Abstract
Background:
Cannabis use is common amongst emerging adults and increasingly linked to negative mood and neurocognitive performance. Aerobic fitness, however, may be positively linked. Therefore we assess the potential moderating influence of aerobic fitness on affective and behavioral functioning associated with cannabis.
Methods:
After 3-weeks of abstinence, 83 16-26 year-olds (38 cannabis, 45 controls) completed self-report inventories (BDI-II, STAI-state, FrSBe, BIS/BAS), an objective emotion functioning measure (PennCNP), and VO2 max testing. Multiple regressions assessed symptoms from past year cannabis use, VO2 max, and cannabis*VO2, controlling for alcohol, cotinine, gender, and BMI.
Results:
Past year cannabis use was associated with increased depressive symptoms (p=.04), BIS/BAS component (p=.002), and emotion recognition (p=.045).
Conclusions:
Results suggest a robust association between past year cannabis use and depressive symptoms and behavioral and affective functioning. Aerobic fitness, however, did not moderate these relationships. Efforts should be made to inform the public of concerns regarding the potential negative impact of cannabis on mood.
Keywords: Cannabis, mood, depression, behavioral approach, VO2 max, aerobic fitness
Introduction
Cannabis use is common in emerging adults, with 45% of 12th graders and 60% of young adults using in their lifetime (Schulenberg et al., 2018). Regular cannabis use downregulates the endogenous cannabinoid system (eCB) (Hirvonen et al., 2012), which is key to cognitive control and emotional functioning (Egerton, Allison, Brett, & Pratt, 2006; Lee & Gorzalka, 2012). Cannabis use has also been associated with greater mood and behavioral difficulties (see Levine, Clemenza, Rynn, & Lieberman, 2017). It is also known that exercise and aerobic fitness level have positive health outcomes, such as increasing positive mood (Cotman, Berchtold, & Christie, 2007) and improving executive functioning and task switching (Hillman, Erickson, & Kramer, 2008; Themanson, Hillman, & Curtin, 2006).
Exercise and relatedly, aerobic fitness, influences cannabinoid receptor 1 (CB1) and endocannabinoid (eCB) mechanisms. Ferreira-Vieira and colleagues (2014) found that one week of exercise on a treadmill in rats upregulated CB1 activity in the hippocampus, which is a key region for both memory and emotion regulation in the limbic system. In humans, aerobic exercise (AE) has been linked to increased circulating endogenous cannabinoid concentrations (Koltyn, Brellenthin, Cook, Sehgal, & Hillard, 2014), and increased eCB as a result of exercise has been shown to increase mood (Brellenthin, Crombie, Hillard, & Koltyn, 2017). Measurement of one’s capacity for AE can be calculated through VO2 max (aka maximal oxygen consumption; Bassett & Howley, 2000), as it indicates the highest amount of oxygen that an individual can consume in order to make energy aerobically. VO2 max also suggests overall fitness level as it provides information on multiple systems, including ventilatory, cardiovascular, hematologic, and muscular systems, and can be improved through AE (e.g., running, walking, biking; Bassett & Howley, 2000). Therefore, the more aerobic activity a person engages in, the higher their VO2 max will be. As exercise and, by conjunction, aerobic fitness may moderate eCB functioning, it is important to consider as a potential influence on the effects of cannabis use.
Though the potential benefits of aerobic exercise and, accordingly, fitness are already at least partially known, and though regular THC use may downregulate CB1 receptor functioning (Hirvonen et al., 2012), scant research has connected the two. A longitudinal study (Henchoz et al., 2014) had young adult males self-report their frequency of exercise and complete a substance use screen. They found fewer cannabis use disorder (CUD) diagnoses and better mental health outcomes in those who maintained regular exercise habits, and lower prevalence of CUD in those who adopted new exercise routines. Another study of adult non-treatment seeking individuals with CUD found reduced cannabis use and craving following two weeks of aerobic exercise (Buchowski et al., 2011). As such exercise regimens can improve aerobic fitness (Bassett & Howley, 2000), these studies need to be supplemented by further research into the potential moderating influence of aerobic fitness on functioning in cannabis users. Indeed, we recently showed a relationship between aerobic fitness level, cannabis use, and objective cognitive functioning (Wade, Wallace, Swartz, & Lisdahl, 2019), but the impact on affective and behavioral functioning is not yet known.
The present study seeks to replicate and expand previous findings of increased depressive symptoms and decreased behavioral approach in cannabis users (Wright, Scerpella, & Lisdahl, 2016). We also aim to explore aerobic fitness level as a possible moderator of affective and behavioral functioning, given its ability to influence underlying cannabinoid receptor mechanisms (Ferreira-Vieira et al., 2014). We hypothesize that greater cannabis use would again be associated with greater depressive symptoms and disinhibition, and decreased behavioral approach and affective processing. We further hypothesize that cannabis use would interact with aerobic fitness, such that individuals with greater past-year cannabis use and higher VO2 max would report better affective and behavioral functioning than less fit cannabis users.
Methods
Participants.
