Abstract
Objective
The progress of accelerated atherosclerosis in systemic lupus erythematosus (SLE) is incompletely understood. Circulating osteopontin (OPN) is increased in autoimmune conditions, e.g. SLE, and its serum concentration was recently reported to associate with subclinical atherosclerosis in SLE, as measured by carotid intima-media thickness. The aim of this study was to investigate whether OPN may be used as a surrogate biomarker of subclinical atherosclerosis in SLE patients with different disease phenotypes.
Methods
We recruited 60 well-characterised SLE cases and 60 age- and sex-matched healthy controls. The SLE cases were divided into three different disease phenotypes: SLE with antiphospholipid syndrome (APS), lupus nephritis, and isolated skin and joint involvement. Plasma OPN was detected by ELISA (Quantikine®, R&D Systems). Common carotid arteries intima media thickness was compared between the studied groups in relation to OPN levels and risk factors for vascular changes. Intima media thickness of common carotid arteries was measured by using a sensitive ultrasound technique (LOGIQ™ E9 ultrasound, GE Healthcare).
Results
OPN levels were significantly higher among the entire SLE group (n = 60) compared to the healthy controls (P = 0.03). SLE cases with concomitant APS (n = 20) showed higher OPN levels than the controls (P = 0.004), whereas none of the other two subgroups differed significantly from the healthy controls. OPN and intima media thickness were correlated to several traditional risk factors of atherosclerosis, as well as to SLE-related factors. Yet, no significant correlation was observed between OPN levels and ultrasound findings of the common carotid arteries.
Conclusions
In line with previous studies, we observed increased OPN levels among SLE patients as compared to matched controls. However, the OPN concentrations did not correlate with intima media thickness of the common carotid arteries. Based on our findings, the use of OPN as a surrogate biomarker of subclinical atherosclerosis in SLE subjects, regardless of clinical phenotypes, cannot be recommended.
Keywords: Osteopontin, carotid intima-media thickness, atherosclerosis, systemic lupus erythematosus, biomarker
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory disease that usually affects young females and may impair several organ systems, with an increased risk of cerebro- and cardiovascular disease (CVD).1–3 Atherosclerosis is an inflammatory process with immune cell activation, leading to plaque formation and risk of subsequent rupture. 4 The systemic inflammation occurring in patients with SLE is thought to accelerate atherosclerosis,1,5,6 but additional mechanisms are probably involved. The autoimmune vascular injury may facilitate the atherosclerotic plaque formation. Furthermore, in SLE patients with or without concomitant antiphospholipid syndrome (APS), the excessive oxidative stress, the apoptotic cell death and the defective clearance of apoptotic materials contribute to tissue damage, and dyslipidaemia can further accelerate atherogenesis.7–9 The pivotal role of the type I interferon (IFN) system may also promote atherosclerosis, by a pro-inflammatory action on the endothelium and by stimulating macrophage recruitment to atherosclerotic lesions.10,11
Traditional risk factors for atherosclerosis, e.g. diabetes, hyperlipidaemia, hypertension, family history CVD, obesity, and tobacco smoking, along with the common need of glucocorticoid treatment, may even more increase the risk of CVD in subjects affected by SLE.12–14 In addition, vasculitis primarily affecting small vessels is not uncommon; medium and large sized vessels are less often engaged by vasculitis in SLE. 15
The extracellular matrix protein osteopontin (OPN) is a mediator of systemic inflammation and has multiple biological functions. 16 Local production and elevated circulating levels of OPN have been observed in several autoimmune diseases, including SLE.17,18 Overexpression of OPN in lupus-prone mice induces B-cell activation and subsequent production of anti-double-stranded (ds) DNA antibodies, a distinctive laboratory finding in subjects with SLE. Intracellular OPN has been implicated in many cellular processes and its expression is required for Toll-like receptor 9-dependent production of IFN-α. 19 Two recent studies by Carbone et al. suggested OPN as a potential predictor of poor outcome in patients with severe carotid atherosclerosis 20 and as a valuable biomarker in SLE, showing a strong association with subclinical atherosclerosis, measured as carotid intima-media thickness (IMT). 21 In contrast, OPN plasma levels (pOPN) and early vascular markers of atherosclerosis in asymptomatic young Scandinavian adults were poorly correlated. 22
The high-frequency ultrasound (HFUS) facilitates the mapping of the vascular damage and gives the clinician the opportunity to distinguish atherosclerosis from inflammation, in individuals with SLE and other inflammatory conditions. Progression of IMT, as well as the presence of carotid plaques, has been associated with traditional cardiovascular risk factors, besides SLE per se. 23 We have recently shown that an extended HFUS protocol focused on multiple arterial areas may provide the clinician with additional information on the vessel wall appearance, in SLE subjects. 24 We recorded that increased IMT (≥0.9 mm) observed in SLE predominantly showed appearance of a medium echogenic, homogenous wall thickening that can be found in inflammatory vascular disease. Concerning early wall changes in SLE, our results indicated other potential mechanisms apart from atherosclerosis. 24
Herein, we aimed to investigate the reliability of pOPN as a surrogate biomarker of atherosclerosis in the common carotid arteries (CCA) of subjects with different SLE phenotypes i.e. in patients with either APS, or nephritis (LN), or isolated skin/joint involvement, as well as in matched control subjects.
Materials and methods
Study population
Patients in this cross-sectional study were recruited from the observational research program KLURING (a Swedish acronym for Clinical LUpus Register in Northeastern Gothia), in which prevalent and incident SLE cases continuously have been included and longitudinally followed since 2008 at the Rheumatology Unit, Linköping University Hospital. 25 Sixty patients (52 women, 8 men; median age 43.0 and mean 42.9 years; range 23–63 years) and 60 age- and sex-matched healthy controls (see below) were recruited. All patients were diagnosed with SLE and fulfilled the 1982 American College of Rheumatology (ACR) and/or the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria as detailed in Table 1. 26 In each patient, the acquired organ damage was assessed by the SLICC/ACR damage index (SDI) 27 and the disease activity by the SLE disease activity index 2000 (SLEDAI-2K). 28
Table 1.
