Abstract
Coronavirus disease 2019 (COVID‐19) predisposes patients to bacterial and fungal superinfections due to the impairment of the immunological system. Among the associated opportunistic fungal infections, mucormycosis is one of the least frequent but with the highest mortality. We describe two cases of mucormycosis in two kidney transplant recipients, while they were hospitalized for SARS‐CoV‐2 pneumonia, with rhinosinusal and musculoskeletal involvement, respectively.
Keywords: COVID‐19, kidney transplant, mucormycosis, transplantation
1. INTRODUCTION
Since the beginning of the coronavirus disease 2019 (COVID‐19) pandemic, there were alarming reports of secondary invasive fungal infections. 1 Although the incidence of fungal infection in COVID‐19 patients is generally low, multiple cases have been reported globally, mainly pulmonary aspergillosis. Among the hypotheses raised to explain this connection, there is the pro‐inflammatory state of the patient and the impairment of cellular immunity, in part due to the immunosuppressive regimens used in patients with severe COVID‐19. 2
Mucormycosis is an angioinvasive fungal infection associated with a high mortality, especially among immunosuppressed patients. 3 Diabetes mellitus is the main underlying disease associated with mucormycosis. Nevertheless, transplantation and hematological malignancies are also associated with this infection. A few case reports of COVID‐19‐associated mucormycosis have recently been published. Here, we present the first two cases of COVID‐19 associated mucormycosis infection in kidney recipients.
2. CASE REPORT
2.1. First case
A 62‐year‐old man with past history of type 2 diabetes mellitus was treated with insulin with non‐optimal control (last HbAc1 9.6%), and kidney transplantation 4 years prior, on immunosuppressive treatment with tacrolimus and prednisone with baseline creatinine of 1.4 mg/dL (Table 1). The patient also had history of disseminated cryptococcosis in 2018 for which he was treated with fluconazole for the first 12 months followed by isavuconazole for an additional 12 months. Three months after finishing the antifungal treatment, he was admitted to the hospital for respiratory failure due to confirmed SARS‐CoV‐2 pneumonia (November 30, 2020).
TABLE 1.
Characteristics of the two kidney transplant patients with mucormycosis
| Case 1 | Case 2 | |
|---|---|---|
| Gender/age | M/62 y | M/48 y |
| Underlying diseases |
Arterial hypertension Diabetes mellitus II (HbAc1 9.6%) End‐stage renal disease with kidney transplant (IS: tacrolimus, prednisone) Disseminated cryptococcosis (2019) Ischemic heart disease |
Arterial hypertension End‐stage renal disease with 4 kidney transplant (IS: prednisone, mycophenolate and tacrolimus). Hypothyroidism |
| COVID‐19 severity | Severe (bilateral pneumonia requiring non‐invasive mechanical ventilation) |
Moderate (FiO2 28%) |
| Systemic corticosteroid therapy for COVID‐19 | Dexametasone 6 mg daily for 10 d | Prednisone 20 mg daily (administered as immunosuppressant) |
| Concomitant treatment |
Ceftriaxone Azithromycin |
Hydroxychloroquine Azithromycin Lopinavir/ritonavir Tocilizumab |
| Time between diagnosis of COVID‐19 and mucormycosis | 1 wk | 3 wk |
| Mucormycosis associated risk factors | Diabetes, previous fungal disease, immunosuppression, steroid therapy | Immunosuppression, steroid therapy |
| Presentation | Rhinosinusal | Musculoskeletal |
| Diagnostics | Culture from the necrotic tissue | Culture from the necrotic tissue |
| Specie aisled | Rizopus oryzae | Lichtheimia ramose |
| Antifungal treatment | Liposomal amphotericin B, isavuconazole and subsequently posaconazole | Liposomal amphrotericin B and isavuconazol |
| Surgical debridement | 7 times | 3 times |
| Outcome | Alive | Alive |
The patient presented severe respiratory failure and he was transferred to the intensive care unit (ICU) requiring non‐invasive mechanical ventilation for 6 days and received treatment with intravenous (IV) dexamethasone 6 mg daily for 10 days and antibiotic empirical therapy with ceftriaxone 1 gr q24h + azithromycin 500 mg q24h. He had initial favorable clinical and radiological evolution. All bacterial cultures were negative. During admission, he did not present diabetic ketoacidosis.
