Abstract
Objective
To test the hypothesis that there is seasonal variation in the rates of gestational diabetes (GDM) diagnosed using a 2‐hour oral glucose tolerance test.
Design
Monthly assessment of the percentage of women screened from 1 April 2016 to the 31 December 2020 who were diagnosed as having gestational diabetes.
Setting
London teaching hospital.
Population
28 128 women receiving antenatal care between 1 April 2016 and 31 December 2020.
Methods
Retrospective study of prospectively collected data.
Main outcome measures
Proportion of women screened diagnosed as having gestational diabetes.
Results
The mean (SD) percentage of women diagnosed with GDM was 14.78 (2.24) in summer (June, July, August) compared with 11.23 (1.62) in winter (P < 0.001), 12.13 (1.94) in spring (P = 0.002) and 11.88 (2.67) in autumn (P = 0.003). There was a highly significant positive correlation of the percentage testing positive for GDM with the mean maximum monthly temperature (R 2 = 0.248, P < 0.001). There was a statistically significant 33.8% increase in the proportion of GDM diagnoses from June 2020 onwards, possibly related to a reduction in exercise secondary to the Covid‐19 pandemic.
Conclusions
There is a 23.3% higher rate of GDM diagnoses in the warmer summer months. There has been a 33.8% rise in GDM diagnoses associated with the Covid‐19 pandemic.
Tweetable abstract
Rates of GDM are higher in summer and since the onset of the Covid‐19 pandemic.
Keywords: Gestational diabetes, screen
Tweetable abstract
Rates of GDM are higher in summer and since the onset of the Covid‐19 pandemic.
Introduction
Gestational diabetes (GDM) is a relatively common disorder which typically affects about one in eight pregnancies in the UK. 1 A meta‐analysis published in 2017 focusing on GDM diagnosed in European countries reported a prevalence of 5.4%. 2 However, it should be noted that many studies included in this meta‐analysis are over 20 years old. 3 , 4 During this time, maternity demographics have changed, with women delaying pregnancy until they are older, there are greater rates of obesity 5 and more multiple pregnancies 6 as a result of assisted conception. All of these are known to be risk factors for gestational diabetes. At present, screening for gestational diabetes in the UK is largely based on a risk factor‐based approach system with guidance set out by the National Institute for Healthcare and Excellence (NICE). 7 In their guidance, the gold standard for testing is a 75‐g oral glucose tolerance test (OGTT) performed at between 24 and 28 weeks (although testing may be done earlier, particularly if there was GDM in a prior pregnancy). A positive result is determined by either a fasting reading of >5.3 mmol/l or a 2‐hour blood level of 7.8 mmol/l. Retesting for GDM in pregnancy may be done if there is high level of clinical suspicion despite the finding of a normal OGTT. The landmark Hyperglycemia and Adverse Pregnancy Events (HAPO) Trial only tested women using a OGTT up 32 completed weeks of pregnancy, 8 beyond this point there are concerns regarding the validity of using a OGTT to diagnose GDM and so home blood glucose monitoring is typically used.
There is increasing evidence to suggest that rates of GDM vary by season. 9 A single‐centre cohort study from Australia showed the prevalence of GDM was 28% higher in summer and 31% lower in winter. 9 Similar findings have been replicated in several other populations. Retnakaran et al. 10 investigated beta cell function and insulin sensitivity in almost 1500 women who were screened for GDM. Their data showed that rising environmental temperature in the 3–4 weeks prior to testing appeared to be associated with beta cell dysfunction and therefore higher rates of GDM. 10 To date there have been few studies in the UK population analysing seasonal variation in the rates of GDM. 11 We therefore conducted a single‐centre study examining rates of GDM diagnosed in our institution over a 4‐year period, examining the hypothesis that the rate of GDM diagnosis would be higher in the summer than in the other seasons. We also took the opportunity to investigate the hypothesis that there had been a change in the rate of GDM diagnosis following the onset of the Covid‐19 (SARS‐CoV‐2) pandemic.
