Abstract
During the COVID‐19 pandemic, there has been wide heterogeneity in the medical management of transplant recipients. We aimed to pragmatically capture immunosuppression practices globally following the early months of the pandemic. From June to September 2020, we surveyed 1267 physicians; 40.5% from 71 countries participated. Management decisions were made on a case‐by‐case basis by the majority (69.6%) of the programs. Overall, 76.8% performed ≥1 transplantation and many commented on avoiding high‐risk transplantations. For induction, 26.5% were less likely to give T‐cell depletion and 14.8% were more likely to give non‐depleting agents. These practices varied by program‐level factors more so than the COVID‐19 burden. In patients with mild, moderate and severe COVID‐19 symptoms 59.7%, 76.0%, and 79.5% decreased/stopped anti‐metabolites, 23.2%, 45.4%, and 68.2% decreased/stopped calcineurin inhibitors, and 25.7%, 43.9%, and 57.7% decreased/stopped mTOR inhibitors, respectively. Also, 2.1%, 30.6%, and 46.0% increased steroids in patients with mild, moderate, and severe COVID‐19 symptoms. For prevalent transplant recipients, some programs also reported decreasing/stopping steroids (1.8%), anti‐metabolites (10.3%), calcineurin inhibitors (4.1%), and mTOR inhibitors (5.5%). Transplant programs changed immunosuppression practices but also avoided high‐risk transplants and increased maintenance steroids. The long‐term ramifications of these practices remain to be seen as programs face the aftermath of the pandemic.
Keywords: COVID‐19 pandemic, COVID‐19 therapeutics, global survey, immunosuppression practices, induction, maintenance, outcomes, transplantation
1. INTRODUCTION
Transplant programs across the world have faced unique challenges during the COVID‐19 pandemic. 1 Initial studies reported that solid organ transplant recipients with SARS‐CoV‐2 were at higher risk for adverse outcomes, 2 , 3 , 4 and mortality rates in transplant recipients with COVID‐19 were reported to be as high as 13%–30%. 2 , 3 , 4 , 5 There was unclear understanding of the pathogenesis of the virus in an immunocompromised host, 6 and wide heterogeneity in the medical management of new and prevalent transplant recipients during the pandemic. However, emerging evidence suggests that after adjusting for age, co‐morbidities, and other variables, the mortality rates might be similar to the general population. 7 , 8 , 9 Also, a recent systematic review of 33 studies reported the mortality rate to be 17.1% in admitted COVID‐19 patients, but 40.5% in studies reporting outcomes in patients with critical illness. 10
Despite the immense amount of literature on COVID‐19 over the past few months, navigating the evidence and applying it to immunosuppressed transplant recipients is a daunting task. Current practice recommendations are limited to expert opinions, which are based on emerging, but low‐quality evidence in transplantation. 11 Existing data are at risk of outcome reporting bias, as not every patient case is being reported, and the nature and direction of the outcomes may determine what is being reported. While no specific data from trials including transplant recipients with COVID‐19 have been published so far, concerns have been raised on the off‐label and potentially harmful use of targeted therapies. 12 , 13 Several variabilities also exist in managing immunosuppression. In the United States, centers were less likely to administer T‐cell depleting agents (TDA) for induction. 14 In terms of maintenance immunosuppression, depending on the patient's symptoms, a stepwise reduction in immunosuppression is recommended. 1 , 12 , 14 , 15 , 16 There is a dearth of literature in practices related to non‐hospitalized transplant recipients with COVID‐19 and prevalent transplant recipients.
While published literature is evolving from case reports to larger multi‐center studies and international registries, 15 sharing of experience worldwide is being called upon to provide a foundation for clinical care. 17 Thus, the aim of our study was to pragmatically capture immunosuppression management practices during the early months of the pandemic.
2. METHODS
From June to September 2020, we conducted a multinational survey of transplant programs during the COVID‐19 pandemic and this manuscript reports the immunosuppression management practices. This study was approved by the Research Ethics Board at the McGill University Health Centre.
2.1. Survey creation
The survey was designed using an iterative process by our team composed of transplant professionals and research methodologists. To do this, we conducted a thorough review of the COVID‐19 literature reported by the Transplantation Society and the American Society of Transplantation. For methodological guidance on survey creation, we sought the works of Boynton, Gillham, and Oppenheim. 18 , 19 , 20 We ensured questions were clear, simple, and neutral. 21 We reviewed all items for relevance, redundancy, and wording. To minimize bias due to predisposition toward socially acceptable answers, that is, social acceptability bias, we formulated the questions to be as neutral as possible. 22 To reduce the risk of acquiescence bias, where applicable, the Likert scale was used. 23 Following modifications and multiple rounds of revisions, the final survey was created and then reviewed by the executive committee of the Transplantation Society. It was self‐administered electronically using the Qualtrics XM platform in English and Mandarin. The survey was first pilot tested using 10 participants who represented four different countries of varied income level. Following this only minor modifications were made, thus these responses were included in the data analysis.
