Summary
The red blood cell distribution width (RDW), an indicator of anisocytosis has emerged as a potential tool for risk stratification of critically ill patients with sepsis. Prognostic predictors are of paramount interest for prompt intervention and optimal utilization of the healthcare system in this ongoing context of the Coronavirus Disease 2019 (COVID‐19) pandemic. The current systematic review and meta‐analysis aims to explore the utility of RDW in the prognosis of COVID‐19 patients. A comprehensive screening of electronic databases was performed up to 30th April 2021 after enrolling in PROSPERO (CRD42020206685). Observational studies or interventional studies, evaluating the impact of RDW in COVID‐19 outcomes (mortality and severity) are included in this meta‐analysis.Our search retrieved 25 studies, with a total of 18,392 and 3,446 COVID‐19 patients for mortality and disease severity outcomes. Deceased and critically ill patients had higher RDW levels on admission in comparison to survivors and non‐severe patients (SMD = 0.46; 95%CI 0.31–0.71; I 2 = 88% and SMD = 0.46; 95%CI 0.26–0.67; I 2 = 60%, respectively). In a sub‐group analysis of 2,980 patients, RDW > 14.5 has been associated with increased risk of mortality (OR = 2.73; 95%CI 1.96–3.82; I 2 = 56%). However, the evidences is of low quality. A higher level of RDW on admission in COVID‐19 patients is associated with increased morbidity and mortality. However, further studies regarding the cut‐off value of RDW are the need of the hour.
Keywords: Coronavirus Disease 2019, red blood cell distribution width, Severe Acute Respiratory Syndrome Coronavirus‐2
1. INTRODUCTION
Even after a year of emergence of the Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS CoV‐2), around 4.6 million new cases and 79,000 deaths are still being reported weekly. 1 The current Coronavirus Disease 2019 (COVID 19) pandemic has overwhelmed the medical infrastructure around the globe. While in the United States, 14% of cases required hospitalization and 2% required ICU care, 2 the incidence of the severe disease in China has been reported as up to 15%, 3 with the mortality rate between 11% and 15% in hospitalized patients, 4 over all‐around 20% of the hospitalized patients required ICU management. 5 Early detection of severe cases is of paramount importance in the context of this pandemic as a method of triage and optimal allocation of resources. Various prognostic markers of COVID‐19 severity are under evaluation since January 2020. 6
Red blood cell distribution width (RDW) is a commonly measured parameter in complete blood count (CBC) panels. It is usually reported as RDW standard deviation (RDW‐SD) or RDW coefficient of variation (RDW‐CV), which provides us with a measure of heterogeneity in the size of red blood cells (RBC), that is, anisocytosis. Traditionally, it is used as a parameter to differentiate various types of anaemia. 7 More recently, it has been established as a marker of inflammation. Various studies have established the predictive value of RDW in the severity of a spectrum of diseases like chronic kidney disease (CKD), 8 preeclampsia, 9 cardiovascular diseases 10 and cancers. 11 Incrementally increasing RDW values are associated with an increased risk of more severe disease and mortality. 12 Systemic inflammation has been associated with increased all‐cause, cancer, cardiovascular and cerebrovascular mortality. 13 Recent studies have demonstrated that increasing RDW represents an increase in the risk of MI and death, independent of anaemia and cardiovascular risk factors. 14 , 15 , 16
Inflammation plays a major role in the pathogenesis and severity of COVID 19 disease, culminating in cytokine release syndrome (CRS) or cytokine storm in its most severe form. 17 Higher baseline CRP and IL‐6 levels were associated with more incidence of ARDS and death. 18 , 19 , 20
RDW as a marker of pre‐existing pro‐inflammatory or chronic inflammatory state can be used as a predictor of COVID 19 disease progression. There have been several studies that have examined the relationship between admission RDW and its ability to predict mortality in COVID 19 disease. In this meta‐analysis, we aim to systematically analyse the current evidence for the utility of elevated RDW on admission as a prognostic indicator of COVID 19 disease, as per the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA‐P) guidelines.
2. METHODS
2.1. Protocol and registration
We prospectively enrolled the protocol of this systematic review and meta‐analyses in PROSPERO (ID: CRD42020206685), and there was no significant deviation from the published protocol.
2.2. Search strategy
Three researchers (Soumya Sarkar [SS], Sundara Kannan [SK] and Puneet Khanna [PK]) independently searched the important electronic databases (PubMed, Medline and Embase), Google Scholar (https://scholar.google.com), preprint platforms MedRxiv (https://www.medrxiv.org) and Clinical trial database (https://ClinicalTrials.gov) from 1st January 2020 to 30th April 2021 with the following keywords: ‘COVID‐19’ or ‘SARS‐CoV‐2’ and ‘RDW’ or ‘Red blood cell distribution width’.
2.3. Inclusion and exclusion criteria
Prospective and retrospective comparative cohort studies, case series with a control group, cross‐sectional studies, controlled clinical trials, case‐control studies and randomized controlled trials (RCT), evaluating RDW on admission in COVID‐19 patients were looked for inclusion.
