Abstract
Aim
The study aim was to map clinical characteristics and the evolution of pregnancies in pregnant women with confirmed diagnosis of SARS‐CoV‐2 infection.
Methods
Searching four databases, studies were investigated that described the evolution of pregnancies in women diagnosed with SARS‐CoV‐2 infection through laboratory tests. A scoping review was undertaken, including 35 articles published in English. Two pairs of independent researchers synthesized the data.
Results
Most studies were case studies or case series and had a low risk of bias. A predominance of cases was found in women over the age of 30 years who got infected in the third term of pregnancy and who had comorbidities. The prematurity index varied with the heterogeneity of the samples, and the cases of abortion occurred in combination with severe forms of infection. Caesarean section deliveries predominated, indicated mainly by respiratory decompensation caused by infection. Most women were discharged.
Conclusion
Based on the reviewed studies, the profile and evolution of pregnant women infected with COVID‐19 could be evaluated.
Keywords: coronavirus infections; COVID‐19; pregnancy; pregnancy complications, infectious; pregnancy maintenance; review
Summary statement
What is already known about this topic?
In 2020, the COVID‐19 pandemic was decreed, currently accounting for more than 105 million cases and more than two million deaths.
Physiological changes of pregnancy predispose pregnant women to COVID‐19 infection.
The characteristics of pregnancy associated with COVID‐19 infection turn obstetric care into a constant challenge.
What this paper adds?
The review presents the profile of pregnant women infected by SARS‐CoV‐2, with laboratory confirmation using the diagnostic reference standard (PCR).
This review presents the profile of pregnant women infected with SARS‐CoV‐2 and the outcomes of the infection and pregnancy.
The implications of this paper:
Based on the knowledge of the profile, public policies can be planned to prevent and manage infection among pregnant and postpartum women.
Further studies are suggested, mainly follow‐up studies of cohorts with pregnant women infected with SARS‐CoV‐2 to expand knowledge about the profile and obstetric outcomes.
1. INTRODUCTION
Since the detection of SARS‐CoV‐2, responsible for the COVID‐19 infection, which was later declared a pandemic by the World Health Organization (WHO), it has infected more than 105 million people and led to more than two million deaths (World Health Organization, WHO, 2021). Pregnancy conveys particular vulnerabilities in relation to this infection. The gestational period is a unique, particular immune status, in which the pregnant woman needs to acquire tolerance to the allogeneic embryo/foetus and, at the same time, protect herself and the concept from pathogens. In this context, three immunological stages are described: pre‐inflammatory (moment of implantation of the placenta), which occurs in the first term; anti‐inflammatory (when the adaptation provides foetal growth and development), in the second term and, finally, pro‐inflammatory, in the third term, which consists in preparing for the expulsion of the foetus (childbirth; Mor et al., 2017).
During pregnancy, the physiological changes in the respiratory tract, such as edema and increased pulmonary expansion, turn the pregnant woman more susceptible to respiratory viral infections. Thus, when SARS‐Cov‐2 infection is associated with pregnancy, increased inflammatory processes are observed, mainly in the first and third trimesters (pre‐and pro‐inflammatory states), exacerbating the severity of the cases (Liu, Wang, et al., 2020).
The particularities of pregnancy associated with COVID‐19 infection turn obstetric care into a constant challenge (Poon et al., 2020). Hence, as this is an emerging disease, hardly explored concerning the effects on pregnant women/foetuses and infants, and given the severe results of previous pandemics, the development of this study is justified.
The aim of this scoping review was to map clinical characteristics and the evolution of pregnancies in pregnant women with confirmed diagnosis of SARS‐CoV‐2 infection.
2. METHOD
2.1. Design
Scoping review according to the method of the Joanna Briggs Institute (JBI), aiming to map the scientific evidence associated with key concepts related to a specific phenomenon (Colquhoun et al., 2014; Lockwood & Tricco, 2020; Peters et al., 2015; Tricco et al., 2018. The review was registered at osf.io/hp25n.
2.2. Search methods
The research question was based on the Population, Concept and Context (PCC) strategy, establishing P for population, C for pregnancy and C for clinical and obstetric aspects of SARS‐Cov‐2/COVID‐19 infection. Based on these definitions, the guiding question was ‘What evidence is available in the literature about the clinical and obstetric aspects of SARS‐Cov‐2 infection during pregnancy?’
Data collection took place on 15 July 2020, and searches were carried out in the U.S. National Library of Medicine National Institutes of Health (PubMed), Latin American and Caribbean Literature in Health Sciences (LILACS), Web of Science and Cumulative Index to Nursing and Allied Health Literature (CINAHL). The choice of the databases was due to the number of primary health articles indexed. PubMed is a free search engine with access to Medline database that registers important publications of American and world literature; CINAHL is a specific database for nursing and health sciences; LILACS contains production from Latin America and the Caribbean, and the Web of Science allows consultation of other databases. The aim of the diversity of bases was to contemplate world production on the theme.
Two reviewers, both holding a PhD, conducted the research independently, using controlled descriptors from the Medical Subject Headings (MeSH), the CINAHL Headings and the Health Sciences Descriptors: ‘COVID‐19’, ‘Coronavirus Infections’ and ‘Pregnancy’. The descriptors were combined in different ways, aiming to broaden the searches. The terminological variations in the different languages, as well as synonyms, were used with the Boolean operators AND for simultaneous occurrence of subjects and OR for the occurrence of their respective synonyms.
In this review, we included studies on the subject of coronavirus infection and pregnancy that addressed clinical, obstetric characteristics and outcomes in women with laboratory proof of infection using PCR (polymerase chain reaction), published in Portuguese, Spanish and English, dated 2020. The exclusion criteria included review studies, editorials, expert opinions and studies whose samples included pregnant women with clinical suspicions of the disease in their analysis. The level of evidence was not considered as an exclusion criterion because it is a new topic, with a reduced possibility of finding articles with a better level of evidence. Thus, 553 articles were identified in the four databases, and one article was added in the search based on the references analysed. The PRISMA method—Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (Tricco et al., 2018)—was adopted to systematize the inclusion process of the studies (Figure 1).
FIGURE 1.
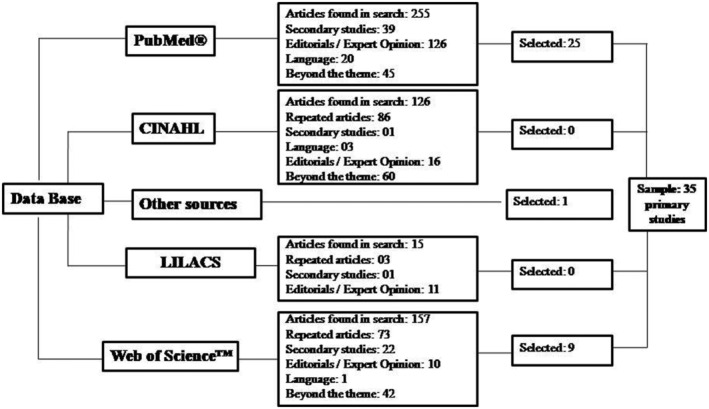
Selection flow of review articles according to PRISMA
2.3. Search outcomes
In the first stage, the choice of the articles was based on the analysis of titles and abstracts. Then, the full version was read for the final selection, extracting 35 studies from the databases. The sources of the analysed databases were PubMed®, CINAHL, LILACS and Web of Science™. Articles were excluded when duplicates; written in non‐established languages; beyond the topic; that did not meet the eligibility criteria (cases without laboratory proof of infection), literature review articles and qualitative research/expert opinion/editorial.
2.4. Quality appraisal
The Joanna Briggs Institute Appraisal Tools (JBI, 2014) were used to evaluate the methodological quality and risk of bias of the included studies. The tools allowed to classify the articles as high risk of bias (scores less than 50%), moderate risk of bias (scores between 50% and 70%) and low risk of bias (scores above 70%). The level of evidence was classified according to the type of study, according to evidence level II of Melnyk and Fineout‐Overholt (2005).
2.5. Data extraction and synthesis
The data were synthesized by two pairs of independent researchers. An instrument structured by the researchers, tested through a pilot study, was used to extract the data from the studies, following the guidelines of the JBI (JBI, 2014), which included the identification of the article, year and place of the study; the methodological characteristics; the evaluation of the methodological rigour and the notes and discussions about the thematic focus of this scoping review. The extracted information was tabulated for data synthesis, and the analysis of the results was descriptive, showing a synthesis of each primary study included in this review.
3. RESULTS
3.1. Characterization of the included studies
After the analysis by the four researchers, given the inclusion and exclusion criteria, 35 articles were selected for qualitative synthesis. All the articles were published in 2020, in English. Among the included studies, 77.1% were case studies, 20% descriptive reports and one cohort study (2.9%).