2.2. Recruitment
We recruited a convenience sample of transplant physicians who were identified as key informants in their programs using publicly available data (congress web pages, program websites) and with the help of regional organizations and individuals (see acknowledgements). We sought one individual per organ transplant program from various centers across the world to take the survey (one center can have up to five different transplant programs, hence, up to five participants from each program could be contacted). Our recruitment goal was 500 different individuals representing 500 different transplant programs. We identified and contacted 1339 physicians from 80 different countries; of these 209 physicians had directly reached out to us with interest in participating in our study. Of the 1267 that were eligible, 513 from 71 different countries completed the survey for a response rate of 40.5%. We also used quota sampling to ensure a heterogenous sample as outlined in Figure 1. 24
FIGURE 1.
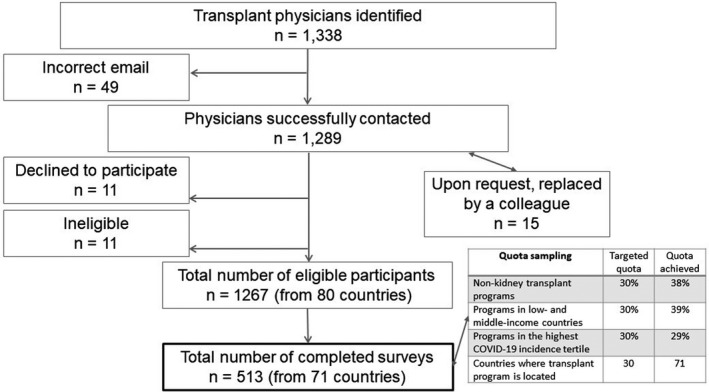
Study flow diagram
2.3. Definitions
New transplantation was one that was performed during the pandemic. Patients with mild COVID‐19 symptoms were those more likely to be treated as an outpatient; patients with moderate COVID‐19 symptoms were those more likely to be treated as an inpatient but not ICU; and patients with severe COVID‐19 symptoms were those who needed care in the ICU. Cumulative COVID‐19 incidence (CCI) was calculated from March 13 to July 15, 2020 using the Johns Hopkins COVID Map and supplemented using data reported at covidindia.org. 25 , 26 For the United States, Canada, Australia, India, and China, CCI was calculated by states/provinces.
2.4. Data
Survey questions pertained to selecting pre‐specified options. A comment box was provided to describe other practices and a descriptive review of these comments was also conducted. The specific outcomes of interest (both descriptive and objective) are as follows:
2.4.1. New transplants performed
We first asked participants whether their program performed any new transplants during the pandemic. If yes, we asked if any recipients were infected with SARS‐CoV‐2. We also asked them to describe their impression for the following short‐term outcomes when compared with their program's norm: acute rejection, infections (excluding COVID), patient death, primary non‐function, and delayed graft function (kidney only). Participants were also asked to rate the likelihood of their program giving COVID‐19 prophylaxis to new transplant recipients on a scale of 1 to 5 (1 being unlikely and 5 being very likely).
2.4.2. Induction and maintenance immunosuppression practices
For induction immunosuppression practices, we asked programs if they made changes to TDA, non‐TDA, and steroids protocols. We then analyzed if four factors influenced the odds of making changes to these induction therapies: the CCI, baseline transplant volume, type of organ transplant, and patient age group. To capture maintenance immunosuppression practices, we presented questions segregated by four clinical situations (prevalent patients and those with mild, moderate, and severe COVID‐19 symptoms). We asked participants to report their program practices for most patients with respect to steroids, anti‐metabolite agents, calcineurin inhibitors, and mammalian target of rapamycin (mTOR) inhibitors.
2.5. Data analysis
Each transplant program, hence, each survey response was treated as a unit of analysis. A descriptive analysis was conducted for each question and associated comments. For the outcome of the likelihood of giving COVID‐19 prophylaxis, Bartlett's test of homogeneity of variances was used to examine variances across survey responses. Multinomial logistic regression was used to determine the association between the variables mentioned above and the odds of making changes to induction immunosuppression. The response “do not know” was excluded for this analysis and when analyzing by the type of organ, and those who picked multiple organs as their scope of practice were also excluded. All analysis was performed using Stata 16.0/MP for Windows, and a significance level using P < .05 was reported.