Participants were recruited for the present analysis from the larger cross-sectional parent imaging study (PI: Lisdahl, R01 DA030354; Wade et al., 2019). Eighty-three participants (38 cannabis users, 45 controls) were recruited through local newspaper advertisements and fliers placed around Milwaukee, Wisconsin. Groups were used only for the selection of covariates. Inclusion criteria included being a fluent English speaker 16-26 years old. Participants were considered cannabis users if they had smoked more than 52 times in the past year, as this represents average weekly cannabis use, which is generally considered to confer risk (Casajuana et al., 2016). Healthy controls had smoked cannabis less than 10 times in the past year. Exclusion criteria for both groups included: being left handed, MRI contraindications, past year co-morbid independent Axis-I disorders, major medical or neurologic disorders, prenatal issues (e.g., gestation <35 weeks) or prenatal alcohol (>4 drinks/day or >7 drinks/week) or illicit drugs (>10 uses), or excessive drug use in lifetime (>50 uses of any drug category except nicotine, alcohol, or cannabis). Participants were required to remain abstinent from alcohol and drug use for 21 days prior to the final session, as confirmed through self-report and drug toxicology screen. The University of Wisconsin Milwaukee and Medical College of Wisconsin IRBs approved all aspects of this study.
Procedure.
Initial Screening. Interested participants called the lab study line in response to community advertisements. Following verbal consent of the participant, or consent of the parent/guardian and assent of the participant if the participant was under 18, study staff completed a 5-10 minute phone screen with each parent and participant separately to determine basic non-sensitive eligibility (including age, ethnicity, MRI contraindications, and yes/no questions regarding psychiatric and substance use history).
Detailed Screening.
Following initial screening, written consent/assent for study was obtained from participants and parents via mail. A 45-minute detailed phone screen was then scheduled and conducted. Participants completed the Customary Drinking and Drug Use Record (CDDR; Brown et al., 1998; Stewart & Brown, 1995) for lifetime use history. Participants and their parents separately completed youth psychiatric history though the Mini International Psychiatric Interview (MINI; Sheehan et al., 1998). If ineligible, youth and their parent were paid $20 for completion of the detailed screen; they were not informed of reason for ineligibility. Eligible participants were scheduled for session visits.
Study Sessions.
Eligible participants attended five study sessions over the course of four weeks. The first three sessions each occurred one week apart and consisted of urinary drug analysis and a brief neuropsychological battery. Session 4 occurred one week later and included self-report measures, a 3-hour neuropsychological battery and VO2 maximum testing. Session 5 was conducted within 24-48 hours of Session 4 and consisted of MRI scanning. Data from objective and self-report measures and VO2 max testing in Session 4 are included in the present study.
Verifying Abstinence.
As participants were expected to remain abstinent from all alcohol and drugs (other than tobacco) throughout the course of the study, abstinence was evaluated at each session through urine toxicology. The ACCUTEST SplitCup 10 Panel drug test measures amphetamines, barbiturates, benzodiazepines, cocaine, ecstasy, methadone, methamphetamines, opiates, PCP, and THC. Urine samples were also tested using NicAlert to test cotinine level, a metabolite of nicotine. Participants also wore PharmChek Drugs of Abuse Patches which continuously monitor sweat toxicology for the presence of cocaine, benzoylecgonine, heroin, 6MAM, morphine, codeine, amphetamines, methamphetamine, THC, and phencyclidine. Participants underwent breathalyzer screens to test for alcohol use at the start of each session. If positive for THC at Sessions 1-3, participants were allowed to remain in the study if their THC level on the PharmChek patch went down over time. If positive for any drug or having a breath alcohol concentration greater than .000 at the start of Session 4 (neuropsychology battery and VO2 max) or Session 5 (MRI scan), participants were deemed ineligible for study participation. This time period was selected to allow for cannabis withdrawal symptoms to subside (Budney & Hughes, 2006; Schlienz, Budney, Lee, & Vandrey, 2017), as a means of examining the chronic effects of cannabis even after abstinence.
Drug Use.
Cannabis, alcohol, and other drug use were measured using a modified version of the Timeline Follow-Back (TLFB; Lisdahl & Price, 2012; Sobell & Sobell, 1992). Utilizing memory cues of common holidays and personal events, participants recounted frequency of drug use over the past year (assessed month-by-month for one year). Additionally, a semi-structured interview was administered to measure frequency/quantity of lifetime drug use (Lisdahl & Price, 2012). For each drug category (e.g., alcohol, cannabis, stimulants, hallucinogens), participants were asked their average weekly use for each year of use. The participant’s drug use was measured in standard units (joints for cannabis; standard drinks for alcohol).
Measurement of VO2 max.
Participants were asked to refrain from food and caffeine for 4 hours prior to the exercise tests. Body height and mass were measured using standard procedures (Pescatello, 2014). Prior to each exercise test, the metabolic measurement system, ParvoMedics TrueOne 2400 (ParvoMedics, Salt Lake City, UT) was calibrated according to the manufacturer’s instructions using a 3 Liter syringe for the pneumotachometer, and a two-point calibration for the gas analyzers (room air and a certified gas 4.08% CO2, 115.98% O2, balance N2).
Participants were fitted with the rubber mouthpiece connected to a Hans Rudolf 2700 series two-way nonrebreathing valve (Kansas City, MO), noseclip, and heart rate strap (Polar Wearlink 31, Finland) for the collection of expired gases and measurement of heart rate. Participants completed a maximal incremental exercise test on a treadmill (Full Vision Inc., TMX425C Trackmaster, Newton, KS) following the Bruce Protocol until volitional fatigue. Expired gases were measured continuously using a ParvoMedics TrueOne 2400 metabolic measurement system (ParvoMedics, Salt Lake City, UT). Criteria for determination of attainment of VO2max were based on those recommended by Howley et al (1995). Metabolic data were averaged over 1 minute and exported into a spreadsheet for analysis.
Self-Report Symptom Inventories.
