Abstract
Background
Gastrointestinal infections represent a risk factor for functional gastrointestinal and somatoform extraintestinal disorders. We investigated the prevalence and relative risk (RR) of gastrointestinal and somatoform symptoms 5 months after SARS‐CoV‐2 infection compared with a control cohort.
Methods
One hundred and sixty‐four SARS‐CoV‐2 infected patients and 183 controls responded to an online questionnaire about symptoms and signs during the acute phase of the infection and after 4.8 ± 0.3 months. Presence and severity of gastrointestinal symptoms, somatization, anxiety, and depression were recorded with standardized questionnaires. Stool form and presence of irritable bowel syndrome (IBS) were also recorded. Any association between exposure to infection and symptoms was evaluated by calculating crude and adjusted RR values and score differences with 95% confidence intervals (CI).
Key Results
Fever, dyspnea, loss of smell/taste/weight, diarrhea, myalgia, arthralgia, and asthenia were reported by more than 40% of patients during the acute phase. Compared with controls, adjusted RRs for loose stools, chronic fatigue, and somatization were increased after infection: 1.88 (95% CI 0.99–3.54), 2.24 (95% CI 1.48–3.37), and 3.62 (95% CI 1.01–6.23), respectively. Gastrointestinal sequelae were greater in patients with diarrhea during the acute phase.
Conclusions & Inferences
Mild gastroenterological symptoms persist 5 months after SARS‐CoV‐2 infection, in particular in patients reporting diarrhea in the acute phase. Infected patients are at increased risk of chronic fatigue and somatoform disorders, thus supporting the hypothesis that both functional gastrointestinal and somatoform disorders may have a common biological origin.
Keywords: chronic fatigue, COVID‐19, functional gastrointestinal disorders, irritable bowel syndrome, SARS‐CoV‐2
Mild gastrointestinal symptoms persist 5 months after SARS‐CoV‐2 infection together with an increased risk of chronic fatigue and somatoform symptoms. These results support the hypothesis that both functional gastrointestinal and somatoform disorders may have a common biological origin.

Key Points.
Gastrointestinal infections represent a risk factor for functional gastrointestinal and somatoform extraintestinal disorders.
Mild gastrointestinal symptoms persist 5 months after severe acute respiratory syndrome coronavirus‐2 infection together with an increased risk of chronic fatigue and somatoform symptoms.
Our results support the hypothesis that both functional gastrointestinal and somatoform disorders may have a common biological origin.
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) is a single‐stranded enveloped RNA beta‐coronavirus, responsible for the first 21st‐century pandemic. 1 SARS‐CoV‐2 infection can be asymptomatic or responsible for coronavirus disease‐2019 (COVID‐19) 2 characterized by a range of pulmonary manifestations from fever, dry cough, and dyspnea to pneumonia and acute respiratory distress syndrome. 3 Additionally, several extrapulmonary manifestations have also been described including neurological, hematological, cardiovascular, renal, dermatological, and gastrointestinal ones. 4 The most frequent gastrointestinal manifestation in COVID‐19 patients is diarrhea, which has been variably reported in 4%–37% of large series. 3 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Less is known about whether gastrointestinal symptoms persist after the resolution of the acute infection. In a recent large Chinese cohort study, 5% of patients reported diarrhea or vomiting 6 months after SARS‐CoV‐2 infection. 13 In another retrospective study, the most common gastrointestinal sequelae 90 days after infection were the loss of appetite, nausea, acid reflux, and diarrhea, that were reported by 24%, 18%, 18%, and 15% of the patients, respectively. 14
Bacterial, protozoal, and viral infections of the gastrointestinal tract represent a recognized risk factor for the development of functional gastrointestinal disorders in the upper and lower gastrointestinal tract, known as post‐infectious dyspepsia 15 , 16 and post‐infectious irritable bowel syndrome (IBS). 17 , 18 , 19 , 20 Gastrointestinal infections have also been reported to increase the risk of chronic fatigue and other extraintestinal symptoms (e.g., headache, articular, and muscle pain), which in absence of organic/biological alterations explaining them are known as functional somatic syndromes or somatoform disorders. 19 , 21 , 22 Whether the origin of these somatoform symptoms was to be searched in a biological, psychological, or social domain is still debated. 23
Since February 2020, SARS‐CoV‐2 has been hitting Italy. 24 This has provided a unique opportunity to assess the long‐term impact of a previously unknown viral infection on the burden of both gastrointestinal and extraintestinal somatoform symptoms. The aim of our study was to assess the frequency and relative risk of gastrointestinal and somatoform symptoms 5 months after the resolution of SARS‐CoV‐2 infection compared with a control cohort.
2. MATERIALS AND METHODS
2.1. Subjects
In February 2020, a SARS‐CoV‐2 outbreak occurred in Italy, with extreme severity in Milan and the surrounding Lombardy region. A first peak was reached at the end of March 2020, while the end of the first wave was recognized in May 2020. 24 Far from that, we launched an online structured questionnaire. All the patients aged between 18 and 60 years who tested positive with a polymerase chain reaction for SARS‐CoV‐2 at nose pharyngeal swab in the laboratories of our hospital between February and April 2020 were contacted by e‐mail. Employees and healthcare professionals who were tested negative at nose pharyngeal swab within the surveillance program of the hospital in the same period were also e‐mailed as a control group. Subjects reporting a previous diagnosis of IBS, inflammatory bowel disease (IBD), or celiac disease were excluded.
