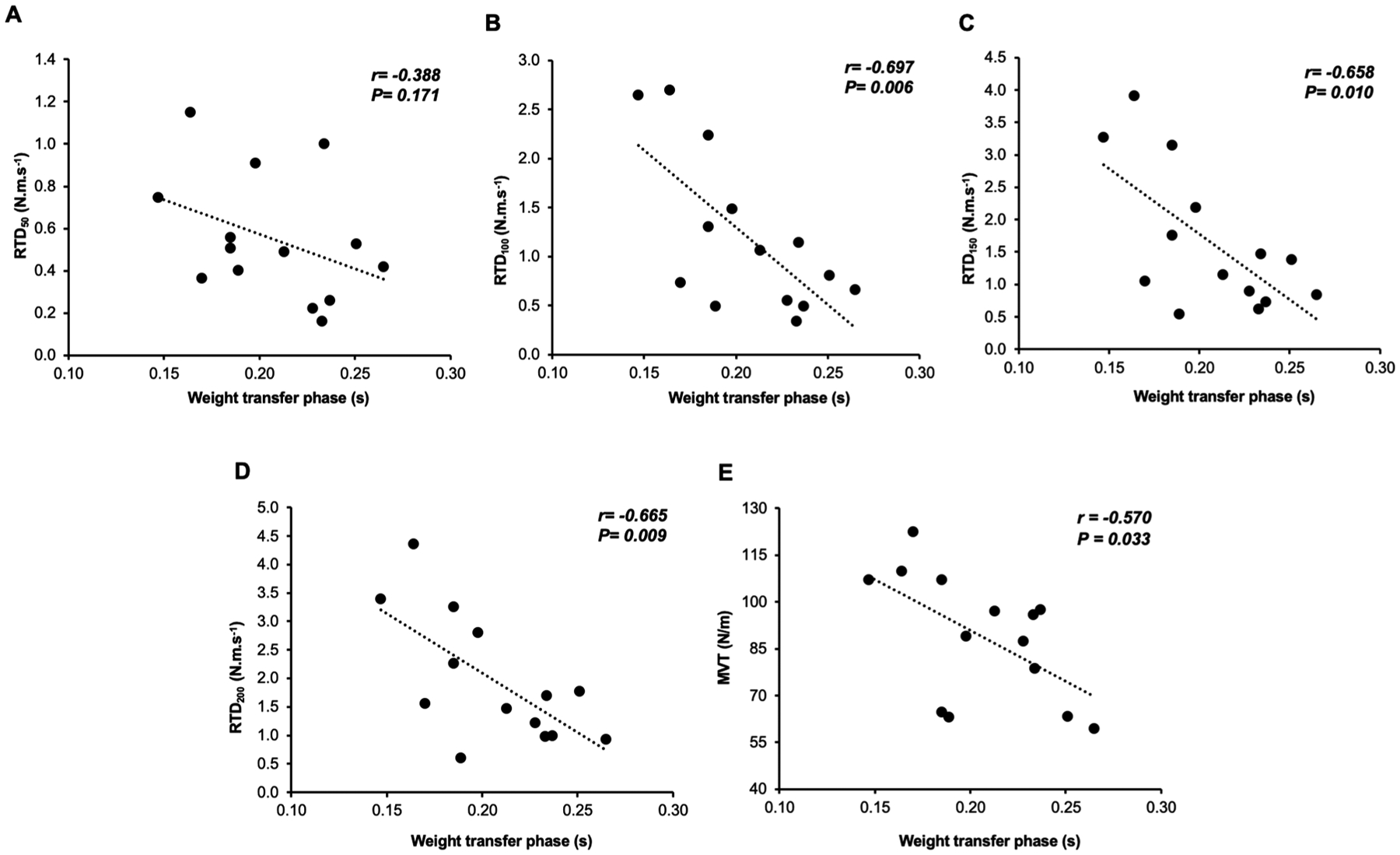
Correlations between weight transfer phase (WTP) of the choice reaction step test and rate of torque development (RTD) at 50, 100, 150 and 200 ms during the maximal isometric voluntary contraction (MIVC) (A-D) and maximum voluntary torque (E) from hip adductors.