Detailed characteristics of the included patients and healthy controls presented as mean ± SD or n (%).
| All SLE(n = 60) | Controls (n = 60) | LN (n = 20) | APS (n = 20) | Skin and joint (n = 20) | |
|---|---|---|---|---|---|
| Variables | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| Background variables | |||||
| Age at examination (years) | 43.2 ± 11.3 | 43.0 ± 11.4 | 41.6 ± 10.4 | 45.2 ± 12.2 | 42.9 ± 11.7 |
| Female gender, n (%) | 52 (87) | 52 (87) | 18 (90) | 15 (75) | 19 (95) |
| Duration of SLE (years) | 12.0 ± 9.4 | NA | 10.7 ± 8.1 | 15.6 ± 12.2 | 9.6 ± 6.3 |
| SDI score | 0.8 ± 1.1 | NA | 0.6 ± 0.9 | 1.5 ± 1.4 | 0.4 ± 0.5 |
| SLEDAI-2K | 2.0 ± 2.1 | NA | 1.6 ± 2.1 | 2.1 ± 2.4 | 2.2 ± 1.7 |
| Traditional risk factors and laboratory data | |||||
| Body mass index (BMI) (kg/m²) | 26.0 ± 4.2a | 24.0 ± 3.3 | 26.5 ± 3.4b | 25.6 ± 4.0 | 25.8 ± 5.1 |
| Ever smoker (former or current), n (%) | 14 (23) | 0 | 4 (20) | 3 (15) | 7 (35) |
| Systolic blood pressure (mm Hg) | 115 ± 26 | 112 ± 18 | 117 ± 17 | 113 ± 32 | 116 ± 29 |
| Diastolic blood pressure (mm Hg) | 73 ± 11b | 68 ± 8 | 74 ± 12 | 73 ± 10 | 72 ± 9 |
| Diabetes mellitus, n (%) | 1 (2) | 0 | 0 | 1 (5) | 0 |
| Raynaud, n (%) | 16 (27) | 9 (15) | 4 (20) | 5 (25) | 7 (35) |
| eGFR (mL/min/1,73m²) | 84 ± 16 | NA | 85 ± 14 | 79 ± 18 | 87 ± 13 |
| Total cholesterol (mmol/L) | 4.7 ± 1.0 | 4.9 ± 1.1 | 4.5 ± 1.0 | 4.7 ± 0.8 | 4.9 ± 1.1 |
| High-density lipoprotein (HDL) (mmol/L) | 1.6 ± 0.5 | 1.7 ± 0.4 | 1.5 ± 0.4 | 1.6 ± 0.5 | 1.6± 0.4 |
| Low-density lipoprotein (LDL) (mmol/L) | 2.6 ± 0.8 | 2.6 ± 0.9 | 2.5 ± 0.9 | 2.5 ± 0.7 | 2.9 ± 0.9 |
| Triglycerides (TG) (mmol/L) | 1.1 ± 0.7 | 1.2 ± 0.6 | 1.2 ± 0.6 | 1.3 ± 1.0 | 0.9 ± 0.4 |
| hsCRP (mg/L) | 2.2 ± 2.8 | 2.0 ± 3.7 | 1.4 ± 1.3 | 2.7 ± 3.4 | 2.5 ± 3.2 |
| Medical treatment, ongoing | |||||
| Antimalarial agents, n (%) | 54 (90) | 0 | 20 (100) | 16 (80) | 18 (90) |
| Glucocorticoid therapy n (%) | 31 (52) | 0 | 12 (60) | 9 (45) | 10 (50) |
| Mean daily Prednisolone dose (mg) | 4.5 | 0 | 5.4 | 3.8 | 4.2 |
| Warfarin therapy, n (%) | 11 (18) | 0 | 1 (5) | 10 (50) | 0 |
| Antiplatelet therapy, n (%) | 11 (18) | 0 | 5 (25) | 6 (30) | 0 |
| Statin therapy n (%) | 5 (8) | 0 | 2 (10) | 3 (15) | 0 |
| DMARD therapy, n (%) | 27 (45) | 0 | 11 (55) | 9 (45) | 7 (35) |
| Mycophenolate mofetil, n (%) | 16 (27) | 0 | 11 (55) | 4 (20) | 1 (5) |
| Methotrexate, n (%) | 5 (8) | 0 | 0 | 1 (5) | 4 (20) |
| Azathioprine, n (%) | 3 (5) | 0 | 0 | 2 (10) | 1 (5) |
| Sirolimus, n (%) | 2 (3) | 0 | 0 | 1 (5) | 1 (5) |
| Dehydroepiandrosterone, n (%) | 1 (2) | 0 | 1 (2) | 0 | 0 |
| Biologics, n (%) | 4 (7) | 0 | 3 (15) | 1 (5) | 0 |
| Bortezomib, n (%) | 1 (2) | 0 | 1 (5) | 0 | 0 |
| Rituximab, n (%) | 1 (2) | 0 | 1 (5) | 0 | 0 |
| Belimumab, n (%) | 2 (3) | 0 | 1 (5) | 1 (5) | 0 |
APS: antiphospholipid syndrome; hsCRP: high-sensitivity C-reactive protein; DMARDs: disease modifying anti-rheumatic drugs; eGFR: estimated glomerular filtration rate; LN: lupus nephritis; N/A: not applicable or available; SDI: SLICC/ACR damage index; SLE: systemic lupus erythematosus.
bp < 0.05.
ap < 0.01.
The selected patients were further divided in three phenotypic subgroups, based on the main clinical manifestations. The subgroups were matched between each other 1:1:1 according to sex and age; 20 cases meeting the renal disorder ACR criterion for LN 29 in the absence of APS, 20 cases meeting APS criteria 30 in the absence of LN, and 20 cases with skin and joint involvement in the absence of LN and APS. Immediately next to the HFUS examination, peripheral venous blood was drawn from each individual, and plasma was prepared and stored at −70°C until analysed.
Sixty healthy Caucasian, age- and sex-matched (1:1 to the 60 SLE cases), non-medicated controls (52 women and 8 men; median age 43.0 and mean 42.9 years; range 23–63 years) were examined with HFUS and blood tests, using the same protocol as for the patients. None of them had clinical signs of inflammatory or atherosclerotic disease.
OPN immunoassay
A serum- and plasma-validated ELISA kit (Quantikine, R&D Systems, Minnesota, USA) was used to analyse pOPN in SLE and control plasma. The samples were randomly applied on the ELISA plates and all assays were performed by the same person (LW) in Linköping, according to the manufacturers’ instructions, as previously described. 31 A correlation coefficient of 0.77 was achieved by measuring OPN in individuals from whom both serum and plasma had been collected simultaneously (Suppl. Figure 1).