Anxiety. The State-Trait Anxiety Inventory (STAI; Spielberger, 1983) State subscale measures temporary or “state” anxiety. Total raw STAI-state was used. Depression. Depression was measured by the Beck Depression Inventory—2nd Edition (BDI; Beck, Steer, & Brown, 1996). The BDI is a 21-item measure of depressive symptoms over the past 2 weeks. Total raw BDI score was used. Self-Reported Executive Functioning. Executive functioning in day-to-day life was measured by the 46-item Frontal Systems Behavioral Scale (FrSBe; Grace & Malloy, 1999). As this measure was designed to assess daily functioning before and after an acute illness or neurological illness, participants only filled out the “after” portion to report on their current levels of functioning. Based on our prior findings (Wright et al., 2016), only the Disinhibition subscale was used in the present analysis. Impulsivity/Behavioral Approach. The Behavioral Inhibition System and Behavioral Approach System (BIS/BAS; Carver & White, 1994) is a 30-item inventory measuring approach and avoidance behaviors of moving towards or away from appetitive or unpleasant stimuli, respectively, with increased BAS and decreased BIS scores being related to increased impulsivity and response to reward cues, while decreased BAS may be indicative of more apathy and decreased response to positive reinforcement. This measure was reduced using a principal components analysis (see Data Analysis below).
Objective Measure of Affective Processing.
Participants completed an affective processing battery (PennCNP: https://penncnp.med.upenn.edu/about.html). Based on prior work (Maple et al., Under Review), an Emotion Recognition and Emotion Discrimination task were included in the present analyses. For the Emotion Regulation task (Gur et al., 2002; Pinkham et al., 2008), participants were instructed to select which emotion each presented face was expression (happy, sad, angry, fearful, or neutral. Variables of measurement included median response time for correct responses for each expressed emotion. For the Emotion Discrimination task (Erwin et al., 1992), participants were presented with two faces that demonstrated subtle variation in emotional facial expression intensity. Participants decided which face demonstrated the expressed emotion with the greatest intensity, or whether they were equally valenced. Variables of measurement included median response time for happy correct, sad correct, and total (happy+sad) correct.
Data Analysis.
To determine selection of covariates, two groups were formed: a cannabis user group with at least an average of one joint per week over the past year, and a control group with less than 10 joints in the past year and no more than 25 joints lifetime; groups were not used in the primary analyses. Between-group differences on demographic and fitness variables were measured with between group t-tests and χ2 tests; variables that differed by group were then used in dose-dependent analyses. In addition, BMI was covaried for due to prior research suggesting higher BMI negatively influences aspects of cognition and emotional processing (Cheke, Simons, & Clayton, 2016; Kennedy, Collins, & Luciana, 2016), and as fitness groups differ by BMI. After controlling for potential confounds (i.e., recent nicotine exposure as measured by cotinine, past year alcohol use, gender, and BMI) in the first block, multiple regressions were run to examine whether past year cannabis use or VO2 max independently related to mood or functioning symptoms. The potential interactive effect of cannabis use and VO2 max was also assessed as a final block in the regression, as a cannabis*VO2 interaction variable was created using the continuous data. Mood and functioning subscales were selected based on prior findings (for depressive symptoms, anxiety symptoms, FrSBe disinhibition; Wright et al., 2016) and through a PCA (for BIS/BAS and PennCNP) to reduce the number of comparisons. All interpretations of statistical significance were made if p<.05.
Three principal components analyses (PCAs) with oblimin rotation were conducted to reduce variables from the measures with multiple variables (BIS/BAS, PennCNP Emotion Recognition, and PennCNP Discrimination). Components were created when eigenvalues > 1.0 and variables with loadings ≥ 0.4 were considered to define a component. Emotion Recognition. The Emotion Recognition median response time variables loaded onto one component (factor loadings: Sad=.75, Happy=.66, Neutral=.65, Anger=.61, Fear=.48) and accounted for 9.47% of the variance. Emotion Discrimination. For the Emotion Discrimination task, three variables loaded onto one component (correct happy and correct sad=.99, correct happy trials=.97, and correct sad trials=.82) and accounted for 27.84% of the variance.
Results
Demographics by Cannabis Grouping.
Drug groups did not differ significantly by age [t(81)=−1.06, p=.29], education [t(81)=.73, p=.47], reading level (from the WRAT-IV) [t(80)=.79, p=.43], race [χ2=5.94, p=.43], or ethnicity [χ2=3.19, p=.20], They marginally differed by gender [χ2=3.97, p=.05], which was therefore included as a covariate in all analyses (see Table I). Drug Use Patterns. As would be expected, drug groups differed significantly in drug use patterns, such as cotinine level [t(80)=−3.56, p=.001], past year alcohol use [t(81)=−4.15, p<.001], and past year cannabis use [t(81)=−6.59, p<.001], Fitness Characteristics. Drug groups did not differ by VO2 max [t(80)=−1.11, p=.27], average moderate-to-vigorous physical activity [t(67)=.39, p=.70], weight [t(75)=. 14, p=.89], height [t(71)=−.25, p=.81], or BMI [t(78)=.37, p=.71]. In addition, VO2 max performance was significantly correlated with average daily physical activity (r=.43, p<.001).
Table I.