Methods
This was a single‐centre study undertaken in a tertiary London hospital where there are approximately 5000 deliveries per year. Within our institution we have offered screening for GDM using a 2‐hour OGTT since 2010, based on the NICE guidelines (which include ethnic/racial origin) as well as additional risk factors including a maternal age of 35 or more, multiple pregnancy and previous late pregnancy loss. The diagnosis of GDM was initially made by either an elevated fasting plasma blood glucose level of ≥5.6 mmol/l or a plasma glucose level of ≥5.67.8 mmol/l in a blood sample taken 2 hours after a polycal drink containing the equivalent of 75 g of glucose. Data have been collected for the number of women tested and diagnosed with GDM by OGTT prospectively each month. An update to NICE guidance in September 2015 recommended a reduction in the fasting threshold for the diagnosis of GDM to 5.3 mmol/l 7 ; this was implemented in our institution from 1 April 2016. We offer screening with OGTT up until 32+6 weeks’ gestation; beyond this gestation we use home glucose testing. The technique used in our laboratory to measure plasma glucose is the Glucose HK Gen.3 produced by COBAS® (Roche Diagnostics, Burgess Hill, West Sussex, UK). The coefficient of variation of the measurement is 0.5–0.7%. We have no reason to consider that the laboratory techniques used vary in their sensitivity by season.
To test the hypothesis that there is a seasonal variation in the rate of positive GDM diagnoses we examined the prevalence of GDM diagnosed by screening using OGTT only before 33 weeks’ gestation to test whether there is a monthly and seasonal variation in the proportion of women tested who receive a positive result.
Because of the changes in diagnostic threshold, we analysed only those women attending for antenatal care from 1 April 2016 until 31 December 2020. We also analysed the demographics of women presenting for antenatal care to assess whether they showed any variation which would explain a seasonal effect.
Data were analysed using SPSS version 26 (IBM, Burgess Hill, West Sussex, UK). Initially we plotted the monthly proportion of screened women testing positive using an individual moving range control chart. We then plotted the distribution of that proportion against the average monthly maximum temperature at Heathrow Airport (17 miles from our institution) for which data are publicly available 12 . We then assessed the mean proportions diagnosed with GDM (± SD) by season, where winter is December to February, spring is March to May, summer is June to August and autumn (fall) is September to November inclusive. We assessed the demographics of women having their antenatal care at our institution on the same seasonal basis. Gaussian (normally distributed) data were analysed using the Student t‐test. Core outcome sets and patient involvement were not involved in this study
Results
The average proportion of women testing positive for GDM was 12.7% (SD 2.60), median 12.78 (interquartile range [IQR] 10.68–13.98). Demographic data for women booked at the institution are displayed in Figure 1. Gaussian distribution was confirmed using the Kolmogorov–Smirnov test. It appeared that there were higher rates of positivity in the summer months; this was confirmed on aggregated analysis by season (Figure 2). The mean (SD) percentage was 14.78 (2.24) in summer compared with 11.23 (1.62) in winter (P < 0.001), 12.13 (1.94) in spring (P = 0.002), and 11.88 (2.67) in autumn (P = 0.003). The average percentage of GDM diagnoses in spring, autumn and winter combined was 11.91% (SD 2.30), so the percentage was almost a quarter (23.3%) higher in the summer than in the other three seasons (P < 0.001).
Figure 1.
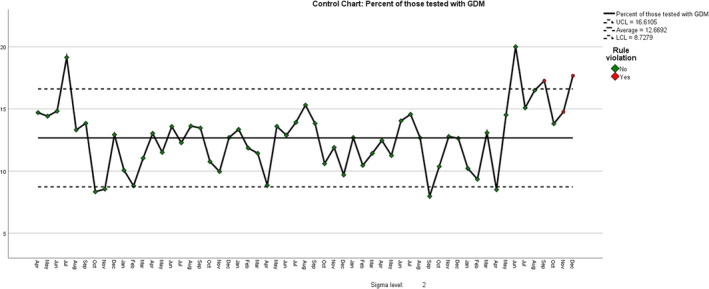
Control chart of proportion of women testing positive by month from 1 April 2016.
| Rule Violations for Run | |
|---|---|
| Month | Violations for Points |
| Sep 2020 | 4 points out of the last 5 above +1 sigma |
| Nov 2020 | 4 points out of the last 5 above +1 sigma |
| Dec 2020 | 4 points out of the last 5 above +1 sigma |
| 3 points violate control rules. | |
Figure 2.