The application of tools to assess the methodological quality and risk of bias from the Joanna Briggs Institute Appraisal Tools revealed a high risk of bias (score = 42%) in one study, moderate risk (scores between 50 and 70%) in seven (20%) and low risk of bias in 27 (77.1%) studies. All of the articles included presented a description of the diagnostic method and used PCR as a reference standard for the diagnosis of COVID‐19 in pregnant women. The most neglected item was the complete description of the treatments employed. Despite the methodological limitations found, it was decided not to exclude the articles as this is a recent topic.
Regarding the producer countries, 34.3% of the articles came from China and 31.4% from the United States, although studies on the subject were produced more widely across the world. When adding up the studies, 685 pregnant women were found who had COVID‐19, ranging from asymptomatic to severe cases of the disease. The flowchart displays the exclusion diagram, according to each database investigated (Figure 2). Table 1 shows the country of origin, the number of pregnant women included (N) and the methodological quality assessment score by JBI Appraisal Tools.
FIGURE 2.
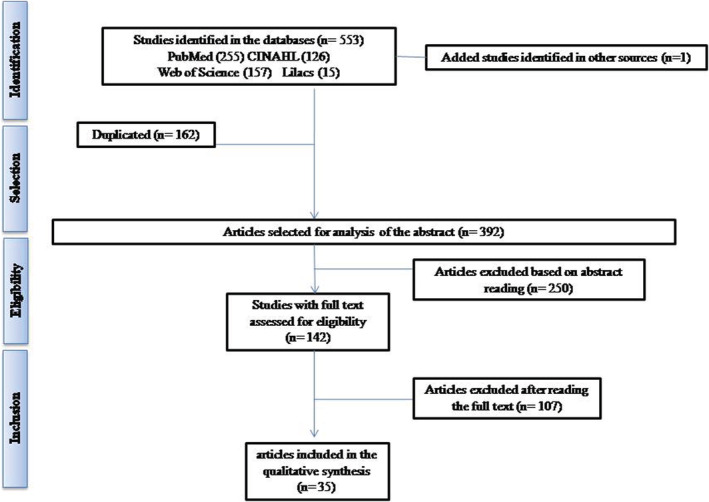
Article selection flow and reasons for exclusion from the review, according to the PRISMA guidelines, 2020
TABLE 1.
Synthesis of outcomes and evidence of selected articles (N = 35), 2020
| Country/reference | Number of participants | Risk of bias a | Evidence b |
|---|---|---|---|
| Australia (Lowe & Bopp, 2020) | 1 | 75% | VI |
| Canada (Kirtsman et al., 2020) | 1 | 87.50% | VI |
| Canada (Koumoutsea et al., 2020) | 2 | 87.50% | VI |
| China (Cao et al., 2020) | 10 | 75% | VI |
| China (Chen, Guo, et al., 2020) | 9 | 87.50% | VI |
| China (Liu et al., 2020) | 15 | 75% | V |
| China (Lu et al., 2020) | 1 | 100% | VI |
| China (Peng et al., 2020) | 1 | 75% | VI |
| China (Wen et al., 2020) | 1 | 87.50% | VI |
| China (Wu et al., 2020) | 13 | 50% | V |
| China (Xia et al., 2020) | 1 | 75% | VI |
| China (Xiong et al., 2020) | 1 | 50% | VI |
| China (Yu, Li, et al., 2020) | 7 | 87.50% | V |
| China (Yu, Fan, et al., 2020) | 1 | 50% | VI |
| China (Zeng et al., 2020) | 16 | 75% | V |
| England (Knight et al., 2020) | 427 | 42% | IV |
| France (Vivanti et al., 2020) | 1 | 87.50% | VI |
| Italy (Ferraiolo et al., 2020) | 1 | 100% | VI |
| Peru (Alzamora et al., 2020) | 1 | 87.50% | VI |
| Portugal (Lyra et al., 2020) | 1 | 100% | VI |
| Spain (Mendoza et al., 2020) | 42 | 50% | V |
| Spain (Pereira et al., 2020) | 60 | 100% | VI |
| The Netherlands (Grimminck et al., 2020) | 1 | 100% | VI |
| Turkey (Kalafat et al., 2020) | 1 | 87.50% | VI |
| United States (Baergen & Heller, 2020) | 20 | 62.50% | V |
| United States (Blauvelt et al., 2020) | 1 | 87.50% | VI |
| United States (Browne et al., 2020) | 1 | 100% | VI |
| United States (Futterman et al., 2020) | 2 | 87.50% | VI |
| United States (Hantoushzadeh et al., 2020) | 9 | 87.50% | VI |
| United States (Iqbal et al., 2020) | 1 | 100% | VI |
| United States (Rosen et al., 2020) | 1 | 87.50% | VI |
| United States (Shena et al., 2020) | 16 | 62.50% | V |
| United States (Silverstein et al., 2020) | 2 | 87.50% | VI |
| United States (Vallejo & Ilagan, 2020) | 1 | 87.50% | VI |
| United States (Qadri & Mariona, 2020) | 16 | 50% | VI |
RB: Risk of bias (JBI critical appraisal).
ELII: Evidence level II (Melnyk & Fineout‐Overholt, 2005).
3.2. Characterization of pregnant women included in the studies
When the maternal age was analysed, a mean of 31.2 ± 4.5 years was found, obtained by analysing the age or mean ages presented in the studies. The stratification by age group showed a predominance in women over the age of 30 years.
Regarding parity, multiparous women were predominant in 13 case studies, and the prevalence of multiparity ranged from 52.4% to 100% of cases. In the studies evaluated, however, no predominance of cases associated with parity was found.
In the evaluation of the gestational term in which the infection by COVID‐19 occurred, infected pregnant women were found in the three trimesters, although with a higher prevalence in the third gestational trimester. The percentage of infection in the third trimester ranged from 38.5% to 100%.
The presence of comorbidities in pregnancy was evaluated in 30 studies (85.7%), and, in eight (26.7%), the pregnant women had no comorbidities. Twenty‐two studies described comorbidities, with prevalence rates ranging from 2.3% to 100% of the pregnant women evaluated. Among the most frequent comorbidities, there was a predominance of endocrine and metabolic diseases and/or alterations; obesity was reported in four case studies, ranging between 43% and 69% of the infected pregnant women. Gestational diabetes (GDM) was described in three cases, ranging from 6.7% to 50% of pregnant women, and Type II diabetes mellitus was reported in three studies; hypothyroidism was evidenced in six studies, with a frequency of 5% to 14.5% of pregnant women included in the studies.
Conditions of the cardiovascular system during pregnancy were also associated with COVID‐19 infection, with cases of pre‐eclampsia. One study pointed out that 15% of the infected pregnant women had hypertensive syndromes. Although less frequent, pregnant women with chronic heart disease infected by COVID‐19 were observed. Two studies described the need for differential diagnosis of severe infection by COVID‐19 in cases of pre‐eclampsia and HELLP.
Other comorbidities such as asthma; chronic neutropenia with recurrent bacterial infections, co‐infection by the Influenza virus; thalassemia trait or thalassemia; anaemia; post‐fertilization pregnancies; twin pregnancy; deep vein thrombosis, ulcerative colitis; systemic lupus erythematosus without renal impairment and migraine have been described in pregnant women affected by COVID‐19.
Pregnancy complications such as intrauterine growth restriction and premature labour have also been reported. One study in particular described Streptococcus B infection; nuchal cord; macrosomal foetus; placenta accreta; postpartum atony and placenta previa with complications. Table 2 describes the variables age, parity, gestational term in which COVID‐19 infection occurred and maternal comorbidities.
TABLE 2.
