Summary
Azithromycin (AZM) is commonly used in Covid‐19 patients based on low‐quality evidence, increasing the risk of developing adverse events and antimicrobial resistance. The current systematic review and meta‐analysis investigated the safety and efficacy of AZM in treating Covid‐19 patients using published randomized controlled trials. Google Scholar, PubMed, Scopus, Cochrane Library, Clinical Trials.gov, MEDLINE, bioRxiv and medRxiv were searched for relevant studies. The random‐effects model was used to pool estimates using the Paule–Mandel estimate for heterogeneity. The odds ratio and raw difference in medians were used for dichotomous and continuous outcomes, respectively. The analysis included seven studies with 8822 patients (median age, 55.8 years; 61% males). The risk of bias was assessed as ‘low’ for five of the seven mortality results and as ‘some concerns’ and ‘high’ in one trial each. There were 657/3100 (21.2%) and 1244/5654 (22%) deaths among patients randomized to AZM and standard of care, respectively. The use of AZM was not associated with mortality in Covid‐19 patients (OR = 0.96, 95% CI 0.88–1.05, p = 0.317 based on the random‐effect meta‐analysis). The use of AZM was not associated with need for invasive mechanical ventilation (OR = 0.96, 95% CI 0.49–1.87, p = 0.85) and length of stay (Δ = 1.11, 95% CI −2.08 to 4.31, p = 0.49). The results show that using AZM as routine therapy in Covid‐19 patients is not justified due to lack of efficacy and potential risk of bacterial resistance that is not met by an increased clinical benefit.
Keywords: azithromycin, Covid‐19, efficacy, meta‐analysis, mortality, safety, systematic review
Abbreviations
- ACE2
Angiotensin‐converting enzyme‐2
- AZM
azithromycin
- Covid‐19
Coronavirus Disease 2019
- CI
confidence interval
- HCQ
hydroxychloroquine
- IV
intravenous
- ITT
intention to treat
- IQR
interquartile range
- LOS
length of stay
- LPV/r
lopinavir/ritonavir
- MiTT
modified intention to treat
- MV
mechanical ventilation
- NCT
National Clinical Trial
- NG
nasogastric
- NIPPV
nasal intermittent positive pressure ventilation
- OR
odds ratio
- PCR
polymerase chain reaction
- QE
quantile estimation
- RCT
randomized controlled trial
- ROB‐2
Risk of Bias‐2
- SARS‐cov‐2
Severe acute respiratory syndrome coronavirus 2
- SOC
standard of care
- SD
standard deviation
- SeTE
standard error for the total effect
- TE
total effect
- WHO
World Health Organization
1. INTRODUCTION
In December 2019, an outbreak was reported in Wuhan, Hubei province, China, caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 On March 11, 2020, the World Health Organization (WHO) declared the severe acute respiratory syndrome caused by SARS‐CoV‐2 a global pandemic due to rapid transmission across countries. 2 On 16 August 2020, more than 20 million cases of Corona Virus Disease 2019 (Covid‐19) and nearly 760,000 deaths from SARS‐CoV‐2 were recorded. 3 Patients with Covid‐19 can experience a range of clinical manifestations, from asymptomatic to severe symptomatic disease. 4 The disease can cause respiratory distress that can progress to hypoxic respiratory failure. Hospital care and prolonged use of breathing support may be needed in such cases. 5 Several studies demonstrated that the leading causes of death in COVID‐19 are acute respiratory failure and sepsis. 6 , 7 , 8 Many pharmacological agents have been utilized to handle the disease and its life‐threatening complications. Most of these agents are repurposed drugs approved for other indications or other viral infections. 9 Among these medications is azithromycin (AZM). AZM is a broad‐spectrum macrolide antibiotic of the azalide class. 10 Macrolide antibiotics have some potentially useful anti‐inflammatory and immunomodulatory effects along with their obvious antibacterial properties. 11 , 12 They have been associated, in many studies, with beneficial outcomes in both acute and chronic inflammatory disorders. 13 , 14 However, the benefits should always be weighed against the potential risk of developing antimicrobial resistance (AMR). 13 , 14
The immunomodulatory properties of macrolides include the ability to down‐regulate prolonged inflammation, inhibiting bacterial biofilm formation, decreasing the production of oxygen free radicals, inhibiting neutrophil chemotaxis, accelerating neutrophil apoptosis and blocking the activation of nuclear transcription factors. 12 , 15 Numerous studies have demonstrated the in vitro antiviral activity of AZM against many viral pathogens such as Zika Ebola, influenza H1N1 virus, enterovirus and rhinovirus. 16 Several mechanisms have been proposed for the potential antiviral action of AZM. 16 One plausible mechanism is that AZM interferes with SARS‐CoV‐2 entry through binding interaction between SARS‐CoV‐2 spike protein and host receptor angiotensin‐converting enzyme‐2 (ACE2) protein. 17 In the early days of the outbreak, AZM was used commonly in combination with HCQ based on insufficiently good quality evidence. 18 , 19 However, later on, the use of HCQ in Covid‐19 was abandoned and halted due to lack of efficacy and increased risk of toxicity like QT prolongation and cardiotoxicity, especially when combined with other QT‐prolonging drugs as AZM. 20 , 21 , 22 , 23 Some cohort studies have reported beneficial outcomes using AZM, 24 , 25 while others could not confirm its efficacy. 26 , 27 Here, we conducted a systematic review and meta‐analysis on randomized controlled trials to evaluate the safety and efficacy of AZM in treating Covid‐19 patients.
2. METHODS
2.1. Development of meta‐analysis protocol
A quick literature search was initially performed (using Google scholar, PubMed, clinicaltrials.gov and Cochrane database) to identify study protocols for randomized trials that assess the efficacy and/or safety of AZM in Covid‐19 patients. These protocols were used to draft the keywords for literature review and sentinel articles that would later be used to ensure the accuracy of the search process. In addition, the protocol for the current systematic review and meta‐analysis (including outcomes and subgroup analysis) was drafted based on these protocols before examining the actual results for the trials (even if trial results were available) to reduce bias. The plans for the prospective meta‐analysis and drafts of the protocol were developed and reviewed by two members of the research team (S.F.F and A.M.K) and delivered to the remaining members of the team to commence the search and data extraction process. The protocol was registered and made publicly available on the PROSPERO database (CRD42021237776) on 17 February 2021, before data collection was started to reduce bias.
2.2. Database search and identification of trials
Pubmed, Cochrane library, Clinicaltrials.gov, Google scholar, Scopus, MEDLINE, bioRxiv and medRxiv were searched for completed clinical trials published in any language evaluating the effect of AZM in patients with Covid‐19. We used the search terms (“Covid.mp.” OR “Covid‐19.mp.” OR “SARS‐CoV‐2.mp.” OR “2019‐nCoV.mp.” OR “coronavirus/” or “CORONAVIRUS.mp.”) AND (“azithromycin.mp.” OR “macrolide.mp.”), filtered by randomized controlled trials according to validated filters. E‐mail alerts were used to identify any articles that were published after the search was concluded.
2.3. Ethics approval
All trials secured institutional review board approval, but approval was not required for the secondary data analysis reported here. Informed consent for participation in each trial was obtained and was consistent with local institutional review board requirements.
2.4. Eligibility criteria
The systematic review and meta‐analysis were conducted according to the PRISMA guidelines. Studies were included/excluded if the following inclusion criteria were met: (a) placebo‐controlled or randomized controlled clinical trial, (b) suspected or confirmed Covid‐19 patients, (c) the intervention group included patients who received AZM and (d) the control group included patients who received only standard of care (SOC) (with or without HCQ) or SOC in addition to placebo. The following exclusion criteria were used: (a) RCTs with a low number of patients (<20 patients), (b) non‐randomized clinical trials (cohort or case‐control studies or (c) RCTs which did not report at least any of the outcomes of interest.