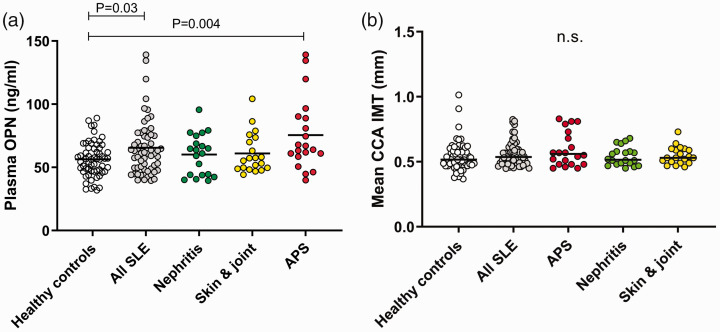
Figure 1.
(a) Plasma osteopontin (OPN) concentrations in healthy controls (n = 60) and the entire group of SLE patients (n = 60), as well as for the three SLE phenotypes of which each include 20 individuals. (b) CCA IMT of healthy controls (n = 60) and the entire group of SLE patients (n = 60), as well as for the three SLE phenotypes of which each include 20 individuals.
n.s. = not significant.
High-frequency ultrasound
For the HFUS measurements, a LOGIQ™ E9 XDclear 2.0 (General Electric Medical Systems Ultrasound, Wauwatosa, WI, USA) ultrasound system was used, with linear transducers L2-9 MHz. The scan was performed in both transverse and longitudinal planes with the patient lying in the supine position with neck extension. The image was carefully optimized with focus on the arterial vessel wall, a preinstalled software with high frequency, medium frame rate and medium dynamic range was used. IMT was measured in CCA. Both sides were investigated. The wall thickness was measured in the longitudinal plane with a 10 mm measurement box placed over the common carotid artery far wall, near (10 mm) the carotid bifurcation. A mean value of all measured far wall points in the box is presented. Two repeated measurements were performed on each side by the same examiner (C.Sv.). Mean CCA IMT bilaterally were used.
Background variables
We obtained data from patients and controls regarding traditional risk factors potentially contributing to atherosclerosis, such as height and weight presented as Body Mass Index (BMI). Variables concerning age, sex, smoking habits, diabetes, presence of Raynaud’s phenomenon and ongoing pharmacotherapy (antimalarial agents, glucocorticoids, warfarin, antiplatelet therapy, statins and disease modifying anti-rheumatic drugs (DMARDs)) were also collected. Blood pressure was determined with oscillometric technique (Dinamap PRO 200 Monitor, Critikon, Tampa, FL, USA).
Laboratory measurements
Blood samples were collected after 12-h overnight fasting at the same day as HFUS examination. Standard measurements of total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), plasma creatinine, and C-reactive protein by high sensitive technique (hsCRP) were performed at the Clinical Chemistry laboratory, at Linköping University Hospital, Sweden. The 4-variable Modification of Diet in Renal Disease Study equation based on plasma creatinine was used to estimate the glomerular filtration rate (eGFR). 32
Presence of anti-dsDNA antibodies (using addressable laser bead immunoassay FIDIS™ Connective profile, Solonium software version 1.7.1.0, Theradiag, Croissy-Beaubourg, France) and plasma levels of complement proteins (C3 and C4) were assessed as serological markers of disease activity. 33
Statistics
A univariate linear regression model was used to evaluate correlations and predictive effects of the investigated risk factors for atherosclerosis in relation to pOPN and CCA IMT. The factors with P values ≤0.05 in the univariate analysis were included in a multivariable regression analysis. Correlations between pOPN and CCA IMT were examined by linear regression analysis. Statistical analyses were performed using SPSS Statistics V.26 (IBM, Armonk, New York, USA) or GraphPad Prism, V.8 (GraphPad Software, La Jolla, CA, USA).
Ethics considerations
Oral and written informed consent was obtained from all patients and healthy controls. The study protocol was approved by the Regional Ethics Review Board in Linköping (Decision No. M75-08).
Results
Descriptive data
A detailed descriptive statistical analysis including demographics, clinical features and medications of the included groups and subgroups is presented in Table 1.
pOPN in different SLE phenotypes versus controls
pOPN levels were significantly higher among patients with SLE (median 61.5 ng/ml) compared to the healthy controls (median 56.6 ng/ml, P = 0.03; Figure 1(a)). No statistically significant differences were observed between the SLE phenotype subgroups. By comparison of each subgroup with the controls, significantly higher pOPN levels were detected among individuals with APS (P = 0.004; Figure 1(a)).
Correlation of pOPN in all patients
For the entire group of patients (n = 60), pOPN was significantly correlated with several variables, including SLE disease duration, SDI, hsCRP, occurrence of Raynaud’s phenomenon and ongoing warfarin therapy (Table 2). No significant associations were found between pOPN levels and any of the studied variables among the controls.
Table 2.
Plasma osteopontin levels related to background variables, traditional risk factors, laboratory tests and pharmacotherapy in the univariate regression model of SLE cases with subgroups compared to healthy controls.