Clinical and obstetric characteristics of pregnant women infected by COVID‐19
| Reference | Number of participants | Maternal age/average | Parity | Gestational term of infection | Comorbidity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primiparous | Multiparous | 1st | 2nd | 3rd | Present | Cardiovascular | Endocrine | etabolic | Others | |||
| Alzamora et al. (2020) | 01 | 41 | No | Yes (G3P2A0) | ‐ | ‐ | Yes | Yes | ‐ | GDM | Obese | ‐ |
| Baergen and Heller (2020) | 20 | 31.5 (16–40) | 20% | 80% | ‐ | ‐ | 100% | Yes | 15% hypertensive syndromes 66% PE | 5% DM II 5% HPT | ‐ | Yes a |
| Blauvelt et al. (2020) | 01 | 34 | No | Yes (G4P3A0) | ‐ | ‐ | Yes | Yes | ‐ | GDM | Obese | Asthma and smoking |
| Browne et al. (2020) | 01 | 33 | Yes | No | ‐ | Yes | ‐ | Yes | ‐ | ‐ | ‐ | Asthma, migraine, twin pregnancy |
| Cao et al. (2020) | 10 | 30.5 (29–35) | NI | NI | ‐ | ‐ | 100% | Yes | 30% PE | 10% HPT | ‐ | 10% anaemia 10% twins |
| Chen, Guo, et al. (2020) | 09 | 29.8 | NI | NI | ‐ | ‐ | 100% | Yes | 11% PIH 11% PE | ‐ | ‐ | 11% influenza |
| Ferraiolo et al. (2020) | 01 | 30 | Yes | No | ‐ | ‐ | Yes | No | ‐ | ‐ | ‐ | ‐ |
| Futterman et al. (2020) | 02 | 36 | No | 100% | ‐ | 50% | 50% | No | ‐ | ‐ | ‐ | ‐ |
| Grimminck et al. (2020) | 01 | 31 | Yes | No | ‐ | ‐ | Yes | Yes | HAC | ‐ | ‐ | Lupus |
| Hantoushzadeh et al. (2020) | 09 | 43%—35 to 39 years | 33% | 67% | ‐ | 12% | 88% | 88% | ‐ | 28% GDM, 14% HPT | 43% obese | 28% pregnancy post IVF |
| Iqbal et al. (2020) | 01 | 34 | No | Yes (G7P5A1) | ‐ | ‐ | Yes | No | ‐ | ‐ | ‐ | ‐ |
| Kalafat et al. (2020) | 01 | 32 | Yes | No | ‐ | ‐ | Yes | Yes | ‐ | ‐ | ‐ | Non‐anaemic thalassemia |
| Kirtsman et al. (2020) | 01 | 40 | No | Yes (G2P1A0) | ‐ | ‐ | Yes | Yes | ‐ | GDM> | ‐ | Neutropenia and recurrent infections |
| Knigth et al. (2020) | 427 | 58%—20 to 34 years | 38% | 62% | 5% | 14% | 81% | Yes | 7% PIH 1% cardiopathy | 12% GDM,3% DM II | 69% obese | 7% asthma |
| Koumoutsea et al. (2020) | 02 | 31.5 | No | Yes | ‐ | ‐ | Yes 100% | 100% | ‐ | 50% GDM | 50% obese | Asthma, chronic neutropenia, recurrent infections |
| Liu, Li et al. (2020) | 15 | 32 | NI | NI | Yes | Yes | Yes | 13.3% | 6.7% Cardiopathy | 6.7% GDM | ‐ | 6.7% thalassemia |
| Lowe and Bopp (2020) | 01 | 31 | Yes | No | ‐ | ‐ | Yes | NI | ‐ | ‐ | ‐ | ‐ |
| Lu et al. (2020) | 01 | 22 | Yes | No | ‐ | ‐ | Yes | NI | ‐ | ‐ | ‐ | ‐ |
| Lyra et al. (2020) | 01 | 35 | Yes | No | ‐ | ‐ | Yes | No | ‐ | ‐ | ‐ | ‐ |
| Mendoza et al. (2020) | 42 | 32 | 47.6% | 52.4% | ‐ | Yes | Yes | 2.3% | ‐ | 2.3% DMII | ‐ | ‐ |
| Peng et al. (2020) | 01 | 25 | Yes | No | ‐ | ‐ | Yes | No | ‐ | ‐ | ‐ | ‐ |
| Pereira et al. (2020) | 60 | 34 | 45% | 55% | 16.7% | 26.7% | 56.6% | 18.3% | 5% PE | ‐ | ‐ | 2%PVT 5% RCIU 5% TPP |
| Qadri and Mariona (2020) | 16 | 20–40 years | NI | NI | ‐ | 6.25% | 93.75% | 12% | ‐ | ‐ | 62.5% obese | ‐ |
| Rosen et al. (2020) | 01 | 26 | NI | NI | Yes | ‐ | ‐ | Yes | ‐ | ‐ | ‐ | Ulcerative colitis |
| Shena et al. (2020) | 16 | NI | NI | NI | ‐ | 6.25% | 93.75% | Yes | 6.3% PIH | 6.3% GDM | ‐ | 12.5% asthma; 6.3% pregnancy post IVF |
| Silverstein et al. (2020) | 02 | 25.5 | 50% | 50% | ‐ | ‐ | Yes | 50% | ‐ | ‐ | Obese | ‐ |
| Vallejo and Ilagan (2020) | 01 | 36 | No | Yes (G5P3A1) | ‐ | ‐ | Yes | Yes | ‐ | ‐ | Obese | ‐ |
| Vivanti et al. (2020) | 01 | 23 | Yes | No | ‐ | ‐ | Yes | No | ‐ | ‐ | ‐ | ‐ |
| Wen et al. (2020) | 01 | 31 | NI | NI | ‐ | ‐ | Yes | NI | ‐ | ‐ | ‐ | ‐ |
| Wu et al. (2020) | 13 | 33 | NI | NI | 38.5% | 23.1% | 38.5% | NI | NI | NI | NI | NI |
| Xia et al. (2020) | 01 | 27 | NI | NI | ‐ | ‐ | Yes | NI | ‐ | ‐ | ‐ | ‐ |
| Xiong et al. (2020) | 01 | 25 | Yes | No | ‐ | ‐ | Yes | No | ‐ | ‐ | ‐ | ‐ |
| Yu, Li, et al. (2020) | 07 | 32 | 42.9% | 57.1% | ‐ | ‐ | 100% | 14.5% | ‐ | 14.5% HPT | ‐ | ‐ |
| Yu, Fan, et al. (2020) | 01 | 35 | NI | NI | ‐ | ‐ | Yes | No | ‐ | ‐ | ‐ | ‐ |
| Zeng et al. (2020) | 16 | 31 | 56.3% | 43.7% | ‐ | ‐ | 100% | 31.3% | 12.5% Cardiopathy | 12.5% HPT | ‐ | 6.3% thalassemia |
Abbreviations: DMII, Diabetes Mellitus Type II; GDM, Gestational Diabetes Mellitus; HAC, Chronic Hypertension; HTD, hypothyroidism; IUGR, intra‐uterine growth restriction; IVF, In‐Vitro Fertilization; NI, Not informed; PE, Pre‐Eclampsia; PIH, Pregnancy‐Induced Hypertension; PL, premature labour; PVT, Profound Venous Thrombosis.
15% infection by streptococcus B; 10% nuchal cord; 10% foetal macrosomia; 5% had received cancer treatment; 5% placenta accreta; 5% postpartum atony; 5% placenta previa and 5% twin pregnancy.
3.3. Characterization of the outcomes of pregnancies of pregnant women included in the studies
In five studies, participants were described who remained pregnant, with discharge and cure of the infection. Between 26.7% and 61.5% of the participants maintained the pregnancy.
Eleven studies had term delivery (31.4%) as the outcome and seven (20%) premature birth. Twelve studies (34.3%) described that the frequency of full‐term births ranged from 23.1% to 100%, against 8.7% to 100% for premature birth, with differences due to the great heterogeneity of the samples studied.
As for the type of delivery, in three (8.6%) case studies, the pregnancies evolved into normal delivery, and, in one of these, delivery was facilitated by the use of a vacuum pump; two studies used a relief forceps in 10% to 17.4% of the cases, and caesarean section was described in 10 (28.5%) articles. In 12 studies, the frequency of delivery types was mentioned, with vaginal births ranging from 6.7% to 100% and caesarean sections from 21.7% to 100%.
Stratifying the indications of caesarean births, we obtained 29 studies, nine of which (31.0%) described the infection as the reason for indicating 16–100% of the caesarean sections, without reporting the severity of the infection. Seven (24.1%) articles found other justifications, such as signs of foetal hypoxemia, detected in cardiotocography; transverse presentation; failure to progress; foetal complications not related to COVID‐19; maternal coagulopathy and one study pointed out parturient choice as the reason.
In five studies, abortion was the gestational outcome, and, in one of them, the pregnancy was discovered after exacerbation of severe ulcerative colitis, and it was not possible to determine the gestational age. In the other studies in which abortion was the outcome, the occurrence ranged from 6.3% to 50%. All pregnant women who experienced abortion had severe forms of COVID‐19.
Most studies indicated good maternal outcomes, whereas only three (8.6%) reported maternal deaths, totalling 12 maternal deaths (1.8%). One of these studies was a cohort with 427 postpartum women which showed 1% maternal death rate (Knight et al., 2020). Another study (Hantoushzadeh et al., 2020) aimed to describe the cases of maternal deaths and those that generated permanent disability in pregnant women with COVID‐19, attended in the United States. Seven pregnant women died, and two remained hospitalized with a definitive tracheostomy and on mechanical ventilation (Hantoushzadeh et al., 2020). The third study (Vallejo & Ilagan, 2020) described a case in which the multiparous and obese postpartum woman, who had a caesarean section for respiratory decompensation by COVID‐19, went on to acute renal failure at 17 h postpartum. Death occurred 36 h after the caesarian section.
