Summary
Globally, infection by seasonal influenza viruses causes 3–5 million cases of severe illness and 290,000–650,000 respiratory deaths each year. Various influenza vaccines, including inactivated split‐ and subunit‐type, recombinant and live attenuated vaccines, have been developed since the 1930s when it was discovered that influenza viruses could be cultivated in embryonated eggs. However, the protection rate offered by these vaccines is rather low, especially in very young children and the elderly. In this review, we describe the history of influenza vaccine development, the immune responses induced by the vaccines and the adjuvants applied. Further, we suggest future directions for improving the effectiveness of influenza vaccines in all age groups. This includes the development of an influenza vaccine that induces a balanced T helper cell type 1 and type 2 immune responses based on the understanding of the immune system, and the development of a broad‐spectrum influenza vaccine that can increase effectiveness despite antigen shifts and drifts, which are characteristics of the influenza virus. A brighter future can be envisaged if the development of an adjuvant that is safe and effective is realized.
Keywords: adjuvants, influenza viruses, vaccines
Abbreviations
- APCs
antigen‐presenting cells
- CTLs
cytotoxic T lymphocytes
- HA
haemagglutinin
- IIVs
inactivated influenza vaccines
- iTregs
induced regulatory T cells
- LAIVs
live‐attenuated influenza vaccines
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- MDCK
Madin–Darby canine kidney
- MHC
major histocompatibility complex
- MPLA
monophosphoryl lipid A
- NA
neuraminidase
- TGF‐β
transforming growth factor‐beta
- Th
T helper
- TLR
Toll‐like receptor
- TNF‐α
tumour necrosis factor alpha
- VPL
virus‐like particle
1. INTRODUCTION
Influenza viruses belong to the family Orthomyxoviridae, and their genomes consist of segments of negative‐sense RNA. 1 They are divided into A, B, C and D types, the latter of which was isolated from pigs exhibiting influenza‐like symptoms in April 2011. 2 In humans, mainly the A and B types cause disease, and the A type causes more severe illness than the B type. 3 , 4 Influenza A viruses are further categorized according to the antigenicity of their surface antigens, haemagglutinin (HA) and neuraminidase (NA), and there are 18 HA and 11 NA serotypes. 5 , 6 Influenza B viruses have diverged into only two antigenically distinguishable lineages, Yamagata and Victoria, since the 1970s. 3 Current influenza viruses circulating in humans are mainly A/H1N1 and A/H3N2 and the B/Yamagata and B/Victoria lineages. 7 Yearly, seasonal influenza causes 3–5 million cases of severe illness and 290,000–650,000 respiratory deaths globally. 8 Mortality rates and severe cases are higher in the elderly (>65 years) and children younger than 5 years, and in immunosuppressed people. 8 , 9 , 10
Antiviral drugs to treat influenza include oseltamivir, zanamivir, peramivir and baloxavir. 11 However, vaccination is considered the most effective method for controlling influenza. 12 Through continuous antigenic drift, that is, the accumulation of point mutations in the surface antigens, influenza viruses can escape immunity, 13 , 14 which is why yearly vaccination is required. Seasonal influenza vaccines have been steadily developed since the 1940s, and currently marketed preventive vaccines differ in type (whole, split, recombinant and subunit inactivated, and live‐attenuated types) and the substrate used for production (embryonated eggs or cells). 15
A study on vaccine effectiveness by the USA Centers for Disease Control and Prevention showed that their protection rate remains low (40%–60%), despite being antigenically matched with circulating strains. 16 Of concern, vaccines do not effectively elicit immune responses among very young children (6–35 months) and elderly people (>65 years), 17 , 18 the two age groups more vulnerable to influenza virus infection and with higher mortality rates. Therefore, there is an urgent need for effective influenza vaccines for all ages.
2. DEVELOPMENTAL HISTORY OF AND IMMUNE RESPONSES INDUCED BY INFLUENZA VACCINES
2.1. History of influenza vaccine development
Methods for culturing influenza viruses were developed in the 1930s. In 1933, the human influenza virus was transmitted to ferrets by intranasal instillation of a specimen obtained from throat washings collected from a patient. 19 In 1935, Wilson Smith suggested a method for cultivating influenza virus in the chorioallantoic membrane of embryonated eggs. 20 This method yielded substantially higher virus concentrations than previous methods based on virus extraction from the lungs of infected animals. Embryonated eggs are still used today to produce influenza vaccines. In addition, cultivation methods using cells and medium have been developed since the mid‐1930s. 21 , 22 With the ability to isolate and culture influenza viruses, research on influenza vaccine development took off. From the mid‐1930s to the early 1940s, vaccine effects of activated and formalin‐inactivated influenza viruses obtained from allantoic fluid of embryonated eggs or extracts of infected animal organs were studied in animals and humans by monitoring antibody production. 23 , 24 , 25 It was found that antigenic matching between the vaccine and circulating strains is important to guarantee vaccine efficacy 26 and that concentrated vaccine is more effective than the unconcentrated vaccine, whether or not the virus is inactivated. 27 , 28 In 1942, immune responses induced by an inactivated whole bivalent influenza vaccine consisting of the PR8 strain of influenza type A and the Lee strain of influenza type B and produced in embryonated eggs were evaluated in a clinical study. 29 In 1943, a larger clinical study by the Commission on Influenza of the U.S. Armed Forces showed that inactivated whole trivalent vaccine including the A‐subtype PR8 and Weiss strains and the B‐type Lee strain protected against influenza. 30 Vaccine doses were established through clinical trials, and in 1945, the first inactivated influenza vaccine (IIV) was licensed in the United States. 31
Since the development of the first inactivated whole virus vaccine in embryonated chicken eggs in the 1940s, production methods for IIVs were continuously improved, and in the 1950s, the current IIV manufacturing process using embryonated eggs was developed. 32 IIVs included whole‐virus, split‐virus disrupted by a detergent and further purified subunit vaccines composed of surface antigen, HA and NA. 33 The whole‐virus vaccine, the first developed IIV, induced good immune responses even in unprimed individuals. 34 However, there were concerns about pyrogenicity and adverse side effects. 35 To overcome these problems, in the 1960s, split virus vaccines were developed by treating the virus with ether or detergent, 36 which made them safe for children. 37 In the 1970s, purified subunit influenza vaccines mainly based on HA and NA were developed, which further improved safety and reduced reactogenicity. 34 , 35 , 38 , 39 , 40 , 41
Live‐attenuated influenza vaccines (LAIVs), prepared by successive passages of influenza virus in ferrets and mice or embryonated eggs, have also been studied since the 1930s. 42 These host‐range variant vaccines protect against influenza without causing flu symptoms in humans. However, they have some drawbacks, including low virus titres and difficulties in maintaining constant attenuation and antigenicity levels. 43 In the 1960s, a new method was adopted to attenuate influenza virus through consecutive passages in embryonated eggs at low temperatures, yielding cold‐adapted, temperature‐sensitive variants. 44 Since cold‐adapted, temperature‐sensitive influenza virus replicates best at lower temperatures, viral replication was enacted in the nasal cavity and not in the respiratory tract. These viruses were safer than those attenuated by previous methods and induced an immune response. Accordingly, they were further developed as donor viruses for LAIVs. 45
As RNA viruses, influenza viruses lack proofreading activity and therefore are genetically unstable; thus, antigenic mutations occur at a high frequency. 46 To increase the protection rate of influenza vaccines, it is important to accurately predict the influenza viruses that will circulate and to manufacture vaccines with those strains. To this end, the World Health Organization's (WHO) Global Influenza Surveillance and Response System has conducted global influenza surveillance since 1952, and based on the monitoring results, the WHO annually announces recommended influenza virus vaccine compositions for the northern (February) and southern (September) hemispheres. 47 , 48 Initially, the WHO recommended three influenza virus strains, including A/H1N1, A/H3N2, and either the B/Victoria or the B/Yamagata lineage for trivalent vaccines; however, since co‐circulation of the two B lineages was observed at a high frequency, B/Victoria as well as B/Yamagata are being recommended for quadrivalent influenza vaccines. 49 However, since IIVs and LAIVs are manufactured using the recommended candidate viruses, they will not be effective because of antigenic mismatch if the prediction is not accurate. Thus, broad‐spectrum or universal influenza vaccines are being actively researched. These vaccine types target a conserved region of the influenza virus, such as the stalk region of HA, M2e, M1 or nucleoprotein instead of the globular head of HA, which is immunodominant, but variable and strain‐specific (Figure 1). 15 , 50 These vaccines are prepared by using a viral vector, DNA vector, virus‐like particle (VLP), nanoparticle or a peptide that directly stimulates T cells. 51 Among them, nanoparticle and VLP platform show the most visible results while completing the phase Ⅲ clinical trial in 2020 (NCT04120194, NCT03301051 and NCT03739112). Both nanoparticle (Novavax) and VLP influenza vaccine (Medicago) showed results inducing cross‐reactive antibody and T‐cell response. 52 , 53
FIGURE 1.
