Abstract
Increasing antimicrobial resistance and limited alternative treatments have led to fluoroquinolone-resistant Shigella strain inclusion on the WHO global priority pathogens list. In this study we characterized multiple Shigella isolates from Malawi with whole genome sequence analysis, identifying the acquirable fluoroquinolone resistance determinant qnrS1.
Keywords: AMR, FQR, WGS, Shigella
Data Summary
The authors confirm all supporting data, code and protocols have been provided within the article or through supplementary data files. The raw sequencing reads from the eight Shigella and Escherichia coli isolates which were subjected to whole genome sequencing have been deposited in the National Center for Biotechnology Information (NCBI) Short Read Archive under accession numbers: ERR2525592, ERR2525594, ERR2525595, ERR2525596, ERR2525597, ERR2525598, ERR2525599 and ERR252600. The accession numbers for all the reference Shigella and E. coli isolates used in the study are provided in the supplementary table.
Impact Statement.
Antimicrobial resistance is a growing threat to our ability to treat bacterial infections. Multidrug resistance is widespread among Shigella , a leading cause of diarrhoeal death globally. The greatest disease burden from shigellosis is on those living in low- and middle-income nations in sub-Saharan Arica and Asia. Emerging resistance to fluoroquinolone, the recommended first-line treatment, has been identified in Asia but is probably being missed in understudied regions. This study characterizes eight shigellae from Malawi, an area where Shigella has been understudied, looking, in particular, at antimicrobial resistance. All the isolates were found to be multidrug resistant, with one isolate probably resistant to fluoroquinolones. This fluoroquinolone resistance element was found to be carried on a plasmid conferring resistance to several other antimicrobials, indicating a probable threat of dissemination of multidrug resistance in the region. This study highlights the importance of studying all high disease burden regions, to ensure ongoing effective treatment of diarrhoeal diseases.
Introduction
Shigella is the second leading cause of diarrhoeal death globally with the greatest disease burdens seen in low- and middle-income countries [1]. Approximately one-third of these deaths are in children under the age of 5 years, with infection potentially causing chronic health effects [1, 2]. Antimicrobial resistance (AMR) increasingly limits treatment options, threatening to reverse hard won reductions in diarrhoeal mortality in high-burden areas [3]. Meanwhile, vaccines are still in development. There is therefore a need for effective public health solutions.
The increasing prevalence of fluoroquinolone-resistant (FQR) strains and limited, widely effective alternatives to this first-line treatment, has led to these strains being included on the WHO global priority pathogens list [4]. Resistance can be acquired de novo through a double mutation in the gyrA gene (amino acids 83 and 87) of the quinolone resistance determining region (QRDR), with a third mutation in the parC gene (AA80) ameliorating the fitness cost [5]. Resistance can also be acquired through horizontal transmission of FQR genes [5].
Shigella is reported as a leading cause of diarrhoea among hospitalized children in Malawi, but there is little information on the circulating strains [6]. Whole genome sequence analysis (WGSA) has been successfully applied to investigate Shigella epidemiology and AMR determinants, and can greatly aid in disease control in high-burden areas, such as Malawi [7]. Here, we have applied WGSA to characterize Shigella strains and AMR determinants in Malawi. Providing important baseline information for public health interventions including antibiotic treatment and deployment of Shigella vaccines, the development of which is a WHO priority [8].
The study
All biochemically confirmed shigellae collected during a rotavirus vaccine evaluation programme between 2012 and 2015, isolated from faecal samples collected from children under the age of 5 years and hospitalized with acute gastroenteritis at the Queen Elizabeth Central Hospital, Blantyre, Malawi, were subjected to WGS (Supplementary methods) [9]. Species were confirmed with a maximum-likelihood phylogeny of the Shigella / Escherichia coli clade, generated from a core single nucleotide polymorphism (SNP) alignment (40 075 SNPs) using quality trimmed reads mapped to a complete reference genome (Supplementary table). Only those genomically confirmed as Shigella or E. coli (8/10) were included in the study. All Shigella isolates were serotyped in silico with ShigaTyper (v1.0.6, https://github.com/CFSAN-Biostatistics/shigatyper).