Table 5.
Pearson correlations between weight transfer phase (WTP) of the stance and stepping leg during the lateral choice reaction step and rate of activation (RoA) in 0–50, 0–100, 0–150 and 0–200 ms of the gluteus medius (GM), tensor fascia latae (TFL) and adductor magnus (ADD) during the maximal isometric voluntary contraction.
| WTP | |||
|---|---|---|---|
| Stance Leg | Stepping Leg | ||
| Gluteus Medius | |||
| RoA0–50 | −0.428 | 0.166 | r |
| 0.127 | 0.570 | P | |
| RoA0–100 | −0.529 | 0.099 | r |
| 0.052 | 0.306 | P | |
| RoA0–150 | −0.521 | 0.262 | r |
| 0.056 | 0.366 | P | |
| RoA0–200 | −0.490 | 0.303 | r |
| 0.075 | 0.292 | P | |
| Tensor Fascia Latae | |||
| RoA0–50 | −0.838 | 0.021 | r |
| < 0.001* | 0.946 | P | |
| RoA0–100 | −0.863 | 0.049 | r |
| < 0.001* | 0.874 | P | |
| RoA0–150 | −0.841 | 0.170 | r |
| < 0.001* | 0.579 | P | |
| RoA0–200 | −0.812 | 0.234 | r |
| 0.001* | 0.442 | P | |
| Adductor Magnus | |||
| RoA0–50 | −0.723 | 0.429 | r |
| 0.033* | 0.126 | P | |
| RoA0–100 | −0.733 | 0.357 | r |
| 0.004* | 0.210 | P | |
| RoA0–150 | −0.707 | 0.405 | r |
| 0.007* | 0.150 | P | |
| RoA0–200 | −0.714 | 0.371 | r |
| 0.006* | 0.212 | P | |
Symbol (*) indicates the Pearson correlation (r) was statistically significant.
Table 6.
Pearson correlations between weight transfer phase (WTP) and muscle cross-section area (CSA), fat percentage relative to CSA (IMAT%), low density lean tissue relative to CSA (LDL%) and high-density lean tissue relative to CSA (HDL %), from gluteus medius/minimum, tensor fascia latae, adductor magnus/longus.
| WTP | |||
|---|---|---|---|
| Stance Leg | Stepping Leg | ||
| Gluteus Medius + Minimus | |||
| CSA | −0.328 | −0.444 | r |
| 0.233 | 0.098 | P | |
| IMAT(%) | 0.187 | 0.043 | r |
| 0.506 | 0.880 | P | |
| LDL(%) | 0.058 | −0.078 | r |
| 0.838 | 0.783 | P | |
| HDL(%) | −0.258 | −0.066 | r |
| 0.354 | 0.816 | P | |
| Tensor Fascia Latae | |||
| CSA | −0.182 | −0.267 | r |
| 0.533 | 0.303 | P | |
| IMAT(%) | 0.347 | 0.280 | r |
| 0.224 | 0.332 | P | |
| LDL(%) | −0.002 | 0.085 | r |
| 0.994 | 0.772 | P | |
| HDL(%) | −0.397 | −0.335 | r |
| 0.160 | 0.241 | P | |
| Adductor Magnus + Longus | |||
| CSA | −0.382 | −0.420 | r |
| 0.177 | 0.135 | P | |
| IMAT(%) | 0.300 | 0.222 | r |
| 0.298 | 0.446 | P | |
| LDL(%) | 0.043 | −0.364 | r |
| 0.884 | 0.201 | P | |
| HDL(%) | −0.318 | −0.041 | r |
| 0.267 | 0.890 | P | |
3.2. Stepping leg
There was no significant relationship between the WTP and RTD at 50, 100, 150, and 200 ms or the peak MVT of the hip abductor or adductor muscles (r ≥ 0.054, P ≥ 0.144). Similarly, no significant relationship was found between the WTP and RoA (Table 4) or muscle structure (CSA, IMAT, and HDL; Table 6) of the stepping leg.
