Abstract
Objectives
To investigate the safety of robotic surgery during COVID‐19 pandemic concerning new‐acquired COVID‐19 infections for patients and healthcare workers.
Patients
We performed a retrospective single‐centre cohort study of patients undergoing robotic surgery in initial period of COVID‐19 pandemic. Patients and healthcare workers COVID‐19 infection status was assessed by structured telephone follow‐up and/or repeated nasopharyngeal swabs.
Results
After 61 robotic surgeries (93,5% cancer surgery), one patient (1.6%) had COVID‐19 infection. Sixty healthcare workers cumulatively exposed to 1187 h of robotic surgery had no infection. One patient with postoperative proof of SARS‐CoV‐2 had complete recovery. After this potentially contagious robotic surgery, eight healthcare workers had no COVID‐19 infection after follow‐up with each three nasopharyngeal swabs.
Conclusions
Early clinical experience of robotic surgery during COVID‐19 pandemic shows that robotic surgery can be safely performed for patients and healthcare workers. Despite our results we recommend elective surgery only for verified COVID‐19 negative patients.
Keywords: coronavirus, infection, robotics, safety, SARS‐CoV‐2, 2019‐nCoV
Abbreviations
- ASA score
American Society of Anesthesiologists Physical Status Classification System
- COVID‐19
coronavirus disease 2019
- ERUS
EAU Robotic Urology Section
- FFP mask
filtering face piece
- SAGES
Society of American Gastrointestinal and Endoscopic Surgeons
- SARS
severe acute respiratory syndrome
- SRS
Society of Robotic Surgery
1. INTRODUCTION
The corona virus disease 2019 (COVID‐19) pandemic is disrupting global urology healthcare. 1 In order to protect patients and healthcare workers and to ensure ventilators and equipment for the flood wave of COVID‐19 patients, elective surgeries had been cancelled worldwide. In urology, global elective surgery cancellations are estimated to be as high as 458,151 (cancellation rate 36.6%) for cancer surgery and 2,492,604 for benign surgery (cancellation rate: 81.7%). 2 While the burden of delayed surgeries is accumulating, 3 strategies to safely restore surgical activity are warranted to clear the backlog of operations resulting from COVID‐19 disruption. In this concern, safety for both patients and healthcare workers during surgery is of utmost importance.
Data on safety for elective surgeries during COVID‐19 show a huge variation ranging from 100% hospital‐acquired COVID‐19 infection rate among general surgery patients in China 4 to 3% 5 and 0% for most recently reported urological surgery patients in the United Kingdom 6 and Germany. 7 While nearly 10%–20% of confirmed COVID‐19 cases worldwide are reported to be healthcare workers, 8 data on safety of surgery for healthcare workers during COVID‐19 pandemic is highly warranted. In this concern, robotic surgery is currently a topic of expert debate since transmission of SARS‐CoV‐2 might be fostered by aerosol generating procedures during CO2 insufflation/evacuation and use of electrocautery or the harmonic scalpel. 9 Therefore, safety recommendations have most recently been published by a number of major surgical societies including ERUS (EAU Robotic Urology Section), SAGES (Society of American Gastrointestinal and Endoscopic Surgeons), Royal College of Surgeons (RCS) and SRS (Society of Robotic Surgery). 10 , 11 , 12 , 13 However, clinical data on safety of robotic surgery during COVID‐19 pandemic for patients and healthcare workers has not been reported yet.
The epidemiological analysis demonstrated for Germany on 31 March 2020 a total of 61,913 confirmed COVID‐19 infections and 583 deaths. The catchment area of the Department of Urology of the University Medical Center Mainz include the federal states Rhineland‐palatinum with 67/1000.000, Saarland 79/100.000 and Hessen with 52/100.000 infections per inhabitants. 14 By Comparison to the European Union Germany was affected by moderate infection and death rates between March and May 2020.
In this study, we investigated the safety of robotic surgery during the pandemic period concerning new‐acquired COVID‐19 infections for both patients, assessed by structured follow‐up telephone interview, and healthcare workers, assessed by structured swab tests through real time PCR on SARS‐CoV‐2.
