Abstract
An otherwise healthy patient, with minimal clinical, biochemical and peroperative signs of infection, was diagnosed with Bartonella quintana prosthetic valve endocarditis by 16S PCR. The patient subsequently developed a post-sternotomy mediastinitis and Bartonella quintana was the only detected pathogen. Bartonella quintana can cause severe infections in individuals not classically at risk, and may be missed in the routine diagnostic work-up of endocarditis.
Keywords: Blood culture negative endocarditis, mediastinitis, 16S PCR sequencing, Bartonella quintana
Case
A 55 year old male, born in Eritrea and living in the Netherlands for over 20 years had undergone a Bentall procedure with a mechanical valve in 2015 for dilatation of the aortic root and aortic valve insufficiency. In July 2018, he was referred by his general practitioner to a nephrologist with progressive tiredness, weight loss, renal insufficiency and anaemia. Laboratory analysis revealed: erythrocyte sedimentation rate 111 mm h–1, serum creatinine 161 μmol l−1, Hb 4.8 mmol l−1 (MCV 85 fl), mild iron deficiency (ferritin 233 µg l−1, transferrin-iron saturation 42 μmol l−1), mild proteinuria with albumin on creatinine ratio of 24 mg mmol–1 and discrete microscopic hematuria with 3–10 erythrocytes per hpf, without any overt signs of glomerulonephritis. The patient initially refused further analysis. In the following months, the patient’s condition deteriorated with marked weight loss and palor. The ESR increased to 95 mm h–1, haemoglobin stabilized at 6.7 mmol l−1, hematuria progressed and the kidney function deteriorated. The differential diagnosis included a vasculitis or a paraneoplastic condition and further diagnostic testing yielded an elevated level of anti-GBM antibodies (40 U ml−1) and a high F-FDG avidity at the prosthetic aortic valve and aortic graft on PET CT, suggesting an infection of the Bentall prosthesis (Fig. 1), with secondary immune complex glomerulonephritis.
Fig. 1.
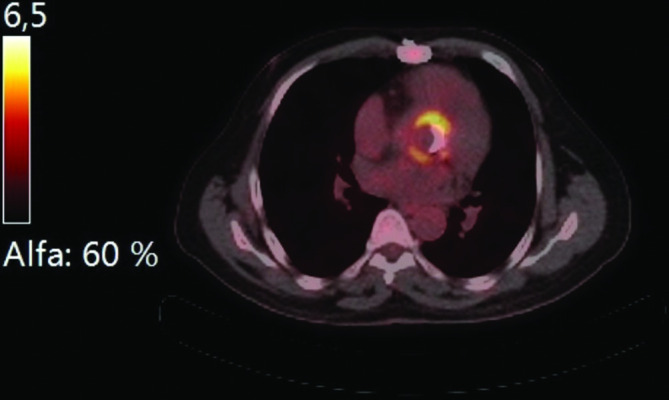
PET-CT image showing high F-FDG uptake around the Bentall prosthesis.
Multiple blood cultures, taken without antibiotics and incubated for 2 weeks, remained negative. A transesophageal echocardiography showed vegetations on the aortic side of the mechanical prosthesis. Vancomycine (6 weeks) and gentamicin (2 weeks) was started for blood culture negative endocarditis (BCNE). Replacement of the aortic graft (not the valve), was performed at day 24 of antibiotic therapy; surprisingly vegetations and insufficiency of the valve were absent during surgery. Three microbiologic samples were taken from tissue surrounding the aortic graft.
Gram-stain and tissue cultures of the three tissue samples were negative and vancomcyin treatment was ceased 3 days after surgery. On postoperative day eight, 16S PCR-analysis returned positive for Bartonella quintana, re-establishing the diagnosis of endocarditis. Antibiotic treatment was re-initiated with doxycycline 100 mg bid and gentamicin 3 mg kg–1 daily.
The patient, initially recovering well, developed a pyogenic discharge from the sternal wound 3 weeks post-surgery (CRP 45 mg l−1, leucocytes 8.8 *109 ml−1). Re-sternotomy was performed and the clinical diagnosis of mediastinitis was macroscopically confirmed. An omentum flap was used to cover the graft and the sternal bone was primarily closed. Again, Gram-stain and cultures from perioperative tissue (one sample) and pus (two samples) yielded no bacteria, but Bartonella quintana was detected using 16S PCR from a tissue biopsy taken during the resternotomy. The patient recovered well and was discharged at day 42 after initial surgery. At 6 months of follow-up, CRP levels had dropped to 2 mg l−1, leucocytes to 5.0×109 ml−1 and creatinine to 113 umol l−1. The haemoglobin returned to 7.8 mmol l−1, and the patient had returned to working full time. He is currently still on doxycycline and experiences no side effects.
We confirmed the diagnosis with serology performed in the expertise centre IHU Méditerranée Infection. The IgG-titre for Bartonella quintana was 1 : 100 (at the cut off), and Western blot confirmed the presence of specific Bartonella quintana (and not B. henselae ) antibodies.