|
All SLE(n = 60) |
Controls(n = 60) |
LN(n = 20) |
APS(n = 20) |
Skin and jointn = 20) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | B | P | B | P | B | P | B | P | B | P |
| Background variables | ||||||||||
| Age | 0.89 | 0.73 | –0.07 | 0.67 | 0.55 | 0.13 | –0.54 | 0.33 | 0.17 | 0.59 |
| Female gender | 8.20 | 0.33 | 3.62 | 0.51 | –3.57 | 0.78 | 9.21 | 0.55 | –6.05 | 0.72 |
| Duration of SLE | 0.63 | 0.04 | 0 | 0 | 0.43 | 0.36 | 0.77 | 0.15 | 0.70 | 0.23 |
| SDI score | 9.74 | <0.001 | 0 | 0 | 9.20 | 0.02 | 10.30 | 0.02 | –6.20 | 0.42 |
| SLEDAI-2K | 2.71 | 0.05 | 0 | 0 | –0.27 | 0.91 | 2.60 | 0.35 | 4.53 | 0.005 |
| Traditional risk factors and laboratory data | ||||||||||
| BMI (kg/m2) | 0.37 | 0.59 | –0.28 | 0.61 | 0.92 | 0.42 | –0.37 | 0.83 | –0.71 | 0.33 |
| Ever smoker (former or current) | 0.83 | 0.90 | 0 | 0 | 2.35 | 0.80 | –7.21 | 0.64 | 6.13 | 0.47 |
| Systolic blood pressure | 0.23 | 0.21 | 0.19 | 0.17 | 0.48 | 0.02 | 0.02 | 0.95 | 0.15 | 0.57 |
| Diastolic blood pressure | 0.27 | 0.32 | 0.16 | 0.48 | 0.40 | 0.20 | 0.13 | 0.85 | 0.20 | 0.62 |
| Raynaud’s phenomenon | 12.37 | 0.05 | –7.85 | 0.12 | 3.21 | 0.80 | 17.76 | 0.19 | 6.90 | 0.37 |
| eGFR | –0.07 | 0.69 | 0 | 0 | 0.12 | 0.61 | –0.45 | 0.35 | 0.01 | 0.95 |
| Total cholesterol | 1.94 | 0.50 | –0.28 | 0.86 | 2.01 | 0.61 | –3.17 | 0.69 | 4.08 | 0.21 |
| High-density lipoprotein (HDL) | 6.73 | 0.32 | 1.37 | 0.76 | 7.33 | 0.46 | –4.31 | 0.74 | 19.15 | 0.06 |
| Low-density lipoprotein (LDL) | –0.81 | 0.81 | –0.40 | 0.83 | –1.71 | 0.70 | –3.30 | 0.74 | 3.57 | 0.38 |
| Triglycerides (TG) | 4.40 | 0.27 | –1.50 | 0.63 | 11.45 | 0.06 | 0.70 | 0.92 | 0.70 | 0.94 |
| hsCRP | 2.16 | 0.03 | 0.19 | 0.71 | 1.66 | 0.59 | 4.10 | 0.03 | –0.51 | 0.66 |
| Medical treatment, ongoing | ||||||||||
| Antimalarial agents | –1.73 | 0.86 | N/A | N/A | NE | NE | –0.33 | 0.98 | 12.57 | 0.30 |
| Glucocorticoid therapy | 4.98 | 0.38 | N/A | N/A | 12.73 | 0.08 | 3.45 | 0.80 | 3.50 | 0.63 |
| Warfarin therapy | 22.0 | 0.002 | N/A | N/A | 9.15 | 0.60 | 18.90 | 0.14 | NE | NE |
| Antiplatelet therapy | –6.60 | 0.37 | N/A | N/A | 4.67 | 0.59 | –26.0 | 0.06 | NE | NE |
| Statin therapy | 7.50 | 0.47 | N/A | N/A | –18.62 | 0.27 | 5.43 | 0.74 | NE | NE |
| DMARD therapy | –1.76 | 0.76 | N/A | N/A | –4.75 | 0.53 | –6.01 | 0.65 | 1.69 | 0.82 |
APS: antiphospholipid syndrome; BMI: body mass index; CRP: C-reactive protein; DMARDs: disease modifying anti-rheumatic drugs; LN: lupus nephritis; N/A: not applicable; NE: Not estimated; SDI: SLICC/ACR damage index; SLE: systemic lupus erythematosus. Note: Statistically significant associations shown in bold.
Correlation of CCA IMT in each subgroup and studied variables
Correlations between background variables and laboratory measurements and mean CCA IMT are shown in Table 3. Statistically significant correlations were mainly observed with SDI, tobacco smoking, triglycerides and use of antimalarial agents. In the control group, age, blood pressure (both systolic and diastolic), cholesterol and LDL levels were correlated with CCA IMT. The analysis was stratified, and the included variables tested in relation to mean CCA IMT right and left sides, as shown in Table 4. A significant association was observed with blood pressure and age in the controls and with the SLE duration, acquired organ damage (SDI score) and lipid levels (TG and total cholesterol) in SLE cases (n = 60).
Table 3.
Mean CCA IMT (bilateral) related to background variables, traditional risk factors, laboratory tests and pharmacotherapy in univariate regression model of SLE cases with subgroups compared to healthy controls.
|
All SLE(n = 60) |
Controls(n = 60) |
LN(n = 20) |
APS(n = 20) |
Skin and joint(n = 20) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | B | P | B | P | B | P | B | P | B | P | |
| Background variables | |||||||||||
| Age | –0.00 | 0.62 | 0.01 | <0.001 | –0.00 | 0.33 | –0.00 | 0.72 | –8.90 | 0.95 | |
| Female gender | 0.06 | 0.15 | –0.00 | 0.99 | 0.07 | 0.19 | 0.06 | 0.44 | –0.08 | 0.29 | |
| Duration of SLE | 0.00 | 0.06 | 0 | 0 | 0.00 | 0.62 | 0.00 | 0.34 | 0.00 | 0.48 | |
| SDI score | 0.03 | 0.01 | 0 | 0 | 0.00 | 0.91 | 0.04 | 0.14 | 0.03 | 0.39 | |
| SLEDAI-2K | 0.00 | 0.76 | 0 | 0 | –0.02 | 0.07 | –0.00 | 0.88 | 0.01 | 0.46 | |
| Traditional risk factors and laboratory data | |||||||||||
| BMI (kg/m2) | 0.00 | 0.79 | 0.01 | 0.05 | 0.00 | 0.58 | 0.01 | 0.60 | –0.01 | 0.63 | |