2. PATIENTS AND METHODS
We performed a retrospective data analysis on patients treated with robotic surgery at our Tertiary Referral Academic Centre after WHO declaration of the pandemic for a 2‐months‐period from March 12 to 11 May 2020. Initially we performed as a high volume center (in total 296 robotic surgeries in 2020) robotic surgery for benign and malign diseases but due to local increasing infections rates we rapidly initiate prioritization towards high‐risk cancer patients according to ERUS (EAU Robotic Urology Section) guidelines. 11 The local ethic board approved the study (2020‐0997).
Demographical information about age and gender of patients was collected. Clinical information about date of surgery, type of surgery, ASA score, risk factors for an adverse COVID‐19 outcome (age ≥ 50 years, circulatory disease, diabetes, respiratory disease, liver disease, renal disease, history of oncological disease, immunosuppression at the time of the interview, nicotine abuse and hypertension), surgery time, preoperative screening with SARS‐CoV‐2 swab or COVID‐19 questionnaire, intraoperative adverse incident complication according to EAU classification, and postoperative complications according to Clavien Dindo Classification were collected.
Patients were followed‐up by structured telephone interviews on 25 May 2020 (minimum follow‐up time: 14 days) for COVID‐19 infection status and SARS‐CoV‐2 swabs performed postoperatively. We implemented preoperative swab PCR testing (maximum 24 h ago) for every robotic surgery ongoing since 26 May 2020. We performed furthermore a 11‐months post‐hoc extension analysis from 12 May 2020 until 11 April 2021 with assessment of COVID‐19 in‐hospital infections for patients undergoing robotic surgery.
All healthcare workers involved in robotic surgery including urology console surgeons, urology bedside surgeons, anaesthetists, urology scrub nurses, and anaesthesia nurses were structurally investigated for presence of SARS‐CoV‐2 in nasopharyngeal swabs at three different time points during the study period on 08/09 April, 15/16 April and 6/7/8/11 May. Additionally, all healthcare workers involved in surgery on the patient in whom COVID‐19 infection was proven on postoperative day two were tested three times negative on day 3, 7 and 11 with nasopharyngeal swabs. During this time period, these healthcare workers were sent to quarantine. Personal protective equipment included besides common surgical gown and gloves the mandatory use of FFP2/3 masks, safety glasses and protective face visor. There was fortunately no shortage of protective equipment in our hospital at any time.
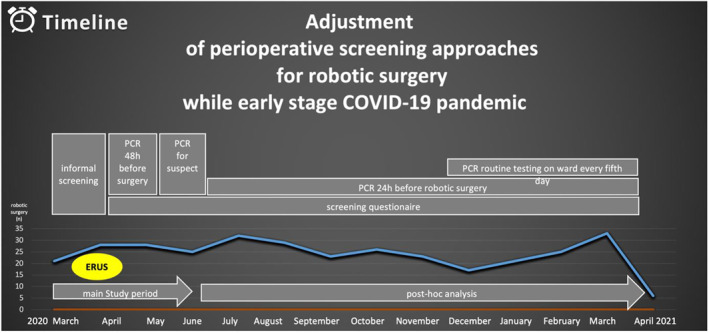
Preoperative COVID‐19 screening was performed by informal history interview on symptoms and travel history in the initial phase from March 16 to March 27. Since March 28, a specific preoperative COVID‐19 screening questionnaire. It contained questions on respiratory symptoms, fever, travel history to a COVID‐19 risk area, history of contact to a COVID‐19 positive patient, and on immunosuppression as most important risk factor for a severe course of COVID‐19. After the above mentioned safety recommendations on robotic surgery had been published, COVID‐19 screening regimen was extended to the inclusion of nasopharyngeal swabs taken 2 days before admission of patients that were scheduled for robotic surgery. This COVID‐19 screening regimen, defined as extended COVID‐19 screening regimen, was applied from 01 April until 20 April. As COVID‐19 swab testing availability within the hospital decreased, swab testing was abolished again and the screening regimen, now defined as limited COVID‐19 screening regimen, was restricted to using the COVID‐19 screening questionnaire. These rapidly changing screening approaches are depicted in Figure 1. Descriptive statistic was used for data reporting of patients and healthcare workers.
FIGURE 1.