4. Discussion
The present study investigated the relationship between RTD, RoA, and muscle structural variables (CSA, IMAT, and HDL) in the hip muscles that control lateral balance with the weight transfer phase that precedes a voluntary step. Our key findings were that older adults produced greater and quicker hip adductor torque, and faster neuromuscular activation of ADD and TFL of the stance leg, and were quicker shifting their body weight prior to a lateral voluntary choice reaction step. These results bring important implications for understanding the factors that underly the ability to react quickly and perform a lateral step. The choice reaction step is an important indicator of fall risk in older adults.
4.1. Rate of torque development (RTD)
The capacity a person has to produce torque quickly is important for quickly transferring the body weight while preparing for the ipsilateral leg to step. In accordance with that, we found that the ability to produce hip adductor torque rapidly was important for the stance leg accepting weight prior to a lateral voluntary step, explaining ~43% up to ~49% of the variance. The maximum hip adduction torque during the MVIC also explained ~38% of the variance during the weight transfer phase. Although the hip abductor torque of the stance leg was not associated with the WTP, there were trends (p values < 0.1 for 200 ms and MVT), possibly indicating a contribution of the hip abductors during WTP of a voluntary step. Hence, maximum torque and the earlier rate of torque development of the hip adductors during a maximal contraction appear to be fundamental to the stance leg accepting weight quickly when taking a lateral step. These findings are supported by other studies that suggest the capacity to develop joint torque rapidly is important for muscle function (Aagaard et al., 2002; Chang et al., 2005; Folland et al., 2014; Izquierdo et al., 1999). Furthermore, hip torque-time capacity, when balance is externally perturbed, is a key determinant of successfully recovering balance with a lateral step (Addison et al., 2017; Inacio et al., 2018, 2019). However, prior studies did not investigate rapid hip joint torque production over different time points of muscle contraction, nor were possible associations with WTP determined (Inacio et al., 2018, 2019). The time to produce peak torque can normally take around 500 ms in younger adults, and can be 26% slower in older compared to younger adults (Klass et al., 2008). Given that the time to transfer the weight to the stance leg for a lateral voluntary step normally takes approximately 200 ms to complete, investigating the earlier phases of rapid and forceful muscle contraction as studied here, provides new insights about the mechanisms that underlie the weight transfer phase. Thus, considering this cumulative information, in addition to the known association between a choice reaction step and falls (Lord and Fitzpatrick, 2001; Pijnappels et al., 2010; Wang et al., 2016), the present study showed that the ability to produce forceful and rapid hip adductor joint torque as early as within the initial 100 ms of contraction, is important for the weight transfer phase prior to stepping.
There were no significant correlations between hip abductors RTD (stepping leg) and the weight transfer phase in the present study. In line with our results, previous research demonstrates the lack of an association between the hip abductor RTD (peak torque) and induced mediolateral balance control using a platform translation (Arvin et al., 2016). In contrast, one study reports that those with a greater hip abduction RTD (peak torque) of the stepping leg had a greater incidence of lateral steps using a lateral waist-pull system (Inacio et al., 2019). Notably, the above-mentioned studies (Arvin et al., 2016; Inacio et al., 2019) used an induced balance perturbation to disrupt balance. The induced perturbation alters the symmetry in limb loading through passive forces pulling at the waist that moves the center of mass outside of the base of support (Martinez et al., 2013; Tisserand et al., 2015) which does not occur during the voluntary step performed in the present study. For instance, the mechanism of the lateral waist-pull system (Inacio et al., 2019) passively transfers the weight in the direction of the pull, loading the limb that would initiate a lateral step to recovery balance. Thus, a greater transfer of weight is necessary, requiring potentially larger forces and muscle activity in similar or different muscles. A similar mechanism occurs with the platform translation, except the base of support moves outside the center of mass as the distal limbs are translated (Arvin et al., 2016). Also, the authors calculated RTD from peak torque, which takes at least 500 ms to occur in older adults (Klass et al., 2008). The present study calculated RTD at a shorter period of time (50–200 ms) to match the weight transfer phase (~200 ms), to potentially provide a more accurate representation of what is occurring during the first stages of the choice step test. Moreover, the choice reaction step test has a sensory factor (visual cue before step) that was suggested to decrease the time to perform an induced lateral step with “visual” or “non-visual” information (Arvin et al., 2016). Furthermore, previous research found that sensory stimulation negatively impacted the weight transfer phase by delaying the initiation time resulting in a longer weight transfer duration (Rogers et al., 2003). The weight transfer was also larger in magnitude, almost twofold in some individuals, compared to cued with a visual stimulus (Rogers et al., 2001). Thus, the sensory input from the waist-pull perturbation to the hips combined with a need to shift weight through a greater distance may require larger forces, and could potentially lead to different sensori-motor responses between conditions (induced vs. voluntary step). Therefore, all these reasons together may help to explain our present results.