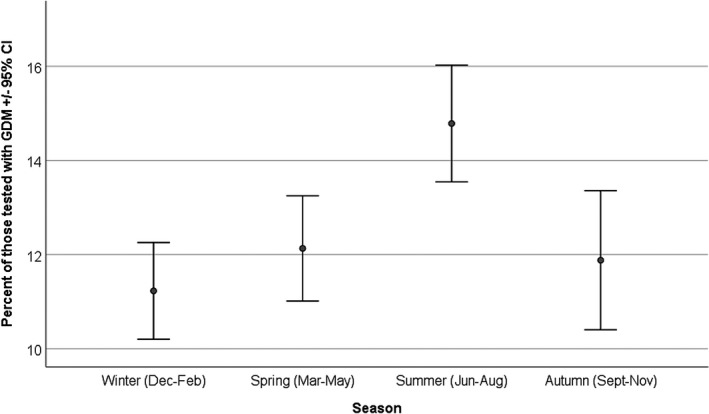
Proportion of women testing positive for GDM by season.
There was a highly significant correlation of the percentage testing positive for GDM with the mean maximum monthly temperature at Heathrow Airport (R = 0.498, R 2 = 0.248, P < 0.001) (Figure 3). This effect is not associated with any significant variation in the demographics of women presenting for antenatal care by season (Table 1), with the exception of autumn, which had a slightly younger mean age and a slightly higher proportion of South Asians compared with the other three seasons. The demographics of the summer population are not significantly different from spring or winter.
Figure 3.
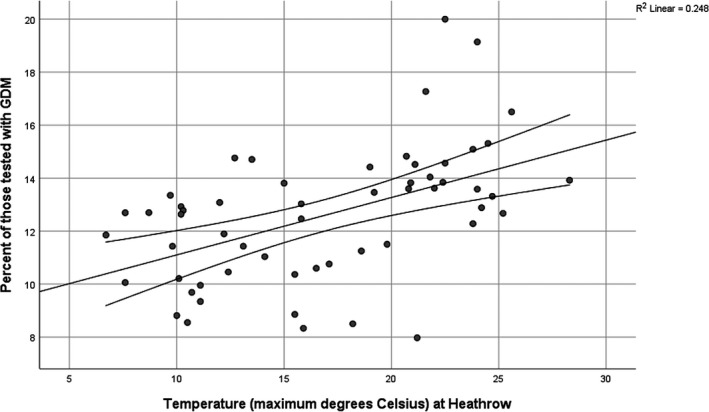
Correlation of percentage of women testing positive for GDM by mean maximum monthly temperature at Heathrow Airport. Percentage of women testing positive for GDM = (temperature × 0.217) + 8.94.
Table 1.
Demographics of women booking for pregnancy care by season
| Spring | Summer | Autumn | Winter | |
|---|---|---|---|---|
| n (% missing) | 6811 (4.2) | 7078 (5.0) | 7242 (3.3) | 5659 (8.2) |
| Age, years | 32.41 (5.28) | 32.29 (5.39) | 31.91 (5.45)* | 32.33 (5.39) |
| Height, m | 1.64 (0.07) | 1.64 (0.07) | 1.64 (0.07) | 1.64 (0.07) |
| Weight, kg | 68.5 (14.8) | 68.3 (15.0) | 68.7 (15.1) | 68.9 (14.9) |
| BMI | 25.6 (5.3) | 25.6 (5.4) | 25.6 (5.5) | 25.7 (5.3) |
| N (no missing cases) | 7108 | 7447 | 7488 | 6165 |
| White European (%) | 3358 (47.2) | 3528 (47.4) | 3610 (48.2) | 2947 (47.8) |
| South Asian (%) | 1023 (14.4)** | 1058 (14.2)*** | 1140 (15.2)**, *** | 879 (14.3) |
| Black African/Caribbean (%) | 700 (9.8) | 793 (10.6) | 812 (10.8) | 675 (10.9) |
| Other or not classified (%) | 2027 (28.5) | 2068 (27.8) | 7685 (25.7) | 1664 (27.0) |
Values are mean (SD) unless otherwise stated.
P < 0.001 compared with the other three seasons.
P = 0.018.
P = 0.045.
An unexpected finding was that, apart a single high proportion in July 2016, there was a consistently higher proportion of GDM positives from June 2020 onwards, following the onset of the COVID‐19 pandemic. We therefore compared the 6 months from June to December 2020 inclusive (period 2, since the beginning of the Covid‐19 pandemic) with the previous 65 months (period 1, pre‐Covid). The mean proportion of GDM diagnoses in period 1 was 12.14% (SD 2.20) but in period 2 it was 16.24% (SD 2.22), P < 0.001, a 33.8% rise (absolute difference 4.1%). There were no significant differences in the mean age, height, weight or body mass index (BMI) of women booking between periods 1 and 2, nor was there a significant change in the booking proportions of White European (47.8% versus 46.7%), Black (10.7% vs 9.8%) or South Asian women (14.7% vs 13.6%), although there was a significant rise in the proportion of ‘others’, from 26.0% to 28.8% (P = 0.001).