| Ever smoker (former or current) | 0.09 | 0.01 | 0 | 0 | 0.10 | 0.02 | –9.40 | 0.96 | 0.02 | 0.66 | |
| Systolic blood pressure | 2.84 | 0.97 | 0.00 | <0.001 | 0.00 | 0.74 | 0.02 | 0.95 | –3.17 | 1.00 | |
| Diastolic blood pressure | –0.00 | 0.59 | 0.00 | 0.02 | 0.00 | 0.86 | –0.00 | 0.44 | 0.00 | 0.87 | |
| Raynaud’s phenomenon | 0.05 | 0.15 | –0.03 | 0.33 | 0.01 | 0.84 | 0.15 | 0.03 | –0.04 | 0.28 | |
| Estimated glomerular filtration rate | 0.00 | 0.45 | 0 | 0 | 0.00 | 0.04 | –0.00 | 0.54 | 0.00 | 0.45 | |
| Total cholesterol | 0.02 | 0.22 | 0.05 | <0.001 | 0.01 | 0.64 | 0.07 | 0.10 | –0.00 | 0.83 | |
| High-density lipoprotein (HDL) | 0.03 | 0.30 | 0.06 | 0.09 | 0.02 | 0.74 | –0.09 | 0.17 | –0.01 | 0.78 | |
| Low-density lipoprotein (LDL) | 0.01 | 0.50 | 0.05 | <0.001 | 0.01 | 0.72 | 0.07 | 0.16 | –0.00 | 0.84 | |
| Triglycerides (TG) | 0.06 | <0.001 | 0.01 | 0.65 | 0.01 | 0.68 | 0.09 | 0.01 | 0.01 | 0.89 | |
| High-sensitivity CRP | 0.01 | 0.34 | 0.00 | 0.57 | 0.00 | 0.96 | 0.01 | 0.40 | –0.00 | 0.90 | |
| Medical treatment, ongoing | |||||||||||
| Antimalarial agents | –0.12 | 0.01 | 0 | 0 | NE | NE | –0.19 | 0.02 | 0.03 | 0.66 | |
| Glucocorticoid therapy | –0.02 | 0.51 | 0 | 0 | 0.05 | 0.15 | –0.06 | 0.36 | –0.03 | 0.39 | |
| Warfarin therapy | 0.07 | 0.05 | 0 | 0 | 0.05 | 0.50 | 0.05 | 0.49 | NE | NE | |
| Antiplatelet therapy | –0.05 | 0.16 | 0 | 0 | –0.01 | 0.84 | –0.14 | 0.06 | NE | NE | |
| Statin therapy | –0.01 | 0.88 | 0 | 0 | –0.16 | 0.04 | –0.00 | 0.96 | NE | NE | |
| DMARD therapy, n (%) | –1.76 | 0.76 | 0 | 0 | 0.01 | 0.88 | 0.14 | 0.03 | 0.10 | 0.001 | |
APS: antiphospholipid syndrome; CRP: C-reactive protein; BMI: body mass index; DMARDs: disease modifying anti-rheumatic drugs; LN: lupus nephritis; N/A: not applicable; NE: Not estimated; SDI: SLICC/ACR damage index; SLE: systemic lupus erythematosus.
Note: Statistically significant associations shown in bold.
Table 4.
Mean CCA IMT in right and left side in all SLE group and controls related to included variables.
| Variables |
Controls: CCA IMT (n = 60) |
All SLE: CCA IMT (n = 60) |
||||||
|---|---|---|---|---|---|---|---|---|
|
Right CCA |
Left CCA |
Right CCA |
Left CCA |
|||||
| B | P | B | P | B | P | B | P | |
| Age | 7.95 | 0.014 | 2.78 | 0.37 | 0.01 | 0.91 | 0.14 | 0.26 |
| Female gender | –110.64 | 0.36 | 72.60 | 0.51 | –2.83 | 0.48 | –5.14 | 0.20 |
| Duration of SLE | 0 | 0 | 0 | 0 | 0.15 | 0.31 | 0.35 | 0.02 |
| SDI score | 0 | 0 | 0 | 0 | 0.35 | 0.77 | 3.07 | 0.01 |
| SLEDAI-2K | 0 | 0 | 0 | 0 | 0.30 | 0.65 | 0.04 | 0.95 |
| Traditional risk factors and laboratory data | ||||||||
| BMI (kg/m2) | 11.6 | 0.30 | 16.42 | 0.12 | –0.16 | 0.64 | –0.30 | 0.37 |
| Ever smoker (former or current) | 0 | 0 | 0 | 0 | –1.05 | 0.74 | –1.10 | 0.74 |
| Systolic blood pressure | 8.60 | 0.002 | 3.03 | 0.26 | –0.03 | 0.76 | 0.10 | 0.25 |
| Diastolic blood pressure | 11.22 | 0.014 | 2.75 | 0.53 | –0.01 | 0.95 | 0.19 | 0.16 |
| Raynaud | –105.00 | 0.32 | –149.94 | 0.11 | –0.87 | 0.78 | 0.58 | 0.85 |
| Estimated glomerular filtration rate | 0 | 0 | 0 | 0 | –0.04 | 0.68 | 0.10 | 0.25 |
| Total cholesterol | 21.21 | 0.53 | 34.38 | 0.27 | 1.37 | 0.32 | 3.07 | 0.03 |
| High-density lipoprotein (HDL) | 138.84 | 0.14 | 5.24 | 0.95 | 0.02 | 1.00 | 5.83 | 0.07 |
| Low-density lipoprotein (LDL) | 3.10 | 0.94 | 51.04 | 0.17 | 0.06 | 0.52 | 1.18 | 0.48 |
| Triglycerides (TG) | 19.14 | 0.77 | –17.60 | 0.77 | 3.10 | 0.09 | 4.81 | 0.01 |
| High-sensitivity CRP | –9.26 | 0.40 | –6.30 | 0.53 | –0.10 | 0.84 | 0.26 | 0.59 |
| Medical treatment, ongoing | ||||||||
| Antimalarial agents | 0 | 0 | 0 | 0 | –5.98 | 0.18 | –2.56 | 0.58 |
| Glucocorticoid therapy | 0 | 0 | 0 | 0 | –3.80 | 0.16 | –0.10 | 0.70 |
| Warfarin therapy | 0 | 0 | 0 | 0 | –1.55 | 0.66 | –1.36 | 0.70 |
| Antiplatelet therapy | 0 | 0 | 0 | 0 | –5.34 | 0.12 | 2.76 | 0.44 |
| Statin therapy | 0 | 0 | 0 | 0 | –4.42 | 0.37 | –4.80 | 0.34 |
| DMARD therapy | 0 | 0 | 0 | 0 | 1.60 | 0.55 | 3.75 | 0.17 |
APS: Antiphospholipid syndrome; CRP: C-reactive protein; BMI: body mass index; DMARDs: disease modifying anti-rheumatic drugs; LN: lupus nephritis; SDI: SLICC/ACR damage index; SLE: systemic lupus erythematosus.
Notes: Statistically significant associations shown in bold.
No obvious differences in bilateral mean CCA IMT between SLE patients and controls, as well as between the SLE subgroups and controls were seen (Figure 1(b)).