Time‐related clinical adjustment of screening approaches for robotic surgery. Upcoming date of ERUS (EAU Robotic Urology Section) recommendations (yellow circle) for robotic surgery. 11 ERUS, EAU Robotic Urology Section
3. RESULTS
Overall, 61 robotic surgery procedures were performed during the 2‐months‐period. Robotic surgeries included robotic‐assisted radical prostatectomy (n = 37, 60.7%), partial nephrectomy (n = 14, 23%), pyeloplasty (n = 3, 4.9%), radical cystectomy (n = 2, 3.3%), radical nephroureterectomy (n = 2, 3.3%), adrenalectomy (n = 1, 1.6%), simple prostatectomy (n = 1, 1.6%), and super‐extended lymphadenectomy (n = 1, 1.6%), resulting in 57 (93.4%) cancer surgeries and 4 (6.6%) surgeries on benign disease. The preoperative screening questionnaire showed for none of these 61 patients symptoms that are suspicious for COVID‐19 infection. Patient and surgery characteristics are listed in Table 1.
TABLE 1.
Clinical data on 61 robotic surgeries performed during COVID‐19 pandemic
| Parameter, unit | Value |
|---|---|
| Patient factors | |
| Age, years, median (IQR) | 68 (63–73) |
| Male gender, n (%) | 53 (86.9%) |
| ASA score, n (%) | |
| 1 | 6 (9.8%) |
| 2 | 34 (55.7%) |
| 3 | 21 (34.4%) |
| COVID‐19 risk factors, n (%) | |
| 0 | 1 (1.6%) |
| 1 | 11 (18%) |
| 2 | 21 (34.4%) |
| 3 | 15 (24.6%) |
| 4 | 9 (14.8%) |
| 5 | 3 (4.9%) |
| 6 | 1 (1.6%) |
| Surgery data | |
| Type of surgery, n (%) | |
| Robot‐assisted radical prostatectomy | 37 (60.7%) |
| Robot‐assisted partial nephrectomy | 14 (23%) |
| Robot‐assisted pyeloplasty | 3 (4.9%) |
| Robot‐assisted radical cystectomy | 2 (3.3%) |
| Robot‐assisted radical nephroureterectomy | 2 (3.3%) |
| Robot‐assisted adrenalectomy | 1 (1.6%) |
| Robot‐assisted simple prostatectomy | 1 (1.6%) |
| Robot‐assisted super‐extended lymphadenectomy | 1 (1.6%) |
| OR time, minutes, median (Q1–Q3) | 171 (138–198) |
| Preoperative COVID‐19 screening, n (%) | |
| COVID‐19 screening form | 46 (75.4%) |
| COVID‐19 swab | 21 (34.4%) |
| Intraoperative adverse events (EAUiaiC), n (%) | |
| None | 54 (90.2%) |
| Grade 1 | 3 (4.9%) |
| Grade 2 | 2 (3.3%) |
| Grade 3 | 1 (1.6%) |
| Grade 4 | 0 (0%) |
| Grade 5 | 0 (0%) |
| Postoperative complications within 7 days, Clavien‐Dindo, n (%) | |
| None | 42 (68.9%) |
| Grade 1 | 10 (16.4%) |
| Grade 2 | 2 (3.3%) |
| Grade 3 | 5 (8.2%) |
| Grade 4 | 2 (3.3%) |
| Postoperative COVID‐19 infection | |
| Follow‐up period, days, median (Q1–Q3) | 48 (28–60) |
| Proven COVID‐19 infection, n (%) | 1 (1.6%) |
| COVID‐19 swab test performed, negative result, n (%) | 6 (9.8%) |
In 1/61 patients (1.6%), a COVID‐19 infection was diagnosed postoperatively. Six patients (9.8%) had negative test results during the postoperative period. Overall, 60 healthcare workers were exposed to robotic surgery a total of 1187 h and 7 min including four urology console surgeons, eight urology bedside surgeons, 21 anaesthetists, 14 urology scrub nurses and 13 anaesthesia nurses (Table 2). All healthcare workers were tested negative for SARS‐CoV‐2 in nasopharyngeal swabs each three times during the study period. Complication rate of robotic surgery was low, with 6 (9.2%) intraoperative and 19 (31.1%) postoperative complications (Table 1). Complication rate of robotic surgery was low, with 1 (1.6%) intraoperative and 7 (11.5%) postoperative complications ≥ grade 3 (Table 1). The 11‐months post‐hoc extension analysis from 12 May 2020 till 11 April 2021 including the 2nd and beginning of the 3rd COVID‐19 infection peak demonstrated among 276 robotic surgeries malign (77,9%) and benign (22,1%) diseases a single COVID‐19 infection (0,36%) after robotic‐assisted radical prostatectomy on postoperative day 10 due to proven contact to a COVID‐19 positive patient on the ward. The post‐hoc analysis discloses no intraoperative COVID‐19 transmission after implementing preoperative testing (real time PCR) within one day before robotic surgery. In addition there was no nosocomial COVID‐19 infection among 293 patients who underwent non‐robotic surgeries (64 major and 229 minor surgery) from March 12 to 11 May 2020. However, 13 nurses on urological ward suffered from a COVID‐19 infection during this time frame and were immediately sent to quarantine after positive swab PCR test.
