Abstract
Objective:
To compare the oral microbiota of Sjögren’s syndrome (SS) with that of healthy subjects (HS).
Methods:
Supragingival and subgingival biofilm samples were collected from the mesial-buccal tooth surfaces of SS patients (n = 57) and age- and sex-matched HS (n = 53). Unstimulated saliva and 8 oral tissue samples were taken using a buccal brush. Caries and periodontal measures were recorded. All supragingival samples and a subgroup of 24 SS and 28 HS subgingival samples, as well as 32 SS and 11 HS saliva and oral tissue samples, were analyzed for their content of 41 bacterial species using checkerboard DNA-DNA hybridization. Mean levels (×105 ± SEM) and percentage of DNA probe counts of each species were determined for each sample site and averaged within subjects in the 2 clinical groups. Kruskal-Wallis tests, adjusting for multiple comparisons and cluster analysis, were used for soft tissue and microbial analysis, and the Mann-Whitney test was used to compare caries and periodontal measures.
Results:
Mean (×105 ± SEM) total DNA probe counts in supragingival samples were significantly lower (P < 0.001) in the SS (13.3 ± .7) compared to the HS (44.1 ± 6.8) group. In supragingival samples, Veillonella parvula, Fusobacterium nucleatum ss vincenti, and Propionibacterium acnes were markedly elevated in the SS compared to the HS group in both mean (×105 ± SEM) and mean (± SEM) percentage DNA probe counts (P < 0.001). In subgingival samples of SS, V. parvula was significantly different compared to HS (P < 0.05). SS was characterized by high levels of purple and low levels of orange and red complexes. Cluster analysis of oral tissues and saliva demonstrated that the mean microbial profiles for SS patients and the HS group clustered separately. Active root caries (P < 0.003) and attachment loss were significantly higher (P < 0.029) in the SS group compared to the HS group.
Conclusion:
These findings indicate that saliva is a major controlling factor of intraoral biofilm. V. parvula may be a unique microbial biomarker for Sjögren’s syndrome.
Knowledge Transfer Statement
The microbiome characterized for Sjögren’s syndrome in salivary hypofunction is shown to be under stress and reduced. Veillonella parvula can be a possible identification of a biomarker for Sjögren’s syndrome.
Keywords: salivary hypofunction, bulk fluid, DNA-DNA hybridization, Veillonella parvula, plaque, mean and percentages of DNA count
Introduction
The oral cavity has the most diverse microbial population in the human body, with moist soft mucosal epithelium and hard tissues for microbial colonization. The biofilms and microbial colonization depend upon the intraoral location, the person’s genetic background, lifestyle, and environmental factors in different individuals (Kilian et al. 2016). In the presence of normal salivary function, the oral surfaces are constantly bathed, primarily by saliva, the major fluid needed for biofilm development.
Sjögren’s syndrome is a progressive autoimmune disorder affecting upward of 4 million Americans according to the Sjögren’s Syndrome Foundation. Sjögren’s syndrome affects mainly middle-aged and older women (female/male 9:1); however, people of all ages and races have been diagnosed with Sjögren’s syndrome. Sjögren’s syndrome patients’ main complaints are xerophthalmia and xerostomia, but extra glandular manifestations may include multiple domains of the human body.
Sjögren’s syndrome represents a unique condition where the effects of the loss of saliva, the bulk fluid that bathes the oral cavity, can be qualitatively and quantitatively studied. Salivary hypofunction alters oral homeostasis and can have a significant negative impact on a person’s quality of life. Sjögren’s syndrome has been reported to cause candidiasis, dental caries, gingival inflammation, clinical attachment loss, gingival recession, plaque accumulation, and bleeding on probing (Najera et al. 1997; Fox et al. 2008). By comparing the microbiota of Sjögren’s to that of healthy subjects, the role of saliva on the development of biofilms in this unique population will be better understood.
Several studies have related oral microbiota to the overall oral health in Sjögren’s subjects (Tanida et al. 2003; Scully 2008). Alterations in the innate and adaptive immune systems may create a shift of microbiota. This in turn plays a fundamental role in the induction, training, and function of the host immune system (Belkaid and Hand 2014). Saliva has a significant role in the initiation, establishment, composition, and metabolism of oral biofilms. It maintains oral homeostasis by providing nutrients to and removing waste products from biofilms (Jakubovics 2015; Marsh et al. 2016). Thus, the low salivary flow rate associated with Sjögren’s can influence the microbial composition of oral biofilms.
Earlier studies focused primarily on Candida or acidogenic species of bacteria in the Sjögren’s population and their associated complications. The aim of this study is to compare the oral microbiota in the various parts of the oral cavity of Sjögren’s syndrome subjects to age and sex-matched healthy subjects. The hypothesis being tested is that low salivary flow will cause major differences in the composition of oral biofilms in subjects with Sjögren’s syndrome compared to healthy subjects.
Materials and Methods
Study Population and Microbial Assessment
This study was conducted jointly at the Forsyth Institute and the Division of Oral Medicine, Tufts University School of Dental Medicine (Boston, MA). This study is the Sjögren’s substudy of the Forsyth Intraoral Biofilm Formation Study. The study was approved by the Tufts Health Science’s Institutional Review Board (IRB). Sjögren’s subjects, with a confirmed diagnosis of primary Sjögren’s syndrome, based on the American-European criteria (Vitali et al. 2002) and healthy subjects with no periodontal disease, normal levels of unstimulated and stimulated salivary flow rates, and taking no medications were recruited from the oral medicine clinic at Tufts University School of Dental Medicine (Boston, MA). All subjects (regardless of group assignment) met the following inclusion criteria: were more than 20 years of age, had 24 or more teeth, and demonstrated a willingness and ability to understand and sign an informed consent form. Those subjects who were on antibiotics or needed premedication were excluded. Healthy subjects were excluded if they were found taking medications, had a history of periodontal disease, had bleeding gingiva, or were found to have periodontal disease after examination.
