Summary
Chloroquine (CQ) and hydroxychloroquine (HCQ) have been used as antiviral agents for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV2) infection. We performed a systematic review to examine whether prior clinical studies that compared the effects of CQ and HCQ to a control for the treatment of non‐SARS‐CoV2 infection supported the use of these agents in the present SARS‐CoV2 outbreak. PubMed, EMBASE, Scopus and Web of Science (PROSPERO CRD42020183429) were searched from inception through 2 April 2020 without language restrictions. Of 1766 retrieved reports, 18 studies met our inclusion criteria, including 17 prospective controlled studies and one retrospective study. CQ or HCQ were compared to control for the treatment of infectious mononucleosis (EBV, n = 4), warts (human papillomavirus, n = 2), chronic HIV infection (n = 6), acute chikungunya infection (n = 1), acute dengue virus infection (n = 2), chronic HCV (n = 2), and as preventive measures for influenza infection (n = 1). Survival was not evaluated in any study. For HIV, the virus that was most investigated, while two early studies suggested HCQ reduced viral levels, four subsequent ones did not, and in two of these CQ or HCQ increased viral levels and reduced CD4 counts. Overall, three studies concluded CQ or HCQ were effective; four concluded further research was needed to assess the treatments' effectiveness; and 11 concluded that treatment was ineffective or potentially harmful. Prior controlled clinical trials with CQ and HCQ for non‐SARS‐CoV2 viral infections do not support these agents' use for the SARS‐CoV2 outbreak.
Keywords: chloroquine, hydroxychloroquine, treatment, viral infection
Abbreviations
- ALT
aspartate aminotransferase
- ART
antiretroviral therapy
- CQ
chloroquine
- EBR
early biochemical response
- EVR
early virological response
- FCT
fever clearance time
- HCQ
hydroxychloroquine
- HPV
human papillomavirus
- IQR
interquartile range
- LFT
liver function test
- LOS
length of hospital stays
- SP
sulfadoxine–pyrimethamine
- TCID
tissue culture infecting doses
- ZEBS
Zambia Exclusive Breast Feeding Study
1. BACKGROUND
Over a short time, the highly transmissible severe acute respiratory syndrome coronavirus 2 (SARS‐CoV2) viral infection went from being an outbreak in Wuhan, China to a worldwide pandemic with a mortality rate roughly 10 times greater than seasonal influenza. 1 , 2 Faced with this rapidly escalating crisis, clinicians sought therapies that could improve outcomes when combined with standard supportive treatments. Based on in vitro findings that aminoquinoline‐4 agents, such as chloroquine (CQ) and hydroxychloroquine (HCQ), inhibited SARS‐CoV2 at clinically achievable concentrations 3 , 4 , 5 and on the widespread use of these agents as anti‐malarial and anti‐inflammatory agents, both were rapidly introduced into patient care. 6 , 7 Early small clinical trials suggested that these agents accelerated SARS‐CoV2 clearance and time to clinical recovery. 6 , 7 However, observational and larger randomised studies have not consistently substantiated these earlier findings, and several indicate CQ and HCQ may have serious adverse effects in some SARS‐CoV2‐infected patients. 8 , 9 , 10 , 11
The antiviral activity of aminoquinoline‐4 agents is thought to be related to inhibition of virus entry and disruption of viral replication. 12 , 13 Based on in vitro and in vivo findings in virus infection models, CQ and HCQ were administered clinically in the past for up to nine different types of noncoronavirus viral infections. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31
But at the outbreak of SARS‐CoV2, neither agent was being recommended for any of these infections, and a recent narrative review, commentary and editorial suggested that prior clinical studies had not provided strong support for aminoquinoline‐4 therapy for viral infection. 32 To comprehensively examine the question of whether prior studies of HCQ and CQ had shown compelling evidence for their benefit in the treatment of other viral infections that would have supported their use for SARS‐CoV‐2, we performed a systematic review and planned a meta‐analysis of clinical studies that were published before the SARS‐CoV‐2 outbreak. This analysis was designed to compare the effects of an aminoquinoline‐4 agent to control for the treatment of any viral infection in earlier studies. Survival, a main therapeutic goal for these agents in COVID‐19 patients, was to be the primary endpoint for the analysis, while viral clearance, biomarkers and organ injury were secondary endpoints.
2. METHODS
This systematic review was registered with PROSPERO on May 1 2020 (CRD42020183429) and prepared using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement guidance for literature review and data extraction (File S1).
2.1. Literature search and study selection
Using published guidelines 33 and search strategies (File S2), two authors (P.T.P. and P.Q.E.) identified relevant studies in PubMed, EMBASE, Scopus and Web of Science from inception through 2 April 2020 without language restrictions. Published clinical studies in adults that compared the effects of CQ, HCQ or related compounds to a control group on survival (primary endpoint), viral clearance, organ injury or other biologic endpoints during viral infection other than coronavirus (secondary endpoint) were retrieved. Recovered reports were scanned for additional relevant ones.
2.2. Data extraction and quality assessment
Four investigators (P.T.P., C.X., Y.L. and P.Q.E.) extracted study data using a standardised extraction form (File S3). If primary and secondary endpoints were not clearly defined in the trial registration, we recorded measures that were most relevant for assessing the efficacy of tested treatments or those emphasised in figures or tables.
Risk of bias was assessed with the Cochrane Collaboration Risk of Bias Assessment Tool for randomised controlled trials and the Newcastle‐Ottawa Tool for observational studies. Three investigators (P.T.P., J.S., P.Q.E.) assessed these risks independently and settled disagreements by consensus. Additional parameters of study weakness were also assessed and tabulated separately.
2.3. Data synthesis and analysis
Unless otherwise noted, levels of significance or non‐significance (p = ns) reported in the text and/or figures and tables, are those provided by the investigators. In some cases, where analysis was not provided in a report but sufficient data available, we calculated the descriptive statistics and/or treatment differences. Median and interquartile ranges (IQRs) were converted to mean and SD using the method of Wan et al. 34 For HIV, which included the greatest number of studies (n = 6), mean differences between treatment and control groups in changes in blood HIV RNA copy levels (log10‐transformed) and CD4+ cell counts from pre‐ to posttreatment were calculated. Heterogeneity among studies was assessed using the Q statistic and I 2 value. 35 The plan was to analyse the mean differences using random‐effect models, 36 if appropriate. However, due to high heterogeneity among studies, we did not combine the studies. Analyses were performed using R 37 (version 4.0.2) with packages meta 38 (version 4.11‐0). In studies also comparing nonaminoquinilone‐4 agents to placebo, data are only presented for the comparison between the control and aminoquinilone‐4 agent.
3. RESULTS
We retrieved 18 studies (File S4) comparing the effects of CQ or HCQ to a control treatment in patients with either chronic HIV infection (n = 6 studies), mononucleosis ([EBV], n = 4), warts ([HPV], n = 2), acute dengue infection (n = 2), chronic HCV (n = 2), acute chikungunya infection (n = 1) and as prophylaxis for influenza A or B infection (n = 1). Tables 1, 2, 3 and Table S1 summarise the following: Table 1, patient populations targeted, study design, the 4‐aminoquinilone and control agent studied, and number of control and treatment patients; Table 2, treatment regimens, length of observation and inclusion and exclusion criteria; Table 3, primary and secondary endpoints or measures investigated; Table S1, enrolment dates, comparisons between groups in baseline parameters and reported adverse events. Seventeen studies were prospective and one retrospective. No study examined the effect of CQ or HCQ on survival, our predefined primary outcome, and only four examined hospitalised patients. The variability of endpoints reported prevented us from proceeding with a meta‐analysis of the studies retrieved. Additionally, because results reported varied dependent on the virus type studied, the studies are presented separately for each virus type.
TABLE 1.