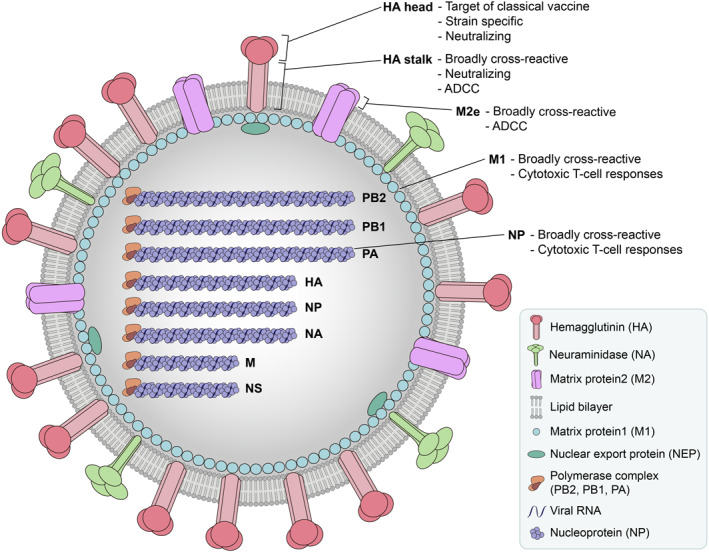
Targets of broad‐spectrum or universal influenza vaccine. Broad‐spectrum or universal influenza vaccines target antigens that can elicit broadly cross‐reactive immune responses. Antibodies induced by HA stalk and ectodomain of the M2 ion channel (M2e), which are highly conserved regions, can mediate antibody‐dependent, cell‐mediated cytotoxicity (ADCC). Antibodies against HA stalk is neutralizing; while antibodies against M2e is not. M1 and NP proteins possess conserved regions and are internal proteins. Therefore, they mainly induce cytotoxic T cell responses
2.2. Substrates for influenza vaccine production
Although the conventional method using embryonated eggs is still predominantly used worldwide, it has significant drawbacks. Egg‐based vaccines may cause an allergic reaction to albumin, and when the demand for embryonated eggs suddenly increases, for example, during a pandemic, the supply may be insufficient, hampering timely vaccine production. 54 , 55 Most importantly, consecutive virus passaging in embryonated eggs, especially in the case of H3N2, can result in egg‐adaptive mutations of the antigenic site, leading to altered antigenicity and thus reduced vaccine effectiveness. 56 , 57 , 58 To overcome these shortcomings, cell‐culture‐based vaccine production technologies were developed. Cell lines used include Madin–Darby canine kidney (MDCK) by Solvay and Seqirus (formerly Novartis), 59 , 60 PER.C6 by Sanofi Pasteur (formerly Crucell), 61 Vero by Baxter 62 and Sf9 insect cells combined with baculovirus vectors by Protein Science (recombinant HA), and Novavax (VLP vaccine). 63 , 64 Thus, influenza vaccine development is gradually moving away from the conventional egg‐based platform to the cell culture (Figure 2), though efforts to increase yield and lower production costs of the latter are needed.
FIGURE 2.
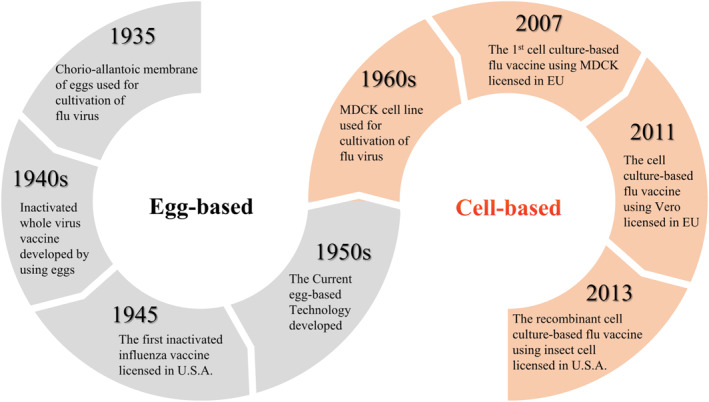
Evolution of influenza vaccine substrates from embryonated eggs to cells. In the 1930s, a method for cultivating influenza viruses in embryonated eggs was developed, and until the 1950s, methods for manufacturing influenza vaccines using embryonated eggs were continuously developed. Since the MDCK cell line was established in the late 1950s, the facts that the influenza viruses could be cultivated in various cells such as MDCK and Vero cells were revealed, and gradually the substrate for manufacturing of the influenza vaccine began to change from embryonated eggs to cells. MDCK, Madin–Darby canine kidney
2.3. FDA‐licensed influenza vaccines and the immune responses they trigger
The immune responses triggered by vaccines differ according to whether the inoculum is an inactivated antigen or a live virus. In the case of an inactivated viral vaccine, the injected inactivated extracellular antigen is engulfed through phagocytosis by antigen‐presenting cells (APCs), such as dendritic cells. The antigen is then degraded to peptides in the lysosomes of APCs, and the peptides are presented on the surface of APCs by major histocompatibility complex (MHC) II molecules. The MHC II–antigen complex is recognized by T helper (Th) cells, also known as CD4+ T cells, which causes the activation of naïve Th cells. The activated Th cells are further differentiated into T‐helper 1 (Th1) cells, Th2 cells, Th17 cells, follicular Th (Tfh) cell, induced regulatory T cells (iTregs), and others. 65
Among the various subsets of CD4+ effector T cells, Th1, Th2 and Th17 cells play major roles in defense against pathogens. Th1 cells, which induce a cell‐mediated immune response, secrete IFN‐γ and tumour necrosis factor‐alpha (TNF‐α), which activate macrophages and neutrophils that eliminate intracellular pathogens through increased phagocytosis and inflammation. Th2 cells, which induce a humoral immune response, mainly secrete IL‐4, IL‐5 and IL‐13. Lastly, Th17 cells secrete IL‐17 and IL‐22, which are pro‐inflammatory cytokines, as well as IL‐21, thereby stimulating immune responses against extracellular pathogens and fungi. 65 , 66 , 67 , 68 , 69
In contrast, a live virus or attenuated live virus vaccine infects cells and produces viral proteins in the cytosol of infected cells. The viral proteins are degraded to peptides by the proteasome, and the peptides are presented on the surface of infected cells in complex with MHC I molecules. The MHC I–antigen complex is recognized by CD8+ T cells, which subsequently differentiate into cytotoxic T lymphocytes (CTLs) with other signals and the help of Th cells. Dendritic cells are not easily infected by viruses, but play an important role in the activation of CD8+ T cells through cross‐presentation. In this pathway, virus‐infected cells are ingested by dendritic cells leading to the release of viral antigens in the cytosol of dendritic cells. Subsequent processes from degradation in the proteasome to the presentation are identical to the MHC I pathway described above. 70 When CTLs recognize the MHC I–antigen complex on infected cells, they release cytotoxic proteins, perforin and granzyme, which enter the infected cells and induce apoptosis. CTLs can also kill infected cells through the interaction of Fas ligand expressed on CTLs and Fas expressed on target cells. 67 , 69 Various types of influenza vaccines have been approved by the FDA (Table 1), and they cause different immune responses. Based on the mechanism of immune response induction, influenza vaccines are largely categorized into IIVs and LAIVs, and IIVs are further classified into whole, split and subunit types.
TABLE 1.