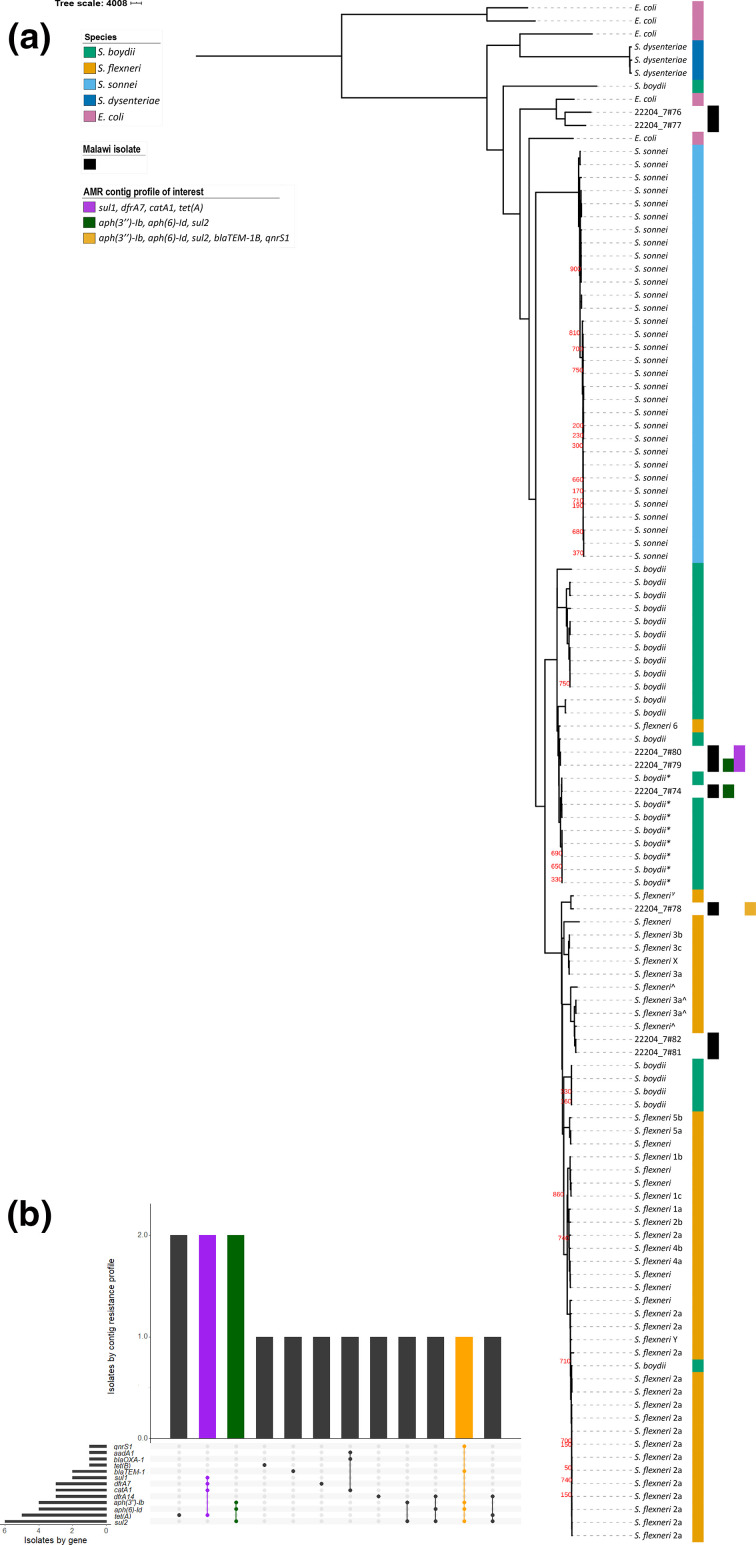
From our eight isolates, four Shigella serotypes were identified: two S. flexneri 3a (Sf3a, Phylogroup 2), one S. flexneri 4av (Sf4av, Phylogroup 7), two S. flexneri 6 (Sf6) and one S. boydii 2 (Sb, Clade 3). Two isolates were of E. coli (Fig. 1a). The lack of Shigella sonnei isolates in our collection was expected as S. sonnei , though highly prevalent globally, is typically associated with high-income nations and industrialization [10]. Prevalence of S. boydii in Africa is thought to be low, isolated in 5.7 % of cases from a multi-national study [11]. However, detection with our small sample size would still be likely by chance (binomial test P=0.375) and may not be due to higher prevalence in Malawi. The strain diversity indicates that several, distinct strains circulate in Malawi; further characterization is needed to aid effective vaccine development for the region.
