Abstract
Carbapenem-hydrolysing enzymes belonging to the OXA-48-like group are encoded by bla OXA-48-like alleles and are abundant among Enterobacterales in the Netherlands. Therefore, the objective here was to investigate the characteristics, gene content and diversity of the bla OXA-48-like carrying plasmids and chromosomes of Escherichia coli and Klebsiella pneumoniae collected in the Dutch national surveillance from 2014 to 2019 in comparison with genome sequences from 29 countries. A combination of short-read genome sequencing with long-read sequencing enabled the reconstruction of 47 and 132 complete bla OXA-48-like plasmids for E. coli and K. pneumoniae , respectively. Seven distinct plasmid groups designated as pOXA-48-1 to pOXA-48-5, pOXA-181 and pOXA-232 were identified in the Netherlands which were similar to internationally reported plasmids obtained from countries from North and South America, Europe, Asia and Oceania. The seven plasmid groups varied in size, G+C content, presence of antibiotic resistance genes, replicon family and gene content. The pOXA-48-1 to pOXA-48-5 plasmids were variable, and the pOXA-181 and pOXA-232 plasmids were conserved. The pOXA-48-1, pOXA-48-2, pOXA-48-3 and pOXA-48-5 groups contained a putative conjugation system, but this was absent in the pOXA-48-4, pOXA-181 and pOXA-232 plasmid groups. pOXA-48 plasmids contained the PemI antitoxin, while the pOXA-181 and pOXA-232 plasmids did not. Furthermore, the pOXA-181 plasmids carried a virB2-virB3-virB9-virB10-virB11 type IV secretion system, while the pOXA-48 plasmids and pOXA-232 lacked this system. A group of non-related pOXA-48 plasmids from the Netherlands contained different resistance genes, non-IncL-type replicons or no replicons. Whole genome multilocus sequence typing revealed that the bla OXA-48-like plasmids were found in a wide variety of genetic backgrounds in contrast to chromosomally encoded bla OXA-48-like alleles. Chromosomally localized bla OXA-48 and bla OXA-244 alleles were located on genetic elements of variable sizes and comprised regions of pOXA-48 plasmids. The bla OXA-48-like genetic element was flanked by a direct repeat upstream of IS1R, and was found at multiple locations in the chromosomes of E. coli . Lastly, K. pneumoniae isolates carrying bla OXA-48 or bla OXA-232 were mostly resistant for meropenem, whereas E. coli bla OXA-48, bla OXA-181 and chromosomal bla OXA-48 or bla OXA-244 isolates were mostly sensitive. In conclusion, the overall bla OXA-48-like plasmid population in the Netherlands is conserved and similar to that reported for other countries, confirming global dissemination of bla OXA-48-like plasmids. Variations in size, presence of antibiotic resistance genes and gene content impacted pOXA-48, pOXA-181 and pOXA-232 plasmid architecture.
Keywords: blaOXA-48, blaOXA-181, blaOXA-232, blaOXA-244, genomes
Data Summary
The Illumina (NGS) sequence data set generated and analysed in this study is available in the European Nucleotide Archive (ENA) with study accession numbers PRJEB42331 (https://www.ebi.ac.uk/ena/browser/view/PRJEB42331) and PRJEB35685 (http://www.ebi.ac.uk/ena/data/view/PRJEB35685), and the Sequence Read Archive (SRA) with the study accession number PRJNA634885 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA634885/). The plasmid and chromosome sequences are deposited in GenBank of the National Center for Biotechnology Information (NCBI) and are available through the accession number PRJNA691727 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA691727). Relevant code was made available through https://github.com/BSR-AMR-RIVM/blaOXA-48-plasmids-Microbial-Genomics. The authors confirm that all supporting data, code, protocols and accession numbers have been provided within the article and through supplementary data files.
Impact Statement.
OXA-48-type carbapenem hydrolysing enzymes encoded by blaOXA-48-like genes from transmissible plasmids or chromosomes of Escherichia coli and Klebsiella pneumoniae have spread world-wide and are of concern. Dissecting the blaOXA-48-like genome architecture at the molecular level by combining short-read and long-read sequencing will lead to understanding trends in the plasmid reservoir of E. coli and K. pneumoniae in the Netherlands and may enhance future international pathogen surveillance.
Introduction
Antimicrobial resistance (AMR) has dispersed among the family Enterobacterales and is a major concern for both hospitalized and non-hospitalized patients [1]. In carbapenemase-producing Enterobacterales (CPE), genes encoding carbapenemases are often located on transmissible plasmids that shuttle between bacterial strains of the same species, but also between distinct bacterial species and often confer resistance to carbapenem antibiotics [2, 3]. The predominant CPE species in the Netherlands from 2014 to 2019 were Klebsiella pneumoniae (43 %), Escherichia coli (30 %) and Enterobacter cloacae complex (13 %) [4]. Carbapenemases are classified in Ambler classes A (i.e. KPC-types), B (i.e. IMP-, NDM- and VIM-types) and D (OXA β-lactamases) of carbapenem antibiotic-degrading enzymes [5]. The KPC, NDM, IMP, VIM and certain OXA-like enzymes are the most commonly identified variant carbapenemases that have spread world-wide among Enterobacterales , including E. coli and K. pneumoniae [6]. The bla OXA-48-like genes make up the most prevalent carbapenemase-encoding genes found in Enterobacterales in the Netherlands (44 %), followed by bla NDM (27 %) [4]. The OXA-48-like carbapenemases are encoded by the bla OXA-48, bla OXA-162, bla OXA-181, bla OXA-204, bla OXA-232 and bla OXA-244 genes. Other OXA-48-likes, such as OXA-245, OXA-484 and OXA-519, are less often reported groups of carbapenemases [6]. The distinction between the OXA-48-like carbapenemases is based on one to five specific amino acid substitutions in the β5–β6 loop of the enzyme that can impact the efficiency of carbapenem hydrolysis [6–8]. OXA-181 differs from OXA-48 by four amino acid substitutions (Thr104Ala, Asn110Asp, Glu168Gln and Ser171Ala), yet both have comparable carbapenem hydrolytic activity [9]. OXA-232 differs from OXA-48 by five amino acid substitutions, four of which are identical to the differential OXA-181 mutations, but OXA-232 contains an additional Arg214Ser substitution [10]. OXA-244 differs only by a single Arg214Gly mutation from OXA-48, and the OXA-244 together with OXA-181 enzymes have reduced carbapenem hydrolysing activity [11].
The most common plasmids that harbour bla OXA-48 belong to the IncL/M family, which are conjugative and have been described for E. coli and K. pneumoniae [12–15]. The bla OXA-181 gene is located on plasmids containing the qnrS1 gene coding for quinolone resistance and either the ColE2, IncX3, IncN1 or IncT type of replicons [16, 17]. Plasmids containing bla OXA-232 have the ColE-type replicon and the backbone is identical to bla OXA-181-containing plasmids [10]. The bla OXA-244 gene is located on an IncL plasmid and is suggested to originate from bla OXA-48 by a point mutation, which possibly occurred during integration in the E. coli ST38 chromosome [6, 11, 15]. Chromosome encoded OXA-48-like carbapenemases have been described previously in globally disseminated E. coli and K. pneumoniae [15, 18, 19]. In these chromosomes, the bla OXA-48-like gene has been found to be inserted at various chromosomal locations [18].
The global emergence of the carbapenem-hydrolysing OXA-48 enzyme and OXA-48-like descendants on transmissible plasmids warrants national surveillance. Currently, a paradigm shift is occurring in national reference laboratories from next-generation sequencing (NGS) towards third generation long-read sequencing (TGS). This allows an in-depth study of CPE antibiotic resistance-plasmid biology and plasmid transmission within and between healthcare institutions and countries, respectively. Therefore, the major goal of this study was to investigate the characteristics and contents of E. coli and K. pneumoniae plasmids and chromosomes carrying bla OXA-48-like genes obtained from isolates submitted to the Dutch national CPE surveillance programme in a global context using a combination of NGS and TGS.