2.5. Quality assessment and risk of bias
For each trial, we assessed the risk of bias (‘low risk’, ‘some concerns’ or ‘high risk’ of bias) in the overall effect of AZM on mortality using version 2 of the Cochrane Risk of Bias Assessment Tool (Risk of Bias 2 or ROB‐2). The tool is structured into five domains through which bias might be introduced into the result (Sterne et al., 2019). The risk of bias for the effect of assignment to the intervention (intention to treat; ITT) was assessed (Table S9).
Risk of bias assessment was based on the following information reported in trial protocols and flowcharts following the Consolidated Standards of Reporting Trials: (a) the methods used to generate the allocation sequence and conceal randomized allocation; (b) whether patients and health professionals were blinded to assigned intervention; (c) the methods used to ensure that patients received their allocated intervention and the extent of deviations from the assigned intervention and (d) the methods used to measure mortality and serious adverse events. The risk of bias assessments was done independently by two of the investigators, and disagreements were resolved through discussion and consulting with a third author.
2.6. Outcomes
The primary outcome of the meta‐analysis was all‐cause mortality up to 30 days after randomization and was determined based on the available literature and the methodology of the included studies that start. The outcomes were specified a priori before data collection and extraction. Shorter‐term mortality (up to 15 days) was acceptable if longer‐term mortality was not available. Secondary categorical outcomes included the need for invasive mechanical ventilation (IMV) (in patients who were not MV at baseline), successful cessation of IMV (in patients who were ventilated at baseline), discharge within the study period, virological clearance and clinical status score (ordinal scale). Secondary outcomes also included the length of the hospital stay (LOS) across survivors and ventilation‐free days. Safety outcomes included the proportion of patients with QT corrected interval (QTc) interval prolongation and cardiac arrhythmia.
2.7. Data extraction
Three research team members screened and agreed on the included studies (N.A, N.M and A.K). Quantitative data were extracted from the included studies by two reviewers (N.M and N.A) and cross‐checked by two reviewers (M.S and A.K) for completeness and accuracy. Full‐text papers for the eligible studies were retrieved. The following relevant information was extracted: the first author or trial name, publication year, demographic characteristics (age and gender), comorbidities, medications used during admission, trial registration number, dose regimen, sample size (ITT population) and outcome data. The counts and percentages were extracted for dichotomous outcomes (mortality, QT‐interval prolongation, arrhythmia, need for IMV and successful cessation of IMV). The median (IQR) or mean ± SD were extracted for LOS.
2.8. Statistical methods
For dichotomous outcomes, the odds ratio (OR) was used as the measure of effect size. The OR and the corresponding 95% confidence intervals (CIs) were calculated from event numbers extracted from each study. Fixed and random‐effects models were used to pool estimates from the included studies. The primary analysis was an inverse variance random fixed‐effect meta‐analysis of ORs for overall mortality. We also conducted fixed‐effects meta‐analyses (with the Paule–Mandel estimate of heterogeneity). 28
For studies with a zero cell count in one of the arms, a treatment arm continuity correction was applied. 29 , 30 This continuity correction was used to calculate individual study results with confidence limits and conduct meta‐analysis based on the inverse variance method. Studies with zero events in both arms were excluded. The Hartung–Knapp adjustment 31 , 32 was applied to account for uncertainty in estimating between‐study variance in the random‐effects meta‐analysis. This variance is imprecisely estimated when few studies are included and when some studies are small (both of which are the case with this meta‐analysis), leading to underestimation of the 95% CIs when the random‐effects model is used.
For the LOS, the pooled raw difference of medians across groups was calculated for each study and subsequently pooled across studies. The meta‐median package was used for the analysis. The quantile estimation method 33 was applied. The method can be used to calculate a pooled effect size even if different measures were used across studies (mean and SD vs. median and IQR). The same approach was used to calculate the pooled median and 95% confidence interval for descriptive purposes.
Heterogeneity between trials was assessed using the I 2 statistic and derived p values for heterogeneity using the Cochran Q statistic. Heterogeneity was measured using the weighted sum of squares test. Thus, it was assumed that the treatment effects were distributed homogeneously; that is, the difference between each treatment effect estimate and the overall treatment effect estimate were normally distributed.
Estimates from subgroups within the same study were pooled using a fixed‐effects model and used in the meta‐analysis. The 95% CI and Z‐statistic were calculated and used for hypothesis testing. Sensitivity (influence) analysis was performed using the leave‐one‐out method to investigate the source and possible causes of heterogeneity, in case of moderate to substantial heterogeneity (I2 > 50%), and to test the robustness of the results. Forest plots were used to visualize the meta‐analysis results. The effect size was estimated using the modified ITT (mITT) population of each trial. p values <0.05 was considered statistically significant. All analyses were performed using R v 3.6.3. 34
2.9. Subgroup analysis
Subgroup analysis was performed based on the severity of the disease (severe vs. non‐severe). The subgroup analyses were defined a priori except for the hospital setting, which was agreed upon during study selection. Covid‐19 was classified as severe if at least one of the following criteria were used for inclusion: use of supplemental oxygen, use of a high‐flow nasal cannula, use of non‐invasive or IMV.
2.10. Meta‐regression analysis
Meta‐regression analysis was initially planned to assess the association of the observed effect size with AZM dose regimen, the severity of the disease, age, the % of patients who used HCQ, % of patients on invasive MV at baseline, cumulative dose of AZM, duration of AZM (10 days vs. < 10 days), setting (hospital vs. community) and severity of the disease. However, the number of available studies was below the recommended threshold of 8–10 studies. 35
3. RESULTS
3.1. Database search
The initial literature search was conducted on February 12, and e‐mail alerts were used to identify additional candidate studies published before data analysis was started (Figure 1). Four studies were identified using the search strategy, 7 , 8 , 9 and two additional studies were identified at a later stage using e‐mail alerts. 36 , 37 One additional trial, the ATOMIC2 trial, was included when it was published in April 2021. 38
FIGURE 1.
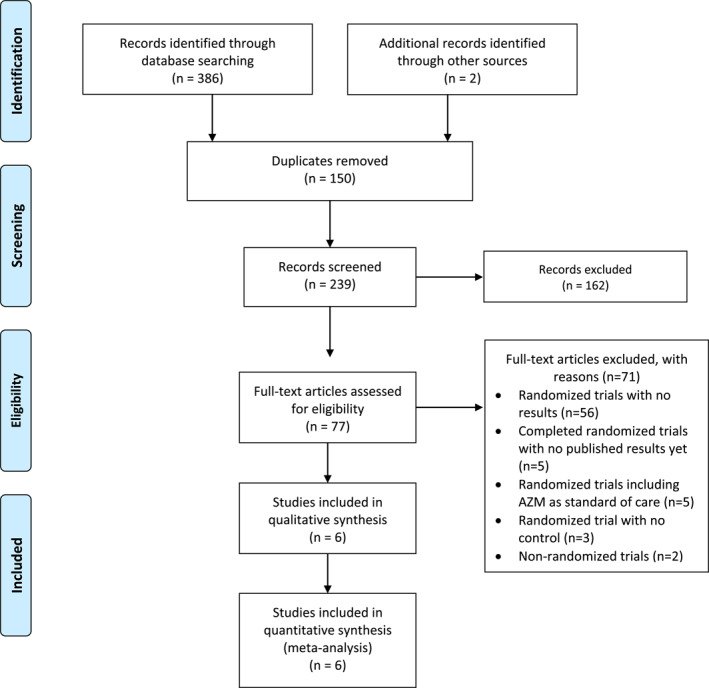
PRISMA flow chart for study selection
3.2. Study characteristics
Details regarding the design of the included studies are shown in Table 1. All seven studies were randomized clinical trials. Only one was a double‐blinded placebo‐controlled RCTs 7 , and all remaining six were open label. Primary outcomes were assessed at 6 days (Q‐PROTECT), 15 days (COALITION I) and 28–30 days (five studies). The secondary outcomes were also assessed at 29 days for the COALITION I trial. All studies reported the results based on pre‐specified protocols, and all were prospectively registered at one of the clinical trials registry platforms. Four trials were conducted in hospital settings, and three (Q‐PROTECT, ATOMIC2 and PRINCIPLE trials) were conducted in community settings. 36 , 37 , 38 Only two studies 39 , 40 included patients with severe Covid‐19.