TABLE 2.
Demographic characteristics and exposure time of 60 healthcare workers involved in 61 robotic surgeries during COVID‐19
| Occupational Group | N | Male (%) | Age, range | Cumulative OR exposure time | COVID‐19 infections |
|---|---|---|---|---|---|
| Urology console surgeons | 4 | 4 (100%) | 39–51 | 175 h 2 min | 0 (0%) |
| Urology bed‐side surgeons | 8 | 4 (50%) | 28–44 | 213 h 29 min | 0 (0%) |
| Anesthesists | 21 | 12 (57%) | 26–61 | 282 h 8 min | 0 (0%) |
| Urology scrub nurses | 14 | 4 (29%) | 27–59 | 246 h 52 min | 0 (0%) |
| Anesthesia nurses | 13 | 3 (23.1%) | 20–59 | 269 h 36 min | 0 (0%) |
| All healthcare workers | 60 | 27 (45%) | 20–61 | 1187 h 7 min | 0 (0%) |
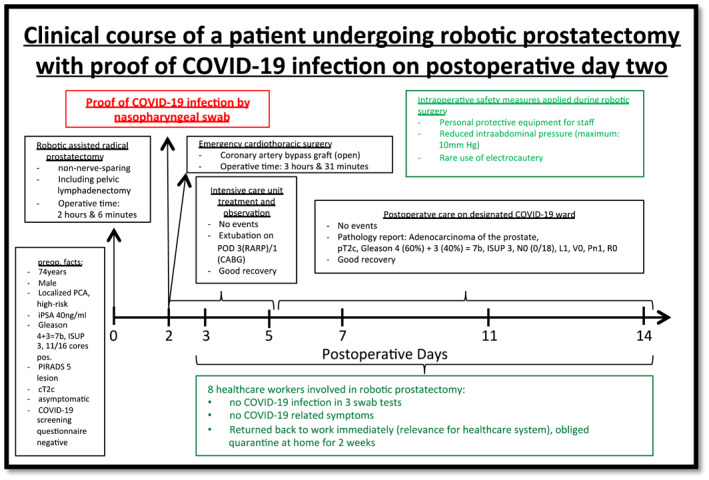
The clinical course of one patient diagnosed with COVID‐19 two days after robotic‐assisted radical prostatectomy is depicted in Figure 2. The patient reported dyspnoea in the night of postoperative day 1. Diagnostic work‐up revealed a myocardial infarction resulting in emergency coronarartery bypass graft surgery. A nasopharyngeal swab taken during diagnostic work‐up on the morning of postoperative day 2, returned SARS‐CoV‐2 positive. Although this patient was high‐risk being contagious at the time of robotic surgery, all eight healthcare workers involved in his robotic surgery had neither COVID‐19 symptoms nor a positive swab on three different time points postoperatively. Beyond that potential postoperative contact persons on regular ward including nurses and patients were tested negative.
FIGURE 2.