Subjects were instructed to refrain from brushing for 12 hours and from drinking, eating, smoking, or chewing gum for 1½ hours before saliva collection. Saliva collections were carried out in the morning to minimize circadian variation. Unstimulated salivary flow rate was measured in each subject. The subjects swallowed immediately before the collection and were seated with their head down for 5 minutes, allowing the saliva to drip off the lower lip into a preweighed vial (Dawes 1987). The weight of the collection vial was recorded before and after collection with the difference representing the salivary volume. Stimulated whole saliva was collected by chewing paraffin wax after all microbial samples were taken, recorded, and discarded.
An oral exam was done to identify obvious periodontal disease or any pathology in the oral cavity. Supragingival and subgingival biofilm samples were collected separately, using sterile Gracey curettes from the mesial-buccal surface of all teeth present (third molars were excluded). Soft tissue samples were taken from Sjögren’s subjects and healthy subjects at 8 different sites: the dorsum (TD), lateral (TL) and ventral tongue (TV), floor of mouth (FOM), buccal mucosa (B), hard palate (HP), vestibule/lip (VL), and attached gingiva (AG) using a buccal brush. Each sample was placed in individual tubes containing 0.15 mL TE (10 mM Tris-HCL, 0.1 mM EDTA, pH 7.6) and had 0.15 mL 0.5 M NaOH added. After removal of any remaining supragingival plaque, subgingival plaque samples were taken separately from the mesial-buccal surface of each tooth and evaluated. Each sample was boiled for 5 min and neutralized using 0.8 mL 5 M ammonium acetate and placed into the extended slots of minislots (Immunetics). They were then concentrated onto a Boehringer Mannheim nylon membrane by vacuum and fixed to the membrane by exposure to ultraviolet light, followed by baking at 120°C for 20 min. Samples were individually processed and analyzed at the Forsyth Institute (Boston, MA) for 41 bacterial species using checkerboard DNA-DNA hybridization. Levels and percentages of bacterial DNA probe counts of each species were determined for each sampled site and averaged within each subject for supra- and subgingival plaque samples separately. Mean microbial profiles of samples from unstimulated saliva and the 8 different intraoral locations from Sjögren’s and healthy subjects were compared using cluster analysis; minimum similarity coefficient was employed, and the profiles were sorted using an average unweighted linkage sort. Microbial profiles were expressed as mean proportions of each species at each sample location from Sjögren’s and healthy subjects separately (Socransky et al. 1994).
After samples were collected, a dental caries exam (using criteria from Pitts et al. 1997) and a periodontal assessment at 6 sites per tooth (mesiobuccal, buccal, distobuccal, mesiolingual, lingual, distolingual) with the exception of third molars were performed by a single examiner. The examiner was trained and calibrated. The frequency of dental visits, brushing, and flossing was self-reported by the study volunteers. Since this was an observational study, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed. All the data were available for supragingival samples. Data are presented for 24 Sjögren’s subjects and 28 periodontally healthy subjects for subgingival microbiological data, as well as 32 Sjögren’s subjects and 11 healthy subjects for oral tissues and salivary samples.
Data Evaluation
A power calculation was done with the log10 (count) of the Sjögren’s subjects, assuming a 20% difference from that of healthy subjects’ bacterial counts; the power value of 99% for 2-tailed comparisons at P = 0.05 was 60 subjects per group (for 80% power in 2-tailed comparisons at P = 0.05, the number of subjects required was 30) for both supragingival and subgingival sites. Counts and the percentage of total DNA probe counts of each of the 41 taxa in each biofilm sample were computed. The mean values for each species in the 2 clinical groups were depicted graphically as “microbial profiles” and ordered according to the microbial complexes (Socransky and Haffajee 2005). The percentage for each of the microbial species was averaged within each individual and averaged across participants in the 2 subject groups separately. Significant differences between subject groups for each species were determined using the Kruskal-Wallis test, adjusting for multiple comparisons. Power-conserving multiple testing procedures were used, such as modified Bonferroni methods or permutation techniques, which preserve more statistical power. Mann-Whitney tests were used to compare caries and periodontal measures. Mean microbial profiles of samples from the different intraoral locations in the groups were compared using cluster analysis. Microbial profiles were expressed as mean proportions of each species at each sample location in the subject groups separately. The minimum similarity coefficient was employed and the profiles sorted using an average unweighted linkage sort.
Results
The clinical characteristics of Sjögren’s (n = 57) and healthy study subjects (n = 53) are presented in the Table. Unstimulated salivary flow was 0.029 ± .042 mL/min for Sjögren’s patients and 0.290 ± 0.295 mL/min for healthy subjects. Stimulated saliva was 0.515 ± 0.705 mL/min for Sjögren’s patients and 1.90 ± .933 mL/min for healthy subjects. This level of flow places the Sjögren’s patients at a higher risk for caries. Despite self-reported increased homecare and dental visits, the prevalence of active root caries was significantly higher in the Sjögren’s group (P < 0.003) compared to the healthy subjects, but coronal carious lesions, filled surfaces, and crowns were not significantly different in the 2 groups. The Sjögren’s group had more clinical attachment loss due to recession compared to the healthy subjects (P > 0.029), but pocket depths did not differ significantly (P > 0.731).