Methods
Bacterial isolates
For the Dutch National CPE Surveillance programme, medical microbiology laboratories from the Netherlands routinely send Enterobacterales isolates with a meropenem minimum inhibitory concentration (MIC) of >0.25 mg l−1 and/or an imipenem MIC of >1 mg l−1 or genotypic or phenotypic evidence of carbapenemase production to the National Institute of Public Health and the Environment through Type-Ned, an online platform [3]. The low MIC threshold for submission was chosen to monitor CPE instead of carbapenem-resistant Enterobacterales (CRE), because CPE represent a reservoir for the spread of antibiotic resistance genes. In this study, 537 carbapenemase-producing E. coli and K. pneumoniae isolates carrying bla OXA-48-like alleles (bla OXA-48, bla OXA-181, bla OXA-232) were included and were collected from 1 January 2014 until 31 December 2019 (Table S1, Suppl. File 1, available in the online version of this article). Only the first submitted E. coli or K. pneumoniae isolate with a bla OXA-48-like allele per person in this study period was included.
Antimicrobial susceptibility testing
Resistance to carbapenem was confirmed by assessing the MIC for meropenem using an Etest (bioMérieux). Based on the clinical breakpoints according to EUCAST, the isolates were classified as sensitive (≤2 mg l−1), intermediate (>2 mg l−1 and ≤8 mg l−1) and resistant (>8 mg l−1) to meropenem. Isolates were analysed for carbapenemase production using the carbapenem inactivation method (CIM) [20].
Next-generation sequencing
E. coli and K. pneumoniae isolates were subjected to NGS using the Illumina HiSeq 2500 (BaseClear). The antibiotic resistance gene profile and plasmid replicon compositions in all of the isolates were determined by interrogating the ResFinder (version 3.1.0) and PlasmidFinder (version 2.0.2) databases available from the Center for Genomic Epidemiology [21, 22]. For ResFinder, a 90 % identity threshold and a minimum length of 60 % were used as criteria, whereas for PlasmidFinder, an identity of 95 % was utilized. The resulting NGS-derived data, such as resistance genes, replicons and whole genome multilocus sequence typing (wgMLST) profiles, were imported into BioNumerics version 7.6.3 for subsequent comparative analyses (Applied Maths).
Long-read third-generation sequencing
High-molecular-weight DNA was isolated using an in-house developed protocol as described previously [3]. The Oxford Nanopore protocol SQK-LSK108 (https://community.nanoporetech.com) and the expansion kit for native barcoding EXP-NBD104 was used (Oxford Nanopore Technologies). A shearing step was performed using g-TUBEs (Covaris) to obtain an average DNA fragment size of 8 kb for isolates from 2014 to 2018. To obtain larger DNA fragments, this shearing step was omitted for isolates from 2019 and subsequently SQK-LSK109 was followed (Oxford Nanopore Technologies). The DNA was repaired using FFPE and end-repair kits (New England BioLabs) followed by ligation of barcodes with 1× bead clean up using AMPure XP (Beckman Coulter Nederland) after each step as described in SQK-LSK108 and SQK-LSK109. Barcoded isolates were pooled and sequencing adapters were added by ligation. The final library was loaded onto a MinION flow cell (MIN-106 R9.4.1). The 48 h sequence run was started without live base calling enabled on a MinION device connected to a desktop computer. After the sequence run, base calling and de-multiplexing were performed using Albacore 2.3.1 and a single FASTA file per isolate was extracted from the FAST5 files using Poretools 0.5.1 [23]. Fifty base pairs were trimmed at both sides and only reads larger than 5000 bp were used in further analyses. Illumina and Nanopore data were used in a hybrid assembly performed by Unicycler v0.4.4 [24]. Illumina data were not trimmed before running Unicycler, which was operated using default settings and verbosity 2. The resulting contig files were annotated using Prokka v1.14.6 and were subsequently loaded into BioNumerics for further analyses [25].
Plasmid content analysis
For annotation a Conda environment was set up with packages to facilitate a Snakemake pipeline which could process samples in bulk, and perform initial annotation with Prokka and enhancement with blast+ [26, 27]. Prokka annotation was executed in two stages: in the first stage it identified the coordinates of candidate genes with Prodigal, and in the second step it predicted these genes by utilizing user-set databases and its default the SWISS-PROT database [28, 29]. SWISS-PROT was used as it is a curated protein sequence database striving to provide a high level of annotation. To preserve the speed of the initial annotation we prepared a small database by combining sequence data from the ResFinder (version 3.1.0) database and the PlasmidFinder (version 2.0.2) database [21, 22]. If Prokka was unable to predict a gene it labelled the coordinate as a hypothetical protein. In order to reduce the hypothetical proteins in our annotation we used a set of custom Python scripts to extract and prepare them for blast+. After alignment with blast+, the supplemented Python code was used to replace the hypothetical proteins in the initial annotation file with their best alignment match (https://github.com/BSR-AMR-RIVM/blaOXA-48-plasmids-Microbial-Genomics). BioNumerics was used to extract and analyse the presence of annotated genes and tranposases in the different plasmids. The data were plotted, analysed and visualized in Excel. The presence of the direct repeat (DR) was analysed by searching for GGTAATGACTCCAAC using the BioNumerics sequence search feature in the sequence viewer.
Plasmid and chromosome comparisons
BioNumerics was used to compare complete plasmid DNA sequence and circular and linear chromosome datasets. Linear assembly contigs were omitted. Plasmid groups were identified based on ‘all-to-all’ primary DNA sequence comparison in BioNumerics in combination with unweighted pair group method with arithmetic mean (UPGMA) clustering. Plasmids with ≥80 % sequence identity were considered to belong to the same plasmid group. The CLC Genomics Workbench version 12.0 software (www.qiagenbioinformatics.com) was used to retrieve bla OXA-48-like plasmids and chromosomes from NCBI (Table S1). These plasmids and chromosomes were stripped from their annotations and re-annotated again using Prokka v1.14.6. All chromosomes have the dnaA gene as a starting point in order to determine relative locations of bla OXA-48-like alleles. For analysis of the plasmid gene content, the bla OXA-48 or bla OXA-48-like allele was set as the starting point.
Minimum spanning tree, UPGMA, MLST and wgMLST analyses
The BioNumerics software was used to generate a minimum spanning tree (MST) or a UPGMA hierarchical clustering as described previously [3]. The categorical coefficient was used to calculate the MST and the MST was based on in-house E. coli and K. pneumoniae wgMLST schemes. The NGS data of the K. pneumoniae and E. coli isolates were used for classical MLST and wgMLST analyses using in-house wgMLST schemes made in the SeqSphere software version 6.0.2 (Ridom). The in-house K. pneumoniae wgMLST scheme comprised 4978 genes (3471 core-genome and 1507 accessory-genome targets) using K. pneumoniae MGH 78,578 (NC_009648.1) as a reference genome. The in-house E. coli wgMLST scheme comprised 4503 genes (3199 core-genome and 1304 accessory-genome targets) using E. coli 536 (CP000247.1) as a reference genome.
Ethics statement
The bacterial isolates belong to the medical microbiological laboratories participating in the Dutch National CPE Surveillance programme and were obtained as part of routine clinical care in recent years. Since no identifiable personal data were collected and data were analysed and processed anonymously, written or verbal patient consent was not required. According to the Dutch Medical Research Involving Human Subjects Act (WMO) this study was exempt from review by an Institutional Review Board.
Results
Resistance to meropemen of CPE carrying bla OXA-48-like plasmids
From 2014 to 2019, the National Institute for Public Health and the Environment (RIVM) received 1503 CPE, of which the majority (n=1106) were E. coli (n=461) and K. pneumoniae (n=645). PCR revealed that 272 E. coli and 338 K . pneumoniae isolates carried bla OXA-48-like alleles. Only the first submitted E. coli or K. pneumoniae isolate with a bla OXA-48-like allele per person in this study period was included. Therefore, 537 carbapenemase-producing E. coli (n=230) and K. pneumoniae (n=307) isolates were sequenced by NGS (Table S1). The majority of the E. coli isolates were carrying bla OXA-48, bla OXA-181 and bla OXA-244 alleles and had MICs for meropenem that were below the clinical breakpoint of 2 mg l−1 for sensitivity according to EUCAST (206/230; 89.6 %) (Table 1). Only 2/157 (1.3 %) of the E. coli isolates with bla OXA-48 reached the clinical breakpoint for resistance (>8 mg l−1) to meropenem. The bla OXA-244 allele was found predominantly in E. coli (30/32; 93.8 %) and was associated with a low MIC for meropenem. K. pneumoniae carried mostly bla OXA-48, bla OXA-181 and bla OXA-232 alleles, of which the bla OXA-48 allele was associated with resistance to meropenem (63/307; 20.5 %). The bla OXA-181 allele was found in both E. coli and K. pneumoniae , and conferred resistance to meropenem in 4/21 (19 %) of the K. pneumoniae isolates and 1/36 (2,8 %) of the E. coli isolates. The bla OXA-232 allele was exclusively found in K. pneumoniae and none of these isolates were meropenem-sensitive (resistant, 17/19; 89.5 %, intermediate, 2/19; 10.5 %). Combinations of bla OXA-48-like alleles with either bla NDM-1 or bla NDM-5 resulted in high MICs for meropenem. For all bla OXA-48-like alleles and double allele combinations, K. pneumoniae was more resistant (123/307; 40.1 %) than E. coli (9/230; 3.9 %). Due to initial limited resources, a subset (220/537; 41 %) of the isolates submitted in 2018 and 2019 were sequenced with Nanopore long-read sequencing enabling the reconstruction of 47 and 132 complete bla OXA-48-like plasmids for E. coli and K. pneumoniae , respectively (Table 2).