After an initial clinical improvement and after being discharged from the ICU, a week later, he presented fever, headache, and left malar region swelling. A facial computed tomography (CT) was performed showing left maxillary sinusitis and empirical treatment with piperacillin/tazobactam 4.5 mg q6h IV was initiated, without any clinical improvement. A new CT scan showed progression, with a greater occupation of the left maxillary sinus with progressive cellulitis, subperiosteal abscess in the lateral wall of the left maxillary sinus and involvement of intraorbital adipose tissue (Figure 1A).
FIGURE 1.
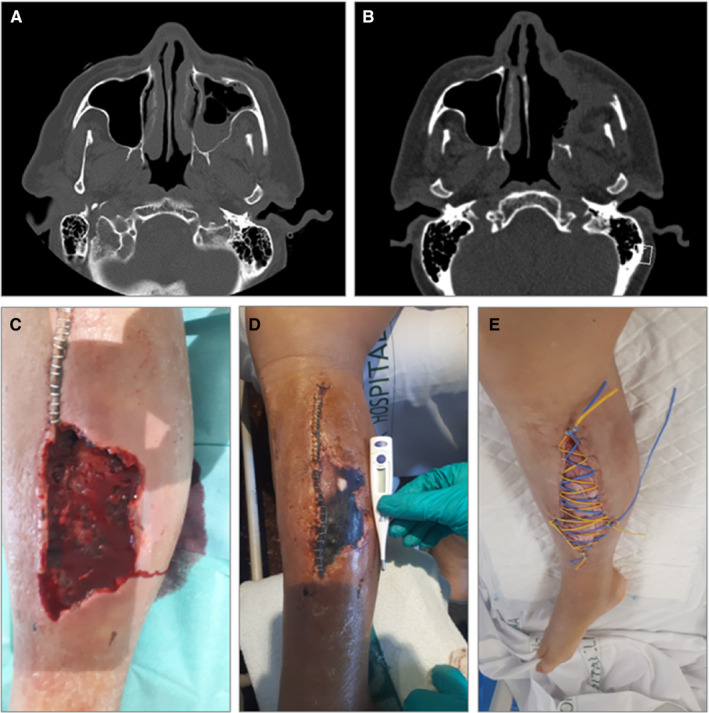
A, Case 1: occupation of the left maxillary sinus and osteomeatal complex, with trabeculation of the left premaxillary facial adipose tissue. B, Case 1: post‐surgical left maxillary, frontal, ethmoidal and sphenoid rhinosinusitis, with collections underlying the anterior and lateral wall of the left maxillary sinus and intraorbital. C–E, Case 2: lower right limb with dorsal hematoma and subsequent compartment syndrome with tissue necrosis, with posterior superinfection by Lichtheimia ramose, which required various debridement surgeries and prolonged antifungal treatment
An endoscopic evaluation and a first ENT debridement were performed. The swab culture showed Rhizopus oryzae resistant to voriconazole and caspofungin, sensitive to isavuconazole and posaconazole. A palate biopsy confirmed the diagnosis. Treatment with amphotericin B and an azole (initially isavuconazole and subsequently posaconazole) was initiated. Although isavuconazole and posaconazole could be used in this case equally, in order to avoid generating resistance to mucor (since the patient had a history of prolonged exposure to isavuconazol, and because it was easier to monitor serum levels of both tacrolimus and posaconazol to control possible interactions), the change from isavuconazol to posaconazol was performed. Within the following two months the patient underwent six subsequent ENT surgical debridement procedures, including a total left maxillectomy (Figure 1B). Prednisone dose was decreased to 2.5 mg, and tacrolimus levels were maintained between 6‐8 ng/mL. As adjuvant treatment, the patient received hyperbaric chamber therapy. Control CT showed no recurrence after 5 months of antifungal therapy.
2.2. Second case
The second case was a 48‐year‐old man with a history of chronic kidney disease due to reflux nephropathy. He received a fourth renal transplant from a cadaveric donor 5 years prior, and was on immunosuppressive treatment with prednisone, mycophenolate and tacrolimus (Table 1). He had chronic graft dysfunction due to transplant glomerulopathy, with basal creatinine of 2.8 mg/dL.