TABLE 1.
Characteristics of the included studies a
| Butler CC et al. (PRINCIPLE) (2021) | Cavalcanti et al. (COALITION I) (2020) | Furtado et al. (COALITION II) (2020) | Hinks at al. (ATOMIC2) (2021) | Horby et al. (RECOVERY) (2021) | Omrani et al. (Q‐PROTECT) (2020) | Sekhavati et al. (2020) | |
|---|---|---|---|---|---|---|---|
| Location | United Kingdom | Brazil | Brazil | United Kingdom | United Kingdom | Qatar | Iran |
| Clinical trial registry identifier | ISRCTN86534580 | NCT04322123 | NCT04321278 | NCT04381962 | NCT04381936 | NCT04349592 | IRCT20200415047092N1 |
| Population | Community PCR‐confirmed or suspected Covid‐19 patients at increased risk of an adverse clinical course | Mild to moderate hospitalized Covid‐19 patients | Severe hospitalized Covid‐19 patients | Mild to moderate adult COVID‐19 patients—managed initially as outpatients | Hospitalized Covid‐19 patients | Non‐hospitalized mild or asymptomatic Covid‐19 patients | Covid‐19 patients with compelling clinical symptoms |
| Planned sample size | 2265 | 667 | 447 | 298 | 7763 | 456 | 111 |
| Blinding | Open label | Open label | Open label | Open label | Open label | Double blinding | Open label |
| Eligibility criteria |
|
|
|
|
|
|
|
| Recruiting site/s |
Recruiting of community patients was through:
|
55 hospitals in Brazil |
57 centres in Brazil |
19 centres in the United Kingdom | 176 hospitals in the United Kingdom |
|
|
| Intervention group | AZM + SOC | AZM + HCQ + SOC | AZM + HCQ + SOC | AZM + SOC | AZM + SOC | AZM + HCQ | AZM + HCQ + LPV/r |
| Comparable Group/s | SOC | HCQ + SOC | HCQ + SOC | SOC | SOC | HCQ | HCQ + LPV/r |
| AZM Dosage | 500 mg (oral) once daily | 500 mg (oral) once daily | 500 mg (oral, NG or IV) once daily | 500 mg (oral) once daily | 500 mg (oral, NG, IV) once daily | 500 mg (oral) day one, 250 mg (oral) daily on days two through five | 500 mg (oral) once daily |
| AZM duration | 3 days | 7 days | 10 days | 14 days | 10 days or until discharge, if sooner | 5 days | 5 days |
| Mortality outcome (days) | 29 days | 15 days | 29 days | 28 days | 29 days | 6 days | 30 days |
Note: Ordered alphabetically by author.
Abbreviations: AZM, azithromycin; Covid‐19, Coronavirus Disease 2019; HCQ, hydroxychloroquine; IV, intravenous; LPV/r, lopinavir/ritonavir; MV, mechanical ventilation; NCT, National Clinical Trial; NG, nasogastric; NIPPV, nasal intermittent positive pressure ventilation; PCR, polymerase chain reaction; SARS‐cov‐2, severe acute respiratory syndrome coronavirus 2; SOC, standard of care.
Table S6: For more details of the characteristics of the included studies.
The PRINCIPLE, ATOMIC2 and RECOVERY trials assessed the effect of AZM as a standalone therapy, while the remaining four studies included HCQ in the SOC arm. Two studies included high‐risk patients or patients with severe Covid‐19, 39 , 40 and two included patients with mild‐moderate Covid‐19. 36 , 41
All studies analysed the results using either the ITT or the mITT result. The COALITION I and COALITION II were conducted by the same research group in two different patient populations (mild‐moderate and severe Covid‐19, respectively). Five of the studies had a multi‐centre setting. The RECOVERY, ATOMIC2 and PRINCIPLE trials were conducted in the United Kingdom; COALITION I and II were conducted in Brazil by the same research group; one was conducted in Qatar (Q‐PROTECT) and one in Iran. The primary outcome in the Q‐PROTECT was virological cure at 6 days. However, it was included as it assessed the mortality and adverse effects (QTc interval prolongation and arrhythmia). Variable AZM regimens were used with a cumulative dose ranging from 1.5 to 7 g. AZM 500 mg was used for 10 days in two studies and 14 days in the ATOMIC2 study. The lowest and highest cumulative doses were used in the PRINCIPLE (1.5 g) and ATOMIC2 (7 g) trials, respectively.
3.3. Patient characteristics
The characteristics of the included patients are shown in Table 2. The median age (Figure S1) across studies was 54.35 years (95% CI 47.88–60.85 years), with males representing 66% of the total population (Figure S2). High heterogeneity was observed for gender and age across studies (I2 = 99%). HCQ was used in all patients in four studies and <1% in the RECOVERY, ATOMIC2 and PRINCIPLE trials. No death cases were observed across the two trials conducted in the community settings (Table S8). All recruited patients were 18 years old and above, except the PRINCIPLE trial, which included only elderly patients above 50 years of age. The pooled median time to symptoms (Figure S3) was 7.24 days (95% CI 6.52–7.95 days).
TABLE 2.