Fig. 1.
Maximum-likelihood phylogeny of eight isolates from Malawi, contextualized among Escherichia coli and Shigella and highlighting some AMR profiles of interest. AMR profiles of interest are those of contiguous sequences which are multi-drug-resistant and shared across multiple isolates, and in one isolate carries additional AMR genes including an FQR gene (qnrS1). They are indicated by columns to the right of the tree (a) and by coloured bars in the AMR profile chart (b). (a) Columns to the right of the phylogeny indicate, from left to right, species, study isolate and AMR profiles of interest. GTR+G substitution model, 1000 bootstrap validation and mid-point rooted. * S. boydii clade 3, ^ S. flexneri phylogroup 2, γ S. flexneri phylogroup 7. All bootstrap values for internal nodes with support <900 are displayed. Bar, SNPs per site. (b) Intersection of individual AMR genes by isolate and AMR gene profile by contig.
Identification of E. coli among the samples is probably due to the close relatedness between the two species. Shigella is a specialized pathovar of E. coli , sharing a disease phenotype with enteroinvasive E. coli (EIEC), which is highly adapted to humans [12]. To determine whether the E. coli isolates were EIEC we looked for the presence of the mxi-spa locus, found on the large virulence plasmid (pINV). This locus encodes a Type 3 Secretion System and secreted effector proteins which produce the distinctive invasive disease phenotype [12, 13]. Contiguous sequences (contigs) from quality-assessed isolate draft genomes, assembled using Unicycler (v0.4.7) [14], were compared against a reference EIEC pINV to identify locus presence (Supplementary table). Using this approach, one E. coli isolate was identified as EIEC (99 % identity and 100 % coverage with the mxi-spa locus).
As resistance is a growing hinderance to effective treatment, we determined genotypic AMR profiles for each isolate using starAMR (v0.5.1; https://github.com/phac-nml/staramr; Supplementary methods). All Shigella isolates were predicted to be multi-drug-resistant (MDR), carrying genes conferring resistance to three or more drug classes, demonstrating MDR Shigella circulate in Malawi (Table 1). While overall resistance was high, we observed limited diversity in AMR genes and predicted resistance profiles; 13 genes encoded resistance to seven antimicrobial classes (Fig. 1b, Table 1). This suggests that there are treatments which remain effective in Malawi, such as azithromycin, though this would need to be confirmed in a larger study and may change with mass drug administration programmes.
Table 1.
AMR genotypic and predicted phenotypic profiles of Malawian Shigella isolates by contiguous sequence
|
Isolate ID |
Contig length (bp) |
Resistance genes |
Predicted resistance drug class |
|---|---|---|---|
|
22204_7#74 ( S. boydii 2) |
9131* |
tet(A) |
Tetracycline |
|
2798 |
aph(3′′)-Ib, aph(6)-Id, sul2 |
Aminoglycoside, sulfonamide |
|
|
2353 |
dfrA7 |
Trimethoprim |
|
|
1831 |
blaTEM-1B |
Aminopenicillin |
|
|
22204_7#78 ( S. flexneri 4av) |
48 082 |
tet(A) |
Tetracycline |
|
17 334 |
aph(3′′)-Ib, aph(6)-Id, sul2, blaTEM-1B, qnrS1 |
Aminoglycoside, sulfonamide, aminopenicillin, fluoroquinolone |
|
|
1588 |
dfrA14 |
Trimethoprim |
|
|
22204_7#79 ( S. flexneri 6) |
34 138 |
tet(A), sul1, dfrA7, catA1 |
Tetracycline, sulfonamide, trimethoprim, chloramphenicol |
|
6200 |
aph(3′′)-Ib, aph(6)-Id, sul2 |
Aminoglycoside, sulfonamide |
|
|
22204_7#80 ( S. flexneri 6) |
25 733 |
tet(A), sul1, dfrA7, catA1 |
Tetracycline, sulfonamide, trimethoprim, chloramphenicol |
|
6200 |
aph(3′′)-Ib, sul2 |
Aminoglycoside, sulfonamide |
|
|
22204_7#81 ( S. flexneri 3a) |
11 392† |
dfrA14, sul2, tet(A) |
Trimethoprim, sulfonamide, tetracycline |
|
22204_7#82 ( S. flexneri 3a) |
45 377 |
tet(B) |
Tetracycline |
|
8773 |
aadA1, blaOXA-1, catA1 |
Aminoglycoside, aminopenicillin, chloramphenicol |
|
|
6790‡ |
aph(6)-Id, dfrA14, sul2 |
Aminoglycoside, trimethoprim, sulfonamide |
*StarAMR identified IncFIB(K) plasmid.