Demographics, Substance Use, and Fitness by Substance Group.
| Controls (n=45) % or M (SD) Range | Cannabis (n=38) % or M (SD) Range | |
|---|---|---|
| Age | 20.89 (2.72) 16-25 |
21.47 (2.20) 17-26 |
| Education | 14.24 (2.30) 9-19 |
13.91 (1.62) 11-18 |
| Reading Score (WRAT-IV) | 106.13 (10.12) 87-133 |
104.13 (12.78) 72-133 |
| Gender (% female) | 53% | 32% |
| % Hispanic | 11% | 21% |
| % Caucasian | 57% | 58% |
| *Past Year Cannabis Use (joints) | 0.56 (1.45) 0-6.67 |
421.68 (429.12) 53-2306 |
| *Past Year Alcohol Use (standard drinks) | 110.53 (177.16) 0-698.50 |
320.71 (279.57) 0-1120.50 |
| *Cotinine Level | 1.09 (.56) 0-3 |
2.08 (1.77) 0-6 |
| VO2 Max Peak Performance (ml/kg/min) | 41.52 (10.65) 20.80-62.90 |
43.98 (9.05) 25.80-62.80 |
| Average Minutes Moderate-to-Vigorous Activity Per Day | 36.86 (23.42) 6.50-110.33 |
39.00 (21.73) 5.83-107.80 |
| Weight (lb) | 151.25 (24.89) 108.8-227.8 |
150.42 (28.11) 103.4-213.4 |
| Height (inches) | 66.89 (3.60) 60.50-76.50 |
67.10 (3.95) 69.0-75.5 |
| BMI | 23.90 (4.39) 17.4-39.1 |
23.54 (4.24) 16.4-33.6 |
Notes: M = mean; SD = standard deviation.
p<.05
Demographics by Fitness Grouping.
For descriptive purposes only, high-fit and low-fit groups were formed by those who were above the 50th percentile in VO2 max by age (Pescatello, 2014), and those who were below the 50th percentile in VO2 max (see Table II). Demographics. Fitness groups did not differ by ethnicity [χ2=3.09, p=.21], race [χ2=7.25, p=.30], age [t(80)=.39, p=.70], level of education [t(80)=1.30, p=.20], estimated IQ [t(80)=−1.73, p=.09]; they differed significantly by gender [χ2=30.21, p<.001]. Drug Use. Fitness groups differed in cotinine level [t(80)=−2.26, p=.03] and past year alcohol use [t(76)=−3.05, p=.003]; they did not differ in past year cannabis use [t(80)=.04, p=.97]. Fitness Characteristics. As would be expected, fitness groups differed in VO2 max [t(80)=−13.05, p<.001] and average moderate-to-vigorous physical activity [t(67)=−4.39, p<.001], as well as by height [t(80)=−7.19, p<.001] and BMI [t(79)=2.86, p=.005]; they did not differ by weight [t(75)=−.59, p=.56]. As calculation of BMI includes height, BMI alone was used as a covariate in all analyses.
Table II.
Demographics, Substance Use, and Fitness Characteristics by Fitness Level.
| Low Fit (n=38) % or M (SD) Range | High Fit (n=44) % or M (SD) Range | |
|---|---|---|
| Age | 21.24 (2.50) 16-26 |
21.02 (2.51) 16-25 |
| Education | 14.37 (1.94) 10-18 |
13.80 (2.03) 9-19 |
| Reading Score (WRAT-IV) | 102.92 (11.23) 72-133 |
107.23 (11.23) 90-133 |
| *Gender (% female) | 76% | 16% |
| % Caucasian | 50% | 73% |
| % Hispanic | 21% | 11% |
| Past Year Cannabis Use (joints) | 192.56 (438.00) 0-2306 |
189.59 (278.26) 0-1394 |
| *Past Year Alcohol Use (standard drinks) | 117.16 (172.43) 0-800 |
279.17 (285.27) 0-1120.50 |
| *Cotinine Level | 1.18 (.77) 0-3 |
1.84 (1.64) 0-6 |
| *VO2 Max Peak Performance (ml/kg/min) | 33.82 (5.43) 20.80-42.40 |
50.23 (5.88) 42.90-62.90 |
| *Average Minutes Moderate-to-Vigorous Activity Per Day | 26.51 (14.75) 5.83-65.71 |
47.67 (23.58) 13.86-110.33 |
| Weight (lb) | 149.07 (29.37) 103.4-227.8 |
152.59 (23.01) 108.2-190.8 |
| *Height (inches) | 64.45 (2.35) 59-70 |
69.14 (3.38) 61-76.50 |
| *BMI | 25.12 (5.15) 18.9-39.1 |
22.49 (2.88) 16.4-27.2 |
Notes: M = mean; SD = standard deviation.
p<.05
Clinical Elevations.
We examined the percentage of controls and cannabis users who demonstrated clinical elevations on each of the anxiety, depressive and the disinhibition scales (see Table III), though it is important to note that no participants met criteria for past-year independent psychiatric diagnoses. Five percent of cannabis users and no controls reached clinical threshold for depressive symptoms (>14 raw score on the BDI-II; χ2=2.49, p=.11). In the cannabis group, three individuals (8%) reached a clinical threshold for anxiety (>39 raw score on STAI-State; Spielberger, 1983) while, in the control group, 4% of healthy controls reached threshold (χ2=.48, p=.49). For self-reported disinhibition, elevations in symptoms were defined as a T-score above 65 (Grace & Malloy, 1999). Thirty-one percent of cannabis users and 20% of controls were elevated in their disinhibition scores (χ2=1.20, p=.27).
Table III.