Clinical course of a patient undergoing robotic prostatectomy with proof of COVID‐19 infection on postoperative day two
Preoperative COVID‐19 screening for this patient had been negative by COVID‐19 screening questionnaire. The patient had been asymptomatic preoperatively, thus not being detected by the limited screening regimen, restricted to using the COVID‐19 screening questionnaire. During limited screening regimen, 1 out of 40 patients had a postoperative COVID‐19 infection (infection rate: 2.5%). During the extended COVID‐19 screening regimen, including nasopharyngeal swabs, none out of 20 patients had a postoperative COVID‐19 infection (infection rate: 0%). In the meantime we changed our preoperative procedure due to the escalating pandemic and implemented preoperative testing since 26 May 2020 for all patients one day before surgery. Beside that we focused on regular intraoperative use of protective equipment and follow strictly the ERUS recommendations (minimum number of staff, reduce surgical smoke, avoid pneumoperitoneum leakage, low intra‐abdominal pressure, short surgery time, disinflation of the pneumoperitoneum at the end of the procedure) 11 for robotic surgery while COVID‐19 pandemic. To date we also initiated coverage through postoperative nasopharyngeal swabs for all stationary patients every fifth day on ward.
4. DISCUSSION
Our single‐center cohort study of 61 consecutive patients undergoing robotic surgery during the 2‐months‐period in early COVID‐19 pandemic showed promising results on the safety of robotic surgery for both patients and healthcare workers. COVID‐19 infection was found in one patient (1.6%) postoperatively and in none of the 60 healthcare workers exposed to robotic surgery. Most importantly, the team of eight healthcare workers involved in robotic surgery of the potentially contagious patient that was diagnosed with COVID‐19 infection two days after surgery, had no symptoms and repeatedly negative SARS‐CoV‐2 swab tests.
COVID‐19 has disrupted urology healthcare in an unprecedented manner including surgery cancellations, 2 reduced emergency room visits for urological conditions 15 , 16 and re‐organisation of urological working practice. 17 , 18 , 19 To clear the backlog of operations resulting from COVID‐19 disruption, safe perpetuation of elective surgery including robotic surgery is highly necessary, aiming to minimize the collateral damage of morbimortality in urology patients.
Safety data on robotic surgery is ultimately warranted since SARS‐CoV‐2 RNA has been detected in the blood, urine, and gastrointestinal tract of infected patients raising the question whether active virus particles can be released and transmitted during robotic surgery on urinary tract and bowel segments 20 Moreover, CO2 insufflation together with cautery may enhance aerosolization of the virus during robotic surgery. 10 , 21 Subsequently, safety recommendations have been published by a number of major surgical societies. For instance, ERUS Guidelines offer recommendations for general health and COVID‐19 screening prior to surgery, necessary protective measures such as wearing goggles and FFP2/3 masks and utilizing intelligent integrated flow systems to reduce intraabdominal pressure (8–10 mmHg), thus preventing aerosol dispersal. 11
Following these recommendations is of utmost importance since preoperative COVID‐19 screening might be insufficient in detecting asymptomatic or paucisymptomatic SARS‐CoV2‐carriers when restricted to history/questionnaire assessment. In our cohort, one postoperative COVID‐19 infection occurred when limited screening regimen was applied (infection rate: 2.5%). When extended COVID‐19 screening regimen including nasopharyngeal swabs was applied, no postoperative COVID‐19 infection occurred ensuring maximal safety for patients and healthcare workers.
According to “COVIDSurg Collaborative” preoperative confirmed COVID‐19 infection is associated with worse postoperative outcome. Our colleagues demonstrated a postoperative pulmonary complication rate of 51,2% and a massive 23,8% 30‐days mortality. 22 Male sex, age > 70 years, ASA grad III–IV, cancer diagnosis, emergency surgery and major surgery were identified as risk factors. These international data including 1128 patients revealed a greatly increased postoperative risk for patients contaminated with COVID‐19 infection, especially for the above‐mentioned vulnerable groups. 22 For preoperative symptomatic and asymptomatic positive SARS‐CoV2‐carriers, we postpone robotic surgery until two repeated nasopharyngeal swabs (real time PCR) verified COVID‐19 negativity. In case of negativity these patients undergo again physical examination and complete resolution of COVID symptoms was presumed. Surgery for patients with an active COVID‐19 infection is considered on an individual risk‐benefit‐analysis in case of symptomas (hematuria) or highest cancer‐risk.