3. RESULTS
3.1. Baseline characteristics
Survey participants listed their primary roles as transplant surgeons (28.8%), transplant physicians (67.1%), administrators (1.6%), infectious disease specialists (0.6%), or others (1.9%). The characteristics of the transplant programs they represented are in Table 1 and the location of the program is illustrated in Figure S1. Management decisions during the pandemic were made on a case‐by‐case basis by 69.6% of the programs or using a standard policy by 27.5% (1.8% picked other and 1.2% picked do not know). Some reported deferring management decisions to the infectious disease specialists.
TABLE 1.
Baseline characteristics of transplant programs
| Type of organ transplant | |
| Heart | 8.6% |
| Kidney | 55.5% |
| Liver | 19.9% |
| Lung | 8.2% |
| Pancreas/Islet | 1.6% |
| Multiple | 6.2% |
| Age group of recipients | |
| Adult only | 64.1% |
| Pediatric only | 10.9% |
| Both | 25.0% |
| Baseline transplant volume | |
| Low (<20) | 27.6% |
| Moderate (21–100) | 45.1% |
| High (>100) | 27.3% |
| Country's income‐level a | |
| Low‐income | 0.8% |
| Lower‐middle‐income | 15.6% |
| Upper‐middle‐income | 23.0% |
| High‐income | 60.6% |
| Region's cumulative COVID‐19 incidence b (ppm) | |
| Low | <2031 |
| Medium | 2031–5400 |
| High | >5400 |
| COVID‐19 patient caseload c | |
| None | 28.8% |
| <5 | 31.0% |
| 5–10 | 16.2% |
| 11–20 | 13.5% |
| 21–50 | 6.4% |
| >50 | 3.5% |
| Do not know | 0.6% |
As defined by the World Bank athttps://www.worldbank.org/
Calculated from March 13 to July 15, 2020 by region and as reported by the Johns Hopkins COVID Map, supplemented by covidindia.org. Reported in person per million population (ppm) and divided into tertiles for the entire cohort.
Self‐reported number of transplant recipients seen/treated with suspected or confirmed COVID‐19 during the early months of the pandemic.
3.2. New transplants performed
Overall, 76.8% of the programs were able to perform ≥1 transplantation during the first few months of the pandemic. Most programs were unlikely to administer any COVID‐19 prophylactic agents to new transplant recipients; the mean score (standard deviation) was 1.37 (1.02). When segregating by organ type, only 68.9% of kidney/pancreas programs performed transplantation, compared with 88.6% of heart transplant programs, 86.3% of liver transplant programs, and 90.5% of lung transplant programs. Of the 394 programs that performed a transplant, 15.7% reported new recipients infected with the SARS‐CoV‐2 virus, and 15 programs described at least one death due to COVID‐19 (Table S1A). Most observed no change in five short‐term outcomes when compared with the norm. (Figure S2). Also, many reported avoiding high‐risk transplants, such as ABO‐incompatible transplantation (Table S1A, B).
3.3. Induction immunosuppression practices
Changes to induction immunosuppression practices were reported by many programs. For TDA, non‐TDA, and steroids, 56.1%, 71.9%, and 87.7% reported no change, 26.5%, 2.3%, and 1.0% reported less likely to give, and 1.9%, 14.8%, and 8.8% reported more likely to give, respectively. The remaining responded do not know or do not use. Some commented on decreasing the dose of TDA administered for induction (Table S1B).
In regression analysis, CCI was not associated with the odds of reporting changes to induction immunosuppression practices, be it less likely or more likely to give, with one exception. When compared with transplant programs in areas with low CCI, those from areas with high CCI had 45% lower odds of reporting “less likely to give TDA” (odds ratio [OR] = 0.55, 95% confidence interval [CI]: 0.32–0.95). Program‐level factors, in particular baseline volume, seemed to influence induction practices, particularly with respect to TDA and non‐TDA. When compared to programs with low baseline transplant volumes, those with moderate and high volumes had higher odds of reporting both “less likely to give TDA” (OR = 2.19, 95%CI:1.24–3.87 and OR = 3.65, 95%CI:2.00–6.69) and “more likely to give non‐TDA” (OR = 2.35 95%CI:1.15–4.80 and OR = 2.87 95%CI:1.35–6.09), respectively. Type of organ transplant program (kidney/pancreas versus liver/lung/heart) was also associated with less likely to give TDA and more likely to give non‐TDA. Last, compared with adult programs, pediatric‐only programs had 66% lower odds of reporting “less likely to give TDA” (OR = 0.34 95%CI:0.14–0.84) (Table 2).
TABLE 2.
