Abstract
The coronavirus (COVID‐19) pandemic is evolving very quickly and has affected healthcare systems worldwide. Many uncertainties remain about transplantation from a SARS‐CoV‐2‐positive donor as only a few cases have been reported. Here, we present the successful transplantation of 2 kidneys from a 52‐year‐old male donor with active (2 weeks of COVID‐19‐like symptoms and positive nasopharyngeal swab SARS‐CoV‐2 polymerase chain reaction on the day of organ recovery) SARS‐CoV‐2 disease. The immediate postoperative course of both recipients was uneventful. This case emphasizes that patients with SARS‐CoV‐2 may be safe organ donors.
Keywords: COVID‐19, kidney transplantation, SARS‐CoV‐2
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- COVID‐19
coronavirus disease 2019
- CT
computed tomography
- Ct
cycle thresholds
- CTA
computed tomography angiography
- GCSF
granulocyte colony‐stimulating factor
- ICU
intensive care unit
- IL
interleukin
- PA
pulmonary artery
- PCR
polymerase chain reaction
- POD
postoperative day
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TNF‐α
tumor necrosis factor‐α
1. INTRODUCTION
The novel coronavirus pandemic has a direct impact on healthcare systems worldwide. COVID‐19 is a new type of infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and the pandemic has an indirect impact in the implementation of extraordinary public health measures to reduce the further spread of the virus. 1 In addition to that, elective procedures remain slowed down, and it is vital to provide continuous health care in other surgical fields, such as transplant surgery. A significant gap exists between the number of patients waiting for organ transplantation and the number of available organs. As the coronavirus pandemic is accelerating, higher numbers of potential organ donors are and will be infected, which will change the circumstances for organ donation. 2 For this reason, it is utmost important to maximize the effort to identify every available organ donor despite the pandemic situation worldwide.
To the best of our knowledge, studies about organ transplantation from SARS‐CoV‐2‐positive donors are scarce. 3 , 4 The current state of recommendations for transplantation and donation during the COVID‐19 pandemic is limited to experts’ opinions, which are based on emerging studies, but currently, the quality of evidence is low.
Here, we report a case of successful transplantation of 2 kidneys from a SARS‐CoV‐2‐positive donor (Figure 1).
FIGURE 1.
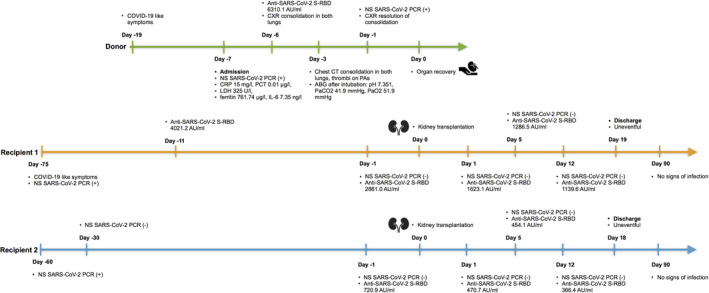
Chronological timeline of the events. Abbreviations: NS, nasopharyngeal swab; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; COVID‐19, COVID‐19, coronavirus disease 2019; PCR, polymerase chain reaction; anti‐SARS‐CoV‐2 S‐RBD, SARS‐CoV‐2 S‐RBD specific IgG antibodies; CXR, chest X‐ray; CT, computed tomography; CRP, C‐reactive protein; IL‐6, interleukin‐6; LDH, lactate dehydrogenase; PCT, procalcitonin
2. DONOR
A 52‐year‐old male with symptoms of headache, facial droop, and left hemiplegia was admitted to a tertiary referral hospital emergency department. The patient was conscious and responsive. He had a medical history of primary arterial hypertension and COVID‐19‐like symptoms (fever and cough) 13 days before admission. The COVID‐19‐like symptoms occurred after close contact with a COVID‐19‐positive‐person; SARS‐CoV‐2 polymerase chain reaction (PCR) testing was not performed until admission. A cerebral computed tomography angiography (CTA), performed on admission, showed a massive acute cerebral infarction of the right middle cerebral artery with signs of edema. Reperfusion therapy was not