The gestational outcomes related to delivery, such as term or preterm, type of delivery and the indication of surgical delivery are described in Table 3.
TABLE 3.
Obstetric outcomes of pregnant women infected by COVID‐19
| Reference | Number of participants | Term birth | Type of birth | Indication for caesarean delivery | Maternal outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Term | Preterm | Vaginal | C‐section | Respiratory decompensation | Other indications | Abortion | Discharge | Death | ||
| Alzamora et al. (2020) | 01 | No | Yes | No | Yes | Yes | ‐ | ‐ | NI | NI |
| Baergen and Heller (2020) | 20 | 75% | 25% | 75% | 25% | NI | NI | NI | NI | NI |
| Blauvelt et al. (2020) | 01 | No | Yes | No | Yes | Yes | ‐ | ‐ | Yes | No |
| Browne et al. (2020) | 01 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Yes | No |
| Cao et al. (2020) | 10 | 70% | 30% | 20% | 80% | No | 100% | ‐ | 100% | No |
| Chen, Li, et al. (2020) | 09 | 66% | 44% | No | 100% | 100% | ‐ | ‐ | 100% | No |
| Ferraiolo et al. (2020) | 01 | Yes | No | No | Yes | No | Transverse position | ‐ | Yes | No |
| Futterman et al. (2020) | 02 | No | Yes | 50% | 50% | ‐ | Foetal complications | 50% | Yes | No |
| Grimminck et al. (2020) | 01 | Yes | No | No | Yes | No | Failed induction | ‐ | Yes | No |
| Hantoushzadeh et al. (2020) | 09 | 88% | 12% | 33% | 67% | 100% | ‐ | 45% | No | 78% |
| Iqbal et al. (2020) | 01 | Yes | No | Yes | No | ‐ | ‐ | ‐ | Yes | No |
| Kalafat et al. (2020) | 01 | No | Yes | No | Yes | Yes | ‐ | ‐ | Yes | No |
| Kirtsman et al. (2020) | 01 | No | Yes | No | Yes | Yes | ‐ | ‐ | Yes | No |
| Knigth et al. (2020) | 427 | 74% | 26% | 40%–10% forceps | 60% | 16% | 44% | ‐ |
93% (6% hospitalized) |
1% |
| Koumoutsea et al. (2020) | 02 | No | 100% | No | 100% | ‐ | Coagulopathy | ‐ | Yes | No |
| Liu, Li, et al. (2020) | 15 | NI | NI | 6.7% a | 66.7% a | NI | NI | ‐ | 100% | No |
| Lowe and Bopp (2020) | 01 | Yes | No | Yes b | No | ‐ | ‐ | ‐ | Yes | No |
| Lu et al. (2020) | 01 | Yes | No | No | Yes | ‐ | Failure to progress | ‐ | Yes | No |
| Lyra et al. (2020) | 01 | Yes | No | No | Yes | Yes | ‐ | ‐ | Yes | No |
| Mendoza et al. (2020) | 42 | NI | NI | 9.5% | 90.5% | 75% | 25% | NI | 100% | No |
| Peng et al. (2020) | 01 | No | Yes | No | Yes | Yes | ‐ | ‐ | Yes | No |
| Pereira et al. (2020) | 60 | 66.7% | 8.7% | 78.3% | 21.7% | 20% | 80% | ‐ | 100% | No |
| Qadri and Mariona (2020) | 16 | 88% | 12% | 78% | 22% | 100% | ‐ | ‐ | 100% | No |
| Rosen et al. (2020) | 01 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Yes | Yes | No |
| Shena et al. (2020) | 16 | 87.5% | 12.5% | NI | NI | NI | NI | 6.3% | 100% | No |
| Silverstein et al. (2020) | 02 | No | Yes | No | Yes | Yes | ‐ | ‐ | Yes | No |
| Vallejo and Ilagan (2020) | 01 | Yes | No | No | Yes | Yes | ‐ | ‐ | No | Yes |
| Vivanti et al. (2020) | 01 | No | Yes | No | Yes | No | Altered CTG | ‐ | Yes | No |
| Wen et al. (2020) | 01 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Yes | No |
| Wu et al. (2020) | 13 | 23.1% | 15.4% | 7.7% a | 38.5% a | 20% |
60% parturient's choice 20% not explicit |
7.7% | Yes | No |
| Xia et al. (2020) | 01 | Yes | No | No | Yes | Yes | ‐ | ‐ | Yes | No |
| Xiong et al. (2020) | 01 | Yes | No | Yes | No | ‐ | ‐ | ‐ | Yes | No |
| Yu, Li, et al. (2020) | 07 | 100% | No | No | 100% | NI | NI | ‐ | 100% | No |
| Yu, Fan, et al. (2020) | 01 | No | 100% | 100% | No | ‐ | ‐ | ‐ | 100% | No |
| Zeng et al. (2020) | 16 | 81.2% | 18.8% | 25% | 75% | NI | NI | ‐ | 100% | No |
Abbreviations: CTG, cardiotocography; NI, not informed.
Remained pregnant after discharge and cure.
With vacuum.
4. DISCUSSION
The results of this review present the profile of pregnant women infected by SARS‐CoV‐2 globally, confirmed by PCR, a reference standard for the diagnosis in pregnant women. It is important to highlight that the physiological changes that are normal in pregnancy predispose pregnant women to acquire viral infections and, if they have any infection, they tend to present more severe forms, including of COVID‐19 (Poon et al., 2020). With the growth of the foetus, the woman starts to breathe faster, and this breathing is called costal breathing, that is, breathing high in the thoracic and non‐abdominal region. The compression of the respiratory system by the growing foetus causes edema (swelling) and increased lung expansion. With this increase and swelling, the pregnant woman becomes more susceptible to respiratory infections (Liu, Li, et al., 2020). Also, there is an increase in volume and blood flow during pregnancy. This increased flow, to transport nutrients to the foetus, means that, when presenting infections, pregnant women may present more severe and intense forms of the infection. The rapid breathing and the increased blood flow of the pregnant woman, which can be observed by the increase in heart rate and decrease in pressure, also make it difficult to assess the cases, especially of the COVID‐19 infection, as these are some of the characteristic signs and symptoms of the infection for the general population, whereas they may just be normal changes in pregnancy (Liu, Li, et al., 2020). Altogether, these physiological events of pregnancy can predispose pregnant women to infection by COVID‐19 and hamper the diagnosis of the infection, deserving to be evaluated and discussed.
Studies conducted in the United States showed universal screening results with PCR for pregnant women corresponding to 1.9% of positive results in symptomatic and 13.5% in asymptomatic cases. Thus, the authors advocate universal screening for all pregnant women, aiming for obstetric/neonatal care planning and the rational use of personal protective equipment—a protective measure for mothers/newborns and health teams (Sutton et al., 2020). Screening is important for the sake of detection and early intervention in COVID‐19 cases during pregnancy, and maintaining prenatal care is important to avoid complications in pregnant women with pre‐existing comorbidities (Quiao, 2020). These data justify the choice of positive PCR as the inclusion criterion of this review.
Like in this review, studies in China (Chen, Li, et al., 2020; Quiancheng et al., 2020; Yang et al., 2020) and in the United States (Ellington et al., 2020) described infections predominantly affecting pregnant women over the age of 30 years, and, to date, there is no description of COVID‐19 cases in pregnant adolescents.
There was no difference in cases associated with parity in the reviewed articles. Only one Chinese study (Chen, Li, et al., 2020), evaluating 118 pregnant women, showed a slight predominance of infections among primiparous women (52%). Similar to the results of the present review, studies point to infection with COVID‐19 being identified throughout the pregnancy period, with a higher incidence in the third trimester (Chen, Li, et al., 2020; Dashraat et al., 2020; Mullins et al., 2020; Quiancheng et al., 2020; Sahin et al., 2021; Stumpfe et al., 2020; Zaigham & Andersson, 2020). Due to the limited number of infections in the first trimester, it cannot be argued whether COVID‐19 is a cause of early‐stage abortion (Li et al., 2020; Schwartz & Graham, 2020).
When comparing information about pregnancy complicated by infection with SARS‐CoV‐2 and comorbidities, the most frequent conditions were obesity, gestational diabetes, hypothyroidism and hypertensive syndromes. A cohort study with 533 pregnant women in Turkey showed a lower percentage of comorbidities in the sample when compared to other studies (30.2%). However, the comorbidities mentioned most frequently were similar to the results from the literature, citing more frequently: obesity; hypothyroidism; hypertensive syndromes and type II diabetes mellitus (Sahin et al., 2021). A Chinese study, involving 17 pregnant women, pointed out a predominance of cases of anaemia (29%), gestational diabetes (12%) and gestational hypertension (6%) (Chen, Zhang, et al., 2020). In another, involving 28 pregnant women, unspecified diabetes (7.1%); hepatitis B (7.1%); unspecified hypertension (3.6%) and hypothyroidism (3.6%) were identified (Quiancheng et al., 2020). American statistics also point to the predominance of pregnant women with comorbidities (88.1%): with chronic lung diseases (21.8%); diabetes (15.3%) and cardiovascular diseases (14%; Ellington et al., 2020).