Summary of study characteristics
| Author (year) | Patient population targeted | Location | Study design | Treatment | a Patients | Reference a | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| In‐ versus out‐Pt | Country | CQ or HCQ | Con | Con | Rx | |||||
| EBV (mononucleosis) | ||||||||||
| Cowley (1962) | Suspected infection | In | US | C, DB | CQ | Plac | 20 | 20 | 15 | |
| Schumacher (1963) | Suspected infection | In | US | C, DB | CQ | Plac | 5 | 5 | 25 | |
| Talstad (1964) | Suspected infection | In | Norway | C | CQ | Plac (CaLac) | 40 | 39 | 29 | |
| Updike (1967) | Suspected infection | Out | US | C, DB | CQ | Plac | 19 | 21 | 31 | |
| Human papilloma virus (HPV, warts) | ||||||||||
| Jacobs (1963) | Warts | Out | US | C, DB | CQ | Plac (Lac) | 25 | 25 | 18 | |
| Murphy (1965) | Warts | Out | US | C, DB | HCQ | Plac | 42 | 48 | 20 | |
| HIV | ||||||||||
| Sperber (1995) | Chronic HIV,CD4 200‐500 cells/mm3 | Out | US | R, C, DB | HCQ | Plac | 19 | 19 | 28 | |
| Sperber (1997) | Chronic HIV,CD4 200‐500 cells/mm3 | Out | US | R, C, DB | HCQ | ZDV | 37 | 35 | 27 | |
| Semrau (2006) | Chronic HIV, breast‐feeding, treated for malaria | Out | Zambia | Observational,CC | CQ | SP | 12 | 18 | 26 | |
| Murray (2010) | Chronic HIV,CD4>250 cells/mm3 | Out | US | R, C, DB | CQ | Plac | 3 | 8 | 21 | |
| Paton (2012) | Chronic HIV,CD4>400 cells/mm3 | Out | US | R, C, DB | HCQ | Plac (Lac) | 41 | 42 | 22 | |
| Jacobson (2016) | Chronic HIV, off or on‐ART | Out | US | R, C, DB, Cx | CQ | Plac | 36 b | 34 b | 19 | |
| Chikungunya virus | ||||||||||
| De Lamballerie (2008) | Acute infection | Out | FRI | R, C, DB | CQ | Plac | 27 | 27 | 16 | |
| Dengue virus | ||||||||||
| Tricou (2010) | Acute infection | In | Vietnam | R, C, DB | CQ | Plac | 154 | 153 | 30 | |
| Borges (2013) | Acute infection | Out | Brazil | R, C, DB | CQ | Plac (Starch) | 18 | 19 | 14 | |
| Hepatitis C virus | ||||||||||
| Helal (2016) | Chronic infection; failed IFN/RBV | Out | Egypt | C, SB | HCQ + standard Rx | Standard Rx# | 60 | 60 | 17 | |
| Peymani (2016) | Chronic infection, failed standard Rx | Out | Iran | R, C, TB | CQ | Plac | 4 | 6 | 24 | |
| Influenza A and B virus | ||||||||||
| Paton (2011) | Subjects at risk of infection | Out | SG | R, C, DB | CQ | Plac (Lac) | 738 | 724 | 23 | |
Abbreviations: ART, antiretroviral therapy; C, controlled; CaLac, calcium lactate; CC, cohort controlled; CD4, CD4+ T cells; Con, control; CQ, chloroquine; Cx, cross over; DB, double blind; FRI, French Reunion Island; HCQ, hydroxychloroquine; Lac, lactose; Plac, placebo; Pt, patient; R, randomised; Rx, treatment; SB, single blind; SG, Singapore; SP, sulfadoxine–pyrimethamine; TB, triple blinded.
Standard treatment = pegylated interferon and ribavirin, no placebo described.
In the initial 12 weeks of study, there were a combination of 36 patients in the off‐ and on‐ART control groups and 34 in the CQ groups, see the text.
TABLE 2.
Summary of treatment regimens, observation, and inclusion and exclusion criteria in studies
| Author (year) | Treatment regimen | Observation time | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|
| EBV (mononucleosis) | ||||
| Cowley (1962) | CQ: 1g ×1; 500 mg at 8 h and daily ×9 days; 250 mg daily × 8 days | LOS | Fever; pharyngitis; ↑ WBC and atypical lymphocytes; lymphadenopathy; heterophile Ab ≥ 1:224 | NR |
| Schumacher (1963) | CQ: 1 g ×1; 500 mg q6 h × 24 h; 250 mg every 6 h × 5 days | NR | >50% Lymphocytes; >20% atypical lymphocytes; heterophile Ab ≥ 1:56 | NR |
| Talstad (1964) | CQ: 1 g ×1; 500 mg 6 h later; 500 mg daily × 2 days | LOS | NR | NR |
| Updike (1967) | CQ: 250 mg twice daily × 7 days | NR | >20% Atypical lymphocytes;+heterophile Ab | Illness besides mononucleosis |
| Human papilloma virus (warts) | ||||
| Jacobs (1963) | CQ: 250 mg daily for up to 60 days | ≤60 days | 2–50 verruca, plantaris, plana or accuminata lesions | NR |
| Murphy (1965) | HCQ: 200 mg twice daily maximum of 9 weeks | ≤9 weeks | NR | NR |
| HIV | ||||
| Sperber (1995) | HCQ: 800 mg daily × 8 weeks | 8 weeks | Asymptomatic; no ART for 4 weeks; 200–500 CD4 cells/mm3; Hb, PMN, PLTS ≥ 8.5 g/dl, 1000 and 75,000 cells/mm3 respectively; ALT/AST and amylase <3X and 1.3X ULN respectively | <18 years; pregnant; AIDS defining condition or malignancy, active ETOH/drug abuse, Stage 2 AIDS dementia, known G6PD deficiency |
| Sperber (1997) | HCQ: 800 mg daily × 16 weeks | 16 weeks | Asymptomatic; no ART for 4 weeks; 200–500 CD4 cells/mm3; Hb ≥ 8.5g/dl, PMN ≥ 1000 cells/mm3 respectively; ALT/AST <3X ULN | <18 years; pregnant; AIDS defining condition or malignancy, Stage 2 AIDS dementia, known G6PD deficiency |
| Semrau (2006) | CQ: 600 mg Days 1, 2; 300 mg Day 3 | ≤16 days | NR | NR |
| Murray (2010) | CQ: 250 mg daily in 6 pts, 500 mg daily in 3 pts, × 2 months | 2 months | Chronic HIV; >250 CD4 cells/mm3; off ART ≥ 16 months | NR |
| Paton (2012) | HCQ: 400 mg daily × 48 weeks | 48 weeks | Chronic HIV; 18–65 years; no ART >12 months; >400 CD4 cells/mm3; HIV > 1000 copies/ml | Psoriasis, epilepsy, arrhythmias, depression, diabetes, CA, chronic liver, retinal ds; pregnancy; primary HIV infection <12 months; infection/vaccination last 2 months; +HBV‐Ag; +HCV PCR |
| Jacobson (2016) | CQ: 250 mg daily × 12 weeks | 12 weeks a | 18–55 years; off‐ART – no ART 6 months, HIV ≥ 1000 copies, ≥400 CD4 cells/mm3; on‐ART – on ART for 24 months, <350 CD4 cells/mm3, HIV copies undetectable | NR |
| Chikungunya virus | ||||
| De Lamballerie (2008) | CQ: 600 mg Day 1; 300 mg twice daily Days 2, 3; 300 mg Days 4 and 5 | 25 days, phone follow‐up at 200 days | 18–65 years; >60 kg; <48 h acute febrile arthralgia; biologically confirmed diagnosis (viraemia quantified by real‐time PCR and seroconversion between 1 and 16 days of study | Pregnancy; renal, retinal, celiac ds; contraindications to CQ |
| Dengue virus | ||||
| Tricou (2010) | CQ: 600 mg Days 1, 2; 300 mg Day 3 | ≥15 days b | >15 years; dengue symptoms <72 h | Pregnant; therapy for chronic ds; CQ hypersensitivity; no consent |
| Borges (2013) | CQ: 500 mg twice daily × 3 days | 7 days c | Enrolled if: fever and at least 2: headache, retro‐orbital pain/muscle/joint pain, nausea/vomiting, rash for <72 h. Confirmed dengue by 2 of either PCR, ELISA or NS1 antigen detection assays | <18 years; pregnant; CV or neurological ds |
| Hepatitis C virus | ||||
| Helal (2016) | HCQ: 200 mg twice daily × 12 weeks | 12 weeks | 18–60 years; HCV genotype‐4; ‐HBV‐Ag; +HCV‐Ab; WBC, PMN, and PLTs > 3000, 1500, and 80,000 cells/mm3 respectively; Hb > 12 g/dl in males and 11 g/dl in females, Cr < 1.2; +HCV liver bx in <12 months. | Pregnant; non‐HCV liver ds; liver bx with F0 and F4 for necrosis, inflammation and fibrosis; BMI > 30; CV, thyroid, retinal ds; antiviral/immunosuppressive therapy past 6 months |
| Peymani (2016) | CQ: 150 mg daily × 8 weeks | 8 weeks | Males; 18–60 years; HCV genotype‐1 unresponsive to pegIFN and RBV | Decompensated cirrhosis; anti‐neoplastic, anti‐viral, immunomodulator therapy in prior 6 months; HAV, HBV, HDV, HIV; active ETOH use; mental impairment; LFTs > 5X ULN; adverse reaction to HCQ; HCC; already on therapy for HCV |
| Influenza A and B virus | ||||
| Patton (2011) | CQ: 500 mg daily × 1 week; once weekly × 11 weeks | 12 weeks | 18–65 years | Pregnancy, breast feeding; psoriasis; porphyria; epilepsy; myopathy; depression; CV, retinal, hepatic, renal, ds; G6PD deficiency; hepatotoxic therapy; flu vaccination past 3 months; flu symptoms at screening |
Abbreviations: Ab, antibody; Ag, antigen; AIDS, acquired immunodeficiency syndrome; ALT, alanine amino‐transferase; ART, antiretroviral therapy; AST, aspartate amino‐transferase; BMI, body mass index; Cr, creatinine; CQ, chloroquine; CV, cardiovascular; ds, disease; ELISA, enzyme‐linked immunosorbent assay; ETOH, alcohol; Flu, influenza; HAV, HBV, HDV, hepatitis A, B and D; Hb, haemoglobin; HCQ, hydroxychloroquine; HPV, human papilloma virus; LFT, liver function test; LOS, hospital length of stay; NR, not reported; pegIFN, pegylated interferon; PCR, polymerase chain reaction; PLT, platelet; PMN, polymorphonuclear cell; RBV, ribavirin; Rx, treatment; ULN, upper limit of normal.