The list of FDA‐licensed seasonal influenza vaccines for use in the United States
| Manufacturing platform | Vaccine type | Trade name | Manufacturer | Dose | Age | Remark |
|---|---|---|---|---|---|---|
| Egg platform | Split | Afluria (TIV, QIV) | Seqirus Pty Ltd. | 15 µg/strain | ≥6 months old | ‐ |
| FluLaval (TIV/QIV) | ID Biomedical Corporation of Quebec (a division of GlaxoSmithKline) | 15 µg/strain | ≥6 months old | ‐ | ||
| Fluarix (TIV/QIV) | GlaxoSmithKline Biologicals | 15 µg/strain | TIV: ≥3 years old, | ‐ | ||
| QIV: ≥6 months old | ||||||
| Fluzone (TIV/QIV) | Sanofi Pasteur, Inc | 15 µg/strain | ≥6 months old | ‐ | ||
| Fluzone intradermal (TIV/QIV) | Sanofi Pasteur, Inc | 9 µg/strain | 18–64 years old | 0.1 ml dose | ||
| Fluzone high‐dose (TIV/QIV) | Sanofi Pasteur, Inc | 60 µg/strain | ≥65 years old | ‐ | ||
| Subunit | Agriflu (TIV) | Seqirus Inc. | 15 µg/strain | ≥18 years old | ‐ | |
| Fluvirin (TIV) | Seqirus vaccines limited | 15 µg/strain | ≥4 years old | ‐ | ||
| FLUAD (TIV/QIV) | Seqirus, Inc. | 15 µg/strain | ≥65 years old | MF59C.1 adjuvant | ||
| Live attenuated | FluMist (TIV/QIV) | MedImmune, LLC | 106.5–7.5 FFU/strain | 2–49 years old | ‐ | |
| Cell platform | Subunit | Flucelvax (TIV/QIV) | Seqirus, Inc. | 15 µg/strain | ≥4 years old | MDCK cells |
| Recombinant | Flublok (TIV/QIV) | Protein Sciences Corporation | 45 µg/strain | ≥18 years old | Insect cells (expresSF+®) |
Abbreviations: FFU, fluorescent focus units; TIV, trivalent; QIV, quadrivalent.
As the active substances of IIVs are three (trivalent IIVs) or four (quadrivalent IIVs) inactivated antigens, inoculated antigens are mainly presented on the surface of APCs through the MHC II pathway and thus CD4+ T cells will be mainly stimulated; however, the immune responses following IIV inoculation are not yet fully understood. 71 As mentioned above, CD4+ T cells can differentiate into several subtypes, but IIVs predominantly stimulate Th2 immune responses, inducing a humoral immune response rather than a cellular immune response. A haemagglutination inhibition titre, which correlates with influenza‐specific antibodies, of more than 1:40 induced by IIV provided a 50% protection rate against influenza infection in adults. 72 , 73 However, IIV does not effectively induce mucosal immunity and cellular immunity, which plays an important role in respiratory viral infection. Unlike IIVs, LAIVs are administered via the intranasal route. They infect cells of the upper respiratory tract and are more efficiently processed through the MHC I pathway, and thus induce mucosal immunity and cellular immunity. Further, Th1 immune responses are well activated by CD4+ T cells in the case of LAIVs. 74 , 75 Although LAIV can be considered as an ideal vaccine type for influenza because it induces balanced Th1/Th2 immune responses, it has safety concerns and cannot be administered in very young children and immunocompromised individuals. 76 Moreover, it is not easy to develop LAIVs for avian influenza viruses since they show greater tropism in the lower than in the upper human respiratory tract. 77
3. STATUS OF INFLUENZA VACCINE ADJUVANT DEVELOPMENT
3.1. The need for adjuvanted influenza vaccines
Adjuvants are agents that boost the immune response of a vaccine, inducing higher antibody production and longer‐lasting protection with lower amounts of antigen per dose, 78 thereby reducing the burden of vaccine production.
Unlike seasonal influenza viruses, where the same subtypes circulate continuously in the human population, influenza viruses that cause pandemics are new reassortant or variant viruses against which humans have no pre‐existing antibodies, thus resulting in higher mortality rates. 79 Non‐adjuvanted pandemic influenza vaccines against H5N1 and H7N9 induced weak immune responses, possibly because most people did not have pre‐existing immunity against those subtypes, and pandemic vaccines are not administered yearly. 80 , 81 Thus, pandemic influenza vaccines are usually adjuvanted, especially because they improve immune responses in individuals who are immunocompromised due to chronic disease, obesity, HIV infection or transplant treatment. 82 , 83 , 84 , 85 Furthermore, unlike unadjuvanted IIV, adjuvanted IIV can confer a balanced Th1/Th2 immune response and mucosal immunity, which protect against influenza infection. 86
3.2. Immune mechanism of licensed adjuvants for influenza vaccine
Adjuvants currently used in influenza vaccines include aluminium phosphate (AlPO4) gel, aluminium hydroxide (Al[OH]3), AS03 and MF59. 86 The oldest and most commonly used adjuvants are AlPO4 and Al(OH)3, commonly called alum (although strictly speaking, alum refers to potassium aluminium sulphate (KAl[SO4]2). Influenza vaccines containing MF59 or AS03, which are oil‐in‐water emulsion adjuvants developed later than alum, were approved in 1997 and 2009, respectively. 87 , 88 These adjuvants are more effective than alum in IIVs. 89 , 90 Two out of the three H5N1 pandemic influenza vaccines that have been licensed by US FDA contain AS03 or MF59 adjuvant. The third vaccine does not contain adjuvant, but contains 12–24 times more HA than the adjuvanted vaccines (Table 2). The FDA‐approved adjuvanted seasonal influenza subunit vaccine (Seqirus, FluAd®) contains MF59 and is intended for use in the elderly (>65 years), who have poorer immune responses than young adults (Table 2). 86 , 91
TABLE 2.
The list of FDA‐licensed adjuvanted influenza vaccines for use in the United States
| Vaccine type | Product name | Trade name | Manufacturer | Dose | Age | Adjuvant |
|---|---|---|---|---|---|---|
| Pandemic | Influenza virus vaccine, H5N1 a | ‐ | Sanofi Pasteur, Inc. | 90 µg/strain | 18–64 years old | ‐ |
| Influenza A (H5N1) virus monovalent vaccine, adjuvanted | ‐ | ID Biomedical Corporation of Quebec | 1.9 µg/strain | 6 months–17 years old | AS03 | |
| 3.75 µg/strain | ≥18 years old | |||||
| Influenza A (H5N1) monovalent vaccine, adjuvanted | AUDENZ | Seqirus Inc. | 7.5 µg/strain | ≥6 months old | MF59C.1 | |
| Seasonal | Influenza vaccine, adjuvanted | FLUAD (TIV/QIV) | Seqirus, Inc. | 15 µg/strain | ≥65 years old | MF59C.1 |
Abbreviations: TIV, Trivalent; QIV, Quadrivalent.
Reference for comparing dose with adjuvanted vaccine.
While alum has since long been widely used as an adjuvant, its mechanism remains unknown. 92 Originally, alum was thought to enhance immune responses by slowly and continuously exposing the antigen to APCs through the so‐called antigen depot effect. 93 However, recent studies showed that alum also recruits various types of innate immune cells, including neutrophils and monocytes, to the injection site, thereby activating innate immune responses. 94 , 95 , 96 Studies on the cellular and molecular mechanisms have reported that alum triggers the activation of the NLRP3 inflammasome through phagosomal destabilization via alum phagocytosis by APCs, resulting in the secretion of the pro‐inflammatory cytokine IL‐1β. 97 , 98 , 99 , 100 In addition, alum causes necrosis at the injection site, resulting in the release of damage‐associated molecular patterns, such as uric acid and DNA, which activate the NLRP3 inflammasome. 95 , 101 Alum can enhance the immune response through prostaglandin E2 production, which is involved in inducing a Th2 immune response, via ITAM‐Syk‐PI3Kδ signalling. 102 These results suggest that alum induces a Th2 response rather than a Th1 response.
MF59 is an oil‐in‐water adjuvant consisting of squalene and two surfactants, polysorbate 80 (Tween 80) and sorbitan trioleate (Span 85). Since its first application in influenza vaccines in Europe in 1997, substantial research has been done to clarify its immune‐boosting mechanism. MF59 was shown to have no depot effect, 103 , 104 which was supported by the finding that it showed an adjuvant effect even when injected 24 h before to 1 h after antigen injection. 105 Instead, MF59 activates monocytes, macrophages and granulocytes at the injection site, resulting in the secretion of chemokines, followed by immune cell recruitment, increased antigen uptake, differentiation of monocytes into immature dendritic cells and enhanced antigen transport to the draining lymph nodes. 106 , 107 The differentiation of monocytes into immature dendritic cells induced by MF59 facilitates their migration to the draining lymph nodes, where they stimulate T cells and B cells, leading to the enhancement of the adaptive immune response with a balanced Th1/Th2 response to the administered vaccine antigen. 108 In addition, MF59‐adjuvanted antigen was presented on various cells, including B cells, monocytes and neutrophils in the lymph nodes of immunized mice, whereas alum‐adjuvanted antigen was presented only on dendritic cells, 96 indicating that MF59 is more efficient in enhancing the immune response than alum. MF59 causes muscle cells at the injection site to release ATP and exerts its adjuvant function through MyD88. 109 , 110 In an animal model, hydrolysis of ATP by locally administered apyrase led to reduced T‐cell responses and haemagglutination inhibition titres in response to MF59‐adjuvanted trivalent IIV. 111 Thus, ATP plays an important role in MF59 adjuvant function; however, the exact signalling pathway requires further study.