The presence of comorbidity is a contradictory result in the literature. However, the fact that the disease is not controlled can predispose to greater risks during the infection. Obesity, hypertensive syndromes and gestational diabetes have been associated with severe cases of COVID‐19 in pregnant women in Sweden (Collin et al., 2020). In Brazil, higher maternal mortality rates occurred in pregnant women with cardiovascular diseases, obesity and gestational diabetes (Takemoto et al., 2020). For these authors, comorbidities can contribute to unfavourable outcomes (Takemoto et al., 2020).
The reduction of unnecessary consultations and examinations, aimed at avoiding the exposure of pregnant women; lifestyle interventions; remote monitoring and risk stratification, maintaining face‐to‐face consultations for more severe cases, are presented as alternatives to control the main complications in pregnancy (Barton et al., 2020; Murphy, 2020; Thangaratinam et al., 2020).
The heterogeneous samples of the studies in this review may explain the divergent results regarding the prematurity outcome, as the percentages ranged from 4% to 66% of births (Chang et al., 2020; Dashraat et al., 2020; Di Mascio et al., 2020; Ferrazzi et al., 2020; Karimi‐Zarchi et al., 2020; Panahi et al., 2020; Quiancheng et al., 2020; Sahin et al., 2021; Schwartz, 2020; Smith et al., 2020; Stumpfe et al., 2020). The high prevalence of premature births associated with COVID‐19 infection can be explained by maternal lung impairment, which decreases the placental flow (Stumpfe et al., 2020).
Abortion was presented as an outcome in five studies, with great variation in frequency, although all of these pregnant women had severe forms of COVID‐19. Studies with pregnancy profiles indicate rates from 2% to 14.3% of abortions secondary to infection (Chen, Zhang, et al., 2020; Dashraat et al., 2020; Quiancheng et al., 2020; Sahin et al., 2021).
The predominant delivery route was caesarean section, justified by maternal respiratory decompensation secondary to COVID‐19. Infection with SARS‐Cov‐2 is not an absolute indication for caesarean delivery, but the clinical conditions of the pregnant woman, gestational age and foetal viability should be evaluated (Gildlof et al., 2020; ISUOG, 2020; Karimi‐Zarchi et al., 2020; Qi et al., 2020; Rasmussen et al., 2020; RCOG & RCM, 2020). The data presented are corroborated by other studies indicating a predominance of caesarean sections, with rates ranging from 60.7% to 100% (Chang et al., 2020; Chen, Zhang, et al., 2020; Di Mascio et al., 2020; Mullins et al., 2020; Panahi et al., 2020; Quiancheng et al., 2020; Sahin et al., 2021; Smith et al., 2020; Stumpfe et al., 2020; Yang et al., 2020; Zaigham & Andersson, 2020). It should be noted that a caesarean section needs precise indications, but in cases of severe infection, it may be one of the most suitable relief and treatment measures, with good postpartum results (Oliva et al., 2020).
Most studies indicated a favourable maternal outcome, with discharge and cure (Chen, Zhang, et al., 2020; Dashraat et al., 2020; Mullins et al., 2020; Quiancheng et al., 2020; Schwartz & Graham, 2020; Smith et al., 2020). The sum of all deaths reached 1.8% of maternal deaths. One case review reported the death of 6.4% of pregnant women infected with SARS‐CoV‐2 (Karimi‐Zarchi et al., 2020), and, based on the death records, a maternal mortality rate of 12.7% was identified in Brazilian pregnant women (Takemoto et al., 2020).
One study pointed out that interrupted routine care and reduced access to healthy eating can have devastating impacts on the lives of women and infants (Roberton et al., 2020), serving as an alert for governments and health professionals. The association between increased maternal mortality and COVID‐19 pandemic can also be explained by the pregnant woman's hesitation to seek care, out of fear of the disease; financial and/or transportation problems that hinder access to health services; maternal isolation of infected pregnant women in distant regions, which compromise timely care in severe conditions and reduction of consultations, supplies and health professionals, compromising the quality of care. Thus, maternal mortality secondary to COVID‐19 demonstrates the disparities of minorities and the vulnerable population (Osanan et al., 2020).
In summary, this review shows that pregnant women are predisposed to infection by COVID‐19 due to the physiological characteristics of pregnancy. As they were not included in the priority group for immunization and can present severe forms of the disease, the importance of adopting preventive measures such as strict hand hygiene, wearing a mask, maintaining social distance and staying at home is reinforced. In case of symptoms, pregnant women need to seek assistance immediately for early treatment and, under no circumstances, should miss prenatal consultations, especially those pregnant women with comorbidities. This profile and information should guide health managers for public health actions.
4.1. Review limitations
Being a novel disease with incipient research on the theme, articles with a moderate risk of bias were included in the analyses to map the theme. With rapid updating of the literature and an increase in the number of cases, different results may arise, and these results may only be relevant at this time.
5. CONCLUSION
Thirty‐five studies were found that assessed cases of pregnant women who had COVID‐19. Overall, cases in women older than 30 years were predominant: who became infected in the third trimester of pregnancy and suffered from comorbidities. The prematurity index varied with the heterogeneity of the samples, and the cases of abortion occurred in combination with severe forms of infection. As for the type of delivery, caesarian section was the most prevalent, the largest number of cases being indicated by respiratory decompensation caused by infection, although most pregnant/postpartum women were discharged in good general condition.
Universal screening of pregnant women and reinforcement of preventive guidelines are recommended, given that this population group is highly vulnerable. Further studies are suggested, particularly longitudinal studies of cohorts of pregnant women infected with SARS‐CoV‐2, to expand knowledge about the profile and obstetric outcomes.
The knowledge of the profile of COVID‐19 infection in pregnant women should guide health managers for public health actions and decision‐making at all levels, aimed at this specific audience. In addition, based on this profile, assistance strategies and elaboration care protocols can be designed for optimize nursing care.
It should be noted that there is no clear profile of infection in pregnant women. Pregnant women are predisposed to infection by COVID‐19 due to the own physiological characteristics of pregnancy. They were not included in the priority group for immunization and can present severe forms of the disease; studies are still incipient about the disease, in general and in the specific audience, with intense production of publications in the period. Thus, this study may reflect a current picture of the pandemic. In this sense, this study is an initial starting point for discussions on the impact of COVID‐19 on pregnancy.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
MTR, KFO, JFO, MW and MCP designed the study; MTR and KFO collected the data; MTR, KFO, JFO and MW analysed the data. All authors prepared the manuscript and approved the final version for submission.
ACKNOWLEDGMENTS
This study was developed with the support of the CNPq—Call MCTIC/CNPq/FNDCT/MS/SCTIE/Decit No. 07/2020—Research to cope with COVID‐19, its consequences and other severe acute respiratory syndromes—Process 141513/2020‐3.