The primary outcome was mortality and disease severity was the secondary outcome. Articles other than those in the English language, without full retrievable text or appropriate control group were excluded (PRISMA flow diagram). 21 , 22
2.4. Study selection
SS, SK and PK screened all the available abstracts independently after removing the duplications to exclude the irrelevant articles. Then, the full‐texts of the eligible studies were screened to check the inclusion criteria. Any disagreements were resolved in consultation with a fourth researcher (AKS).
2.5. Data extraction
SS and SK used a pre‐conceived data extraction sheet individually for extracting the following data from all included studies: first author, year of publication, type of study, place, sample size, coefficient of variation of the red cell distribution width (RDW‐CV) expressed in percentage on admission, disease severity and mortality in COVID‐19 patients.
For dichotomous data, the number of incidents and the total number of patients in each group were noted and for continuous data, means and SD are extracted. Studies with missing data have been reported descriptively.
Due to lack of consensus regarding defining the severity of the disease among studies, any patient either requiring mechanical ventilation or with a ratio of the partial pressure of arterial blood oxygen (PaO2)/oxygen concentration (FiO2) ≤300 mm Hg is considered as severe/critically ill, and the rest of the patient's are defined as mild/moderate ill patients.
2.6. Risk of bias assessment
SS and PK independently assessed any potential bias in selected studies. The difference of opinion was resolved by consulting with AKS. The Risk Of Bias In Non‐randomized Studies, of Interventions (ROBINS‐I) 23 tool was used for assessing the risk of bias in non‐randomized studies. It includes the following seven domains: ‘bias due to confounding’, ‘selection of participants, classification of interventions’, ‘deviations from intended interventions’, ‘missing data’, ‘measurement of outcomes’ and ‘selection of the reported result’. Every domain is graded as ‘Low’, ‘Moderate’, ‘Serious’ and ‘Critical’.
2.7. Quality of the evidence
The quality of evidence was judged independently by PK and SS with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool, which has five downgrading factors (study limitations, indirectness, imprecision, consistency of effect and publication bias) and three upgrading factors (dose‐response relation, large magnitude of the effect and plausible confounders or biases). 24 , 25 The quality of evidence of every outcome is assorted as ‘High’, ‘Moderate’, ‘Low’ or ‘Very low’. 26 , 27 , 28 , 29 , 30 , 31 Any difference of opinion was resolved after consulting with AKS.
2.8. Data synthesis
We (PK and SS) used Review Manager version 5 to conduct this frequentist meta‐analysis. The odds ratio (OR) for dichotomous data and mean differences (MDs) for continuous data along with the 95% confidence intervals (CIs), respectively, were assessed as per the Cochrane Handbook for Systematic Reviews of Interventions. 32 The I 2 statistic was used for evaluating the statistical heterogeneity, a value of >50% was accepted as significant heterogeneity. A funnel plot was used to assess publication bias.
3. RESULTS
3.1. Basic characteristics
25 studies 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 out of 1,008 identified publications were incorporated according to the inclusion criteria (Figure 1; Table 1). 19 articles were peer‐reviewed and 6 were preprints. 35 , 36 , 37 , 40 , 45 , 53 While nine articles evaluated RDW on admission to assess the severity of COVID‐19 patients, others addressed RDW on admission between survivors and non‐survivors. Among the included studies, nine studies had a moderate degree of bias (Figure 2).
FIGURE 1.
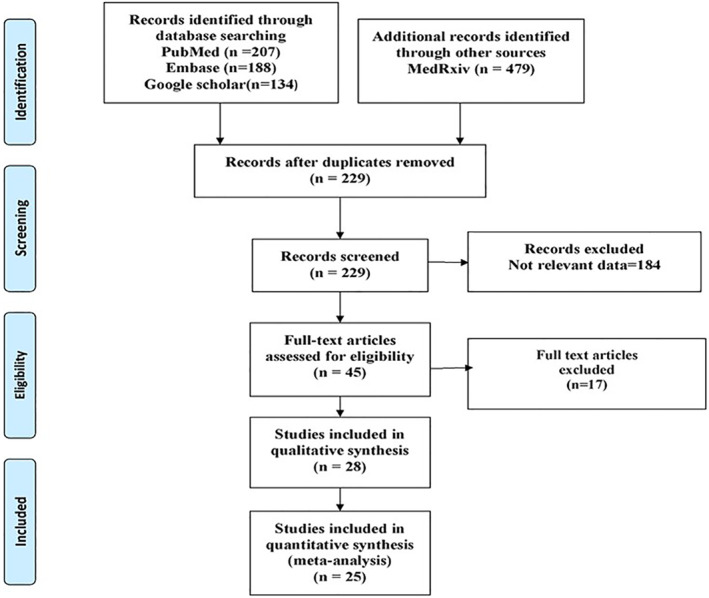
PRISMA‐2009‐Flow‐Diagram
TABLE 1.