Table 1.
Resistance to meropenem per E. coli or K. pneumoniae isolate carrying bla OXA-48-like alleles in 2014–2019
|
Total |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
bla OXA-48-like allele |
S |
I |
R |
All |
S |
I |
R |
All |
|
|
bla OXA-48 |
145 |
10 |
2 |
157 |
102 |
52 |
63 |
217 |
374 |
|
bla OXA-181 |
33 |
2 |
1 |
36 |
14 |
3 |
4 |
21 |
57 |
|
bla OXA-232 |
2 |
17 |
19 |
19 |
|||||
|
bla OXA-244 |
28 |
2 |
30 |
1 |
1 |
2 |
32 |
||
|
bla NDM-1, bla OXA-48-like |
1 |
3 |
21 |
25 |
25 |
||||
|
bla NDM-5, bla OXA-48-like |
4 |
4 |
14 |
14 |
18 |
||||
|
Other |
1 |
2 |
3 |
2 |
3 |
4 |
9 |
12 |
|
|
Total |
206 |
15 |
9 |
230 |
120 |
64 |
123 |
307 |
537 |
Based on the clinical breakpoints according to EUCAST, the isolates were classified as sensitive (S: ≤2 mgl−1), intermediate (I: >2 to 8 mg l−1) or resistant (R: >8 mg l−1) for meropenem.
Table 2.
bla OXA-48-like plasmids and chromosomes analysed in this study
|
Carbapenemase allele |
||||||
|---|---|---|---|---|---|---|
|
Plasmid/chromosome |
Species |
bla OXA-48 |
bla OXA-181 |
bla OXA-232 |
bla OXA-244 |
Total |
|
Plasmids |
30 |
16 |
1 |
47 |
||
|
|
108 |
10 |
14 |
132 |
||
|
Plasmids NCBI |
14 |
35 |
49 |
|||
|
|
81 |
10 |
22 |
1 |
114 |
|
|
Chromosomes |
30 |
10 |
40 |
|||
|
|
4 |
4 |
||||
|
Chromosomes NCBI |
6 |
1 |
7 |
|||
|
|
1 |
1 |
||||
|
Total |
|
274 |
71 |
37 |
12 |
394 |
Plasmids included in this study are complete and circular only, while the chromosomes were either circular or linear DNA.
NCBI indicates plasmids or chromosomes retrieved from the National Center for Biotechnology Information.
bla OXA-48-like plasmids cluster in distinct genogroups
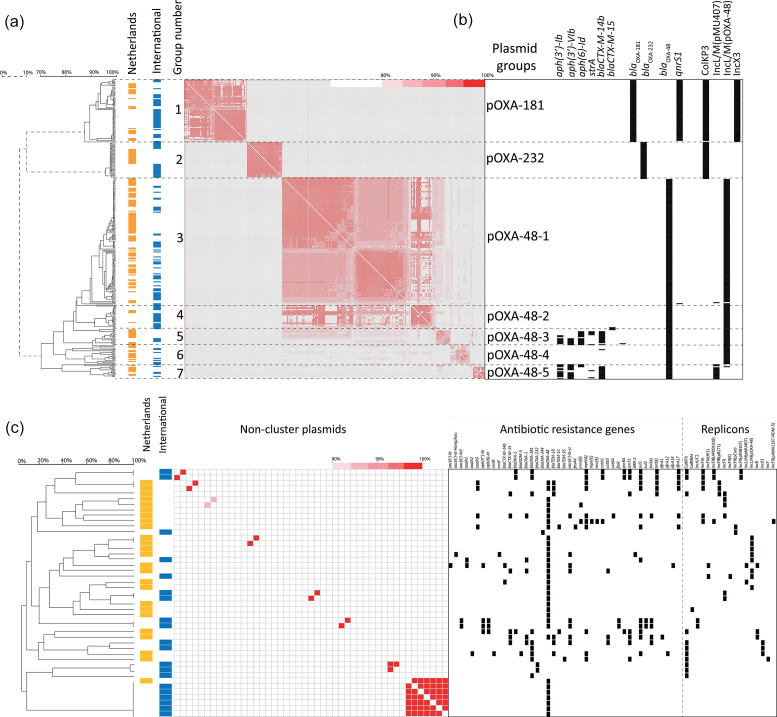
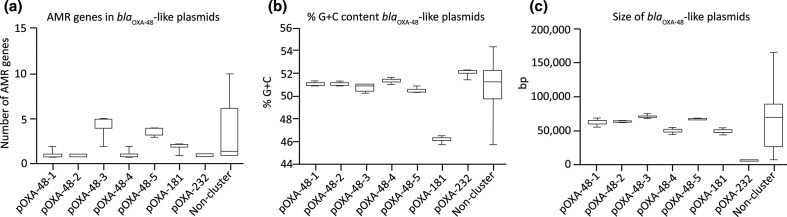
Comparison of the bla OXA-48-like plasmid sequences retrieved from the Netherlands with internationally reported bla OXA-48-like plasmids revealed clustering of the plasmids in a pOXA-232 group, a pOXA-181 group and five distinct pOXA-48 groups (Fig. 1a). A number of plasmids did not cluster with any of the other plasmids and were designated as the ‘non-cluster’ group (Fig. 1c). Plasmids identified in the Netherlands were similar to internationally reported plasmids that were obtained from 29 different countries from North and South America, Europe, Asia and Oceania (File. S1). In general, there was a paucity of antibiotic resistance genes in most of the bla OXA-48-like-containing plasmids (Fig. 1a, b). UPGMA clustering based on plasmid sequence comparison showed that the pOXA-232 plasmids containing the ColKP3 replicon were highly conserved (96–100 % similarity). At 6.2 kb in size, the pOXA-232 plasmids were the smallest bla OXA-48-like plasmids and carried a single replicon, but had the highest average G+C content of 52.2 % (Fig. 2a–c). In contrast, pOXA-181 plasmids carried the qnrS1 allele and ColKP3 and IncX3 replicons and were also conserved (90–100 %) (Fig. 1a, b). The pOXA-181 plasmids were on average 51.3 kb in size and had the lowest G+C content of 46.4 % (Fig. 2b, c). Despite the high sequence conservation of pOXA-181 and pOXA-232 plasmids, they were found in CPE with distinct chromosomal backgrounds (Table S2). The largest and most variable group comprised bla OXA-48-containing plasmids with an IncL/M(pOXA-48) type of replicon and could be divided into five subgroups, pOXA-48-1 to pOXA-48-5. The sequence conservation among pOXA-48-1 plasmids ranged from 80 to 100 % (Fig. 1a). pOXA-48-1 plasmids were on average 64 kB with a G+C content of 51.2 % and differed only from pOXA-48-2 plasmids by 0.1 kb. pOXA-48–3 was characterized by the presence of the aminoglycoside resistance genes aph(3′)-Ib, aph(3′)-VIb, aph(6′)-Id and the extended spectrum beta-lactamase (ESBL) gene bla CTXM-14b (Fig. 1b). pOXA-48-3 plasmids resembled pOXA-48-5 plasmids, but most of the pOXA-48-5 plasmids lacked the aph(6′)-Id gene and contained a distinct IncL/M(pMU407) replicon (Fig. 1b). pOXA-48-4 plasmids lacked these aminoglycoside resistance genes and these plasmids were smaller in size (Fig. 2). pOXA-48-3 and pOXA-48-5 had on average four AMR genes, one replicon per plasmid, a highly similar G+C content of 50.9 and 50.7 %, respectively, but differed by 3.4 kB in size (Fig. 2). Non-cluster bla OXA-48-like plasmids were distinct from those in the other groups and carried a wide variety of AMR genes resulting in distinct resistomes (Fig. 2a). These plasmids had either non-IncL/M-type replicons (e.g. IncR, IncY, IncF or IncA) or no known replicons (Fig. 1c). In addition, they had plasmid sizes that differed from those in the different plasmid groups and in G+C content and predominantly originated from isolates from the Netherlands (Fig. 2b, c).