Characteristics of the patients included in the perspective meta‐analysis
| Butler CC et al. (PRINCIPLE) (2021) | Cavalcanti et al. (COALITION I) (2020) | Furtado et al. (COALITION II) (2020) | Hinks at al. (ATOMIC2) (2021) | Horby et al. (RECOVERY) (2021) | Omrani et al. (Q‐PROTECT) (2020) | Sekhavati et al. (2020) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AZM | Control | AZM | Control | AZM | Control | AZM | Control | AZM | Control | AZM | Control | AZM | Control | ||
| Randomized patients | 526 | 862 | 217 | 221 | 237 | 210 | 147 | 148 | 2582 | 5181 | 152 | 152 | 56 | 55 | |
| Age (years) (mean ± SD or median [IQR]) | 60.9 ± 7.9 | 60.5 ± 7.8 | 49.6 ± 14.2 | 51.3 ± 14.5 | 59.9 (49.3–70.3) | 60.2 (51.8–70.6) | 45.53 (14.23) | 46.30 (15.53) | 65·4 ± 15·6 | 65.2 ± 15.7 | 42 (38–48) | 40 (31–47) | 54.38 ± 15.92 | 59.89 ± 15.55 | |
| Male sex, no. (%) | 224 (43%) | 375 (44%) | 123 (56.7%) | 142 (64.3%) | 152 (64.13%) | 134 (63.80%) | 76 (51.7%) | 76 (51.4%) | 1604 (62%) | 3215 (62%) | 150 (98·7%) | 149 (98·0%) | 28 (50%) | 23 (41.82%) | |
| PCR‐confirmed SARS‐CoV‐2 infection, no. (%) | 189 (36%) | 245 (28%) | 172 (79.3%) | 159 (71.9%) | 214 (90.30%) | 183 (87.14%) | 76 (51.7%) | 76 (51.4%) | 2350 (91%) | 4743 (92%) | 152 (100%) | 152 (100%) | 56 (100%) | 55 (100%) | |
| Treatment at randomization, no./total (%) | Any antiviral | NA | NA | NA | Not specific | NA | NA | NA | |||||||
| Oseltamivir | 51 (23.5%) | 64 (29%) | 95/214 (44%) | 88/183 (48%) | NA | NA | NA | NA | |||||||
| Remdesivir | NA | NA | NA | 407 (20%) | 862 (22%) | NA | NA | ||||||||
| LPV/r | 0 | 0 | 1/214 (<1%) | 1/183 (1%) | NA | 3 (<1%) | 6 (<1%) | NA | 56 (100%) | 55 (100%) | |||||
| Favipravir | NA | NA | NA | NA | NA | NA | |||||||||
| HCQ | 165 (95.9%) | 154 (96.9%) | 237 (100%) a | 210 (100%) a | 0 | 0 | 2 (<1%) | 8 (<1%) | 152 (100%) | 152 (100%) | 56 (100%) | 55 (100%) | |||
| Corticosteroids | 6 (2.8%) | 6 (2.7%) | 48 (20.25%) | 36 (17.14%) | Not specific | 918 (46%) | 1925 (49%) | NA | NA | ||||||
| Antibiotics | 137 (63.13%) b | 140 (63.35%) b | 195 (82.27%) | 180 (85.71%) | 23/147 (15·6%) | 38/148 (25·7%) | NA | NA | NA | ||||||
| Convalescent plasma | NA | NA | NA | 336 (17%) | 689 (18%) | NA | NA | ||||||||
| Tocilizumab or sarilumab | NA | NA | Not specific | 126 (6%) | 305 (8%) | NA | NA | ||||||||
| Duration of symptoms at enrolment (days), median (IQR) | 7 (4–10) | 7 (5–9) | 8 (6–10) | 6 (6) | 8 (5–11) | Not specific c | Not specific | ||||||||
Note: Studies are ordered alphabetically by author.
Abbreviations: AZM, azithromycin; HCQ, hydroxychloroquine; IQR, interquartile range; LPV/r, Lopinavir/Ritonavir; NA, not applicable; NG: , nasogastric; PCR, polymerase chain reaction; SARS‐cov‐2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
HCQ 400 mg twice daily for 10 days (oral or NG).
Including piperacillin/tazobactam, quinolone, ceftriaxone and ceftaroline.
Participants were involved within 24 h of testing positive for the virus, whatever with or without symptoms.
3.4. Risk of bias assessment
The risk of bias was assessed as ‘low’ in five of the seven mortality results (Table 3) and as ‘some concerns’ in the PRINCIPLE trial as the analyses used might not have been sufficient to estimate the effect of assignment to intervention. 37 The risk of bias was assessed as ‘High’ in the study conducted by Sekhavati et al. as more severe symptoms were observed in the control group than the case group at baseline. 42
TABLE 3.
Summary of risk of bias assessment in the estimated effect of azithromycin on mortality in each trial, with brief explanation of judgements
| Studies | Risk of bias domains (assessment for the effect of assignment to intervention) | |||||
|---|---|---|---|---|---|---|
| 1. Randomization process | 2. Deviation from the intended interventions | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported results | Overall risk of bias | |
| Results of mortality outcome | ||||||
| Butler CC et al. (PRINCIPLE) (2021) | Low | Some concerns a | Low | Low | Low | Some Concerns |
| Cavalcanti et al. (COALITION I) (2020) | Low | Low | Low | Low | Low | Low |
| Furtado et al. (COALITION II) (2020) | Low | Low | Low | Low | Low | Low |
| Hinks at al. (ATOMIC2) (2021) | Low | Low | Low | Low | Low | Low |
| Horby et al. (RECOVERY) (2021) | Low | Low | Low | Low | Low | Low |
| Omrani et al. (Q‐PROTECT) (2020) | Low | Low | Low | Low | Low | Low |
| Sekhavati et al. (2020) | High b | Low | Low | Low | Some concerns c | High |
Note: Studies ordered alphabetically by author.
Deviation from the intended interventions: concerns about the different analyses used to estimate the effect of assignment to intervention.
Randomization process: symptoms were more severe (significant more frequent symptoms) in the control group.
Selection of the reported results: mortality rate was not among the protocol's outcomes.
3.5. Primary outcome
Five studies 38 , 39 , 40 , 41 , 42 reported the estimates for mortality (n = 8754). Two of the studies conducted in the community settings did not report any deaths and were not included in the analysis. The random‐effects model was used to pool estimates (Figure 2A). No statistically significant difference was observed between groups (OR = 0.96, 95% CI = 0.88–1.05, p = 0.317). Results did not change when the fixed‐effects model was used. No heterogeneity was observed between studies (I2 = 0, p = 0.87). Subgroup analysis by severity did not affect the mortality estimate. The analysis was not stratified by setting as only two deaths occurred in the community setting. Sensitivity analysis (using the leave‐one‐out method) did not alter the significance of the results (Figure S4).
FIGURE 2.
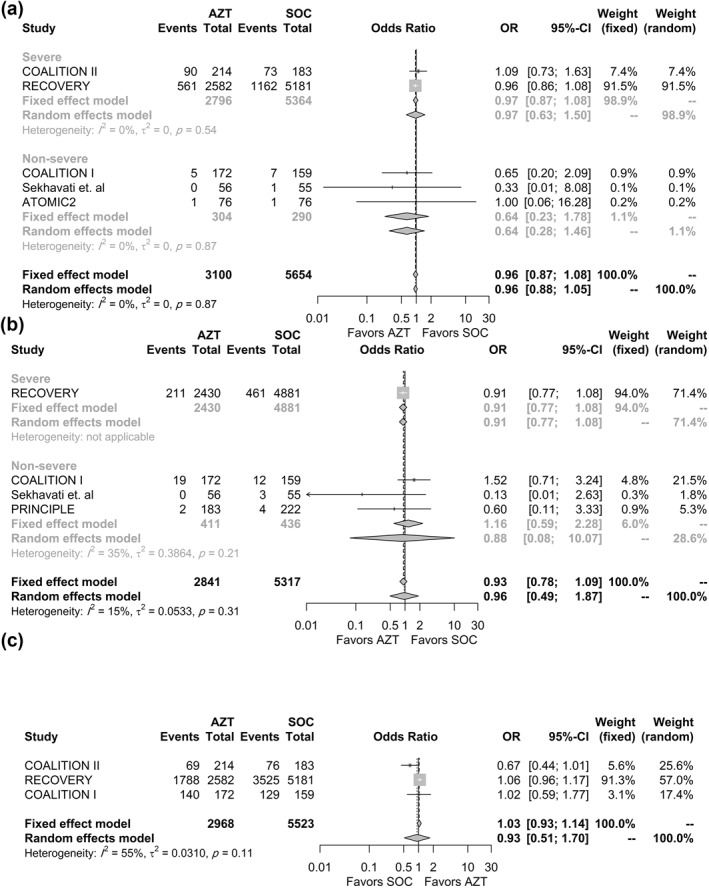
Association between AZM use and (a) 28‐day all‐cause mortality; (b) Need for invasive mechanical ventilation; (c) Discharge within the study period effect size is shown for each trial, overall and stratified by the severity. AZM, azithromycin
3.6. Secondary efficacy outcomes
The need for IMV was reported in four studies (n = 8158). The percentage was calculated from patients who were not ventilated at baseline to reduce bias. The number of patients who required IMV during hospital stay could not be retrieved in the COALITION II study and were not applicable in the Q‐PROTECT trial. Pooled results from four studies (three studies were excluded as no events occurred in both arms) showed that the need for IMV at up to 30 days (Figure 2B) was not significantly different between groups (OR = 0.96, 95% CI 0.49–1.87, p = 0.85) with low heterogeneity observed between studies (I 2 = 15%, p = 0.21). Results did not change when the fixed‐effects model was used. Omitting any of the trials did not affect the significance of the results (Figure S5).
