Abstract
The clinical spectrum of COVID‐19 in heart transplant recipients has not been fully defined, because asymptomatic and sub‐clinical cases are difficult to capture. Seroprevalence surveys are an important tool to identify not just cases that have come to clinical attention, but all previously infected recipients. We performed a seroprevalence survey of the adult heart transplant program at a large New York City Hospital System. A total of 232 (87% of recipients being followed) subjects were tested, of whom 37 (15.9%) were found to be previously infected. This is comparable to the overall rate of prior infection in the NYC metro area. Disease course tended to be more severe than in the general population; however, this was at least partially driven by traditional risk factors of age and comorbidities. Lastly, 9 of 10 recipients who were initially found to be PCR positive subsequently tested positive for antibodies, confirming the ability of this population to mount a humoral response. In conclusion, prevalence of COVID‐19 in heart transplant recipients on immunosuppression was comparable to that in the general population of NYC, and 90% of those with an initially positive viral swab developed antibodies. In those who are infected, disease course tends to be more severe.
Keywords: complication: infectious, heart (allograft) function/dysfunction, immunosuppressant
1. INTRODUCTION
Heart transplant (HTX) recipients may be at an increased risk of complications from COVID‐19 due to obligatory immunosuppression as well as the high prevalence of comorbidities. Preliminary data have indicated a significant risk of death comparable to other high‐risk groups among HTX recipients requiring hospitalization; however, the overall clinical spectrum of COVID‐19 in heart transplant recipients ranging from the risk of infection to need for hospitalization and death has not been defined. 1 , 2 This is due to a lack of information on asymptomatic and subclinical cases, an issue prevalent among all COVID analyses.
Seroprevalence surveys are an important epidemiological tool that can overcome this problem by identifying subjects who were previously infected but clinically unrecognized. 3 In addition, serologic surveillance can assess the antibody response in specific populations and identify high‐risk features. Therefore, in the current analysis, we performed a seroprevalence survey from a large heart transplant program in New York City, one of the early epicenters in the United States.
2. MATERIALS AND METHODS
The first cases of COVID‐19 were detected in New York after March 1, 2020, at which time the Montefiore/Einstein HTX program was actively following 268 adult heart transplant recipients. By June 1, the pandemic had receded in New York; therefore, at this time we initiated serological testing on all recipients followed by our program.
For the purposes of this study, any recipient who either tested positive for COVID antibody (Ab) or previously had a positive COVID polymerase chain reaction (PCR) nasal swab was considered as having been infected. Clinical outcomes and symptoms were retrieved by both chart review and phone call to all subjects who were found to be infected. Infected recipients were subsequently categorized according to their clinical course as asymptomatic, mild (managed at home), or severe (hospitalized).
The baseline characteristics were described as frequencies (percentage) for categorical variables and mean ± standard deviation. Comparison between groups was made using the chi‐square test for categorical variables and ANOVA for continuous variables. All statistical analysis was performed with MedCalc version 19.5.6, and a two‐sided p values <.05 was considered significant. The Montefiore Medical Center IRB approved this study.
3. RESULTS
Two hundred and thirty‐two patients (87%) underwent testing between June 1 and August 1, 2020, of whom 37 (15.9%) were COVID Ab and/or PCR positive. The total of 37 COVID‐19‐positive subjects was comprised of 31 with a detectable Ab plus 1 who was PCR positive but did not develop Ab and five others who were PCR positive but died before Ab testing could be obtained (Figure 1). The average age of the infected recipients was 54 ± 21 years, 27% were female, 57% Black race, mean time since transplant was 5 ± 3 years, BMI was 28 ± 5 kg/m2, 84% were hypertensive, and 73% were diabetic. Immunosuppression was tacrolimus in 97%, mycophenolate in 73%, prednisone in 32%, and rapamycin in 14%. Comparison of COVID‐positive vs COVID‐negative heart transplant recipients is displayed in Table 1.
FIGURE 1.
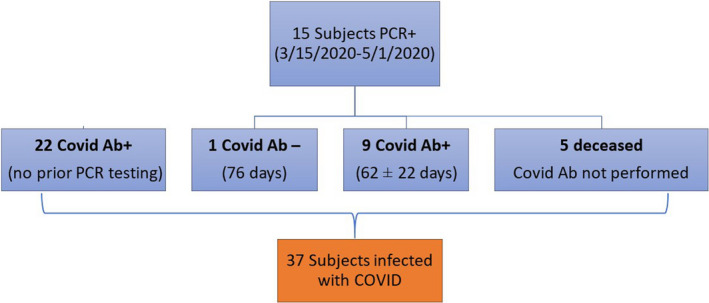
Consort diagram depicting final cohort of infected heart transplant recipients
TABLE 1.