Fig. 1.
Genetic clustering of bla OXA-48-like plasmids in seven groups. (a) UPGMA clustering based on plasmid DNA sequences revealed seven distinct groups of bla OXA-48-like plasmids. These groups were designated as pOXA-xxx (e.g. pOXA-48-1). Plasmids retrieved from the Netherlands are indicated in orange and international plasmids in blue. Group numbers are indicated. A heatmap shows the percentage of sequence identity, where red is 100 % identical and white 0 % identical. (b) The presence of AMR genes and replicons among the plasmids is indicated with black squares. Plasmids are depicted in rows and the AMR genes and replicons in columns. (c) As in (a) and (b) for the non-cluster plasmids.
Fig. 2.
bla OXA-48-like plasmids have distinct molecular characteristics. (a) The number of AMR genes among the pOXA-48-like plasmid groups, (b) the G+C content (%) of the distinct pOXA-48-like plasmid groups and (c) the size (kb) of the pOXA-48-like plasmid groups. Bars, the variation per group.
Gene content determines distinct bla OXA-48-like plasmid architecture
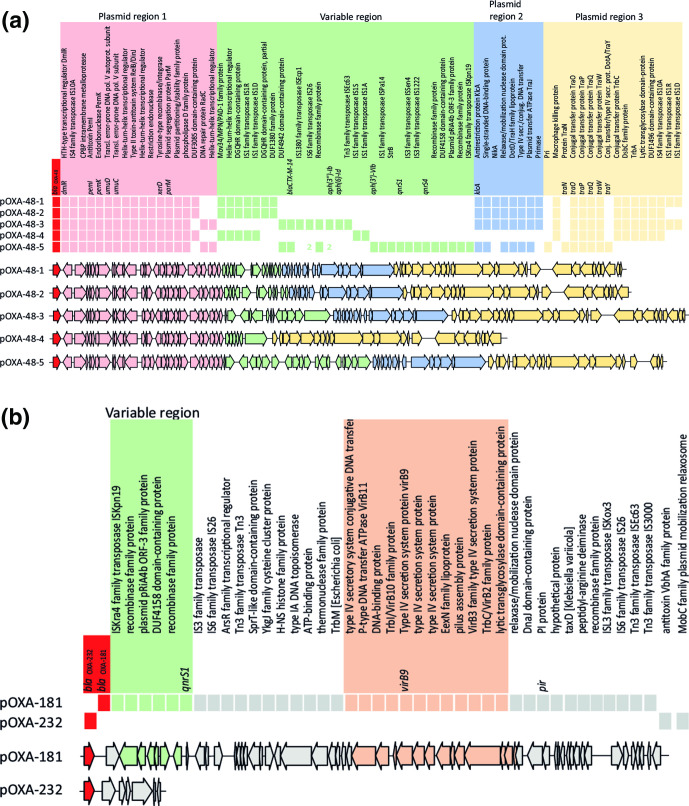
Analysis of the gene content of representative plasmids from the seven distinct plasmid groups revealed a group-associated gene content (Fig. 3). pOXA-48 plasmids had conserved plasmid regions, designated as regions 1, 2 and 3 and a central variable region (VR) which displayed variability in gene content and length (Fig. 3a). Plasmid region 2 was absent in pOXA-48-4 plasmids. The variations in pOXA-48 plasmid gene content such as the presence or absence of AMR genes shaped the primary bla OXA-48-like plasmid architecture, and varied among the different plasmid groups. While the pOXA-48-1, pOXA-48-2, pOXA-48-3 and pOXA-48-5 groups contained the klcA anti-restriction gene and a putative conjugation system, these features were absent in the pOXA-48-4, pOXA-181 and pOXA-232 plasmid groups (Fig. 3b). In pOXA-48-4 plasmids, the tra conjugation system was incomplete, while pOXA-48-5 plasmids contained a full conjugation system. pOXA-48 plasmids contained the PemI antitoxin, while the pOXA-181 and pOXA-232 plasmids did not. pOXA-181 plasmids carried a virB2-virB3-virB9-virB10-virB11 type IV secretion system, while the other pOXA-48 plasmids and pOXA-232 lacked this system. IS1 family transposases IS1R and IS1D, and IS4 family transposase IS10A were predominantly found in the pOXA-48 plasmids, and pOXA-181 plasmids were characterized by a variety of Tn3 family transposases. pOXA-232 plasmids did not contain IS or Tn3 elements.
Fig. 3.
Differences in bla OXA-48-like plasmid architecture. (a) Diversity in pOXA-48-1 to pOXA-48-5 plasmid gene content. Complete plasmids were visualized in a linear way with the bla OXA-48-like allele at starting position 1. The presence and absence of genes is indicated among representative plasmids from the plasmid groups. Colours indicate different groups of genes corresponding to different regions in the plasmid, or the variable region. Plasmid regions are labelled above the plasmid sequence. (b) Similar to (a) but displaying diversity in pOXA-181 and pOXA-232 plasmid gene content.
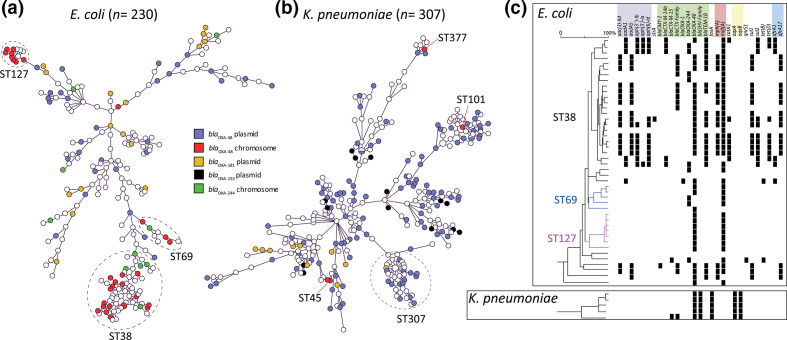
Distribution of isolates harbouring plasmid or chromosomally localized bla OXA-48 and bla OXA-244 alleles
A fraction of the bla OXA-48 (30/230; 13%) and bla OXA-244 (10/230; 4.3%) alleles were located in the chromosomes of E. coli isolates, respectively (Table 2). Chromosomal bla OXA-48 or bla OXA-244 occurred in E. coli isolates with the MLST sequence types ST38, ST69 and ST127 among other STs (Fig. 4a, Table S2). The STs were all unrelated and were multiple locus variants from ST38. The chromosome-localized bla OXA-48 and bla OXA-244 were non-randomly distributed in the MST and restricted to specific STs (Fig. 4a, Table S2). In contrast, plasmid-localized bla OXA-48 occurred in E. coli isolates from a variety of non-related STs and were found randomly dispersed among the MST, except in ST38, ST69 and ST127. In four K. pneumoniae isolates, bla OXA-48 was found to be integrated in the chromosome (4/307; 1.3%), while none of the bla OXA-181 or bla OXA-232 alleles were located chromosomally. K. pneumoniae with either chromosome- or plasmid-localized bla OXA-48-like were randomly distributed in the MST (Fig. 4b). The presence of the bla OXA-48 allele in the E. coli ST38 chromosomes was associated with the presence of the macrolide, trimethoprim and sulphonamide AMR genes mph(A), dfrA and sul, while ST69 and ST127 were lacking the dfrA and sul genes (Fig. 4c). In contrast to E. coli ST38, the bla OXA-48-containing K. pneumoniae chromosomes were mostly devoid of AMR genes, with the exception of the fosfomycin and quinolone resistance genes fosA, oqxA and oqxB.