de Oliveira, K. F. , de Oliveira, J. F. , Wernet, M. , Carvalho Paschoini, M. , & Ruiz, M. T. (2021). COVID‐19 and pregnancy: A scoping review on pregnancy characteristics and outcomes. International Journal of Nursing Practice, 27(5), e12956. 10.1111/ijn.12956
Funding information CNPq, Grant/Award Number: 141513/2020‐3
REFERENCES
- Alzamora, M. C. , Paredes, T. , Caceres, D. , Webb, C. M. , Valdez, L. M. , & La Rosa, M. (2020). Severe COVID‐19 during pregnancy and possible vertical transmission. American Journal of Perinatology, 37, 861–865. 10.1055/s-0040-1710050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baergen, N. , & Heller, D. S. (2020). Placental pathology in COVID‐19 positive mothers: Preliminary finding. Pediatric and Developmental Pathology, 23, 177–180. 10.1177/1093526620925569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton, J. R. , Saade, G. R. , & Sibai, B. H. (2020). A proposed plan for prenatal care to minimize risks of COVID‐19 to patients and providers: Focus on hypertensive disorders of pregnancy. American Journal of Perinatology, 37, 837–844. 10.1055/s-0040-1710538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauvelt, C. A. , Chiu, C. , Donovan, A. L. , Prahl, M. , Shimotake, T. K. , George, R. B. , Schwartz, B. S. , Farooqi, N. A. , Ali, S. S. , Cassidy, A. , Gonzalez, J. M. , & Gaw, S. L. (2020). Acute respiratory distress syndrome in a preterm pregnant patient with coronavirus disease 2019 (COVID‐19). Obstetrics & Gynecology, 136, 46–51. 10.1097/AOG.0000000000003949 [DOI] [PubMed] [Google Scholar]
- Browne, P. C. , Linfert, J. B. , & Perez‐Jorge, E. (2020). Sucessful treatment of preterm labor in association with acute COVID‐19 infection. American Journal of Perinatology, 37, 866–868. 10.1055/s-0040-1709993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, D. , Yin, H. , Chen, J. , Tang, F. , Peng, M. , Li, R. , Xie, H. , Wei, X. , Zhao, Y. , & Sun, G. (2020). Clinical analysis of ten pregnant women with COVID‐19 in Wuhan, China: A retrospective study. International Journal of Infectious Diseases, 95, 294–300. 10.1016/j.ijid.2020.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, T. H. , Wu, J. L. , & Chang, L. Y. (2020). Clinical characteristics and diagnostic challenges of pediatric COVID‐19: A systematic review and meta‐analysis. Journal of the Formosan Medical Association, 119, 982–989. 10.1016/j.jfma.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Guo, J. , Wang, C. , Lou, F. , Yu, X. , Zhang, W. , Li, J. , Zhao, D. , Xu, D. , Gong, Q. , Liao, J. , Yang, H. , Hou, W. , & Zhang, Y. (2020). Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: A retrospective review of medical records. The Lancet, 395, 809–815. 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Li, Q. , Zheng, D. , Jiang, H. , Wei, Y. , Zou, L. , Feng, L. , Xiong, G. , Sun, G. , Wang, H. , Zhao, Y. , & Quiao, J. (2020). Clinical characteristics of pregnant women with COVID‐19 in Wuhan, China. The New England Journal of Medicine, 382, e100. 10.1056/NEJMc2009226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. , Zhang, Y. , Huang, L. , Cheng, B. , Xia, Z. , & Meng, Q. (2020). Safety and efficacy of different anesthetic regimens for parturients with COVID‐19 undergoing cesarean delivery: A case series of 17 parturients. Canadian Journal of Anesthesia, 67, 655–663. 10.1007/s126300-020-01630-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin, J. , Bystrom, E. , Carnahan, A. S. , & Ahrme, M. (2020). Public Health Agency of Sweden's brief report: Pregnant and postpartum women with SARS‐CoV‐2 infection in intensive care in Sweden. Acta et Obstetricia Gynecology Scandinavica, 99, 819–822. 10.1111/aogs.13901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun, H. L. , Levac, D. , O'Brien, K. K. , Straus, S. , Tricco, A. C. , Perrier, L. , Kastner, M. , & Moher, D. (2014). Scoping reviews: Time for clarity in definition, methods, and reporting. Journal of Clinical Epidemiology, 67, 1291–1294. 10.1016/j.jclinepi.2014.03.013 [DOI] [PubMed] [Google Scholar]
- Dashraat, P. , Wong, J. L. J. , Lim, M. X. K. , Lim, L. M. , Li, S. , Biswas, A. , Choolani, M. , Mattar, C. , & Su, L. L. (2020). Coronavirus disease 2019 (COVID‐19) pandemic and pregnancy. American Journal of Obstetrics and Gynecology, 222, 521–531. 10.1016/j.ajog.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mascio, D. , Khalil, A. , Saccone, G. , Rizzo, G. , Buca, D. , Liberati, M. , Vecchiet, J. , Nappi, L. , Scambia, G. , Berghella, V. , & D'Antonio, F. (2020). Outcome of coronavirus spectrum infections (SARS, MERS, COVID‐19) during pregnancy: A systematic review and meta‐analysis. American Journal of Obstetrics & Gynecology MFM, 2, 100107. 10.1016/jajogmfm.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington, S. , Strid, P. , Tong, V. T. , Woodworth, K. , Galang, R. R. , & Zambrano, L. D. (2020). Characteristics of women of reproductive age with laboratory‐confirmed SARS‐Cov‐2 infection by pregnant status, United States, January 22‐June7, 2020. Morbidity and Mortality Weekly Report, 69, 769–775. https://www.cdc.gov/mmwr/volumes/69/wr/mm6925a1.htm. 10.15585/mmwr.mm6925a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraiolo, A. , Barra, K. , Kratochwila, C. , Paudice, M. , Vellone, V. G. , Godano, E. , Varesano, S. , Noberasco, G. , Ferrero, S. , & Arioni, C. (2020). Report of positive placental swabs for SARS‐CoV‐2 in an asymptomatic pregnant woman with COVID‐19. Medicina, 56, 306. 10.3390/medicina56060306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrazzi, E. M. , Frigerio, L. , Cetin, I. , Vergani, P. , Spirillo, A. , Prefumo, F. , Pellegrini, E. , & Gargantini, G. (2020). COVID‐19 obstetrics task force, Lombardy, Italy: Executive management summary and short report of outcome. International Journal of Gynecology & Obstetrics, 149, 377–378. 10.1002/ijgo.13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterman, I. , Toaff, M. , Navi, L. , & Clare, C. A. (2020). COVID‐19 and HELLP: Overlapping clinical pictures in two gravid patients. American Journal of Perinatology Reports, 10, e179–e182. 10.1055/s-0040-1712978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildlof, S. , Savchenko, J. , Brune, T. , & Joefson, H. (2020). COVID‐19 in pregnancy with comorbidities: More liberal testing strategy is needed. Acta et Obstetricia Gynecology Scandinavica, 99, 948–949. 10.1111/aogs.13862 [DOI] [PubMed] [Google Scholar]
- Grimminck, K. , Santegoets, L. A. M. , Siemens, F. C. , Fraaij, P. L. A. , Reiss, I. K. M. , & Schoenmakers, S. (2020). No evidence of vertical transmission of SARS‐CoV‐2 after induction of labour in an immune‐suppressed SARS‐CoV‐2‐positive patient. BMJ, 13, e235581. 10.1136/bcr-2020-235581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantoushzadeh, S. , Shamshirsaz, A. A. , Aleyasin, A. , Seferovic, M. D. , Aski, S. K. , Arian, S. E. , Pooransari, P. , Ghotbizadeh, F. , Aalipour, S. , Soleimani, Z. , Naemi, M. , Molaei, B. , Ahangari, R. , Salehi, M. , Oskoei, A. D. , Pirozan, P. , Darkhaneh, R. F. , Laki, M. G. , Farani, A. K. , … Aagard, K. (2020). Maternal death due COVID‐19. American Journal of Obstetrics and Gynecology, 223, 109.e1–109.e16. 10.1016/j.ajog.2020.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal, S. N. , Overcash, R. , Mokhtari, N. , Saeed, H. , Gold, S. , Auguste, T. , Mirza, M. U. , Ruiz, M. E. , Chahine, J. J. , Waga, M. , & Wortmann, G. (2020). An uncomplicated delivery in a patient with COVID‐19 in the United States. The New England Journal Medicine, 382, e34. 10.1056/nejmc2007605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISUOG . (2020). ISUOG interim guidance on 2019 novel coronavirus infection during pregnancy and puerperium: Informations for healthcare professionals. Ultrasound in Obstetrics & Gynecology, 55, 700–708. 10.1002/uog.22013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanna Briggs Institute . (2014). Joanna Briggs institute reviewers' manual (2014th ed.). Adelaide: The University of Adelaide. https://nursing.lsuhsc.edu/JBI/docs/ReviewersManuals/ReviewersManual.pdf [Google Scholar]
