Abstract

The application of cyclical microwave modification for accelerating the extraction of coalbed methane (CBM) from anthracite is limited. In this study, the apparent permeability of anthracite samples before and after each microwave treatment (three in total) for 120 s was measured by a self-built permeability-testing platform. Microcomputed tomography (micro-CT) technology and image-processing technology were employed to analyze the 3D micron-scale pore structures, especially the quantitative characterization of connected pores and throats. After modification, the average apparent permeability increased from 0.6 to 5.8 × 10–3 μm2. The generation, expansion, and connection of micron-scale pores and fractures became more obvious with each treatment. The total porosity increased from 3.5 to 6.2%, the connected porosity increased from 0.9 to 4.8%, and the porosity of isolated pores decreased from 2.5 to 1.4% after three cycles. The number, volume, and surface area of the connected pores as well as the number, radius, and surface area of the throats were significantly increased. In addition, the release of alkyl side chains from the anthracite surface reduced the capacity of the anthracite to adsorb CH4 and the decomposition of minerals promoted the development and connectivity of pores. As a result, the gas seepage channels have been greatly improved. This work provides a basis for micron-scale pore characterization after cyclical microwave modification and contributes to CBM extraction.
1. Introduction
Coalbed methane (CBM) provides a clean energy supply for the world.1 Since the 1970s, CBM has developed into a sustainable commodity with a great economic value in the United States and Canada.2 The CBM reserves in China are approximately 36.81 × 1012 m3 and rank third after Russia and Canada.3 In the past, CBM was only discharged to avoid coal/gas outbursts or gas explosions during coal production.4−6 It was not until 2003 that the first commercial well for CBM extraction in China was reported.7 Subsequently, China’s CBM extraction industry developed rapidly. However, the efficiency of CBM extraction is extremely low in most areas due to poor geological conditions such as low permeability and low porosity, which restricts the commercialization process.
Many approaches have been considered in the effort to stimulate coal reservoirs. Methods other than CO2 substitution and N2 displacement will have a significant effect on reservoir permeability.8 One approach is fluid injection, which includes hydraulic,9 high energy gas,10,11 supercritical CO2,12,13 liquid nitrogen,14−16 and steam injection fracturing.17,18 Another approach is the application of external acoustic,19−21 electric,22 electromagnetic,23−26 or electrochemical fields.27−33 Although these methods have achieved enhancements, there are still some limitations, such as water locking damage, construction costs, complex operations, ecological environmental pollution, and even the possibility of inducing earthquakes by hydraulic fracturing.34,35 At present, microwave modification technology has been successfully used for coking,36,37 reduction of energy for pulverization,38 lignite dehydration,39 coal desulfurization,40 low-rank coal pyrolysis,41 biomass pyrolysis,42 enhancing floatation,43 auxiliary rock breaking,44 heavy oil exploitation,45 and oil shale exploitation.46
Microwave modification can increase the temperature of the coal or rock stratum and aid in moisture removal. Microwave radiation heating is a highly efficient and environmentally benign method for reservoir stimulation due to its unique instantaneous effects, overall penetrability, selectivity, and controllability. Microwaves are electromagnetic waves with frequency in the 300 MHz to 300 GHz range. The essence of the heating effect is dielectric relaxation; that is, under the effect of an electric field, the dipole moments of polar molecules rotate. When the electric field frequency is equal to the microwave frequency, the rotation speed of electric dipoles cannot keep up with the frequency of the microwave electric field, resulting in a hysteresis phenomenon.47 Hong et al.,48 Xu et al.,49 and Huang et al.50 carried out simulations of the heating behavior of coal under microwave radiation using COMSOL and reported that microwaves can rapidly heat coal. Cai et al.,51 Teng et al.,52 and Zhao et al.53 found that the pore structures and permeability of coal change under high-temperature conditions. Wang et al.54 found that high temperatures can induce cracks in the hot dry rock and improve its brittleness index and even increase its permeability by an order of magnitude.
Micro-CT is a high-efficiency method to analyze micron-scale pores, fractures, cracks, and cleats without causing any structural damage in coal samples.55−57 Feng and Zhao58 observed the characteristics of mesocrack evolution in lignite and gas coal with temperature variation. Kang et al.59 observed and analyzed the thermal cracking process of oil shale from 20 to 600 °C. The structural parameters of micron-size pores can be characterized quantitatively by the postprocessing of images. Kong et al.60 measured the pore-fracture features of anthracite such as pore number, porosity, and average pore diameter before and after electrochemical modification combined with MATLAB software. Huang et al.61 studied the connectivity of the pores and fractures in oil shale at different steam temperatures by digitization of cores. Kumar et al.38 determined the cleat frequency and distribution in two cores and confirmed that new fractures were induced by exposure to high-energy microwaves. Yao et al.62 demonstrated the capability of micro-CT to characterize the development of coal porosity and fractures and found that the distribution characteristics of porosity were highly anisotropic. Cai et al.63 examined the evolution of a 3D fracture network under stress until failure occurred by micro-CT and acoustic emission.
In this paper, the apparent permeability was studied for anthracite samples before and after they underwent cyclical microwave modification; measurements were performed with a permeability-testing platform that was built in this laboratory. The structure of micron-scale pores before and after modification, especially the connected pores that make the main contribution to gas seepage in anthracite, were quantitatively characterized by micro-CT combined with the image-processing technology. Moreover, the surface groups on anthracite and the minerals in the coal were investigated by Fourier transform infrared (FTIR) spectroscopy.
2. Results and Discussion
2.1. Influence of Cyclical Microwave Modification on the Apparent Permeability of Anthracite
Apparent permeability plays an important role in the evaluation of recovery efficiency of CBM extraction.2 Li et al. found that the apparent permeability of anthracite in Qinshui Basin is in the range of 0.01 × 10–3 to 10 × 10–3 μm2.64 The variation law of apparent permeability of anthracite after cyclical microwave modification is shown in Figure 1a, and the variation fitting results are shown in Figure 1b. It can be seen that the apparent permeability of the raw anthracite sample was in the range of 0.5–0.7 × 10–3 μm2, and the results were consistent with Li et al.2 After microwave exposures for 1, 2, and 3 cycles, the average apparent permeability increased from 0.6 to 3.6, 5.0, and 5.8 × 10–3 μm2, increasing by 5.1, 7.4, and 8.8 times, respectively. The results showed that improving apparent permeability by cyclical microwave modification can effectively accelerate the extraction of CBM. The modification mechanism would be analyzed by the changes in microscale pore structures, surface groups, and mineral materials in anthracite.