Discharge within the study period (Figure 2C) was reported in three studies (n = 8491) and was not significantly different between groups (OR = 0.93, 95% CI 0.5–1.7, p = 0.67). Cessation of IMV was reported in two studies 39 , 40 that included patients who were ventilated at baseline. Thus, a meta‐analysis of this outcome was not performed.
The length of hospital stay was reported in five studies. Three studies reported the mean and the standard deviation, 38 , 41 , 42 and two reported the median and IQR. 39 , 40 The pooled raw difference in medians from the random‐effects model did not reveal a statistically significant difference in the median LOS between patients who received AZM and patients who received the SOC (Δ = 1.11, 95% CI −2.08 to 4.31, p = 0.49), and results did not change when the fixed‐effects model was used (Figure 3). High heterogeneity was observed between studies (I2 = 88%, p < 0.01). Stratifying the analysis by severity did not affect the statistical significance of the pooled estimate. Sensitivity analysis was performed to investigate the source of heterogeneity and assess the robustness of the results. The results were robust to the leave‐one‐out sensitivity analysis, although the heterogeneity between studies decreased to 65% when the COALITION II study was removed (Figure S6).
FIGURE 3.
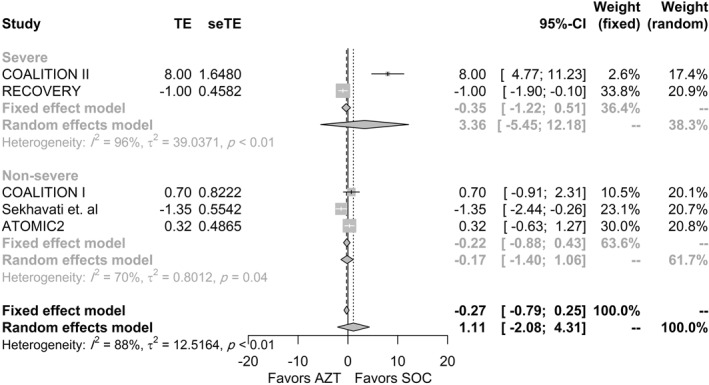
Association between AZM use and length of stay in each trial, overall and according to the severity. AZM, azithromycin; SeTE, standard error for the total effect; TE, total effect (raw difference in medians)
3.7. Secondary safety outcomes
Six and five studies reported the proportion of patients who experienced arrhythmia and QTc interval prolongation, respectively. Only three trials reported at least one event for each outcome and were included in the meta‐analysis. Two studies, 40 , 41 that reported the incidence of arrhythmia were using either chloroquine (CQ) or hydroxychloroquine (HCQ) compared to all three that reported the incidence of QTc interval prolongation. 36 , 40 , 41 Nonetheless, the use of these drugs was not significantly different between the arms of any of the included studies (Tables S7 and S8). The pooled estimate for arrhythmia (Figure 4A) was not statistically significant (OR = 0.91, 95% CI 0.67–1.25, p = 0.34). Leave‐one‐out sensitivity analysis did not affect the statistical significance of the estimate. Similarly, the pooled estimate, using a random‐effects model, for the association between AZM use and QTc interval prolongation incidence (Figure 4B) was not statistically significant (OR = 1.06, 95% CI 0.65–1.72, p = 0.67).
FIGURE 4.
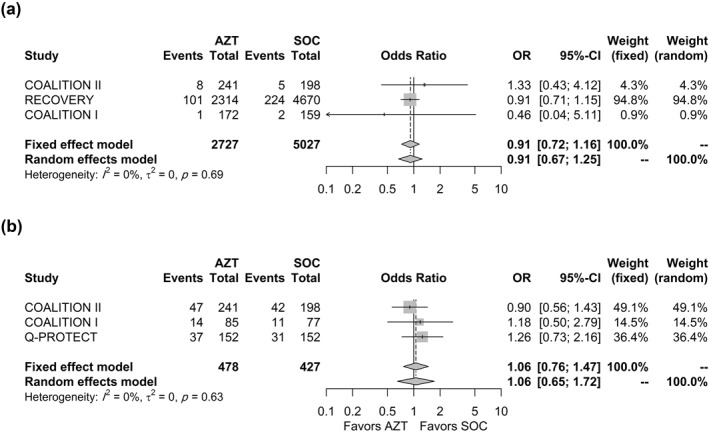
Association between AZM use and (a) incidence of arrhythmia and (b) incidence of QTc interval prolongation. AZM, azithromycin; QTC, Q‐T corrected interval
4. DISCUSSION
COVID‐19 is a life‐threatening condition caused by the novel SARS‐CoV‐2. Generally, the symptoms resemble viral Pneumonia with main clinical manifestations of fever, cough, myalgia, fatigue and dyspnoea. 43 , 44 Besides the respiratory system, other organ systems can also be involved to a lesser extent, including the gastrointestinal, neurological and hematopoietic systems. 45 Regarding life‐threatening complications of SARS‐CoV‐2 infection, dyspnoea is the main symptom predictive of severe COVID‐19 and ICU admission. 46
Outbreaks of such epidemics often present unique challenges for healthcare workers and beg for selecting the appropriate clinical treatments with no time available to discover new drugs. 9 Several trials are becoming increasingly interested in reusing existing drugs that can directly enter phase III or IV clinical trials to save cost and time. 9 , 47 These drugs include AZM, CQ, HCQ, lopinavir/ritonavir, Remdesivir, Tocilizumab, Interferon and Dexamethasone. 9 Many of them, such as Remdesivir, HCQ, lopinavir/ritonavir and interferon, later on, are recognized to have little or no effect on overall mortality, need for ventilation and LOS. 48 Only dexamethasone 49 and tocilizumab 50 , 51 , 52 , 53 have been shown to decrease mortality, need for ventilation and length of hospital stay compared to usual supportive care.
AZM is a broad‐spectrum antibiotic indicated for the treatment of susceptible bacterial infections of respiratory, enteric and genitourinary tract systems. 16 In addition to its antibacterial activity, it has well‐established anti‐inflammatory and immunomodulatory effects. 54 , 55 AZM is considered safe and well‐tolerated by adult patients of all ages. 56 Nonetheless, several studies in recent years have found a correlation between macrolides and cardiotoxicity. 57 QT‐interval prolongation, torsades de pointes (TdP), ventricular tachycardia and sudden cardiac death have all been identified as side effects of macrolides in vulnerable patients. 57 , 58
Additionally, it has been proven that HCQ can prolong the QT interval and potentially initiate ventricular arrhythmias. 53 Hence, the combination of these medications poses a considerable safety risk. 23 , 59 , 60 Thereby, current clinical evidence does not recommend using HCQ alone or in combination with AZM for COVID‐19. 61
Initially, few studies have assessed AZM alone in the management of COVID‐19. 62 However, most of these studies were observational and with inconclusive results. A meta‐analysis of observational studies showed that HCQ combined with AZM did not improve mortality risk compared with HCQ alone. 63 Moreover, Das and colleagues reported a substantial increase in mortality with the addition of AZM. 64 In this study, we assessed the efficacy and safety of AZM in the management of COVID‐19 to provide sufficient conclusive evidence regarding the use of AZM in COVID‐19.