Each of two cross‐over periods was 12 weeks.
Blood drawn BID for 5 days in hospital and then at 10–14 days after discharge.
7 days following confirmed infection.
TABLE 3.
Summary of endpoints or measures
| Author (year) | Primary and secondary endpoints prospectively defined | Endpoints or measures | Prospective power analysis performed |
|---|---|---|---|
| EBV (mononucleosis) | |||
| Cowley (1962) | UC |
Followed patients from hospital admission to discharge. Factors assessed for degree of improvement—general condition, throat condition, lymphadenopathy, spleen and liver size, skin eruption, percent diet eaten and drug reaction. Discharged 3–4 days after last temperature elevation and if no further symptoms occurred. Lab data obtained but not presented—WBC and differential, haematocrit, ESR, serologic syphilis test, urinalysis, CXR, throat and blood cultures at admission and then later if needed; LFT twice weekly. |
NR |
| Schumacher (1963) | UC |
Followed patients from hospital admission to discharge. Oral temperature > 99.6°F checked four times per day and signs or symptoms including sore throat, lymphadenopathy, hepatosplenomegaly, exanthem, enanthem reported over the 5 days after starting treatment. Duration of illness prior to therapy, total hospitalisation time and time after therapy. |
NR |
| Talstad (1964) | UC | Mean temperature (°C) 1, 4 and 7 days; mean WBC and ESR 1–3 and 6–9 days; hospital LOS. | NR |
| Updike (1967) | UC | Disabling days—a combination of fully or partially disabling days students recorded in a log while on therapy based on whether they were too sick to attend class (1 point) or attended class but had symptoms (½ point) respectively, during the 7 days of treatment. | NR |
| Human papilloma virus (warts) | |||
| Jacobs (1963) | UC | Wart clearance over 9 weeks; Warts mapped as to size and location at first visit;Baseline and monthly urinalysis, CBC and LFT. | NR |
| Murphy (1965) | UC | Wart clearance over 60 days. | NR |
| HIV | |||
| Sperber (1995) | UC |
Compared changes within groups over 8 weeks of treatment. Successful treatment defined as a reduction in levels of plasma HIV RNA, serum p24, or cultured virus from PBMC. Other measures—CD4 count and %, CBC, β‐microglobulin, IgG, IgM, IgA, IL‐1α, IL‐1β, IL‐6 and TNF; antigen stimulation assays. |
NR |
| Sperber (1997) | UC |
Compared changes within and between groups over 16 weeks of treatment. Successful treatment defined as a reduction in levels of plasma HIV RNA, serum p24, or cultured virus from PBMC. Other measures—CD4 count and %, CBC, β‐microglobulin, IgG, IL‐6. |
NR |
| Semrau (2006) | UC | Compared breast milk and plasma HIV RNA, and CD4 counts in blood obtained 3–16 days after patients received treatment. | NR |
| Murray (2010) | UC |
Blood sampled at baseline, 1 and 2 months of treatment. Measured CD8CD38+HLA+ cell frequency and Ki‐67 expression, CD4 counts, plasma HIV RNA, preservation of CD4 and CD8 cell producing TNF, IFN‐γ, and IL‐2 in response to Gag, Pol, Enc or NEF HIV antigens, LPS levels |
NR |
| Paton (2012) | Yes |
Primary endpoint—Change in proportion of activated T‐cells measured by expression of CD38 and HLA‐DR surface markers from baseline to 48 weeks. Secondary endpoint—Change in CD4 cell activation and counts, plasma HIV RNA and IL‐6 levels from baseline to 48 weeks. Changes calculated based on samples obtained at baseline, 4, 12, 24, 26 and 48 weeks. |
Yes:80 patients |
| Jacobson (2016) | Yes for 1° endpoint | Primary endpoint—changes in %CD8CD38+HLA‐DR+ T‐cells from baseline to the first 12 weeks of study.Other measures—%CD8CD38+HLA‐DR+ T‐cells, over the second 12 weeks of study and CD4 counts, HIV RNA, IL‐6 and LPS over the first and second 12 weeks of study and over the two 12 weeks periods combined. | NR |
| Chikungunya virus | |||
| De Lamballerie (2008) | UC |
Primary (‘main criterion’) duration of febrile arthralgia. Other measures—change in viral genome level from 1 to 3 days and presence of viraemia. Clinical exam was performed by a general practitioner at Days 1, 7 and 25 and patients monitored their symptoms twice daily on Days 1–5 and qd on Days 6–14. Biologic investigations were performed at Days 1, 3, 6 and 16. |
Yes:250 patients required. Only 54 enrolled due to decrease in epidemic |
| Dengue virus | |||
| Tricou (2010) | Yes |
Primary endpoint—time to resolution of viraemia or antigenemia defined as the time from the initiation of treatment until the first two consecutive plasma samples were RT‐PCR negative or NS1 ELISA negative, respectively. Blood samples obtained at hospital admission before drug administration and then twice daily for a minimum of 5 days and again 10–14 days after hospital discharge. Secondary endpoints—fever clearance time defined as the time from the initiation of treatment to the beginning of the first 48‐h period the temperature remained <37.5°C, platelet count nadir, mean maximum % haemoconcentration based on haematocrit values, proportion of patients in either arm requiring intravenous fluids or developing dengue hemorrhagic fever (DHF). |
Yes:213 patients |
| Borges (2013) | UC |
Duration of dengue disease and duration and intensity of fever up to 7 days after treatment. Possible dengue symptoms including fever (axillary temperature ≥ 37.8°C), headache, and retro‐orbital, muscle, bone or joint pain were determined at baseline and after 1 week of therapy. |
NR |
| Hepatitis C virus | |||
| Helal (2016) | Yes |
Primary—early virological response (EVR) defined as either undetectable HCV RNA tested at 12 weeks (complete EVR) or ≥2 log drop in HCV RNA from baseline to 12 weeks (partial EVR). Other measure—early biochemical response (EBR) defined as an aspartate aminotransferase (ALT) < 40 IU after 12 weeks of therapy. |
NR |
| Peymani (2016) | UC | Plasma HCV RNA and ALT were measured at baseline, 4, 8 and 12 weeks. | NR |
| Influenza virus A and B | |||
| Paton (2011) | Yes |
Primary endpoint—combination of laboratory‐confirmed and clinical influenza.Laboratory‐confirmed influenza infection based on one of the following test results—PCR confirmation on a nasal swab taken by the participant, PCR confirmation or positive influenza culture on a nasal swab taken by a health‐care worker, or serological confirmation by at least a fourfold increase in antibody titre on a haemoagglutinin‐inhibition or microneutralisation assay for H1N1, H3N2 or influenza B infection from baseline to 12 weeks. Clinical influenza based on the development of influenza‐like symptoms including reported temperature of at least 37.2°C with at least one respiratory symptom (sneezing, runny nose, blocked nose, sore throat, dry cough, coughing up phlegm, wheezing, shortness of breath) and at least one constitutional symptom (feverish feeling, muscle aches, fatigue, headache, diarrhoea) occurring on the same day. Main secondary endpoint—laboratory confirmed influenza alone. Patients completed weekly diaries if asymptomatic or daily ones if symptomatic via a password‐protected trial website that presented symptom checklists. Blood was drawn at baseline and at 12 weeks; patients were followed by their primary care givers. |
Yes:1500 patients |
Abbreviations: ALT, alanine aminotransferase; CBC, complete blood count; CQ, chloroquine; CXR, chest x‐ray; ELISA, enzyme‐linked immune‐absorbent assay; ESR, erythrocyte sedimentation rate; HCQ, hydroxychloroquine; IgG, M and A, immunoglobulins g, M and A; IL‐1, 2, 6, interleukin 1,2, and 6; IFN, interferon; IU, international units; LFT, liver function tests; LOS, length of stay; LPS, lipopolysaccharide; NR, not reported; PBMC, peripheral blood mononuclear cells; RT‐PCR, real time polymerase chain reaction; UC, unclear; WBC, white blood cell count.
3.1. Mononucleosis (EBV)
Three controlled double‐blind and one controlled trial compared CQ to placebo in patients with suspected mononucleosis. Only one study provided baseline data. 15 Cowley 15 enrolled 220 hospitalised patients and presented data on 40 hospitalised patients. No descriptive statistics or analysis were reported but based on data shown in the figures, CQ patients had shorter mean (days ± SEM) length of hospital stays (LOS) and duration of subjective symptoms (p ≤ 0.02), but not of fever, pharyngitis or abnormal liver function tests (LFTs; p ≥ 0.07; Figure 1). Schumacher 25 investigated hospitalised patients treated with either CQ (n = 5) or placebo (n = 5) for up to 6 days. No descriptive statistics are provided. Compared to placebo, CQ patients had similar LOS after starting treatment (16.2 vs. 17.8, no p‐value reported), mean temperatures over 5 days, and temperatures at 2 and 5 days (p > 0.1). Numbers of patients with fever, signs or symptoms at 5 days did not differ (p ≥ 0.40). No adverse events were noted. Talstad 29 studied hospitalised patients treated for 3 days. Of 93 patients enrolled, 39 received CQ and 40 placebo. No descriptive statistics are presented but CQ reportedly had no significant effect (no p‐values reported) on LOS, temperatures, white blood cell counts or erythrocyte sedimentation rates. One patient in the CQ group had a cardiac arrest and died but this was attributed to mononucleosis after autopsy. Finally, Updike 31 studied outpatient college students treated for 7 days. Of 60 screened patients, 21 received CQ and 19 placebo. The only measure presented, the mean (±SEM) number of disability days, was not significantly different between groups (no p‐value reported; Figure 1). No adverse event data were provided.