AS03 is an oil‐in‐water adjuvant consisting of polysorbate 80 (Tween 80) and two biodegradable oils, squalene and α‐tocopherol, which is the most bioavailable form of vitamin E. 112 Dietary vitamin E supplementation has an immunostimulatory effect. 113 Accordingly, α‐tocopherol exhibits an adjuvant‐like function, whereas the MF59 components do not have an adjuvant effect per se. A study in mice showed that immune responses were weakened when the antigen was administered with AS03 lacking α‐tocopherol. 114 Unlike MF59, AS03 requires spatiotemporal co‐localization with the antigen to function as adjuvant. Similar to MF59, AS03 promotes immune cell recruitment to the injection site, and antigen uptake and transport to the draining lymph nodes. 114 In detail, AS03 induces a local and transient increase in NF‐κB and enhances cytokine release and immune cell recruitment. 115 , 116 Subsequently, activated and antigen‐loaded APCs activate CD4+ T cells in the draining lymph nodes, which directly stimulate antigen‐specific B cells. Thus, AS03 strengthens the immune response by inducing a high number of memory B cells and antibody‐secreting plasma cells. 116
3.3. Development status of influenza vaccine adjuvants
Adjuvants are widely studied worldwide. In what follows, we discuss the mechanisms and immune responses of influenza vaccine adjuvants other than alum, MF59 and AS03 that have reached clinical trials, including TLR ligands, cytokines, and micro‐ and nanoemulsions. In addition, there are immunostimulators of which the mechanisms are not precisely known. Among these, TLR agonists and formulations are the most studied (Table 3).
TABLE 3.
Adjuvants for influenza vaccine in clinical trials
| Adjuvant type | Adjuvant name | Adjuvant description | Effect on immune response | Status | Registration number | Reference |
|---|---|---|---|---|---|---|
| TLRs ligand | dsRNA | TLR3 agonist | Improved protection effect in challenge study compared to non‐adjuvanted group | Phase II | NCT02918006 | 117 |
| GLA‐AF | TLR4 agonist | Increased HI titer | Phase I | NCT01657929 | 118 | |
| MPLA | TLR4 ligand monophosphoryl lipid A | Dose‐sparing effect | Phase I | NCT01111968 | 119 | |
| ND002 | Pantoea agglomerance‐derived LPS (TLR4 agonist) | Data not shown | Phase I | NCT02955030 | N/A | |
| Vax128 | Haemagluttinin‐flagellin fusion (TLR5 agonist) | Induced immune response (no non‐adjuvanted group) | Phase I | NCT01172054 | 120 | |
| Imiquimod | a synthetic TLR7 agonist | Significantly improved immunogenicity | Phase Ⅲ | NCT02103023 | 121 | |
| CpG7909 | TLR9 agonist | Dose‐sparing effect | Phase I | NCT00559975 | 122 | |
| Micro‐ and nanoemulsion | JVRS‐100 | Cationic liposome‐DNA complexes | Data not shown | Phase II | NCT00936468 | N/A |
| IB160 | Squalene based oil‐in‐water emulsion | Data not shown | Phase I | NCT03330899 | N/A | |
| MAS‐1 | Nanoparticular, emulsion‐based | Data not shown | Phase I | NCT02500680 | N/A | |
| Matrix‐M1 | Saponins formulated with cholesterol and phospholipids into nanoparticles | Enhanced antibody response | Phase Ⅲ | NCT04120194 | 123 | |
| CCS/C | Polycationic sphingolipid complexed with cholesterol | Data not shown | Phase II | NCT00915187 | N/A | |
| ISCOMATRIX™ | A particulate adjuvant comprising cholesterol, phospholipid and saponin | Data not shown | Phase I | NCT00851266 | N/A | |
| AS25, AS50, | Oil‐in‐water emulsion containing MPL, | Increased influenza‐specific | Phase II | NCT00318149 | N/A | |
| AS01B, AS01E | Liposomal adjuvant containing MPL‐A | CD4 T cell responses | ||||
| SE | 2% oil‐in‐water stable emulsion | Dose‐sparing effect | Phase I/II | NCT02464163 | 124 | |
| Montanide ISA‐51 | Water‐in‐oil | Induced immune response (no non‐adjuvanted group) | Phase II | NCT03180801, NCT02962908 | 125 | |
| W805EC | Nanoemulsion‐based adjuvant | Improved mucosal immunity | Phase I | NCT01333462 | 126 | |
| EndocineTM | Liposome‐based adjuvant | Data related to immune response not shown | Phase I/II | NCT02998996 | N/A | |
| PAL a | Papaya mosaic virus nanoparticle | Dose‐sparing, improved CMI response | Phase I | NCT02188810 | 127 | |
| Immuno‐stimulator | LT Adjuvant patch | Heat labile enterotoxin from E. coli | Enhanced immune response | Phase I/II | NCT00532792 | 128 |
| AD07010 | Heat‐labile enterotoxin (LT)‐derived from E. coli | Improved mucosal immunity | Phase II | NCT03784885 | 129 | |
| Advax‐CpG55.2 | Combination adjuvant (Advax + CpG55.2) | N/A (active, not recruiting) | Phase I | NCT03945825 | N/A | |
| Advax | Polysaccharide adjuvant based on delta inulin | Increased seroprotection rate | Phase I/II | ACTRN 12609000674235& | 130 | |
| Cytokine | IFN‐α | Type I interferon | No adjuvant effect | Phase I | NCT00436046 | 131 |
Note: Searched at ClinicalTrials.gov (Search criteria: Condition or disease is influenza vaccine and other terms is adjuvant) except for &, Australia New Zealand Clinical Trial Registry. When there were multiple studies using the same adjuvant, only the higher clinical stage was indicated.
Although PAL is not an emulsion type, it has a similar form as nanoparticle, so it is classified in this category.
TLRs stimulate innate immune responses by recognizing pathogen‐associated molecular patterns. Based on their localization, they are classified into cell‐surface TLRs and intracellular TLRs. Cell‐surface TLRs, including TLR1, TLR2, TLR4, TLR5, TLR6 and TLR10, mostly recognize components derived from microbial membranes. Intracellular TLRs, which are localized in the endosome, including TLR3, TLR7, TLR8, TLR9, TLR11, TLR12 and TLR13, recognize self and non‐self nucleic acids. 132 Given that various TLRs can recognize unique ligands, several TLR agonists are in clinical trials as adjuvants. The TLR4 agonists AS01 and AS04 are licensed, while other TLR4 agonists, including glucopyranosyl lipid adjuvant, monophosphoryl lipid (MPLA), and LPS, are actively being studied. 118 , 119 In addition, dsRNA, flagellin, imiquimod and CpG are being developed as TLR3, TRL5, TLR7 and TLR9 agonists, respectively. 117 , 120 , 121 , 122
Emulsion particles such as MF59 and AS03 are sometimes combined with other substances such as saponin, DNA and MPLA. Various emulsion‐type adjuvants with different components and ratios are in clinical trials (Table 3). 123 , 124 , 125 , 126 Other particle types, such as virosome or VLP, may also have an adjuvant function. 127 Several immunostimulators of which the mechanisms of action are not yet known are also being studied. 128 , 129 , 130 By the way, cytokine, which stimulates the Th1 immune response and B lymphocyte differentiation in mice, showed no adjuvant effect in phase I clinical trials. 131 Thus, it is important to develop a non‐clinical system that can accurately predict adjuvant effects. In a recent study, an IIV formulated with a single‐stranded RNA adjuvant induced cross‐protection against heterologous influenza virus infection and mucosal immune response. 133 The detailed mechanism and safety aspects remain to be studied.
4. DIRECTIONS FOR INFLUENZA VACCINE DEVELOPMENT
4.1. Current status and adverse events associated with influenza vaccines
The influenza vaccines currently on the market can be administered to very young children (≥6 months of age), although the recommended age for vaccination differs for each product, and most of them are inactivated vaccines. They primarily induce Th2 immune responses and lead to the production of specific antibodies against the administered influenza virus strains, thereby conferring immune protection. However, the effectiveness of the influenza vaccines investigated over the last decade is not high, with an average protection rate of 42% (range, 19%–60%), 16 and generally is even lower in young children and the elderly.