4.2. Rate of activation (RoA)
The present study showed the weight transfer phase of the stance leg was largely explained by the earlier activation of the TFL (~70% up to ~74%) and ADD (~50% up to ~54%) muscles. Additionally, even though no significant correlations were found in this study between GM RoA and the weight transfer phase, there were trends (p values < 0.1 for 100 up to 200 ms), which might indicate a possible contribution of GM activation during the weight transfer phase at the stance leg. The lack of association between the GM RoA and weight transfer phase, maybe due to the inherently large variability in EMG measurements, especially in the earliest phase of the rapid contraction (Folland et al., 2014; Lanza et al., 2019b). Previous research using an induced waist-pull perturbation system, demonstrated greater RoA of the TFL, GM, and ADD (from stepping leg) was associated with better balance control when stepping laterally (Inacio et al., 2019). Thus, appears to have difference in the RoA between voluntary and induced step tasks.
The increase in muscle activation in the present study (50 up to 200 ms) might be explained by increases in motor unit recruitment, recruitment threshold, firing frequency and/or synchronization of motor units that occur when greater EMG amplitude is detected (Hunter et al., 2004; Miller et al., 2019). Considering the present investigation did not aim to comprehend the mechanisms behind RoA, it is not possible to conclude which factors contribute to the observed differences. However, evidence suggests the motor unit characteristics (e.g., firing rate) of a rapid contraction may be a key factor (Miller et al., 2019). For example, while motor unit firing rates were lower, larger action potential amplitudes were found during isometric contractions performed at a slower RTD compared to contractions performed at a faster RTD (Miller et al., 2019). Additionally, the rapid increases in force appear to be accompanied by increases in the discharge rate at the beginning of the contraction with a subsequent decline (Enoka and Duchateau, 2017). This may explain the brief decline in RoA100 we found in our study. Hence, these mechanisms together may support the RoA results reported here. Future interventions that focus on improving not only muscle strength but also rapid hip adduction and abduction torque, would be important for enhancing the neuromuscular system [e. g., exercise until muscle failure (Sundstrup et al., 2012)]. This could elicit benefits which may be necessary for improving lateral balance control.
4.3. Muscle structure measures
There was no correlation between any of the muscle structure measures (CSA, IMAT, or HDL) and the weight transfer phase in this study. This was surprising because IMAT and HDL have consistently been shown to negatively impact muscle function (Addison et al., 2017, 2014; Inacio et al., 2014; Ryan et al., 2011; Tuttle et al., 2012; Visser et al., 2005), while HDL is positively correlated (Addison et al., 2014). It may indicate that, overall, muscle size and composition (IMAT and HDL) are not critical factors that influence the weight transfer phase of the choice reaction step test, indicating that neuromuscular activation might be more critical. A previous study with a large cohort (3075 older individuals) found a correlation between a reduction in thigh muscle strength, but not thigh muscle size (or IMAT), and mobility limitations (Visser et al., 2005). Our results also demonstrate a reduced ability to produce torque rapidly with no relationship between CSA and WTP. Given the previous negative (IMAT) and positive (HDL) associations between gluteus (medius and minimus) muscle composition and balance (Addison et al., 2014), we hypothesized a relationship with the weight transfer phase. Contrary findings may indicate that the influence of muscle composition on the weight transfer phase could be dependent on the task (e.g., dynamic vs. isometric) or the particular muscle. These results suggest that the RoA, and not muscle structure, is an important factor for a quick reaction on the choice reaction step test. Therefore, further investigation of how muscle composition, and different muscles, influence the ability to perform functional tasks appear to be warranted.