2.2. Ethics
The study was approved by the local Ethics Committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan (approval no. 106876 on June 23rd 2020). All the subjects received an e‐mail explaining the rationale of the study for their informed consent to participate. As they agreed, the subjects were directed via a link to an online structured questionnaire on the EU‐Survey platform (https://ec.europa.eu/eusurvey/) supported by the European Commission, which allows to collect sensible data with no user identification via IT tracking, profiling cookies, or geographical location or personal/socio‐demographic/health data.
2.3. Symptom questionnaires
The structured questionnaire contained: (i) demographic characteristics and medical history including: age, sex, level of education, current job, past surgery, chronic medications, smoking habits, psychiatric disorders, and a previous diagnosis of IBS, IBD, and celiac disease; (ii) symptoms and signs in the acute phase of SARS‐CoV‐2 infection including: presence/absence of fever, dyspnea, expectorated phlegm, hemoptysis, rhinitis, sore throat, conjunctivitis, loss of smell, loss of taste, nausea, sickness, diarrhea, abdominal pain, weight loss, headache, myalgia, arthralgia, and asthenia; (iii) severity of SARS‐CoV‐2 infection scored according to the need and type of hospitalization: as mild (no hospitalization or discharge from emergency department), moderate (hospitalization in non‐intensive care unit), or severe (hospitalization in intensive care unit); (iv) the concomitant use of antibiotic or antiviral therapy. (v) gastrointestinal and selected non‐gastrointestinal symptoms recorded according to the Structured Assessment of Gastrointestinal Symptoms (SAGIS) questionnaire 25 at the time of the survey. The SAGIS questionnaire includes 22 gastrointestinal symptoms scored on a five‐point Likert scale as 0: no problem, 1: mild (a symptom can be ignored when you do not think about it) 2: moderate (it cannot be ignored, but does not influence daily activities), 3: severe (influencing your concentration on daily activities), and 4: very severe (it markedly influences your daily activities and/or requires rest). Symptoms were grouped in five symptoms domains: 1‐ Abdominal pain/discomfort including: post‐prandial pain, epigastric pain, bloating, fullness, early satiety, retrosternal discomfort, and abdominal cramps. 2‐ Diarrhea/incontinence including: diarrhea, loose stools, urgency to defecate, pain/discomfort prior to defecation, excessive gas flatulence, and incontinence. 3‐ Gastroesophageal reflux disease/regurgitation including: dysphagia, excessive belching, and acid eructation. 4‐ Nausea/vomiting including: sickness, nausea, vomiting, and loss of appetite. 5‐ Constipation including: constipation and difficult defecation. The scores of each symptom domain are reported as the arithmetic mean of the scores for the symptoms of the given domain. The SAGIS questionnaire also asked subjects to describe in their own words their first and second most important health concern/problem and the presence/absence of 6 selected non‐gastrointestinal symptoms including: headache, back pain, sleep disturbances, chronic fatigue, and self‐reported depression and anxiety (vi) A yes/no question summarizing the Rome IV criteria for IBS 26 (vii) The visual chart of the Bristol Stool scale 27 (viii) Symptom Checklist (SCL)‐12 for somatoform disorders 28 including the 11 “non‐gastrointestinal” questions (headache, faintness or dizziness, pains in the heart or chest, pains in the lower back, soreness of your muscles, trouble getting your breath, hot or cold spells, numbness or tingling in parts of your body, a lump in your throat, feeling weak in parts of your body, heavy feelings in your arms or legs) scored on how much that problem bothered or distressed during the past week (from 0: not at all to 4: extremely). The “gastrointestinal” question: “nausea or upset stomach” was excluded from the analysis to avoid the overlap with the symptoms recorded with the SAGIS questionnaire. The individual raw scores were converted to standard area t‐scores based on a non‐psychiatric patient normative sample. An area t‐score of 60 places the individual in the 84th centile of the normative or referent population and an area t‐score of 70 in the 98th centile; according to Derogatis et al., 29 area t‐scores ≥63 have been applied for making an operational definition of “positive cases” when using the SCL90‐R as a screening measure for psychiatric disorders. (ix) Hospital Anxiety and Depression Scale (HADS) 30 composed of 14 items, 7 of which are related to anxiety (‐A) and seven to depression (‐D). Each item of the questionnaire was scored from 0 to 3. The scores of each domain are reported as the arithmetic mean of the scores of the symptoms composing the domain in each subject. The final score ranged from 0 to 21 for each anxiety or depression with a cutoff value ≥11 suggestive of the condition.
2.4. Statistical analysis
Data were automatically collected on EU‐Survey. The sample size was calculated assuming the frequency of IBS‐like symptoms in the study and control groups of 25% and 5%, respectively; accordingly, 140 subjects in each group would be needed to have a 80% power with a 0.05 type‐I error. At univariate analysis, the differences between the two groups for categorical variables and continuous variables were analyzed by Chi‐square and Mann‐Whitney test, respectively. Adjusted score differences and 95% confidence intervals (CI) between SARS‐CoV‐2‐positive patients and negative controls were obtained from multiple linear regression models containing covariates selected a priori as potential confounders: sex, age, level of education, past surgery, chronic medications, smoking habits, and psychological comorbidity. The same covariates were entered in multiple Poisson regression models with robust variance to compare symptoms frequencies in the two groups and to calculate adjusted risk ratios (RR). 31 Statistical analysis was carried out by software: Stata 16 (StataCorp. 2019).