Characteristics of studies
| SN | Author, Year | Type of study, centre | Country | Total no. of patients | Outcome |
|---|---|---|---|---|---|
| 1. | Wang Y et al., 2020 33 | Retrospective, SC | China | 344 | Non survivors had a higher RDW in comparison to the surviving COVID‐19 patients (p < 0.001). |
| 2. | Foy et al., 2020 34 | Retrospective, MC | USA | 1,641 | The mortality rate of COVID‐19 patients with RDW ≥14.5 at admission was higher (31%) in comparison to those with an RDW <14.5 (11%). |
| 3. | Levy et al., 2020 35 | Retrospective, MC | USA | 11,095 | High RDW value was associated with disease severity, progression and an overall poor prognosis |
| 4. | Santos‐Lozano et al., 2020 36 | Retrospective, SC | Spain | 1,369 | High RDW associated with risk of in hospital death in persons with COVID‐19 |
| 5. | Nicholson et al., 2020 37 | Retrospective, MC | USA | 1,042 | Non survivors had a high level of RDW (14.84) in comparison to survivors (13.9) at admission. |
| 6. | Rizo‐Téllez et al., 2020 38 | Retrospective, SC | Mexico | 54 | No significant differences between survivors and non‐survivors were found for most of the haematological parameters |
| 7. | Allahverdiyev et al., 2020 39 | Retrospective, SC | Turkey | 455 | The mortality rate of COVID‐19 positively correlated with higher neutrophil‐to‐lymphocyte ratio, RDW |
| 8. | Wei Y et al., 2020 40 | Retrospective, SC | China | 112 | Mortality is associated with higher variation of RDW (HR, 2.63; 95%CI, 1.10‐6.30; p = 0.0297) |
| 9. | Lorente et al., 2020 41 | Prospective, MC | Spain | 143 | The deceased patients had a higher RDW (p = 0.001) in compare to surviving patients. |
| 10. | Wang c et al., 2020 42 | Retrospective, MC | China | 98 | RDW is a prognostic predictor for patients with severe COVID‐19 |
| 11. | Henry et al., 2020 43 | Prospective, SC | USA | 49 | Progressive increase in RDW was associated with advancing COVID‐19 severity |
| 12. | Gong et al., 2020 44 | Retrospective, MC | China | 189 | Higher red blood cell distribution width was associated with severe COVID‐19. |
| 13. | Jans et al., 2020 45 | Retrospective, SC | Netherlands | 254 | Patients with severe disease had a higher RDW on admission. |
| 14. | Wang C et al., 2020 46 | Retrospective, SC | China | 161 | NLR and RDW‐SD parameter helps to predict the severity of COVID‐19 patients. |
| 15. | Gowda et al., 2020 47 | Retrospective, SC | India | 100 | RDW is an early predictive marker of mortality in COVID‐19 |
| 16. | Kaufmann et al., 2020 48 | Retrospective, SC | Austria | 423 | Raised RDW was an important predictor of 28 days mortality [crude odds ratio (OR) 1.717, 95% confidence interval (CI) 1.462–2.017; P = <0.001] |
| 17. | Paliogiannis et al., 2020 49 | Case series, SC | Italy | 30 | Increased RDW associated with mortality |
| 18. | Sema Yağc et al., 2020 50 | Cross‐sectional, SC | Turkey | 59 | Elevated RDW in COVID‐19 patients had a higher rate of in‐hospital mortality* |
| 19. | Tocoglu et al., 2020 51 | Retrospective, SC | Turkey | 55 | In critically ill COVID‐19 patients with AKI low RDW may be associated with mortality. |
| 20. | Soni et al., 2020 52 | Retrospective, SC | India | 622 | Non survivors had a high level of RDW (15.45) in comparison to survivors (14.49) at admission. |
| 21. | Ramchandran et al., 2020 53 | Retrospective, SC | USA | 294 | COVID‐19 patients with elevated RDW value had a higher frequency of in‐hospital mortality |
| 22. | De La Rica R et al., 2020 54 | Case series, SC | Spain | 48 | No significant differences between survivors and non‐survivors were found for RDW |
| 23. | Lin S et al., 2020 55 | Retrospective, SC | China | 68 | No significant differences in haematological parameters between patients with mild and severe illness at the time of admission, |
| 24. | Solmaz et al., 2020 56 | Retrospective, SC | Turkey | 1,950 | Majority of the COVID‐19 patients with elevated RDW on admission required ICU care. |
| 25. | Asan et al., 2020 57 | Retrospective, SC | Turkey | 695 | Initial elevated RDW was associated with the severity of COVID‐19 and ICU requirement. |
Abbreviations: AKI, Acute kidney injury; CI, confidence interval; COVID‐19, Coronavirus Disease 2019; MC, Multi centre; OR, odds ratio; RDW, Red blood cell distribution width; RDW‐SD, Red blood cell distribution width standard deviation; SC, Single centre.