Fig. 4.
Distribution of chromosome- or plasmid-localized bla OXA-48 or bla OXA-244. (a) MST of E. coli in which chromosome- or plasmid-localized bla OXA-48 or bla OXA-244 alleles are indicated by different colours. (b) Similar to (a) but for K. pneumoniae . (c) The presence of AMR genes among the chromosomes analysed in this study is indicated with black squares. Chromosomes are depicted in rows and the AMR genes in columns. Antibiotic classes are indicated above the AMR genes in different colours.
Architecture of chromosome-localized bla OXA-48 or bla OXA-244 allelic regions
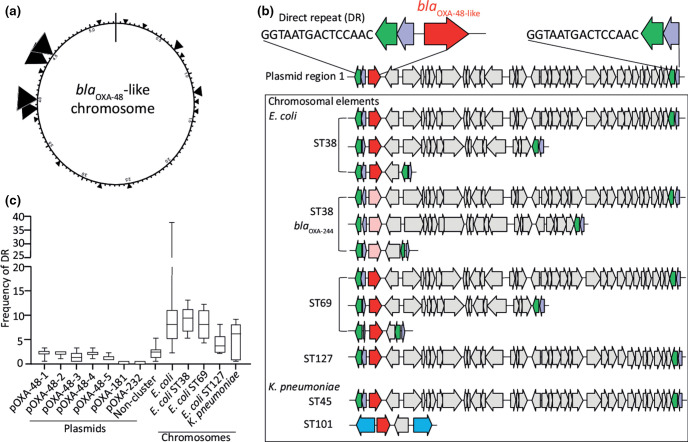
In E. coli , the bla OXA-48 or bla OXA-244 alleles were positioned in distinct regions in the chromosome relative to dnaA (Fig. 5a, Table 3). Chromosomally residing bla OXA-48 or bla OXA-244 were located on different genetic elements with variable sizes of ~2.6,~11 or ~20 kb. The bla OXA-48-like genetic element was flanked by IS1 family transposases IS1R and IS1D and had the IS1R-IS1D-bla OXA-48-insert-IS1R-IS1D structure or variants thereof (Fig. 5b). The sizes of the genetic elements were determined as the sequence in between the flanking IS1R and IS1D, thereby excluding the size of the IS1R/1D sequence. The chromosomal insertion sites of bla OXA-48-like genes and length of the insertion element varied per sequence type. In contrast to the bla OXA-48 allele, the chromosomally residing bla OXA-244 allele was not found in the ST127 genetic background. In K. pneumoniae , bla OXA-48 was also found to be embedded between two IS4 family transposase IS10A genes. Comparison of pOXA-48 plasmids with the chromosomal bla OXA-48 insertions revealed that these chromosomal insertions resembled variable regions of plasmid region 1 (Fig. 5b). A 15-nt DR ggtaatgactccaac was typically located directly upstream IS1R, thereby flanking the bla OXA-48-like insertion element. This DR sequence occurs on average once or twice in pOXA-48-1 to pOXA-48-5 plasmids and non-cluster plasmids, except in pOXA-181 and pOXA-232 plasmids (Fig. 5c). The DR was found on average 9× in the 47 E. coli chromosomes with bla OXA-48-like, compared to 4.6× in the five K . pneumoniae chromosomes containing bla OXA-48. The DR occurred on average 9, 8 and 4× in E. coli ST38, ST69 and ST127, respectively. In only four of the 52 chromosomes analysed, the bla OXA-48 region was flanked by one single DR sequence if the orientation of the carbapenemase allele was in reverse orientation (Table 3). In one chromosome, no DR sequence or truncates thereof were found. In K. pneumoniae , in two of the four ST45 isolates bla OXA-48 was inserted in the same location in the chromosome through a highly comparable genetic element (Fig. 5b). In a more distantly related K. pneumoniae ST101 isolate, a mobile genetic element of ~2.4 kb bla OXA-48 was localized in a distinct region, as also for the chromosomes retrieved from NCBI (Fig. 5b).
Fig. 5.
Distinct integration sites of variable bla OXA-48 and bla OXA-244 elements in the chromosome. (a) Artificial chromosome in which the different bla OXA-48-like insertion positions are indicated by triangles. (b) Comparison of plasmid region 1 with chromosomal insertion sites of bla OXA-48-like. Arrows indicate ORFs of which bla OXA-48 is depicted in red and bla OXA-244 in light red. DR indicates the direct repeat sequence ggtaatgactccaac located upstream of IS1R. Sequence types are depicted by ST and sizes of the different insertion sequences are indicated in kilobases (kb). (c) Frequency of the DR sequence in bla OXA-48-like plasmids and chromosomes of E. coli and K. pneumoniae .
Table 3.
Characteristics of the bla OXA-48-like chromosomal insertion site, direct repeat and insertion element
|
Species |
MLST ST |
Carba allele |
Location of bla OXA-48-like |
Location of bla OXA-48-like fragment |
Size |
No. of DRs in chromosome |
DRs flanking bla OXA-48-like |
||
|---|---|---|---|---|---|---|---|---|---|
|
Start |
End |
Start |
End |
||||||
|
cRIVM_C012087 |
38 |
bla OXA-48 |
1226615 |
1227412 |
1227631 |
1216632 |
−10999 |
6 |
1 |
|
cRIVM_C014115 |
38 |
bla OXA-48 |
837858 |
838655 |
838907 |
818476 |
−20431 |
10 |
2 |
|
cRIVM_C014187 |
38 |
bla OXA-48 |
3322083 |
3322880 |
3323099 |
3321208 |
−1891 |
5 |
2 |
|
cRIVM_C017997 |
38 |
bla OXA-48 |
1271878 |
1272675 |
1272927 |
1261895 |
−11032 |
7 |
2 |
|
cRIVM_C018220 |
38 |
bla OXA-48 |
4497614 |
4498411 |
4497362 |
4517799 |
20437 |
11 |
2 |
|
cRIVM_C018563 |
38 |
bla OXA-48 |
1225857 |
1226654 |
1226873 |
1215874 |
−10999 |
5 |
2 |
|
cRIVM_C018567 |
38 |
bla OXA-48 |
4429086 |
4429883 |
4428834 |
4449271 |
20437 |
11 |
2 |
|
cRIVM_C018583 |
38 |
bla OXA-244 |
5316678 |
5317475 |
5317694 |
5297296 |
−20398 |
9 |
2 |
|
cRIVM_C018699 |
38 |
bla OXA-48 |
4450722 |
4451519 |
4450470 |
4470907 |
20437 |
13 |
2 |
|
cRIVM_C018707 |
38 |
bla OXA-48 |
4437017 |
4437814 |
4436798 |
4457202 |
20404 |
11 |
2 |
|
cRIVM_C028536 |
38 |
bla OXA-48 |
1293474 |
1294271 |
1294490 |
1283491 |
−10999 |
7 |
2 |
|
cRIVM_C028568 |
38 |
bla OXA-48 |
4379381 |
4380178 |
4378887 |
4390834 |
11947 |
12 |
2 |
|
cRIVM_C028613 |
38 |
bla OXA-244 |
102972 |
103769 |
102478 |
104598 |
2120 |
8 |
2 |
|
cRIVM_C028803 |
38 |
bla OXA-48 |
4458895 |
4459692 |
4458676 |
4479080 |
20404 |
9 |
2 |
|
cRIVM_C029020 |
38 |
bla OXA-48 |
5148725 |
5149522 |
5149741 |
5129343 |
−20398 |
5 |
2 |
|
cRIVM_C029033 |
38 |
bla OXA-244 |
102972 |
103769 |
102433 |
104975 |
2542 |
12 |
2 |
|
cRIVM_C029042 |
38 |
bla OXA-48 |
4342207 |
4343004 |
4341988 |
4362392 |
20404 |
13 |
2 |
|
cRIVM_C029951 |
38 |
bla OXA-244 |
106793 |
107590 |
106574 |
126972 |
20398 |
6 |
2 |
|
cRIVM_C029952 |
38 |
bla OXA-48 |
4455075 |
4455872 |
4454823 |
4475254 |
20431 |
8 |
2 |
|
cRIVM_C030197 |
38 |
bla OXA-48 |
3998627 |
3999424 |
3998408 |
4001066 |
2658 |
7 |
2 |
|
cRIVM_C030300 |
38 |
bla OXA-48 |
3998582 |
3999379 |
3998088 |
4001021 |
2933 |
7 |
2 |
|
cRIVM_C030371 |
38 |
bla OXA-244 |
5246611 |
5247408 |
5247627 |
5227229 |
−20398 |
5 |
2 |
|
cRIVM_C030453 |
38 |
bla OXA-48 |
1219470 |
1220267 |
1209487 |
1220734 |
−11247 |
10 |
2 |
|
CP032145_1 |
38 |
bla OXA-48 |
844693 |
845490 |
845709 |
827237 |
−18472 |
9 |
2 |
|
CP040390_1 |
38 |
bla OXA-48 |
4461613 |
4462410 |
4461394 |
4479866 |
18472 |
11 |
2 |
|
cRIVM_C010151 |
69 |
bla OXA-48 |