3. RESULTS
The rate of response to the structured questionnaire was 34.6% (177 out of 511) among SARS‐CoV‐2‐positive patients and 10.1% (201 out of 1,987) among the control group: 13 patients and 18 controls were excluded because of a pre‐existing gastrointestinal disease, 9 (5%) and 16 (8%) with IBS, respectively; thus 164 SARS‐CoV‐2‐positive patients and 183 control subjects were finally included. The flowchart of the recruited subjects is reported in Figure S1.
The questionnaire was completed by the enrolled patients 4.8 ± 0.3 months after the nose pharyngeal swab. The demographic characteristics of the compared groups are provided in Table 1. SARS‐CoV‐2‐positive patients smoked less than control subjects and were older, with lower frequency of women and lower education level. Job activity, past surgery, chronic medications, and psychiatric disorders were not different in the compared groups.
TABLE 1.
Demographic characteristics and clinical history of the recruited subjects
| Characteristic | SARS‐CoV‐2 | p‐value | |
|---|---|---|---|
| Positive (n = 164) | Negative (n = 183) | ||
| Age, years. mean (range) | 44.1 (23–60) | 39.6 (22–60) | <0.001 |
| Female, n (%) | 66 (40.2%) | 111 (60.7%) | <0.001 |
| Educational level, n (%) | |||
| Middle school | 17 (10.4%) | 5 (2.7%) | <0.001 |
| High school | 64 (39%) | 37 (20.2%) | |
| Degree | 81 (49.4%) | 138 (75.4%) | |
| Current job, n (%) | |||
| Unemployed | 6 (3.7%) | 1 (0.55%) | 0.05 |
| Employed | 80 (48.8%) | 100 (54.6%) | |
| Freelance | 22 (13.4%) | 23 (12.6%) | |
| Retired | 5 (3.0%) | 0 | |
| Student | 3 (1.8%) | 0 | |
| Other | 47 (28.7%) | 49 (26.8%) | |
| Past surgery, n (%) | 103 (62.8%) | 102 (55.7%) | 0.19 |
| Chronic medications, n (%) | 59 (36%) | 53 (29%) | 0.25 |
| Smoking, n (%) | |||
| Never smoked | 112 (68.3%) | 99 (54.1%) | <0.001 |
| Former smoker | 30 (18.3%) | 20 (10.9%) | |
| Current smoker | 11 (6.7%) | 35 (19.1%) | |
| Psychiatric disorders, n (%) | |||
| Depression | 3 (1.8%) | 0 | 0.25 |
| Anxiety disorder | 8 (4.8%) | 9 (4.9%) | |
| Other psychiatric disorders | 0 | 1 (0.5%) | |
3.1. Characteristics of acute SARS‐CoV‐2 infection
The frequency of symptoms and signs of SARS‐CoV‐2‐positive patients compared to those reported by negative controls are shown in Figure 1. Fever, dyspnea, loss of smell, loss of taste, diarrhea, weight loss, myalgia, arthralgia, and asthenia were reported by more than 40% of patients and with a greater frequency (p < 0.001) than in the control group. Half of patients received antibiotic treatment, mainly beta‐lactams, cephalosporins, and macrolides; 3 patients received antiviral treatments; among control subjects, 11 (6%) received antibiotic treatment, mainly amoxicillin/clavulanate; none received antiviral treatments. Most of the patients suffered of a mild (53%) or moderate (33%) form of SARS‐CoV‐2 infection; 22 patients (13%) experienced a severe form; five control subjects were hospitalized for issues other than SARS‐CoV‐2 infection in the same period.
FIGURE 1.
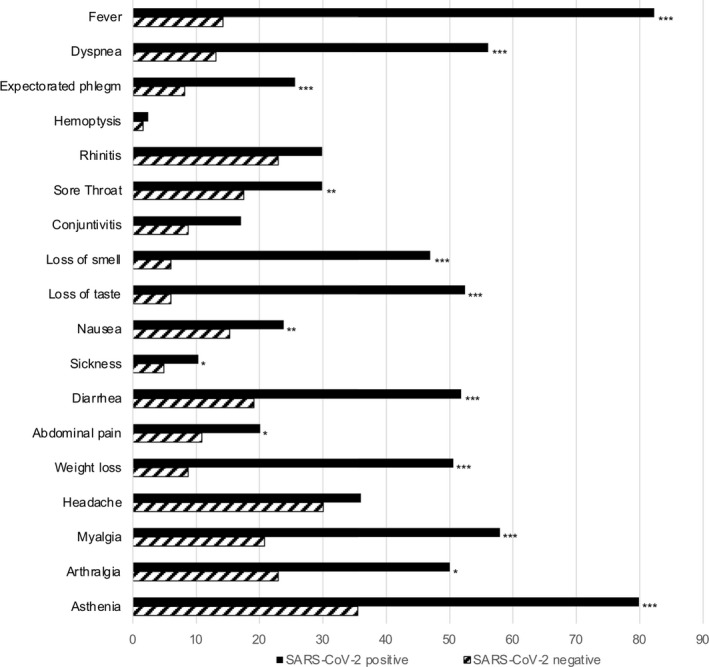
Symptoms and signs during the acute phase of SARS‐CoV‐2 infection and during the same period in SARS‐CoV‐2‐nesgative subjects. Data are expressed as percentage of subjects reporting the condition. *p < 0.05, **p < 0.01, ***p < 0.001
3.2. Characteristics after acute SARS‐CoV‐2 infection
Gastrointestinal symptoms summarized according to the 5 domains of the SAGIS questionnaire are reported in Table 2. At univariate analysis, the symptoms in the abdominal pain/discomfort, diarrhea/incontinence, and gastroesophageal reflux disease/regurgitation domains were more severe in patients with previous SARS‐CoV‐2 infection than in control subjects with a score difference of +0.16, +0.13, and +0.13, respectively. These differences were lower at multivariable analysis (Table 2). Similar scores of nausea/vomiting and of constipation domains were reported in the two groups at both univariate and multivariable analysis.