FIGURE 2.
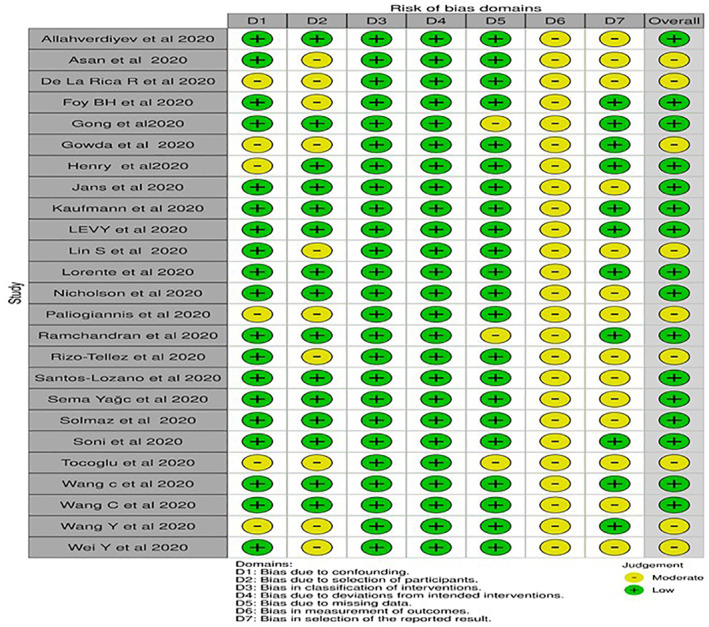
ROBINS‐I assesment for the included non‐randomized cohort studies
3.2. Meta‐analyses
3.2.1. Mortality
15 articles with a total of 18,392 patients were evaluated for mortality in COVID‐19. Significantly, RDW on admission was higher among the deceased in comparison to the survivors (SMD = 0.46; 95%CI 0.31–0.61; I 2 = 88%) (Figure 3a).
FIGURE 3.
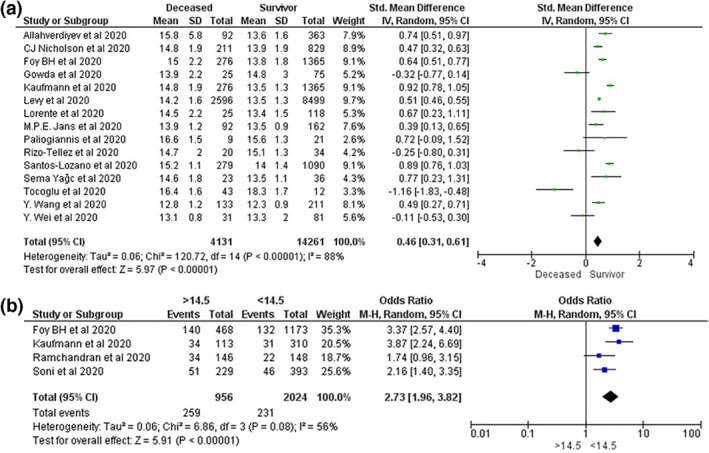
(a) The impact of the Red blood cell distribution width (RDW) on mortality in Coronavirus Disease 2019 (COVID‐19) patients. (b) Subgroup analysis of impact of the RDW >14.5 on mortality in COVID‐19 patients
In a subgroup analysis of four studies (n = 2980), COVID‐19 patients with RDW >14.5 on admission had a significantly higher risk of mortality in comparison to the patients with RDW <14.5 (OR = 2.73; 95%CI 1.96–3.82; I 2 = 56%) (Figure 3b).
3.2.2. Severity
Nine studies with a total of 3,446 patients were assessed for the severity of COVID‐19. Critically ill patients are associated with increased RDW on admission (SMD = 0.46; 95%CI 0.26–0.67; I 2 = 60%) (Figure 4). Significant heterogeneity is found among studies assessing mortality and severity
FIGURE 4.
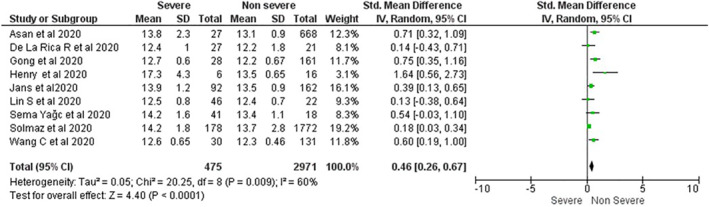
The impact of Red blood cell distribution width on disease severity in Coronavirus Disease 2019 patients
3.3. Quality of evidence
We found a low quality of evidence on the impact of raised RDW on COVID‐19 mortality and severity (Table 2).
TABLE 2.