Odds of reporting changes to induction immunosuppression practices by four program‐level factors (significant values in bold)
| T‐cell depleting agents | Non–T‐cell depleting agents | Steroids | ||||
|---|---|---|---|---|---|---|
| Less likely | More likely | Less likely | More likely | Less likely | More likely | |
| Cumulative COVID‐19 incidence (ref: low) a | ||||||
| Medium | 0.72 1.16 1.85 | 0.02 0.20 1.71 | 0.11 0.46 1.89 | 0.51 0.90 1.57 | 0.13 0.92 6.62 | 0.37 0.74 1.47 |
| High | 0.32 0.55 0.95 | 0.21 0.82 3.14 | 0.14 0.57 2.35 | 0.28 0.55 1.08 | 0.05 0.57 6.37 | 0.23 0.51 1.17 |
| Baseline transplant volume (ref: low) b | ||||||
| Moderate | 1.24 2.19 3.87 | 0.05 0.23 1.17 | 0.19 0.68 2.41 | 1.15 2.35 4.80 | 0.03 0.31 3.40 | 0.57 1.22 2.61 |
| High | 2.00 3.65 6.69 | 0.09 0.46 2.35 | 0.09 0.47 2.46 | 1.35 2.87 6.09 | 0.14 1.01 7.27 | 0.47 1.10 2.59 |
| Organ (ref: kidney/pancreas) | ||||||
| Liver | 0.04 0.10 0.26 | 0 | 0.26 1.05 4.15 | 0.03 0.11 0.36 | 0.38 2.72 19.58 | 0.16 0.42 1.12 |
| Heart | 0.05 0.15 0.42 | 0.06 0.52 4.32 | 0.08 0.71 5.91 | 0.01 0.07 0.56 | 0 | 0.09 0.37 1.61 |
| Lung | 0.00 0.04 0.27 | 0 | 0 | 0.01 0.08 0.59 | 0 | 0.03 0.19 1.43 |
| Age group (ref: adult or adult/ped) | ||||||
| Pediatric | 0.14 0.34 0.84 | 0.79 3.20 12.97 | 0.09 0.73 5.78 | 0.22 0.56 1.48 | 0.21 1.91 17.45 | 0.08 0.36 1.51 |
"Less likely" (/"more likely") indicates that a program reported less likely (more likely) to use the agent in question during the early months of the pandemic, when compared to before the pandemic.
calculated from March 13 to July 15, 2020 as reported by the Johns Hopkins COVID Map, supplemented by covidindia.org. Reported in person per million population (ppm) and divided into tertiles for the entire cohort. Low: <2031 ppm, Medium: 2032‐5400 ppm, High: >5400 ppm
Volume was defined as conducting the following number of transplants/year: Low<20, moderate 20‐100, high >100. "0" with no confidence interval indicates that zero respondents in this category gave this response.
3.4. Maintenance immunosuppression practices
For prevalent transplant patients, the majority made no changes to steroids (95.3%), anti‐metabolite agents (86.7%), calcineurin inhibitors (94.1%), and mTOR inhibitors (85.4%) (Figure 2). Although some did report decreasing/stopping/tapering them: 1.8% for steroids, 10.3% for anti‐metabolite agents, 4.1% for calcineurin inhibitors, and 5.5% for mTOR inhibitors. In patients with COVID‐19, as shown in Figure 2, the symptoms of the patient influenced practice. Across the spectrum of mild, moderate, and severe COVID‐19 symptoms, the percentage of programs that reported decreasing or stopping these agents increased from 59.7%–76.0%–79.5% for anti‐metabolite agents, from 23.2%–45.4%–68.2% for calcineurin inhibitors, and from 25.7%–43.9%–57.7% for mTOR inhibitors. For steroids, there was a parallel increase in dose instead, 2.1%–30.6%–46.0%. Some programs also reported switching agents, such as, from tacrolimus to cyclosporine or from mycophenolate to azathioprine (Table S1C).
FIGURE 2.