To overcome the low antibody production rate in young children and the elderly, vaccine manufacturers have increased the standard HA antigen content of seasonal influenza vaccines two times for young children (≥6 months; Flulaval Trivalent/Quadrivalent, Fluarix Quadrivalent, Fluzone Quadrivalent) and four times for the elderly (≥65 years; Fluzone High‐Dose) or have applied an adjuvant (only for the elderly; FLUAD). These products are FDA‐licensed, and the improved efficacy or effectiveness has been proven. 134 , 135 , 136 However, the required increase in antigen production can pose a burden to the manufacturers, especially in emergencies such as pandemics.
Alternatively, immune responses to influenza vaccines can be enhanced by administering LAIV instead of IIV as mentioned earlier, or by applying new strategies, such as using the intradermal route instead of injection (Sanofi Pasteur's Fluzone® Intradermal influenza vaccine), as the skin is rich in APCs. Despite the same IIV, the intradermal route induced a non‐inferior protective immune response with a smaller amount of HA antigen (9 µg per strain) compared to an intramuscular route. 137 , 138 However, FDA‐licensed products using these approaches are also not yet applicable to young children or the elderly (Table 1). The LAIV FluMist is approved for use in persons 2–49 years of age, and Fluzone intradermal, which is injected intradermally, is approved for use in persons 18–64 years of age.
The adverse events associated with the influenza vaccine vary from mild symptoms, such as erythema from the shot, headache, fever, nausea, and myalgia to unusual events, such as severe allergic reaction, Guillain–Barré syndrome and oculo‐respiratory syndrome. Most of the adverse events associated with influenza vaccines are mild and easy to recover. 139 , 140 However, the Pandemrix vaccine against the 2009 H1N1 influenza pandemic is considered to be associated with narcolepsy, 141 , 142 and this narcolepsy caused by Pandemrix is linked to autoimmune disease, but the mechanism remains unknown. 143 , 144
4.2. Directions for influenza vaccine development
For influenza vaccines, seroprotection, seroconversion and geometric mean titre ratio are criteria considered for obtaining approval as a commercial product. When evaluating the efficacy of a vaccine, the focus is on a Th2 immune response rather than a Th1 immune response. However, Th1 and mucosal immune responses also play important roles in the defence against respiratory viruses such as influenza virus. 145 Therefore, an influenza vaccine that induces balanced Th1/Th2 immune responses, including cell‐mediated and antibody responses and mucosal immune responses involving secretory IgA in all age groups, including infants and the elderly, could significantly reduce the influenza mortality and morbidity rates.
As mentioned earlier, many attempts have been made to increase the efficacy of influenza vaccines using various platforms, including viral vectors, DNA vectors, VLP or peptide vaccines, which are expected to overcome the limitations of current influenza vaccines, for example, by inducing a more dominant Th1 response and a memory response. Moreover, considering that the antigenicity of influenza vaccine strains changes almost yearly and a wide variety of influenza subtypes can cause outbreaks due to antigen shift and drift, universal influenza vaccines are being actively studied. 146 One candidate, a recombinant protein M‐001 containing nine conserved epitopes from influenza A and B, is currently in a phase III clinical trial (NCT03450915), which is expected to be completed in December 2020. mRNA‐based vaccines established their potential during the SARS‐CoV‐2 pandemic 147 , 148 ; therefore, they are worth considering against an influenza pandemic as well. mRNA vaccines for influenza virus exhibited protective effects in mice, immunogenic responses and safety in human clinical trials, and cross‐protection effect by eliciting influenza virus HA stalk‐specific antibodies. 149 , 150 Adjuvants, such as AS03 and MF59, are already applied in influenza vaccines. However, influenza vaccines are inoculated yearly, and therefore, substantial effort should be made to develop new adjuvants with good safety for repeated administration and efficacy, such as RNA‐based adjuvants. These are as easily degradable as TLR3 or TLR7/8 ligands 133 and are relatively safe, when compared to other adjuvants that remain in the body for long time.
5. CONCLUSIONS
Influenza vaccines are somewhat complex. They induce different immune responses depending on the vaccine type, such as IIV versus LAIV, and the age at vaccination and their mechanisms in inducing immune responses have not been completely clarified. Most of the licensed vaccines induce mainly Th2‐type immune responses, and in young children and the elderly, they induce weaker immune responses than in adults. Therefore, efforts are needed to develop influenza vaccines that induce stronger and more balanced Th1/Th2 immune responses, based on our understanding of the immune system, which differs according to age. Current approval criteria for influenza vaccines, which focus on Th2 responses, will have to be modified accordingly to include confirmation of T‐cell‐mediated protection. Improved immune responses can also be achieved by using adjuvants, and thus, safer and more effective adjuvants should be developed. Finally, effective and reliable tools for predicting immune responses to vaccines and adjuvants would greatly help increase the protection rate against influenza virus infection, not only in adults but also in young children and the elderly.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Yun‐Hee Kim wrote and designed the first draft, Kee‐Jong Hong and Hun Kim provided advice for improving the manuscript, and Jae‐Hwan Nam edited and re‐wrote the manuscript. All authors have read and approved the submitted version.
ACKNOWLEDGEMENTS
This work was supported by the Catholic University of Korea, Research Fund, 2020, and partially supported by the Brain Korea 21 Plus Program. We thank Sunghwan Bae for the graphic illustration. Data sharing is not applicable to this article because no new data were created or analysed in this study.
Kim Y‐H, Hong K‐J, Kim H, Nam J‐H. Influenza vaccines: past, present, and future. Rev Med Virol. 2022;32(1):e2243. 10.1002/rmv.2243
REFERENCES
- 1. Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. 2008;26(Suppl 4):49‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hause BM, Ducatez M, Collin EA, et al. Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses. PLoS Pathog. 2013;9(2):e1003176. 10.1371/journal.ppat.1003176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharabi S, Drori Y, Micheli M, et al. Epidemiological and virological characterization of influenza B virus infections. PLoS One. 2016;11:e0161195. 10.1371/journal.pone.0161195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verma R, Khanna P, Chawla S. Influenza vaccine: an effective preventive vaccine for developing countries. Hum Vaccines Immunother. 2012;8:675‐678. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention , National Center for Immunization and Respiratory Diseases (NCIRD), Influenza Type A Viruses. 2017. https://www.cdc.gov/flu/avianflu/influenza‐a‐virus‐subtypes.htm. Accessed April 19, 2017. [Google Scholar]
- 6. Tong S, Zhu X, Li Y, et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9:e1003657. 10.1371/journal.ppat.1003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petrova VN, Russell CA. The evolution of seasonal influenza viruses. Nat Rev Microbiol. 2018;16:47‐60. [DOI] [PubMed] [Google Scholar]
- 8. WHO Ask The Expert: Influenza Q&A. 2018. https://www.who.int/news‐room/fact‐sheets/detail/influenza‐(seasonal) [Google Scholar]
- 9. Thompson WW, Weintraub E, Dhankhar P, et al. Estimates of US influenza‐associated deaths made using four different methods. Influenza and other respiratory viruses. 2009;3:37‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta‐analysis. Lancet. 2011;378:1917‐1930. [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention , National Center for Immunization and Respiratory Diseases (NCIRD), Influenza Antiviral Medications: Summary for Clinicians. 2021. https://www.cdc.gov/flu/professionals/antivirals/summary‐clinicians.htm. Accessed January 25, 2021. [Google Scholar]
- 12. Onions D, Egan W, Jarrett R, et al. Validation of the safety of MDCK cells as a substrate for the production of a cell‐derived influenza vaccine. Biologicals. 2010;38:544‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Mol Biol Rev. 1992;56:152‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science. 2008;320:340‐346. [DOI] [PubMed] [Google Scholar]