FIGURE 1.
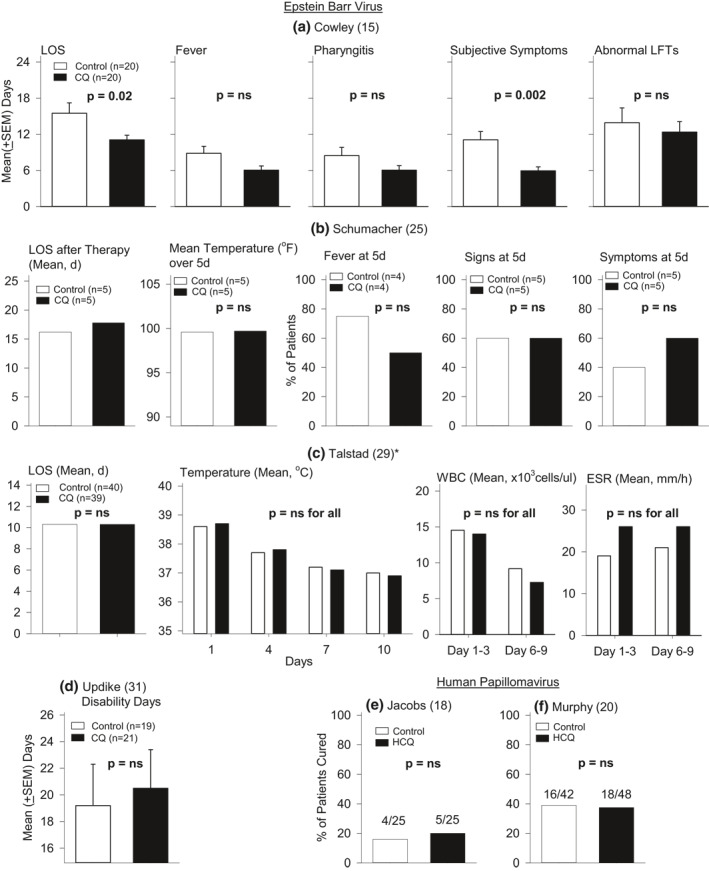
The effect of CQ or HCQ compared to control on measures reported in four studies of mononucleosis and EBV infection ((a)Cowley et al., (b) Schumacher et al., (c), Talstad et al. (d) and Updike et al.) and two studies of warts and human papilloma virus infection ((e) Jacobs et al. and (f) and Murphy et al.). Mean (±SEM) data and levels of significance were provided in the reports or were calculated based on data provided in the reports. Except for the effect of CQ on the length of hospital stay (LOS) and changes in subjective symptoms in the study by Cowley et al., CQ did not have significant (p = ns and p > 0.05) effects compared to placebo on any other measure in that or any other of the studies shown. CQ, chloroquine; HCQ, hydroxychloroquine
3.2. Warts (HPV)
Two controlled double‐blind studies compared the effects of CQ or HCQ to placebo administered for up to 63 days on wart clearance. Partial or no baseline data were presented. Jacobs 18 reported that 4 of 25 placebo (16%) versus 5 of 25 CQ (20%) CQ patients had wart clearance (p = 0.71). Murphy 20 reported that 16 of 42 (39%) placebo versus 18 of 48 (37.5%) HCQ patients had wart clearance (p = 0.95; Figure 1). Adverse events were not different between groups in either study.
3.3. Chronic HIV infection
Five randomised controlled double‐blind and one retrospective study examined CQ or HCQ in patients with chronic HIV infection (Tables 1, 2, 3 and S1). Sperber compared the effect of HCQ to placebo over 8 weeks in one study 28 and to Zidovudine 27 over 16 weeks in a subsequent one. Limited baseline patient demographic data appeared similar between study groups in each trial. In the first study 28 with 19 patients per group, possible differences in baseline viral level measures (mean ± SD) in the HCQ versus placebo groups were not analysed (plasma PCR HIV RNA 311 ± 331 vs. 222 ± 215 counts/min and 5136 ± 836 vs. 835 ± 136 copies/ml; and serum p24 16 ± 19 vs. 1 ± 3 pg/ml, control vs. treatment group, respectively). Eleven HCQ and nine placebo patients had no detectable serum p24 levels and 19 patients (no group assignment reported) had negative viral cultures. Compared to pretreatment levels, HCQ decreased plasma HIV RNA counts/min (p = 0.02) and placebo did not (p = ns). Neither HCQ nor placebo significantly altered plasma HIV RNA copies/ml, CD4 counts (Table 4), serum p24 levels, or tissue culture infecting doses (TCIDs) of virus. We extracted the fold change data for individual patients from their figure and calculated the mean difference (95% confidence interval) between treatment and control. When comparing changes in blood HIV RNA copy levels from pre‐ to posttreatment, HCQ appeared to decrease viral loads compared to control, as shown in Figure 2. HCQ decreased IL‐6 levels (p = 0.02) but not placebo (p = ns). In the subsequent study, 27 HCQ (n = 35 patients) and Zidovudine (n = 37) both decreased plasma HIV RNA copies/ml, serum p24 and TCID (p ≤ 0.05 for each) but neither altered CD4 counts. Levels of IL‐6 decreased with HCQ (p = 0.03) but not zidovudine (p = ns). There were no adverse events with treatment in either study.
TABLE 4.
HIV studies: Blood viral copy numbers and CD4+ T‐cell counts comparing pre‐ and postcontrol versus chloroquine or hydroxychloroquine treatment
| Author (year) | Control | p‐Value pre versus post | CQ or HCQ | p‐Value pre versus post | p‐ValueCQ or HCQ versus control | ||
|---|---|---|---|---|---|---|---|
| Pre | Post (value or change from baseline) | Pre | Post (value or change from baseline) | ||||
| Blood viral copy numbers/ml | |||||||
| Sperber (1995) | 835 ± 136 | 988 ± 455 | NS | 5136 ± 836 | 1334 ± 899 | NS | NR |
| Sperber (1997) a | 42,709 ± 33050 | 11,228 ± 7459 | 0.001 | 39,456 ± 31000 | 16,434 ± 11373 | 0.02 | NR |
| Semrau (2006) b | NR | 4.58 ± 0.78 | NR | NR | 4.65 ± 0.73 | NR | 0.75 |
| Murray (2010) (log10/ml) | 3.80 ± 0.99 | 3.75 ± 1.27 | NR | 4.77 ± 0.32 | 4.81 ± 0.55 | NS | NR |
| Paton (2012) (log10/ml) | 4.11 ± 0.53 | +0.23 (0.08, 0.38) c | NR | 4.33 ± 0.48 | +0.61 (0.37, 0.85) c | NR | 0.003 |
| Jacobson (2016) off‐ART d (log10/ml) | 4.42 (4.03, 4.83) | −0.20 (‐0.27, −0.02) c | 0.08 | 4.48 (4.02, 4.74) | +0.29 (0.15, 0.35) c | <0.001 | <0.001 |
| Jacobson (2016) on‐ART d | NA | NA | NA | NA | NA | NA | NA |
| CD4 cells/mm3 | |||||||
| Sperber (1995) | 312 ± 121 | 321 ± 124 | NS | 263 ± 166 | 251 ± 163 | NS | NR |
| Sperber (1997) a | 275 ± 145 | 262 ± 131 | NS | 276 ± 140 | 272 ± 134 | NS | NR |
| Semrau (2006) b | NR | 272 ± 166 | NR | NR | 392 ± 178 | NR | 0.07 |
| Murray (2010) | 389 ± 62 | 431 ± 143 | NR | 402 ± 183 | 361 ± 136 | NR | NR |
| Paton (2012) | 509 ± 121 | −23 (−60, 14) c | NR | 492 ± 114 | −85 (−125, −45) c | NR | 0.03 |
| Jacobson (2016) off‐ART d | 493 (448, 541) | −11 (−38, 71) c | NS | 641 (561, 755) | −27 (−150, 4) c | NS | 0.21 |
| Jacobson (2016) on‐ART d | 270 (218, 296) | 7 (−17, 23) c | NS | 259 (224, 281) | −6 (−21, 10) c | NS | 0.25 |
| Jacobson (2016) on‐ART | −1.2 (−3.3, 1.4) | −3.1 (−4.3, 0.4) | 0.077 | 0.25 | |||
| Jacobson (2016) on‐ART:combined 2 12‐weeks treatment cycles | −3.0 Median decrease | 0.003 | |||||
Note: When data from the full 24 weeks of study was analysed together, chloroquine treatment was associated with a significant increase in median viral levels (+0.30 log10/ml, p < 0.001) in the off‐ART groups and reductions in the median CD4 counts (cells/mm3) in both the off‐ART (−39, p = 0.04) and on‐ART (−15, 0.02) groups.