GRADE evidence profile of COVID‐19 studies
| Out come | No. of participants | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Quality of evidence (Grade) | Relative effect | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. | Elevated RDW | Control | ||||||||
| Mortality | 18,392 | 4,131 | 14,261 | No | No | Yes | No | None | Low ⊕⊕⊝⊝ | MD = 0.66 (95%CI 0.41–0.91) |
| Severity | 3,446 | 475 | 2,971 | No | No | Yes | No | None | Low ⊕⊕⊝⊝ | MD = 0.41 (95%CI 0.26–0.55) |
Abbreviations: COVID‐19, Coronavirus Disease 2019; RDW, red blood cell distribution width.
3.4. Publication bias
The publication bias was assessed for the studies on COVID‐19 mortality. As per the Funnel plot qualitatively a publication bias is implausible (Figure 5).
FIGURE 5.
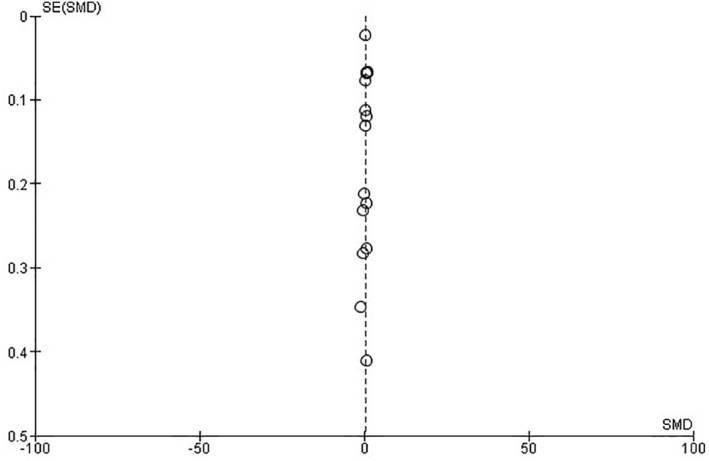
Funnel plot of the included studies for assessment of publication bias
4. DISCUSSION
We have identified low‐quality evidence with variability that RDW value on admission has the potential ability of discrimination in COVID‐19 patients predicting the mortality and severity.
RDW, generated automatically in the majority of haematological analysers, is a low‐cost parameter. It increases in response to many acute and chronic proinflammatory conditions. Raised RDW implies a large burden of anisocytosis in circulating erythrocytes. It is associated with mortality in patients with nonspecific ARDS (i.e., without COVID‐19). A median RDW value of 14.1% (IQR: 13.3%–15.2%) on admission was associated with increased morbidity and mortality in patients with community‐acquired pneumonia (CAP). 58
A recent systematic review also echoed that a higher RDW is associated with severely ill COVID‐19 patients with severe illness than in those with mild disease (SMD = 0.69,95%CI 0.40–0.98). 59
Zinellu & Mangoni also found that the critically ill and expired COVID‐19 patients had significantly elevated RDW (SMD = 0.56, 95%CI 0.31 to 0.81). 60
Similarly, a meta‐analysis of 10 studies found that the deceased COVID‐19 patients had significantly elevated RDW in comparison to the surviving patients (MD = 0.93; 95%CI = 0.63–1.23; I2 = 85.58%). They also reported that the elevated RDW is associated with disease severity (MD = 0.61; 95%CI = 0.28–0.94; I2 = 82.18%). 61
In the subgroup analysis, we found COVID‐19 patients with initial RDW >14.5 are associated with almost double the risk of mortality.
Another recent observational multicentric study with 193 hospitalized COVID‐19 patients, also found that RDW ≥14.5% were also significantly associated with increased risk of mortality (HR: 4.1, 95%CI: 0.88–19.23, p = 0.02). 62
An elevated RDW, a marker of anisocytosis has been implicated in a wide spectrum of diseases, particularly in patients with nonspecific ARDS (non‐ COVID‐19). 63 , 64 However, the particular mechanism for altered RDW with SARS‐COV‐2 is still under evaluation.
While a recent study reported about the structural change of lipid and proteins in the membrane of circulating RBCs due to SARS‐CoV‐2 infection, 65 there are reports of bone marrow injury secondary to SARS‐CoV‐2 infection. 66
The development of micro‐and macro‐thrombi, due to intravascular coagulopathy is commonly seen in critically ill COVID‐19 patients, may also lead to erythrocyte injury resulting in morphological abnormalities. 67
However, there is no consensus regarding the optimum cut‐off for RDW. While Pan Y et al. 68 suggested a cut off value of 13.35 (sensitivity: 79.8%, specificity: 84.6%), Lorente et al. 41 advocated for cut off of >13 (sensitivity: 63%, specificity: 78%), Gowda et al. 47 have reported RDW ≤15% is a potential predictive value (sensitivity: 92%, negative predictive value: 95%), and Wang c found a cut off value 12.85 had 73.95 sensitivity along with 81.9% specificity. 42
Irrespective of different cut‐off values of RDW at admission, it cannot be ignored that elevated RDW is associated with increased morbidity and mortality in SARS‐COV2 infection.