indicated. The patient was hospitalized to the COVID‐19 general ward on neurologist surveillance. On admission, SARS‐CoV‐2 PCR via nasopharyngeal swab was positive (BioFire SARS‐CoV‐2), C‐reactive protein was 15 mg/L, procalcitonin 0.01 µg/L, lactate dehydrogenase 325 U/L, ferritin 761.74 µg/L, and interleukin (IL)‐6 7.35 ng/L. On the next day, SARS‐CoV‐2 IgG antibodies (Abbott Architect SARS‐CoV‐2 IgG‐assay) were detected (6310.1 AU/mL). The CT scan showed the zones of consolidation in both lungs, thrombi on both sides of the main, lobed and smaller branches of the pulmonary arteries, and the saddle thrombus. The main pulmonary artery (PA) diameter was 36 mm, the right PA diameter was 26 mm and the left PA diameter 27 mm. The donor has received dexamethasone since the admission to the hospital. As thrombolytic therapy was not appropriate in this case, anticoagulant therapy was initiated with low molecular weight heparin. After 3 days, his neurological status and respiratory function worsened, and the patient was moved to the COVID‐19 intensive care unit (ICU) and placed on a ventilator. Blood gas analysis after intubation was pH 7.351, PaCO2 41.9 mm Hg, PaO2 51.9 mm Hg. Seventy‐two hours after admission to the ICU, brain death was determined. The SARS‐CoV‐2 PCR via nasopharyngeal swab was repeated and remained positive (gene E Ct =32.0; gene N Ct =33.8; GeneXpert SARS‐CoV‐2; Cepheid). There were signs of resolution of consolidation in the lungs on the chest X‐ray. Laboratory investigations revealed the following: C‐reactive protein 129.1 mg/L, procalcitonin 0.16 µg/L, aspartate transaminase 145 U/L, alanine transaminase 112 U/L, alkaline phosphatase 95 U/L, lactate dehydrogenase 684 U/L, D‐dimer 9775 µg/L, a serum creatinine level of 107 µmol/L, blood urea 9.9 mmol/L, with urine output of 2.34 L in the last 24 hours (Table 1). Abdominal ultrasound showed kidneys of a structurally normal size and shape. A multidisciplinary team meeting decided to proceed with organ donation. We have questioned all potential recipients from the waiting list with compatible blood type and have enrolled for further testing only those who have had the COVID‐19 disease and had developed immunity. There was no specific protocol at that time, the principle of precedence was maintained. Upon arrival SARS‐CoV‐2 antibodies of the recipients were measured and only those with positive antibody threshold (>50 AU/mL) were eligible for transplantation. Both kidneys were transplanted.
TABLE 1.
Inflammatory markers in the donor
| Variable | Reference range | Day ‐7 | Day ‐3 | Day ‐2 | Day ‐1 | Day 0 |
|---|---|---|---|---|---|---|
| WBC (×109/L) | 4.0‐9.8 | 6.93 | 12.25 | 15.68 | 13.61 | 16.44 |
| Lymphocytes (×109/L) | 1.0‐4.0 | 1.6 | 1.9 | 2.8 | 2.9 | 3.0 |
| Neutrophils (×109/L) | 1.5‐6.0 | 4.6 | 9.4 | 11.4 | 9.3 | 11.9 |
| CRP (mg/L) | 0‐5 | 15.0 | 64.1 | 83.0 | 77.9 | 129.1 |
| PCT (µg/L) | < 0.05 | 0.01 | 0.07 | 0.16 |
Abbreviations: CRP, C‐reactive protein; PCT, procalcitonin; WBC, white blood cells.
3. SURGERY
Kidney recovery was performed without complications. Fifteen glomeruli were found in two renal biopsies. There was no glomerular sclerosis. Fibrinous blood clots were seen in the lumen of small blood vessels. There were red blood cells in the majority of tubules and hemosiderin in the tubular epithelium. Warm ischemia time was minimal (about 20 minutes). First recipient kidney total ischemia time was 16 hours. Urine output started within 1.5 hours after surgery. Second recipient kidney total ischemia time was 26 hours. Urine output started within 2 hours after surgery. No infarct zones were noticed in both kidneys.