Figure 1.
Change in apparent permeability of anthracite with the number of treatment cycles. (a) Experimental results. (b) Fitting results.
2.2. Influence of Cyclical Microwave Modification on the Porosity of Anthracite
The change in pore structures after three cycles of microwave exposure is exhibited in a 3D representative volume element (3D-REV) in Figure 2. The porosity of the total pores and connected pores increased, while the porosity of the isolated pores decreased with cyclical microwave modification, as shown in Figure 3. The total porosity increased from 3.5 to 6.2% and the connected porosity increased from 0.9 to 4.8%, increases of 77.6 and 409.6%, respectively. The porosity of isolated pores decreased from 2.5 to 1.4%, a reduction of 45.3%.
Figure 2.
Schematic diagram of the total pores, connected pores, and isolated pores of the 3D-REV before and after cyclical microwave modification. (a–c) Total pores, connected pores, and isolated pores of the unmodified sample, respectively. (d–f) Total pores, connected pores, and isolated pores of the modified sample, respectively.
Figure 3.

Porosity of the 3D-REV before and after cyclical microwave modification.
This phenomenon indicated that microwave radiation had an obvious effect on the generation, expansion, and connection of micron-sized pores and fractures, which could be attributed to several factors. Images were obtained using micro-CT scanning at the same positions before and after three cycles of microwave modification, as shown in Figure 4. New micron-scale pores developed in zone A1, and the micron-scale pores and fractures in zone A2 became wider. The main reason for these changes was that the moisture in pores quickly evaporates and expands when stimulated by microwave radiation; this behavior may open some closed pores. The residual water in the coal matrix and part of the water bound to the minerals evaporate and is removed under microwave radiation; the high-pressure steam creates new pores, widens the original pores, and increases the pore connectivity. It can be seen that in zone A3, new cracks formed at the coal–mineral interfaces because the thermal conductivities and thermal expansion coefficients differ for coal and minerals, causing different temperature increases in coal and minerals under microwave irradiation. In addition, the high temperature (locally up to 369 °C after 120 s of microwave irradiation) causes thermolysis in the macromolecular structure of coal. We can see in zone B that some minerals that were removed under microwave irradiation led to the formation of new micron-scale pores. The reason for the formation of new pores may have been that the microwaves catalyzed the chemical reaction of pyrite (FeS2) in the coal with the surrounding H2, O2, or small molecules such as H2O, CO, and CO2 adsorbed in the coal; these reactions may have released gases such as H2S, SO2, and carbonyl sulfide (COS).65 Besides, some minerals may have been displaced and fallen into the fractures in more highly fractured regions.38
Figure 4.
Illustration of the expansion and development of micron-scale pores. The gray zone, the black zone, and the white zone represent the coal matrix, the micron-scale pores/fractures, and the minerals contained in the coal sample, respectively.
2.3. Quantitative Characterization of Connected Pores and Throats before and after Cyclical Microwave Modification
The pores and throats of connected pores in the 3D-REV were characterized quantitatively based on a pore network model (PNM).66Figure 5 shows the changes in pore parameters in the 3D-REV before and after cyclical microwave modification. The pore number, pore volume, and pore surface area all increased at first and then decreased with increasing pore radius. The total number of connected pores increased from 2651 to 10,020, the total volume increased from 8.9 to 45.3 mm3, and the total area increased from 609.2 to 1899.6 mm2, the increase being 278, 410, and 212%, respectively. The number of pores with a radius of 70 μm corresponded to the maximum in the pore size distribution before and after modification, 417 and 1394, respectively. The maximum values of the pore volume and surface area occurred for pores with the maximum radii of 130 and 90–130 μm, respectively, after modification and 90–100 μm (both) before modification. The increase due to modification was the largest for the total volume of connected pores, which indicated that some of the pores became larger under the action of microwave radiation. The increase was larger for the total number of connected pores than the surface area, indicating that some isolated pores developed into connected pores during the modification process.
Figure 5.
Changes in pore parameters of the 3D-REV before and after cyclical microwave modification. The statistical results for (a) pore quantity, (b) pore volume, and (c) pore surface area, respectively.
The throat parameters can essentially reflect the connectivity of pores. Figure 6 shows the throat parameters of the 3D-REV before and after cyclical microwave modification. The number of throats increased from 6607 before modification to 33,120 after modification; similarly, the maximum throat radius increased from 110 to 260 μm, and the maximum throat length decreased from 2710 to 1430 μm. The largest contributions to the throat surface area were throats with radii of 0–100 μm before modification and radii of 0–200 μm after modification. The decrease in throat length and increase in throat radius and surface area all indicated that the pore connectivity was better after modification.
Figure 6.
Throat parameters of the 3D-REV before and after cyclical microwave modification. The statistical results for (a) throat quantity, (b) throat length, and (c) throat surface area, respectively.
The pore coordination number represents the mutual configuration relationship between pores and throats, which is numerically equal to the number of throats connected to a pore. Figure 7 shows the relationship between the pore number and the pore coordination number before and after modification. The number of pores with coordination numbers less than 10 was 2591 (98% of the total) before modification and 9427 (94%) after modification. The maximum pore coordination number increased from 22 before modification to 29 after modification. The number of connected pores increased and that their connectivity improved due to cyclical microwave modification.
Figure 7.

Relationship between pore quantity and pore coordination number before and after cyclical microwave modification.