Characteristics of Infected vs Non‐Infected Recipients
| Non‐Infected (n = 195) | Infected (n = 37) | p value | |
|---|---|---|---|
| Age (years) | 59.4 ± 13.8 | 54.0 ± 20.9 | .047 |
| Sex (% female) | 28 | 27 | .884 |
| BMI (kg/m2) | 28.6 ± 6.1 | 28.3 ± 5.4 | .753 |
| Time since transplant (years) | 5.6 ± 9.3 | 5.2 ± 3.6 | .826 |
| HTN (%) | 77 | 84 | .390 |
| DM (%) | 50 | 73 | .047 |
| CKD (%) | 53 | 65 | .034 |
| CAV (%) | 19 | 16 | .505 |
| LVEF (%) | 61 ± 7 | 58 ± 10 | .028 |
| Tacrolimus | 94 | 97 | .404 |
| Mycophenolate | 79 | 73 | .860 |
| Prednisone | 26 | 32 | .393 |
Nine of 10 (90%) of subjects who were PCR+ subsequently developed COVID Ab at 62 ± 22 days. The lone subject who was Ab negative (tested at day 76) initially was hospitalized at the time of PCR+ with low‐grade fever and subjective dyspnea. This individual was on heightened immunosuppression (rapamycin, tacrolimus, and prednisone) for chronic AMR.
In regard to the clinical course, the overall asymptomatic rate was 32%, 11 (30%) had documented mild symptoms and were managed at home, and 14 (38%) had severe symptoms requiring hospitalization.
As shown in Table 2, those with a more severe course were more likely to be male and had more CKD with a trend toward older age. Five of the hospitalized subjects died from COVID‐19 complications for an infection fatality rate (IFR) of 13.5% (Figure 2). Patients who died were older at 65, 73, 75, 75, and 76 years, and two were nursing home residents. Stratified by age, there were no deaths in 22 subjects <65 years, but mortality was 33% in those ≥65 (p = .007).
TABLE 2.
Characteristics of COVID‐19‐Infected Recipients by Severity of Disease Course
| Asymptomatic (n = 12) | Mild Disease (n = 11) | Severe Disease (n = 14) | p value | |
|---|---|---|---|---|
| Age (years) | 52.7 ± 14.3 | 54.9 ± 12.1 | 62.9 ± 11.7 | .109 |
| Sex (% female) | 50.0 | 37.5 | 7.7 | .049 |
| BMI (kg/m2) | 28.3 ± 3.0 | 30.1 ± 6.3 | 26.4 ± 5.9 | .244 |
| Time since transplant (years) | 5.8 ± 3.4 | 5.0 ± 3.0 | 4.9 ± 4.5 | .834 |
| HTN (%) | 92 | 73 | 86 | .719 |
| DM (%) | 50 | 73 | 72 | .266 |
| CKD (%) | 25 | 18 | 64 | .034 |
| Creatinine (mg/dl) | 1.2 ± 0.3 | 1.1 ± 0.2 | 1.5 ± 0.6 | .109 |
| CAV (%) | 25 | 18 | 14 | .489 |
| LVEF (%) | 61 ± 9 | 60 ± 8 | 60 ± 8 | .917 |
FIGURE 2.
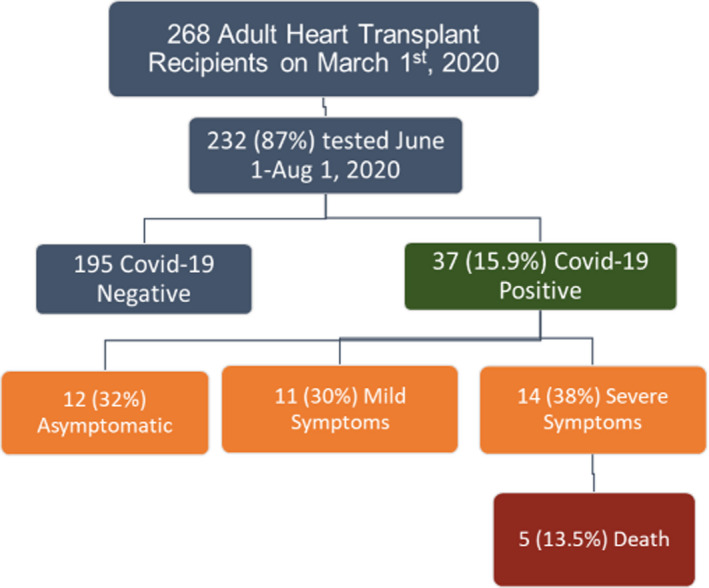
Outcomes of adult heart transplant recipients with prior COVID‐19
4. DISCUSSION
In this seroprevalence survey of COVID‐19 in heart transplant recipients, we made the following observations: (a) 90% of subjects on immunosuppression developed antibodies to SARS‐CoV‐2 after confirmed COVID‐19 infection, (b) the infection rate was not higher than the general population, and (c) after developing infection, heart transplant recipients tended to have a more severe course, which was largely dictated by the presence of traditional risk factors.