Finally, we checked to see whether there had been a change in the proportion of the bookings tested (Figure 4). There was a drop in March and April 2020 due to a change in the screening policy in response to the COVID‐19 pandemic, as recommended by the Royal College of Obstetricians and Gynaecologists, followed by a high value in June as the previous testing regimen was reinstated, including catch‐up for those not tested in March–April, following which the proportions returned to previous levels. We have reported this previously. 13 However, the overall proportion tested from 1 April 2016 to 31 May 2020 (63.75%, SD 7.99) was not significantly different from that during 1 June 2020 to 31 December 2020 (69.49%, SD 20.5) (P = 0.526, unequal variances).
Figure 4.
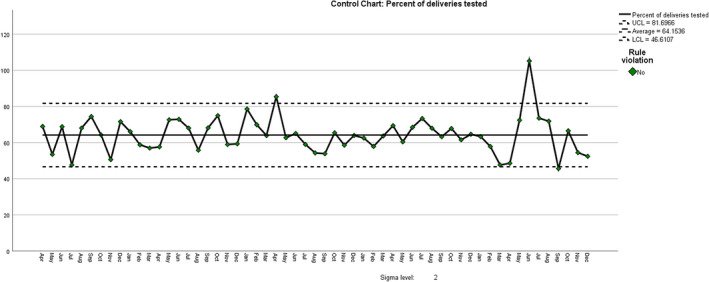
Percent of bookings tested for gestational diabetes. Rule: 4 points out of the last 5 above +1 sigma.
Discussion
Main findings
Our study demonstrates there is a significant seasonal variation with regard to women receiving a positive screening result for gestational diabetes through the OGTT, with more women being diagnosed in summer months than in winter months. The proportion of women testing positive is strongly correlated with the mean maximum monthly temperature. Furthermore, since the beginning of the Covid‐19 pandemic there has been a significant increase in the proportion of women screened for GDM who receiving a positive result.
The findings from our study in England agree with others who have examined how seasonality influences rates of GDM. 9 , 14 , 15 , 16 In their review paper, Pace and colleagues 16 report higher rates of GDM in the warmer months in Italy, Greece, Sweden, Brazil, Canada and Taiwan, although not in two of three studies in Australia (perhaps because of limited seasonal differences). The only previous study we have identified in the UK reported a GDM prevalence of 2.9% in June compared with 1.1% in November, 11 but they concluded that there was no significant seasonal effect, probably because of small numbers of positive diagnoses (they only studied 4942 women who were all White European).
Pace et al. 17 outline several different mechanistic ways in which this effect of temperature may be explained. One possible pathway is through brown adipose tissue metabolism. Data suggest that exposure to cold temperatures improves insulin sensitivity in those with Type 2 diabetes. 18 Conversely, with rising temperatures, brown adipose tissue is less activated; 19 this may partially explain the higher rates of GDM witnessed in warmer months.
Interestingly, our data did not show quite such a striking variation in rates of GDM as found the Australian study by Moses et al. 9 They found that the prevalence of GDM was 29% higher in summer than in winter. The variation witnessed in our study was 23.3%. Although average seasonal temperature differences were similar in both cohorts, the mean summer temperatures they reported were much higher. There is compelling evidence that GDM is an important indicator that women are likely to develop Type 2 diabetes later in life, with rates being ten times greater for those with a prior history of GDM compared with healthy controls. 20 Our data confirm that the rate of diagnosis of GDM is higher in the summer months but it is not known whether this is associated with long‐term changes in beta cell function or temporary changes in beta cell function and/or insulin sensitivity. It will therefore be important in future long‐term follow‐up studies to document the season in which the GDM diagnosis was made. If diagnosis in the summer is associated with a lower rate of long‐term Type 2 diabetes, this would favour the elevated rate of GDM diagnosis being due to temporary changes in insulin sensitivity rather than a permanent effect on beta cell function. Future studies should also investigate whether the season at diagnosis alters the significance of the diagnosis in relation to the clinical outcome of the affected pregnancy.