Table.
Demographics and Characteristics of Sjögren’s and Healthy Subjects.
| Characteristic | Sjögren’s | Healthy | P (Mann-Whitney) |
|---|---|---|---|
| Age, y | 57 ± 10.7 | 54 ± 10.6 | NS |
| Number of missing teeth | 3.0 ± 3.4 | 2.2 ± 2.3 | NS |
| Number of sites with pocket depth ≤4 mm | 7.9 ± 1.5 | 6.0 ± 1.1 | NS |
| Number of sites with Attachment loss ≤4 mm | 43.9 ± 4.7 | 29.4 ± 3.6 | 0.029 |
| Bleeding on probing | 30.4 ± 26.1 | 30.7 ± 24.6 | NS |
| Frequency of dental visits, per year | 2.3 | 1.7 | 0.0001 |
| Frequency of brushing, per day | 2.2 | 2 | NS |
| Number of medications | 5.4 ± 3.8 | 0 | 0.0001 |
| DMFS (including crowns) a | 56.6 ± 30.7 | 40 ± 27.6 | 0.007 |
| Mean number of incipient root carious surfaces | 0.4 ± 1.1 | 0.1 ± 0.3 | 0.027 |
| Mean number of cavitated root surfaces | 0.7 ± 0.7 | 0.0 ± 0.2 | 0.003 |
| Mean number of filled root surfaces | 2.2 ± 3.8 | 0.8 ± 1.6 | 0.003 |
DMFS, Decayed, Missing, Filled surfaces; NS, not significant.
No difference in number of coronal surfaces with caries or the number of crowns; DMFS without crowns is not significant.
Supragingival
The microbial results of this study indicate that there are differences in the composition of microbiota in oral biofilms in subjects with Sjögren’s syndrome compared to healthy subjects. Mean (×105 ± SEM) total DNA probe counts were significantly higher (P < 0.001) in healthy subjects (44.1 ± 6.8) compared to Sjögren’s patients (13.3 ± .7). When the levels of the 41 bacterial taxa examined were analyzed, in the supragingival plaque samples, the mean counts (×105 ± SEM) of 30 species differed significantly between the groups in supragingival samples after adjusting for multiple comparisons. Several species were present in significantly higher levels in healthy subjects, including Aggregatibacter actinomycetemcomitans, Campylobacter rectus, Eubacterium nodatum, and Fusobacterium nuceatum ss polymorphum (P < 0.001). The mean counts (×105 ± SEM) of Veillonella parvula demonstrated that this was the only species with levels significantly higher in Sjögren’s patients than in healthy subjects (P < 0.001).
When the microbiota was analyzed based on the percent DNA probe count in supragingival samples, the proportions of some species differed significantly between the groups after adjusting for multiple comparisons. The healthy subjects had a greater percentage of DNA probe counts for Campylobacter showae and Capnocytophaga sputigena (P < 0.001) and Eikenella corrodens (P < 0.01) compared to the Sjögren’s subjects. The Sjögren’s subjects harbored significantly higher proportions of V. parvula, Fusobacterium nucleatum ss vincenti, and Propionibacterium acnes (P < 0.001). The supragingival microbiota of Sjögren’s subjects was characterized by lower counts of most species examined, as well as very high proportions of V. parvula (Fig. 1).
Figure 1.
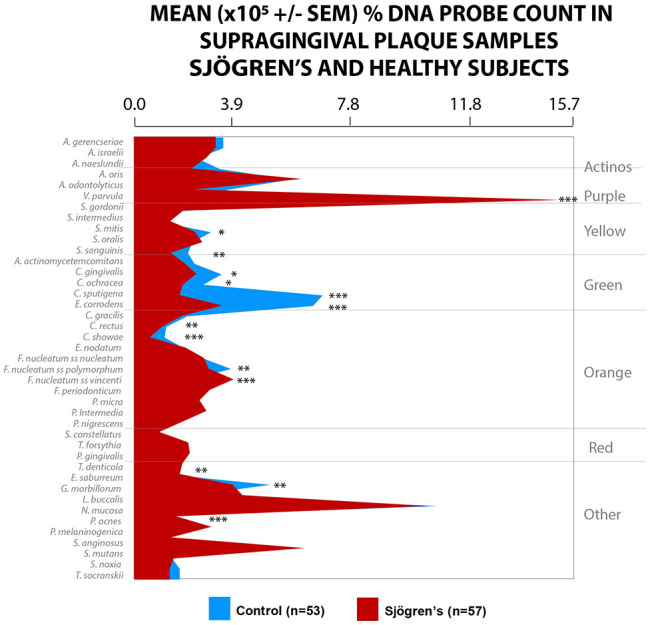
Mean ± SEM × 105 probe count of 41 taxa in supragingival plaque samples from 57 Sjögren’s subjects and 53 healthy subjects. Data for each species were averaged within a subject and then across subjects in the 2 clinical groups separately. The significance of differences among groups was sought using the Kruskal-Wallis test and adjusted for multiple comparisons (Socransky et al. 1991). *P < 0.05. **P < 0.01. ***P < 0.001. Species were ordered according to the complexes described by Socransky et al. (1998).