4. RECIPIENT 1
A 38‐year‐old woman with many years of type 1 diabetes mellitus, causing diabetic nephropathy and end‐stage renal disease on hemodialysis, had been placed on the waiting list for a kidney transplant in 2020. Due to febrile fever, general weakness, and gastrointestinal tract symptoms, she was hospitalized 2.5 months earlier. The SARS‐CoV‐2 PCR on nasopharyngeal swab was positive. In the chest X‐ray, bilateral infiltration was present, however there was no need of oxygen supply. Treatment with amoxicillin and clavulanic acid was administered. The patient was successfully discharged after 10 days. On the day of transplantation, SARS‐CoV‐2 IgG antibodies were positive (2861.0 AU/mL), and SARS‐CoV‐2 PCR on the nasopharyngeal swab was negative. Thus, recipient informed consent was obtained, and a standard kidney transplantation was performed. After uneventful surgery, the patient was transferred to the ICU. There was no need for supplemental oxygen or hemodialysis and the immune‐suppressing therapy was initiated. The patient was successfully discharged from the ICU on the next day to the nephrology department. In the early postoperative period, transplant function was sufficient.
5. RECIPIENT 2
The second recipient was a 36‐year‐old man who had been suffering from IgA nephropathy since 2008 and was on hemodialysis for the past 3 years. Due to contact with a relative suffering from COVID‐19 infection, SARS‐CoV‐2 PCR on the nasopharyngeal swab was performed and resulted positive 2 months earlier. There were no signs of infiltration on the chest X‐ray. Three days later, in the absence of any special treatment he was released for outpatient care. Before transplantation, repeated SARS‐CoV‐2 IgG antibodies were positive (720.9 AU/mL) and SARS‐CoV‐2 PCR on nasopharyngeal swab was negative; thus, recipient informed consent was obtained, and a standard kidney transplantation was performed. After uneventful surgery, the patient was transferred to the recovery room and monitored as regained consciousness. There was no need for supplemental oxygen and the patient was successfully transferred to the nephrology department. The next day the immune‐suppressing therapy was initiated. As there was a delayed graft function, 2 hemodialysis procedures were performed. On the same day, the recipient developed fever, accordingly blood and urine cultures were collected. Initial empirical therapy with amoxicillin and clavulanic acid was initiated for 10 days. However, no bacterial infection was documented from blood and urine cultures. In addition, 2 consecutive SARS‐CoV‐2 PCR tests remained negative. The early postoperative period went uneventful with an adequate urine output. Duplex ultrasound imaging of the transplant kidney was unremarkable.
Both recipients received Basiliximab and prednisolone induction. The maintenance immunosuppression consisted of tacrolimus and mycophenolate mofetil. Tacrolimus concentration was measured, and the dosage was modified accordingly. Both SARS‐CoV‐2 PCR via nasopharyngeal swab and SARS‐CoV‐2 IgG antibody tests were performed several times over the hospitalization. Table 2 shows the laboratory results of the recipients. After 18 days, patients were discharged from hospital with no evidence of any active infection, including COVID‐19. The graft is well functioning in both recipients 3 months later.
TABLE 2.
Laboratory results of the recipients
| Variable | Reference range | Recipient | Day 0 | Day 1 | Day 5 | Day 12 |
|---|---|---|---|---|---|---|
| WBC (×109/L) | 4.0‐9.8 | 1 | 6.25 | 15.65 | 7.49 | 10.31 |
| 2 | 14.1 | 12.41 | 5.81 | 9.83 | ||
| Lymphocytes (×109/L) | 1.0‐4.0 | 1 | 3.0 | 2.4 | 1.4 | 2.7 |
| 2 | 0.2 | 0.3 | 1.2 | 1.9 | ||
| CRP (mg/L) | 0‐5 | 1 | 3.47 | 37.2 | 8.5 | 2.25 |
| 2 | 8.7 | 54.4 | 4.99 | 0.47 | ||
| Tacrolimus (ng/mL) | 5‐8 | 1 | 10.6 | 10.2 | 6.5 | |
| 2 | 1.2 | 8.6 | 4.7 |
Abbreviations: CRP, C‐reactive protein; WBC, white blood cells.