4.4. Study limitations
The current results should be taken with caution considering the small number of participants, although, importantly, part of the results are consistent with other studies. Also, the current cohort was higher functioning as demonstrated by the high scores on the functional tests (Four Square Step Test and Short Physical Performance Battery). Hence, the results may differ in older individuals with significant health conditions (e.g., neurological disorder), and/or fallers, requiring further investigation. Multiple regression analysis would provide valuable information to understand the contribution of all measures associated with the weight transfer phase. Although the use of computed tomography scan to measure IMAT and HDL is considered the “gold-standard” (Klopfenstein et al., 2012), CSAs of muscle groups rather than individual muscle areas (e.g., GM and gluteus minimus) were determined. Additionally, despite the fact we provide a large rest between contractions, fatigue may have influenced the present results. Moreover, further exploration of the contributions of muscle contractile properties, activation, and composition during the stepping test could provide useful information on preventing falls.
4.5. Study relevance
The present study brings to light how different physiological mechanisms involved in strength production (maximal or explosive) may contribute to the ability to perform weight transfer that precedes a choice reaction step. The choice reaction step is a simple and quick test associated with fall risk in older adults (Lord and Fitzpatrick, 2001; Pijnappels et al., 2010). Thus, the current findings provides valuable information on the mechanisms that contribute to better performance, ultimately informing the development of training interventions that target fall prevention in older adults. For instance, the significant positive relationship reported here between TFL and adductor magnus muscles with the weight transfer phase, indicates the important role of these muscles during weight transfer. Additionally, the results of the present study may also be extended to other voluntary tasks that involve weight transfer, such as gait and/or stair walk, although further research is necessary to confirm that.
5. Conclusion
In conclusion, the present study demonstrated that rapid hip torque adductor development, and a faster and earlier activation of the hip adductor (ADD) and hip abductor (TFL) muscles during a maximal isometric contraction, is important weight transfer phase prior to a voluntary choice reaction step. These results provide insight to information regarding overall muscle function during weight transfer and the importance of the stance leg during the control of lateral balance.
Acknowledgments
We would like to thank all the participants in the present study for their valuable time. This work was supported by and the Baltimore VA Geriatric, Research, Education and Clinical Center and The University of Maryland Claude D. Pepper Older Americans Independence Center (P30-AG028747) O.A was supported by a VA Career Development Award (IK2RX001788), A.S.R was supported by a VA Senior Research Career Scientist Award from Department of Veterans Affairs. M.B.L. is supported by a grant from the U.S. Administration for Community Living, National Institute of Disability, Independent Living, and Rehabilitation Research post-doctoral training grant (90AR5028).