TABLE 2.
Gastrointestinal manifestation after resolution of SARS‐CoV‐2 infection according to the five domains of the Structured Assessment of Gastrointestinal Symptoms Scale (SAGIS) questionnaire
| SARS‐CoV‐2 | p‐value | Adjusted score difference | p‐value | ||
|---|---|---|---|---|---|
| Mean score ± SD | (95% confidence interval) | ||||
| Positive | Negative | ||||
| SAGIS domain | |||||
| Abdominal pain/discomfort | 0.49 ± 0.60 | 0.33 ± 0.53 | 0.009 | 0.11 (−0.04; 0.26) | 0.15 |
| Diarrhea/incontinence | 0.41 ± 0.55 | 0.28 ± 0.40 | 0.03 | 0.07 (−0.05; 0.19) | 0.27 |
| Gastroesophageal reflux disease/regurgitation | 0.39 ± 0.51 | 0.26 ± 0.44 | 0.06 | 0.07 (−0.05; 0.20) | 0.28 |
| Nausea/vomiting | 0.20 ± 0.33 | 0.17 ± 0.35 | 0.76 | 0.06 (−0.03; 0.16) | 0.20 |
| Constipation | 0.31 ± 0.62 | 0.35 ± 0.68 | 0.82 | −0.01 (−0.17; 0.16) | 0.95 |
Adjusted score differences between SARS‐CoV‐2‐positive and negative subjects were obtained from multiple linear regression models containing the covariates sex, age, level of education, past surgery, chronic medications, smoking habits, and psychological comorbidity.
The frequency of IBS according to the Rome IV criteria was similar in patients with previous SARS‐CoV‐2 infection and control subjects (26.2% vs. 25.1%; p = 0.81) with an adjusted RR of 1.07 (0.72–1.60). Loose stools, defined as a Bristol stool score ≥6, were more frequent (17.8% vs. 9.3%; p = 0.02) in patients with previous SARS‐CoV‐2 infection than control subjects with an adjusted RR of 1.88 (0.99–3.54).
The extraintestinal symptoms recorded by the SAGIS questionnaire are reported in Table 3. Chronic fatigue was more than twice more frequent in patients with previous SARS‐CoV‐2 infection than in control subjects both at univariate (31.7% vs. 13.7%; p < 0.001) and multivariable robust Poisson analysis with an adjusted RR of 2.24 (1.48–3.37). Chronic fatigue was not associated with the severity of the infection (mild 33.3% vs. moderate 25.9% vs. severe 40.1%, p = 0.41). The frequency of headache, back pain, sleep disturbances, depression, and anxiety disorders was similar in the two groups (Table 3).
TABLE 3.
Extraintestinal symptoms after resolution of SARS‐CoV‐2 infection according to the Structured Assessment of Gastrointestinal Symptoms Scale (SAGIS) questionnaire
| SARS‐CoV‐2 | p‐value | Adjusted risk ratio | p‐value | ||
|---|---|---|---|---|---|
| n (%) | (95% confidence interval) | ||||
| Positive | Negative | ||||
| SAGIS extraintestinal | |||||
| Headache | 33 (20.1%) | 61 (33.3%) | 0.017 | 0.68 (0.46; 1.0) | 0.05 |
| Back pain | 46 (28.0%) | 68 (37.2%) | 0.185 | 0.65 (0.47; 0.90) | 0.01 |
| Sleep disturbances | 63 (38.4%) | 59 (32.2%) | 0.483 | 1.23 (0.90; 1.68) | 0.19 |
| Chronic fatigue | 52 (31.7%) | 25 (13.7%) | <0.001 | 2.24 (1.48; 3.37) | <0.001 |
| Depression | 13 (7.9%) | 7 (3.8%) | 0.262 | 1.35 (0.53; 3.45) | 0.52 |
| Anxiety disorder | 30 (18.3%) | 30 (16.4%) | 0.745 | 0.88 (0.53; 1.48) | 0.64 |
Adjusted risk ratios were obtained from multiple Poisson regression models with robust variance containing the covariates sex, age, level of education, past surgery, chronic medications, smoking habits, and psychological comorbidity.
The first most important health concern/problem among gastrointestinal symptoms was abdominal pain and bloating in both patients with previous SARS‐CoV‐2 infection and control subjects (n = 16 and n = 10), while chronic fatigue (n = 14) or headache (n = 6) was the most important non‐gastrointestinal symptoms. Similar trends were reported for the second most important health concern/problem (data not reported).