Whereas a lot of countries still face an escalating pandemic, COVID‐19 infection rates are decreasing in some countries, allowing to enter presently into a phase post COVID‐19 pandemic. During this phase, safely ramping up surgical activity in order to reduce the backlog of operations that had been delayed is a major goal. In urology, global elective surgery cancellations in urology are estimated to be as high as 458,151 (cancellation rate 36.6%) for cancer surgeries. 2 Of these urology cancer surgeries, the proportion of robotic surgery was reported to be 92% for prostate cancer, 61% for renal cell carcinoma, 40% for bladder cancer, and 48% for upper tract urothelial carcinoma. 3
Our study has strengths and limitations. To the best of our knowledge, this cohort is an early report of clinical data on safety of robotic surgery for both patients and healthcare workers during COVID‐19 pandemic. In particular, healthcare workers were structurally followed‐up by repeated nasopharyngeal swabs three times during the study period, inheriting the potential to detect the transmission of asymptomatic or paucisymptomatic SARS‐CoV‐2 patients onto healthcare workers. Limitations include the restriction of follow‐up to telephone interview assessment and inter‐individual differences in length of follow‐up time for patients. Beside the limited sample size and restricted benefit of screening questionnaires for definite exclusion of COVID‐19 infection, we particulary need to indicate the heterogeneous preoperative screening approaches due to rapidly changing recommendations while the pandemic.
In conclusion, early clinical experience of robotic surgery during COVID‐19 pandemic on 61 patients shows that robotic surgery was safely performed for most patients (COVID‐19 infection rate: 1/61; 1.6%) and all healthcare workers (no COVID‐19 infections). In particular, there was no COVID‐19 infection among 60 healthcare workers with direct contact during robotic surgery, performed using COVID‐19 safety precautions, on a patient for whom COVID‐19 infection was proven two days after surgery. Limited COVID‐19 screening regimen, restricted to questionnaire use and withholding nasopharyngeal swabs for real time PCR, did not detect this patient who was potentially contagious during robotic surgery. According to several colleagues we also state that patients with confirmed COVID‐19 infection carry high perioperative risk. Despite our results without a single infection in the group of healthcare workers we recommend further analysis of potential SARS‐CoV‐2 transmission during robotic surgery and recommend robotic surgery only for verified COVID‐19 negative patients.
CONFLICT OF INTEREST
The authors have no potential conflict of interest.
FUNDING
No specific funding was obtained for this study.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
None.
DATA AVAILABILITY STATEMENT
All original study data is available and can be provided by the corresponding author upon request.
REFERENCES
- 1. Puliatti S, Eissa A, Eissa R, et al. COVID‐19 and urology: A comprehensive review of the literature. BJU Int. 2020;125(6):E7‐E14. 10.1111/bju.15071. Epub 2020 May 12. PMID: 32249538 [DOI] [PubMed] [Google Scholar]
- 2. COVIDSurg Collaborative. Elective surgery cancellations due to the COVID‐19 pandemic: Global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107(11):1440‐1449. 10.1002/bjs.11746. Epub 2020 Jun 13. PMID: 32395848; PMCID: PMC7272903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campi R, Amparore D, Capitanio U, et al. Assessing the burden of nondeferrable major uro‐oncologic surgery to guide prioritisation strategies during the COVID‐19 pandemic: insights from three Italian high‐volume referral centres. Eur Urol. 2020;78(1):11‐15. 10.1016/j.eururo.2020.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID‐19 infection. EClinicalMedicine. 2020;21, 100331. 10.1016/j.eclinm.2020.100331. PMID: 32292899; PMCID: PMC7128617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDermott A, O'Kelly J, de Barra E, Fitzpatrick F, Little DM, Davis NF. Perioperative outcomes of urological surgery in patients with SARS‐CoV‐2 infection. Eur Urol. 2020;78(1):118‐120. 10.1016/j.eururo.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paramore L, Yang B, Abdelmotagly Y, et al. Delivering urgent urological surgery during the COVID‐19 pandemic in the UK: Outcomes from our initial 52 patients. BJU Int. 2020;126(2):248‐251. 