An unexpected finding was the significant increase in the proportion of women testing positive for GDM since the onset of the Covid pandemic. We hypothesise that this may be related to restricted activity during the various lockdowns 21 , 22 without a commensurate reduction in dietary intake. 23 A review of 11 studies of the effect of the pandemic in India 24 found that restriction of travel outside the home and workplace has led to an increase in snacking and meal frequency, paralleled by a reduction in physical activity and exercise duration, resulting in weight gain. Another study of 307 diabetics in India found an increment in mean HBA1c levels of 0.51%, from a mean of 7.92% pre‐lockdown to a mean of 8.43%, and concluded that lockdown had produced a significant derangement of glycaemic control. 25 A similar finding has been reported from Italy 26 and was attributed to ‘a dysfunctional adaptive reaction to lockdown‐induced stress’.
Strengths and limitations
One of the key strengths of our study is that data regarding the incidence of GDM in women screened have been collected prospectively. Although we did not record the maternal demographics specifically of those tested, we do have them for the total population, and there have been no significant temporal changes in the characteristics of the population served. We have longitudinal data spanning more than 4.5 years, with large numbers making our findings highly statistically significant. Although our data are limited to a single centre and may not be generalisable to other maternity units, our cohort includes over 25 000 women; furthermore, our population is highly diverse, with just over half the women booking describing their ethnicity as being something other than White European.
Conclusion
The diagnosis of GDM is significantly temperature‐sensitive, with the incidence being 23.3% higher in the summer months. This may affect its significance in relation to outcome, which would have management implications. There has been a significant 33.8% increase in the proportion of GDM diagnoses since the onset of the COVID‐19 pandemic, which may be due to reduced exercise levels during lockdown or, alternatively, may be secondary to stress‐induced hyperglycaemia.
Disclosure of interests
None declared. Completed disclosure of interests forms are available to view online as supporting information.
Contribution to authorship
YDVL: abstraction of data and study design. PJS: analysed data and edited the manuscript. IWC: study design. MC: wrote the manuscript.
Details of ethics approval
As this study analysed routinely collated data as part of a service evaluation, ethical approval was not required.
Funding
None.
Acknowledgements
We would like to acknowledge the staff working in the diabetes service who worked tirelessly to arrange retesting of women for GDM.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Cauldwell M, van‐de‐L'Isle Y, Watt Coote I, Steer PJ. Seasonal and SARS‐CoV‐2 pandemic changes in the incidence of gestational diabetes. BJOG 2021; 10.1111/1471-0528.16779.128:1881–1887.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Pregnancy DU‐WigDa . Diabetes UK‐ "What is gestational diabetes? 2020. https://www.diabetes.org.uk/diabetes‐the‐basics/gestational‐diabetes
- 2. Eades CE, Cameron DM, Evans JMM. Prevalence of gestational diabetes mellitus in Europe: a meta‐analysis. Diabetes Res Clin Pract 2017;129:173–81. [DOI] [PubMed] [Google Scholar]
- 3. Breschi MC, Seghieri G, Bartolomei G, Gironi A, Baldi S, Ferrannini E. Relation of birthweight to maternal plasma glucose and insulin concentrations during normal pregnancy. Diabetologia 1993;36:1315–21. [DOI] [PubMed] [Google Scholar]
- 4. Griffin ME, Coffey M, Johnson H, Scanlon P, Foley M, Stronge J, et al. Universal vs. risk factor‐based screening for gestational diabetes mellitus: detection rates, gestation at diagnosis and outcome. Diabet Med 2000;17:26–32. [DOI] [PubMed] [Google Scholar]
- 5. McKeating A, Maguire PJ, Daly N, Farren M, McMahon L, Turner MJ. Trends in maternal obesity in a large university hospital 2009–2013. Acta Obstet Gynecol Scand 2015;94:969–75. [DOI] [PubMed] [Google Scholar]