Subgingival
Only 32 Sjögren’s subjects and 28 periodontally healthy subjects for subgingival microbiological data could be reported in this substudy. The mean total DNA probe counts (×105 ± SEM) of subgingival microbiota of the Sjögren’s subjects (15.1 ± 1.7) exhibited low counts of most species compared with healthy subjects (37.4 ± 7.3). V. parvula was elevated in Sjögren’s subjects compared to healthy subjects, mean percent ± SEM of 14.3 ± 1.4 versus 8.4 ± 0.9, P < 0.05, respectively. Mean counts of 19 species and proportions of 16 species differed significantly among groups after adjusting for multiple comparisons. Mean counts of Streptococci, Capnocytophaga, E. corrodens, and Neisseria mucosa were comparable in the 2 groups. Mean counts of Tannerella forsythia, Porphyromonas gingivalis, Treponema denticola, and Treponema socranskii were extremely low in both healthy and Sjögren’s subjects. The Sjögren’s subjects were characterized by high levels of the purple complex and the lowest levels of the orange and red complex (Fig. 2).
Figure 2.
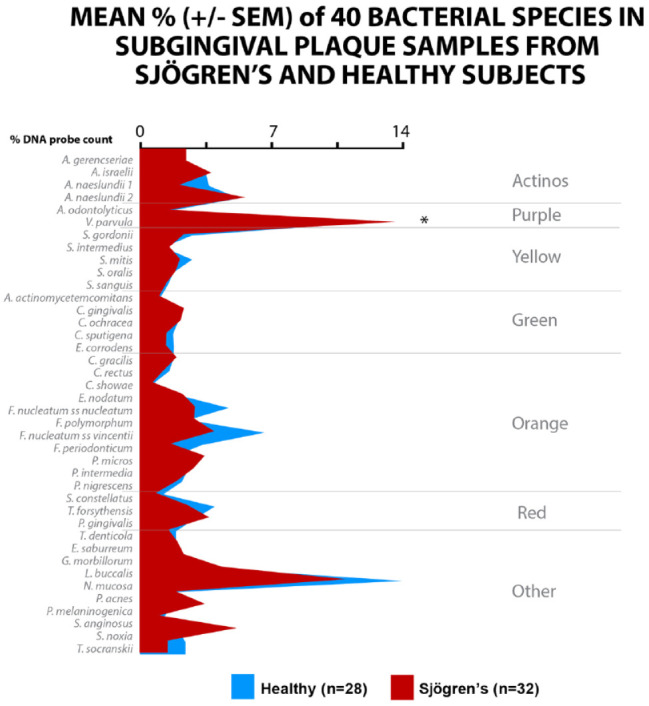
Mean DNA probe count of 41 taxa in supragingival plaque samples from 57 Sjögren’s subjects and 53 healthy subjects. Data for each species were averaged within a subject and then across subjects in the 2 clinical groups separately. The significance of differences among groups was sought using the Kruskal-Wallis test and adjusted for multiple comparisons (Socransky et al. 1991). *P < 0.05. **P < 0.01. ***P < 0.001. Species were ordered according to the complexes described by Socransky et al. (1998).
Oral Soft Tissues and Saliva
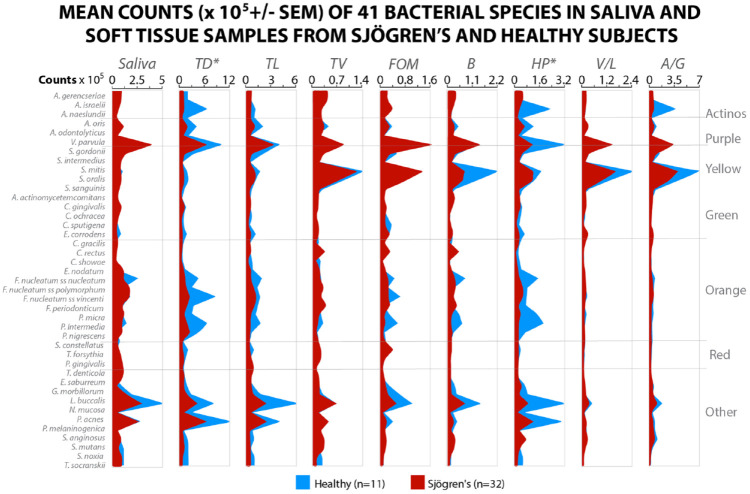
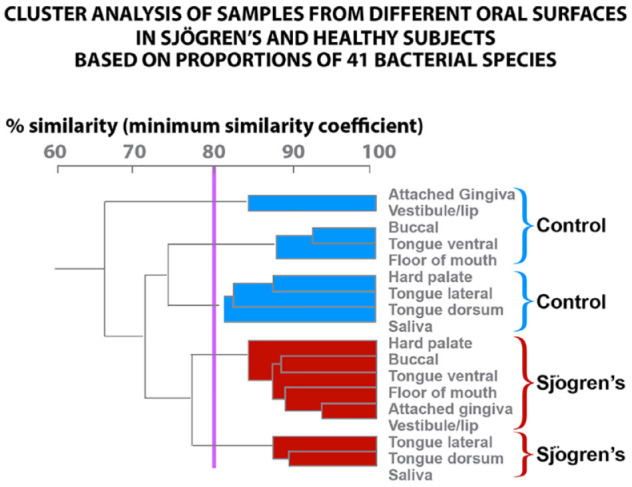
We can only report on 32 Sjögren’s and 11 healthy subjects for oral tissues and salivary samples. There were significantly lower mean total DNA probe counts for Sjögren’s subjects compared with healthy subjects in the samples from the dorsal tongue, TD (46.6 ± 10.1 vs. 97.5 ± 21.7), B (6.2 ± 1.2 vs. 14.8 ± 4.2), and HP (7.6 ± 1.9 vs. 32.5 ± 15.1). For these locations, species that were significantly elevated in the healthy subjects were Streptococcus oralis, E. corrodens, N. mucosa, P. acnes, and Prevotella melaninogenica. V. parula and N. mucosa were high in Sjögren’s but not significantly different from healthy subjects (Fig. 3). Our data indicate that the microbial species on the soft tissues and in saliva of Sjögren’s subjects differ in percent DNA probe counts from that detected in healthy subjects. The cluster analysis demonstrated that the mean microbial profiles for Sjögren’s and healthy subjects clustered separately. A total of 4 clusters were formed. Each subject group provided 2 clusters; 1 for each subject group contained the saliva and tongue dorsum profiles. The supragingival and subgingival species cluster together (Fig. 4).