10.1111/bju.15110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Würnschimmel C, Maurer T, Knipper S, et al. Martini‐Klinik experience of prostate cancer surgery during the early phase of the COVID‐19 pandemic. BJU Int. 2020;126(2):252‐255. 10.1111/bju.15115. Epub 2020 Aug 4. PMID: 32424990; PMCID: PMC7276763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The Lancet . COVID‐19: Protecting health‐care workers. Lancet. 2020;395(10228):922. 10.1016/S0140-6736(20)30644-9. PMID: 32199474; PMCID: PMC7138074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: Lessons learned in China and Italy. Ann Surg. 2020;272(1):e5‐e6. 10.1097/SLA.0000000000003924. PMID: 32221118; PMCID: PMC7188059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Porter J, Blau E, Gharagozloo F, et al. Society of robotic surgery review: Recommendations regarding the risk of COVID‐19 transmission during minimally invasive surgery. BJU Int. 2020;126(2):225‐234. 10.1111/bju.15105. PMID: 32383520; PMCID: PMC7267386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mottrie A, Puliatti S, Mazzone E, ERUS . ERUS‐EAU robotic urology section ERUS (EAU robotic urology section) guidelines during COVID‐19 emergency. (last update 27.4.2021) EAU Robotic Urology Section; 2020. https://uroweb.org/wp‐content/uploads/ERUS‐guidelines‐for‐COVID‐def.pdf [Google Scholar]
- 12. Francis N, Dort J, Cho E, et al. SAGES and EAES recommendations for minimally invasive surgery during COVID‐19 pandemic. Surg Endosc. 2020;34(6):2327‐2331. 10.1007/s00464-020-07565-w. Epub 2020 Apr 22. PMID: 32323016; PMCID: PMC7175828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Royal College of Surgeons . Updated Intercollegiate General Surgery Guidance on COVID‐19. Available from (last date 27.4.2021). https://www.rcseng.ac.uk/coronavirus/joint‐guidance‐for‐surgeons‐v2/ [Google Scholar]
- 14. RKI ‐ Robert Koch Institut Germany – (last update 27.4.2021) https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Archiv_Maerz.html; March 31 2020. [Google Scholar]
- 15. Madanelo M, Ferreira C, Nunes‐Carneiro D, et al. The impact of the coronavirus disease 2019 pandemic on the utilisation of emergency urological services. BJU Int. 2020;126(2):256‐258. 10.1111/bju.15109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Motterle G, Morlacco A, Iafrate M, et al. The impact of COVID‐19 pandemic on urological emergencies: A single‐center experience [published online ahead of print, 2020 May 23]. World J Urol. 2020:1‐5. 10.1007/s00345-020-03264-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Popert R, Kum F, MacAskill F, et al. Our first month of delivering the prostate cancer diagnostic pathway within the limitations of COVID‐19 using local anaesthesia transperineal biopsy. BJU Int. 2020;126(3):329‐332. 10.1111/bju.15120. Epub 2020 Aug 5. PMID: 32455471; PMCID: PMC7283663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novara G, Bartoletti R, Crestani A, et al. Porpiglia F; members of the Research Urology Network (RUN) (see appendix). Impact of the COVID‐19 pandemic on urological practice in emergency departments in Italy. BJU Int. 2020;126(2):245‐247. 10.1111/bju.15107. Epub 2020 May 30. PMID: 32407585; PMCID: PMC7273082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmed K, Hayat S, Dasgupta P. Global challenges to urology practice during the COVID‐19 pandemic. BJU Int. 2020;125(6):E5‐E6. 10.1111/bju.15082. Epub 2020 May 15. PMID: 32275792; PMCID: PMC7262148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peng L, Liu J, Xu W, et al. SARS‐CoV‐2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J Med Virol. 2020;92(9):1676‐1680. 10.1002/jmv.25936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vigneswaran Y, Prachand VN, Posner MC, Matthews JB, Hussain M. What is the appropriate use of laparoscopy over open procedures in the current COVID‐19 climate? J Gastrointest Surg. 2020;24(7):1686‐1691. 10.1007/s11605-020-04592-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: An international cohort study. Lancet. 2020;396(10243):27‐38. 10.1016/S0140-6736(20)31182-X. Epub 2020 May 29. Erratum in: Lancet. 2020 Jun 9; PMID: 32479829; PMCID: PMC7259900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All original study data is available and can be provided by the corresponding author upon request.