3898487 |
3899284 |
3900200 |
3879422 |
−20778 |
4 |
2 |
|
cRIVM_C018576 |
69 |
bla OXA-48 |
749018 |
749815 |
750731 |
747656 |
−3075 |
8 |
1 |
|
cRIVM_C030256 |
69 |
bla OXA-48 |
749016 |
749813 |
750729 |
747654 |
−3075 |
8 |
1 |
|
cRIVM_C030443 |
69 |
bla OXA-244 |
102728 |
103525 |
102509 |
116091 |
13582 |
12 |
2 |
|
cRIVM_C036689 |
99 |
bla OXA-48 |
79721 |
80518 |
79502 |
99900 |
20398 |
5 |
2 |
|
cRIVM_C014046 |
127 |
bla OXA-48 |
nd |
nd |
nd |
nd |
20673 |
3 |
2 |
|
cRIVM_C017887 |
127 |
bla OXA-48 |
4003811 |
4004608 |
4003592 |
4023990 |
20398 |
4 |
2 |
|
cRIVM_C018150 |
127 |
bla OXA-48 |
nd |
nd |
nd |
nd |
nd |
8 |
2 |
|
cRIVM_C028497 |
127 |
bla OXA-48 |
3939883 |
3940680 |
3939664 |
3960068 |
20404 |
4 |
2 |
|
cRIVM_C028620 |
127 |
bla OXA-48 |
3968348 |
3969145 |
3968129 |
3988533 |
20404 |
3 |
2 |
|
cRIVM_C028724 |
127 |
bla OXA-48 |
3884417 |
3885214 |
3884198 |
3904602 |
20404 |
2 |
2 |
|
cRIVM_C028786 |
127 |
bla OXA-48 |
4000159 |
4000956 |
3999940 |
4020344 |
20404 |
6 |
2 |
|
cRIVM_C029324 |
127 |
bla OXA-48 |
3910758 |
3911555 |
3910539 |
3930943 |
20404 |
2 |
2 |
|
cRIVM_C018249 |
349 |
bla OXA-244 |
89993 |
90790 |
89454 |
91619 |
2165 |
15 |
2 |
|
cRIVM_C011532 |
361 |
bla OXA-244 |
80789 |
81568 |
80376 |
100968 |
20592 |
36 |
2 |
|
cRIVM_C018404 |
940 |
bla OXA-48 |
3841159 |
3841956 |
3840907 |
3859484 |
18577 |
25 |
2 |
|
cRIVM_C029494 |
1722 |
bla OXA-244 |
102501 |
103298 |
101585 |
115864 |
14279 |
2 |
2 |
|
CP38505_1 |
nd |
bla OXA-244 |
102956 |
103753 |
102737 |
123135 |
20398 |
10 |
2 |
|
CP050382_1 |
nd |
bla OXA-244 |
80907 |
81704 |
80688 |
82533 |
1845 |
25 |
2 |
|
cRIVM_C014073 |
45 |
bla OXA-48 |
4405354 |
4406151 |
4404860 |
4425910 |
21050 |
6 |
2 |
|
cRIVM_C018500 |
45 |
bla OXA-48 |
4348845 |
4349642 |
4348626 |
4369024 |
20398 |
7 |
2 |
|
cRIVM_C015657 |
101 |
bla OXA-48 |
262228 |
263025 |
262095 |
264541 |
2446 |
0 |
0 |
|
cRIVM_C015043 |
377 |
bla OXA-48 |
2534772 |
2535569 |
2535788 |
2515390 |
-20398 |
9 |
2 |
|
NZ-CP040023_1 |
nd |
bla OXA-48 |
1406533 |
1407330 |
1405017 |
1407743 |
2726 |
1 |
1 |
Size of the blaOXA-48-like insertion element excludes the IS1R-IS1D elements. If a number denotes a ‘−’, the bla OXA-48-like element is in reverse orientation. The location of bla OXA-48-like and the bla OXA-48-like fragment in the chromosome is indicated by locations relative to dnaA.
Discussion
We dissected the architecture of 179 complete plasmids carrying bla OXA-48-like and 44 bla OXA-48-like alleles containing chromosomes of E. coli and K. pneumoniae isolates obtained from the Dutch national CPE surveillance programme in comparison with bla OXA-48-like plasmids and chromosomes reported in the NCBI databank. The overall bla OXA-48-like plasmid population in the Netherlands is conserved and compares to internationally reported plasmids. Most of the bla OXA-48-like plasmids from both E. coli and K. pneumoniae could be clustered into seven distinct genotypic plasmid groups, which were characterized by a paucity in AMR genes, marked differences in gene content, replicon family, size and G+C content. This suggests the plasmids studied here have distinct origins and have transferred horizontally among CPE world-wide. In contrast to pOXA-181 and pOXA-232 plasmids, which were highly conserved, a group of pOXA-48 plasmids were diverse in genetic composition with sequence variation as high as 20 %. The presence of a variety of transposases and insertion sequences, in addition to conjugation machinery, may be attributable to the genetic diversity of the pOXA-48 plasmids, in particular in the pOXA-48-3 and pOXA-48-5 plasmid subgroups.
There was an additional group of genetically highly diverse bla OXA-48-like plasmids obtained in the Netherlands with a large range in G+C content, a variety of IncL and non-IncL-type replicons (IncR, IncFII or IncY), AMR genes and low inter-plasmid similarity. This suggests the presence of a potentially recently introduced set of plasmids that have not yet widely spread in the Netherlands. OXA-48 plasmids with either an IncR, IncFII or IncY replicon have only recently been described and are relatively rare [30–32]. The presence of these variable and rare bla OXA-48-like plasmids suggest that the current OXA-48 plasmid reservoir may larger than currently reported. bla OXA-48-like plasmids occurred in globally disseminated E. coli and K. pneumoniae isolates with known genetic backgrounds such as E. coli ST38 and K. pneumoniae ST307, but also multiple new STs, demonstrating continuous dissemination of AMR plasmids to new genetic backgrounds. To date, no double combinations of bla OXA-48-like of alleles have been detected in one strain, although combinations with other carbapenemase alleles such as either bla NDM-1 or bla NDM-5 exist.
In this study, we also detected chromosomally localized bla OXA-48 and bla OXA-244 alleles, but not chromosomal bla OXA-181 and bla OXA-232 alleles. This is in contrast to reports from other countries, where chromosomally localized bla OXA-181 and bla OXA-232 alleles have been described and found occasionally [6, 33]. Possibly, fragments containing bla OXA-181 and bla OXA-232 alleles failed to integrate by the lack of appropriate transposases, direct repeat target sequences in the plasmids or a suitable genetic background. Chromosomal insertion of bla OXA-48 or bla OXA-244 may have occurred through IS1R-mediated transposition and recombination of OXA-48 plasmid sequences into E. coli and K. pneumoniae chromosomes with distinct genetic compositions [15]. The various lengths and compositions of bla OXA-48-like segments and a variety of locations in the chromosome suggest that multiple transposition and recombination events have occurred. The chromosomal bla OXA-48 segment probably originated from plasmids belonging to the pOXA-48-1 to pOXA-48-5 groups. A potential insertion target site, a 15 bp direct repeat, was present in multiple copies in the chromosome and was found only in pOXA-48-1 to pOXA-48-5 plasmids and non-cluster plasmids, but not in pOXA-181 or pOXA-232 plasmids. This direct repeat was also found more frequently in E. coli than in K. pneumoniae chromosomes, which may explain why more E. coli than K. pneumoniae isolates harbour chromosomal bla OXA-48/bla OXA-244 and not bla OXA-181/bla OXA-232.