6. DISCUSSION
It is not completely known whether it is safe to transplant kidney in terms of virus transmission and how the SARS‐CoV‐2 virus can affect graft function.
The lungs are the first and primary target of SARS‐CoV‐2. Autopsies of COVID‐19 have shown that virus apart from the respiratory system can be detected in the heart, liver, brain, and kidneys as well. 5 , 6 Proposed mechanisms of kidney injury are direct viral‐induced tubular or glomerular injury, sepsis‐associated acute kidney injury, and thrombotic disease. 4 There is scientific proof that SARS‐CoV‐2 spike (S) protein binds to angiotensin‐converting enzyme 2 (ACE2) receptors on host cells. 7 Moreover, there is evidence that RNA for ACE2, transmembrane serine protease 2 (TMPRSS2), and cathepsin L (CTSL) are upregulated and enriched in multiple kidney‐cell types in patients with COVID‐19. 5 In kidneys, ACE2 receptors are found in podocytes, mesangial cells, parietal epithelium of Bowma''s capsule, proximal cell brush border, and collecting ducts. Despite that SARS‐CoV‐2 viral or particles are seen in all kidney compartments in histopathology examinations, the detection rate of virus in urine is very low up to 0.8% with very low viral load 79 ± 30 copy/μL. 8
The SARS‐CoV‐2 may directly damage the cells of the kidney. Some studies discovered fragments of the virus in blood and urine of COVID‐19 patients, using PCR. 9 Other studies showed that many patients with COVID‐19 have collapsing glomerular disease due to damage to podocytes caused by cytokines and a direct toxic viral effect. 10 , 11 , 12
A virus‐induced immune response is another factor that may contribute to acute kidney injury. In a disproportionate response, tissue immune cells release significant amounts of cytokines and chemokines, which leads to cytokine storm. Several clinical studies have indicated that the levels of inflammatory mediators' IL‐2, IL‐7 and IL‐10, IFN‐inducible protein 10, granulocyte colony‐stimulating factor (GCSF), macrophage inflammatory protein 1α, tumor necrosis factor‐α (TNF‐α), and monocyte chemoattractant protein 1 were significantly higher in patients with severe COVID‐19 than in mild cases. 9 , 13
The factors described above may impact the transplant function in the future, despite successful transplantation and a well‐functioning graft. Therefore, further monitoring of the recipients is needed. This may help to improve the current understanding of the impacts of the pandemic on transplantation practices. 14
The current state of recommendations for transplantation and donation during the COVID‐19 pandemic is limited to experts’ opinions, which are based on emerging evidence; however, currently, the quality of evidence is low.
Therefore, we can count on the experience of the cases with influenza A (H1N1) during the pandemic in 2009. In the literature, we found that overall, during the pandemic in 2009, 19 organs were transplanted from nine donors infected by the H1N1 virus. Most of them received incomplete treatment with oseltamivir before organ recovery; moreover, three donors did not receive oseltamivir at all. None of the recipients developed clinical signs of viral illness. 15 , 16 , 17
However, a review of available data and the biology of SARS‐CoV‐2 and related RNA respiratory viruses suggests that the risk for transplant transmission is low, although it cannot be excluded. 18 , 19 There is an incomplete understanding of the mode of transmission of SARS‐CoV‐2 and whether blood‐borne transmission through solid organ transplants is a risk. There are no data on the transmission of the virus through the blood, although a theoretical possibility exists. The rate of viremia in the literature varies substantially from 1% to 78%. It seems that this is associated with the severeness of the COVID‐19 disease. 20 There are concerns about the effects of de novo immunosuppression on COVID‐19 severity. Transplant centers also need to better understand the role of serology screening. It is not known whether positive serology means that the recipient is protected from SARS‐CoV‐2 infection, and if so, how long the protection will last. Likewise, if a vaccine becomes available, research is needed to determine whether immunosuppressed patients will respond to it and the duration of the immune response. The most recent data show that immune response to vaccine is significantly lower in immunocompromised patients. 21 , 22 , 23 With progression of scientific evidence, future revisions of recommendations for organ donation and transplantation during the COVID‐19 pandemic will be warranted. 24
7. CONCLUSION
In conclusion, we report a successful transplantation of 2 kidneys from a SARS‐CoV‐2‐positive donor. Both kidneys were transplanted for recipients who have had the COVID‐19 disease and had developed immunity after COVID‐19 disease. The immediate postoperative course of recipients was uneventful. This case shows that patients with SARS‐CoV‐2 may be safe organ donors.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHORS' CONTRIBUTION
LP: concept, design, data collection, manuscript preparation and editing; MS: manuscript preparation and editing; AK: analysis and editing; JS: analysis and editing; MM: analysis and editing; MMJ: manuscript preparation and editing; EA: analysis and editing; EM: manuscript preparation and editing; AZ: analysis and editing; OA: manuscript preparation and editing; LJ: analysis and editing.