- 6. Smith LK, Manktelow BN, Draper ES, Boyle EM, Johnson SJ, Field DJ. Trends in the incidence and mortality of multiple births by socioeconomic deprivation and maternal age in England: population‐based cohort study. BMJ Open 2014;4:e004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Institute for Health and Care Excellence . Clinical Guidelines. Diabetes in Pregnancy: Management from Preconception to the Postnatal Period. London: National Institute for Health and Care Excellence (UK). Copyright © NICE 2019; 2015. [Google Scholar]
- 8. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 9. Moses RG, Wong VC, Lambert K, Morris GJ, San GF. Seasonal changes in the prevalence of gestational diabetes mellitus. Diabetes Care 2016;39:1218–21. [DOI] [PubMed] [Google Scholar]
- 10. Retnakaran R, Ye C, Kramer CK, Hanley AJ, Connelly PW, Sermer M, et al. Impact of daily incremental change in environmental temperature on beta cell function and the risk of gestational diabetes in pregnant women. Diabetologia 2018;61:2633–42. [DOI] [PubMed] [Google Scholar]
- 11. Janghorbani M, Stenhouse E, Jones RB, Millward A. Gestational diabetes mellitus in Plymouth, U.K.: prevalence, seasonal variation and associated factors. J Reprod Med 2006;51:128–34. [PubMed] [Google Scholar]
- 12. https://www.metoffice.gov.uk/pub/data/weather/uk/climate/stationdata/heathrowdata. txt
- 13. van‐de‐l’Isle Y, Steer PJ, Watt Coote I, Cauldwell M. Impact of changes to national UK Guidance on testing for gestational diabetes screening during a pandemic: a single‐centre observational study. BJOG 2021;128:917–20. [DOI] [PubMed] [Google Scholar]
- 14. Chiefari E, Pastore I, Puccio L, Caroleo P, Oliverio R, Vero A, et al. Impact of seasonality on gestational diabetes mellitus. Endocr Metab Immune Disord Drug Targets 2017;17:246–52. [DOI] [PubMed] [Google Scholar]
- 15. Vasileiou V, Kyratzoglou E, Paschou SA, Kyprianou M, Anastasiou E. The impact of environmental temperature on the diagnosis of gestational diabetes mellitus. Eur J Endocrinol 2018;178:209–14. [DOI] [PubMed] [Google Scholar]
- 16. Booth GL, Luo J, Park AL, Feig DS, Moineddin R, Ray JG. Influence of environmental temperature on risk of gestational diabetes. CMAJ 2017;189:E682–E689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pace NP, Vassallo J, Calleja‐Agius J. Gestational diabetes, environmental temperature and climate factors—from epidemiological evidence to physiological mechanisms. Early Hum Dev 2020;155:105219. [DOI] [PubMed] [Google Scholar]
- 18. Hanssen MJ, Hoeks J, Brans B, van der Lans AA , Schaart G, van den Driessche JJ , et al. Short‐term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 2015;21:863–5. [DOI] [PubMed] [Google Scholar]
- 19. Symonds ME, Farhat G, Aldiss P, Pope M, Budge H. Brown adipose tissue and glucose homeostasis—the link between climate change and the global rise in obesity and diabetes. Adipocyte 2019;8:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta‐analysis. BMJ 2020;369:m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davenport MH, Ruchat SM, Poitras VJ, Jaramillo Garcia A, Gray CE, Barrowman N, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta‐analysis. Br J Sports Med 2018;52:1367–75. [DOI] [PubMed] [Google Scholar]
- 22. Ruiz‐Roso MB, Knott‐Torcal C, Matilla‐Escalante DC, Garcimartín A, Sampedro‐Nuñez MA, Dávalos A, et al. COVID‐19 lockdown and changes of the dietary pattern and physical activity habits in a cohort of patients with type 2 diabetes mellitus. Nutrients 2020;12:2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park S, Kim MY, Baik SH, Woo JT, Kwon YJ, Daily JW, et al. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Eur J Clin Nutr 2013;67:196–201. [DOI] [PubMed] [Google Scholar]
- 24. Rawat D, Dixit V, Gulati S, Gulati S, Gulati A. Impact of COVID‐19 outbreak on lifestyle behaviour: a review of studies published in India. Diabetes Metab Syndr 2021;15:331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khare J. Observational study on effect of lock down due to COVID 19 on HBA1c levels in patients with diabetes: experience from Central India. Prim Care Diabetes 2021;S1751‐9918(20)30362‐4. 10.1016/j.pcd.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barchetta I, Cimini FA, Bertoccini L, Ceccarelli V, Spaccarotella M, Baroni MG, et al. Effects of work status changes and perceived stress on glycaemic control in individuals with type 1 diabetes during COVID‐19 lockdown in Italy. Diabetes Res Clin Pract 2020;170:108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