Biography
Dr. Marcel B. Lanza graduated in Physical Education from Federal University of Acre (2005, Brazil), obtained his M.Sc. in Mechanical Engineering from Federal University of Minas Gerais (2011, Brazil), and his Ph.D. in Neuromechanics at Loughborough University (2018, UK). He was a postdoctoral fellow at the Federal University of Minas Gerais (2019, Brazil), and is currently a postdoctoral fellow at the University of Maryland Baltimore. His main areas of research are focused on the neural and morphological mechanisms of force production.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P, 2002. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J. Appl. Physiol 93, 1318–1326. 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- Addison O, Inacio M, Bair W-N, Beamer BA, Ryan AS, Rogers MW, 2017. Role of hip abductor muscle composition and torque in protective stepping for lateral balance recovery in older adults. Arch. Phys. Med. Rehabil 98, 1223–1228. 10.1016/j.apmr.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addison O, Young P, Inacio M, Bair W-N, Prettyman MG, Beamer BA, Ryan AS, Rogers MW, 2014. Hip but not thigh intramuscular adipose tissue is associated with poor balance and increased temporal gait variability in older adults. Curr. Aging Sci 7, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin M, van Dieën JH, Faber GS, Pijnappels M, Hoozemans MJM, Verschueren SMP, 2016. Hip abductor neuromuscular capacity: a limiting factor in mediolateral balance control in older adults? Clin. Biomech 37, 27–33. 10.1016/j.clinbiomech.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Bento PCB, Pereira G, Ugrinowitsch C, Rodacki ALF, 2010. Peak torque and rate of torque development in elderly with and without fall history. Clin. Biomech 25, 450–454. 10.1016/j.clinbiomech.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Bouche KGW, Vanovermeire O, Stevens VK, Coorevits PL, Caemaert JJ, Cambier DC, Verstraete K, Vanderstraeten GG, Danneels LA, 2011. Computed tomographic analysis of the quality of trunk muscles in asymptomatic and symptomatic lumbar discectomy patients. BMC Musculoskelet Disord 12. 10.1186/1471-2474-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-H, Mercer VS, Giuliani CA, Sloane PD, 2005. Relationship between hip abductor rate of force development and mediolateral stability in older adults. Arch. Phys. Med. Rehabil 86, 1843–1850. [DOI] [PubMed] [Google Scholar]
- Dite W, Temple VA, 2002. A clinical test of stepping and change of direction to identify multiple falling older adults. Arch. Phys. Med. Rehabil 83, 1566–1571. 10.1053/apmr.2002.35469. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J, 2017. Rate coding and the control of muscle force. Cold Spring Harb Perspect Med 7, a029702. 10.1101/cshperspect.a029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WJ, Lexell J, 1995. Human aging, muscle mass, and fiber type composition. J. Gerontol. Ser. A: Biol. Sci. Med. Sci 50A, 11–16. 10.1093/gerona/50A.Special_Issue.11. [DOI] [PubMed] [Google Scholar]
- Folland JP, Buckthorpe MW, Hannah R, 2014. Human capacity for explosive force production: neural and contractile determinants: determinants of explosive force production. Scand. J. Med. Sci. Sports 24, 894–906. 10.1111/sms.12131. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB, 1994. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol 49, M85–M94. 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- Hannah R, Minshull C, Smith SL, Folland JP, 2014. Longer electromechanical delay impairs hamstrings explosive force versus quadriceps. Med. Sci. Sports Exerc 46, 963–972. 10.1249/MSS.0000000000000188. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Rios DA, Edelberg HK, 2001. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch. Phys. Med. Rehabil 82 (8), 1050–1056. 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G, 2000. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol 10, 361–374. 10.1016/S1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Duchateau J, Enoka RM, 2004. Muscle fatigue and the mechanisms of task failure. Exerc. Sport Sci. Rev 32, 44–49. 10.1097/00003677-200404000-00002. [DOI] [PubMed] [Google Scholar]