- 15. BARBERIS I, MYLES P, Ault SK, Bragazzi NL, Martini M. History and evolution of influenza control through vaccination: from the first monovalent vaccine to universal vaccines. J Prev Med Hygiene. 2016;57:115‐120. [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention , National Center for Immunization and Respiratory Diseases (NCIRD) . CDC Seasonal Flu Vaccine Effectiveness Studies. 2020. https://www.cdc.gov/flu/vaccines‐work/effectiveness‐studies.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fflu%2Fprofessionals%2Fvaccination%2Feffectiveness‐studies.htm. Accessed December 11, 2020. [Google Scholar]
- 17. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159‐1169. [DOI] [PubMed] [Google Scholar]
- 18. Luo SY, Zhu JL, Lyu MZ, et al. Evaluation of the influenza vaccine effectiveness among children aged 6 to 72 months based on the test‐negative case control study design. Zhonghua Yu Fang Yi Xue Za Zhi [Chin J Prev Med]. 2019;53:576‐580. [DOI] [PubMed] [Google Scholar]
- 19. Smith W, Andrewes CH, Laidlaw PP. A virus obtained from influenza patients. Lancet. 1933; 66‐68. [Google Scholar]
- 20. Smith W, Cultivation of the virus of influenza. Br J Exp Pathol. 1935;16:508. [Google Scholar]
- 21. Francis T, Magill TP. Cultivation of human influenza virus in an artificial medium. Science. 1935;82:353‐354. [DOI] [PubMed] [Google Scholar]
- 22. Magill TP, Francis T, Jr , Studies with human influenza virus cultivated in artificial medium. J Exp Med. 1936;63:803‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stokes J, Chenoweth AD, Waltz AD, Gladen RG, Shaw D. Results of immunization by means of active virus of human influenza. J Clin Investig. 1937;16:237‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stokes J, Jr , McGuinness AC, Langner PH, Jr , Shaw DR. Vaccination against epidemic influenza with active virus of human influenza. A two‐year study. Am J Med Sci. 1937;194:757‐768. [Google Scholar]
- 25. Stuart‐Harris CH, Andrewes CH, Smith W, Chalmers DKM, Cowen EGH, Hughes DL. A study of epidemic influenza: with special reference to the 1936‐7 epidemic. Special report series. Medi Res Council. 1938;228:151. [Google Scholar]
- 26. Taylor RM, Dreguss M. An experiment in immunization against influenza with a formaldehyde‐inactivated virus. Am J Hyg. 1940;31:31‐35. [Google Scholar]
- 27. Hirst GK, Rickard ER, Whitman L, Horsfall FL, Jr . Antibody response of human beings following vaccination with influenza viruses. J Exp Med. 1942;75:495‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirst GK, Rickard ER, Whitman L. A new method for concentrating influenza virus from allantoic fluid. PSEBM (Proc Soc Exp Biol Med). 1942;50:129‐133. [Google Scholar]
- 29. Salk JE, Pearson HE, Brown PN, Smyth CJ, Francis T, Jr . Immunization against influenza with observations during an epidemic of influenza A one year after vaccination. Am J Epidemiol. 1945;42:307‐322. [DOI] [PubMed] [Google Scholar]
- 30. Salk JE, Menke WJ, Jr , Francis T, Jr . A clinical, epidemiological and immunological evaluation of vaccination against epidemic influenza. Am J Hygiene. 1945;42:57‐93. [Google Scholar]
- 31. Centers for Disease Control and Prevention , National Center for Immunization and Respiratory Diseases (NCIRD) . Influenza Historic Timeline. 2019. https://www.cdc.gov/flu/pandemic‐resources/pandemic‐timeline‐1930‐and‐beyond.htm. Accessed January 30, 2019. [Google Scholar]
- 32. Barrett AD, Stanberry LR. Vaccines for Biodefense and Emerging and Neglected Diseases. 1st ed. Elsvier Inc. 2009:498‐518. [Google Scholar]
- 33. Sekiya T, Mifsud EJ, Ohno M, et al. Inactivated whole virus particle vaccine with potent immunogenicity and limited IL‐6 induction is ideal for influenza. Vaccine. 2019;37:2158‐2166. [DOI] [PubMed] [Google Scholar]
- 34. Parkman PD, Hopps HE, Rastogi SC, Meyer HM, Jr . Summary of clinical trials of influenza virus vaccines in adults. J Infect Dis. 1977;136(Suppl_3):722‐730. [DOI] [PubMed] [Google Scholar]
- 35. Brady MI, Furminger IGS. A surface antigen influenza vaccine: 2. Pyrogenicity and antigenicity. Epidemiol Infect. 1976;77:173‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duxbury AE, Hampson AW, Sievers JGM. Antibody response in humans to deoxycholate‐treated influenza virus vaccine. J Immunol. 1968;101:62‐67. [PubMed] [Google Scholar]
- 37. Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015;14:167‐182. [DOI] [PubMed] [Google Scholar]
- 38. Bachmayer H, Liehl E, Schmidt G. Preparation and properties of a novel influenza subunit vaccine. Postgrad Med J. 1976;52:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brady MI, Furminger IGS. A surface antigen influenza vaccine. 1. Purification of haemagglutinin and neuraminidase proteins. Epidemiol Infect. 1976;77:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gross PA, Ennis FA, Gaerlan PF, Denson LJ, Denning CR, Schiffman D. A controlled double‐blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole‐virus and split‐product influenza vaccines in children. J Infect Dis. 1977;136:623‐632. [DOI] [PubMed] [Google Scholar]
- 41. Gross PA, Ennis FA. Influenza vaccine: split‐product versus whole‐virus types—how do they differ? N Engl J Med. 1977;296:567‐568. [DOI] [PubMed] [Google Scholar]
- 42. Smorodintseff AA, Tushinsky MD, Drobyshevskaya AI, Korovin AA, Osetroff AI. Investigation on volunteers infected with the influenza virus. Am J Med Sci. 1937;194:159‐170. [Google Scholar]
- 43. Beare AS, Bynoe ML, Tyrrell DAJ. Investigation into the attenuation of influenza viruses by serial passage. Br Med J. 1968;4:482‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maassab HF. Adaptation and growth characteristics of influenza virus at 25 C. Nature. 1967;213:612‐614. [DOI] [PubMed] [Google Scholar]
- 45. Isakova‐Sivak I, Rudenko L. Safety, immunogenicity and infectivity of new live attenuated influenza vaccines. Expert Rev Vaccine. 2015;14:1313‐1329. [DOI] [PubMed] [Google Scholar]
- 46. Boivin S, Cusack S, Ruigrok RW, Hart DJ. Influenza A virus polymerase: structural insights into replication and host adaptation mechanisms. J Biol Chem. 2010;285:28411‐28417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ziegler T, Mamahit A, Cox NJ. 65 years of influenza surveillance by a World Health Organization‐coordinated global network. Influenza Other Respir Viruses. 2018;12:558‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hay AJ, McCauley JW. The WHO global influenza surveillance and response system (GISRS)—a future perspective. Influenza Other Respir Viruses. 2018;12:551‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine. 2012;30:1993‐1998. [DOI] [PubMed] [Google Scholar]
- 50. Darricarrère N, Qiu Y, Kanekiyo M, et al. Broad neutralization of H1 and H3 viruses by adjuvanted influenza HA stem vaccines in nonhuman primates. Sci Transl Med. 2021;13:eabe5449. 10.1126/scitranslmed.abe5449 [DOI] [PubMed] [Google Scholar]
- 51. Nachbagauer R, Krammer F. Universal influenza virus vaccines and therapeutic antibodies. Clin Microbiol Infect. 2017;23:222‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shinde V, Cho I, Plested JS, et al. Comparison of the Safety and Immunogenicity of a Novel Matrix‐M‐Adjuvanted Nanoparticle Influenza Vaccine with a Quadrivalent Seasonal Influenza Vaccine in Older Adults: A Randomized Controlled Trial. medRxiv. 2020. 10.1101/2020.08.07.20170514 [DOI] [PubMed] [Google Scholar]
- 53. Pillet S, Aubin É, Trépanier S, et al. A plant‐derived quadrivalent virus like particle influenza vaccine induces cross‐reactive antibody and T cell response in healthy adults. Clin Immunol. 2016;168:72‐87. [DOI] [PubMed] [Google Scholar]
- 54. Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev. 2013;26:476‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hannoun C. The evolving history of influenza viruses and influenza vaccines. Expert Rev Vaccine. 2013;12:1085‐1094. [DOI] [PubMed] [Google Scholar]
- 56. Wu NC, Lv H, Thompson AJ, et al. Preventing an antigenically disruptive mutation in egg‐based H3N2 seasonal influenza vaccines by mutational incompatibility. Cell Host Microbe. 2019;25:836‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg‐adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PloS One. 2014;9:e92153. 10.1371/journal.pone.0092153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parker L, Wharton SA, Martin SR, et al. Effects of egg‐adaptation on receptor‐binding and antigenic properties of recent influenza A (H3N2) vaccine viruses. J general virology. 2016;97:1333‐1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Palache AM, Brands R, Scharrenburg GV. Immunogenicity and reactogenicity of influenza subunit vaccines produced in MDCK cells or fertilized chicken eggs. J Infect Dis. 1997;176(Suppl_1):20‐23. [DOI] [PubMed] [Google Scholar]