Figure 3.
Mean percent DNA probe counts of 40 bacterial species were determined for each sampled site and averaged within each subject for subgingival plaque samples separately.
Figure 4.
Mean count (×105 ± SEM) of 41 microbial species in saliva, dorsal, lateral and ventral tongue, floor of the mouth, buccal mucosa, hard palate, labial vestibule, and attached gingiva.
Discussion
The results of this study strongly support the hypothesis that there are major differences in the composition of oral biofilms in subjects with Sjögren’s syndrome when compared with healthy subjects with normal salivary flow throughout the entire oral cavity. The microbiota of the oral cavity consists of highly regulated, structurally and functionally organized communities that attach to hard and soft tissue surfaces (Li and Tian 2012). Our findings highlight the importance of saliva in governing the composition of the oral microbiota. Sjögren’s subjects who have minimal salivary flow had markedly lower supragingival and subgingival biofilm accumulation characterized by lower mean (×105 ± SEM) and percentage DNA total counts compared to healthy subjects with normal salivary flow. Mean bacterial counts in subgingival biofilms of Sjögren’s subjects, which are fed by a different bulk fluid, were lower than in healthy subjects. In addition, the composition of the microbiota was dramatically altered in the Sjögren’s compared with healthy subjects and was characterized by lack of the red complex implicated in periodontitis (Socransky and Haffajee 2005). In addition, the composition of the microbiota was dramatically altered in the Sjögren’s subjects compared with the other 2 groups in the 3 biofilm categories studied: the supragingival, the subgingival, and the soft tissue and saliva. These data indicate that saliva is a major controlling factor in intraoral biofilm development. Thus, the hypothesis that a decrease in salivary flow would significantly affect counts and composition of the microbiota on the teeth and oral tissues was strongly supported.
The microbial species on the soft tissues and in saliva of Sjögren’s subjects differ in quantity and proportions from that detected in healthy subjects. Reduced levels of saliva, the bulk fluid for the oral cavity, have a major impact on biofilm formation on the soft tissues, as well as hard tissues. Saliva is the major source of nutrients for the microorganisms in the oral cavity. A reduction of saliva results in a decrease in the availability of nutrients for the development of the microbiome (Jakubovics 2015). Our study found lower microbial counts in all the samples taken in Sjögren’s subjects compared to healthy subjects. In addition, saliva, the bulk fluid for the oral cavity, removes deleterious metabolic products (Kilian et al. 2016). An altered microbiome diversity with a decreased microbial population was also found in mucosal surfaces in Sjögren’s compared to the controls by Paiva et al. (2016) and Mandl et al. (2017). Pulukool et al. (2015) found increased levels of Capnocytophaga, Dialister, Fusobacterium, Helicobacter, and Streptococcus in Sjögren’s subjects. P. gingivalis and Aggregatibacter actinomycetemcomitans were not detected in any subjects with Sjögren’s, again similar to our findings. More important, they did detect higher levels of V. parvula, which confirms our results. The presence of high levels of Helicobacter is not a surprising finding, since a Sjögren’s syndrome Harris interactive survey showed that 45% of the Sjögren’s patients had gastroesophageal reflux. Zhou et al. (2018) also confirmed our findings using an oral rinse that was analyzed with high-throughput sequencing. They found Veillonella to be 4 times higher in primary Sjögren’s, and Actinomyces, Haemophilus, Neisseria, Rothia, Porphyromonas, and Peptostreptococcue were significantly lower in Sjögren’s subjects compared to healthy age- and sex-matched subjects. Similar to our findings, they observed that S. mutans and Lactobacillus, known to cause caries, were similar in Sjögren’s and control subjects. Rusthen et al. (2019) found salivary microbiota in xerostomic and primary Sjögren’s syndrome patients to be dysbiotic. They also confirmed that Veillonella was higher in Sjögren’s patients than control subjects. When Sembler-Møller et al. (2019) compared hyposalivation patients, defined as unstimulated whole saliva ≤0.1 mL/min and stimulated whole saliva ≤0.7 mL/min, to Sjögren’s subjects, they found the microbial species reduced and similar in composition. They attributed these changes to low salivary flow.
Leung et al. (2007) found significantly lower proportions of gram-negative species in primary Sjögren’s subjects compared to secondary Sjögren’s and healthy control subjects (P < 0.047). Anaerobic gram-negative rods were uncommon. In addition, they also isolated nonoral species of bacteria in greater proportions from primary Sjögren’s supragingival plaque (P = 0.007). Our study did detect P. gingivalis and A. actinomycetemcomitans, but the levels were not significantly different from the healthy subjects.