4.1. Strengths and Limitation
Our study is one of the extensive & comprehensive systematic review of the effectiveness of RDW on admission in patients with COVID‐19 for predicting the mortality and severity, and may be considered at this moment as the pre‐eminent evidence for decision‐making. The Majority of the included studies are retrospective in nature, and six studies are not peer‐reviewed. Although in the current scenario, the prognostic role of RDW in COVID‐19 is promising, our findings are heterogeneous, medium in effect and of low‐quality evidence. We also acknowledged that the cut‐off value of RDW and the point of evaluation is yet to be standardized and information in this regard is still evolving.
5. CONCLUSION
RDW may be a useful tool for stratifying the risk and prompt decision about an escalation of management, further large‐scale prospective studies for assessing the appropriate cut‐off points for determining healthcare allocation during this pandemic is the need of the hour.
AUTHOR CONTRIBUTION
Dr. Soumya Sarkar (SS): Conceptualization, Search strategy, Study selection, Data extraction, Data synthesis, Risk of bias assessment, and Draughted the manuscript.
Dr. Sundara Kannan (SK): Study selection, and Data extraction.
Dr. Puneet Khanna (PK): Conceptualization, Search strategy, Study selection, Risk of bias assessment, Quality of the evidence assessment, and Editing.
Dr. Akhil Kant Singh (AKS): Study selection, Data extraction, Risk of bias assessment, Quality of the evidence assessment, and Editing.
Sarkar S, Kannan S, Khanna P, Singh AK. Role of red blood cell distribution width, as a prognostic indicator in COVID‐19: a systematic review and meta‐analysis. Rev Med Virol. 2022;32(2):e2264. 10.1002/rmv.2264
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Weekly Epidemiological Update – 22 December 2020. 2020. [Internet]. [cited 2020 Dec 28]. Available from: https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐‐‐22‐december‐2020
- 2. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance – United States, 22 January–30 May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu W, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72,314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020;323(13):1239‐1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 4. Harapan H, Itoh N, Yufika A, et al. Coronavirus disease 2019 (COVID‐19): a literature review. J Infect Public Health. 2020;13(5):667‐673. 10.1016/j.jiph.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Trav Med Infect Dis. 2020;34:101623. 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balcells ME, Rojas L, Le Corre N , et al. Early anti‐SARS‐CoV‐2 convalescent plasma in patients admitted for COVID‐19: a randomized phase II clinical trial. medRxiv. Published online January 1, 2020: 2020. 10.1101/2020.09.17.20196212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salvagno GL, Sanchis‐Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86‐105. [DOI] [PubMed] [Google Scholar]
- 8. Vashistha T, Streja E, Molnar MZ, et al. Red cell distribution width and mortality in hemodialysis patients. Am J Kidney Dis Off J Natl Kidney Found. 2016;68:110‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adam I, Mutabingwa TK, Malik EM. Red cell distribution width and preeclampsia: a systematic review and meta‐analysis. Clin Hypertens. 2019. [Internet] [cited 2020 Dec 28];25. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6628484/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu L, Li M, Ding Y, et al. Prognostic value of RDW in cancers: a systematic review and meta‐analysis. Oncotarget. 2016;8:16027‐16035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen Y. High red blood cell distribution width is closely associated with risk of carotid artery atherosclerosis in patients with hypertension. Exp Clin Cardiol. 2010;15:37‐40. [PMC free article] [PubMed] [Google Scholar]
- 12. Skjelbakken T, Lappegård J, Ellingsen TS, et al. Red cell distribution width is associated with incident myocardial infarction in a general population: the Tromsø Study. J Am Heart Assoc. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Proctor MJ, McMillan DC, Horgan PG, Fletcher CD, Talwar D, Morrison DS. Systemic inflammation predicts all‐cause mortality: a Glasgow inflammation outcome study. PLOS ONE. 2015;10:e0116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borné Y, Smith JG, Melander O, Engström G. Red cell distribution width in relation to incidence of coronary events and case fatality rates: a population‐based cohort study. Heart Br Card Soc. 2014;100:1119‐1124. [DOI] [PubMed] [Google Scholar]
- 15. Zalawadiya SK, Veeranna V, Panaich SS, Afonso L, Ghali JK. Gender and ethnic differences in red cell distribution width and its association with mortality among low risk healthy United state adults. Am J Cardiol. 2012;109:1664‐1670. [DOI] [PubMed] [Google Scholar]