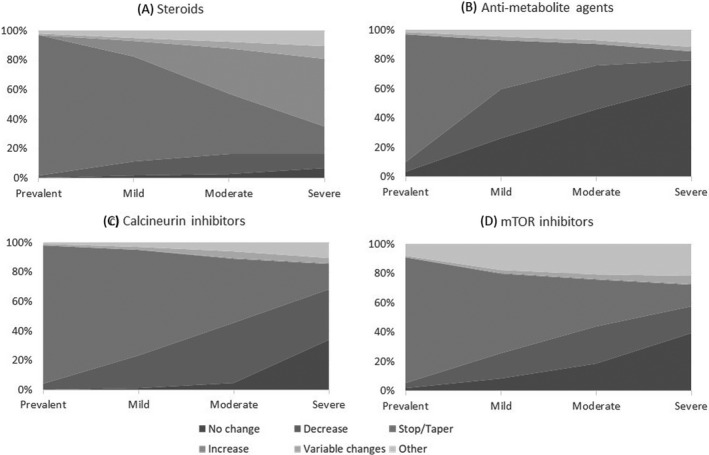
Changes to maintenance immunosuppression regimens: Respondents were asked to report their program practices in most patients within each category of patient symptomatology (Prevalent transplant recipients; patients with mild COVID‐19 symptoms were those more likely to be treated as an outpatient; patients with moderate COVID‐19 symptoms were those more likely to be treated as an inpatient but not ICU; and patients with severe COVID‐19 symptoms were those needing care in the ICU)
3.5. Outcomes in patients with COVID‐19
The majority of transplant programs (70.6%) reported seeing or treating transplant recipients with suspected or confirmed COVID‐19 (31.00% saw <5, 16.2% saw 5–10, 13.5% saw 11–20, 6.4% saw 21–50, and 3.5% saw >50 cases). Figure 3 demonstrates the responses of only those who reported seeing at least one transplant recipient with COVID‐19. From these responses, 94.2%, 90.6%, 87.8%, 69.3%, and 51.4% agreed or strongly agreed that baseline co‐morbidities, severity of symptoms, recipient age, baseline graft function, and type of solid organ determined their patients’ outcomes, respectively. For the latter two, many picked neutral or do not know.
FIGURE 3.
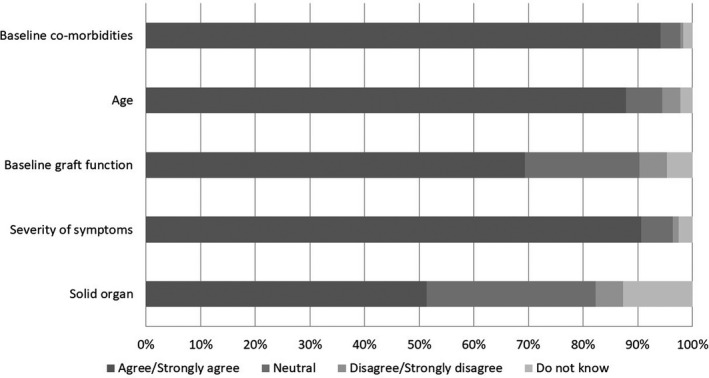
Determinants of outcome in patients with COVID‐19: Only participants from programs that saw or treated transplant recipients with suspected or confirmed COVID‐19 were asked to rate their level of agreement
4. DISCUSSION
In this global survey, we report the early immunosuppression practices of 513 transplant programs from 71 different countries. Of the 76.8% programs that performed a transplant during the first few months of the pandemic, 15.7% reported new transplant recipients with COVID‐19 and the majority were unlikely to administer COVID‐19 prophylaxis. For induction, 26.5% were less likely to give TDA, 14.8% were more likely to give non‐TDA and many commented on avoiding high‐risk transplants. For maintenance immunosuppression, the proportion of programs that reported increasing steroids and decreasing other maintenance immunosuppression increased linearly across the spectrum of transplant recipients with mild‐moderate‐severe COVID‐19 symptoms. However, 4%–10% did report decreasing immunosuppression in prevalent transplant recipients. To our knowledge, this is the first study to capture management practices of transplant programs during the COVID‐19 pandemic on a global scale including in non‐hospitalized recipients with COVID‐19 and prevalent transplant recipients.
Currently, other than expert opinions and case reports, we found only one study that commented on induction practices. For kidney transplant recipients from the United States, Bae et. al. reported that recipients in the pandemic era were 47% less likely to receive TDA compared to their pre‐pandemic counterparts. 14 We report that many transplant programs across the world are indeed making changes to induction immunosuppression practices. Also, the type of solid organ and baseline transplant volumes influenced these changes more so than the COVID‐19 burden. Higher volume centers were less likely to give TDA and more likely to give non‐TDA perhaps speaks to their experience in transplantation or was done to mitigate the risk associated with nosocomial COVID‐19 transmission. Also, the COVID‐19 burden (as defined by CCI) did not influence induction immunosuppression practices except in areas with high CCI where programs had 45% lower odds of reporting “less likely to give TDA.” This is counterintuitive, to what one might expect and the reason for this is unclear. One of the main reasons to use TDA is to enable high immunologic risk transplants and to decrease the risk and severity of acute rejection. 27 , 28 In our survey, 3.1% did observe higher acute rejection, but many also reported avoiding high‐risk transplants. None of these numbers were large enough or granular enough to conduct a robust analysis. However, future work should focus on comparing pre‐pandemic and post‐pandemic graft and patient outcomes due to these variable practices.