Abbreviations: ART, antiretroviral therapy; CQ, chloroquine; HCQ, hydroxychloroquine; NA, not applicable; NR, not reported; NS, reported to be non‐significant; pre, pretreatment; post, posttreatment.
Control = Zidovudine.
Control = Sulfadoxine–pyrimethamine.
Change from pre to post.
Data from the first 12 weeks of study which was the primary end point of the study.
FIGURE 2.
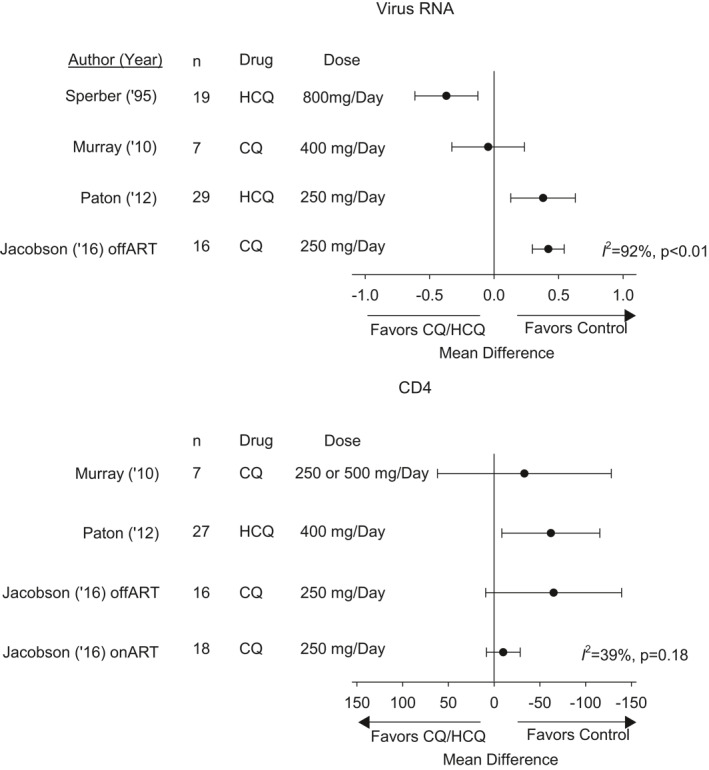
The effect of CQ or HCQ compared to control on the change (calculated as the mean difference [95% CI]) from pre‐ to posttreatment on plasma human immunodeficiency viral RNA levels (log10 HIV RNA copies/ml) and on blood CD4 cell counts (cells/mm3) in the four studies each these data could be calculated in. Also noted are the drug regimens used in each trial. There was heterogeneity across studies for both measures. Notably, while HCQ appeared to decrease viral levels in Sperber, 28 it increased these in Paton and in patients off antiretroviral therapy (off‐ART) in Jacobson (on‐ART patients had negative viral levels pretreatment). Neither treatment was associated with an increase in CD4 cell counts and in Paton it decreased them. ART, antiretroviral therapy; CI, confidence interval; CQ, chloroquine; HCQ, hydroxychloroquine
Semrau 26 compared the effects of CQ to sulfadoxine–pyrimethamine (SP; thought to lack anti‐HIV activity) treatment on breast milk and plasma HIV RNA in patients with HIV receiving acute anti‐malaria treatment. Chart review from the Zambia Exclusive Breast Feeding Study (ZEBS) identified breast feeding women treated for malaria with CQ (n = 18 patients) or SP (n = 12 patients) who had breast milk and plasma samples within 3–16 days of anti‐malarial treatment. Compared to SP, CQ‐treated subjects did not have significantly different breast milk HIV RNA levels (mean ± SD, log10 copies/ml) (2.59 ± 1.04 vs. 3.14 ± 1.03, respectively, p = 0.21), plasma HIV RNA levels (p = 0.75) or CD4 counts (p = 0.07) (Table 4). After adjustment for viral loads and CD4 counts at ZEBS enrolment, the authors reported breast milk viral levels were possibly lower with CQ (p = 0.053). Adverse event data were not recorded.
Murray 21 compared the effects of 2 months of daily CQ (n = 9) versus placebo (n = 4) on immune activation, viral loads and CD4 counts in patients who had been off antiretroviral therapy (ART) ≥ 16 months. Data were presented for seven or eight CQ and two or three placebo patients depending on the measure. CQ treatment decreased %CD8CD38+HLA‐DR+ cells at 1 and 2 months (p ≤ 0.05) and CD8+Ki67+ cells at 1 (p = 0.03) but not 2 months (p = 0.4) and placebo did not alter either significantly. Neither treatment reportedly altered plasma HIV RNA levels significantly at 1 or 2 months, and a mean difference we calculated indicated the effects of CQ and placebo did not differ significantly, as shown in Figure 2. Similarly, CQ did not change CD4 counts (Table 4, Figure 2). Adverse event data were not reported.
Paton 22 compared the effects of a 48‐weeks regimen of HCQ to placebo in patients off ART > 12 months. The primary end point was change in the frequency of CD8+CD38+HLA‐DR+ T‐cells, and secondary endpoints were changes in HIV viral loads, CD4 counts and activation and IL‐6 levels. Based on an a priori power calculation of 80 patients, 42 HCQ and 41 placebo‐treated patients were enrolled. Baseline data were similar between groups. Nine HCQ and one placebo patient were started on ART therapy for decreasing CD4 counts and their data after ART initiation were excluded. Three placebo and one HCQ patient dropped out due to adverse events. Changes in the %CD8+Cd38+HLA‐DR+ cells and IL‐6 from baseline to 48 h did not differ between groups (p ≥ 0.80). When we assessed the effect of HCQ therapy compared to control on the change from pre‐ to posttreatment in HIV viral load and CD4 count, HCQ led to increases in viral loads and decreases in CD4 counts that were significantly greater than placebo (p ≤ 0.03, as reported by the authors; Table 4, Figure 2). Adverse events did not differ between groups except for increased influenza‐like illnesses with HCQ treatment (p = 0.03).
Jacobson 19 compared CQ to placebo over two 12 weeks periods in HIV patients either not on ART (off‐ART group) or on ART (on‐ART group). There was a cross‐over after the first 12 weeks, in which patients initially receiving CQ received placebo for the subsequent 12 weeks and vice versa for patients initially receiving placebo. The primary endpoint was change in %CD8+CD38+HLA‐DR+ cells over the first 12 weeks, with additional endpoints of HIV RNA levels, CD4 counts, and IL‐6 levels. Sixteen off‐ART patients initially received CQ (Arm A) and 17 received placebo (Arm B). Eighteen on‐ART patients initially received CQ (Arm C) and 19 received placebo (Arm D). Three patients, one each from Arms A, B and C discontinued the study prematurely. Compared to placebo CQ did not significantly decrease the %CD8+CD38+HLA‐DR+ cells over the first 12 weeks periods in off‐ART or on‐ART patients (p ≥ 0.25), but produced a decrease in the on‐ART group for the combined 12 weeks periods following cross‐over (p = 0.003). In off‐ART patients, CQ increased HIV RNA levels over the first and combined 12 weeks periods (p < 0.001), also shown in our analysis of the effect of CQ on changes from pre‐ to posttreatment in viral loads compared to control (Figure 2). On‐ART patients had negative viral loads. CQ did not alter CD4+ counts significantly over the first 12 weeks period in either off‐ART or on‐ART groups (Table 4, Figure 2) but differences for the combined 12 weeks periods could not be determined. CQ was not associated with significant changes in IL‐6 levels in either group. No adverse events were reported.
3.4. Acute chikungunya infection
In a randomised double‐blind trial, De Lamballerie 16 compared the effect of 5 days of CQ therapy to placebo over 25 days on febrile arthralgia in patients with acute biologically confirmed chikungunya infection‐associated febrile arthralgia. The primary endpoint was febrile arthralgia duration. An a priori power calculation required 250 patients, but the infection outbreak ended and only 27 patients were randomised to each group. No baseline data or full descriptive statistics are provided. The mean duration of febrile arthralgia (4.7 days with CQ vs. 3.9 days with placebo) and mean reduction in viraemia (genomes/ml blood) between Days 1 and 3 (1.6 × 109 with CQ vs. 6.8 × 108 with placebo) were reportedly not significantly different between groups (no p‐value provided; Figure 3). Eleven patients in each group by 3 days, and all patients by 6 days had negative viraemia. At a 200 days follow‐up interview that most enrollees reportedly participated in, 61% of CQ and 25% of placebo patients described persistent arthralgia (p < 0.0001). Seven CQ and no placebo patients described mild adverse events (p < 0.01).
FIGURE 3.