Somatization and psychological characteristics according to the Hospital Anxiety and Depression Scale (HADS) are reported in Table 4. Somatization scores were higher in patients with previous SARS‐CoV‐2 infection than in control subjects both at univariate (p = 0.0006) and multivariable analysis with an adjusted score difference of 3.62 (1.01–6.23). Positive cases, namely with a normalized t‐score ≥63, were more frequent (24.4% vs. 14.2%, p = 0.02) in patients with previous SARS‐CoV‐2 infection than in control subjects. Somatization was not associated with the severity of SARS‐CoV‐2 infection (mild 53.8% vs. moderate 54.5% vs. severe 57.9% normalized scores, p = 0.31). Anxiety and depression scores were similar in the two groups (Table 4); positive cases, with a score equal or greater than the operational cutoff value of 11 for anxiety were 17 (10%) in patients with previous SARS‐CoV‐2 infection and 8 (4%) in controls and for depression 9 (5%) and 4 (2%), respectively.
TABLE 4.
Psychological characteristics according to the Symptom Checklist (SCL) −12 for Somatization and the Hospital Anxiety and Depression Scale (HADS)
| SARS‐CoV‐2 | p‐value | Adjusted score difference | p‐value | ||
|---|---|---|---|---|---|
| Mean score ± SD | (95% confidence interval) | ||||
| Positive | Negative | ||||
| SCL‐12 | 54.6 ± 10.8 | 50.5 ± 10.8 | 0.0006 | 3.6 (1.0; 6.2) | 0.007 |
| HADS‐A | 4.68 ± 3.97 | 4.47 ± 3.38 | 0.87 | 0.39 (−0.52; 1.31) | 0.40 |
| HADS‐D | 3.81 ± 3.53 | 3.53 ± 3.34 | 0.47 | 0.47 (−0.38; 1.34) | 0.27 |
The adjusted score differences between SARS‐CoV‐2‐positive and negative subjects were obtained from multiple linear regression models containing the covariates sex, age, level of education, past surgery, chronic medications, smoking habits, and psychological comorbidity.
3.3. Post hoc analysis
The presence of diarrhea was associated (a) in the acute phase of SARS‐CoV‐2 infection with an increased frequency of hospitalization (64.7% vs. 47.9%; p = 0.03); among the hospitalized patients the frequency of diarrhea tended to be greater in moderate than in severe patients (64.2% vs. 45.2%; p = 0.13); and (b) after the acute phase with higher scores of gastrointestinal symptoms in the abdominal pain/discomfort, diarrhea/incontinence, and gastroesophageal reflux disease/regurgitation symptoms domains of the SAGIS questionnaire with a score difference of +0.17, +0.19 and +0.14 in comparison to patients without diarrhea (Table 5), together with an increased frequency of IBS (32.9% vs. 19.2%, p = 0.05) and of loose stools (21.2% vs. 9.6%, p = 0.04). The frequency of chronic fatigue (p = 0.05) and elevated somatization scores (p = 0.003) were higher in patients with diarrhea than those without it (Table 5). Antibiotic treatments tended to be more frequent in patients with diarrhea (58.8%) than in those without (41.1%) (p=0.03).
TABLE 5.
Gastrointestinal and extraintestinal manifestations and psychological profile after resolution of SARS‐CoV‐2 infection in patients who reported diarrhea vs. no diarrhea at the time of acute infection
| SARS‐CoV‐2 Positive | p‐value | ||
|---|---|---|---|
| Diarrhea n = 85 | No Diarrhea n = 73 | ||
| SAGIS domain, (mean ± SD) | |||
| Abdominal pain/discomfort | 0.46 ± 0.69 | 0.29 ± 0.38 | 0.02 |
| Diarrhea/incontinence | 0.39 ± 0.63 | 0.20 ± 0.26 | 0.006 |
| Gastroesophageal reflux disease/Regurgitation symptoms | 0.36 ± 0.55 | 0.22 ± 0.40 | 0.009 |
| Nausea/vomiting | 0.17 ± 0.36 | 0.11 ± 0.29 | 0.56 |
| Constipation | 0.32 ± 0.59 | 0.31 ± 0.62 | 0.59 |
| SAGIS extraintestinal, n (%) | |||
| Headache | 23 (27.0%) | 9 (12.3%) | 0.02 |
| Back pain | 34 (40.0%) | 10 (13.7%) | <0.001 |
| Sleep disturbances | 38 (44.7%) | 22 (30.1%) | 0.06 |
| Chronic fatigue | 32 (37.6%) | 17 (23.3%) | 0.05 |
| Depression | 7 (8.2%) | 5 (6.8%) | 0.74 |
| Anxiety disorder | 15 (17.6%) | 13 (17.8%) | 0.98 |
| SCL‐12, (mean ± SD) | 56.5 ± 10.18 | 52.1 ± 11.49 | 0.003 |
| HADS‐A, (mean ± SD) | 5.03 ± 4.16 | 4.09 ± 3.59 | 0.15 |
| HADS‐D, (mean ± SD) | 4 ± 3.66 | 3.33 ± 3.33 | 0.19 |
Data are expressed as mean ± SD or n (percentages).
Among SARS‐CoV‐2‐positive patients, the subjects with chronic fatigue reported higher somatization scores than those without (61.7 ± 10.8 vs. 50.9 ± 10.9, p = <0.001).
4. DISCUSSION
To our knowledge, this is the first controlled cohort study investigating the frequency and relative risk of gastrointestinal and somatoform symptoms 5 months after SARS‐CoV‐2 infection. Our results show that gastroenterological symptoms may persist after SARS‐CoV‐2 infection but with mild intensity. Regarding the somatoform symptoms, that are often associated with functional gastrointestinal disorders, our results show that SARS‐CoV‐2 infection increases the risk of chronic fatigue together with elevated scores for somatization. Both gastrointestinal and extraintestinal sequelae tended to be greater in patients who reported diarrhea at the time of acute infection.