- 16. Pilling LC, Atkins JL, Kuchel GA, Ferrucci L, Melzer D. Red cell distribution width and common disease onsets in 240,477 healthy volunteers followed for up to 9 years. PloS One. 2018;13:e0203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. García LF. Immune response, inflammation, and the clinical spectrum of COVID‐19. Front Immunol. 2020. [Internet]. 2020 [cited 2021 Jan 1];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7308593/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu T, Zhang J, Yang Y, et al. The role of interleukin‐6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12:e12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomized studies of interventions. BMJ. 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383‐394. [DOI] [PubMed] [Google Scholar]
- 25. Norris SL, Meerpohl JJ, Akl EA, et al. The skills and experience of GRADE methodologists can be assessed with a simple tool. J Clin Epidemiol. 2016;79:150‐158. [DOI] [PubMed] [Google Scholar]
- 26. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64:407‐415. [DOI] [PubMed] [Google Scholar]
- 27. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64:1277‐1282. [DOI] [PubMed] [Google Scholar]
- 28. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283‐1293. [DOI] [PubMed] [Google Scholar]
- 29. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294‐1302. [DOI] [PubMed] [Google Scholar]
- 30. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64:1303‐1310. [DOI] [PubMed] [Google Scholar]
- 31. Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311‐1316. [DOI] [PubMed] [Google Scholar]
- 32. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID‐19. Am J Respir Crit Care Med. 2020;201(11):1430‐1434. 10.1164/rccm.202003-0736LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Foy BH, Carlson JCT, Reinertsen E, et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS‐CoV‐2 infection. JAMA Netw Open. 2020;3(9):e2022058. 10.1001/jamanetworkopen.2020.22058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Levy TJ, Richardson S, Coppa K, et al. Development and validation of a survival calculator for hospitalized patients with COVID‐19. medRxiv. Published online January 1, 2020. 10.1101/2020.04.22.20075416 [DOI] [Google Scholar]
- 36. Santos‐Lozano A, Calvo‐Boyero F, López‐Jiménez A, et al. Can routine laboratory variables predict survival in COVID‐19? An artificial neural network‐based approach. Clin Chem Lab Med. 2020;58(12):e299‐e302. 10.1515/cclm-2020-0730 [DOI] [PubMed] [Google Scholar]
- 37. Nicholson CJ, Wooster L, Sigurslid HH, et al. Estimating risk of mechanical ventilation and mortality among adult COVID‐19 patients admitted to mass general Brigham: the VICE and DICE scores. medRxiv. Published online January 1, 2020: 2020. 10.1101/2020.09.14.20194670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rizo‐Téllez SA, Méndez‐García LA, Flores‐Rebollo C, et al. The neutrophil‐to‐monocyte ratio and lymphocyte‐to‐neutrophil ratio at admission predict in‐hospital mortality in Mexican patients with severe SARS‐CoV‐2 infection (Covid‐19). Microorganisms. 2020;8(10):1560. 10.3390/microorganisms8101560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Allahverdiyev S, Quisi A, Harbalioglu H, et al. The neutrophil to lymphocyte ratio and in‐hospital all‐cause mortality in patients with COVID‐19. Eur J Therapeut. 2020;26(3):251+. [Google Scholar]
- 40. Wei Yongyue, He Jieyu, Chen Jiao, et al. Dynamic Prognosis Model for Predicting Survival in Severe and Critically Ill COVID‐19 Patients Using Machine Learning, 2020, PREPRINT (Version 1) available at Research Square. 10.21203/rs.3.rs-64083/v1 [DOI] [Google Scholar]
- 41. Lorente L, Martín MM, Argueso M, et al. Association between red blood cell distribution width and mortality of COVID‐19 patients [published online ahead of print, 2020 Nov 7]. Anaesth Crit Care Pain Med. 2020;40:100777. 10.1016/j.accpm.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang C, Zhang H, Cao X, et al. Red cell distribution width (RDW): a prognostic indicator of severe COVID‐19. Ann Transl Med. 2020;8(19):1230. 10.21037/atm-20-6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Henry BM, Benoit JL, Benoit S, et al. Red blood cell distribution width (RDW) predicts COVID‐19 severity: a prospective, observational study from the cincinnati SARS‐CoV‐2 emergency department cohort. Diagn. 2020;10(9):618. 10.3390/diagnostics10090618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID‐19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833‐840. 10.1093/cid/ciaa443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jans MPE, Kuijper TM, den Hollander JG, et al. Predicting severe COVID‐19 at presentation, introducing the COVID severity score (6/8/2020). 2020. Available at SSRN 10.2139/ssrn.3627260 [DOI] [Google Scholar]
- 46. Wang C, Deng R, Gou L, et al. Preliminary study to identify severe from moderate cases of COVID‐19 using combined hematology parameters. Ann Transl Med. 2020;8(9):593. 10.21037/atm-20-3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bommenahalli Gowda S, Gosavi S, Ananda Rao A, et al. Prognosis of COVID‐19: red cell distribution width, platelet distribution width, and C‐reactive protein. Cureus. 2021;13(2):e13078. 10.7759/cureus.13078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaufmann CC, Ahmed A, Brunner U, et al. Red cell distribution width upon hospital admission predicts short‐term mortality in hospitalized patients with COVID‐19: a single‐center experience. Front Med. 2021;8:652707. 10.3389/fmed.2021.652707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paliogiannis P, Zinellu A, Scano V, et al. Laboratory test alterations in patients with COVID‐19 and non COVID‐19 interstitial pneumonia: a preliminary report. J Infect Dev Ctries. 2020;14(7):685‐690. Published 2020 Jul 31. 10.3855/jidc.12879 [DOI] [PubMed] [Google Scholar]