- Kalafat, E. , Yaprak, E. , Cinar, G. , Varli, B. , Ozisik, S. , Uzun, C. , Azap, A. , & Koc, A. (2020). Lung ultrasound and computed tomographic findings in pregnant woman with COVID‐19. Ultrasound in Obstetrics & Gynecology, 55, 835–837. 10.1002/uog.22034 [DOI] [PubMed] [Google Scholar]
- Karimi‐Zarchi, M. , Neamatzadeh, H. , Datgheib, A. S. , Abbasi, H. , Mirjalili, S. R. , Behforouz, A. , Ferdosian, F. , & Bahrami, R. (2020). Vertical transmission of coronavirus disesase 19 (COVID‐19) from infected pregnant mothers to neonates: A review. Fetal and Pediatric Pathology, 39, 246–250. 10.1080/15513815.2020.1747120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtsman, M. , Diambomba, Y. , Poutanen, S. M. , Malinowski, A. K. , Vlachodimitropoulou, E. , Parks, T. , Erdman, L. , Morris, S. K. , & Shah, P. S. (2020). Probable congenital SARS‐CoV‐2 infection in a neonate born to a woman with active SARS‐CoV‐2 infection. CMAJ, 192, E647–E650. 10.1503/cmaj.200821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, M. , Bunch, K. , Vousden, N. , Morris, E. , Simpson, N. , Gale, C. , O'Brien, P. , Quigley, M. , Brocklehurst, P. , Kurinczuk, J. J. , & UK Obstetric Surveillance System SARS‐CoV‐2 Infection in Pregnacy Collaborative Group . (2020). Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS‐CoV‐2 infection in UK: National population based cohort study. BMJ, 2020. 10.1101/2020.05.08.20089268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumoutsea, E. V. , Vivanti, A. J. , Shehata, N. , Benachi, A. , Gouez, A. L. , & Desconclois, C. (2020). COVID‐19 and acute coagulopathy in pregnancy. Journal of Thrombosis and Haemostasis, 18, 1648–1652. 10.1111/jth.14856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Chen, L. , Zhang, J. , Xiong, C. , & Li, X. (2020). The SARS‐CoV‐2 receptor ACE2 expression of maternal‐fetal interface and fetal organs by single‐cell transcriptione study. PLoS ONE, 15, e0230295. 10.1371/jounal.pone.023095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D. , Li, L. , Wu, X. , Zheng, D. , Wang, J. , Yang, L. , & Zheng, C. (2020). Pregnancy and perinatal outcomes of women with coronavirus disease (COVID‐19) pneumonia: A preliminary analysis. American Journal of Roentgenology, 215, 127–132. 10.2214/AJR.20.23072 [DOI] [PubMed] [Google Scholar]
- Liu, H. , Wang, L. L. , Zhao, S. J. , Kwak‐Kim, J. , Mor, G. , & Liao, A. H. (2020). Why are pregnant women susceptible to COVID‐19? An immunological viewpoint. Journal of Reproductive Immunology, 139, 103122. 10.1016/j.jri.2020.103122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood, C. , & Tricco, A. C. (2020). Preparing scoping reviews for publication using methodological guides and reporting standards. Nursing & Health Sciences, 22, 1–4. 10.1111/nhs.12673 [DOI] [PubMed] [Google Scholar]
- Lowe, B. , & Bopp, B. (2020). COVID‐19 vaginal delivery—A case report. Australian and New Zealand Journal of Obstetricians and Gynaecologists, 60, 1–2. 10.1111/ajo.13173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, D. , Sang, L. , Du, S. , Li, T. , Chang, Y. , & Yang, X. (2020). Asymptomatic COVID‐19 infection in late pregnancy indicated no vertical transmission. Journal of Medical Virology, 92, 1660–1664. 10.1002/jmv.25927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyra, J. , Valente, R. , Rosario, M. , & Guimarães, M. (2020). Cesarean section in a pregnant woman with COVID‐19: First case in Portugal. Acta Médica Portuguesa, 33, 429–431. 10.20344/amp.13883 [DOI] [PubMed] [Google Scholar]
- Melnyk, B. M. , & Fineout‐Overholt, E. (2005). Making the case for evidence‐based practice. In Melnyk B. M. & Fineout‐Overholt E. (Eds.), Evidence‐based practice in nursing and healthcare: A guide to best practice (pp. 3–24). Philadelphia: Lippincott Williams and Wilkins. [Google Scholar]
- Mendoza, M. , Garcia‐Ruiz, I. , Maiz, N. , Rodo, C. , Garcia‐Manau, P. , Serrano, B. , Lopez‐Martinez, R. M. , Balcells, J. , Fernandez‐Hidalgo, N. , Carreras, E. , & Suy, A. (2020). Pre‐eclampsia‐like syndrome induced by severe COVID‐19: A prospective observational study. BJOG, 127, 1374–1380. 10.1111/1471-0528.16339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor, G. , Aldo, P. , & Alvero, A. B. (2017). The unique immunological and microbial aspects of pregnancy. Nature Reviews Immunology, 17, 469–482. 10.1038/nri.2017.64 [DOI] [PubMed] [Google Scholar]
- Mullins, E. , Evans, D. , Viner, R. M. , O'Brien, P. , & Morris, E. (2020). Coronavirus in pregnancy and delivery: Rapid review. Ultrasound in Obstetrics & Gynecology, 55, 586–592. 10.1002/uog.22014 [DOI] [PubMed] [Google Scholar]
- Murphy, H. R. (2020). Managing diabetes in pregnancy before, during and after COVID‐19. Diabetes Technology & Therapheutics, 22, 454–461. 10.1089/dia.2020.0223 [DOI] [PubMed] [Google Scholar]
- Oliva, M. , Hsu, K. , Alsamari, S. , de Chavez, V. , & Ferrara, L. (2020). Clinical improvement of severe COVID‐19 pneumonia in pregnant patient after caesarean delivery. BMJ Case Reports, 13, e236290. 10.1136/bcr-2020-236290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanan, G. C. , Vidarte, M. F. E. , & Ludmir, J. (2020). Do not forget our pregnant women during the COVID‐19 pandemic. Women & Health, 60, 959–962. 10.1080/03630242.2020.1789264 [DOI] [PubMed] [Google Scholar]
- Panahi, L. , Amiri, M. , & Pouy, S. (2020). Risks of novel coronavirus disease (COVID‐19) in pregnancy, a narrative review. Archives of Academic Emergencies Medicine, 8, e34. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7092922/pdf/aaem-8-e34.pdf [PMC free article] [PubMed] [Google Scholar]
- Peng, Z. , Wang, J. , Mo, Y. , Duan, W. , Xiang, G. , Yi, M. , Bao, L. , & Shi, Y. (2020). Unlikely SARS‐CoV‐2 vertical transmission from mother to child: A case report. Journal of Infection and Public Health, 13, 818–820. 10.1016/j.jiph.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, A. , Cruz‐Melguizo, S. , Adrien, M. , Fuentes, L. , Marin, E. , & Perez‐Medina, T. (2020). Clinical course of coronavirus disease—2019 (COVID‐19) in pregnancy. Acta Obstetricia et Gynecologica Scandinavica, 99, 839–847. 10.1111/AOGS.13921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, M. D. , Godfrey, C. M. , Khalil, H. , Mc Inerney, P. , Parker, D. , & Soares, C. B. (2015). Guidance for conducting systematic scoping reviews. International Journal of Evidence‐Based Healthcare, 13, 141–146. 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- Poon, L. C. , Yang, H. , Kapur, A. , Melamed, N. , Dao, B. , Divakar, H. , McIntyre, H. D. , Kihara, A. B. , Ayres‐de‐Campos, D. , Ferrazzi, E. M. , di Renzo, G. C. , & Hod, M. (2020). Global interim guidance on coronavirus disease 2019 (COVID‐19) during pregnancy and puerperium from FIGO and allied partners: Information for healthcare professionals. International Journal of Gynecology & Obstetrics, 149, 273–286. 10.1002/ijgo.1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri, F. , & Mariona, F. (2020). Pregnancy affected by SARSCoV‐2 infection: A flash report from Michigan. The Journal of Maternal‐ Fetal Neonatal Medicine, 20, 1–3. 10.1080/14767058.2020.1765334 [DOI] [PubMed] [Google Scholar]
- Qi, H. , Luo, X. , Zheng, Y. , Zhang, H. , Li, J. , Zou, L. , Feng, L. , Chen, D. , Shi, Y. , Tong, C. , & Baker, P. N. (2020). Safe delivery for pregnancies affected by COVID‐19. BJOG, 127, 927–929. 10.1111/1471-0528.16231 [DOI] [PubMed] [Google Scholar]