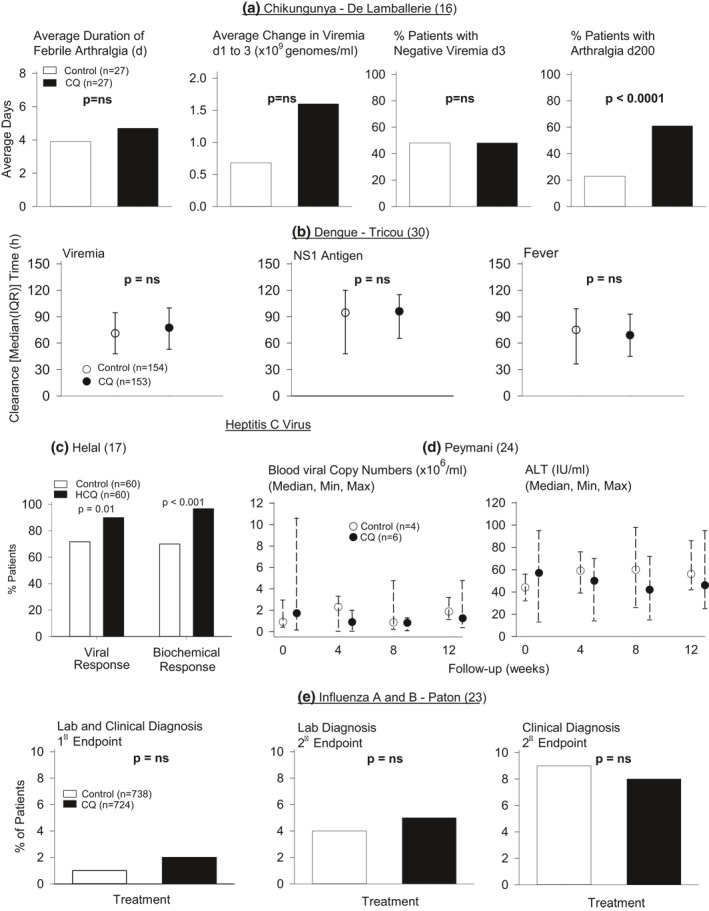
The effect of CQ or HCQ compared to control on measures reported in one study each of acute chikungunya ((a) Lamballerie et al.) and dengue ((b) Tricou et al.), two studies of chronic hepatitis C ((c) Helal et al. and (d) Peymani et al.) and one study of influenza A and B ((e) Paton et al.). Median (IQR) data and levels of significance were provided in the report by Tricou et al. Comparison between the CQ and control group based on the median with maximum and minimum data provided was not possible in the Peymani study. Except for the effect of CQ on increasing the percentage of patients with a viral or biochemical response (i.e., a reduction in alanine aminotransferase level) in the study by Helal et al. CQ did not have significant (p = ns and p > 0.05) effects compared to placebo on any other in the studies shown except that it increased the percentage of patients reported to have late stage arthralgia following chikungunya infection. CQ, chloroquine; HCQ, hydroxychloroquine; IQR, interquartile renge
3.5. Acute dengue infection
Two randomised double‐blind trials compared 3 days of CQ to placebo for patents presenting within 72 h of the onset of suspected dengue fever. In the study by Tricou, 30 the primary endpoint was time to resolution of viraemia or antigenemia. Secondary endpoints included fever clearance time (FCT), platelet count nadir, haemoconcentration, intravenous fluid requirement and dengue hemorrhagic fever development. A required sample size of 213 patients was calculated a priori. Of 307 patients randomised (n = 153 CQ and 154 placebo), 248 were viraemic (n = 124 per group). Baseline characteristics did not differ between groups except dengue viraemia was higher in CQ‐treated patients. In intention‐to‐treat analysis, CQ did not alter median (IQR) viral clearance time (h) (p = 0.10), time to negative NS1 antigenemia (p = 0.70), or, when adjusted for baseline covariates, FCT (p = 0.28; Figure 3). These measures did not differ significantly comparing the per‐protocol populations and secondary endpoints did not differ between groups (p ≥ 0.07). There were more adverse events reported with CQ (p = 0.01).
In the study by Borges, 14 investigators obtained data regarding possible dengue symptoms including fever (axillary temperature ≥ 37.8°C), headache, and retro‐orbital, muscle, bone or joint pain in patients with laboratory confirmed infection. Patients were enrolled if they presented with dengue‐like symptoms. Of 129 randomised patients, 54 had confirmed dengue infection, of which only 18 placebo and 19 CQ patients completed the study. Baseline data appeared similar between groups. There were reportedly no significant differences in the duration of disease symptoms or the intensity and days of fever comparing groups but no descriptive statistics or analysis are provided. Twelve CQ and no placebo patients described reduced pain intensity and increased ability to do daily chores (p < 0.001). Adverse effects by treatment group were not provided.
3.6. Chronic active HCV infection
Helal 17 compared 12 weeks of HCQ and placebo treatment in patients with chronic active HCV receiving interferon plus ribavirin therapy in a randomised single‐blind study. The primary endpoint was early virological response (EVR) based on plasma HCV RNA reductions. Early biochemical response (EBR) based on aspartate aminotransferase (ALT) reductions was also investigated. From 120 patients, 60 were randomised to each group. Baseline characteristics were similar between groups. Compared to placebo, the percentage of patients with EVR or EBR was greater in the HCQ‐treated group (p ≤ 0.01) (Figure 3). Adverse events did not differ significantly between groups (p ≥ 0.08).
Peymani 24 compared 8 weeks of CQ to placebo treatment in patients with chronic HCV unresponsive to standard therapy in a pilot, randomised triple‐blind placebo‐controlled trial. Median, maximum and minimum HCV RNA and ALT levels were reported. Of 12 randomised patients, data are reported for six CQ and four placebo patients. Baseline data were reportedly not different between groups. The authors reported that HCV viral and ALT levels varied over time in the CQ group but did not compare changes in these levels in the CQ versus control groups, or provide sufficient information to allow this comparison (Figure 3). Adverse events were reportedly not different between groups.
3.7. Influenza A and B prophylaxis
Paton 23 compared 12 weeks of CQ to placebo treatment on preventing influenza A or B infection in healthy individuals during the 2009 H1N1 outbreak in a randomised double‐blind placebo‐controlled trial. The primary endpoint was the incidence of a combination of laboratory‐confirmed and clinical influenza. The main secondary endpoint was laboratory confirmed influenza. Clinical influenza was based on the development of influenza like symptoms. An a priori sample size of 1500 patients was calculated. A total of 759 patients were randomised to placebo and 757 to CQ. Baseline data were similar between groups. More CQ than placebo patients withdrew (33 vs. 21, p = 0.048). There were no significant differences between groups in laboratory‐confirmed clinical influenza (p = 0.376), laboratory only diagnosis (p = 0.261) or clinical diagnosis (p = 0.70; Figure 3). One or more adverse events in patients occurred more frequently with CQ (p < 0.0001).
3.8. Summary of study conclusions
Overall, only three of these eighteen studies concluded that treatment with CQ or HCQ was effective (Table S2). Four concluded that study results indicated further research was necessary to assess the tested agent's effectiveness. Eleven studies concluded that CQ or HCQ or the regimens employed had been ineffective. None of the studies included mortality as an endpoint.
3.9. Potential risk of bias
Potential risk of bias appeared high among most studies (Table 5). Randomisation sequence generation and/or allocation concealment were frequently not reported. Although most studies indicated there was blinding of subjects and personnel at the outset of the study, only one study 22 reported methods to blind outcome assessment from the well‐known bitterness and gastrointestinal upset CQ and HCQ produce. 39 , 40 This latter weakness is of particular concern since many recorded measures were subjective ones (LOS without discrete discharge criteria and signs and symptoms [pharyngitis, wart clearance, arthralgia, ability to do work]). Eight of the eighteen studies did not report on all randomised patients. Absence of a priori power analyses, incomplete or absent baseline data, and incomplete or absent descriptive statistics weakened many studies as well (Table 6).
TABLE 5.
Assessment of potential risk of bias in prospective controlled trials
| Author (year) | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | |
|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding of personnel and subjects a | Blinding of outcome assessment | Incomplete outcome data or incomplete outcome data addressed | Selective reporting | |
| EBV (mononucleosis) | ||||||
| Cowley (1962) | High | Low | UC | UC | High | UC |
| Schumacher (1962) | High | High | High | High | High | UC |
| Talstad (1964) | UC | High | UC | UC | High | UC |
| Updike (1967) | UC | UC | UC | UC | Low | UC |
| Human papilloma virus (warts) | ||||||
| Jacobs (1963) | High | UC | UC | UC | Low | UC |
| Murphy (1965) | UC | UC | UC | UC | Low | UC |
| HIV | ||||||
| Sperber (1995) | UC | UC | UC | UC | High | UC |
| Sperber (1997) | UC | UC | UC | UC | High | UC |
| Murray (2010) | UC | UC | UC | UC | High | UC |
| Paton (2012) | Low | Low | Low b | Low | Low | Low |
| Jacobson (2016) c | UC | UC | UC | UC | High | High |
| Chikungunya | ||||||
| De Lamballerie (2008) | UC | UC | UC | UC | Low | UC |
| Dengue | ||||||
| Tricou (2010) d | High | High | UC | Low | Low | UC |
| Borges (2013) d | High | High | UC | UC | Low | Low |
| Hepatitis C virus | ||||||
| Helal (2016) | UC | UC | High | High | Low | UC |
| Peymani (2016) | Low | Low | UC | Low | High | High |
| Influenza A and B | ||||||
| Paton (2011) | Low | Low | Low e | Low | Low | High |
Abbreviation: UC, unclear.