- 60. Milián E, Kamen AA. Current and emerging cell culture manufacturing technologies for influenza vaccines. BioMed Res Int. 2015;2015:504831. 10.1155/2015/504831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pau MG, Ophorst C, Koldijk MH, et al. The human cell line PER. C6 provides a new manufacturing system for the production of influenza vaccines. Vaccine. 2001;19:2716‐2721. [DOI] [PubMed] [Google Scholar]
- 62. Kistner O, Barrett PN, Mundt W, Reiter M, Schober‐Bendixen S, Dorner F. Development of a mammalian cell (Vero) derived candidate influenza virus vaccine. Vaccine. 1998;16:960‐968. [DOI] [PubMed] [Google Scholar]
- 63. King JC, Jr , Cox MM, Reisinger K, Hedrick J, Graham I, Patriarca P. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok® trivalent recombinant baculovirus‐expressed hemagglutinin influenza vaccine administered intramuscularly to healthy children aged 6–59 months. Vaccine. 2009;27:6589‐6594. [DOI] [PubMed] [Google Scholar]
- 64. López‐Macías C, Ferat‐Osorio E, Tenorio‐Calvo A, et al. Safety and immunogenicity of a virus‐like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo‐controlled trial of adults in Mexico. Vaccine. 2011;29:7826‐7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+ T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. 10.1155/2012/925135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 9th ed. Elsevier Inc. 2017:225‐242. [Google Scholar]
- 68. Jiskoot W, Kersten GFA, Mastrobattista E, Slütter B. Vaccines. Pharm Biotechnol. 2019:281‐304. 10.1007/978-3-030-00710-2 [DOI] [Google Scholar]
- 69. Yang L, Li W, Kirberger M, Liao W, Ren J. Design of nanomaterial based systems for novel vaccine development. Biomaterials Sci. 2016;4:785‐802. [DOI] [PubMed] [Google Scholar]
- 70. Joffre OP, Segura E, Savina A, Amigorena S. Cross‐presentation by dendritic cells. Nat Rev Immunol. 2012;12:557‐569. [DOI] [PubMed] [Google Scholar]
- 71. Sridhar S, Brokstad KA, Cox RJ. Influenza vaccination strategies: comparing inactivated and live attenuated influenza vaccines. Vaccines. 2015;3:373‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hobson DRAA, Curry RL, Beare AS, Ward‐Gardner A. The role of serum haemagglutination‐inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. Epidemiol Infect. 1972;70:767‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103:133‐138. [DOI] [PubMed] [Google Scholar]
- 74. Belshe R, Lee MS, Walker RE, Stoddard J, Mendelman PM. Safety, immunogenicity and efficacy of intranasal, live attenuated influenza vaccine. Expert Rev Vaccine. 2004;3:643‐654. [DOI] [PubMed] [Google Scholar]
- 75. Lanthier PA, Huston GE, Moquin A, et al. Live attenuated influenza vaccine (LAIV) impacts innate and adaptive immune responses. Vaccine. 2011;29:7849‐7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Centers for Disease Control and Prevention , National Center for Immunization and Respiratory Diseases (NCIRD) . Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). 2021. https://www.cdc.gov/flu/prevent/nasalspray.htm. Accessed January 25, 2021. [Google Scholar]
- 77. Shibya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;6:435‐436. [DOI] [PubMed] [Google Scholar]
- 78. Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006;12:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Clegg CH, Roque R, Van Hoeven N, et al. Adjuvant solution for pandemic influenza vaccine production. Proc Natl Acad Sci. 2012;109:17585‐17590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Castrucci MR. Factors affecting immune responses to the influenza vaccine. Hum Vaccines Immunother. 2018;14:637‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Paich HA, Sheridan PA, Handy J, et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity. 2013;21:2377‐2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liao Z, Tang H, Xu X, Liang Y, Xiong Y, Ni J. Immunogenicity and safety of influenza vaccination in systemic lupus erythematosus patients compared with healthy controls: a meta‐analysis. PLoS One. 2016;11:e0147856. 10.1371/journal.pone.0147856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ceravolo A, Orsi A, Parodi V, Rosselli R, Ansaldi F. Influenza vaccination in HIV‐positive subjects: latest evidence and future perspective. J Prev Med Hygiene. 2013;54:1‐10. [PMC free article] [PubMed] [Google Scholar]
- 85. Blumberg EA, Albano C, Pruett T, et al. The immunogenicity of influenza virus vaccine in solid organ transplant recipients. Clin Infect Dis. 1996;22:295‐302. [DOI] [PubMed] [Google Scholar]
- 86. Tregoning JS, Russell RF, Kinnear E. Adjuvanted influenza vaccines. Hum Vaccines Immunother. 2018;14:550‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. O’Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. The history of MF59® adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccine. 2013;12:13‐30. [DOI] [PubMed] [Google Scholar]
- 88. Laupèze B, Hervé C, Di Pasquale A, Da Silva FT. Adjuvant systems for vaccines: 13 years of post‐licensure experience in diverse populations have progressed the way adjuvanted vaccine safety is investigated and understood. Vaccine. 2019;37:5670‐5680. [DOI] [PubMed] [Google Scholar]
- 89. Ko EJ, Kang SM. Immunology and efficacy of MF59‐adjuvanted vaccines. Hum Vaccines Immunother. 2018;14:3041‐3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lin YJ, Shih YJ, Chen CH, Fang CT. Aluminum salts as an adjuvant for pre‐pandemic influenza vaccines: a meta‐analysis. Sci Rep. 2018;8:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Siegrist CA, Aspinall R. B‐cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185‐194. [DOI] [PubMed] [Google Scholar]
- 92. Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Glenny AT, Buttle GAH, Stevens MF. Rate of disappearance of diphtheria toxoid injected into rabbits and guinea pigs: toxoid precipitated with alum. J Pathol Bacteriol. 1931;34:267‐275. [Google Scholar]
- 94. Erdohazi M, Newman RL. Aluminium hydroxide granuloma. Br Med J. 1971;3:621‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kool M, Soullié T, van Nimwegen M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Calabro S, Tortoli M, Baudner BC, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29:1812‐1823. [DOI] [PubMed] [Google Scholar]
- 97. Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122‐1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase‐1 and induce IL‐1β and IL‐18 release. J Immunol. 2007;178:5271‐5276. [DOI] [PubMed] [Google Scholar]
- 99. Franchi L, Núñez G. The Nlrp3 inflammasome is critical for aluminium hydroxide‐mediated IL‐1β secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085‐2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Goto N, Kato H, Maeyama JI, et al. Local tissue irritating effects and adjuvant activities of calcium phosphate and aluminium hydroxide with different physical properties. Vaccine. 1997;15:1364‐1371. [DOI] [PubMed] [Google Scholar]
- 102. Kuroda E, Ishii KJ, Uematsu S, et al. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome‐independent mechanisms. Immunity. 2011;34:514‐526. [DOI] [PubMed] [Google Scholar]
- 103. Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59 design and evaluation of a safe and potent adjuvant for human vaccines. Vaccine Des. 1995;6:277‐296. [DOI] [PubMed] [Google Scholar]
- 104. Dupuis M, McDonald DM, Ott G. Distribution of adjuvant MF59 and antigen gD2 after intramuscular injection in mice. Vaccine. 1999;18:434‐439. [DOI] [PubMed] [Google Scholar]
- 105. Ott G, Barchfeld GL, Van Nest G. Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. Vaccine. 1995;13:1557‐1562. [DOI] [PubMed] [Google Scholar]
- 106. Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180:5402‐5412. [DOI] [PubMed] [Google Scholar]
- 107. Dupuis M, Murphy TJ, Higgins D, et al. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 1998;186:18‐27. [DOI] [PubMed] [Google Scholar]
- 108. O'hagan DT, Wack A, Podda A. MF59 is a safe and potent vaccine adjuvant for flu vaccines in humans: what did we learn during its development? Clin Pharmacol Therap. 2007;82:740‐744. [DOI] [PubMed] [Google Scholar]
- 109. Ellebedy AH, Lupfer C, Ghoneim HE, DeBeauchamp J, Kanneganti TD, Webby RJ. Inflammasome‐independent role of the apoptosis‐associated speck‐like protein containing CARD (ASC) in the adjuvant effect of MF59. Proc Natl Acad Sci. 2011;108:2927‐2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hui GS, Hashimoto CN. Adjuvant formulations possess differing efficacy in the potentiation of antibody and cell mediated responses to a human malaria vaccine under selective immune genes knockout environment. Int Immunopharmacol. 2008;8:1012‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vono M, Taccone M, Caccin P, et al. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc Natl Acad Sci. 2013;110:21095‐21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Brigelius‐Flohé R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145‐1155. [PubMed] [Google Scholar]