†StarAMR identified MDR IncQ1 plasmid.
‡Possible multi-copy plasmid.
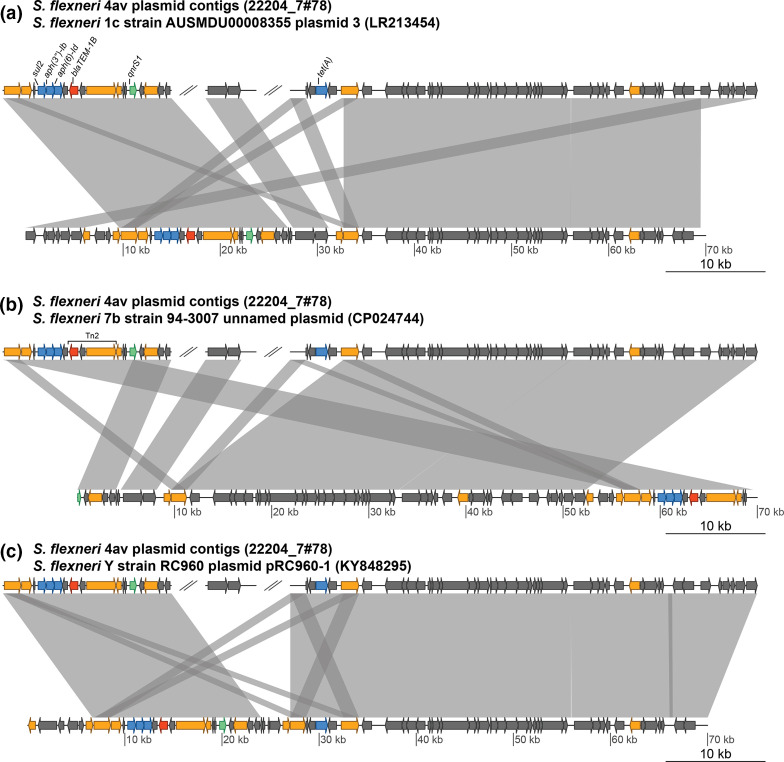
Mobile genetic elements (MGEs) are important drivers of AMR dissemination, so we explored the genetic context of our AMR genes to look for evidence of them being within MGEs [15]. Resistance contigs from two isolates were identified as likely plasmid contigs, also using starAMR (Supplementary methods): one Sf3a isolate (22204_7#81) carried an MDR IncQ1 plasmid, and the Sf4av isolate carried a tet(A) encoding IncFIB(K) plasmid (Table 1). This contig was probably part of a MDR plasmid in combination with two other contigs, discussed below (Fig. 2). Another MDR contig had a read depth 5.29-fold higher than the chromosomal contigs, providing possible evidence of being part a multi-copy plasmid (Table 1). The same AMR gene profile (aph(3′′)-Ib, aph(6)-Id and sul2) was identified in contigs from multiple isolates (22204_7#74, 22204_7#78 and 22204_7#79) across distinct phylogroups (Sb2 clade 3, Sf4av Phylogroup 7 and Sf6 respectively), possibly indicating an MGE (Fig. 1, Table 1). A comparison of these contigs showed that they had similarity (≥99 % sequence identity) to only the AMR gene-encoding region (2620–3184bp) (Supplementary methods). In one case (Sb2), however, this was the entire contig. The limited similarity across the whole contigs, and the presence of insertion sequences and transposase genes surrounding the AMR gene-encoding region in the largest of these contigs (Sf4av), suggests that these genes are more likely to have been spread via transposition than carriage on plasmids (Fig. 2b). One AMR gene (blaTEM-1B) was identified as being carried within a Tn2 transposon (Fig. 2b). Together, our data support a role for MGEs in the spread of MDR Shigella in Malawi.
Fig. 2.