Multivariable regression analysis for pOPN and CCA IMT
Next, variables showing significant associations with pOPN or CCA IMT in the univariate regression model were analysed in a multivariable regression model. As shown in Table 5, only the global SDI score remained significantly associated with pOPN levels, whereas smoking (ever) and triglycerides were significantly associated with CCA IMT.
Table 5.
Multivariable regression analysis for significant associations between evaluated variables and plasma osteopontin (pOPN), as well as CCA IMT in the SLE patients (n = 60).
|
Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Risk factors | B | P value | B | P value |
| pOPN | ||||
| SLE duration | 0.63 | 0.04 | –0.24 | 0.50 |
| SDI score | 9.74 | 0.00 | 7.02 | 0.04 |
| hsCRP | 2.16 | 0.03 | 1.55 | 0.10 |
| Raynaud | 12.37 | 0.05 | 8.12 | 0.15 |
| Warfarin | 22.0 | 0.002 | 13.27 | 0.08 |
| CCA IMT | ||||
| SDI score | 0.03 | 0.01 | 0.010 | 0.44 |
| Tobacco smoking (ever) | 0.09 | 0.01 | 0.06 | 0.05 |
| Triglycerides | 0.06 | 0.00 | 0.04 | 0.04 |
| Antimalarials | –0.12 | 0.01 | –0.07 | 0.10 |
| Warfarin | 0.07 | 0.05 | 0.01 | 0.81 |
hsCRP: high-sensitivity C-reactive protein; SDI: SLICC/ACR damage index; SLE: systemic lupus erythematosus.
Note: Statistically significant associations shown in bold.
Correlation between pOPN and CCA IMT
Finally, the potential role of pOPN as a surrogate marker of CCA IMT was evaluated. No significant correlations between pOPN levels and CCA IMT were found in any of the included groups (Table 6).
Table 6.
Correlations between plasma osteopontin (pOPN) and CCA IMT in the studied groups and subgroups in a univariate regression model.
| pOPN levels |
CCA IMT |
|
|---|---|---|
| B | P | |
| Healthy controls | 0.00 | 0.72 |
| All SLE cases | 0.00 | 0.38 |
| SLE with lupus nephritis | 0.00 | 0.91 |
| SLE with antiphospholipid syndrome | 0.00 | 0.85 |
| SLE with skin & joint involvement | 0.00 | 0.79 |
Discussion
The aims of the present study were to evaluate the potential of pOPN as a surrogate marker mirroring the CCA IMT, assessed by using HFUS, to investigate subclinical atherosclerosis in well-characterized SLE subjects and healthy controls. Entirely in line with previous reports,18,31,34,35 OPN was significantly higher in patients with SLE than in healthy controls, but its association with subclinical atherosclerosis was poor, which is not consistent with the findings reported by Carbone et al. 21
The discrepant findings may be explained by several factors, such as the higher frequency we utilized in our HFUS investigations, the fact that OPN was measured in plasma (instead of serum) and the presence of males (13%) in our study populations. Sex bias could be relevant since male SLE patients often have worse prognosis than women. 36 Furthermore, our data were adjusted for SDI, disease phenotypes and ongoing pharmacotherapy, including treatments with important effects on both atherogenesis and vessels changes, e.g. corticosteroids and DMARDs, especially antimalarials. We believe it is critical to contemplate damage accrual (assessed by the SDI) in such a study, as it has been recognized that damage predicts further damage in SLE.37,38
We did not detect any considerable differences in pOPN levels between the SLE phenotypic subgroups. Previous reports on larger study populations have shown that LN is associated with higher OPN levels compared to other disease phenotypes, but the impact of SLE disease activity cannot be excluded.31,34,35 Herein, most patients had clinically quiescent disease and the SLEDAI-2K did not differ significantly between the phenotypic subgroups.
In the subgroup with isolated skin and joint involvement, pOPN was significantly correlated with some SLE-related factors, such as disease duration, SDI and disease activity. These observations are mainly in agreement with previous findings, where elevated levels of OPN preceded increased cumulative SLE disease activity and organ damage. 31
OPN has been implicated as a mediator of Th17 regulation via type I IFN receptor signalling, in macrophage activity at sites of tissue repair and in bone homoeostasis.18,39,40 OPN contributes to macrophage chemotaxis, activation, survival and pro-inflammatory M1 polarization. In addition, OPN promotes neutrophil recruitment and activation. 41 Regarding SLE and atherosclerosis, OPN plays a role through regulation of type I IFN response, which is considered as part of pathophysiology in both conditions.42,43
Warfarin therapy showed a prominent positive association with pOPN levels. Warfarin is a vitamin K antagonist and may give vitamin K deficiency causing vascular calcification through inhibition of calcification inhibitors, including gamma-carboxyglutamic acid, Gla protein, fetuin and OPN.44,45 CCA IMT had significant correlations in our study with traditional factors such as age, smoking, blood pressure and lipid (TG, LDL) levels in controls, SLE patients and subgroups. SDI of all SLE cases were also slightly correlated with CCA IMT. A negative low association was observed between CCA IMT and treatment with statins, as well as with antimalarial agents.
Antimalarials have a known cholesterol-lowering effect, especially in SLE patients with concomitant corticosteroid treatment.46,47 In the SLE subgroups with APS and skin and joints involvement, a minor positive association was observed between DMARD therapy and CCA IMT. Similarly to this finding, long-term use of DMARDs in patients with rheumatoid arthritis was reported to develop incident hyperlipidaemia. 48 Following a multivariable analysis of the studied variables or risk factors against both elevated pOPN or increased CCA IMT, only a few factors still had significant associations (as shown in Table 5).
We could not find any associations between pOPN and CCA IMT in the SLE group, not even after stratifying into different disease phenotypes. Despite the multiple possible mechanistic roles of OPN in atherogenesis in SLE patients,17,19,31 pOPN levels did not mirror the wall thickness of CCA. This may be due to the younger age of the patients in our study, 22 differences in SLE duration, or possibly obscured by warfarin treatment in approximately one fifth of the patients. Yet, although warfarin can cause arterial wall calcification, the vascular wall changes in atherosclerosis are different from vascular calcification. Arteriosclerosis makes the artery wall thicker as a result of invasion and accumulation of white blood cells (foam cells) and the proliferation of intimal smooth muscle cells, creating fibro-fatty plaques, while vascular calcification is present mainly in the smooth muscle layer of arteries leading to impairment of the vascular tone and consequent arterial stiffness.49,50
The limitations in the present study are mainly due to the relatively small number of included subjects. The low age of the selected patients may have affected the limited vascular changes. The well-characterized SLE patients’ group and the study design with stratification of data according to different clinical phenotypes and well-matched controls represent major strengths of our investigation. Moreover, the accuracy of the HFUS examination is high with two repeated measurements in each side in every subject performed by the same examiner.