He was admitted to the hospital on April 14, 2020 with bilateral SARS‐CoV‐2 pneumonia. The patient was started on hydroxychloroquine, azithromycin and lopinavir/ritonavir and anticoagulation with prophylactic low weight heparin according to our hospital protocol at that time. Due to worsening respiratory failure, a unique dose of tocilizumab 400 mg was administered during the hospitalization. Tacrolimus and mycophenolate were stopped, and prednisone was maintained at a dose of 20 mg/d. During admission, he did not present diabetic ketoacidosis. The patient had a favorable respiratory outcome.
Three weeks after admission, the patient presented with pain and an increase of lower right limb diameter and was diagnosed with a hematoma in the dorsal area with compartment syndrome, probably secondary to anticoagulation. A fasciotomy and surgical debridement were performed, and surgical findings showed necrotic muscle tissue (Figure 1C‐E). A culture from the necrotic tissue smears showed Lichtheimia ramosa and the patient was diagnosed with musculoskeletal mucormycosis infection and treatment was started with liposomal amphotericin B 5 mg/kg q24h together with isavuconazole 200 mg/8 h for 24 days. Two other surgical debridements were performed, with negative surgical cultures. The patient received a total of 3 months of isavuconazole, with favorable outcome.
3. DISCUSSION
Immunosuppressed patients such as solid organ transplant recipients with SARS‐CoV‐2 infection have a high risk of developing severe COVID‐19, 4 and they present high mortality. 5 , 6 About 29% of these patients present diffuse alveolar damage requiring mechanical ventilation and admission to the ICU which prolongs hospital stay and increases the chances of developing fungal co‐infections. 3 , 7
In patients with severe COVID‐19 infection, there have been reports of an impairment of immunity with up balance of proinflammatory cytokines (IL‐1, IL2, IL6, TNF‐α), disturbance in the lymphocyte Th1 and Th2 responses and lower number of CD4 and CD8 cell counts. 8 In addition, these patients usually receive high‐dose corticosteroids and immunomodulatory drugs as part of COVID‐19 treatment. In this context, these patients are especially predisposed to the development of fungal infections, including mucormycosis. Maoorthy et al reported 16 cases of mucormycosis in diabetic patients treated for COVID‐19, but none of them were transplant recipients. The main risk factor in that cohort of patients was poor control of their diabetes and concomitant use of corticosteroids. 9 In our cases, the severity of SARS‐CoV‐2 infection and the specific SARS‐CoV‐2 treatment such as corticosteroids therapy were probably associated with the development of mucormycosis.
Mucormycosis is a rare invasive fungal infection, with an incidence of up to two of 1000 transplanted patients. 10 Commonly described risk factors are diabetes mellitus, re‐transplantation (probably due to multiple periods of induction with immunosuppressive therapies), chronic graft dysfunction, history of invasive fungal disease as well as long‐term use of antifungal, and chronic corticosteroid therapy. 11 On the other hand, a beneficial effect has been described with the use of calcineurin inhibitors (CNI) 12 since the calcineurin pathway plays a key role in the yeast‐hyphal and spore‐size dimorphic transitions that contributes to virulence of this uncommon fungal pathogen. Also, the inhibition of this pathway reduces the antifungal resistance. 13 , 14 An in vitro study reported that tacrolimus increased the effectiveness of posaconazole against Rhizopus oryzae. 15 In our cases, both patients had several risk factors for mucormycosis, and despite being under treatment with CNI, they developed the disease. It has been suggested that the protective effect of CNI was not patent since immunosuppressants are usually stopped or reduced as part of COVID‐19 management. Nevertheless, different mucormycosis mutations that confer resistance against CNI have been identified. 16
Rhinosinusal disease is the most common presentation of mucormycosis in solid organ transplant recipients, 3 , 10 , 17 and similar locations have been reported in COVID‐19‐associated cases. 9 However, pulmonary infection is also frequent. 18 , 19 , 20 , 21 , 22 , 23 In these cases, the differential diagnosis with invasive pulmonary aspergillosis should be considered, since these two entities have similar risk factors and may present similar radiological patterns and clinical course. 24 The diagnosis of mucormycosis is made after isolating the pathogen in cultures obtained after surgery or bronchoalveolar lavage, tissue biopsy, or PCR‐amplification. 3 , 10 The lack of clinical suspicion and the difficulty in isolating the fungus could lead to a delay in diagnosis and treatment initiation.