2.4. Change in Surface Groups on Anthracite and Minerals in Anthracite
The surface groups and minerals can affect the gas seepage behavior by influencing the adsorption/desorption of methane and pore structures in anthracite. The chemical bonds in sulfur-containing groups such as mercaptans (−SH), thioethers (−S−), and thiophenes (−C4H4S) in the coal macromolecular structure break when they resonate with the electromagnetic microwaves, and some alkyl side chains and oxygen-containing functional groups in coal are pyrolyzed and released as gas.65Figure 8 shows the FTIR results for surface groups on anthracite samples before and after cyclical microwave modification. The peaks near 2925 and 2855 cm–1 are identified as the stretching vibrations of −CH2 and −CH3, respectively.67,68 The peaks near 2515 and 460 cm–1 are attributed to the vibrations of S–H.69 The peak at 1600 cm–1 corresponds to the vibrations of C=O and C=C.70 The peak at 1430 cm–1 is identified as the bending vibration of −CH3 and the antisymmetric stretching vibration of carbonate groups.32 The peak near 1030 cm–1 is attributed to the stretching vibrations of Si–O–Si and Si–O–C.33 The peak near 540 cm–1 corresponds to the vibrations of S–S.33 The adsorption peaks near 2925, 2855, and 1430 cm–1 are slightly smaller for modified samples than unmodified samples, indicating that some of the methyl and methylene groups were removed and the adsorption capacity of coal was weaker.71 The decrease in CH4 adsorption indicated that the gas seepage performance in coal was improved because of the coal matrix shrinkage effect.72,73 The intensities of absorption peaks near 2515, 1030, 540, and 460 cm–1 decreased notably after modification of the samples, indicating that microwave radiation broke some sulfur-containing bonds and decomposed some minerals, such as sulfur, carbonate, and silicate. The decomposition of minerals would increase the pores in anthracite. This phenomenon is also observed in the micron-scale pores and fractures shown in Figure 4 (zone B).
Figure 8.

FTIR spectra of anthracite samples before and after cyclical microwave modification.
3. Application and Significance
Microwaves can heat the reservoir rapidly within the distance that microwaves penetrate. The penetrability and controllability of microwaves make their heating efficiency higher and more convenient than traditional conduction heating. More importantly, the whole modification process causes limited pollution of the environment. Therefore, in the field of engineering applications, the cyclical microwave modification method can not only inhibit methane adsorption and accelerate methane seepage but also make a significant contribution to environmental protection.
Figure 9 shows a schematic diagram of the method for accelerating CBM extraction by microwave radiation combined with water injection. Microwave treatment and water injection are performed alternately by the system. High-pressure steam produced by rapid vaporization of water by microwaves can cause cracks to grow rapidly and relieve water locking damage caused by water injection. The cyclical temperature impact makes the coal reservoir expand and contract repeatedly, which is conducive to the development of pores and fractures. Yang and Liu15 used experiments and modeling to study the changes in the pore structure of coal that were induced by cyclic nitrogen injections, they found that the total volume of mesopores (2–50 nm) and macropores (>50 nm) increased with cryogenic treatment, while the growth rate of pore volume decreased with increasing numbers of freeze–thaw times.
Figure 9.
Schematic diagram of enhanced CBM extraction by microwave radiation combined with water injection.
4. Conclusions
-
(1)
For accelerating CBM extraction, the influence of cyclical microwave modification on the apparent permeability of anthracite in Sihe mine, China, was studied, and the change in microscale pore structures, surface groups, and minerals in anthracite before and after microwave modification was measured.
-
(2)
With the increase in cyclical microwave modification times, the apparent permeability of anthracite increased continuously due to the continuous increase in the quantity and connectivity of micron-scale pores.
-
(3)
The gasified release of alkyl side chains on anthracite surface reduced the CH4 adsorption capacity, and the decomposition of mineral materials in anthracite increased the micron-scale pores.
-
(4)
The changing law of anthracite apparent permeability after three times of single power microwave modification has been researched. The effect of cyclical microwave modification on various metamorphic degree coals with different electric powers and different cycles will be further studied to achieve more parameters for in situ engineering application.
5. Experimental Section
5.1. Sample Preparation
The anthracite coal samples for the cyclical microwave treatment experiment were obtained from the 15,303 freshly exposed working face of the Sihe mine of the Qinshui coalfield, Shanxi Province, China. The large lumps of coal were carefully selected and immediately packed in plastic wrap and sealed in bags, and then, they were sent to the laboratory as soon as possible to avoid changes in their physicochemical properties due to oxidation. The coring direction was perpendicular to the bedding of the large lumps of coal, and the cylindrical samples were polished to a size of 50 mm diameter and 50 mm height. In addition, powdered samples with a size of 60–80 mesh were prepared. The mean maximum vitrinite reflectance (Ro,max), the proximate analysis, the elemental composition, and the maceral composition of the anthracite were determined following the standards GB/T 6948-2008, GB/T 212-2008, GB/T 476-2001, and GB/T 8899-2013, respectively.32,33 The analysis results are listed in Table 1.
Table 1. Petrologic Characteristics, Elemental Composition, and Proximate Analysis of Coal Samplesa.
| proximate analysis (wt %) |
ultimate analysis (%, daf) |
maceral groups (vol %) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| samples | Ro,max (%) | moisture, ad | ash yield, ad | volatile matter, daf | C | H | O | S | vitrinite | inertinite | liptinite |
| anthracite | 2.86 | 1.65 | 5.21 | 6.12 | 86.52 | 2.64 | 6.83 | 3.32 | 86.3 | 13.7 | 0.0 |
ad: air dried basis; daf: dry ash-free basis.
5.2. Experimental Apparatus
The tests of apparent permeability of the anthracite samples before and after cyclical microwave modification were conducted using a self-built permeability-testing platform. Figure 10 shows the schematic of the experimental apparatus. The device is mainly composed of a vacuum-pumping system, a confining pressure-loading system, an inlet/outlet system, and a measuring system. Before the test, the coal samples and the porous metal gaskets at both ends were placed together in the sealing sleeve and were sealed between the base and the axial piston with two sealing rings.
Figure 10.
Schematic of the seepage experiment apparatus.
The cyclical microwave modification experiment of the anthracite samples was carried out in a P70F20CL-DG(B0) microwave oven produced by Guangdong Galanz company. The dimensions of the resonator in the oven were 180 mm high, 315 mm wide, and 329 mm deep. The frequency of the microwave oven was 2.45 × 109 ± 50 Hz. The maximum power was 700 W and could be adjusted.