Clinical Levels of Symptom Scales
| Cannabis Users | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | Range | Elevated Score* | M | SD | Range | Elevated Score* | |
| BDI-II | 5.32 | 4.60 | 0-19 | 5% | 2.78 | 3.31 | 0-11 | 0% |
| STAI-State | 27.70 | 6.99 | 20-47 | 8% | 26.20 | 6.21 | 20-42 | 4% |
| Disinhibition+ | 30.72 | 6.60 | 19-45 | 31% | 26.44 | 6.98 | 15-43 | 20% |
Disinhibition is a subscale from the Frontal Systems Behavior Scale (FrSBe); raw scores are presented.
Elevated scores denote the percentage of individuals who demonstrated scores above 65 T-score on the FrSBe scales, raw scores >39 on STAI-State, and raw scores ≥14 on the BDI-II.
Primary Analyses.
Multiple regressions were run to investigate whether past year cannabis use, VO2 max, or cannabis*VO2 interactions predicted participant’s affective and behavioral functioning, while controlling for cotinine level, past year alcohol use, gender, and BMI.
Cannabis Use Results.
Past year cannabis use was significantly associated with increased depressive symptoms [beta=.31, t=2.35, p=.02; see Figure 1]. On the BIS/BAS component, overall scores were significantly positively associated with past year cannabis use [beta=.42, t=3.17, p=.002], suggesting greater approach tendencies toward appetitive stimuli with greater cannabis use. Past year cannabis use positively related to the emotion recognition component [beta=.33, t=2.04, p=.045], as it took cannabis users longer on average to respond to queries.
Figure 1.
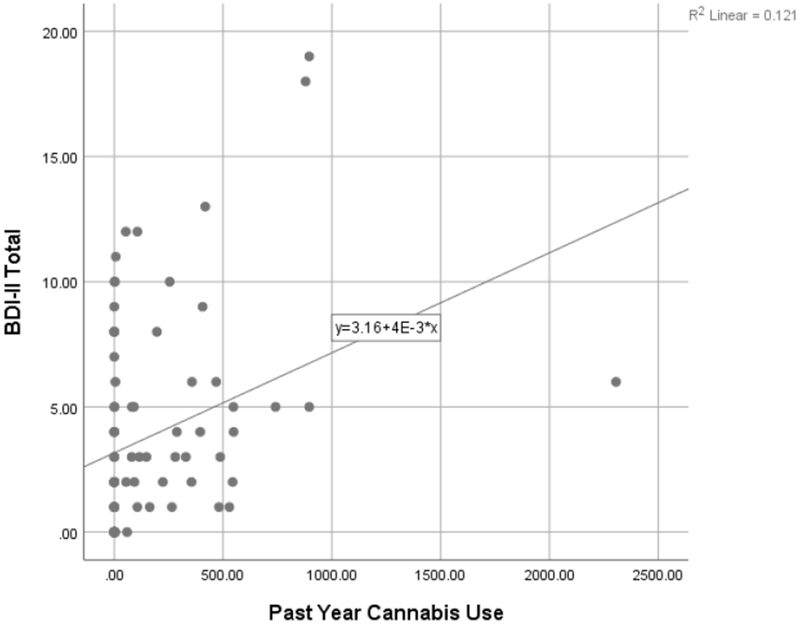
Self-reported depressive symptoms by past year cannabis use.
VO2 Max Results.
There were no significant relationships between VO2 Max interactions and any assessed variables.
Cannabis*VO2 Results.
There were no significant relationships between cannabis*VO2 interactions and any assessed variables.
Covariate Results.
Past year alcohol use, cotinine level, gender, and BMI were not significantly related to any measures.
Discussion
The present study replicated and extended prior findings regarding the relationship between cannabis use and psychological symptomatology (Wright et al., 2016). In particular, we found that cannabis use was associated with increased depressive symptoms and slower emotion recognition. However, in contrast to our prior findings, the current results suggest an opposite pattern on the BIS/BAS scales, with cannabis use being related to increased drive, reward responsiveness, fun-seeking, and behavioral approach system total. Contrary to our hypothesis, we did not find that aerobic fitness level was independently associated with any variables, nor that aerobic fitness interacted with cannabis use in predicting self-reported mood or behavioral functioning.
Given the number of times cannabis use has been associated with increased depressive symptoms in prior studies (Medina, Nagel, Park, McQueeny, & Tapert, 2007; Medina & Shear, 2007; Troup, Andrzejewski, Braunwalder, & Torrence, 2016; Wright et al., 2016), evidence of cannabis use increasing depressive symptoms in a rat model (Khadrawy, Sawie, Abdel-Salam, & Hosny, 2017), the links between cannabis use and onset of major depressive disorder (Chen, Wagner, & Anthony, 2002; Lev-Ran et al., 2014; van Laar, van Dorsselaer, Monshouwer, & de Graaf, 2007), and the perceived benefit of using cannabis to cope with psychological distress and depressions symptoms (Moitra, Christopher, Anderson, & Stein, 2015; Patrick, Schulenberg, O’Malley, Johnston, & Bachman, 2011), this negative relationship between cannabis use and depressive symptoms needs to be better communicated to lay audiences. There is a significant public health concern that individuals are using cannabis as a means to cope, while in reality cannabis use may be leading to worsening depressive symptoms. However, given the cross-sectional nature of the present results, it is not possible to determine directionality between cannabis use and depressive symptoms. Future longitudinal studies (such as the Adolescent Brain Cognitive Development [ABCD] Study, https://abcdstudy.org/) will be beneficial in assessing causal relationships.