- 113. Wu D, Meydani SN. Age‐associated changes in immune and inflammatory responses: impact of vitamin E intervention. J Leukoc Biol. 2008;84:900‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Morel S, Didierlaurent A, Bourguignon P, et al. Adjuvant System AS03 containing α‐tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461‐2473. [DOI] [PubMed] [Google Scholar]
- 115. Hatada EN, Krappmann D, Scheidereit C. NF‐κB and the innate immune response. Curr Opin Immunol. 2000;12:52‐58. [DOI] [PubMed] [Google Scholar]
- 116. Garçon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an Adjuvant System containing α‐tocopherol and squalene in an oil‐in‐water emulsion. Expert Rev Vaccine. 2012;11:349‐366. [DOI] [PubMed] [Google Scholar]
- 117. Liebowitz D, Gottlieb K, Kolhatkar NS, et al. Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo‐controlled and active‐controlled phase 2 human challenge study. Lancet Infect Dis. 2020;20:435‐444. [DOI] [PubMed] [Google Scholar]
- 118. Carter D, van Hoeven N, Baldwin S, et al. The adjuvant GLA‐AF enhances human intradermal vaccine responses. Sci Adv. 2018;4:eaas9930. 10.1126/sciadv.aas9930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Precioso AR, Miraglia JL, Campos LMA, et al. A phase I randomized, double‐blind, controlled trial of 2009 influenza A (H1N1) inactivated monovalent vaccines with different adjuvant systems. Vaccine. 2011;29:8974‐8981. [DOI] [PubMed] [Google Scholar]
- 120. Taylor DN, Treanor JJ, Sheldon EA, et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza‐flagellin fusion vaccine with improved safety and immune response. Vaccine. 2012;30:5761‐5769. [DOI] [PubMed] [Google Scholar]
- 121. Hung IFN, Zhang AJ, To KKW, et al. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non‐vaccine and antigenically drifted viruses: a single‐centre, double‐blind, randomised, controlled phase 2b/3 trial. Lancet Infect Dis. 2016;16:209‐218. [DOI] [PubMed] [Google Scholar]
- 122. Cooper CL, Davis HL, Morris ML, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine. 2004;22:3136‐3143. [DOI] [PubMed] [Google Scholar]
- 123. Cox RJ, Pedersen G, Madhun AS, et al. Evaluation of a virosomal H5N1 vaccine formulated with Matrix M™ adjuvant in a phase I clinical trial. Vaccine. 2011;29:8049‐8059. [DOI] [PubMed] [Google Scholar]
- 124. Stadlbauer D, Rajabhathor A, Amanat F, et al. Vaccination with a recombinant H7 hemagglutinin‐based influenza virus vaccine induces broadly reactive antibodies in humans. Msphere. 2017;2:e00502‐17. 10.1128/mSphere.00502-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pleguezuelos O, James E, Fernandez A, et al. Efficacy of FLU‐v, a broad‐spectrum influenza vaccine, in a randomized phase IIb human influenza challenge study. NPJ Vaccines. 2020;5:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Stanberry LR, Simon JK, Johnson C, et al. Safety and immunogenicity of a novel nanoemulsion mucosal adjuvant W805EC combined with approved seasonal influenza antigens. Vaccine. 2012;30:307‐316. [DOI] [PubMed] [Google Scholar]
- 127. Langley J, Macdonald L, Weir G, et al. A phase I randomized, observer‐blind, controlled, dose escalation trial of the safety and tolerability of a single intramuscular dose of a PAL adjuvant (laboratory code, FB‐631) Co‐administered with seasonal TIV (2013–2014) to healthy Adults ≥18–50 Years of age. Open Forum Infect Dis. 2016;3:1270. 10.1093/ofid/ofw172.973 [DOI] [Google Scholar]
- 128. Glenn GM, Thomas DN, Poffenberger KL, et al. Safety and immunogenicity of an influenza vaccine A/H5N1 (A/Vietnam/1194/2004) when coadministered with a heat‐labile enterotoxin (LT) adjuvant patch. Vaccine. 2009;27:60‐66. [DOI] [PubMed] [Google Scholar]
- 129. Pan SC, Hsu WT, Lee WS, et al. A double‐blind, randomized controlled trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with the adjuvant LTh (αK): a phase II study. Vaccine. 2020;38:1048‐1056. [DOI] [PubMed] [Google Scholar]
- 130. Gordon DL, Sajkov D, Woodman RJ, et al. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax™ polysaccharide adjuvant. Vaccine. 2012;30:5407‐5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Couch RB, Atmar RL, Cate TR, et al. Contrasting effects of type I interferon as a mucosal adjuvant for influenza vaccine in mice and humans. Vaccine. 2009;27:5344‐5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kawasaki T, Kawai T. Toll‐like receptor signaling pathways. Front Immunol. 2014;5:461. 10.3389/fimmu.2014.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kim YH, Bang YJ, Park HJ, et al. Inactivated influenza vaccine formulated with single‐stranded RNA‐based adjuvant confers mucosal immunity and cross‐protection against influenza virus infection. Vaccine. 2020;38:6141‐6152. [DOI] [PubMed] [Google Scholar]
- 134. DiazGranados CA, Robertson CA, Talbot HK, Landolfi V, Dunning AJ, Greenberg DP. Prevention of serious events in adults 65 years of age or older: a comparison between high‐dose and standard‐dose inactivated influenza vaccines. Vaccine. 2015;33:4988‐4993. [DOI] [PubMed] [Google Scholar]
- 135. Robertson CA, Mercer M, Selmani A, Klein NP, Jeanfreau R, Greenberg DP. Safety and immunogenicity of a full‐dose, split‐virion, inactivated, quadrivalent influenza vaccine in healthy children 6‐35 months of age: a randomized controlled clinical trial. Pediatr Infect Dis J. 2019;38:323‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Van Buynder PG, Konrad S, Van Buynder JL, et al. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine. 2013;31:6122‐6128. [DOI] [PubMed] [Google Scholar]
- 137. Romani N, Flacher V, Tripp CH, Sparber F, Ebner S, Stoitzner P. Targeting skin dendritic cells to improve intradermal vaccination. Intradermal Immun. 2012;351:113‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ansaldi F, De Florentiis D, Durando P, Icardi G. Fluzone® Intradermal vaccine: a promising new chance to increase the acceptability of influenza vaccination in adults. Expert Rev Vaccine. 2012;11:17‐25. [DOI] [PubMed] [Google Scholar]
- 139. Centers for Disease Control and Prevention , National Center for Immunization and Respiratory Diseases (NCIRD) . Flu Vaccine Safety Information. 2019. https://www.cdc.gov/flu/prevent/general.htm. Accessed September 17, 2019. [Google Scholar]
- 140. Cross JW, Joy M, McGee C, Akinyemi O, Gatenby P, de Lusignan S. Adverse events of interest vary by influenza vaccine type and brand: sentinel network study of eight seasons (2010–2018). Vaccine. 2020;38:3869‐3880. [DOI] [PubMed] [Google Scholar]
- 141. Nohynek H, Jokinen J, Partinen M, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PloS One. 2012;7:e33536. 10.1371/journal.pone.0033536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Miller E, Andrews N, Stellitano L, et al. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. Br Med J. 2013;346:f794. 10.1136/bmj.f794 [DOI] [PubMed] [Google Scholar]
- 143. Sarkanen T, Alakuijala A, Julkunen I, Partinen M. Narcolepsy associated with Pandemrix vaccine. Curr Neurol Neurosci Rep. 2018;18:1‐10. [DOI] [PubMed] [Google Scholar]
- 144. Wallenius M, Lind A, Akel O, et al. Autoantibodies in Pandemrix®‐induced narcolepsy: nine candidate autoantigens fail the conformational autoantibody test. Autoimmunity. 2019;52:185‐191. [DOI] [PubMed] [Google Scholar]
- 145. Brandtzaeg P. Role of mucosal immunity in influenza. Dev Biol. 2003;115:39‐48. [PubMed] [Google Scholar]
- 146. Soema PC, Kompier R, Amorij JP, Kersten GF. Current and next generation influenza vaccines: formulation and production strategies. Eur J Pharm Biopharm. 2015;94:251‐263. [DOI] [PubMed] [Google Scholar]
- 147. Mahase E. Covid‐19: moderna vaccine is nearly 95% effective, trial involving high risk and elderly people shows. BMJ Br Med J (Online). 2020;371:m4471. 10.1136/bmj.m4471 [DOI] [Google Scholar]
- 148. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID‐19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589‐593. [DOI] [PubMed] [Google Scholar]
- 149. Pardi N, Parkhouse K, Kirkpatrick E, et al. Nucleoside‐modified mRNA immunization elicits influenza virus hemagglutinin stalk‐specific antibodies. Nat Commun. 2018;22:3361‐3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Feldman RA, Fuhr R, Smolenov I, et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326‐3334. [DOI] [PubMed] [Google Scholar]


