DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose.
To the Editor,
Immunocompromised patients, including solid organ transplant recipients (SOTRs), were not included in the two large trials of Pfizer/BioNTech and Moderna mRNA vaccines, and therefore, safety and efficacy data are lacking in this population. 1 , 2 Recently, immunogenicity, at a median of 29 days, after dose 2 of the mRNA SARS‐CoV‐2 vaccines have been studied among 658 SOTRs. 3 Forty‐six percentage of participants did not mount anti spike antibody responses suggesting that such patients may remain at high risk for COVID‐19 despite vaccination. 3
Herein we report a case of COVID‐19 in a SOTR, a 49‐year‐old female kidney transplant recipient, after 23 days of a complete cycle of vaccination with the BNT162b2 mRNA vaccine. At the onset of symptoms, the patient was on maintenance immunosuppression therapy with tacrolimus, mycophenolate mofetil and prednisone. Genomic analysis by Next Generation Sequencing (NGS) identified the SARS‐CoV‐2 B.1.1.29 variant which carries the N440K mutation on the receptor‐binding domain (RBD) of the spike protein, described as possibly associated with immune escape. 4 , 5
Testing for SARS‐CoV‐2 antibodies after 1 week since the onset of symptoms revealed the absence of IgG anti‐nucleoprotein (Architect SARS‐CoV‐2 IgG, Roche) and IgM anti‐spike protein (Architect SARS‐CoV‐2 IgM, Roche), and the presence of 91 BAU/mL of IgG anti‐trimeric Spike protein (LIAISON® SARS‐CoV‐2 Trimeric S IgG, Diasorin). Neutralizing antibodies (Nabs) blocking the interaction between ACE2 and RBD were not detectable (ACE2‐RBD Neutralization Assay, Diagnostic Bioprobes). After further 2 weeks, testing for SARS‐CoV‐2 antibodies revealed the presence of IgG anti‐nucleoprotein and IgM anti‐spike protein, and the presence of 1630 BAU/mL of IgG anti‐trimeric Spike protein. Nabs blocking the interaction between ACE2 and RBD were also detectable (index 1∙9 vs a cut‐off value of 1). At this time, we also investigated the presence of SARS‐CoV‐2‐specific cell‐mediated immune response. Flow cytometric analysis performed on peripheral blood mononuclear cells (PBMNCs) showed the presence of SARS‐CoV‐2 spike‐specific B cells (0∙06% of total CD19+ B cells) and SARS‐CoV‐2 specific CD4+ T cells. In particular, we found CD4+T cells specific for S, N, and M‐ protein peptide pools expressing CD154 and producing IFN‐g and IL‐2 (Figure 1).
FIGURE 1.
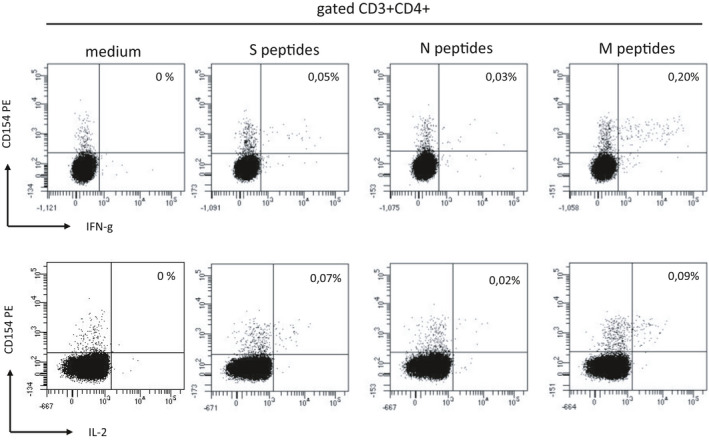
PBMCs were stimulated in vitro with medium (RPMI plus 5% human AB serum) or with SARS‐CoV‐2 S, N, and M proteins peptide pool (Miltenyi Biotech) for 6 h. Then CD3+CD4+ T cells were evaluated by flow cytometry for CD154 expression and IFN‐γ and IL‐2 intracellular production
These findings, together with longitudinal data obtained on antibody levels, led us to speculate a weak vaccine‐induced immunity in this patient. Indeed, exposure to SARS‐CoV‐2 led to unrestricted viral replication, sufficient to drive the development of primary immune responses vs non‐spike antigens (known to be elicited by natural infection). The occurrence of a symptomatic infection after vaccination could have also been related with the exposure to a viral variant with spike alteration possibly associated with immune‐escape (B.1.1.29), although the specific contribution played by each condition remains uncertain at this stage. Based on our report, further studies are needed to evaluate the immunogenicity of SARS‐CoV‐2 vaccines in SOTRs.
ACKNOWLEDGEMENTS
The author(s) received no financial support for the research, authorship, and/or publication of this article.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2‐dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jolly B, Rophina M, Shamnath A, et al. Genetic epidemiology of variants associated with immune escape from global SARS‐CoV‐2 genomes. bioRxiv. 2020. [Google Scholar]
- 5. Weisblum Y, Schmidt F, Zhang F, et al. Escape from neutralizing antibodies by SARS‐CoV‐2 spike protein variants. eLife. 2020;9:e61312. Published 2020 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]