In addition, affective processing more broadly appears to be impacted in relation to cannabis use. Our results suggest that it takes adolescents and young adults with more past-year cannabis use longer to recognize facial emotions. This is consistent with prior work of slowed affect recognition in adult cannabis users (Platt, Kamboj, Morgan, & Curran, 2010) and altered neural response to affective facial and picture cues in adolescents and young adults (Spechler et al., 2015; Wesley, Lile, Hanlon, & Porrino, 2016). Given the fact that adolescence is a critical period for maturation of brain regions associated with affective processing (Yurgelun-Todd, 2007), it is important to limit any factor (e.g., cannabis) that may be impairing emotional development.
The findings regarding behavioral approach are intriguing and in contrast to our previous results (Wright et al., 2016), though results are consistent with other groups (Kim-Spoon et al., 2016; Prince van Leeuwen, Creemers, Verhulst, Ormel, & Huizink, 2011). Carver and White (1994) proposed that the BIS/BAS, and specifically the BAS scale, may most aptly be described as a measure of reward response, while others (Taubitz, Pedersen, & Larson, 2015) have suggested the BIS/BAS to be reflective of positive and adaptive functioning. Perhaps this sample of cannabis users was more sensitive to reward than our previous sample, or this sample had more adaptive functioning. Given the increased past year cannabis use in our previous sample (current sample=422 joints, SD=429; previous sample=644 joints, SD=1272), it may be that the previous participants had less adaptive functioning and had moved further along in the addiction cycle beyond that of reward seeking.
Aerobic fitness is a promising potential mediator of improved mental and neurocognitive health. Here, however, no significant relationships were revealed between cannabis use and aerobic fitness. This is despite the fact that prior research has indicated that higher aerobic fitness level was related to decreased depressive symptoms in non-cannabis samples (Carter, Rees, Hale, Bhattacharjee, & Paradkar, 2016), and better objective measures of neurocognition in cannabis users (Wade et al., 2019). We (Lisdahl, Wright, Kirchner-Medina, Maple, & Shollenbarger, 2014) and others (Brellenthin & Koltyn, 2016) have also suggested exercise as a potentially beneficial intervention for cannabis use disorder, though notably the present study investigated aerobic fitness level, rather than the use of an aerobic exercise routine. Better understanding of how and when aerobic fitness influences functioning, particularly in cannabis users, is needed.
Limitations are noted. Most of the data presented relies on self-report, though a notable strength is inclusion of an objective measure of emotional processing in addition to self-reported symptoms. As a cross-sectional study, causality cannot be established. Our study excluded for psychiatric co-morbidity, which may limit generalizability to the typical cannabis user (Rosen, Sodos, Hirst, Vaughn, & Lorkiewicz, 2018), given the high rates of depression within cannabis users. As such, few participants met clinical threshold for self-reported symptoms. However, as previous research indicates cannabis users report lower life satisfaction (Fergusson & Boden, 2008; Grevenstein & Kroninger-Jungaberle, 2015), even slight changes in mood and behavioral functioning may be important targets for prevention and intervention. As noted, there is some lack of clarity on what the BIS/BAS precisely measures. This could be elucidated through supplemental measures, such as a measure of sensitivity to reward, though no such measures were used in the present study. Aerobic fitness level can be influenced by numerous factors, including exercise routines, genetics, and gender; therefore fitness level alone does not likely explain these results.
In conclusion, our results confirm a relationship between cannabis use and depressive symptoms. Aerobic fitness level, on the other hand, was not associated with any measures of affective or behavioral functioning in cannabis users, despite prior evidence of being associated with better cognitive performance (Wade et al., 2019). As the negative relationship between cannabis and depressive symptoms has often been found, there is a clear need to make known to lay audiences that cannabis is not likely beneficial, and may actually be harmful, in treating even subclinical depression. Finally, as this was a cross-sectional study, future research should assess whether such mood and behavioral symptoms precede or are potentially caused by cannabis use through longitudinal studies.
Acknowledgements:
KML’s work was supported by R01 DA030354 and manuscript preparation was supported by funding by the National Institutes of Health (U01DA041025; P.I.: Lisdahl, K.M.). NEW’s manuscript preparation was supported by the National Institute On Alcohol Abuse And Alcoholism of the National Institutes of Health under Award Number T32AA013525. The funding sources had no further role in the study design, data collection, analysis, interpretation, the writing of the report, or in the decision to submit the article for publication. NEW, ERG, AMS, and KML all declare that they have no conflict of interest.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Bassett DR, & Howley ET (2000). Limiting factors for maximum oxygen uptake and determinants of endurance performance. Medicine and Science in Sports and Exercise, 31(1), 70–84. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. . San Antonio, TX: Psychological Corporation. [Google Scholar]
- Brellenthin AG, Crombie KM, Hillard CJ, & Koltyn KF (2017). Endocannabinoid and Mood Responses to Exercise in Adults with Varying Activity Levels. Medicine and Science in Sports and Exercise, 49(8), 1688–1696. doi: 10.1249/MSS.0000000000001276 [DOI] [PubMed] [Google Scholar]