The micro-CT scanning of the anthracite samples was conducted with a nanoVoxel-4000 open tube reflective high penetration CT system produced by Sanying Precision Instruments Co., Ltd. The scanning voltage was 150 kV, the scanning current was 150 μA, the exposure time was 3.5 s, and the spatial resolution was 19.6 μm. Sixteen-bit images with 3000 × 3000 × 3000 voxels were obtained after the whole sample was scanned and processed.
The analysis of the surface groups of the anthracite samples was performed using a Nicolet iS5 FTIR instrument produced by Thermo Fisher company. The detection spectral range of the instrument was 350–7800 cm–1. The resolution and accuracy of the instrument were better than 0.5 and 0.01 cm–1, respectively, and the signal-to-noise ratio was 40,000:1. Before the test, the dried fine anthracite particles and potassium bromide (KBr) were fully ground in an agate bowl at a ratio of 1:150 wt %; then, this powder was loaded into a mold, and tablets were pressed under a pressure of 10 MPa.
5.3. Experimental Process
Figure 11 shows the schematic of the cyclical microwave modification experimental procedure. Before the experiment, the powdered anthracite samples were dried to a constant weight in a vacuum-drying oven at 373–378 K. First, the micro-CT scanning and permeability measurements of the cylindrical raw anthracite sample were carried out. At the same time, the infrared spectrum of anthracite powder was measured. Second, the sample was irradiated in the microwave oven for 120 s with a power of 700 W. Then, the micro-CT scans, seepage measurements, and FTIR measurements were repeated with the modified sample. According to this procedure, the experiment was completed after three microwave modification cycles. The temperature of the sample was detected using an AS852B infrared thermometer produced by Smart sensor company. The temperature detection range was −50–750 °C with an accuracy of ±2%, a resolution of 0.1 °C, and a response time of 500 ms.
Figure 11.

Schematic of the cyclical microwave modification experimental procedure.
5.4. Permeability Data
The apparent permeability of anthracite can be calculated using eq 1.74
| 1 |
where ka is the apparent permeability of the anthracite sample (10–3 μm2), q is the flow rate of the CH4 (cm3/s), μ is the dynamic viscosity of the CH4 at a pressure of (P1 + P2)/2 (mPa·s), L is the length of the anthracite sample (cm), A is the cross-sectional area of the anthracite sample (cm2), P0 is the standard atmospheric pressure (MPa), P1 is the methane pressure at the inlet (MPa), and P2 is the methane pressure at the outlet (MPa). The confining pressure was set at 2 MPa, and the outlet pressure was set at atmospheric pressure. The inlet pressure was set at 0.5, 0.9, 1.5, 1.9, and 2.5 MPa, respectively. Effective stress is the difference between the confining stress and the average pore fluid pressure70
| 2 |
where σe is the effective stress (MPa), σc is the confining stress (MPa), P is the average pore fluid pressure (MPa), and α is the effective stress coefficient (dimension-less, approximate to 1).
The relationship between the effective stress and confining pressure, inlet pressure, and outlet pressure is listed in Table 2. The calculation method is consistent with Li et al.75
Table 2. Calculation Results of Effective Stressa.
| P1 (MPa) | P2 (MPa) | P (MPa) | σc (MPa) | σe (MPa) |
|---|---|---|---|---|
| 0.5 | 0.1 | 0.3 | 2 | 1.7 |
| 0.9 | 0.1 | 0.5 | 2 | 1.5 |
| 1.5 | 0.1 | 0.8 | 2 | 1.2 |
| 1.9 | 0.1 | 1.0 | 2 | 1.0 |
| 2.5 | 0.1 | 1.3 | 2 | 0.7 |
P1: inlet pressure; P2 outlet pressure; P: average pore fluid pressure; σc: confining stress; and σe: effective stress.
5.5. Image Processing
The 3D images before and after cyclical microwave modification were reconstructed to visualize and analyze the changes in the internal pore-fracture characteristics. The brightness and contrast of the images were adjusted to the appropriate ranges. Then, processes for denoising and enhancement were carried out, and edge detection and binarization segmentation were conducted. The coal matrix, minerals, and pores were divided by interactive thresholding combined with an interactive top-hat transform.
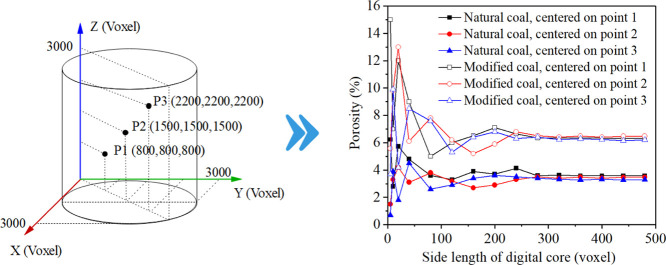
The 3D-REV is a small cube that can reflect the pore structure of the whole coal sample. More petrophysical properties of coal samples can be reflected when more voxels are contained in the 3D-REV. However, the selected 3D-REV cannot be too large due to the limited computing capacity.76Figure 12 shows the change in porosity of the cube with the change in side length (voxels) before and after cyclical microwave modification with point 1 (800, 800, 800), point 2 (1500, 1500, 1500), and point 3 (2200, 2200, 2200) as central points. When the side length was greater than 280 voxels, the porosity tended to be a certain value and was approximately equal to the porosity of the whole sample. In this study, a cube with point 2 (1500, 1500, 1500) as the center point and a side length of 500 voxels (9.8 mm) was selected as the 3D-REV, as shown in Figure 13. The 3D-REV was divided into 500 layers along the vertical Z-axis direction, and the porosity of each XY section was calculated to observe the internal pore structure of the 3D-REV more accurately, as shown in Figure 14. The total pores and connected pores of all XY planes increased irregularly after cyclical microwave modification, which was caused by the heterogeneous distribution of molecular structures, water, and minerals in the coal.
Figure 12.
Change in cube porosity with side length at different central positions.
Figure 13.

3D-REV of coal samples before and after cyclical microwave modification. (a) Unmodified and (b) modified. The yellow, red, and green zones represent the coal matrix, the pores/fractures, and the minerals, respectively.