The impact of SARS‐CoV‐2 infection on the gastrointestinal tract during the acute phase has been already reported in several studies. Acute gastrointestinal manifestations include diarrhea, nausea, vomiting, abdominal pain, and rare cases of mesenteric ischemia with gastrointestinal bleeding. 4 , 5 , 6 Diarrhea has been the most common symptom with high variability among the published studies ranging from 4% to 37% of large series. 3 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 In line with these studies, acute diarrhea was reported by more than 50% of our SARS‐CoV‐2 patients. In our cohort, the presence of diarrhea was associated with an increased hospitalization rate, but among the hospitalized patients it tended to be associated with a less severe disease in line with the results of a recent study reporting a less severe COVID‐19 in patients with diarrhea. 32
Less is known about whether gastrointestinal symptoms persist after the resolution of the acute infection. 13 , 14 Our results show that abdominal pain/discomfort, diarrhea/incontinence, and gastroesophageal reflux disease/regurgitation symptoms do persist after SARS‐CoV‐2 infection, but with very low severity; the relative increase on the mean score of each domain was minimal (up to +0.16) and the absolute score failed to achieve even the level of 1, which identifies the severity of a symptom (mild) that can be ignored when you do not think about it according to the SAGIS classification. In line with the mild impact of the viral infection on gastrointestinal symptoms, we did not found an increased risk of IBS in our patients. On the other hand, our patients had an increased risk of about twofold of loose stools 5 months after the infection and the gastrointestinal sequelae seemed greater in the subgroup of patients with diarrhea during the acute phase. In addition, patients with acute infections other than SARS‐CoV‐2 might have been included in our control group increasing the probability of post‐infectious functional gastrointestinal disorders at the time of the comparison and thus reducing the magnitude of the difference between groups. Whether mild gastrointestinal symptoms and diarrhea seemed to persist after SARS‐CoV‐2 infection, previous studies on post‐infectious IBS after viral infections reported a not increased risk of IBS 6 months after a Norwalk‐like virus food‐borne outbreak 33 but an increased IBS risk 12 months after Norovirus infection. 34
Fatigue is reported during acute viral infections and is known to persist after the resolution of infection with several different viral and non‐viral pathogens. 22 The risk of chronic fatigue increases threefold after Giardia infection 19 and, in a population‐based analysis, it increased 1.35‐ to 1.82‐fold after a previous gastrointestinal infection. 21 Our results indicate that SARS‐CoV‐2 infection elevates the risk of chronic fatigue more than two times according to a recent large Chinese cohort study 13 and with the reports of severe cases of chronic fatigue syndrome/myalgic encephalomyelitis described after SARS infection in the earlier coronavirus epidemics. 35 , 36 In line with the increased risk in chronic fatigue, the scores for somatization were higher in our patients following their SARS‐CoV‐2 infection than in control subjects both at univariate and multivariable analysis.
Somatic symptoms are greater in patients with IBS than in patients with functional diarrhea, 37 are usually associated with anxiety and depression, 37 , 38 and are interpreted on the basis of psychological or social disturbances. Interestingly, the increased risk of chronic fatigue and somatization scores in our study was not associated with significant changes in anxiety and depression; moreover, somatization but not anxiety and depression scores were significantly increased in patients with diarrhea during the acute phase. According to the “biology first” hypothesis by Enck and Mazurak, 23 it is conceivable that chronic fatigue and somatization might also have a post‐infectious origin to begin with and that anxiety might develop at a further step following the bi‐directional brain‐to‐gut and gut‐to‐brain interplay over time. 39 If this hypothesis stands true also for SARS‐CoV‐2 infection, further studies will be needed to understand the biological mechanisms contributing to the development of the final phenotype including genetic predisposition in response to the infection, 40 , 41 the alterations of the neuro‐immune response that controls the penetration of the virus into the central nervous system through the olfactory pathway 35 , 42 as well as studies with longer follow‐up on the long‐term changes in the microbiota, 43 , 44 in the gut mucosal barrier, 41 and in the evolution of somatic symptoms, anxiety, and depression over time. 39
This study comes with some limitations that should be acknowledged. (1) The response rate to the questionnaire was far from optimal in particular for the control group and younger subjects, more prone to respond to an online survey, and female subjects perhaps more interested to take part in a study on functional bowel disorders, might have eventually been selected. To obviate this, we performed the analysis adjusted for gender, age, and other potential confounders. (2) More anxious subjects might have been selected by internet survey assessment. However, positive cases for anxiety in our patients with previous SARS‐CoV‐2 infection (10%) and in controls (4%) were comparable to those reported in the Italian general population before the COVID‐19 pandemic (10.3%) 45 and lower than those reported in two recent internet‐based survey (20.8% and 32.1%) in the Italian population during the pandemic. 46 , 47 (3) Gastrointestinal symptoms and the Bristol Stool Scale were not recorded before the nose pharyngeal swab, raising the possibility that patients with undiagnosed gastrointestinal diseases might have been included; on the other hand, subjects with a diagnosis of IBS, IBD, or celiac disease were carefully excluded. (4) A recall bias might have influenced the assessment of the symptoms during the acute phase of the infection; however, the major aim of the study was to assess the persistence of the symptoms 5 months after the acute infection, that is, at the time when the questionnaire was completed. (5) The study was monocentric and accordingly the results should be extrapolated with cautions in other geographical areas and institutions. Conversely, the single‐center study design reduced the heterogeneity for both patients and controls and the environmental confounders, which have high impact on functional gastrointestinal disorders. 48 (6) A standardized questionnaire 25 was used, albeit not previously validated for an online survey on a target Italian population. However, this questionnaire has shown to represent a reliable valid tool for the assessment of the severity and impact of a wide spectrum of gastrointestinal and selected extraintestinal symptoms in an outpatient settings and, in comparison to other questionnaires, 49 , 50 it is characterized by a limited number of questions that better suit an internet‐based survey, as already shown. 51
In conclusion, our study shows that acute SARS‐CoV‐2 infection may affect the brain‐gut axis. Five months after the acute infection, mild gastroenterological symptoms persist, in particular in patients reporting diarrhea in the acute phase of the infection. Infected patients are also at increased risk of chronic fatigue and somatoform disorders, thus supporting the hypothesis that both functional gastrointestinal and somatoform disorders may have a common biological origin.