Pairwise comparisons of S. flexneri 4av study isolate plasmid contigs against previously identified MDR plasmids. Pairwise comparisons of the S. flexneri 4av isolate plasmid contigs (upper sequence) against the previously identified plasmids (lower sequences). Transposase genes are shown in orange and AMR genes in blue, except the FQR gene qnrS1 which is in green and β-lactamase gene blaTEM-1B which is in red and is part of a Tn2 transposon. (a) Comparison against the S. flexneri 1c, strain AUSMDU00008355 plasmid 3, which lacks the tet(A) gene. (b) Comparison against the S. flexneri 7b, strain 94-3007 unnamed plasmid, which also lacks a tet(A) gene. (c) Comparison against the S. flexneri Y, strain RC690 plasmid pRC960-1.
To predict FQR, we looked for the presence of either point mutations in the QRDR, or FQR-associated genes. Point mutations were identified by comparing the gyrA and parC amino acid sequences across all isolates, with amino acid identity at resistance-associated sites confirmed as expected based on the literature. One isolate (Sf4av) was predicted to be FQR, due to the presence of qnrS1 (Table 1). A mutation at parC R91Q was also identified, but the same variation was present in all isolates, E. coli and Shigella , and probably represents natural variation rather than a resistance adaptation. Unfortunately, phenotype data were unavailable to confirm these findings.
Detection of qnrS1 indicates acquirable FQR is present among Shigella in Malawi. As this gene is typically carried on a plasmid, a blast comparison of the qnrS1-containing contig (17 334 bases) against the NCBI nt database was performed [5]. Three top equivocal hits against S. flexneri plasmids (KY848295.1, CP024474.1, LR213454.1) were found (e-score=0.0, query sequence coverage and identity ≥99.9 %), suggesting that this gene might be carried on a related plasmid. To investigate this possibility, the contigs of the draft genome were compared (by blast) to identify other contigs which probably belong to this plasmid, and three contigs were identified in total (e-value=0.0) (Supplementary methods). Together, the contigs showed high sequence similarity and sequence coverage against all three plasmids, which were themselves highly similar to each other (Fig. 2). It is likely that our isolate was carrying a plasmid very similar to these other previously identified plasmids. This is supported by the isolate sequence read mapping coverage (mean depth=53 reads) to the S. flexneri 1c strain AUSMDU00008355 plasmid 3 (LR213454) (Supplementary methods). The probable presence of an qnrS1-carrying plasmid means a high risk of widespread FQR in Malawi and neighbouring regions; further study into the prevalence and nature of FQR in the region is needed.
All four of these highly similar plasmids are MDR, encoding sul2, aph(3′′)-Ib, aph(6)-Id, blaTEM-1B and qnrS1 genes. Our isolate and one other also carry an additional resistance gene tet(A) (Fig. 2). Each was identified in a different S. flexneri serotype (1c, Y, 7b and 4av), suggesting the dissemination of this MDR plasmid across multiple lineages. They were also identified across multiple continents [specifically in Australia, China, America (unpublished) and Malawi] [16–18]. This suggests the plasmid has been successfully horizontally transmitted between strains and spread intercontinentally. It also provides evidence for an interaction between MDR Shigella globally and MDR Shigella in Malawi, though further research is needed to characterize this.
Conclusions
Together, the high proportion of MDR Shigella, the acquirable FQR and the MDR plasmids detected in this study show that, without intervention, controlling shigellosis in Malawi will be increasingly difficult, which will probably have global consequences. We highlight the importance of further research into the epidemiology of Shigella in the region to ensure effective disease control.
Funding information
This work was funded by a studentship from the MRC Discovery Medicine North (DiMeN) Doctoral Training Partnership (MR/N013840/1), an MRC NIRG award (MR/R020787/1), a Wellcome Trust Clinical Research Career Development Award (106690/A/14/Z) and Wellcome Trust Grant number 098051. Kate Baker and Nigel Cunliffe are affiliated to the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Gastrointestinal Infections at the University of Liverpool in partnership with Public Health England (PHE), in collaboration with the University of Warwick. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health and Social Care or Public Health England. AKC was supported by an Australian Research Council (ARC) DECRA fellowship (DE180100929).
Acknowledgements
The authors would like to acknowledge the technical assistance of Trevor Wilson and Chikondi Jassi, and the bioinformatic assistance of Caisey Pulford and Lewis Fisher. The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the institutions with which the authors are affiliated.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
Ethical approval was obtained from the National Health Sciences Research Committee, Lilongwe, Malawi (#867), and the Research Ethics Committee of the University of Liverpool, Liverpool, UK (000490).