The majority of the bla OXA-48-containing K. pneumoniae isolates in this study had MICs for meropenem above the clinical breakpoint, in contrast to E. coli , which were mostly sensitive. The bla OXA-48-like alleles had different meropenem susceptibilities in K. pneumoniae and E. coli isolates, indicating that not all alleles result in the same resistance phenotype. In particular, K. pneumoniae containing bla OXA-232 were highly resistant, which can possibly be attributed to a high copy number of pOXA-232 plasmids [34]. Alternatively, OXA-48 enzyme production, an altered affinity for meropenem, or other determinants such as outer membrane proteins, porins, efflux pumps or the presence of additional ESBLs can be responsible for this phenomenon as well [35, 36].
In conclusion, long-read sequencing of isolates from the Dutch National CPE surveillance contributed to the dissection of the architecture of bla OXA-48-like plasmids and bla OXA-48-like chromosome insertions of CPE in the Netherlands. Conjugation machinery, transposable elements and/or virulence determinants may contribute to plasmid diversification and dissemination, and represent important features that warrant future investigation. Additional long-read sequencing efforts of plasmids of CPE are required to monitor the changing plasmid reservoir involved in the spread of antibiotic resistance determinants in the Netherlands and beyond.
Supplementary Data
Funding information
This work received no specific grant from any funding agency.
Acknowledgements
We thank all the members of the Dutch CPE surveillance study Group and the Dutch medical microbiology laboratories for submitting CPE isolates to the RIVM for the national CPE surveillance programme. We also thank Professor Dr E. Kuijper and Dr D. W. Notermans for critical reading of the manuscript. Members of the Dutch CPE surveillance Study Group: A. Maijer-Reuwer, ADRZ medisch centrum, Department of Medical Microbiology, Goes; M. A. Leversteijn-van Hall, Alrijne Hospital, Department of Medical Microbiology, Leiden; J. A. J. W. Kluytmans, Amphia Hospital, Microvida Laboratory for Microbiology, Breda; I. J. B. Spijkerman, Amsterdam UMC - location AMC, Department of Medical Microbiology, Amsterdam; K. van Dijk, Amsterdam UMC - location Vumc, Department of Medical Microbiology and Infection Control, Amsterdam; T. Halaby, Analytical Diagnostic Center N.V.Curaçao, Department of Medical Microbiology, Willemstad (Curaçao); B. Zwart, Atalmedial, Department of Medical Microbiology, Amsterdam; B. M. W. Diederen, Bravis Hospital/ZorgSaam Hospital Zeeuws-Vlaanderen, Department of Medical Microbiology, Roosendaal/Terneuzen; A. Voss, Canisius Wilhelmina Hospital, Department of Medical Microbiology and Infectious Diseases, Nijmegen; J. W. Dorigo-Zetsma, CBSL, Department of Medical Microbiology, Hilversum; D. W. Notermans, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven; A. Ott, Certe, Department of Medical Microbiology, Groningen; J. H. Oudbier, Comicro, Department of Medical Microbiology, Hoorn; M. van der Vusse, Deventer Hospital, Department of Medical Microbiology, Deventer; A. L. M. Vlek, Diakonessenhuis, Department of Medical Microbiology and Immunology, Utrecht; A. G. M. Buiting, Elisabeth-TweeSteden (ETZ) Hospital, Department of Medical Microbiology and Immunology, Tilburg; L. Bode, Erasmus University Medical Center, Department of Medical Microbiology, Rotterdam; S. Paltansing, Franciscus Gasthuis and Vlietland, Department of Medical Microbiology and Infection Control, Rotterdam; A. J. van Griethuysen, Gelderse Vallei Hospital, Department of Medical Microbiology, Ede; M. den Reijer, Gelre Hospitals, Department of Medical Microbiology and Infection prevention, Apeldoorn; M. van Trijp, Groene Hart Hospital, Department of Medical Microbiology and Infection Prevention, Gouda; E. P. M. van Elzakker, Haga Hospital, Department of Medical Microbiology,’s-Gravenhage; A. E. Muller, HMC Westeinde Hospital, Department of Medical Microbiology,’s-Gravenhage; M. P. M. van der Linden, IJsselland hospital, Department of Medical Microbiology, Capelle a/d IJssel; M. van Rijn, Ikazia Hospital, Department of Medical Microbiology, Rotterdam; M. J. H. M. Wolfhagen, Isala Hospital, Laboratory of Medical Microbiology and Infectious Diseases, Zwolle; K. Waar, Izore Centre for Infectious Diseases Friesland, Department of Medical Microbiology, Leeuwarden; E. Kolwijck, Jeroen Bosch Hospital, Department of Medical Microbiology and Infection Control,’s-Hertogenbosch; W. Silvis, LabMicTA, Regional Laboratory of Microbiology Twente Achterhoek, Hengelo; T. Schulin, Laurentius Hospital, Department of Medical Microbiology, Roermond; M. Damen, Maasstad Hospital, Department of Medical Microbiology, Rotterdam; S. Dinant, Maasstad Hospital, Department of Medical Microbiology, Rotterdam; S. P. van Mens, Maastricht University Medical Centre, Department of Medical Microbiology, Maastricht; D. C. Melles, Meander Medical Center, Department of Medical Microbiology, Amersfoort; J. W. T. Cohen Stuart, Noordwest Ziekenhuisgroep, Department of Medical Microbiology, Alkmaar; M. L. van Ogtrop, Onze Lieve Vrouwe Gasthuis, Department of Medical Microbiology, Amsterdam; I. T. M. A. Overdevest, PAMM, Department of Medical Microbiology, Veldhoven; A. P. van Dam, Amsterdam Health Service, Public Health Laboratory, Amsterdam; H. Wertheim, Radboud University Medical Center, Department of Medical Microbiology, Nijmegen; H. M. E. Frénay, Regional Laboratory Medical Microbiology (RLM), Department of Medical Microbiology, Dordrecht; J. C. Sinnige, Regional Laboratory of Public Health, Department of Medical Microbiology, Haarlem; E. E. Mattsson, Reinier de Graaf Groep, Department of Medical Microbiology, Delft; R. W. Bosboom, Rijnstate Hospital, Laboratory for Medical Microbiology and Immunology, Velp; A. Stam, Saltro Diagnostic Centre, Department of Medical Microbiology, Utrecht; E. de Jong, Slingeland Hospital, Department of Medical Microbiology, Doetinchem; N. Roescher, St Antonius Hospital, Department of Medical Microbiology and Immunology, Nieuwegein; E. Heikens, St Jansdal Hospital, Department of Medical Microbiology, Harderwijk; R. Steingrover, St. Maarten Laboratory Services, Department of Medical Microbiology, Cay Hill (St. Maarten); A. Troelstra, University Medical Center Utrecht, Department of Medical Microbiology, Utrecht; E. Bathoorn, University of Groningen, Department of Medical Microbiology, Groningen; T. A. M. Trienekens, VieCuri Medical Center, Department of Medical Microbiology, Venlo; D. W. van Dam, Zuyderland Medical Centre, Department of Medical Microbiology and Infection Control, Sittard-Geleen; E. I. G. B. de Brauwer, Zuyderland Medical Centre, Department of Medical Microbiology and Infection Control, Heerlen; F. S. Stals, Zuyderland Medical Centre, Department of Medical Microbiology and Infection Control, Heerlen.
Author contributions
Conceptualization and methodology, A.P.A.H. and L.M.S.; visualization, A.P.A.H.; data curation, F.L., S.W. and M.V.S.V.; formal analysis, A.P.A.H. and L.M.S.; funding, not applicable; sample collection, Dutch CPE surveillance study Group; laboratory experiments, F.L., A.D.H., M.V.S.V.; supervision, A.P.A.H. and L.M.S.; manuscript preparation – original draft, A.P.A.H.; review and editing, A.P.A.H., L.M.S., F.L.; review and approval of final manuscript, all authors.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: AMR, antimicrobial resistance; CPE, carbapenemase-producing Enterobacterales; CRE, carbapenemase-resistant Enterobacterales; DR, direct repeat; ESBL, extended spectrum beta-lactamase; MIC, minimum inhibitory concentration; MST, minimum spanning tree; NGS, next-generation sequencing; ST, sequence type; TGS, third-generation sequencing; UPGMA, unweighted pair group method with arithmetic mean; VR, variable region; wgMLST, whole genome multilocus sequence typing.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Two supplementary tables and supplementary files are available with the online version of this article.