- Brown, Myers MG, Lippke K, Tapert SF, Stewart DG, & Vik PW (1998). Psychometric evaluation of the customary drinking and durg use record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol, 59(4), 427–438. [DOI] [PubMed] [Google Scholar]
- Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, & Martin PR (2011). Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS One, 6(3), e17465. doi: 10.1371/journal.pone.0017465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, & Hughes JR (2006). The cannabis withdrawal syndrome. Current Opinions in Psychiatry, 19, 233–238. [DOI] [PubMed] [Google Scholar]
- Carter B, Rees P, Hale L, Bhattacharjee D, & Paradkar MS (2016). Association Between Portable Screen-Based Media Device Access or Use and Sleep Outcomes: A Systematic Review and Meta-analysis. JAMA Pediatrics, 170(12), 1202–1208. doi: 10.1001/jamapediatrics.2016.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67(2), 319–333. [Google Scholar]
- Casajuana C, Lopez-Pelayo H, Balcells MM, Miquel L, Colom J, & Gual A (2016). Definitions of Risky and Problematic Cannabis Use: A Systematic Review. Substance Use and Misuse, 51(13), 1760–1770. doi: 10.1080/10826084.2016.1197266 [DOI] [PubMed] [Google Scholar]
- Cheke LG, Simons JS, & Clayton NS (2016). Higher body mass index is associated with episodic memory deficits in young adults. Quarterly Journal of Experimental Psychology, 69(11), 2305–2316. doi: 10.1080/17470218.2015.1099163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Wagner FA, & Anthony JC (2002). Marijuana use and the risk of major depressive episode: Epidemiological evidence from the United States National Comorbidity Study. Social Psychiatry and Psychiatric Epidemiology, 37, 199–206. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, & Christie LA (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in Neuroscience, 30(9), 464–472. doi: 10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Egerton A, Allison C, Brett RR, & Pratt JA (2006). Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neuroscience and Biobehavioral Reviews, 30(5), 680–695. doi: 10.1016/j.neubiorev.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, & Smailis J (1992). Facial emotion discrimination: I. Task construction and behavioral findings from normal subjects. Psychiatry Research, 42(3), 231–240. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, & Boden JM (2008). Cannabis use and later life outcomes. Addiction, 103(6), 969–976. doi: 10.1111/j.1360-0443.2008.02221.x [DOI] [PubMed] [Google Scholar]
- Ferreira-Vieira TH, Bastos CP, Pereira GS, Moreira FA, & Massensini AR (2014). A role for the endocannabinoid system in exercise-induced spatial memory enhancement in mice. Hippocampus, 24(1), 79–88. doi: 10.1002/hipo.22206 [DOI] [PubMed] [Google Scholar]
- Grace J, & Malloy PF (1999). Frontal Systems Behavior Scale (FrSBe). Odessa, FL: : Psychological Assessment Resources, Inc. [Google Scholar]
- Grevenstein D, & Kroninger-Jungaberle H (2015). Two patterns of cannabis use among adolescents: results of a 10-year prospective study using a growth mixture model. Substance Abuse, 36(1), 85–89. doi: 10.1080/08897077.2013.879978 [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, … Gur RE (2002). A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. Journal of Neuroscience Methods, 115(2), 137–143. [DOI] [PubMed] [Google Scholar]
- Henchoz Y, Dupuis M, Deline S, Studer J, Baggio S, N’Goran AA, … Gmel G (2014). Associations of physical activity and sport and exercise with at-risk substance use in young men: a longitudinal study. Preventive Medicine, 64, 27–31. doi: 10.1016/j.ypmed.2014.03.022 [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, & Kramer AF (2008). Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience, 9(1), 58–65. doi: 10.1038/nrn2298 [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, … Innis RB (2012). Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Molecular Psychiatry, 17(6), 642–649. doi: 10.1038/mp.2011.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley ET, Bassett DR, & Welch HG (1995). Criteria for maximal oxygen uptake: Review and commentary. Medicine and Science in Sports and Exercise, 27(9), 1292–1301. [PubMed] [Google Scholar]
- Kennedy JT, Collins PF, & Luciana M (2016). Higher Adolescent Body Mass Index Is Associated with Lower Regional Gray and White Matter Volumes and Lower Levels of Positive Emotionality. Frontiers in Neuroscience, 10, 413. doi: 10.3389/fnins.2016.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadrawy YA, Sawie HG, Abdel-Salam OME, & Hosny EN (2017). Cannabis exacerbates depressive symptoms in rat model induced by reserpine. Behavioural Brain Research, 324, 41–50. doi: 10.1016/j.bbr.2017.02.015 [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J, Deater-Deckard K, Holmes C, Lee J, Chiu P, & King-Casas B (2016). Behavioral and neural inhibitory control moderates the effects of reward sensitivity on adolescent substance use. Neuropsychologia, 91, 318–326. doi: 10.1016/j.neuropsychologia.2016.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, & Hillard C (2014). Mechanisms of exercise-induced hypoalgesia. Journal of Pain, 15(12), 1294–1304. doi: 10.1016/j.jpain.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TT, & Gorzalka BB (2012). Timing is everything: evidence for a role of corticolimbic endocannabinoids in modulating hypothalamic-pituitary-adrenal axis activity across developmental periods. Neuroscience, 204, 17–30. doi: 10.1016/j.neuroscience.2011.10.006 [DOI] [PubMed] [Google Scholar]
- Lev-Ran S, Roerecke M, Le Foil B, George TP, McKenzie K, & Rehm J (2014). The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychological Medicine, 44(4), 797–810. doi: 10.1017/S0033291713001438 [DOI] [PubMed] [Google Scholar]
- Levine A, Clemenza K, Rynn M, & Lieberman J (2017). Evidence for the Risks and Consequences of Adolescent Cannabis Exposure. Journal of the American Academy of Child and Adolescent Psychiatry, 56(3), 214–225. doi: 10.1016/j.jaac.2016.12.014 [DOI] [PubMed] [Google Scholar]