In recipients with COVID‐19, there is no agreement on how best to manage these patients. 1 , 5 , 15 A registry analysis of 482 solid organ transplant recipients reported that transplant‐related measures of immunosuppression intensity were not associated with mortality. 5 Despite this, decreasing immunosuppression is the mainstay of therapeutic management with the dominant practice being to hold or reduce the anti‐metabolite agents or mTOR inhibitors. 1 , 5 , 15 Indeed, we demonstrate that in patients with moderate and severe symptoms, 76.0% and 79.5% reported decreasing or stopping anti‐metabolite agents, respectively. We also demonstrate that 10.3% of the programs reported doing this in prevalent transplant recipients and 59.7% reported doing this in patients with mild symptoms. Similar relationships were noted for calcineurin and mTOR inhibitors as well. These practices may influence graft outcomes, as a reduction in maintenance immunosuppression is associated with rejection and graft loss. 29 , 30 However, some programs reported increasing doses of steroids, which may have been done in parallel to decreasing other immunosuppression or as a treatment modality. This may offset the risks of rejection and graft loss, but the long‐term ramifications of these practices remain to be seen.
Two notable findings merit further discussion. Kidney transplant programs may be more disproportionately affected by the pandemic. Given the alternative of dialysis and reliance on the ethical principle of nonmaleficence, many programs globally suspended kidney transplantation in the initial months. 31 We report that only 69% of the kidney transplant programs performed a transplant during the initial months of the pandemic when compared with >86% of the liver/heart/lung programs. Global transplant numbers are heavily driven by kidney transplantations, 32 and we expect dramatic declines in the number of transplants performed in 2020. Second, baseline co‐morbidities strongly determine the outcomes of patients with COVID‐19, 5 , 33 and the majority of the respondents in our survey reported them to be the strongest determinant of the outcome of patients with COVID‐19. The prevalence of baseline co‐morbidities in transplant recipients is high, and these co‐morbidities may be independently associated with mortality in patients with COVID‐19. 5 , 8 , 34 , 35 Thus, the perceived higher morbidity and mortality in transplant recipients may have more to do with the higher prevalence of baseline co‐morbidities than their immunosuppressed state. In fact, immunosuppression is thought to represent a “protective factor” for COVID‐19, 36 and more recent studies are reporting that transplantation in itself does not seem to be a risk factor for inferior outcomes. 7 , 8 Thus, comparing the outcomes of transplant recipients with an appropriate control group of patients with similar co‐morbidities might help solve this conundrum.
We have pragmatically captured induction and maintenance immunosuppression practices and therapeutics on a global scale. The knowledge generated complements the current literature reporting expert opinion or consensus recommendations. The strength of our study is that we were able to include and synthesize the input of transplant leaders from 71 different countries; this has practice implications. Also, we were able to present practice patterns in areas where currently no consensus exists, such as practices related to non‐hospitalized patients with COVID‐19 and prevalent transplant recipients. We, however, acknowledge the following limitations. We employed several best practices in survey design, recruitment, and dissemination. Despite this our response rate was only 40.5%; however, this is at par with the response rate of physician surveys in the literature. 37 Some of our questions pertained to subjective opinions of transplant physicians and objective data is needed to confirm our findings. However, we did employ several best practices in survey design to minimize the risk of acquiescence and social desirability bias. Also, while we had representation from 71 countries that represented a broad range of COVID‐19 incidence, our findings may not be applicable to all. We only captured macro, program‐level practices and we acknowledge there are many micro, patient‐level factors that were not accounted for.
In conclusion, we report immunosuppression practices of solid organ transplant programs from 71 different countries during the early months of the COVID‐19 pandemic. Some transplant programs are making changes to their induction and maintenance immunosuppression practices, avoiding high‐risk transplants, and increasing steroids to mitigate the risks associated with these practices. The long‐term ramifications of these practices remain to be seen as programs face the aftermath of the pandemic. Our findings may inform transplant practice and policy during future pandemics, and in regions that are currently experiencing second and third waves of the pandemic.
CONFLICT OF INTEREST
Dr Segev receives speaking honoraria from Sanofi and Novartis. Dr Sandal has received an education grant from Amgen Canada. The rest of the authors have no disclosures.
AUTHOR CONTRIBUTIONS
Sandal conceived and designed the work, acquired the data, analyzed and interpreted the data; drafted the manuscript; approved the final version. Massie and Chiang involved with data analysis and interpretation; critically revised the manuscript, approved the final version. Boyarsky and Segev involved with design of the study, data analysis and interpretation; critically revised the manuscript, approved the final version. Cantarovich conceived and designed the work, assisted with data collection; critically revised the manuscript, approved the final version.