References
- 1.Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017;215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopotsa K, Osei Sekyere J, Mbelle NM. Plasmid evolution in carbapenemase-producing Enterobacteriaceae: a review. Ann N Y Acad Sci. 2019 doi: 10.1111/nyas.14223. [DOI] [PubMed] [Google Scholar]
- 3.Hendrickx APA, Landman F, de Haan A, Borst D, Witteveen S, et al. Plasmid diversity among genetically related Klebsiella pneumoniae bla KPC-2 and bla KPC-3 isolates collected in the Dutch national surveillance. Sci Rep. 2020;10:16778. doi: 10.1038/s41598-020-73440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Zwaluw K, Witteveen S, Wielders L, van Santen M, Landman F, et al. Molecular characteristics of carbapenemase-producing Enterobacterales in the Netherlands; results of the 2014-2018 national laboratory surveillance. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2020 doi: 10.1016/j.cmi.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Ambler RP, Coulson AF, Frère JM, Ghuysen JM, Joris B, et al. A standard numbering scheme for the class A beta-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitout JDD, Peirano G, Kock MM, Strydom K-A, Matsumura Y. The global ascendency of OXA-48-Type carbapenemases. Clin Microbiol Rev. 18 2019;33 doi: 10.1128/CMR.00102-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oueslati S, Nordmann P, Poirel L. Heterogeneous hydrolytic features for OXA-48-like β-lactamases. J Antimicrob Chemother. 2015;70:1059–1063. doi: 10.1093/jac/dku524. [DOI] [PubMed] [Google Scholar]
- 8.Poirel L, Héritier C, Tolün V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae . Antimicrob Agents Chemother. 2004;48:15–22. doi: 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potron A, Nordmann P, Lafeuille E, Al Maskari Z, Al Rashdi F, et al. Characterization of OXA-181, a carbapenem-hydrolyzing class D β-lactamase from Klebsiella pneumoniae . Antimicrob Agents Chemother. 2011;55:4896–4899. doi: 10.1128/AAC.00481-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potron A, Rondinaud E, Poirel L, Belmonte O, Boyer S, et al. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae . Int J Antimicrob Agents. 2013;41:325–329. doi: 10.1016/j.ijantimicag.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Oteo J, Hernández JM, Espasa M, Fleites A, Sáez D, et al. Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain. J of Antimicrobial Chemother. 2013;68:317–321. doi: 10.1093/jac/dks383. [DOI] [PubMed] [Google Scholar]
- 12.Carattoli A. Resistance plasmid families in Enterobacteriaceae . Antimicrob Agents Chemother. 2009;53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carattoli A, Seiffert SN, Schwendener S, Perreten V, Endimiani A. Differentiation of IncL and IncM plasmids associated with the spread of clinically relevant antimicrobial resistance. PLoS One. 2015;10:e0123063. doi: 10.1371/journal.pone.0123063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poirel L, Bonnin RA, Nordmann P. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother. 2012;56:559–562. doi: 10.1128/AAC.05289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyrouthy R, Robin F, Delmas J, Gibold L, Dalmasso G, et al. IS 1R -mediated plasticity of IncL/M plasmids leads to the insertion of bla OXA-48 into the Escherichia coli chromosome. Antimicrob Agents Chemother. 2014;58:3785–3790. doi: 10.1128/AAC.02669-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potron A, Poirel L, Nordmann P. Origin of OXA-181, an emerging carbapenem-hydrolyzing oxacillinase, as a chromosomal gene in Shewanella xiamenensis . Antimicrob Agents Chemother. 2011;55:4405–4407. doi: 10.1128/AAC.00681-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Feng Y, Wu W, Xie Y, Wang X, et al. First report of OXA-181-producing Escherichia coli in China and characterization of the isolate using whole-genome sequencing. Antimicrob Agents Chemother. 2015;59:5022–5025. doi: 10.1128/AAC.00442-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turton JF, Doumith M, Hopkins KL, Perry C, Meunier D, et al. Clonal expansion of Escherichia coli ST38 carrying a chromosomally integrated OXA-48 carbapenemase gene. J Med Microbiol. 2016;65:538–546. doi: 10.1099/jmm.0.000248. [DOI] [PubMed] [Google Scholar]
- 19.Mataseje LF, Boyd DA, Fuller J, Haldane D, Hoang L, et al. Characterization of OXA-48-like carbapenemase producers in Canada, 2011–14. J Antimicrob Chemother. 2018;73:626–633. doi: 10.1093/jac/dkx462. [DOI] [PubMed] [Google Scholar]
- 20.van der Zwaluw K, de Haan A, Pluister GN, Bootsma HJ, de Neeling AJ, et al. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS One. 2015;10:e0123690. doi: 10.1371/journal.pone.0123690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance. 2017;22:30589. doi: 10.2807/1560-7917.ES.2017.22.31.30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, et al. Identification of acquired antimicrobial resistance genes. Journal of Antimicrobial Chemotherapy. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loman NJ, Quinlan AR. Poretools: a toolkit for analyzing nanopore sequence data. Bioinformatics. 2014;30:3399–3401. doi: 10.1093/bioinformatics/btu555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 26.Köster J, Rahmann S. Snakemake-a scalable bioinformatics workflow engine. Bioinformatics. 2018;34:3600. doi: 10.1093/bioinformatics/bty350. [DOI] [PubMed] [Google Scholar]
- 27.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyatt D, Chen G-L, LoCascio PF, Land ML, Larimer FW, et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boutet E, Lieberherr D, Tognolli M, Schneider M, Bairoch A. UniProtKB/Swiss-Prot. Methods Mol Biol Clifton NJ. 2007;406:89–112. doi: 10.1007/978-1-59745-535-0_4. [DOI] [PubMed] [Google Scholar]
- 30.Palmieri M, D’Andrea MM, Pelegrin AC, Mirande C, Brkic S, et al. Genomic epidemiology of carbapenem- and colistin-resistant Klebsiella pneumoniae isolates from serbia: predominance of ST101 strains carrying a novel OXA-48 plasmid. Front Microbiol. 2020;11:294. doi: 10.3389/fmicb.2020.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jousset AB, Dabos L, Bonnin RA, Girlich D, Potron A. Ctx-M-15-producing Shewanella species clinical isolate expressing OXA-535, a Chromosome-Encoded OXA-48 variant, putative progenitor of the plasmid-encoded OXA-436. Antimicrob Agents Chemother. 2018 doi: 10.1128/AAC.01879-17. https://aac.asm.org/content/62/1/e01879-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moussa J, Panossian B, Nassour E, Salloum T, Abboud E, et al. Detailed characterization of an IncFII plasmid carrying blaOXA-48 from Lebanon. J Antimicrob Chemother. 2020;75:2462–2465. doi: 10.1093/jac/dkaa181. [DOI] [PubMed] [Google Scholar]
- 33.Rojas LJ, Hujer AM, Rudin SD, Wright MS, Domitrovic TN, et al. NDM-5 and OXA-181 beta-lactamases, a significant threat continues to spread in the Americas. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Z, Zhang H, Gao Q, Qin J, Zhang C, et al. Increased plasmid copy number contributes to the elevated carbapenem resistance in OXA-232-producing Klebsiella pneumoniae . Microb Drug Resist. 2020;26:561–568. doi: 10.1089/mdr.2018.0407. [DOI] [PubMed] [Google Scholar]
- 35.Sugawara E, Kojima S, Nikaido H. Klebsiella pneumoniae major porins OmpK35 and OmpK36 allow more efficient diffusion of β-Lactams than their Escherichia coli homologs OmpF and OmpC. J Bacteriol. 2016;198:3200–3208. doi: 10.1128/JB.00590-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davin-Regli A, Bolla J-M, James C, Lavigne J-P, Chevalier J, et al. Membrane permeability and regulation of drug ‘influx and efflux’ in enterobacterial pathogens. Curr Drug Targets. september. 2008;9:750–759. doi: 10.2174/138945008785747824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.