The current meta‐analysis included seven randomized clinical trials to compare mortality based on the use of AZM. Some studies such as the ATOMIC2 and Q‐PROTECT might have been underpowered to detect a meaningful difference due to the low number of events in both arms, while studies such as the RECOVERY and COALITION II were sufficiently powered. Nonetheless, none of these trials showed a favourable effect for AZM on mortality, suggesting that AZM does not improve mortality in COVID‐19 patients. In addition, the length of stay and need for IMV were not significantly different between groups which even adds more evidence to discourage the use of AZM as a SOC in COVID‐19 patients.
The duration of AZM use ranged from 3 to 14 days with inconsistent dose regimens across studies. Nonetheless, the lack of benefit was consistent across studies. As discussed by Zimmermann and colleagues, 55 the immunomodulatory effect of macrolides is thought to start at a lower dose and last for a longer duration than their antibacterial effect. This may be attributed to drug accumulation within immune cells. The authors further stated that AZM was less frequently associated with changes in immunological markers than other macrolides and attributed that to the shorter duration of AZM use in the included studies. 55 In the current meta‐analysis, the ATOMIC2 trial did not report any benefit for AZM, although the cumulative dose (7 g) and duration (500 mg daily for 14 days) were higher than the remaining studies, which suggests that even higher dose regimens of AZM would not yield clinically relevant effects in Covid‐19 patients.
In the current meta‐analysis, the incidence of arrhythmia and QTc interval prolongation was not significantly different between groups. Some studies were not focused, nor powered to assess, clinical endpoints (including therapeutic risks) or rare events such as TdP. 36 , 42 The varying definitions for QTc interval prolongation and deviations from the study protocol may have also introduced bias into the results. For example, the Q‐PROTECT's initial protocol called for withdrawing participants for QT prolongation exceeding 30 ms, but the protocol was modified to increase the cut‐off to 60 ms as the study staff was not following the protocol and were retaining participants whose QT prolongation did not reach 60 m.
We did not assess outcomes such as virological cure as the meta‐analysis was focussed on clinical outcomes such as mortality and length of stay. Nonetheless, trials such as the Q‐PROTECT assessed virological cure as a primary outcome, and results showed that virological cure at Day 14 was more favourable for placebo than for either HCQ or HCQ + AZM, which even adds more evidence to discourage the use of AZM in patients with Covid‐19. 36 We also did not include the recent study conducted by Johnston and colleagues as it assessed the virological cure, which was not one of the outcomes for the current systematic review. Nonetheless, the authors concluded that neither HCQ nor HCQ/AZM shortened the clinical course of outpatients with Covid‐19. 65
It should be noted that AZM is an antibacterial agent, and its irrational use in COVID‐19 patients may lead to increased bacterial resistance and adverse events. Widespread use of AZM leads to a dilemma of bacterial resistance risk that is not limited to the individuals using it and affects the whole community level in the first place. 66 Additionally, the risk is actually beyond resistance against AZM only and extends to other antibiotic classes leading to multidrug resistance. 67 The association between AZM use, penicillin use 68 , 69 and multidrug resistance 70 , 71 has been reported in Streptococcus Pneumonia patients. As discussed by García‐Rey, 68 macrolides and β‐lactams had similar global contributions to penicillin resistance. Moreover, a marked increase in macrolide resistance has been observed in many countries with both long and short courses of use. As discussed by Serisier, 67 the widespread use of macrolides such as AZM in chronic obstructive pulmonary disease and bronchiectasis patients can substantially influence AMR rates of a range of respiratory microbes.
The emergence of AMR is a major clinical problem worldwide. Improper use of antibiotics during the pandemic might increase the long‐term threat of AMR. 72 , 73 Pandemic stress on healthcare systems may jeopardize antibiotic stewardship programs designed to help hospitals reduce AMR risks. Furthermore, there is no reported evidence that AZM has any anti‐inflammatory effect against Covid‐19 disease, as is suggested by other disease states. 74 Thus, there is no convincing clinical evidence to suggest that the benefits of AZM for Covid‐19 surpass the risks of treatment, and the routine use of AZM in Covid‐19 patients should be discontinued.
5. LIMITATIONS
The current study had several limitations. First, the current meta‐analysis included only seven studies. Nonetheless, the included trials had a large sample size to pool and reach reliable conclusions for the primary and secondary efficacy outcomes. Second, there was a high risk of bias in assessing outcomes such as arrhythmia and QTc interval prolongation, and the effect might have been confounded by the use of chloroquine and HCQ, which are associated with a higher incidence of arrhythmia. Some studies also lacked sufficient power to detect a statistically significant difference in mortality and the incidence of arrhythmia due to the low number of events in both arms. Defining clinical outcomes other than all‐cause mortality such as arrhythmia and QTc interval was insufficient and inconsistent across studies. Third, the data were insufficient to perform a meaningful meta‐regression and subgroup analysis. Fourth, two of the seven RCTs had some concerns or a high risk of bias for the mortality outcome. However, the weights of the meta‐analysis on all‐cause mortality were dominated by data from the RECOVERY trial, which had a low risk of bias. Fifth, three studies were conducted in hospitalized settings, and two of them included only patients with severe Covid‐19. Thus, the generalizability of the results is unclear.
6. CONCLUSION
The current meta‐analysis is the first to explore the association between AZM use and outcomes in Covid‐19 patients using sufficiently powered, low risk of bias randomized clinical trials. The results showed that the use of AZM was not associated with mortality, time to discharge, length of stay or the need for IMV in Covid‐19 patients. Based on the results, the use of AZM in clinical practice as a component of the SOC in Covid‐19 patients is not recommended and should be discontinued due to the increased risk of bacterial resistance that is not justified by evidence of benefit.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Samar Farid: Protocol formatting, and revision, final approval for submission. Ahmed Kamel: Statistical analysis and manuscript revision. Mona Sobhy: Manuscript drafting, literature review and data extraction. Nada Magdy: Manuscript drafting and data extraction. Nour A. Sharaf: Risk of bias assessment and literature review.
Supporting information
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENT
None.