- Inacio M, Creath R, Rogers MW, 2019. Effects of aging on hip abductor-adductor neuromuscular and mechanical performance during the weight transfer phase of lateral protective stepping. J. Biomech 82, 244–250. 10.1016/j.jbiomech.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inacio M, Creath R, Rogers MW, 2018. Low-dose hip abductor-adductor power training improves neuromechanical weight-transfer control during lateral balance recovery in older adults. Clin. Biomech 60, 127–133. 10.1016/j.clinbiomech.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inacio M, Ryan AS, Bair W-N, Prettyman M, Beamer BA, Rogers MW, 2014. Gluteal muscle composition differentiates fallers from non-fallers in community dwelling older adults. BMC Geriatr 14. 10.1186/1471-2318-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo M, Aguado X, Gonzalez R, Lopez JL, Hakkinen K, 1999. Maximal and explosive force production capacity and balance performance in men of different ages. Eur. J. Appl. Physiol. Occup. Physiol 79, 260–267. 10.1007/s004210050504. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R, 2002. Low relative skeletal muscle mass (Sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc 50, 889–896. 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Johnson ME, Mille M-L, Martinez KM, Crombie G, Rogers MW, 2004. Age-related changes in hip abductor and adductor joint torques. Arch. Phys. Med. Rehabil 85, 593–597. 10.1016/j.apmr.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Kannus P, Parkkari J, Niemi S, Sievänen H, 2018. Continuously declining incidence of fall injuries in older adults: nationwide statistics from Finland between 1970 and 2016. Eur Geriatr Med 9 (3), 371–375. 10.1007/s41999-018-0053-3. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J, 2008. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J. Appl. Physiol 104, 739–746. 10.1152/japplphysiol.00550.2007. [DOI] [PubMed] [Google Scholar]
- Klopfenstein BJ, Kim MS, Krisky CM, Szumowski J, Rooney WD, Purnell JQ, 2012. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br. J. Radiol 85, e826–e830. 10.1259/bjr/57987644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza MB, Balshaw TG, Folland JP, 2019a. Explosive strength: effect of knee-joint angle on functional, neural, and intrinsic contractile properties. Eur. J. Appl. Physiol 119, 1735–1746. 10.1007/s00421-019-04163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza MB, Balshaw TG, Folland JP, 2019b. Is the joint-angle specificity of isometric resistance training real? And if so, does it have a neural basis? Eur J Appl Physiol 119, 2465–2476. 10.1007/s00421-019-04229-z. [DOI] [PubMed] [Google Scholar]
- Lord SR, Fitzpatrick RC, 2001. Choice stepping reaction time: a composite measure of falls risk in older people. J. Gerontol. Ser. A: Biol. Sci. Med. Sci 56, M627–M632. 10.1093/gerona/56.10.M627. [DOI] [PubMed] [Google Scholar]
- Lord SR, Ward JA, Williams P, Anstey KJ, 1993. An epidemiological study of falls in older community-dwelling women: the Randwick falls and fractures study. Aust. J. Public Health 17, 240–245. [DOI] [PubMed] [Google Scholar]
- Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J, 2016. Rate of force development: physiological and methodological considerations. Eur J Appl Physiol 116, 1091–1116. 10.1007/s00421-016-3346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez KM, Mille M-L, Zhang Y, Rogers MW, 2013. Stepping in persons poststroke: comparison of voluntary and perturbation-induced responses. Arch. Phys. Med. Rehabil 94, 2425–2432. 10.1016/j.apmr.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Melzer I, Oddsson LIE, 2004. The effect of a cognitive task on voluntary step execution in healthy elderly and young individuals. J. Am. Geriatr. Soc 52, 1255–1262. 10.1111/j.1532-5415.2004.52353.x. [DOI] [PubMed] [Google Scholar]
- Mille M-L, Johnson-Hilliard M, Martinez KM, Zhang Y, Edwards BJ, Rogers MW, 2013. One step, two steps, three steps more … directional vulnerability to falls in community-dwelling older people. J. Gerontol. Ser. A 68, 1540–1548. 10.1093/gerona/glt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Lund CJ, Gingrich MD, Schtul KL, Wray ME, Herda TJ, 2019. The effect of rate of torque development on motor unit recruitment and firing rates during isometric voluntary trapezoidal contractions. Exp Brain Res 237, 2653–2664. 10.1007/s00221-019-05612-0. [DOI] [PubMed] [Google Scholar]