HCQ and CQ are reported to be bitter. Bitterness of these tablets can lead to lack of appropriate blinding.
In this report the authors use an encapsulation process to mask the bitterness of HCQ.
This is a cross‐over study and the authors do not specify a washout period before crossing from one arm of the study to the other.
In these two reports, randomisation takes place before eligibility is confirmed thus leading to high selection bias.
In this study the authors used capsules.
TABLE 6.
Assessment of study design weaknesses in prospective controlled trials
| Author (year) | Weakness | ||||
|---|---|---|---|---|---|
| Prospective power analysis reported | Comprehensive baseline data provided | Subjective measures reported | Complete descriptive statistics provided | Statistical analysis reported | |
| EBV (mononucleosis) | |||||
| Cowley (1962) | No | No | Yes | No | No |
| Schumacher (1963) | No | Yes | Yes | No | No |
| Talstad (1964) | No | No | No | No | No |
| Updike (1967) | No | No | Yes | No | No |
| Human papilloma virus (warts) | |||||
| Jacobs (1963) | No | No | Yes | No | No |
| Murphy (1965) | No | No | Yes | No | No |
| HIV | |||||
| Sperber (1995) | No | Yes | No | Yes | Yes |
| Sperber (1997) | No | Yes | No | Yes | Yes |
| Murray (2010) | No | No | No | No | Yes |
| Paton (2012) | Yes | Yes | No | Yes | Yes |
| Jacobson (2016) | No | Yes | No | Yes | Yes |
| Chikungunya | |||||
| De Lamballerie (2008) | Yes | No | Yes | No | Yes |
| Dengue | |||||
| Tricou (2010) | Yes | Yes | No | Yes | Yes |
| Borges (2013) | No | Yes | Yes | No | Yes |
| Hepatitis C virus | |||||
| Helal (2016) | No | Yes | No | Yes | Yes |
| Peymani (2016) | No | Yes | No | No | Yes |
| Influenza A and B | |||||
| Paton (2011) | Yes | Yes | No | Yes | Yes |
4. DISCUSSION
Compelling factors prompted the use of CQ and HCQ during the early stages of the SARS‐CoV2 outbreak. Mortality rates associated with this novel coronavirus were exceptionally high and these agents had had anti‐viral effects in in vitro studies with SARS‐CoV2 and appeared relatively safe as anti‐malarial and anti‐inflammatory therapies. 3 , 4 , 5 However, this systematic review shows that for several reasons, the prior controlled clinical experience with CQ or HCQ would not have supported their use as antiviral agents.
First, while the primary goal with CQ and HCQ therapy for SARS‐CoV2 was to reduce mortality rates in acutely hospitalised patients, none of the 18 studies retrieved with any of the viral infections investigated tested these agents' effects on survival. Only four studies examined hospitalised patients, and only seven examined treatment for an acute viral infection (i.e., mononucleosis, dengue and chikungunya), while the majority evaluated the effectiveness of these agents in chronic infections.
Second, while HIV, chikungunya, dengue and HCV trials were also based in part on in vitro studies reporting the respective antiviral effects of CQ or HCQ, clinical trials provided little data that these agents actually reduced viral levels in patients. In contrast to two early studies in chronic HIV infection reporting an antiviral effect with HCQ, the more recent ones found that CQ did not decrease viral levels in two and that either HCQ or CQ actually increased viral levels in two others. CQ or HCQ also did not alter viral levels with acute chikungunya or dengue infection in the two studies providing these data. While HCQ produced an antiviral response in one study with chronic HCV infection, CQ's effects in another HCV study with only 10 patients were uninterpretable. Finally, as a prophylactic agent, CQ did not alter evidence of influenza A or B infection.
Third, these were generally small studies and the potential risk of bias appeared increased in many. Nine studies included 50 or fewer subjects and only three had more than 100 patients. While 17 of the 18 studies were described as prospective and controlled, only three adequately described both random sequence generation and allocation methods. Due to the bitterness and gastrointestinal upset with these agents, inadequate outcome blinding may have been a problem, a particular concern in studies presenting subjective findings. Eight studies had high attrition bias due to not providing data on all enrolled patients or high rates of losing patients to follow‐up. Only two studies explicitly described the primary and secondary endpoints that we were able to confirm by checking their trial registration. Only a minority of studies provided descriptive statistics along with the results. Finally, no study appeared to be without any of the weaknesses outlined in Table 6.
Fourth, and most importantly, only 3 of the 18 studies concluded that CQ or HCQ was effective (one study each for mononucleosis, HIV and HCV) and these effects were not reproduced or tested adequately in subsequent studies (Table S2). Four concluded that further research was necessary to determine the benefits of the agent investigated and the other 11 concluded that treatment was ineffective.
While the studies examined here provided little clinical evidence that CQ or HCQ were effective antiviral agents, whether they demonstrated these agents had minimal risk with viral infection is unclear. Four studies did not report adverse event data or presented insufficient data to compare groups. Six simply reported there were no adverse events in groups. One study in mononucleosis patients reported a cardiac arrest and fatality in one patient that was thought to be related to the infection itself. However, QT interval prolongation is known to occur with these agents and may underlie some of the adverse cardiac events reported in SARs‐CoV2 patients. 41 Of the remaining seven studies, three indicated there were no significant differences in adverse events comparing groups (p ≥ 0.08) while four reported significant increases in one or more adverse events in the CQ or HCQ group. Notably, HCQ or CQ were associated with significant increases in viral levels with chronic HIV infection in two studies and CQ worsened later stage arthralgia in acute chikungunya infection.
It is noteworthy that, as Table 2 shows, the regimens of CQ and HCQ differed across studies and these differences were sometimes substantial. Some data from the present SARS‐CoV‐2 outbreak suggest that dosages used in some countries may have only partially achieved maximal tissue concentrations. 42 , 43 CQ doses recommended in China (e.g., 1000 mg/day) where this agent has been strongly recommended for SARS‐CoV‐2 were higher than doses from the non‐SARS‐CoV‐2 virus studies examined here. However, while differing dose regimens of CQ and HCQ may have influenced the effects these agents across the studies we examined, the endpoints measured in studies were sufficiently different and the number of studies for each virus type too small to allow stratification and sensitivity analysis based on drug‐dosing. However, for HIV, the virus with the greatest number of studies, the treatment regimens are provided in Figure 2 for comparison.
A recent narrative review, editorial and commentary concluded that the prior clinical experience with CQ or HCQ as antiviral agents did not provide strong evidence supporting their use for SARS‐CoV2 and that these agents could pose risks for some SARS‐CoV2 patients. 32 Another recent systematic review that did not report a PROSPERO registration was consistent with these prior publications. 44 The present systematic review, which included all of the controlled clinical studies presented in these prior reports (except for one paediatric study 45 ) and seven additional ones with EBV, HPV and HIV, further supports these conclusions.
The present study has limitations. First, the differing measures reported and limited number and quality of studies examining treatment for the individual viruses investigated prevented a synthesis and meta‐analysis of most available data. Of note, for HIV, the one virus‐type a limited quantitative analysis was possible, CQ and HCQ had highly variable effects on CD4 counts, and may have actually increased viral load, although these findings were also variable across studies. Second, it did not include one published randomised open label study of CQ in HIV children, 45 but that study also did not report benefit with treatment. Finally, parameters reported regarding possible severity of disease at baseline differed across studies (e.g., fever and subjective symptoms for EBV; wart number for HPV; CD4 counts and viral load for HIV; fever and joint symptoms for chikungunya; viraemia and antigenemia for acute dengue; LFTs and histology for HCV) and could not be succinctly summarised in a table. However, when comparisons were presented for such baseline parameters between treated and control patients, these were not reportedly different in studies.
The benefits and risks of HCQ and CQ for SARS‐CoV2 either administered prophylactically or for acute disease remain controversial. 8 , 9 , 10 , 11 , 46 Several recent systematic reviews of this clinical experience have thus far suggested these agents lack clear benefit. 47 , 48 On 15 June 2020, the United States Food and Drug Administration revoked its Emergency Use Authorization for either HCQ or CQ. 49 In early July 2020, the World Health Organization accepted the Solidarity Trial's International Steering Committee's recommendation to discontinue the trial's HCQ arm. 50 The trial's interim results had shown that HCQ produced little or no reduction in the mortality of hospitalised COVID‐19 patients and safety was a potential concern. Finally, a recent trial comparing the efficacy of HCQ with or without azithromycin to standard of care failed to detect any improvement in clinical status in patients hospitalised with mild to moderate SARS‐CoV2 infection. 11 Although endpoints are difficult to compare, the limited beneficial effects HCQ and CQ have demonstrated clinically with SARS‐CoV2 are consistent with these agents' effects with the other types of viral infections examined in the present report.