V. parvula species was markedly elevated in the Sjögren’s group compared to the healthy group, both in mean counts (P < 0.001) and mean percent DNA probe counts (P < 0.001). Siddiqui et al. (2016) found significantly increased levels of V. parvula in Sjögren’s patients (P < 0.001) even with normal salivary flow. V. parvula is strictly anaerobic and is isolated from the dental plaque, the vagina, and the gastrointestinal (GI) tract. It is regarded as commensal (Bhatti and Frank 2000) and has a unique physiology that uses organic acids (malate and lactate) produced by S. mutans, lactobacilli, and Actinomyces (Delwiche et al. 1985) for its metabolism. It cannot metabolize carbohydrates directly (Ng and Hamilton 1971). The survival of V. parvula is through coaggregation and has an intricate metabolic complementation with S. mutans (Luppens et al. 2008), which could help explain why Sjögren’s subjects have more prevalent carious lesions. P. acne also has been identified as an opportunistic pathogen, which may contribute to the endodontic pathology (Niazi et al. 2016). Induction of proinflammatory cytokines, interleukin (IL)–1α, IL-1β, IL-8, and tumor necrosis factor–α (TNF-α) in acne by P. acnes has been reported (Vowels et al. 1995). Therefore, although P. acnes is usually regarded as a harmless commensal, it possesses many attributes of a disease-causing organism.
Proctor et al. (2108) described that both the unstimulated and stimulated whole saliva flow rate were the contributing factors that distinguished the microbial communities observed in xerostomic and healthy subjects. They also noted that acidophilic and caries-associated taxa, such as Catonella sp., S. mutans, Lactobacillus fermentum, Scardovia wiggsiae, 2 Atopobium parvulum strains, and Veillonella sp., were associated with positive scores for 67% of Sjögren’s samples mapped. Furthermore, it was determined that the composition of the microbial community, modulated by low salivary flow, selects for acidophilic and acidogenic species. This may be due to the homogenization of intraplaque pH. The greatest shift in microbial differences is observed in patients who have a stimulated saliva secretion rate of <0.5 mL/min−1 (Paiva et al. 2016). As the whole salivary flows were lowered, a shift in the microbial population was also described by Almståhl and Wikström (1999).
It has been shown that soft tissues, especially the tongue in healthy subjects, are predominantly colonized by Gram-negative species, including P. melaninogenica, V. parvula, and C. gingivalis, and may serve as a reservoir for the red complex to reinfect periodontal pockets after treatment (Mager et al. 2003). Our data indicate that the microbial species on the soft tissues and in saliva of Sjögren’s subjects differ in quantity and proportions from that detected in control subjects and differ from that found on hard tissues, with saliva and tongue microbiota being similar. A limitation of the study is that we did not have sufficient data on oral soft tissue and saliva samples. Sjögren’s subjects had significantly lower levels of all species and especially the orange and red complex, which might explain why they did not have periodontitis. The recession they had may be due to excessive brushing or gingivitis. This suggests that Sjögren’s subjects were less affected in the subgingival area, possibly due to the fact that gingival crevice fluid rather than saliva provides the bulk fluid to the area. Also, the low number of periodontal pockets in Sjögren’s and healthy subjects provides less surface area for microbial colonization.
Additional potential causes for the microbial differences observed are changes in the pH, buffering capacity, protein, and immunological profiles of the saliva found in Sjögren’s syndrome. In addition, food debris is retained longer, prolonging the pH drop. We have found increased levels of proline-rich phosphoproteins in the saliva of Sjögren’s subjects (Zoukhri et al. 2012). Saliva delivers statherin, proline-rich proteins, and other mucinous proteins that bind to negatively charged hydroxyapatite to immediately form an acquired enamel pellicle. The pellicle consists of various salivary glycoproteins that function as adhesion sites for bacteria and control bacterial levels.
Data on the periodontal health of Sjögren’s syndrome patients have been scarce. The imbalance caused by reduced salivary flow affects the commensal microorganisms, the hosts’ defense, and debris clearance that could result in gingival inflammation. Maarse et al. (2019), in a systematic review of the literature on Sjögren’s patients and periodontal disease, found no significant differences in the gingival index, periodontal index, clinical attachment loss, and probing pocket depth in patients with Sjögren’s compared to controls. The clinical attachment loss in our study was due to gingival recession and not to pocket depth. This can also explain this group having the lowest level of red complex bacteria. They did find, as we have, that Sjögren’s patients had higher dental caries compared to non–Sjögren syndrome patients.
A higher caries rate as well as total DMFS has been found in the Sjögren’s patients compared with the healthy subjects (Pedersen et al. 2005; Zhou et al. 2018). Pedersen et al. (2005) found that there was an inverse correlation between the salivary flow rate and higher decayed surfaces. Salivary flow is negatively correlated with caries in the Sjögren’s syndrome subjects (r2 = −0.511, P < 0.05). In our study, root surfaces lesions were found to be significantly higher in Sjögren’s compared to the healthy subjects. This is consistent with the finding of greater recession in Sjögren’s subjects, in whom exposed dentin is more susceptible to caries than enamel.
Saliva acting as a gatekeeper for protecting both the gastrointestinal and respiratory tracts preserves a beneficial oral biofilm and eliminates potential pathogens that enter the oral cavity, preventing them from causing disease in other parts of the body (Ruhl 2012). The changes caused by the Sjögren’s B-cell destruction of the salivary glands probably initiates a dysbiosis in the oral cavity. Alternatively, Nikitakis et al. (2017) suggested that a dysregulated immune response against the oral microbiome in Sjögren’s patients could be responsible for initiating an autoimmune response in Sjögren’s or involved in its amplification. A Taiwanese case-control study of chronic periodontitis patients, identified from the National Health Insurance Database frequency matched by age, sex, and index year to a cohort from the general population, showed the incidence of Sjögren’s (8.5 y later) was significantly higher in the chronic periodontitis cohort (hazard ratio, 1.79; Lin et al. 2018). Szymula et al. (2014) in a laboratory study found that the von Willebrand factor type A (vWFA) domain protein produced by Capnocytophaga ochracea was the most potent activator of SSA (Ro). In addition, SSA-reactive T cells could be activated in vitro by recombinant vWFA protein. They suggest that a microbial trigger might initiate autoimmunity in Sjögren’s. In our study, there was no difference in C. ochracea in healthy and Sjögren’s subjects. The presence of elevated levels of normally commensal organisms that become dysbiotic, such as V. parvula, P. acne, and N. mucosa, found in this study, can initiate the progression of Sjögren’s. Further studies could explore the interrelationship of the microbiome and the pathogenesis of this disease.