Currently, liposomal amphotericin B, posaconazole, isavuconazole, and itraconazole, although the latter with limited activity, are the main available therapeutic options. 25 Some studies have reported a lower mortality with the use of a combination of two antifungal compared to monotherapy. 26 More importantly one of the pillars of treatment is the surgical debridement of the necrotic tissue, which often has to be performed several times, as reflected in both of our cases. Hyperbaric chambers have been described as adjuvant therapy; although there are no randomized clinical trials, some case series data have shown a potential benefit. 27 , 28
Transplant physicians must be aware that patients with COVID‐19 are at risk of developing unusual opportunistic infections. Some authors suggest reducing immunosuppressant therapy in these patients, such as reducing or stopping CNI and antimetabolites (mycophenolate or azathioprine) in moderate or severe cases, leaving corticosteroids as monotherapy. However, in case of developing mucormycosis, different strategies other than steroids should be considered. 9 , 10 , 11 , 29
The mortality of mucormycosis is high, with an estimate of around 30%‐50%, 3 , 12 mostly attributed to impossibility to perform surgical debridement. In the published COVID‐19 related cases, an even higher mortality rate was reported, up to even 90% of the patients, and in some, the diagnosis was made post‐mortem. 18 , 19 , 20 , 21 , 22 , 23 , 30 , 31 , 32 , 33 , 34 However, lower rates have also been described in a recent review with an in‐hospital mortality of 49% in a series of 41 cases. 35 Our cases had a more favorable outcome. This is explained in part due to the presentation, since the musculoskeletal forms are associated with a better prognosis, in part due to the accessibility to obtain histological samples allowing an early diagnosis. In both cases, the patients were able to benefit from surgical debridement.
In conclusion, we believe these two cases reflect the importance of suspicion by transplant physicians of opportunistic infections in solid organ transplant recipients, particularly mucormycosis, in patients with severe COVID‐19 infection, where patients have an impaired immune response and are commonly treated with high doses of corticosteroids. It is possible that this entity might be under diagnosed since these patients often have overlapping or non‐specific symptoms, the complexity of the diagnosis, especially in cases with lung involvement.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
CA and RC: data collection, data analysis and manuscript writing. MX and SH: conceptualization, data collection, data analysis, and manuscript editing. JC: data collection. AM, MB, FC, FD, and NE: conceptualization and manuscript editing. All authors approved the latest version of the manuscript.
Arana C, Cuevas Ramírez RE, Xipell M, et al. Mucormycosis associated with COVID‐19 in two kidney transplant patients. Transpl Infect Dis. 2021;23:e13652. 10.1111/tid.13652
Carolt Arana and Cuevas Ramírez contributed equally to this manuscript.
Funding information
This work has not received funding.
Contributor Information
Carolt Arana, @AranaCarolt.
Rafael E. Cuevas Ramírez, @emmanuel_cr3.
Marc Xipell, @mxipell.
Joaquim Casals, @QCasals.
Marta Bodro, @MartaBodro.
Fritz Diekmann, Email: fdiekman@clinic.cat, @, FritzDiekmann.