5. CONCLUSIONS
CQ and HCQ have been suggested and tested as anti‐viral agents for the treatment of patients hospitalised with COVID‐19. This systematic review of the literature evaluating the effects of these agents in the treatment of nine different non‐SARS‐CoV2 viral infections suggests that well‐designed randomised controlled trials are needed to evaluate the use of these agents for the treatment of COVID‐19. At the present time, the only therapies that data suggest may be beneficial for the treatment of COVID‐19 are remdesivir 51 and dexamethasone. 52 A recent randomised controlled trial provides promising data supporting the use of inhaled nebulised interferon beta‐1a. 53
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Parizad Torabi‐Pariziand and Peter Q. Eichacker conceived and designed the study with contributions from Junfeng Sun. Diane Cooper conducted the initial search and contributed to the manuscript. Parizad Torabi‐Pariziand and Peter Q. Eichacker reviewed search results and Parizad Torabi‐Pariziand, Xizhong Cui, Yan Li and Peter Q. Eichacker extracted data with support from Zoe Couse. Parizad Torabi‐Pariziand, Junfeng Sun, Samuel J. Minkove, and Peter Q. Eichacker wrote and edited the manuscript. All authors reviewed and approved the final version of this manuscript for submission.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGMENT
Intra‐mural funding from the National Institutes of Health (NIH) supported this work. The NIH had no role in the design of the study or the collection, analysis and interpretation of the data. The opinions expressed in this manuscript reflect those of the authors and do not reflect the views of the National Institutes of Health, the Department of Health and Human Services or of the United States Government.
Cui X, Sun J, Minkove SJ, et al. Effects of chloroquine or hydroxychloroquine treatment on non‐SARS‐CoV2 viral infections: a systematic review of clinical studies. Rev Med Virol. 2021;31(6):e2228. 10.1002/rmv.2228
REFERENCES
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. J Am Med Assoc. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yao X, Ye F, Zhang M, et al. Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;71(15):732‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov. 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J, Liu D, Liu L, et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID‐19. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):215‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with COVID‐19. N Engl J Med. 2020;382(25):2411‐2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in‐hospital mortality in patients with COVID‐19 in New York State. J Am Med Assoc. 2020;323(24):2493‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID‐19. N Engl J Med. 2020;383(6):517‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild‐to‐moderate COVID‐19. N Engl J Med. 2020;383:2041‐2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3(11):722‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oscanoa TJ, Romero‐Ortuno R, Carvajal A, Savarino A. A pharmacological perspective of chloroquine in SARS‐CoV‐2 infection: an old drug for the fight against a new coronavirus? Int J Antimicrob Agents. 2020;56(3):106078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borges MC, Castro LA, da Fonseca BAL. Chloroquine use improves dengue‐related symptoms. Mem Inst Oswaldo Cruz. 2013;108(5):596‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cowley RG, Myers JE, Jr. Chloroquine in the treatment of infectious mononucleosis. Ann Intern Med. 1962;57:937‐945. [DOI] [PubMed] [Google Scholar]
- 16. De Lamballerie X, Boisson V, Reynier JC, et al. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 2008;8(6):837‐839. [DOI] [PubMed] [Google Scholar]
- 17. Helal GK, Gad MA, Abd‐Ellah MF, Eid MS. Hydroxychloroquine augments early virological response to pegylated interferon plus ribavirin in genotype‐4 chronic hepatitis C patients. J Med Virol. 2016;88(12):2170‐2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacobs PH, Tromovitch TA, Lucasg, Puzak HP. Effect of chlorquine and placebo on warts. Arch Dermatol. 1963;87:89‐90. [DOI] [PubMed] [Google Scholar]
- 19. Jacobson JM, Bosinger SE, Kang M, et al. The effect of chloroquine on immune activation and interferon signatures associated with HIV‐1. AIDS Res Hum Retroviruses. 2016;32(7):636‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murphy JC, Petty S. Chloroquine treatment of warts. A double‐blind clinical study. Rocky Mt Med J. 1965;62(1):25‐26. [PubMed] [Google Scholar]
- 21. Murray SM, Down CM, Boulware DR, et al. Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol. 2010;84(22):12082‐12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paton NI, Goodall RL, Dunn DT, et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV‐infected patients not receiving antiretroviral therapy: a randomized controlled trial. J Am Med Assoc. 2012;308(4):353‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paton NI, Lee L, Xu Y, et al. Chloroquine for influenza prevention: a randomised, double‐blind, placebo controlled trial. Lancet Infect Dis. 2011;11(9):677‐683. [DOI] [PubMed] [Google Scholar]
- 24. Peymani P, Yeganeh B, Sabour S, et al. New use of an old drug: chloroquine reduces viral and ALT levels in HCV non‐responders (a randomized, triple‐blind, placebo‐controlled pilot trial). Can J Physiol Pharmacol. 2016;94(6):613‐619. [DOI] [PubMed] [Google Scholar]
- 25. Schumacher HR, Jacobson WA, Bemiller CR. Treatment of infectious mononucleosis. Ann Intern Med. 1963;58:217‐228. [DOI] [PubMed] [Google Scholar]
- 26. Semrau K, Kuhn L, Kasonde P, et al. Impact of chloroquine on viral load in breast milk. Trop Med Int Health. 2006;11(6):800‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sperber K, Chiang G, Chen H, et al. Comparison of hydroxychloroquine with zidovudine in asymptomatic patients infected with human immunodeficiency virus type 1. Clin Therapeut. 1997;19(5):913‐923. [DOI] [PubMed] [Google Scholar]
- 28. Sperber K, Louie M, Kraus T, et al. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin Therapeut. 1995;17(4):622‐636. [DOI] [PubMed] [Google Scholar]
- 29. Talstad I. Chloroquine treatment of infectious mononucleosis. Nord Med. 1964;72:1327‐1328. [PubMed] [Google Scholar]
- 30. Tricou V, Minh NN, Van TP, et al. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis. 2010;4(8):e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Updike SJ, Eichman PL. Infectious mononucleosis treated with chloroquine. A double‐blind study of 40 cases. Am J Med Sci. 1967;254(1):69‐70. [DOI] [PubMed] [Google Scholar]
- 32. Touret F, de Lamballerie X. Of chloroquine and COVID‐19. Antivir Res. 2020;177:104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items For Systematic Reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. [DOI] [PubMed] [Google Scholar]
- 34. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 36. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 37. R. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 38. Schwarzer G. meta: an R package for Meta‐Analysis. R News. 2007;7(3):40‐45. [Google Scholar]
- 39. Wallace DJ, Tse K, Hanrahan L, Davies R, Petri MA. Hydroxychloroquine usage in US patients, their experiences of tolerability and adherence, and implications for treatment: survey results from 3127 patients with SLE conducted by the Lupus Foundation of America. Lupus Sci Med. 2019;6(1):e000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jimenez‐Alonso J, Sabio JM, Carrillo‐Alascio PL, et al. Intolerance to hydroxychloroquine marketed in Spain (Dolquine) in patients with autoimmune conditions. Rev Clin Esp. 2004;204(11):588‐591. [DOI] [PubMed] [Google Scholar]
- 41. Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3(4):e208857. [DOI] [PubMed] [Google Scholar]
- 42. Tarek M, Savarino A. Pharmacokinetic basis of the hydroxychloroquine response in COVID‐19: implications for therapy and prevention. Eur J Drug Metab Pharmacokinet. 2020;45(6):715‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Neves FS. Dose‐response effects of hydroxychloroquine on prophylaxis against SARS‐CoV‐2/COVID‐19 infection. Clin Infect Dis. 2020;ciaa1807. 10.1093/cid/ciaa1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodrigo C, Fernando SD, Rajapakse S. Clinical evidence for repurposing chloroquine and hydroxychloroquine as antiviral agents: a systematic review. Clin Microbiol Infect. 2020;26(8):979‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Engchanil C, Kosalaraksa P, Lumbiganon P, et al. Therapeutic potential of chloroquine added to zidovudine plus didanosine for HIV‐1 infected children. J Med Assoc Thai. 2006;89(8):1229‐1236. [PubMed] [Google Scholar]
- 46. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID‐19: a randomized trial. Ann Intern Med. 2020;173(8):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID‐19. J Crit Care. 2020;57:279‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cortegiani A, Ippolito M, Ingoglia G, Iozzo P, Giarratano A, Einav S. Update I. A systematic review on the efficacy and safety of chloroquine/hydroxychloroquine for COVID‐19. J Crit Care. 2020;59:176‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coronavirus (COVID‐19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. 2020. https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐update‐fda‐revokes‐emergency‐use‐authorization‐chloroquine‐and [Google Scholar]
- 50. “Solidarity” Clinical Trial for COVID‐19 Treatments. 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/global‐research‐on‐novel‐coronavirus‐2019‐ncov/solidarity‐clinical‐trial‐for‐covid‐19‐treatments. [Google Scholar]
- 51. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID‐19‐final report. N Engl J Med. 2020;383(19):1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID‐19. N Engl J Med. 2020;384(8):693–704. 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Monk PD, Marsden RJ, Tear VJ, et al. Safety and efficacy of inhaled nebulised interferon beta‐1a (SNG001) for treatment of SARS‐CoV‐2 infection: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Respir Med. 2021;9:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