Kamel AM, Monem MSA, Sharaf NA, Magdy N, Farid SF. Efficacy and safety of azithromycin in Covid‐19 patients: a systematic review and meta‐analysis of randomized clinical trials. Rev Med Virol. 2022;32(1):e2258. 10.1002/rmv.2258
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. WHO . Listings of WHO's Response to COVID‐19. Geneva: World Heal Organization. [Google Scholar]
- 2.WHO. Coronavirus Disease (COVID‐19) Situation Report 51. Geneva: World Health Organization. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200311‐sitrep‐51‐covid‐19.pdf?sfvrsn=1ba62e57_10
- 3. WHO . Coronavirus Disease (COVID‐19) Situation Report 165. Geneva: World Health Organization. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200816‐covid‐19‐sitrep‐209.pdf?sfvrsn=5dde1ca2_2 [Google Scholar]
- 4. Clinical Spectrum | COVID‐19 Treatment Guidelines. [Google Scholar]
- 5. Nicholson TW, Talbot NP, Nickol A, Chadwick AJ, Lawton O. Respiratory failure and non‐invasive respiratory support during the Covid‐19 pandemic: an update for re‐deployed hospital doctors and primary care physicians. BMJ. 2020;30:m2446. 10.1136/bmj.m2446 [DOI] [PubMed] [Google Scholar]
- 6. Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 cases of death from COVID‐19. PLoS One. 2020;15(7):e0235458. 10.1371/journal.pone.0235458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang K, Qiu Z, Liu J, et al. Analysis of the clinical characteristics of 77 COVID‐19 deaths. Sci Rep. 2020;10(1):16384. 10.1038/s41598-020-73136-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ketcham SW, Bolig T, Molling DJ, Sjoding MW, Flanders SA, Prescott HC. Causes and circumstances of death among patients hospitalized with COVID‐19: A retrospective cohort study [published online ahead of print 2020]. Ann Am Thorac Soc. 10.1513/AnnalsATS.202011-1381RL [DOI] [PMC free article] [PubMed]
- 9. Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK. Drug repurposing approach to fight COVID‐19. Pharmacol Rep. 2020;72(6):1479‐1508. 10.1007/s43440-020-00155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ballow CH, Amsden GW. Azithromycin: the first azalide antibiotic. Ann Pharmacother. 1992;26(10):1253‐1261. 10.1177/106002809202601014 [DOI] [PubMed] [Google Scholar]
- 11. Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23(3):590‐615. 10.1128/CMR.00078-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: proposed mechanisms of action. Pharmacol Ther. 2008;117(3):393‐405. 10.1016/j.pharmthera.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 13. Crosbie PAJ, Woodhead MA. Long‐term macrolide therapy in chronic inflammatory airway diseases. Eur Respir J. 2009;33(1):171‐181. 10.1183/09031936.00042208 [DOI] [PubMed] [Google Scholar]
- 14. Reijnders TDY, Saris A, Schultz MJ, van der Poll T. Immunomodulation by macrolides: therapeutic potential for critical care. Lancet Respir Med. 2020;8(6):619‐630. 10.1016/S2213-2600(20)30080-1 [DOI] [PubMed] [Google Scholar]
- 15. Healy DP. Macrolide immunomodulation of chronic respiratory diseases. Curr Infect Dis Rep. 2007;9(1):7‐13. 10.1007/s11908-007-0016-1 [DOI] [PubMed] [Google Scholar]
- 16. Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID‐19. Clin Pharmacol Ther. 2020;108(2):201‐211. 10.1002/cpt.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sandeep S, McGregor K. Energetics based modeling of hydroxychloroquine and azithromycin binding to the SARS‐CoV‐2 spike (S)Protein ‐ ACE2 complex [published online ahead of print 2020]. ChemRxiv. 10.26434/chemrxiv.12015792.v2 [DOI]
- 18. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Million M, Lagier J‐C, Gautret P, et al. Early treatment of COVID‐19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Trav Med Infect Dis. 2020;35:101738. 10.1016/j.tmaid.2020.101738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghazy RM, Almaghraby A, Shaaban R, et al. A systematic review and meta‐analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID‐19 treatment. Sci Rep. 2020;10(1):1‐18. 10.1038/s41598-020-77748-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maraolo AE, Grossi A. Safety of hydroxychloroquine for treatment or prevention of SARS‐CoV‐2 infection: a rapid systematic review and meta‐analysis of randomized clinical trials. Immun Inflamm Dis. 2021;9(1):31‐36. 10.1002/iid3.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen LS, Dolladille C, Drici M‐D, et al. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin. Circulation. 2020;142(3):303‐305. 10.1161/CIRCULATIONAHA.120.048238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020;5(9):1036‐1041. 10.1001/jamacardio.2020.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Albani F, Fusina F, Giovannini A, et al. Impact of azithromycin and/or hydroxychloroquine on hospital mortality in COVID‐19. J Clin Med. 2020;9(9):2800. 10.3390/jcm9092800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guérin V, Lévy P, Thomas J‐L, et al. Azithromycin and hydroxychloroquine accelerate recovery of outpatients with mild/moderate COVID‐19. Asian J Med Health. 2020;18(7):45‐55. 10.9734/ajmah/2020/v18i730224 [DOI] [Google Scholar]
- 26. Fujihashi A, Jones J. Association of treatment with hydroxychloroquine or azithromycin with in‐hospital mortality in patients with COVID‐19 in New York state. J Emerg Med. 2020;59(2):333‐334. 10.1016/j.jemermed.2020.07.042 [DOI] [Google Scholar]
- 27. Rodríguez‐Molinero A, Pérez‐López C, Gálvez‐Barrón C, et al. Observational study of azithromycin in hospitalized patients with COVID‐19. PLoS One. 2020;15(9):e0238681. 10.1371/journal.pone.0238681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Langan D, Higgins JPT, Simmonds M. Comparative performance of heterogeneity variance estimators in meta‐analysis: a review of simulation studies. Res Synth Methods. 2017;8(2):181‐198. 10.1002/jrsm.1198 [DOI] [PubMed] [Google Scholar]
- 29. J. Sweeting M, J. Sutton A, C. Lambert P. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Stat Med. 2004;23(9):1351‐1375. [DOI] [PubMed] [Google Scholar]
- 30. Böhning D, Mylona K, Kimber A. Meta‐analysis of clinical trials with rare events. Biom J. 2015;57(4):633‐648. 10.1002/bimj.201400184 [DOI] [PubMed] [Google Scholar]
- 31. Hartung J, Knapp G. A refined method for the meta‐analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20(24):3875‐3889. http://www.ncbi.nlm.nih.gov/pubmed/11782040 [DOI] [PubMed] [Google Scholar]
- 32. Inthout J, Ioannidis JP, Borm GF. The Hartung‐Knapp‐Sidik‐Jonkman method for random effects meta‐analysis is straightforward and considerably outperforms the standard DerSimonian‐Laird method. BMC Med Res Methodol. 2014;14(1):25. 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McGrath S, Sohn H, Steele R, Benedetti A. Meta‐analysis of the difference of medians. Biom J. 2020;62(1):69‐98. 10.1002/bimj.201900036 [DOI] [PubMed] [Google Scholar]
- 34. R Core Team. R . A Language and Environment for Statistical Computing. Austria: R A Lang Environ Stat Comput R Found Stat Comput Vienna; 2020. [Google Scholar]
- 35. Jenkins DG, Quintana‐Ascencio PF. A solution to minimum sample size for regressions. PLoS One. 2020;15:e0229345. 10.1371/journal.pone.0229345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Omrani AS, Pathan SA, Thomas SA, et al. Randomized double‐blinded placebo‐controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non‐severe Covid‐19. EClinicalMedicine. 2020;29:100645. 10.1016/j.eclinm.2020.100645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Butler CC, Dorward J, Yu L‐M, et al. Azithromycin for community treatment of suspected COVID‐19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open‐label, adaptive platform trial. Lancet. 397:1063‐1074. [published online ahead of print 2021]. 10.1016/S0140-6736(21)00461-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hinks TS, Cureton L, Knight R, et al. A randomised clinical trial of azithromycin versus standard care in ambulatory COVID‐19 – the ATOMIC2 trial [published online ahead of print 2021:1‐22]. medRxiv. 10.1101/2021.04.21.21255807 [DOI]