To summarize, we evaluated the vessel wall appearance of CCA using sensitive HFUS technique in well-characterized SLE subjects and matched controls. Although pOPN levels were significantly increased among the patients and associated with both traditional and SLE-related risk factors, the pOPN concentrations did not correlate significantly with CCA IMT findings. We propose that the pOPN levels should not be used as a surrogate marker of atherosclerosis in patients with SLE, regardless of disease phenotype.
Supplemental Material
Supplemental material, sj-pdf-1-lup-10.1177_09612033211013898 for Plasma osteopontin versus intima media thickness of the common carotid arteries in well-characterised patients with systemic lupus erythematosus by Lina Wirestam, Muna Saleh, Christina Svensson, Michele Compagno, Helene Zachrisson, Jonas Wetterö and Christopher Sjöwall in Lupus
Acknowledgements
We thank Marianne Petersson for biobank administration and all the clinicians at the Rheumatology Unit, Linköping University Hospital for their efforts.
Data availability statement: The raw data supporting the conclusions of this article will be made available by the authors, upon reasonable request, without undue reservation.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by grants from the Swedish Rheumatism Association, the Region Östergötland (ALF Grants), the King Gustaf V’s 80-year Anniversary Foundation and the King Gustaf V and Queen Victoria’s Freemasons’ Foundation.
ORCID iDs: Muna Saleh https://orcid.org/0000-0002-4836-6373
Jonas Wetterö https://orcid.org/0000-0002-6916-5490
Christopher Sjöwall https://orcid.org/0000-0003-0900-2048
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Hahn BH. Systemic lupus erythematosus and accelerated atherosclerosis. N Engl J Med 2003; 349: 2379–2380. [DOI] [PubMed] [Google Scholar]
- 2.Bengtsson C, Öhman M-L, Nived O, Dahlqvist SR. . Cardiovascular event in systemic lupus erythematosus in Northern Sweden: incidence and predictors in a 7-year follow-up study. Lupus 2012; 21: 452–459. [DOI] [PubMed] [Google Scholar]
- 3.Arkema EV, Svenungsson E, Von Euler M, Sjöwall C, Simard JF. Stroke in systemic lupus erythematosus: a Swedish population-based cohort study. Ann Rheum Dis 2017; 76: 1544–1549. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352: 1685–16–95. [DOI] [PubMed] [Google Scholar]
- 5.Bruce IN, Gladman DD, Urowitz MB. Premature atherosclerosis in systemic lupus erythematosus. Rheum Dis Clin North Am 2000; 26: 257–278. [DOI] [PubMed] [Google Scholar]
- 6.Kostopoulou M, Nikolopoulos D, Parodis I, Bertsias G. Cardiovascular disease in systemic lupus erythematosus: recent data on epidemiology, risk factors and prevention. Curr Vasc Pharmacol 2020; 18: 549–565. [DOI] [PubMed] [Google Scholar]
- 7.Hasunuma Y, Matsuura E, Makita Z, Katahira T, Nishi S, Koike T. Involvement of beta 2-glycoprotein I and anticardiolipin antibodies in oxidatively modified low-density lipoprotein uptake by macrophages. Clin Exp Immunol 1997; 107: 569–573. [DOI] [PubMed] [Google Scholar]
- 8.Gómez-Zumaquero JM, Tinahones FJ, De Ramón E, Camps M, Garrido L, Soriguer FJ. Association of biological markers of activity of systemic lupus erythematosus with levels of anti-oxidized low-density lipoprotein antibodies. Rheumatology (Oxford) 2004; 43: 510–513. [DOI] [PubMed] [Google Scholar]
- 9.Matsuura E, Kobayashi K, Inoue K, Lopez LR, Shoenfeld Y. Oxidized LDL/beta2-glycoprotein I complexes: new aspects in atherosclerosis. Lupus 2005; 14: 736–741. [DOI] [PubMed] [Google Scholar]
- 10.Goossens P, Gijbels MJJ, Zernecke A, et al. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab 2010; 12: 142–153. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Fu Q, Cui H, et al. Interferon-α priming promotes lipid uptake and macrophage-derived foam cell formation: a novel link between interferon-α and atherosclerosis in lupus. Arthritis Rheum 2011; 63: 492–502. [DOI] [PubMed] [Google Scholar]
- 12.Rahman P, Urowitz MB, Gladman DD, Bruce IN, Genest J. Jr. Contribution of traditional risk factors to coronary artery disease in patients with systemic lupus erythematosus. J Rheumatol 1999; 26: 2363–2368. [PubMed] [Google Scholar]
- 13.Pons-Estel GJ, González LA, Zhang J, et al. Predictors of cardiovascular damage in patients with systemic lupus erythematosus: data from LUMINA (LXVIII), a multiethnic US cohort. Rheumatology (Oxford) 2009; 48: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tselios K, Sheane BJ, Gladman DD, Urowitz MB. Optimal monitoring for coronary heart disease risk in patients with systemic lupus erythematosus: a systematic review. J Rheumatol 2016; 43: 54–65. [DOI] [PubMed] [Google Scholar]
- 15.Barile-Fabris L, Hernández-Cabrera MF, Barragan-Garfias JA. Vasculitis in systemic lupus erythematosus. Curr Rheumatol Rep 2014; 16: 440. [DOI] [PubMed] [Google Scholar]
- 16.Lok ZSY, Lyle AN. Osteopontin in vascular disease. Arterioscler Thromb Vasc Biol 2019; 39: 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clemente N, Raineri D, Cappellano G, et al. Osteopontin bridging innate and adaptive immunity in autoimmune diseases. J Immunol Res 2016; 2016: 7675437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YH, Song GG. Correlation between circulating osteopontin level in systemic lupus erythematosus and disease activity and associations between osteopontin polymorphisms and disease susceptibility: a meta-analysis. Lupus 2017; 26: 132–138. [DOI] [PubMed] [Google Scholar]
- 19.Shinohara ML, Lu L, Bu J, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nature Immunol 2006; 7: 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbone F, Rigamonti F, Burger F, et al. Serum levels of osteopontin predict major adverse cardiovascular events in patients with severe carotid artery stenosis. Int J Cardiol 2018; 255: 195–199. [DOI] [PubMed] [Google Scholar]
- 21.Carbone F, Dallegri F, Montecucco F, et al. Serum osteopontin negatively impacts on intima-media thickness in patients with systemic lupus erythematosus. Eur J Clin Invest 2019; 49: e13089. [DOI] [PubMed] [Google Scholar]