Figure 14.
Porosity distribution of the 3D-REV along the Z-axis. (a) Total pores. (b) Connected pores.
The PNM was used to quantitatively characterize the pore and throat parameters before and after cyclical microwave modification. The PNM uses regular shapes to characterize the complex space in coal or rock. In this model, the maximal inscribed sphere algorithm was used to idealize the connected pores into two parts: pores and throats. Figure 15 shows the PNM of the 3D-REV before and after cyclical microwave modification. The calculation method was consistent with Silin and Patzek,77 Al-Kharusi and Blunt,78 Ngom et al.,79 Lin et al.,66 and Zhao et al.80
Figure 15.

PNM of the 3D-REV before and after cyclical microwave modification. (a) Unmodified and (b) modified.
Acknowledgments
This research was supported financially by the National Natural Science Foundation of China (U1810102, 41902179, and 42072203).
The authors declare no competing financial interest.
Notes
The data used to support the findings of this study are available from the corresponding author upon request.
References
- Liu X.; Nie B.; Wang W.; Wang Z.; Zhang L. The use of AFM in quantitative analysis of pore characteristics in coal and coal-bearing shale. Mar. Pet. Geol. 2019, 105, 331–337. 10.1016/j.marpetgeo.2019.04.021. [DOI] [Google Scholar]
- Qin Y.; Moore T. A.; Shen J.; Yang Z.; Shen Y.; Wang G. Resources and geology of coalbed methane in China: a review. Int. Geol. Rev. 2018, 60, 777–812. 10.1080/00206814.2017.1408034. [DOI] [Google Scholar]
- Liu C.; Che C.; Zhu J.; Yang H.; Fan M. Methodologies and results of the latest assessment of coalbed methane resources in China. Nat. Gas Ind. 2009, 29, 130–132. [Google Scholar]
- Wang Z.; Cheng Y.; Wang L.; Zhou H.; He X.; Yi M.; Xi C. Characterization of pore structure and the gas diffusion properties of tectonic and intact coal: Implications for lost gas calculation. Process Saf. Environ. 2020, 135, 12–21. 10.1016/j.psep.2019.12.020. [DOI] [Google Scholar]
- Zhao W.; Cheng Y.; Pan Z.; Wang K.; Liu S. Gas diffusion in coal particles: A review of mathematical models and their applications. Fuel 2019, 252, 77–100. 10.1016/j.fuel.2019.04.065. [DOI] [Google Scholar]
- Ma Y.-k.; Nie B.-s.; He X.-q.; Li X.-c.; Meng J.-q.; Song D.-z. Mechanism investigation on coal and gas outburst: An overview. Int. J. Miner., Metall. Mater. 2020, 27, 872–887. 10.1007/s12613-019-1956-9. [DOI] [Google Scholar]
- Qin Y. Situation and challenges for coalbed methane industrialization in China (I): at the current stage of growing period. Nat. Gas Ind. 2006, 26, 4–7. [Google Scholar]
- Busch A.; Gensterblum Y. CBM and CO2-ECBM related sorption processes in coal: a review. Int. J. Coal Geol. 2011, 87, 49–71. 10.1016/j.coal.2011.04.011. [DOI] [Google Scholar]
- Duan K.; Li Y.; Yang W. Discrete element method simulation of the growth and efficiency of multiple hydraulic fractures simultaneously-induced from two horizontal wells. Geomech. Geophys. Geo-energ. Geo-resour. 2021, 7, 3. 10.1007/s40948-020-00196-4. [DOI] [Google Scholar]
- Zhang R.; Zhang X.; Kang T.; He F. Dynamic fracture propagation model for oriented perforation steering fracturing in low permeability reservoir based on microelement method. J. Nat. Gas Sci. Eng. 2020, 74, 103105. 10.1016/j.jngse.2019.103105. [DOI] [Google Scholar]
- Zhang R.; Kang T.; Kang J.; Guo J.; Li L.; Zhao G. Influence of horizontal control holes on fracture height containment in low permeability layered coal measure gas reservoirs. J. Pet. Sci. Eng. 2019, 179, 104–111. 10.1016/j.petrol.2019.04.066. [DOI] [Google Scholar]
- Su E.; Liang Y.; Chang X.; Zou Q.; Xu M.; Sasmito A. P. Effects of cyclic saturation of supercritical CO2 on the pore structures and mechanical properties of bituminous coal: An experimental study. J. CO2 Util. 2020, 40, 101208. 10.1016/j.jcou.2020.101208. [DOI] [Google Scholar]
- Su E.; Liang Y.; Li L.; Zou Q.; Niu F. Laboratory study on changes in the pore structures and gas desorption properties of intact and tectonic coals after supercritical CO2 treatment: implications for coalbed methane recovery. Energies 2018, 11, 3419. 10.3390/en11123419. [DOI] [Google Scholar]
- Su S.; Gao F.; Cai C.; Du M.; Wang Z. Experimental study on coal permeability and cracking characteristics under LN2 freeze-thaw cycles. J. Nat. Gas Sci. Eng. 2020, 83, 103526. 10.1016/j.jngse.2020.103526. [DOI] [Google Scholar]
- Yang Y.; Liu S. Laboratory study of cryogenic treatment induced pore-scale structural alterations of Illinois coal and their implications on gas sorption and diffusion behaviors. J. Pet. Sci. Eng. 2020, 194, 107507. 10.1016/j.petrol.2020.107507. [DOI] [Google Scholar]
- Liu S.; Li X.; Wang D. Numerical simulation of the coal temperature field evolution under the liquid nitrogen cold soaking. Arabian J. Geosci. 2020, 13, 1215. 10.1007/s12517-020-06237-2. [DOI] [Google Scholar]