Supporting information
Fig S1
Fig S2
Table S1
ACKNOWLEDGEMENTS
We thank Dr Lorraine Bell, Dr Andrey Cybulsky, Ms Patricia Hales, Dr Andrea Herrera‐Gayol, Dr Rachel Massicotte, Dr Tomoko Takano, and the executive committee of The Transplantation Society for reviewing our survey. We thank Ms Sijia Wu, Ms Anika Vankooten, and Ms Maricel Hope for their assistance with data collection. We would also like to thank the following individuals and organizations for helping us with the recruitment list: Dr Curie Ahn, Dr Helmut PD Arbogast, Dr Marcelo Barrios, Dr Abdelhadi AL Breizat, Dr Mirela Bušić, Mr Robert Caruso, Dr Toby Coates, Dr Beatriz Domínguez‐Gil, Dr Hiroto Egawa, Dr Susumu Eguchi, Dr Maria Gerbase de Lima, Dr Rodrigo López Falcony, Dr John Forsyth, Dr Gabriel Gondolesi, Dr Mehmet Haberal, Dr Eric Hooste, Dr Tania Imran, Dr Jong Cheol Jeong, Agustín Iturregui, Dr Refaat Kamel, Dr Alvaro Kompatzki, Dr Dirk Kuypers, Dr Vivek Kute, Dr Alexandre Loupy, Dr Maggie Ma, Dr Nancy Kwan Man, Dr Maria A. Matamoros, Dr Raul Mizraji, Dr Alejandro Niño‐Murcia, Ms Nieves Piaggio, Dr Helen Pilmore, Dr Marlies Reinders, Dr Adam Remport, Dr Gamal Saadi, Dr Dennis P. Serrano, Prof. Dra. Idalina Stanley, Dr Anikka Tibell, Dr Luciola Vasquez Flores, Dr Haibo Wang, Dr Karl Martin Wissing, Dr Aysegul Yesilkaya, Dr ShiongShiong Yew, and Dr Bengt von Zur‐Mühlen. The organizations are as follows: the Belgian Transplantation Society, the Brazilian Association for Organ Transplantation, the Chilean Society of Transplantation, the Colombian Organ Transplantation Association, the Mexican Society of Transplantation, the Paraguayan Transplant Society, and the Swedish Transplant Society. Finally, we thank the following orders and organization for supporting/endorsing our study: African Society of Organ Transplantation, Asian Society of Transplantation, Canadian Donation and Transplantation Research Program, China Organ Transplant Response System, European Society of Transplantation, Indian Society of Transplantation, International Pancreas and Islet Transplant Association, International Pediatric Transplant Association, International Society for Heart and Lung Transplantation, International Society of Nephrology, Japan Society of Transplantation, Korean Society of Transplantation, Middle East Society for Organ Transplantation, National Transplant Organization of Spain, The Transplantation Society, Transplantation Society of Australia and New Zealand and Transplant Society of Latin America and the Caribbean.
Sandal S, Boyarsky BJ, Massie A, Chiang TP‐Y, Segev DL, Cantarovich M. Immunosuppression practices during the COVID‐19 pandemic: A multinational survey study of transplant programs. Clin Transplant. 2021;35:e14376. 10.1111/ctr.14376
DATA AVAILABILITY STATEMENT
Data sharing requests for de‐identified data reported in this article, will be considered upon written request to the corresponding author for up to 36 months following publication of this work. Data will be available subject to a written proposal, approval by an independent review committee, and a signed data sharing agreement.
REFERENCES
- 1. Ahn C, Amer H, Anglicheau D, et al. Global transplantation COVID report March 2020. Transplantation. 2020;104(10):1974‐1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Azzi Y, Bartash R, Scalea J, Loarte‐Campos P, Akalin E. Covid‐19 and solid organ transplantation: a review article. Transplantation. 2020;105(1):37‐55. [DOI] [PubMed] [Google Scholar]
- 3. Boyarsky BJ, Massie AB, Love AD, et al. Early experiences with COVID‐19 testing in transplantation. Transplantation Direct. 2020;6(7):e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Messika J, Eloy P, Roux A, et al. COVID‐19 in lung transplant recipients. Transplantation. 2020;105(1):177‐186. [DOI] [PubMed] [Google Scholar]
- 5. Kates OS, Haydel BM, Florman SS, et al. COVID‐19 in solid organ transplant: a multi‐center cohort study. Clin Infect Dis. 2020:ciaa1097. 10.1093/cid/ciaa1097 [DOI] [Google Scholar]
- 6. Fishman JA. The immunocompromised transplant recipient and SARS‐CoV‐2 infection. J Am Soc Nephrol. 2020;31(6):1147‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Webb GJ, Marjot T, Cook JA, et al. Outcomes following SARS‐CoV‐2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avery RK, Chiang TP, Marr KA, et al. Inpatient COVID‐19 outcomes in solid organ transplant recipients compared to non‐solid organ transplant patients: a retrospective cohort. Am J Transplant. 2020. 10.1111/ajt.16431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chavarot N, Gueguen J, Bonnet G, et al. COVID‐19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am J Transplant. 2021;21(3):1285‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macedo A, Gonçalves N, Febra C. COVID‐19 fatality rates in hospitalized patients: systematic review and meta‐analysis. Ann Epidemiol. 2021;57:14‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weiss MJ, Lalani J, Patriquin‐Stoner C, et al. Summary of International recommendations for donation and transplantation programs during the Coronavirus Disease (COVID‐19) pandemic. Transplantation. 2020;105(1):14‐17. [DOI] [PubMed] [Google Scholar]