- 22.Wendelin-Saarenhovi M, Oikonen M, Loo BM, et al. Plasma osteopontin is not associated with vascular markers of subclinical atherosclerosis in a population of young adults without symptoms of cardiovascular disease. The cardiovascular risk in young Finns study. Scand J Clin Lab Invest 2011; 71: 683–689. [DOI] [PubMed] [Google Scholar]
- 23.Henrot P, Foret J, Barnetche T, et al. Assessment of subclinical atherosclerosis in systemic lupus erythematosus: a systematic review and meta-analysis. Joint Bone Spine 2018; 85: 155–163. [DOI] [PubMed] [Google Scholar]
- 24.Svensson C, Eriksson P, Zachrisson H, Sjöwall C. High-frequency ultrasound of multiple arterial areas reveals increased intima media thickness, vessel wall appearance, and atherosclerotic plaques in systemic lupus erythematosus. Front Med (Lausanne) 2020; 7: 581336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ighe A, Dahlstrom O, Skogh T, Sjowall C. Application of the 2012 systemic lupus international collaborating clinics classification criteria to patients in a regional Swedish systemic lupus erythematosus register. Arthritis Res Ther 2015; 17: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64: 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the systemic lupus international collaborating clinics/American college of rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996; 39: 363–369. [DOI] [PubMed] [Google Scholar]
- 28.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29: 288–291. [PubMed] [Google Scholar]
- 29.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 30.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4: 295–306. [DOI] [PubMed] [Google Scholar]
- 31.Wirestam L, Enocsson H, Skogh T, et al. Osteopontin and disease activity in patients with recent-onset systemic lupus erythematosus: results from the SLICC inception cohort. J Rheumatol 2019; 46: 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53: 766–772. [DOI] [PubMed] [Google Scholar]
- 33.Enocsson H, Wirestam L, Dahle C, et al. Soluble urokinase plasminogen activator receptor (suPAR) levels predict damage accrual in patients with recent-onset systemic lupus erythematosus. J Autoimmun 2020; 106: 102340. [DOI] [PubMed] [Google Scholar]
- 34.Quaglia M, Chiocchetti A, Cena T, et al. Osteopontin circulating levels correlate with renal involvement in systemic lupus erythematosus and are lower in ACE inhibitor-treated patients. Clin Rheumatol 2014; 33: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 35.Wirestam L, Frodlund M, Enocsson H, Skogh T, Wetterö J, Sjöwall C. Osteopontin is associated with disease severity and antiphospholipid syndrome in well characterised Swedish cases of SLE. Lupus Sci Med 2017; 4: e000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sepúlveda JIR, Bolin K, Mofors J, et al.; the DISSECT consortium. Sex differences in clinical presentation of systemic lupus erythematosus. Biol Sex Differ 2019; 10: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alarcón GS, Roseman JM, McGwin G, Jr, et al. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004; 43: 202–205. [DOI] [PubMed] [Google Scholar]
- 38.Bruce IN, O’Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the systemic lupus international collaborating clinics (SLICC) inception cohort. Ann Rheum Dis 2015; 74: 1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rullo OJ, Woo JM, Parsa MF, et al. Plasma levels of osteopontin identify patients at risk for organ damage in systemic lupus erythematosus. Arthritis Res Ther 2013; 15: R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolak T. Osteopontin – a multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis 2014; 236: 327–337. [DOI] [PubMed] [Google Scholar]
- 41.Singh R, Hui T, Matsui A, et al. Modulation of infection-mediated migration of neutrophils and CXCR2 trafficking by osteopontin. Immunology 2017; 150: 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol 2014; 192: 5459–5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somers EC, Zhao W, Lewis EE, et al. Type I interferons are associated with subclinical markers of cardiovascular disease in a cohort of systemic lupus erythematosus patients. PloS One 2012; 7: e37000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siltari A, Vapaatalo H. Vascular calcification, vitamin K and warfarin therapy – possible or plausible connection? Basic Clin Pharmacol Toxicol 2018; 122: 19–24. [DOI] [PubMed] [Google Scholar]
- 45.Poterucha TJ, Goldhaber SZ. Warfarin and vascular calcification. Am J Med 2016; 129: 635.e1–635.e4. [DOI] [PubMed] [Google Scholar]
- 46.Rahman P, Gladman DD, Urowitz MB, Yuen K, Hallett D, Bruce IN. The cholesterol lowering effect of antimalarial drugs is enhanced in patients with lupus taking corticosteroid drugs. J Rheumatol 1999; 26: 325–330. [PubMed] [Google Scholar]
- 47.Tam LS, Gladman DD, Hallett DC, Rahman P, Urowitz MB. Effect of antimalarial agents on the fasting lipid profile in systemic lupus erythematosus. J Rheumatol 2000; 27: 2142–2145. [PubMed] [Google Scholar]
- 48.Desai RJ, Eddings W, Liao KP, Solomon DH, Kim SC. Disease-modifying antirheumatic drug use and the risk of incident hyperlipidemia in patients with early rheumatoid arthritis: a retrospective cohort study. Arthritis Care Res 2015; 67: 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu D, Mackenzie NC, Farquharson C, Macrae VE. Mechanisms and clinical consequences of vascular calcification. Front Endocrinol (Lausanne) 2012; 3: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation 2008; 117: 2938–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-lup-10.1177_09612033211013898 for Plasma osteopontin versus intima media thickness of the common carotid arteries in well-characterised patients with systemic lupus erythematosus by Lina Wirestam, Muna Saleh, Christina Svensson, Michele Compagno, Helene Zachrisson, Jonas Wetterö and Christopher Sjöwall in Lupus