CONFLICT OF INTEREST
The authors have no competing interests related to this study.
DISCLOSURES
AC received lecturer fees from Takeda, a sponsorship from Bracco. MV served as a consultant to Abbvie, MSD, Takeda, Janssen‐Cilag, and Celgene. He received lecturer fees from Abbvie, Ferring, Takeda, MSD, Janssen‐Cilag, and Zambon.
AUTHOR CONTRIBUTIONS
DN contributed to conceptualization, collection and analysis of data, writing, and review and editing. AC contributed to conceptualization, analysis of data, and review and editing. AM and AB contributed to review and editing. DC contributed to statistical analysis and review and editing. MV contributed to conceptualization and review and editing. GB contributed to conceptualization, analysis of data, writing, review and editing, and supervision.
Supporting information
Fig S1
Noviello D, Costantino A, Muscatello A, et al. Functional gastrointestinal and somatoform symptoms five months after SARS‐CoV‐2 infection: A controlled cohort study. Neurogastroenterology & Motility.2022;34:e14187. 10.1111/nmo.14187
Funding information
None.
REFERENCES
- 1. Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Naming the coronavirus disease (COVID‐ 19) and the virus that causes it. 2019. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/technical‐guidance/naming‐the‐coronavirus‐disease‐(covid‐2019). Accessed March 16, 2020.
- 3. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID‐19. Nat Med. 2020;26:1017‐1032. [DOI] [PubMed] [Google Scholar]
- 5. D’Amico F, Baumgart DC, Danese S, Peyrin‐Biroulet L. Diarrhea during COVID‐19 infection: pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol. 2020;18:1663‐1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marasco G, Lenti MV, Cremon C, et al. Implications of SARS‐CoV‐2 infection for neurogastroenterology. Neurogastroenterol Motil. 2021;33:e14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jin XI, Lian J‐S, Hu J‐H, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut. 2020;69:1002‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo S, Zhang X, Xu H. Don’t overlook digestive symptoms in patients With 2019 Novel Coronavirus Disease (COVID‐19). Clin Gastroenterol Hepatol. 2020;18:1636‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han C, Duan C, Zhang S, et al. Digestive symptoms in COVID‐19 patients with mild disease severity. Am J Gastroenterol. 2020;115:916‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pan L, Mu MI, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am J Gastroenterol. 2020;115:766‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of gastrointestinal symptoms in patients with COVID‐19. Gastroenterology. 2020;158:2294‐2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weng J, Li Y, Li J, et al. Gastrointestinal sequelae 90 days after discharge for COVID‐19. Lancet Gastroenterol Hepatol. 2021;1253:9‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mearin F, Pérez‐Oliveras M, Perelló A, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: One‐year follow‐up cohort study. Gastroenterology. 2005;129:98‐104. [DOI] [PubMed] [Google Scholar]
- 16. Ford AC, Thabane M, Collins SM, et al. Prevalence of uninvestigated dyspepsia 8 years after a large waterborne outbreak of bacterial dysentery: a cohort study. Gastroenterology. 2010;138:1727‐1736. [DOI] [PubMed] [Google Scholar]
- 17. Barbara G, Grover M, Bercik P, et al. Rome foundationa working team report on post‐infection irritable bowel syndrome. Gastroenterology. 2019;156:46‐58.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cremon C, Stanghellini V, Pallotti F, et al. Salmonella gastroenteritis during childhood is a risk factor for irritable bowel syndrome in adulthood. Gastroenterology. 2014;147:69‐77. [DOI] [PubMed] [Google Scholar]
- 19. Litleskare S, Rortveit G, Eide GE, Hanevik K, Langeland N, Wensaas K‐A. Prevalence of irritable bowel syndrome and chronic fatigue 10 years after giardia infection. Clin Gastroenterol Hepatol. 2018;16:1064‐1072.e4. [DOI] [PubMed] [Google Scholar]
- 20. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta‐analysis. Gastroenterology. 2017;152:1042‐1054.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donnachie E, Schneider A, Mehring M, Enck P. Incidence of irritable bowel syndrome and chronic fatigue following GI infection: a population‐level study using routinely collected claims data. Gut. 2018;67:1078‐1086. [DOI] [PubMed] [Google Scholar]
- 22. Hickie I, Davenport T, Wakefield D, et al. Post‐infective and chronic fatigue syndromes precipitated by viral and non‐viral pathogens: prospective cohort study. Br Med J. 2006;333:575‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Enck P, Mazurak N. The, “Biology‐First” Hypothesis: Functional disorders may begin and end with biology—a scoping review. Neurogastroenterol Motil. 2018;30:e13394. [DOI] [PubMed] [Google Scholar]