Puodziukaite L, Serpytis M, Kundrotaite A, et al. Kidney transplantation from a SARS‐CoV‐2‐positive donor for the recipients with immunity after COVID‐19. Transpl Infect Dis. 2021;23:e13666. 10.1111/tid.13666
Funding information
This work has not received funding.
REFERENCES
- 1. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moris D, Shaw BI, Dimitrokallis N, Barbas AS. Organ donation during the coronavirus pandemic: an evolving saga in uncharted waters. Transpl Int. 2020;33(7):826‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kates OS, Fisher CE, Rakita RM, Reyes JD, Limaye AP. Use of SARS‐CoV‐2‐infected deceased organ donors: should we always “just say no?”. Am J Transplant. 2020;20(7):1787‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farouk SS, Fiaccadori E, Cravedi P, Campbell KN. COVID‐19 and the kidney: what we think we know so far and what we don't. J Nephrol. 2020;33(6):1213‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS‐CoV‐2. N Engl J Med. 2020;383(6):590‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID‐19 in China. Kidney Int. 2020;98(1):219‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 dell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim J‐M, Kim HM, Lee EJ, et al. Detection and isolation of SARS‐CoV‐2 in serum, urine, and stool specimens of COVID‐19 patients from the Republic of Korea. Osong Public Health Res Perspect. 2020;11(3):112‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2021;12(1):2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington State. JAMA. 2020;323(16):1612‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Z, Wu M, Yao J, et al. Caution on kidney dysfunctions of COVID‐19 patients. medRxiv. 2020. 10.1101/2020.02.08.20021212. [DOI] [Google Scholar]
- 13. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581(7809):465‐469. [DOI] [PubMed] [Google Scholar]
- 14. Neidlinger NA, Smith JA, D'Alessandro AM, et al. Organ recovery from deceased donors with prior COVID‐19: a case. Transpl Infect Dis. 2021;23(2);e13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cockbain AJ, Jacob M, Ecuyer C, et al. Transplantation of solid organs procured from influenza a H1N1 infected donors. Transpl Int. 2011;24(12):e107‐e110. [DOI] [PubMed] [Google Scholar]
- 16. Lattes R, Jacob N, de la Fuente J , Fragale G, Massari P. Pandemic influenza A/H1N1 and organ donation. Transpl Infect Dis. 2010;12(2):169‐172. [DOI] [PubMed] [Google Scholar]
- 17. Halliday N, Wilmore S, Griffiths PD, et al. Risk of transmission of H1N1 influenza by solid organ transplantation in the United Kingdom. Transplant. 2012;93(5):551‐554. [DOI] [PubMed] [Google Scholar]
- 18. Kumar D, Humar A, Keshavjee S, Cypel M. A call to routinely test lower respiratory tract samples for SARS‐CoV‐2 in lung donors. Am J Transplant. 2021. 10.1111/ajt.16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS‐CoV‐2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021. 10.1111/ajt.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bermejo‐Martin JF, González‐Rivera M, Almansa R, et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID‐19. Crit Care. 2020;24(1):691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS‐CoV‐2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS‐Cov‐2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS‐CoV‐2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ASTS COVID‐19 strike force initial guidance. Asts.org. https://asts.org/advocacy/covid‐19‐resources/asts‐covid‐19‐strike‐force/asts‐covid‐19‐strike‐force‐initial‐guidance#.YDPy_S0Rrfa. Accessed February 10, 2021