- 12. Zaidan M, Legendre C. Solid organ transplantation in the era of COVID‐19: lessons from the past six months. Transplantation. 2020;105(1):61‐66. 10.1097/TP.0000000000003536 [DOI] [PubMed] [Google Scholar]
- 13. Laracy JC, Verna EC, Pereira MR. Antivirals for COVID‐19 in solid organ transplant recipients. Curr Transplant Rep. 2020;7(4):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bae S, McAdams‐DeMarco MA, Massie AB, et al. Early changes in kidney transplant immunosuppression regimens during the COVID‐19 pandemic. Transplantation. 2021;105(1):170‐176. 9000;Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahalingasivam V, Craik A, Tomlinson LA, et al. COVID‐19 and kidney transplantation: a systematic review. Kidney Int Rep. 2020;6(1):24‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avery RK. COVID‐19 therapeutics for solid organ transplant recipients; 6 months into the pandemic: where are we now? Transplantation. 2021;105(1):56‐60. [DOI] [PubMed] [Google Scholar]
- 17. Fishman JA, Grossi PA. Novel Coronavirus‐19 (COVID‐19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant. 2020;20(7):1765‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boynton PM, Greenhalgh T. Selecting, designing, and developing your questionnaire. BMJ. 2004;328(7451):1312‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oppenheim AN. Questionnaire Design, Interviewing and Attitude Measurement. London; NY: Pinter Publishers; 1992. [Google Scholar]
- 20. Gillham B. Developing a Questionnaire.. London, UK: Continuum International Publishing Group Ltd; 2000. [Google Scholar]
- 21. Oppenheim A. Questionnaire Design, Interviewing and Attitude Measurement. London, UK: Bloomsbury Publishing PLC; 2000. [Google Scholar]
- 22. de Leeuw ED, vdZJ . Data Quality in Telephone and Face‐To‐Face Surveys: A Comparative Meta‐Analysis. In: Groves RMBP, Lyberg LE, et al. (eds.). Telephone Survey Methodology. hoboken, NJ: John Wiley and Sons; 1998. [Google Scholar]
- 23. Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health. 2005;27(3):281‐291. [DOI] [PubMed] [Google Scholar]
- 24. Encyclopedia of survey research methods. 2008.
- 25. Coronavirus COVID‐19 global cases by the center for systems science and engineering. 2020. https://coronavirus.jhu.edu/map.html. Accessed September, 2020.
- 26. Open repository of all COVIDIndia.org's historical data. 2020. https://covidindia.org/#. Accessed October 15, 2020.
- 27. Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355(19):1967‐1977. [DOI] [PubMed] [Google Scholar]
- 28. Hill P, Cross NB, Barnett AN, Palmer SC, Webster AC. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev. 2017;1(1):CD004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pelletier RP, Akin B, Henry ML, et al. The impact of mycophenolate mofetil dosing patterns on clinical outcome after renal transplantation. Clin Transplant. 2003;17(3):200‐205. [DOI] [PubMed] [Google Scholar]
- 30. McAdams‐DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99(4):805‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stock PG, Wall A, Gardner J, et al. Ethical Issues in the COVID Era: Doing the Right Thing Depends on Location, Resources, and Disease Burden. Transplantation. 2020;104(7):1316‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Global observatory on donation and transplantation. 2020; http://www.transplant‐observatory.org/download/2017‐activity‐data‐report/. Accessed April 24, 2020
- 33. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: A systematic literature review and meta‐analysis. J Infect. 2020;81(2):e16‐e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang Y, Lu Y, Huang YM, et al. Obesity in patients with COVID‐19: a systematic review and meta‐analysis. Metabolism. 2020;113:154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID‐19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Romanelli A, Mascolo S. Immunosuppression drug‐related and clinical manifestation of coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant. 2020;20(7):1947‐1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. International Covenant on Civil and Political Rights . https://www.ohchr.org/en/professionalinterest/pages/ccpr.aspx. Accessed January 9, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Data Availability Statement
Data sharing requests for de‐identified data reported in this article, will be considered upon written request to the corresponding author for up to 36 months following publication of this work. Data will be available subject to a written proposal, approval by an independent review committee, and a signed data sharing agreement.