Discussion
Bartonella quintana is a facultative intracellular and zoonotic Gram-negative bacillus which can cause various, mainly self-limiting diseases. It is transmitted primarily through body lice, known for causing Trench fever in soldiers in World War I. Nowadays transmitting lice are mostly found in people living in unsanitary conditions [1].
Prior cases
Bartonella species are a rare cause of native valve endocarditis, however with increasing awareness and progressed molecular diagnostics, more than several hundred cases have been reported in literature to date.
Bartonella quintana endocarditis has only been described once in the Netherlands in a post-mortem patient without risk factors [2] and no cases had been found in a Swedish cohort of 71 suspected episodes of BCNE (zero patients had IgG-antibodies to B. quintana and B. henselae ) [3]. However, in Egypt, four out of 92 cases of BCNE were caused by Bartonella species (positive serology and/or tissue PCR), and prevalences reported from Tunesia and Algeria are 9.8 and 11.4% respectively [4–6]. In Brazil, Bartonella was diagnosed in two out of 17 (11.7 %) cases of BCNE by serology; both patients did not survive and had pathologic signs of endocarditis on autopsy [7]. There appears to be an north-south gradient in the prevalence of this zoonotic disease.
The most common species implicated are Bartonella henselae and to a lesser extent Bartonella quintana . The majority of the described cases of Bartonella quintana endocarditis occurred in homeless, immunosuppressed, or alcoholic patients and patients with IV drug abuse [8]. Although it is suggested that the majority of Bartonella endocarditis occurs in patients with pre-existing valvular abnormalities, epidemiological data is limited and case reports have been written about both native and prosthetic valve endocarditis, both in the presence and absence of pre-existing heart disease. Although unsanitary conditions and lice infestation are associated with Bartonella quintana endocarditis, it has been described in four Ethiopian children with congenital or rheumatic heart disease; in all cases without recent lice infestation, suggesting patients might had been infected for months or years [9].
Pathophysiology
There are two interesting aspects about this case.
First, the aetiology of Bartonella quintana infection. There were no risk factors for body lice infestation and the patient had last visited Eritrea, where Bartonella infections are more common, 2 years prior to presentation. We hypothesize that our patient may either have had a chronic asymptomatic bacteremia with Bartonella quintana for years, and that it might also have been the cause of the aortic root dilatation for which he underwent the original Bentall procedure; Bartonella has a tropism for erythrocytes and vascular endothelium and can stay dormant in these tissue for longer periods of time [10, 11]. Alternatively, he acquired the infection in the Netherlands through a yet unknown route. Human body lice are not detected in the healthy Dutch population, however it has been suggested that Bartonella quintana can also occur in head lice, ticks and other hematophagous arthropods [10, 12]. Both scenarios are unusual and cannot be proven at this point.
A second interesting aspect of our case is the development of culture negative post-sternotomy mediastinitis after which Bartonella quintana DNA was the only bacterial DNA found, suggesting that this bacteria possibly contributed to, or even caused the mediastinitis. Bartonella quintana mediastinitis has not been described in the literature before. Inadequate antibiotic coverage during the first operation (this disease episode) and the first eight postoperative days may have played a role in the development of post-sternotomy mediastinitis.
Diagnosis
Bartonella species is a known cause of classical BCNE, causing 0–3 % of these cases in Europe [3, 8]. It is invisible in Gram-stain and a very fastidious organism, and hence not easily detected in routine diagnostics when not suspected, such as in countries with very low prevalence. As demonstrated here, the clinical presentation may be very non-specific and its course indolent, with only marginal signs of infection on laboratory examination.
The European Society of Cardiology suggests a screening strategy for suspected endocarditis when blood cultures remain negative for >48 h, using serology for Bartonella spp., Coxiella burnetii, Legionella pneumophiliae, Brucella spp., Mycoplasma pneumoniae and Aspergillus spp. [13]. For Bartonella species, serum testing has a sensitivity of 58 % with IgG titres >=1 : 800, and additionally cross-reactivity may occur with Chlamydia spp. and C. burnetii [8]. Western blotting can confirm the diagnosis in 100 % of cases and allows distinction between different species of Bartonella [8]. When tissue is available molecular analysis can be performed, using either broad-spectrum PCR (16S rRNA) or specific PCR, with the latter being significantly more sensitive [8]. Bartonella species specific PCR can be applied on both serum and valve tissue, with sensitivities for Bartonella quintana of 41 % and ~96 % respectively. The 16S rRNA PCR on valve tissue has a sensitivity of 60 % for Bartonella spp. endocarditis, and should not be performed on serum/blood (sensitivity 0 %) [8]. Therefore, the diagnosis of Bartonella endocarditis is especially complex in non-operated non-suspect patients, when tissue is not available for molecular testing and when serology is not easily available (such as in the Netherlands, where serology is only available for B. henselae ).