- 39. Abaleke E, Abbas M, Abbasi S, et al. Azithromycin in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. Lancet. 2021;397(10274):605‐612. 10.1016/s0140-6736(21)00149-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Furtado RHM, Berwanger O, Fonseca HA, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID‐19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396(10256):959‐967. 10.1016/S0140-6736(20)31862-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild‐to‐moderate covid‐19. N Engl J Med. 2020;383(21):2041‐2052. 10.1056/nejmoa2019014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sekhavati E, Jafari F, SeyedAlinaghi S, et al. Safety and effectiveness of azithromycin in patients with COVID‐19: an open‐label randomised trial. Int J Antimicrob Agents. 2020;56(4):106143. 10.1016/j.ijantimicag.2020.106143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID‐19). J Gen Intern Med. 2020;35(5):1545‐1549. 10.1007/s11606-020-05762-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bchetnia M, Girard C, Duchaine C, Laprise C. The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): a review of the current global status. J Infect Public Health. 2020;13(11):1601‐1610. 10.1016/j.jiph.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Behzad S, Aghaghazvini L, Radmard AR, Gholamrezanezhad A. Extrapulmonary manifestations of COVID‐19: radiologic and clinical overview. Clin Imaging. 2020;66:35‐41. 10.1016/j.clinimag.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jain V, Yuan J‐M. Predictive symptoms and comorbidities for severe COVID‐19 and intensive care unit admission: a systematic review and meta‐analysis. Int J Public Health. 2020;65(5):533‐546. 10.1007/s00038-020-01390-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2018;18(1):41‐58. 10.1038/nrd.2018.168 [DOI] [PubMed] [Google Scholar]
- 48. Charrez B, Charwat V, Siemons B, et al. In vitro safety “clinical trial” of the cardiac liability of hydroxychloroquine and azithromycin as COVID19 polytherapy [published online ahead of print 2020]. bioRxiv Prepr Serv Bio. 10.1101/2020.12.21.423869 [DOI]
- 49. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid‐19—preliminary report. N Engl J Med. 2020;384(8):693‐704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Landray M. Tocilizumab in patients admitted to hospital with COVID‐19 (RECOVERY): preliminary results of a randomised, controlled, open‐label, platform trial [published online ahead of print February 11, 2021]. medRxiv. 10.1101/2021.02.11.21249258 [DOI]
- 51. Kow CS, Hasan SS. The effect of tocilizumab on mortality in hospitalized patients with COVID‐19: a meta‐analysis of randomized controlled trials. Eur J Clin Pharmacol. 2021; 10.1007/s00228-021-03087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. Lancet (London, England). 2021;397(10285):1637‐1645. 10.1016/S0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kamp TJ, Hamdan MH, January CT. Chloroquine or hydroxychloroquine for COVID‐19: is cardiotoxicity a concern? J Am Heart Assoc. 2020;9(12):e016887. 10.1161/JAHA.120.016887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K. Macrolides: from in vitro anti‐inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol. 2012;68(5):479‐503. 10.1007/s00228-011-1161-x [DOI] [PubMed] [Google Scholar]
- 55. Zimmermann P, Ziesenitz VC, Curtis N, Ritz N. The immunomodulatory effects of macrolides‐A systematic review of the underlying mechanisms. Front Immunol. 2018;9:302. 10.3389/fimmu.2018.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hopkins S. Clinical toleration and safety of azithromycin. Am J Med. 1991;91(3A):40S‐45S. 10.1016/0002-9343(91)90401-I [DOI] [PubMed] [Google Scholar]
- 57. Abo‐Salem E, Fowler JC, Attari M, et al. Antibiotic‐induced cardiac arrhythmias. Cardiovasc Ther. 2014;32(1):19‐25. 10.1111/1755-5922.12054 [DOI] [PubMed] [Google Scholar]
- 58. Cheng Y‐J, Nie X‐Y, Chen X‐M, et al. The role of macrolide antibiotics in increasing cardiovascular risk. J Am Coll Cardiol. 2015;66(20):2173‐2184. [DOI] [PubMed] [Google Scholar]
- 59. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID‐19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol 2020;5(9):1067‐1069. 10.1001/jamacardio.2020.1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nguyen LS, Dolladille C, Drici M‐D, et al. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin. Circulation. 2020;142(3):303‐305. 10.1161/circulationaha.120.048238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Axfors C, Schmitt AM, Janiaud P, et al. Mortality outcomes with hydroxychloroquine and chloroquinein COVID‐19 from an international collaborative meta‐analysis of randomizedtrials. Nat Commun. 2021;12(1):2349. 10.1038/s41467-021-22446-z [DOI] [PMC free article] [PubMed]
- 62. Gyselinck I, Janssens W, Verhamme P, Vos R. Rationale for azithromycin in COVID‐19: an overview of existing evidence. BMJ Publ Group. 2021;8:e000806. 10.1136/bmjresp-2020-000806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mega TA, Feyissa TM, Bosho DD, Goro KK, Negera GZ. The outcome of hydroxychloroquine in patients treated for COVID‐19: systematic review and meta‐analysis. Can Respir J. 2020;2020:4312519. 10.1155/2020/4312519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Das RR, Jaiswal N, Dev N, Jaiswal N, Naik SS, Sankar J. Efficacy and safety of anti‐malarial drugs (chloroquine and hydroxy‐chloroquine) in treatment of COVID‐19 infection: a systematic review and meta‐analysis. Front Med. 2020;7. 10.3389/fmed.2020.00482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Johnston C, Brown ER, Stewart J, et al. Hydroxychloroquine with or without azithromycin for treatment of early SARS‐CoV‐2 infection among high‐risk outpatient Adults: a randomized clinical trial [published online ahead of print 2021]. SSRN J. 10.2139/ssrn.3745831 [DOI] [PMC free article] [PubMed]
- 66. Doern GV. Macrolide and ketolide resistance with Streptococcus pneumoniae. Med Clin N. Am. 2006;90(6):1109‐1124. 10.1016/j.mcna.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 67. Serisier DJ. Risks of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. Lancet Respir Med. 2013;1(3):262‐274. 10.1016/S2213-2600(13)70038-9 [DOI] [PubMed] [Google Scholar]
- 68. García‐Rey C, Aguilar L, Baquero F, Casal J, Dal‐Ré R. Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumoniae. J Clin Microbiol. 2002;40(1):159‐164. 10.1128/JCM.40.1.159-164.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dias R, Caniça M. Trends in resistance to penicillin and erythromycin of invasive pneumococci in Portugal. Epidemiol Infect. 2008;136(7):928‐939. 10.1017/S0950268807009405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Barkai G, Greenberg D, Givon‐Lavi N, Dreifuss E, Vardy D, Dagan R. Community prescribing and ResistantStreptococcus pneumoniae. Emerg Infect Dis. 2005;11(6):829‐837. 10.3201/eid1106.050198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hicks LA, Chien Y‐W, Taylor TH, Haber M, Klugman KP. Outpatient antibiotic prescribing and nonsusceptible streptococcus pneumoniae in the United States, 1996‐2003. Clin Infect Dis. 2011;53(7):631‐639. 10.1093/cid/cir443 [DOI] [PubMed] [Google Scholar]
- 72. Sabry NA, Farid SF, Dawoud DM. Antibiotic dispensing in Egyptian community pharmacies: an observational study. Res Soc Adm Pharm. 2014;10(1):168‐184. 10.1016/j.sapharm.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 73. Abdel GE, Samir MB, Abdou AKA, et al. Pattern of antibiotic abuse—a population based study in Cairo. Egypt J Chest Dis Tuberc. 2013;62(1):189‐195. [Google Scholar]
- 74. Lighter J, Raabe V. Azithromycin should not Be used to treat COVID‐19. Open Forum Infect Dis. 2020;7(6):ofaa207. 10.1093/ofid/ofaa207 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