DATA AVAILABILITY STATEMENT
Data partially available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Koehler P, Cornely OA, Böttiger BW, et al. COVID‐19 associated pulmonary aspergillosis. Mycoses. 2020;63:528‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pemán J, Ruiz‐Gaitán A, García‐Vidal C, et al. Fungal co‐infection in COVID‐19 patients: Should we be concerned? Rev Iberoam Micol. 2020;37:41‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song Y, Qiao J, Giovanni G, et al. Mucormycosis in renal transplant recipients: review of 174 reported cases. BMC Infect Dis. 2017;17:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cravedi P, Mothi SS, Azzi Y, et al. COVID‐19 and kidney transplantation: results from the TANGO international transplant consortium. Am J Transplant. 2020;20:3140‐3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linares L, Cofan F, Diekmann F, et al. A propensity score‐matched analysis of mortality in solid organ transplant patients with COVID‐19 compared to non‐solid organ transplant patients. PLoS One. 2021;16.e0247251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Regele F, Oberbauer R. COVID‐19 and renal transplantation. Nephrologe. 2021;1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID‐19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 8. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moorthy A, Gaikwad R, Krishna S, et al. SARS‐CoV‐2, uncontrolled diabetes and corticosteroids‐An unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi‐centric analysis. J Maxillofac Oral Surg. 2021;1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Almyroudis NG, Sutton DA, Linden P, Rinaldi MG, Fung J, Kusne S. Zygomycosis in solid organ transplant recipients in a tertiary transplant center and review of the literature. Am J Transplant. 2006;6:2365‐2374. [DOI] [PubMed] [Google Scholar]
- 11.[11]Serris A, Danion F, Lanternier F. Disease entities in mucormycosis. J Fungi (Basel). 2019;5(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh N, Aguado JM, Bonatti H, et al. Zygomycosis in solid organ transplant recipients: a prospective, matched case‐control study to assess risks for disease and outcome. J Infect Dis. 2009;200:1002‐1011. [DOI] [PubMed] [Google Scholar]
- 13. Cowen LE. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathog. 2009;5.e1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SC, Li A, Calo S, Heitman J. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 2013;9.e1003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewis RE, Ben‐Ami R, Best L, Albert N, Walsh TJ, Kontoyiannis DP. Tacrolimus enhances the potency of posaconazole against Rhizopus oryzae in vitro and in an experimental model of mucormycosis. J Infect Dis. 2013;207:834‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vellanki S, Billmyre RB, Lorenzen A, et al. A novel resistance pathway for calcineurin inhibitors in the human‐pathogenic Mucorales Mucor circinelloides. MBio. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant‐Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101‐1111. [DOI] [PubMed] [Google Scholar]
- 18. Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID‐19: a post‐mortem study. Lancet Microbe. 2020;1:e245‐e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garg D, Muthu V, Sehgal IS, et al. Coronavirus disease (Covid‐19) Associated Mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pasero D, Sanna S, Liperi C, et al. A challenging complication following SARS‐CoV‐2 infection: a case of pulmonary mucormycosis. Infection. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Placik DA, Taylor WL, Wnuk NM. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS‐CoV‐2 pneumonia. Radiol Case Rep. 2020;15:2378‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bellanger A‐P, Navellou J‐C, Lepiller Q, et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a Severe Acute Respiratory syndrome Coronavirus 2 (SARS‐CoV‐2) patient. Infect Dis Now. 2021. 10.1016/j.idnow.2021.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zurl C, Hoenigl M, Schulz E, et al. Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically Ill COVID‐19 Patient with Underlying Hematological Malignancy. J Fungi (Basel). 2021;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia‐Vidal C, Sanjuan G, Moreno‐García E, et al. Incidence of co‐infections and superinfections in hospitalized patients with COVID‐19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cornely OA, Alastruey‐Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405‐e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene‐caspofungin treatment of rhino‐orbital‐cerebral mucormycosis. Clin Infect Dis. 2008;47:364‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaide CG, Khandelwal S. Hyperbaric oxygen: applications in infectious disease. Emerg Med Clin North Am. 2008;26(2):571‐595. [DOI] [PubMed] [Google Scholar]
- 28. Ferguson BJ, Mitchell TG, Moon R, Camporesi EM, Farmer J. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10:551‐559. [DOI] [PubMed] [Google Scholar]
- 29. López V, Vázquez T, Alonso‐Titos J, et al. Recommendations on management of the SARS‐CoV‐2 coronavirus pandemic (Covid‐19) in kidney transplant patients. Nefrologia. 2020;40:265‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Werthman‐Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID‐19. Am J Emerg Med. 2021;42:264.e5–264.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Monte Junior ESD, Santos MELD, Ribeiro IB, et al. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID‐19 Patient: a case report. Clin Endosc. 2020;53:746‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mekonnen ZK, Ashraf DC, Jankowski T, et al. acute invasive rhino‐orbital mucormycosis in a patient with COVID‐19‐associated acute respiratory distress syndrome. Ophthal Plast Reconstr Surg. 2021;37:e40‐e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mehta S, Pandey A. Rhino‐orbital mucormycosis associated with COVID‐19. Cureus. 2020;12.e10726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Waizel‐Haiat S, Guerrero‐Paz JA, Sanchez‐Hurtado L, Calleja‐Alarcon S, Romero‐Gutierrez L. A case of fatal rhino‐orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID‐19. Cureus. 2021;13.e13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID‐19 converge: the perfect storm for mucormycosis. J Fungi (Basel). 2021;7(4):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data partially available on request due to privacy/ethical restrictions.