- Quiancheng, X. , Jian, S. , Lingling, P. , Lei, H. , Xiaogan, J. , Weihua, L. , Gang, Y. , Shirong, L. , Zhen, W. , GuoPing, X. , Lei, Z. , & The six batch of Anhui Medical Team Aiding Wuhan for COVID‐19 . (2020). Coronavirus disease 2019 in pregnancy. International Journal of Infectious Diseases, 95, 376–383. 10.1016/j.ijid.2020.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiao, J. (2020). What are the risks of COVID‐19 infection in pregnant women? Lancet, 395, 760–762. 10.1016/S140-6736(20).30365-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, A. S. , Smulian, J. C. , Lidnicky, J. A. , Wen, T. S. , & Jamieson, D. J. (2020). Coronavirus disease 2019 (COVID‐19) and pregnancy: What obstetricians need to know. American Journal of Obstetrics and Gynecology, 222, 415–426. 10.1016/j.ajog.2020.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton, T. , Carter, E. D. , Chou, V. B. , Stegmuller, A. R. , Jackson, B. D. , Tam, Y. , Sawadogo‐Lewis, T. , & Walker, N. (2020). Early estimates of the indirect effects of the COVID‐19 pandemic on maternal and child mortality in low income and middle‐income countries: A modeling study. The Lancet Global Health, 8, e901–e908. 10.1016/S2214-109X(20)30229-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, M. H. , Axelrad, J. , Hudesman, D. , Rubin, D. T. , & Chang, S. (2020). Management of acute severe ulcerative colitis in a pregnant woman with COVID‐19 infection: A case report and review of the literature. Inflammatory Bowel Diseases, 26, 971–973. 10.1093/ibd/izaa109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal College of Obstetricians & Gynaecologists & The Royal College of Midwives . (2020). Coronavirus (COVID‐19) Infection in pregnancy. rcog.org.uk/globalassets/documents/guidelines/2020‐04‐03‐coronavirus‐covid‐19‐infection‐in‐pregnancy.pdf
- Sahin, D. , Tanacan, A. , Erol, S. A. , Anuk, A. T. , Yetinskin, F. D. Y. , Keskin, H. L. , Ozcan, N. , Ozgu‐Erdinc, A. S. , Eyi, E. G. Y. , Yucel, A. , Tayman, C. , Unlu, S. , Dinc, B. , Sari, E. , Surel, A. A. , & Moraglu, O. T. (2021). Update experience of a tertiary pandemic center on 533 pregnant women with COVID‐19 infection: A prospective cohort study from Turkey. International Journal of Gynecology & Obstetrics, 152, 328–334. 10.1002/ijgo.13460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, D. A. (2020). An analysis of 38 pregnant women with of COVID‐19, their newborn infants and maternal‐fetal transmission of SARS‐CoV‐2: Maternal coronavirus infections and pregnancy outcomes. Archives of Pathology & Laboratory Medicine, 144, 799–805. 10.5858/arpa.2020-0901-AS [DOI] [PubMed] [Google Scholar]
- Schwartz, D. A. , & Graham, A. L. (2020). Potential maternal and infant outcomes from coronavirus 2019‐n‐CoV (SARS‐CoV‐2) infecting pregnant women: Lessons from SARS, MERS and other human coronavirus infections. Viruses, 12, 154. 10.3390/v.12020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shena, E. D. , Mithal, L. B. , Otero, S. , Azad, H. A. , Miller, E. S. , & Goldstein, J. A. (2020). Placental pathology in COVID‐19. American Journal of Clinical Pathology, 154, 23–32. 10.1093/ajcp/aqaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein, J. S. , Limaye, M. A. , Brubaker, S. G. , Roman, A. S. , Bautista, J. , Chervenak, J. , Ratner, A. J. , Sommer, P. M. , Roselli, N. M. , Gibson, C. D. , Ellenberg, D. , & Penfield, C. A. (2020). Acute respiratory decompensation requiring intubation in pregnant women with SARS‐CoV‐2 (COVID‐19). American Journal of Perinatology Reports, 10, e169–e175. 10.1055/s-0040-1712925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, V. , Seo, D. , Warty, R. , Payne, O. , Salih, M. , Chin, K. L. , Ofori‐Asenso, R. , Krishnan, S. , da Silva Costa, F. , Vollenhoven, B. , & Wallace, E. (2020). Maternal and neonatal outcomes associated with COVID‐19 infection: A systematic review. PLoS ONE, 15, e0234187. 10.1371/jornal.pone.0234187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpfe, F. M. , Titzmann, A. , Schneider, M. O. , Stelzl, P. , Kehl, S. , Fasching, P. A. , Beckmann, M. W. , & Ensser, A. (2020). SARS‐CoV‐2 infection in pregnancy—A review of the current literature and possible impact on maternal and neonatal outcome. Geburtshilfe Und Frauenheilkunde, 80, 380–390. 10.1055/a-1134-5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, D. , Fucks, K. , D'Alton, M. , & Goffman, D. (2020). Universal screening for SARS‐CoV‐2 in women admitted for delivery. The New England Journal of Medicine, 382, 2163–2164. 10.1056/NEJMe2009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto, L. S. , Menezes, M. O. , Andreucci, C. B. , Nakamura‐Pereira, M. , Amorim, M. M. R. , Katz, L. , & Knobel, R. (2020). The tragedy of COVID‐19 in Brazil: 124 maternal deaths and counting. International Journal of Gynecology & Obstetrics, 151, 154–156. 10.1002/ijgo.13300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaratinam, S. , Cooray, S. D. , Sukuimar, N. , Huda, M. S. B. , Devlieger, R. , Benhalima, K. , McAuliffe, F. , Saravanan, P. , & Teede, H. J. (2020). Endocrinology in time of COVID‐19: Diagnosis and management of gestational diabetes mellitus. European Journal of Endocrinology, 183, 49–56. 10.1530/EJE-20-0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco, A. C. , Lillie, E. , Zarin, W. , O'Brien, K. K. , Colquhoun, H. L. , Levac, D. , Moher, D. , Peters, M. D. J. , Horsley, T. , Weeks, L. , Hempel, S. , Akl, E. A. , Chang, C. , McGowan, J. , Stewart, L. , Hartling, L. , Aldcroft, A. , Wilson, M. G. , Garritty, C. , … Straus, S. E. (2018). PRISMA extension for scoping reviews (PRISMA‐ScR): Checklist and explanation. Annals of Internal Medicine, 169, 467–473. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- Vallejo, V. , & Ilagan, J. G. (2020). A postpartum death due to coronavirus disease 2019 (COVID‐19) in the United States. Obstetrics & Gynecology, 136, 52–55. 10.1097/AOG.0000000000003950 [DOI] [PubMed] [Google Scholar]
- Vivanti, A. J. , Vauloup‐Fellous, C. , Prevot, S. , Zupan, V. , Suffee, C. , Cao, J. D. , Benachi, A. , & De Luca, D. (2020). Transplacental transmission of SARS‐CoV‐2 infection. Nature, 11, 3572. 10.1038/s41467-020-17436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, R. , Sun, Y. , & Xing, Q. S. (2020). A patient with SARS‐CoV‐2 infection during pregnancy in Qingdao, China. Journal of Microbiology, Immunology and Infection, 53, 499–500. 10.1016/j.jmii.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2021). Coronavirus disease (COVID‐19) situation dashboard. https://covid19.who.int/
- Wu, Y. , Liu, C. , Dong, L. , Zhang, C. , Chen, Y. , Liu, J. , Zhang, C. , Duan, C. , Zhang, H. , Mol, B. W. , Dennis, C.‐. L. , Yin, T. , Yang, J. , & Huang, H. (2020). Coronavirus disease 2019 among pregnant Chinese women: Case series data on the safety of vaginal birth and breastfeeding. BJOG, 127, 1109–1115. 10.1111/1471-0528.16276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, H. , Zhao, S. , Wu, Z. , Luo, H. , Zhou, C. , & Chen, X. (2020). Emergency caesarean delivery in a patient with confirmed COVID‐19 under spinal anaesthesia. Bristish Journal of Anaesthesia, 124, e216–e218. 10.1016/j.bja.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, X. , Wei, H. , Zhang, Z. , Chang, J. , Ma, X. , Gao, X. , Chen, Q. , & Pang, Q. (2020). The SARS‐CoV‐2 receptor ACE2 expression of maternal‐fetal interface and fetal organs by single‐cell transcriptome study. Journal of Medical Virology, 92, 1657–1659. 10.1002/jmv.25857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. , Sun, G. , Tang, F. , Peng, M. , Gao, Y. , Peng, J. , Xie, H. , Zhao, Y. , & Jin, Z. (2020). Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. Journal of Infection, 81, e40–e44. 10.1016/j.jinf.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, N. , Li, W. , Kang, Q. , Xiong, Z. , Wang, S. , Lin, X. , Liu, Y. , Xiao, J. , Liu, H. , Deng, D. , Chen, S. , Zeng, W. , Feng, L. , & Wu, J. (2020). Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID‐19 in Wuhan, China: A retrospective, single‐centre, descriptive study. The Lancet Infectious Diseases, 20, 559–564. 10.1016/S1473-3099(20)30176-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. , Fan, C. , Bian, J. , & Shen, Y. (2020). Severe COVID‐19 in a pregnant patient admitted to hospital in Wuhan. International Journal of Gynecology & Obstetrics, 150, 262–263. 10.1002/ijgo.13232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaigham, M. , & Andersson, O. (2020). Maternal and perinatal outcomes with COVID‐19: A systematic review of 108 pregnancies. Acta Obstetricia et Gynecologica Scandinavica, 99, 823–829. 10.1111/aogs.13867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Y. , Lin, L. , Yan, Q. , Wei, W. , Yang, B. X. , Huang, R. , & Chen, D. (2020). Update on clinical outcomes of women with COVID‐19 during pregnancy. International Journal of Gynecology & Obstetrics, 150, 264–266. 10.1002/ijgo.13236 [DOI] [PMC free article] [PubMed] [Google Scholar]