We are aware that the checkerboard technique is confined to species for which probes are available and that newer techniques are now available. But the technique has high sensitivity and specificity, and it can use entire samples, can investigate large numbers of species, and is inexpensive.
The significance of this study resides on the observation that Sjögren’s syndrome patients harbor lower levels of the most common oral microorganisms, collectively and individually, but they have a high percentage of V. parvula and do not have the red and orange complex. In fact, most studies report on the microbiota of saliva for the Sjögren’s population, and only a few studies exist from the supragingival area (Leung et al. 2007). Thus, the high levels of V. parvula and its potential contribution to caries development are important clinical findings that can help diagnose, treat, and monitor Sjögren’s syndrome patients.
Conclusion
These findings indicate that saliva is a major controlling factor of intraoral biofilm development.
The microbial species of the Sjögren’s group differ in quantity and proportions from that detected in healthy subjects, with the mean and percentage of total DNA probe counts of microbial profiles being significantly lower compared to that of the healthy group. Our findings, which have been corroborated by other researchers, are suggestive that V. parvula may be a unique microbial biomarker for Sjögren’s syndrome.
Author Contributions
M. Singh, F. Teles, A. Papas, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; N.G. Uzel, contributed to data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
This article is dedicated in memory and honor of Drs. Sigmund Socransky, Anne Haffejee, and Ricardo Teles from The Forsyth Institute (Boston, MA), who were involved in conception, design, data acquisition, and interpretation but could not complete the manuscript because they passed away. They continue to inspire many researchers by their examples and dedication to the betterment of science. We also thank Adriane Kilar, DMD, for reading the manuscript and providing critical suggestions; Elizabeth Tzavaras and Gay Torresyap for their help coordinating this study; and Karun Thapa for the professional touch on the graphs and tables.
Footnotes
This work was supported in part by National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) grant R01-DE-14368.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Almståhl A, Wikström M. 1999. Oral microflora in subjects with reduced salivary secretion. J Dent Res. 78(8):1410–1416. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell. 157(1):121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti MA, Frank MO. 2000. Veillonella parvula meningitis: case report and review of veillonella infections. Clin Infect Dis. 31 (3): 839–840. [DOI] [PubMed] [Google Scholar]
- Dawes C. 1987. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J Dent Res. 66 Spec No:648–653. [DOI] [PubMed] [Google Scholar]
- Delwiche EA, Pestka JJ, Tortorello ML. 1985. The veillonellae: gram-negative cocci with a unique physiology. Annu Rev Microbiol. 39:175–193. [DOI] [PubMed] [Google Scholar]
- Fox PC, Bowman SJ, Segal B, Vivino FB, Murukutla N, Choueiri K, Ogale S, McLean L. 2008. Oral involvement in primary Sjögren syndrome. J Am Dent Assoc. 139(12):1592–1601. [DOI] [PubMed] [Google Scholar]
- Jakubovics NS. 2015. Saliva as the sole nutritional source in the development of multispecies communities in dental plaque. Microbiol Spectr. 3(3). doi: 10.1128/microbiolspec.MBP-0013-2014 [DOI] [PubMed] [Google Scholar]
- Kilian M, Chapple ILC, Hannig M, Marsh PD, Meuric V, Pedersen AML, Tonetti MS, Wade WG, Zaura E. 2016. The oral microbiome—an update for oral healthcare professionals. Br Dent J. 221(10):657–666. [DOI] [PubMed] [Google Scholar]
- Leung KCM, Leung WK, McMillan AS. 2007. Supra-gingival microbiota in Sjögren’s syndrome. Clin Oral Investig. 11(4):415–423. [DOI] [PubMed] [Google Scholar]
- Li YH, Tian X. 2012. Quorum sensing and bacterial social interactions in biofilms. Sensors (Basel). 12(3):2519–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TC, Tseng CF, Wang YH, Yu HC, Chang YC. 2018. Patients with chronic periodontitis present increased risk for primary Sjögren syndrome: a nationwide population-based cohort study. PeerJ. 6:e5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppens SB, Kara D, Bandounas L, Jonker MJ, Wittink FR, Bruning O, Breit TM, Ten Cate JM, Ten Crielaard W. 2008. Effect of Veillonella parvula on the antimicrobial resistance and gene expression of Streptococcus mutans grown in a dual-species biofilm. Oral Microbiol Immunol. 23(3):183–189. [DOI] [PubMed] [Google Scholar]
- Maarse F, Jager DHJ, Alterch S, Korfage A, Forouzanfar T, Vissink A, Brand HS. 2019. Sjögren’s syndrome is not a risk factor for periodontal disease: a systematic review. Clin Exp Rheumatol. 37(Suppl 118):S225–S233. [PubMed] [Google Scholar]
- Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. 2003. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol. 30(7):644–654. [DOI] [PubMed] [Google Scholar]
- Mandl T, Marsal J, Olsson P, Ohlsson B, Andréasson K. 2017. Severe intestinal dysbiosis is prevalent in primary Sjögren’s syndrome and is associated with systemic disease activity Arthritis Res Ther. 19(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD, Do T, Beighton D, Devine DA. 2016. Influence of saliva on the oral microbiota. Periodontol 2000. 70(1):80–92. [DOI] [PubMed] [Google Scholar]