- Minetti AE, 2002. On the mechanical power of joint extensions as affected by the change in muscle force (or cross-sectional area), ceteris paribus. Eur. J. Appl. Physiol 86, 363–369. 10.1007/s00421-001-0554-4. [DOI] [PubMed] [Google Scholar]
- Morcelli MH, Rossi DM, Karuka AH, Crozara LF, Hallal CZ, Marques NR, Gonçalves M, Navega MT, 2016. Peak torque, reaction time, and rate of torque development of hip abductors and adductors of older women. Physiotherapy Theory Pract. 32, 45–52. 10.3109/09593985.2015.1091870. [DOI] [PubMed] [Google Scholar]
- Orr R, de Vos NJ, Singh NA, Ross DA, Stavrinos TM, Fiatarone-Singh MA, 2006. Power training improves balance in healthy older adults. J. Gerontol. Ser. A: Biol. Sci. Med. Sci 61 (1), 78–85. 10.1093/gerona/61.1.78. [DOI] [PubMed] [Google Scholar]
- Phelan EA, Mahoney JE, Voit JC, Stevens JA, 2015. Assessment and management of fall risk in primary care settings. Med. Clin. North Am 99, 281–293. 10.1016/j.mcna.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnappels M, Delbaere K, Sturnieks DL, Lord SR, 2010. The association between choice stepping reaction time and falls in older adults–a path analysis model. Age Ageing 39, 99–104. 10.1093/ageing/afp200. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Hedman LD, Johnson ME, Martinez KM, Mille M-L, 2003. Triggering of protective stepping for the control of human balance: age and contextual dependence. Cognit. Brain Res 16, 192–198. 10.1016/S0926-6410(02)00273-2. [DOI] [PubMed] [Google Scholar]
- Rogers M, Kukulka C, Brunt D, Cain T, Hanke T, 2001. The influence of stimulus cue on the initiation of stepping in young and older adults. Archives of Physical Medicine and Rehabilitation 82 (5), 619–624. 10.1053/apmr.2001.20833. [DOI] [PubMed] [Google Scholar]
- Ryan AS, Buscemi A, Forrester L, Hafer-Macko CE, Ivey FM, 2011. Atrophy and intramuscular fat in specific muscles of the thigh: associated weakness and hyperinsulinemia in stroke survivors. Neurorehabil Neural Repair 25, 865–872. 10.1177/1545968311408920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettino L, Luz CPN, de Oliveira LEG, de Assunção PL, da Silva Coqueiro R, Fernandes MH, Brown LE, Machado M, Pereira R, 2014. Comparison of explosive force between young and elderly women: evidence of an earlier decline from explosive force. AGE (Omaha) 36, 893–898. 10.1007/s11357-013-9612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchomel T, Stone M, 2017. The relationships between hip and knee extensor cross-sectional area, strength, power, and potentiation characteristics. Sports 5, 66. 10.3390/sports5030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrup E, Jakobsen MD, Andersen CH, Zebis MK, Mortensen OS, Andersen LL, 2012. Muscle activation strategies during strength training with heavy loading vs. repetitions to failure. J. Strength Condition. Res 26, 1897–1903. 10.1519/JSC.0b013e318239c38e. [DOI] [PubMed] [Google Scholar]
- Tieland M, Trouwborst I, Clark BC, 2018. Skeletal muscle performance and ageing: skeletal muscle performance and ageing. J. Cachexia, Sarcopenia Muscle 9, 3–19. 10.1002/jcsm.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillin NA, Pain MTG, Folland JP, 2013. Identification of contraction onset during explosive contractions. Response to Thompson et al. “Consistency of rapid muscle force characteristics: Influence of muscle contraction onset detection methodology” [J Electromyogr Kinesiol 2012;22(6):893–900]. J. Electromyogr. Kinesiol. 23, 991–994. 10.1016/j.jelekin.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Tisserand R, Robert T, Chabaud P, Livet P, Bonnefoy M, Chèze L, 2015. Comparison between investigations of induced stepping postural responses and voluntary steps to better detect community-dwelling elderly fallers. Neurophysiologie Clinique/Clin. Neurophysiol 45, 269–284. 10.1016/j.neucli.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Tuttle LJ, Sinacore DR, Mueller MJ, 2012. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J. Aging Res 2012, 1–7. 10.1155/2012/172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB, 2005. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. Ser. A: Biol. Sci. Med. Sci 60, 324–333. 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhang J, Sun Y, Zhu W, Tian S, Liu Y, 2016. Evaluating the fall risk among elderly population by choice step reaction test. Clin. Interv. Aging 11, 1075–1082. 10.2147/CIA.S106606. [DOI] [PMC free article] [PubMed] [Google Scholar]


