CONFLICT OF INTEREST
All authors declare that they have no conflict of interests.
AUTHORS' CONTRIBUTIONS
All the authors have positively contributed to the study. The study was designed by DB and TEJ. DS, SD, SB, CG, TM, KK, NR, AK, NS, AP, ST, and AEP, were involved in the data collection and performance of the study. DS and GN were involved in conducting the laboratory tests. DB and TEJ did the data analysis. DB and TEJ prepared the manuscript. Final manuscript was read and approved by all the authors.
To the editors,
The development and longevity of antibodies against SARS‐CoV‐2 in kidney transplant recipients (KTRs) is not clear. Although seroconversion has been documented, 1 the persistence of this response with time is not established. We longitudinally studied the presence of anti‐SARS‐CoV‐2 IgG antibodies in KTRs after symptomatic coronavirus disease‐2019 (COVID‐19).
Forty‐seven patients with a positive SARS‐CoV‐2 RTPCR between May 2020 and March 2021 were followed up in the transplant clinic for 5.55 ± 2.32 months. Sera was initially tested at day 15 from diagnosis for the presence of anti‐SARS‐CoV‐2 total IgG antibodies using COVID KAWACH IgG MICROLISA (J. Mitra Pvt. Ltd; approved by the Indian Council of Medical Research 2 ). It is a qualitative assay using SARS CoV‐2 virus whole‐cell antigen (sensitivity = 96.33%, specificity = 100%). Patients negative on day 15, were retested until they seroconverted.
Nineteen of 47 (40.4%) patients developed severe COVID‐19 as defined by WHO (Table S1). None of the patients developed reinfection. None of these patients were vaccinated against SARS‐CoV‐2 till data collection.
Forty‐one (87.2%) patients achieved seroconversion with majority (63.4%) achieving within 2 weeks (Figure 1A). Six (12.7%) patients who did not develop antibodies were repeatedly tested for 3.29 ± 1.38 months (Figure 1B). Patients who seroconverted had higher body mass index (P = .036) but there were no differences in the age, time post‐transplant, baseline immunosuppression, graft function, severity of COVID‐19, and immunosuppression modifications (Table S1).
FIGURE 1.
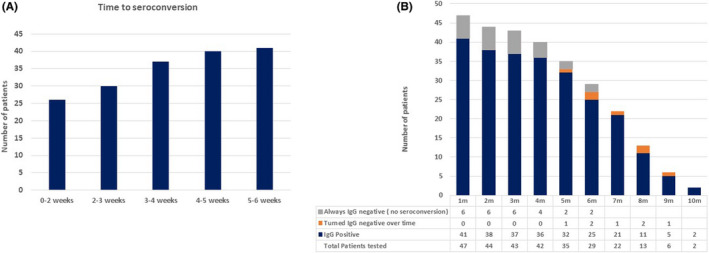
(A) Time to achieve initial seroconversion (n = 41, remaining 6 patients did not achieve seroconversion). (B) Evolution of antibody response in recipients over time. (n = 47). Patients with who failed to achieve initial seroconversion were repeatedly tested over 1‐6 months. For patients who turned negative with time, the test was not repeated any further, thus they are counted only once here
Patients with positive IgG were repeatedly tested at an interval of 1‐3 months till the last follow‐up (5.62 ± 2.37 months). Seven of 41 (17.1%) patients became IgG negative with time (Figure 1B). With respect to persistence of antibody response, there was no difference associated with age (P = .90), time post‐transplantation (P = .085), use of antibody induction (P = .70), baseline antimetabolite use (P = .99), and use of anti‐rejection therapy in past (P = .99). 44.1% of patients with persistent seroconversion had a history of severe COVID‐19 (vs 28.6% of those who become IgG negative, P = .68). Patients with persistent antibody response were more likely to have withdrawal of antimetabolite (71.9% vs 57.1% but insignificant P = .72) and reduction/withdrawal of calcineurin inhibitors (60% vs 42.9%, but insignificant P = .43) during COVID‐19. None of the factors predicted persistent immunological response in multivariate analysis.
This study confirms that the majority of KTRs (87%) develop serological response after symptomatic COVID‐19 and it is sustained with time in 34/41 patients (83%). These results are in concurrence with Chavarot et al 3 who have reported seroconversion in 71.4% KTRs. However, in contrast, 60% of patients in their cohort, turned equivocal/negative at 6 months. This can be attributed to the lesser severity of COVID‐19 in their cohort (76% patients hospitalized) and higher age (57.7 years 4 ). Data from the general population suggest that antibody response is blunted in mild COVID‐19 and a decline can occur over 60‐90 days. 4 , 5 In KTRs, this can be impacted by baseline immunosuppression, the severity of COVID‐19, immunosuppression modifications during COVID‐19. In our cohort, there is a signal of persistent seroconversion in patients with severe COVID‐19, we could not establish statistical significance probably due to a smaller sample size, which remains a limitation of this study. However, to our knowledge, this remains the largest study evaluating immunological response in KTRs longitudinally and merits meticulous clinical correlation and follow‐up.
In conclusion, these data are reassuring about the development and persistence of seroconversion against SARS‐CoV‐2 in KTRs despite majority being on standard immunosuppression. The factors affecting this response warrant further evaluation.
Supporting information
Table S1
Bajpai D, Shah D, Bose S, et al. Development and longevity of antibodies against SARS‐CoV‐2 in kidney transplant recipients after symptomatic COVID‐19. Transpl Infect Dis. 2021;23:e13646. 10.1111/tid.13646
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Prendecki M, Clarke C, Gleeson S, et al. Detection of SARS‐CoV‐2 antibodies in kidney transplant recipients. J Am Soc Nephrol. 2020;31(12):2753‐2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. https://www.icmr.gov.in/pdf/covid/kits/ELISA_CLIA_Kits_List_03112020_v1.pdf. Accessed May 31, 2021.
- 3. Chavarot N, Leruez‐Ville M, Scemla A, et al. Decline and loss of anti‐SARS‐CoV‐2 antibodies in kidney transplant recipients in the 6 months following SARS‐CoV‐2 infection. Kidney Int. 2021;99(2):486‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bölke E, Matuschek C, Fischer JC. Loss of anti‐SARS‐CoV‐2 antibodies in mild Covid‐19. N Engl J Med. 2020;383(17):1694‐1695. [DOI] [PubMed] [Google Scholar]
- 5. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, et al. Rapid decay of anti‐SARS‐CoV‐2 antibodies in persons with mild Covid‐19 [published correction appears in N Engl J Med. 2020 Jul 23]. N Engl J Med. 2020;383(11):1085‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