- Najera MP, al-Hashimi I, Plemons JM, Rivera-Hidalgo F, Rees TD, Haghighat N, Wright JM. 1997. Prevalence of periodontal disease in patients with Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 83(4):453–457. [DOI] [PubMed] [Google Scholar]
- Ng SKC, Hamilton IR. 1971. Lactate metabolism by Veillonella parvula. J Bacteriol. 105(3):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazi SA, Al Kharusi HS, Patel S, Bruce K, Beighton D, Foschi F, Mannocci F. 2016. Isolation of Propionibacterium acnes among the microbiota of primary endodontic infections with and without intraoral communication. Clin Oral Invest. 20(8):2149–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitakis NG, Papaioannou W, Sakkas LI, Kousvelari E. 2017. The autoimmunity-oral microbiome connection: distribution of selected bacterial species on intraoral surfaces. Oral Dis. 3(7):828–839. [DOI] [PubMed] [Google Scholar]
- Paiva CSD, Jones DB, Stern ME, Bian F, Moore QL, Corbiere S, Streckfus CF, Hutchinson DS, Ajami NJ, Petrosino JF, et al. 2016. Altered mucosal microbiome diversity and disease severity in Sjögren syndrome. Sci Rep. 6:23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AML, Bardow A, Nauntofte B. 2005. Salivary changes and dental caries as potential oral markers of autoimmune salivary gland dysfunction in primary Sjögren’s syndrome. BMC Clin Pathol. 5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts NB, Evans DJ, Pine CM. 1997. British Association for the Study of Community Dentistry (BASCD) diagnostic criteria for caries prevalence surveys—1996/97. Community Dent Health. 14(Suppl 1):6–9. [PubMed] [Google Scholar]
- Proctor DM, Fukuyama JA, Loomer PM, Armitage GC, Lee SA, Davis NM, Ryder MI, Holmes SP, Relman DA. 2018. A spatial gradient of bacterial diversity in the human oral cavity shaped by salivary flow. Nat Commun. 9(1):681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulukool S, Sharma D, Vellarikkal SK, Surin AK, Jayarajan R, Verma A, Dixit V, Sivasubbu S, Danda D, Scaria V. 2015. AB0188. Systematic analysis of the oral microbiome in primary Sjögren’s syndrome suggest enrichment of distinct microbes. Ann Rheum Dis. 74:953–954. [Google Scholar]
- Ruhl S. 2012. The scientific exploration of saliva in the post-proteomic era: from database back to basic function. Expert Rev Proteomics. 9(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusthen S, Kristoffersen AK, Young A, Galtung HK, Petrovski BÉ, Palm Ø, Enersen M, Jensen JL. 2019. Dysbiotic salivary microbiota in dry mouth and primary Sjögren’s syndrome patients. PLoS ONE. 14(6):e0218319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully C. 2008. Oral and maxillofacial medicine: the basis of diagnosis and treatment. 2nd ed. Edinburgh (UK): Churchill Livingstone Elsevier. [Google Scholar]
- Sembler-Møller ML, Belstrøm D, Locht H, Enevold C, Pedersen AML. 2019. Next-generation sequencing of whole saliva from patients with primary Sjögren’s syndrome and non-Sjögren’s sicca reveals comparable salivary microbiota. J Oral Microbiol. 11(1):1660566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui H, Chen T, Aliko A, Mydel PM, Jonsson R, Olsen I. 2016. Microbiological and bioinformatics analysis of primary Sjögren’s syndrome patients with normal salivation. J Oral Microbiol. 8:31119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. 2005. Periodontal microbial ecology. Periodontol 2000. 38:135–187. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. 1998. Microbial complexes in subgingival plaque. J Clin Periodontol. 25:134–144. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Smith C, Dibart S. 1991. Relation of counts of microbial species to clinical status at the site. J Clin Periodontol. 18:766–775. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. 1994. “Checkerboard" DNA-DNA hybridization. Biotechniques. 17(4):788–792. [PubMed] [Google Scholar]
- Szymula A, Rosenthal J, Szczerba BM, Bagavant H, Fu SM, Deshmukh US. 2014. T cell epitope mimicry between Sjögren’s syndrome antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin Immunol. 152(1–2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida T, Okamoto T, Okamoto A, Wang H, Hamada T, Ueta E, Osaki T. 2003. Decreased excretion of antimicrobial proteins and peptides in saliva of patients with oral candidiasis. J Oral Pathol Med. 32(10):586–594. [DOI] [PubMed] [Google Scholar]
- Vitali C, Bombardieri S, Jonsson R, et al. 2002. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 61(6):554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowels BR, Yang S, Leyden JJ. 1995. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 63(8):3158–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Ling G, Ding N, Xun Z, Zhu C, Hua H, et al. 2018. Molecular analysis of oral microflora in patients with primary Sjögren’s syndrome by using high-throughput sequencing. PeerJ. 6:e5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Rawe I, Singh M, Brown A, Kublin C, Dawson K, Haddon WF, White EL, Hanley KM, Tusé D, et al. 2012. Discovery of putative salivary biomarkers for Sjögren’s syndrome using high resolution mass spectrometry and bioinformatics. J Oral Sci. 54(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]