- 50. Yağcı S, Serin E, Acicbe Ö, Zeren Mİ, Odabaşı MS. The relationship between serum erythropoietin, hepcidin, and haptoglobin levels with disease severity and other biochemical values in patients with COVID‐19 [published online ahead of print, 2021 Feb 7]. Int J Lab Hematol. 2021. 10.1111/ijlh.1347910.1111/ijlh.13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tocoglu A, Dheir H, Bektas M, Acikgoz S, Karabay O, Sipahi S. Predictors of mortality in patients with COVID‐19 infection‐associated acute kidney injury. J Coll Physicians Surg Pak. 2021;31(1):S60‐S65. 10.29271/jcpsp.2021.01.s60 [DOI] [PubMed] [Google Scholar]
- 52. Soni M, Gopalakrishnan R. Significance of RDW in predicting mortality in COVID‐19‐An analysis of 622 cases [published online ahead of print, 2021 Mar 27]. Int J Lab Hematol. 2021. 10.1111/ijlh.1352610.1111/ijlh.13526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ramachandran P, Gajendran M, Perisetti A, et al. Red blood cell distribution width (RDW) in Hospitalized COVID‐19 Patients. medRxiv. Published online January. 2020:1, 20143081. 10.1101/2020.06.29.20143081 [DOI] [Google Scholar]
- 54. de la Rica R , Borges M, Aranda M, et al. Low albumin levels are associated with poorer outcomes in a case series of COVID‐19 patients in Spain: a retrospective cohort study. Microorganisms. 2020;8(8):1106. 10.3390/microorganisms8081106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lin S, Mao W, Zou Q, Lu S, Zheng S. Associations between hematological parameters and disease severity in patients with SARS‐CoV‐2 infection. J Clin Lab Anal. 2021;35:e23604. 10.1002/jcla.23604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Solmaz I, Özçaylak S, Alakuş ÖF, et al. Risk factors affecting ICU admission in COVID‐19 patients; Could air temperature be an effective factor? Int J Clin Pract. 2021;75(3):e13803. 10.1111/ijcp.13803 [DOI] [PubMed] [Google Scholar]
- 57. Asan A, UstUnda GY, Koca N, et al. Do initial hematologic indices predict the severity of COVID‐19 patients? Turk J Med Sci. 2020:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee JH, Chung HJ, Kim K, et al. Red cell distribution width as a prognostic marker in patients with community‐acquired pneumonia. Am J Emerg Med. 2013;31:72‐79. [DOI] [PubMed] [Google Scholar]
- 59. Lippi G, Henry BM, Sanchis‐Gomar F. Red blood cell distribution is a significant predictor of severe illness in coronavirus disease 2019 [published online ahead of print, 2020 aug 25]. Acta Haematol 2020:1‐5. 10.1159/000510914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zinellu A, Mangoni AA. Red blood cell distribution width, disease severity, and mortality in hospitalized patients with SARS‐CoV‐2 infection: a systematic review and meta‐analysis. J Clin Med. 2021;10(2):286. 10.3390/jcm10020286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee JJ, Montazerin SM, Jamil A, et al. Association between red blood cell distribution width and mortality and severity among patients with COVID‐19: a systematic review and meta‐analysis. J Med Virol. 2021;93:2513‐2522. 10.1002/jmv.26797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Karampitsakos T, Akinosoglou K, Papaioannou O, et al. Increased red cell distribution width is associated with disease severity in hospitalized adults with SARS‐CoV‐2 infection: an observational multicentric study. Front Med. 2020;7:616292. 10.3389/fmed.2020.616292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yu XS, Chen ZQ, Hu YF, et al. Red blood cell distribution width is associated with mortality risk in patients with acute respiratory distress syndrome based on the Berlin definition: a propensity score matched cohort study. Heart Lung. 2020;S0147‐9563:30143‐30146. [DOI] [PubMed] [Google Scholar]
- 64. Alkhatib A, Price LL, Esteitie R, LaCamera P. A predictive model for acute respiratory distress syndrome mortality using red cell distribution width. Crit Care Res Pract. 2020;2020:3832683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thomas T, Stefanoni D, Dzieciatkowska M, et al. Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID‐19 patients. J Proteome Res. 2020;19(11):4455‐4469. 10.1021/acs.jproteome.0c00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Prieto‐Pérez L, Fortes J, Soto C, et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID‐19 infection. Mod Pathol. 2020:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Martinelli N, Montagnana M, Pizzolo F, et al. A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thromb Res. 2020;193:170‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pan Y, Ye G, Zeng X, et al. Can routine laboratory tests discriminate SARS‐CoV‐2‐infected pneumonia from other causes of community‐acquired pneumonia? Clin Transl Med. 2020;10(1):161‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