Footnotes
Abbreviations: AA, amino acid; AMR, antimicrobial resistance; EIEC, enteroinvasive E. coli; FQR, fluoroquinolone-resistant; MDR, multi-drug-resistant; MGE, mobile genetic element; NCBI, National Centre for Biotechnology Information; pINV, invasion plasmid; QRDR, quinolone resistance determining region; Sb, Shigella boydii; Sf, Shigella flexneri; SNP, Single Nucleotide Polymorphism; WGS, Whole genome sequencing; WGSA, whole genome sequencing analysis.
References
- 1.Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A. Morbidity and mortality due to Shigella and enterotoxigenic Escherichia coli diarrhoea: the global burden of disease study 1990–2016. Lancet Infectious Disease. 2018;2018:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C, Forouzanfar M, Rao PC, lI K, Brown AE, et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the global burden of disease study 2015. Lancet Infect Dis. 2017;17:909–948. doi: 10.1016/S1473-3099(17)30276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu B, Cao Y, Pan S, Zhuang L, Yu R, et al. Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Shigella between Europe-America and Asia-Africa from 1998 to 2009. Int J Antimicrob Agents. 2012;40:9–17. doi: 10.1016/j.ijantimicag.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 4.WHO Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO press. 2017;7 [Google Scholar]
- 5.Nüesch-Inderbinen M, Heini N, Zurfluh K, Althaus D, Hächler H, et al. Shigella antimicrobial drug resistance mechanisms, 2004-2014. Emerg Infect Dis. 2016;22:1083–1085. doi: 10.3201/eid2206.152088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iturriza-Gomara M, Jere KC, Hungerford D, Bar-Zeev N, Shioda K, et al. Etiology of diarrhea among hospitalized children in Blantyre, Malawi, following rotavirus vaccine introduction: a case-control study. J Infect Dis. 2019;220:213–218. doi: 10.1093/infdis/jiz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker KS, Campos J, Pichel M, Della Gaspera A, Duarte-Martinez F, et al. Whole genome sequencing of Shigella sonnei through PulseNet Latin America and Caribbean: advancing global surveillance of foodborne illnesses. Clin Microbiol Infect. 2017;23:845–853. doi: 10.1016/j.cmi.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Draft who preferred product characteristics for vaccines against Shigella . WHO press. 2020 [Google Scholar]
- 9.Bar-Zeev N, Kapanda L, Tate JE, Jere KC, Iturriza-Gomara M, et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis. 2015;15:422–428. doi: 10.1016/S1473-3099(14)71060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AKM. Shigellosis. The Lancet. 2018;391:801–812. doi: 10.1016/S0140-6736(17)33296-8. [DOI] [PubMed] [Google Scholar]
- 11.Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis. 2014;59:933–941. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan R, Lumb B, Ryan D, Reeves PR. Molecular evolution of large virulence plasmid in Shigella clones and enteroinvasive Escherichia coli . Infect Immun. 2001;69:6303–6309. doi: 10.1128/IAI.69.10.6303-6309.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeder GN, Hilbi H. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev. 2008;21:134–156. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker KS, Dallman TJ, Field N, Childs T, Mitchell H, et al. Horizontal antimicrobial resistance transfer drives epidemics of multiple Shigella species. Nat Commun. 2018;9:1462. doi: 10.1038/s41467-018-03949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingle DJ, Easton M, Valcanis M, Seemann T, Kwong JC, et al. Co-circulation of multidrug-resistant Shigella among men who have sex with men in Australia. Clin Infect Dis. 2019;69:1535–1544. doi: 10.1093/cid/ciz005. [DOI] [PubMed] [Google Scholar]
- 17.Liang B, Roberts AP, Xu X, Yang C, Yang X, et al. Transferable Plasmid-Borne mcr-1 in a Colistin-Resistant Shigella flexneri isolate. Appl Environ Microbiol. 2018;84:e02655–17. doi: 10.1128/AEM.02655-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder MR, Juieng P, Batra D, Knipe K, Rowe LA, et al. High-Quality complete and draft genome sequences for three Escherichia spp. and three Shigella spp. generated with Pacific biosciences and illumina sequencing and optical. Mapping Genome Announcements. 2018;Ja;6:e01384–17. doi: 10.1128/genomeA.01384-17. [DOI] [PMC free article] [PubMed] [Google Scholar]