- Xie J.; Zhao Y. A mathematical model to study the coupling effect of deformation-seepage-heat transfer on coalbed methane transport and its simulative application. Math. Probl. Eng. 2020, 2020, 1247240. 10.1155/2020/1247240. [DOI] [Google Scholar]
- Wang L.; Zhao Y.; Yang D.; Kang Z.; Zhao J. Effect of pyrolysis on oil shale using superheated steam: A case study on the Fushun oil shale, China. Fuel 2019, 253, 1490–1498. 10.1016/j.fuel.2019.05.134. [DOI] [Google Scholar]
- Jia Q.; Liu D.; Cai Y.; Fang X.; Li L. Petrophysics characteristics of coalbed methane reservoir: A comprehensive review. Front. Earth Sci. 2020, (2), 1–22. 10.1007/s11707-020-0833-1. [DOI] [Google Scholar]
- Shi Q.; Qin Y.; Zhou B.; Wang X. Porosity changes in bituminous and anthracite coal with ultrasonic treatment. Fuel 2019, 255, 115739. 10.1016/j.fuel.2019.115739. [DOI] [Google Scholar]
- Shi Q.; Qin Y.; Li J.; Wang Z.; Zhang M.; Song X. Simulation of the crack development in coal without confining stress under ultrasonic wave treatment. Fuel 2017, 205, 222–231. 10.1016/j.fuel.2017.05.069. [DOI] [Google Scholar]
- Zhang J.; Si L.; Chen J.; Kizi M.; Wang C.; Chen Z. Stimulation techniques of coalbed methane reservoirs. Geofluids 2020, 2020, 5152646. 10.1155/2020/5152646. [DOI] [Google Scholar]
- Lan W.; Wang H.; Zhang X.; Fan H.; Feng K.; Liu Y.; Sun B. Investigation on the mechanism of micro-cracks generated by microwave heating in coal and rock. Energy 2020, 206, 118211. 10.1016/j.energy.2020.118211. [DOI] [Google Scholar]
- Li H.; Tian L.; Huang B.; Lu J.; Shi S.; Lu Y.; Huang F.; Liu Y.; Zhu X. Experimental study on coal damage subjected to microwave heating. Rock Mech. Rock Eng. 2020, 53, 5631–5640. 10.1007/s00603-020-02230-z. [DOI] [Google Scholar]
- Li H.; Shi S.; Lu J.; Ye Q.; Lu Y.; Zhu X. Pore structure and multifractal analysis of coal subjected to microwave heating. Powder Technol. 2019, 346, 97–108. 10.1016/j.powtec.2019.02.009. [DOI] [Google Scholar]
- Hu G.; Yang N.; Xu G.; Xu J. Experimental investigation on variation of physical properties of coal samples subjected to microwave irradiation. J. Appl. Geophys. 2018, 150, 118–125. 10.1016/j.jappgeo.2017.12.011. [DOI] [Google Scholar]
- Kang G.; Kang T.; Guo J.; Kang J.; Zhang R.; Zhang X.; Zhao G.; Zhang B.; Li L.; Zhang L. Effect of electric potential gradient on methane adsorption and desorption behaviors in lean coal by electrochemical modification: implications for coalbed methane development of Dongqu mining, China. ACS Omega 2020, 5, 24073–24080. 10.1021/acsomega.0c03496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Kang T.; Hou M.; Kang J.; Guo J.; Li L.; Zhang R.; Hu Y. Experimental research on the effects of electrode materials on methane adsorption and desorption in anthracite modified by electrochemical treatment. Chin. J. Geophys. 2020, 63, 2466–2477. [Google Scholar]
- Guo J.; Kang T.; Kang J.; Chai Z.; Zhao G. Accelerating methane desorption in lump anthracite modified by electrochemical treatment. Int. J. Geol. 2014, 131, 392–399. 10.1016/j.coal.2014.06.011. [DOI] [Google Scholar]
- Guo J.; Kang T.; Kang J.; Zhao G.; Huang Z. Effect of the lump size on methane desorption from anthracite. J. Nat. Gas Sci. Eng. 2014, 20, 337–346. 10.1016/j.jngse.2014.07.019. [DOI] [Google Scholar]
- Zhang X.; Zhang R.; Kang T.; Hu Y.; Li C. Experimental and mechanistic research on methane adsorption in anthracite modified by electrochemical treatment using selected electrode materials. Sci. Rep. 2019, 9, 17163. 10.1038/s41598-019-53840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Zhang R.; Kang T.; Hu Y. The adsorption and desorption behavior of CH4 on Jincheng anthracite modified in Fe3+ and Cu2+ ion electrolytes. Energy Fuels 2020, 34, 1251–1258. 10.1021/acs.energyfuels.9b02622. [DOI] [Google Scholar]
- Zhang L.; Kang T.; Kang J.; Zhang X.; Zhang R.; Kang G. Effects of electrolyte pH on the electro-osmotic characteristics in anthracite. ACS Omega 2020, 5, 29257–29264. 10.1021/acsomega.0c04013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R.; Atkinson G.; Eaton D. W.; Gu Y. J.; Kao H. Hydraulic fracturing volume is associated with induced earthquake productivity in the Duvernay play. Science 2018, 359, 304–308. 10.1126/science.aao0159. [DOI] [PubMed] [Google Scholar]
- Ellsworth W. L. Injection-induced earthquakes. Science 2013, 341, 1225942. 10.1126/science.1225942. [DOI] [PubMed] [Google Scholar]
- Binner E.; Lester E.; Kingman S.; Dodds C.; Robinson J.; Wu T.; Wardle P.; Mathews J. P. A review of microwave coal processing. J. Microwave Power 2014, 48, 35–60. 10.1080/08327823.2014.11689870. [DOI] [Google Scholar]
- Binner E.; Mediero-Munoyerro M.; Huddle T.; Kingman S.; Dodds C.; Dimitrakis G.; Robinson J.; Lester E. Factors affecting the microwave coking of coals and the implications on microwave cavity design. Fuel Process. Technol. 2014, 125, 8–17. 10.1016/j.fuproc.2014.03.006. [DOI] [Google Scholar]