- Lisdahl KM, & Price JS (2012). Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. Journal of the International Neuropsychological Society, 18(4), 678–688. doi: 10.1017/S1355617712000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, & Shollenbarger S (2014). Considering Cannabis: The Effects of Regular Cannabis Use on Neurocognition in Adolescents and Young Adults. Current Addiction Reports, 1(2), 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, & Tapert SF (2007). Depressive symptoms in adolescents: Associations with white matter volume and marijuana use. Journal of Child Psycholology and Psychiatry, 48(6), 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, & Shear PK (2007). Anxiety, depression, & behavioral symptoms of executive dysfunction in ecstasy users: Contributions of polydrug use. Drug and Alcohol Dependence, 87(2-3), 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra E, Christopher PP, Anderson BJ, & Stein MD (2015). Coping-motivated marijuana use correlates with DSM-5 cannabis use disorder and psychological distress among emerging adults. Psychology of Addictive Behavior, 29(3), 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, O’Malley PM, Johnston LD, & Bachman JG (2011). Adolescents’ reported reasons for alcohol and marijuana use as predictors of substance use and problems in adulthood. Journal of Studies on Alcohol and Drugs, 72, 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescatello LS (2014). ACSM’s guidelines for exercise testing and prescription. 9th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health. [Google Scholar]
- Pinkham AE, Sasson NJ, Calkins ME, Richard J, Hughett P, Gur RE, & Gur RC (2008). The other-race effect in face processing among African American and Caucasian individuals with schizophrenia. American Journal of Psychiatry, 165(5), 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B, Kamboj S, Morgan CJ, & Curran HV (2010). Processing dynamic facial affect in frequent cannabis-users: evidence of deficits in the speed of identifying emotional expressions. Drug and Alcohol Dependence, 112(1-2), 27–32. doi: 10.1016/j.drugalcdep.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Prince van Leeuwen A, Creemers HE, Verhulst FC, Ormel J, & Huizink AC (2011). Are adolescents gambling with cannabis use? A longitudinal study of impulsivity measures and adolescent substance use: The TRAILS study. Journal of Studies on Alcohol and Drugs, 72, 70–78. [DOI] [PubMed] [Google Scholar]
- Rosen AS, Sodos LM, Hirst RB, Vaughn D, & Lorkiewicz SA (2018). Cream of the Crop: Clinical Representativeness of Eligible and Ineligible Cannabis Users in Research. Subst Use Misuse, 52(12), 1937–1950. doi: 10.1080/10826084.2018.1441312 [DOI] [PubMed] [Google Scholar]
- Schlienz NJ, Budney AJ, Lee DC, & Vandrey R (2017). Cannabis Withdrawal: A Review of Neurobiological Mechanisms and Sex Differences. Curr Addict Rep, 4(2), 75–81. doi: 10.1007/s40429-017-0143-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, & Patrick ME (2018). Monitoring the Future national survey results on drug use, 1975-2017: Volume II, college students and adults ages 19-55. Ann Arbor: University of Michigan. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, & Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In Allen J & Litten RZ (Eds.), Measuring Alcohol Consumption: Psychosocial and Biological Methods (pp. 41–72). Totowa, NJ: Humana Press. [Google Scholar]
- Spechler PA, Orr CA, Chaarani B, Kan KJ, Mackey S, Morton A, … Consortium, I. (2015). Cannabis use in early adolescence: Evidence of amygdala hypersensitivity to signals of threat. Dev Cogn Neurosci, 16, 63–70. doi: 10.1016/j.dcn.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1983). Manual for the State-Trait Anxiety Inventory (For Y). Palo Alto, CA: Mind Garden. [Google Scholar]
- Stewart DG, & Brown SA (1995). Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction, 90, 627–635. [DOI] [PubMed] [Google Scholar]
- Taubitz LE, Pedersen WS, & Larson CL (2015). BAS Reward Responsiveness: A unique predictor of positive psychological functioning. Pers Individ Dif, 80, 107–112. doi: 10.1016/j.paid.2015.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Themanson JR, Hillman CH, & Curtin JJ (2006). Age and physical activity influences on action monitoring during task switching. Neurobiol Aging, 27(9), 1335–1345. doi: 10.1016/j.neurobiolaging.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Troup LJ, Andrzejewski JA, Braunwalder JT, & Torrence RD (2016). The relationship between cannabis use and measures of anxiety and depression in a sample of college campus cannabis users and non-users post state legalization in Colorado. Peer J, 4, e2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Laar M, van Dorsselaer S, Monshouwer K, & de Graaf R (2007). Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction, 102(8), 1251–1260. [DOI] [PubMed] [Google Scholar]
- Wade NE, Wallace AL, Swartz AM, & Lisdahl KM (2019). Aerobic Fitness Level Moderates the Association Between Cannabis Use and Executive Functioning and Psychomotor Speed Following Abstinence in Adolescents and Young Adults Journal of the International Neuropsychological Society, 25(2), 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Lile JA, Hanlon CA, & Porrino LJ (2016). Abnormal medial prefrontal cortex activity in heavy cannabis users during conscious emotional evaluation. Psychopharmacology (Berl), 233(6), 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NE, Scerpella D, & Lisdahl KM (2016). Marijuana Use Is Associated with Behavioral Approach and Depressive Symptoms in Adolescents and Emerging Adults. PLoS One, 11(11), e0166005. doi: 10.1371/journal.pone.0166005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd D (2007). Emotional and cognitive changes during adolescence. Curr Opin Neurobiol, 17(2), 251–257. doi: 10.1016/j.conb.2007.03.009 [DOI] [PubMed] [Google Scholar]