- 24. Alicandro G, Remuzzi G, La Vecchia C. Italy’s first wave of the COVID‐19 pandemic has ended: no excess mortality in May, 2020. Lancet. 2020;396:e27‐e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koloski NA, Jones M, Hammer J, et al. The validity of a new structured assessment of gastrointestinal symptoms scale (SAGIS) for evaluating symptoms in the clinical setting. Dig Dis Sci. 2017;62:1913‐1922. [DOI] [PubMed] [Google Scholar]
- 26. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393‐1407.e5. [DOI] [PubMed] [Google Scholar]
- 27. Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea‐predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693‐703. [DOI] [PubMed] [Google Scholar]
- 28. Derogatis L. SCL‐90‐R. Symptom Checklist‐90‐R. Administration, Scoring, and Procedures Manual, 3rd edn. Minneapolis, MN: National Computer Systems; 1997. [Google Scholar]
- 29. Derogatis LR, Cleary PA. Confirmation of the dimensional structure of the scl‐90: a study in construct validation. J Clin Psychol. 1977;33:981‐989. [Google Scholar]
- 30. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361‐370. [DOI] [PubMed] [Google Scholar]
- 31. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702‐706. [DOI] [PubMed] [Google Scholar]
- 32. Livanos AE, Jha D, Cossarini F, et al. Intestinal host response to SARS‐CoV‐2 infection and COVID‐19 outcomes in patients with gastrointestinal symptoms. Gastroenterology. 2021. 10.1053/j.gastro.2021.02.056. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marshall JK Marroon T, Borgaonkar MR, James C. Postinfectious irritable bowel syndrome after a food‐borne outbreak of acute gastroenteritis attributed to a viral pathogen. Clin Gastroenterol Hepatol. 2007;5:457‐460. [DOI] [PubMed] [Google Scholar]
- 34. Zanini B, Ricci C, Bandera F, et al. Incidence of post‐infectious irritable bowel syndrome and functional intestinal disorders following a water‐borne viral gastroenteritis outbreak. Am J Gastroenterol. 2012;107:891‐899. [DOI] [PubMed] [Google Scholar]
- 35. Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post‐SARS syndrome; a case‐controlled study. BMC Neurol. 2011;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perrin R, Riste L, Hann M, Walther A, Mukherjee A, Heald A. Into the looking glass: post‐viral syndrome post COVID‐19. Med Hypotheses. 2020;144:110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shiha MG, Asghar Z, Thoufeeq MO, et al. Increased psychological distress and somatization in patients with irritable bowel syndrome compared with functional diarrhea or functional constipation, based on Rome IV criteria. Neurogastroenterol Motil. 2021;e14121. 10.1111/nmo.14121. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38. Arsiè E, Coletta M, Cesana BM, Basilisco G. Symptom‐association probability between meal ingestion and abdominal pain in patients with irritable bowel syndrome. Does somatization play a role? Neurogastroenterol Motil. 2015;27:416‐422. [DOI] [PubMed] [Google Scholar]
- 39. Koloski NA, Jones M, Talley NJ. Evidence that independent gut‐to‐brain and brain‐to‐gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1‐year population‐based prospective study. Aliment Pharmacol Ther. 2016;44:592‐600. [DOI] [PubMed] [Google Scholar]
- 40. McCaffery JM, Snieder H, Dong Y, de Geus E. Genetics in psychosomatic medicine: research designs and statistical approaches. Psychosom Med. 2007;69:206‐216. [DOI] [PubMed] [Google Scholar]
- 41. Villani A, Lemire M, Thabane M, et al. Genetic risk factors for post‐infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology. 2010;138:1502‐1513. [DOI] [PubMed] [Google Scholar]
- 42. DosSantos MF, Devalle S, Aran V, et al. Neuromechanisms of SARS‐CoV‐2: a review. Front Neuroanat. 2020;14:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dinan TG, Cryan JF. Brain‐gut‐microbiota axis and mental health. Psychosom Med. 2017;79:920‐926. [DOI] [PubMed] [Google Scholar]
- 44. Yeoh YK, Zuo T, Lui G‐Y, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID‐19. Gut. 2021;70:698‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Girolamo G, Polidori G, Morosini P, et al. Prevalence of common mental disorders in Italy. Soc Psychiatry Psychiatr Epidemiol. 2006;41:853‐861. [DOI] [PubMed] [Google Scholar]
- 46. Casagrande M, Favieri F, Tambelli R, Forte G. The enemy who sealed the world: effects quarantine due to the COVID‐19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Med. 2020;75:12‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rossi R, Socci V, Talevi D, et al. COVID‐19 Pandemic and lockdown measures impact on mental health among the general population in Italy. Front Psychiatry. 2020;11:7‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology. 2021;160:99‐114.e3. [DOI] [PubMed] [Google Scholar]
- 49. Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456‐1479. [DOI] [PubMed] [Google Scholar]
- 50. Palsson OS, Whitehead WE, van Tilburg MAL, et al. Development and validation of the Rome IV diagnostic questionnaire for adults. Gastroenterology. 2016;150:1481‐1491. [Google Scholar]
- 51. Goncharova M, Grey J, Druce M. Impact of gastrointestinal symptoms on quality of life in MEN2. Clin Endocrinol (Oxf). 2020;94(4):606‐615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