- Kumar H.; Lester E.; Kingman S.; Bourne R.; Avila C.; Jones A.; Robinson J.; Halleck P. M.; Mathews J. P. Inducing fractures and increasing cleat apertures in a bituminous coal under isotropic stress via application of microwave energy. Int. J. Coal Geol. 2011, 88, 75–82. 10.1016/j.coal.2011.07.007. [DOI] [Google Scholar]
- Wang W.-D.; Yang X.; Sun Y.; Sun J.-T.; Zhu L.-F. Lignite dewatering rule and related influencing factors in the microwave. J. China Coal Soc. 2014, 39, 1159–1163. [Google Scholar]
- Mesroghli S.; Yperman J.; Jorjani E.; Vandewijngaarden J.; Reggers G.; Carleer R.; Noaparast M. Changes and removal of different sulfur forms after chemical desulfurization by peroxyacetic acid on microwave treated coals. Fuel 2015, 154, 59–70. 10.1016/j.fuel.2015.03.058. [DOI] [Google Scholar]
- Liu S.; Tuo K.; Wang L.; Chen G.; Ma W.; Fang M. Microwave-assisted metal-catalyzed pyrolysis of low-rank coal: promising option towards obtaining high-quality products. J. Energy Inst. 2020, 93, 1602–1614. 10.1016/j.joei.2020.01.022. [DOI] [Google Scholar]
- Wang G.; Dai Y.; Yang H.; Xiong Q.; Wang K.; Zhou J.; Li Y.; Wang S. A review of recent advances in biomass pyrolysis. Energy Fuels 2020, 34, 15557–15578. 10.1021/acs.energyfuels.0c03107. [DOI] [Google Scholar]
- Luo L.; Wu H.; Yang J.; Tang Z.; Shu K.; Xu Y.; Yan W.; Xu L. Effects of microwave pre-treatment on the flotation of ilmenite and titanaugite. Miner. Eng. 2020, 155, 106452. 10.1016/j.mineng.2020.106452. [DOI] [Google Scholar]
- Gao F.; Shao Y.; Zhou K. Analysis of microwave thermal stress fracture characteristics and size effect of sandstone under microwave heating. Energies 2020, 13, 3614. 10.3390/en13143614. [DOI] [Google Scholar]
- Zhang Y.; Adam M.; Hart A.; Wood J.; Rigby S. P.; Robinson J. P. Impact of oil composition on microwave heating behavior of heavy oils. Energy Fuels 2018, 32, 1592–1599. 10.1021/acs.energyfuels.7b03675. [DOI] [Google Scholar]
- Zhu J.; Yi L.; Yang Z.; Duan M. Three-dimensional numerical simulation on the thermal response of oil shale subjected to microwave heating. Chem. Eng. J. 2021, 407, 127197. 10.1016/j.cej.2020.127197. [DOI] [Google Scholar]
- Li H.; Shi S.; Lin B.; Lu J.; Lu Y.; Ye Q.; Wang Z.; Hong Y.; Zhu X. A fully coupled electromagnetic, heat transfer and multiphase porous media model for microwave heating of coal. Fuel Process. Technol. 2019, 189, 49–61. 10.1016/j.fuproc.2019.03.002. [DOI] [Google Scholar]
- Hong Y.-d.; Lin B.-q.; Li H.; Dai H.-m.; Zhu C.-j.; Yao H. Three-dimensional simulation of microwave heating coal sample with varying parameters. Appl. Therm. Eng. 2016, 93, 1145–1154. 10.1016/j.applthermaleng.2015.10.041. [DOI] [Google Scholar]
- Xu G.; Huang J.; Hu G.; Yang N.; Zhu J.; Chang P. Experimental study on effective microwave heating/fracturing of coal with various dielectric property and water saturation. Fuel Process. Technol. 2020, 202, 106378. 10.1016/j.fuproc.2020.106378. [DOI] [Google Scholar]
- Huang J.; Xu G.; Chen Y.; Chen Z. Simulation of microwave’s heating effect on coal seam permeability enhancement. Int. J. Min. Sci. Technol. 2019, 29, 785–789. 10.1016/j.ijmst.2018.04.017. [DOI] [Google Scholar]
- Cai Y.; Liu D.; Pan Z. Partial coal pyrolysis and its implication to enhance coalbed methane recovery: a simulation study. Energy Fuels 2017, 31, 4895–4903. 10.1021/acs.energyfuels.7b00219. [DOI] [Google Scholar]
- Teng T.; Wang J. G.; Gao F.; Ju Y. Complex thermal coal-gas interactions in heat injection enhanced CBM recovery. J. Nat. Gas Sci. Eng. 2016, 34, 1174–1190. 10.1016/j.jngse.2016.07.074. [DOI] [Google Scholar]
- Zhao Y.; Meng Q.; Feng Z.; Feng Z.; Yang D.; Zhang Y. Evolving pore structures of lignite during pyrolysis observed by computed tomography. J. Porous Media 2017, 20, 143–153. 10.1615/jpormedia.v20.i2.40. [DOI] [Google Scholar]
- Wang D.; Zhou F.; Dong Y.; Sun D.; Yu B. Experimental investigation of thermal effect on fracability index of geothermal reservoirs. Nat. Resour. Res. 2020, 30, 273–288. 10.1007/s11053-020-09733-0. [DOI] [Google Scholar]
- Li Z.; Liu D.; Cai Y.; Ranjith P. G.; Yao Y. Multi-scale quantitative characterization of 3-D pore-fracture networks in bituminous and anthracite coals using FIB-SEM tomography and X-ray μ-C. Fuel 2017, 209, 43–53. 10.1016/j.fuel.2017.07.088. [DOI] [Google Scholar]
- Nie B.; Fan P.; Li X. Quantitative investigation of anisotropic characteristics of methane-induced strain in coal based on coal particle tracking method with X-ray computer tomography. Fuel 2018, 214, 272–284. 10.1016/j.fuel.2017.10.084. [DOI] [Google Scholar]
- Lin W.; Xiong S.; Liu Y.; He Y.; Chu S.; Liu S. Spontaneous imbibition in tight porous media with different wettability: Pore-scale simulation. Phys. Fluids 2021, 33, 032013. 10.1063/5.0042606. [DOI] [Google Scholar]