Treatment and prognosis
Little evidence is available about the optimal treatment of prosthetic valve endocarditis with Bartonella species. Guidelines suggest a 2 week course of aminoglycosides (gentamicin 3 mg/24 h IV), alongside a 4 week treatment with doxycycline (100 mg/12 h PO) for native valve endocarditis [13]. Case reports, mostly of Bartonella henselae prosthetic valve endocarditis, report good clinical outcomes with and without surgery. In a case series of 99 Bartonella species endocarditis, of which 53 were caused by B. quintana and 17 by B. henselae endocarditis, 75 % of patients underwent valve replacement, 84 % received antibiotic therapy including an aminoglycoside, and the mortality rate was 7 % [14].
Future implications
This case demonstrates that in a patient with BCNE with low infection parameters and absent macroscopic signs of infection perioperatively, 16S PCR on tissue was pivotal in identifying an infectious aetiology and in supporting the aetiology of the post-sternotomy mediastinitis.
Funding information
This work received no specific grant from any funding agency.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
The patient has consented to publication of this Case Report.
Footnotes
Abbreviations: BCNE, blood culture negative endocarditis; bid, bis in die (twice a day); F-FDG, fluorodeoxyglucose; GBM, glomerular basement membrane; hpf, high-power field; IV, intravenous; PET-CT, positron emission tomography – computed tomography; PO, per os (oral).
References
- 1.Anstead GM. The centenary of the discovery of Trench fever, an emerging infectious disease of World War 1. Lancet Infect Dis. 2016;16:e164–e172. doi: 10.1016/S1473-3099(16)30003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmans AMC, Coenen JL, Bakhuizen R, Mooi BW, Ramdat Misier AR, et al. Endocarditis in a Dutch patient caused by Bartonella quintana . Clin Microbiol Infect. 1997;3:692–695. doi: 10.1111/j.1469-0691.1997.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 3.Werner M, Fournier P-E, Andersson R, Hogevik H, Raoult D. Bartonella and Coxiella antibodies in 334 prospectively studied episodes of infective endocarditis in Sweden. Scand J Infect Dis. 2003;35:724–727. doi: 10.1080/00365540310015980. [DOI] [PubMed] [Google Scholar]
- 4.El-Kholy AA, El-Rachidi NGE-din, El-Enany MG, AbdulRahman EM, Mohamed RM, et al. Impact of serology and molecular methods on improving the microbiologic diagnosis of infective endocarditis in Egypt. Infection. 2015;43:523–529. doi: 10.1007/s15010-015-0761-2. [DOI] [PubMed] [Google Scholar]
- 5.Benslimani A, Fenollar F, Lepidi H, Raoult D. Bacterial zoonoses and infective endocarditis, Algeria. Emerg Infect Dis. 2005;11:216–224. doi: 10.3201/eid1102.040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Znazen A, Rolain J-M, Hammami N, Kammoun S, Hammami A, et al. High prevalence of Bartonella quintana endocarditis in Sfax, Tunisia. Am J Trop Med Hyg. 2005;72:503–507. doi: 10.4269/ajtmh.2005.72.503. [DOI] [PubMed] [Google Scholar]
- 7.Siciliano RF, Strabelli TM, Zeigler R, Rodrigues C, Castelli JB, et al. Infective endocarditis due to Bartonella spp. and Coxiella burnetii: experience at a cardiology hospital in Sao Paulo, Brazil. Ann N Y Acad Sci. 2006;1078:215–222. doi: 10.1196/annals.1374.123. [DOI] [PubMed] [Google Scholar]
- 8.Edouard S, Nabet C, Lepidi H, Fournier P-E, Raoult D. Bartonella, a common cause of endocarditis: a report on 106 cases and review. J Clin Microbiol. 2015;53:824–829. doi: 10.1128/JCM.02827-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tasher D, Raucher-Sternfeld A, Tamir A, Giladi M, Somekh E. Bartonella quintana, an unrecognized cause of infective endocarditis in children in Ethiopia. Emerg Infect Dis. 2017;23:1246–1252. doi: 10.3201/eid2308.161037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harms A, Dehio C. Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin Microbiol Rev. 2012;25:42–78. doi: 10.1128/CMR.05009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foucault C, Raoult D, Brouqui P. Randomized open trial of gentamicin and doxycycline for eradication of Bartonella quintana from blood in patients with chronic bacteremia. Antimicrob Agents Chemother. 2003;47:2204–2207. doi: 10.1128/AAC.47.7.2204-2207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangaré AK, Boutellis A, Drali R, Socolovschi C, Barker SC, et al. Detection of Bartonella quintana in African body and head lice. Am J Trop Med Hyg. 2014;91:294–301. doi: 10.4269/ajtmh.13-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of cardiology (ESC). endorsed by: European association for Cardio-Thoracic surgery (EACTS), the European association of nuclear medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 14.Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine. 2005;84:162–173. doi: 10.1097/01.md.0000165658.82869.17. [DOI] [PubMed] [Google Scholar]