There has been speculation that immunosuppressed individuals are more likely to contract COVID‐19. Our data indicate that this is not likely the case. The overall prevalence in our cohort of 15.9% is lower than that reported by an analysis of over one million tests by the NYC Dept of Health, which documented a prevalence of 27% over a similar time frame. 4 While such a comparison between heart transplant recipients and the general population is biased, these preliminary data are reassuring. Of note, patients who had infection were slightly younger that is not surprising since younger individuals are more likely to have responsibilities that prevent effective quarantine.
In our study, we found 32% were asymptomatic, 30% had mild symptoms, 38% had severe symptoms, and the overall IFR was 13.5% (Figure 2). In comparison, the most useful data on the general population describe an asymptomatic rate of 45%, hospitalization rate of 5%–15%, and IFR <1%. 5 , 6 , 7 , 8 It is important to note that in the study group those who had a more severe course tended to have more comorbidities and were older. This is consistent with the COVID literature that demonstrates age and comorbidities, including CKD and diabetes, drive disease severity. 9 For age in particular, the CDC reports a 220X higher rate of mortality in age 75‐84, while a recent antibody survey from England documented a mortality rate of 11.64% in those >75. 10 , 11 These findings are consistent with a recent meta‐analysis that concluded the higher mortality found in solid organ transplant recipients is driven by age and comorbidities. 12 Regardless, the data on whether transplant immunosuppression alone is a risk factor for disease severity in COVID‐19 remain unclear and require further investigation.
In 10 subjects who had PCR confirmed COVID‐19, 90% developed antibodies at a median of 2 months. This is comparable to the 91% rate of sero‐conversion found in a large population study in Iceland and suggests that heart transplant recipients on immunosuppression are able to mount an antibody response. 13 Interestingly, the one individual who did not develop Ab was on higher immunosuppression for chronic AMR.
Limitations of the study include the small sample and a cohort of older age and high comorbidities. In addition, the results may have been effected by the varying performance characteristics of different Ab tests as well as the possibility that Ab levels may have waned prior to detection.
In conclusion, this sero‐survey of heart transplant recipients demonstrates that post‐infection seroconversion rates approach that of the general population. In addition, we found that for these individuals, the risk of acquiring infection is not greater than the general population, while on the other hand, the clinical spectrum tended to more severe disease, which was at least partially driven by age and comorbidities.
AUTHOR CONTRIBUTIONS
Dr Patel and Miss Gjelaj contributed to research design, performance of research, data analysis, and drafting of the manuscript. Drs Jorde, Vukelic, and Saeed contributed to data analysis and manuscript drafting. Dr Farooq, Miss Luke, Paschenko, and Fletcher participated in performance of the research.
Patel SR, Gjelaj C, Fletcher R, et al. COVID‐19 in heart transplant recipients—A seroprevalence survey. Clin Transplant. 2021;35:e14329. 10.1111/ctr.14329
Funding information
This manuscript was completed with departmental funds.
DATA AVAILABILITY STATEMENT
Data Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Latif F, Farr MA, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020;5(10):1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bottio T, Bagozzi L, Fiocco A, et al. COVID‐19 in heart transplant recipients: a multicenter analysis of the Northern Italian outbreak. JACC. Heart Fail. 2020;9(1):52‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng MP, Yansouni CP, Basta NE, et al. Serodiagnostics for severe acute respiratory syndrome‐related coronavirus 2: a narrative review. Ann Intern Med. 2020;173:450‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. NYC Health . COVID‐19: Data. https://www1.nyc.gov/site/doh/covid/covid‐19‐data‐testing.page. Accessed September 15, 2020.
- 5. Oran DP, Topol EJ. Prevalence of asymptomatic SARS‐CoV‐2 infection. Ann of Int Med. 2020;173(5):362‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The COVID Tracking Project . https://covidtracking.com/data. Accessed October 16, 2020.
- 7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 8. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. The Lancet Infectious diseases. 2020;20(6):669‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID‐19: a comprehensive systematic review and meta‐analysis of 77 studies and 38,000 patients. PLoS One. 2020;15:e0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. NCHS Provisional Death Counts . https://www.cdc.gov/nchs/nvss/vsrr/COVID19/index.htm. Accessed September 15, 2020.
- 11. Ward H, Atchison CJ, Whitaker M, et al. https://www.medrxiv.org/content/ 10.1101/2020.08.12.20173690v2. 2020. [DOI]
- 12. Corse T, Dayan L, Kersten S, Battaglia F, Terlecky SR, Han Z. Clinical outcomes of COVID‐19 patients with pre‐existing, compromised immune systems: a review of case reports. Int J Med Sci. 2020;17:2974‐2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS‐CoV‐2 in iceland. N Engl J Med. 2020;383:1724‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.