- Feng Z.; Zhao Y. Pyrolytic cracking in coal: Meso-characteristics of pore and fissure evolution observed by micro-CT. J. China Coal Soc. 2015, 40, 103–108. 10.13225/j.cnki.jccs.2014.0113. [DOI] [Google Scholar]
- Kang Z.-Q.; Zhao Y.-S.; Meng Q.-R.; Yang D.; Xi B. Micro-CT experimental research of oil shale thermal cracking laws. Chin. J. Geophys. 2009, 52, 842–848. [Google Scholar]
- Kong X.; Guo J.; Kang T. Change of pore-fracture structure of anthracite modified by electrochemical treatment using micro-CT. Adv. Mater. Sci. Eng. 2018, 2018, 2651424. 10.1155/2018/2651424. [DOI] [Google Scholar]
- Huang X.; Yang D.; Kang Z. Study on the pore and fracture connectivity characteristics of oil shale pyrolyzed by superheated steam. Energies 2020, 13, 5716. 10.3390/en13215716. [DOI] [Google Scholar]
- Yao Y.; Liu D.; Che Y.; Tang D.; Tang S.; Huang W. Non-destructive characterization of coal samples from China using microfocus X-ray computed tomography. Int. J. Coal Geol. 2009, 80, 113–123. 10.1016/j.coal.2009.08.001. [DOI] [Google Scholar]
- Cai Y.; Liu D.; Mathews J. P.; Pan Z.; Elsworth D.; Yao Y.; Li J.; Guo X. Permeability evolution in fractured coal - Combining triaxial confinement with X-ray computed tomography, acoustic emission and ultrasonic techniques. Int. J. Coal Geol. 2014, 122, 91–104. 10.1016/j.coal.2013.12.012. [DOI] [Google Scholar]
- Li H.; Lau H. C.; Huang S. China’s coalbed methane development: A review of the challenges and opportunities in subsurface and surface engineering. J. Pet. Sci. Eng. 2018, 166, 621–635. 10.1016/j.petrol.2018.03.047. [DOI] [Google Scholar]
- Weng S. In-situ microwave chemical reaction of pyrite inclusions in bituminous coal. J. Nat. Sci. Huadong Normal Univ. 1996, 3, 46–50. [Google Scholar]
- Lin W.; Li X.; Yang Z.; Lin L.; Xiong S.; Wang Z.; Wang X.; Xiao Q. A new improved threshold segmentation method for scanning images of reservoir rocks considering pore fractal characteristics. Fractals 2018, 26, 1840003. 10.1142/s0218348x18400030. [DOI] [Google Scholar]
- Zhou H.; Wu C.; Pan J.; Wang Z.; Niu Q.; Du M. Research on molecular structure characteristics of vitrinite and inertinite from bituminous coal with FTIR, micro-Raman, and XRD spectroscopy. Energy Fuels 2021, 35, 1322–1335. 10.1021/acs.energyfuels.0c03586. [DOI] [Google Scholar]
- Meng D.; Yue C.; Wang T.; Chen X. Evolution of carbon structure and functional group during Shenmu lump coal pyrolysis. Fuel 2021, 287, 119538. 10.1016/j.fuel.2020.119538. [DOI] [Google Scholar]
- Cai S.; Zhang S.; Wei Y.; Sher F.; Wen L.; XuDang J. J.; Hu L. A novel method for removing organic sulfur from high-sulfur coal: Migration of organic sulfur during microwave treatment with NaOH-H2O2. Fuel 2021, 289, 119800. 10.1016/j.fuel.2020.119800. [DOI] [Google Scholar]
- Shang L.; Guanhua N.; Baisheng N.; Shouqing L.; Xijian L.; Gang W. Microstructure characteristics of lignite under the synergistic effect of oxidizing acid and ionic liquid [Bmim][Cl][J]. Fuel 2021, 289, 119940. 10.1016/j.fuel.2020.119940. [DOI] [Google Scholar]
- Li H.; Shi S.; Lin B.; Lu J.; Ye Q.; Lu Y.; Wang Z.; Hong Y.; Zhu X. Effects of microwave-assisted pyrolysis on the microstructure of bituminous coals. Energy 2019, 187, 115986. 10.1016/j.energy.2019.115986. [DOI] [Google Scholar]
- Xue S.; Zheng C.; Kizil M.; Jiang B.; Wang Z.; Tang M.; Chen Z. Coal permeability models for enhancing performance of clean gas drainage: A review. J. Pet. Sci. Eng. 2021, 199, 108283. 10.1016/j.petrol.2020.108283. [DOI] [Google Scholar]
- Long Q.; Zhao X.; Sun D.; Zou Y. Experimental study on coal permeability by adsorption. J. China Coal Soc. 2008, 33, 1030–1034. [Google Scholar]
- Fu X.; Li D.; Qin Y.; Jiang B.; Wang W.; Li G. Experimental research of influence of coal matrix shrinkage on permeability. J. China Univ. Min. Technol. 2002, 31, 129–137. [Google Scholar]
- Li J.; Liu D.; Yao Y.; Cai Y.; Chen Y. Evaluation and modeling of gas permeability changes in anthracite coals. Fuel 2013, 111, 606–612. 10.1016/j.fuel.2013.03.063. [DOI] [Google Scholar]
- Liu X.-J.; Zhu H.-L.; Liang L.-X. Digital rock physics of sandstone based on micro-CT technology. Chin. J. Geophys. 2014, 57, 1133–1140. 10.6038/cjg20140411. [DOI] [Google Scholar]
- Silin D.; Patzek T. Pore space morphology analysis using maximal inscribed spheres. Phys. A 2006, 371, 336–360. 10.1016/j.physa.2006.04.048. [DOI] [Google Scholar]
- Al-Kharusi A. S.; Blunt M. J. Network extraction from sandstone and carbonate pore space images. J. Pet. Sci. Eng. 2007, 56, 219–231. 10.1016/j.petrol.2006.09.003. [DOI] [Google Scholar]
- Ngom N. F.; Garnier P.; Monga O.; Peth S. Extraction of three-dimensional soil pore space from microtomography images using a geometrical approach. Geoderma 2011, 163, 127–134. 10.1016/j.geoderma.2011.04.013. [DOI] [Google Scholar]
- Zhao X.; Blunt M. J.; Yao J. Pore-scale modeling: Effects of wettability on waterflood oil recovery. J. Pet. Sci. Eng. 2010, 71, 169–178. 10.1016/j.petrol.2010.01.011. [DOI] [Google Scholar]