Summary
Despite over 140 million SARS‐CoV‐2 infections worldwide since the beginning of the pandemic, relatively few confirmed cases of SARS‐CoV‐2 reinfection have been reported. While immunity from SARS‐CoV‐2 infection is probable, at least in the short term, few studies have quantified the reinfection risk. To our knowledge, this is the first systematic review to synthesise the evidence on the risk of SARS‐CoV‐2 reinfection over time. A standardised protocol was employed, based on Cochrane methodology. Electronic databases and preprint servers were searched from 1 January 2020 to 19 February 2021. Eleven large cohort studies were identified that estimated the risk of SARS‐CoV‐2 reinfection over time, including three that enrolled healthcare workers and two that enrolled residents and staff of elderly care homes. Across studies, the total number of PCR‐positive or antibody‐positive participants at baseline was 615,777, and the maximum duration of follow‐up was more than 10 months in three studies. Reinfection was an uncommon event (absolute rate 0%–1.1%), with no study reporting an increase in the risk of reinfection over time. Only one study estimated the population‐level risk of reinfection based on whole genome sequencing in a subset of patients; the estimated risk was low (0.1% [95% CI: 0.08–0.11%]) with no evidence of waning immunity for up to 7 months following primary infection. These data suggest that naturally acquired SARS‐CoV‐2 immunity does not wane for at least 10 months post‐infection. However, the applicability of these studies to new variants or to vaccine‐induced immunity remains uncertain.
Keywords: COVID‐19, SARS‐CoV‐2, reinfection
Abbreviations
- Covid‐19
coronavirus disease 2019
- CI
confidence interval
- Ct
cycle threshold
- HIQA
Health Information and Quality Authority
- IgG
immunoglobulin G
- NAAT
nucleic acid amplification technology
- RNA
ribonucleic Acid
- RT‐PCR
reverse transcription polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus type 2
- WHO
World Health Organization
1. INTRODUCTION
Following the emergence of a novel coronavirus (SARS‐CoV‐2) in China in December 2019 and the declaration by WHO of a public health emergency of international concern on 30 January 2020, countries worldwide have experienced epidemics of Covid‐19. While much is yet unknown about the immune response following infection with SARS‐CoV‐2, evidence is emerging at a fast pace. The Health Information and Quality Authority (HIQA) of Ireland has conducted a series of rapid reviews on various public health topics relating to SARS‐CoV‐2 infection. These reviews arose directly from questions posed by policy makers and expert clinicians supporting the National Public Health Emergency Team to inform the national response to the pandemic in Ireland.
Our team at HIQA previously concluded that SARS‐CoV‐2 infection produces detectable immune responses in most cases. 1 However, the extent to which previously infected people are immune to reinfection is uncertain. In the short term, protection against reinfection is probable, as few confirmed SARS‐CoV‐2 reinfections have been reported despite over 140 million infections worldwide since the beginning of the pandemic. 2
The objective of this systematic review was to evaluate the risk and relative risk of SARS‐CoV‐2 reinfection over time, comparing previously infected individuals to those without evidence of prior infection. The review informed a range of policy questions relating to the duration of protective immunity (as in, prevention of reinfection) following SARS‐CoV‐2 infection.
2. METHODS
A standardised protocol was employed 3 based on Cochrane methodology. 4 Electronic databases (PubMed, EMBASE and EuropePMC) were searched from 1 January 2020 to 19 February 2021 (Data S1). Table 1 outlines the Population, Outcome, Study design (POS) criteria for study selection.
TABLE 1.
Population outcome Study design criteria for systematic search
| Population | Individuals (of any age) with evidence of prior SARS‐CoV‐2 infection, who subsequently recovered a |
| Evidence of prior infection includes diagnosis by RT‐PCR or antigen testing, or evidence of an immune response through antibody detection (seropositivity) | |
| Outcomes |
|
| Types of studies |
|
Abbreviation: RT‐PCR, reverse transcription polymerase chain reaction.
‘Recovered’ refers to molecular or clinical evidence of viral clearance following initial infection; definitions of recovery in primary studies were used. Common definitions include two consecutive negative respiratory RT‐PCR tests 24 h apart and WHO clinical criteria of viral clearance (27 May 2020). 5
Reinfection was defined as any reverse transcription polymerase chain reaction (RT‐PCR) or antigen‐confirmed SARS‐CoV‐2 infection in an individual with evidence of a prior SARS‐CoV‐2 infection. Evidence of prior infection included a previously documented immune response through antibody detection (seropositivity) and/or a prior SARS‐CoV‐2 diagnosis by RT‐PCR or antigen testing followed by recovery (molecular or clinical evidence of viral clearance). No minimum time interval was defined between primary and secondary infections; however, cases within 90 days of initial infection were considered suggestive of prolonged viral shedding following the primary infection.
All potentially eligible papers, including preprints, were exported to Endnote x8.2 and screened for relevance by one reviewer. Following removal of irrelevant citations, two reviewers independently reviewed the full text of potentially relevant articles. For each included study, data on study design, participant demographics and relevant clinical and laboratory data were extracted by two reviewers. Quality appraisal was undertaken using the National Heart, Lung and Blood Institute (NIH) quality assessment tool for observational cohort studies. 6 The findings of the research question were synthesised narratively due to the heterogeneity of study designs and outcome data.
3. RESULTS
The collective database search resulted in 1893 citations, with four citations retrieved from other sources (grey literature search). Following removal of duplicates, 1771 citations were screened for relevance. This resulted in 105 studies eligible for full text review (Figure 1), where a further 94 studies were excluded (Table S1).
FIGURE 1.
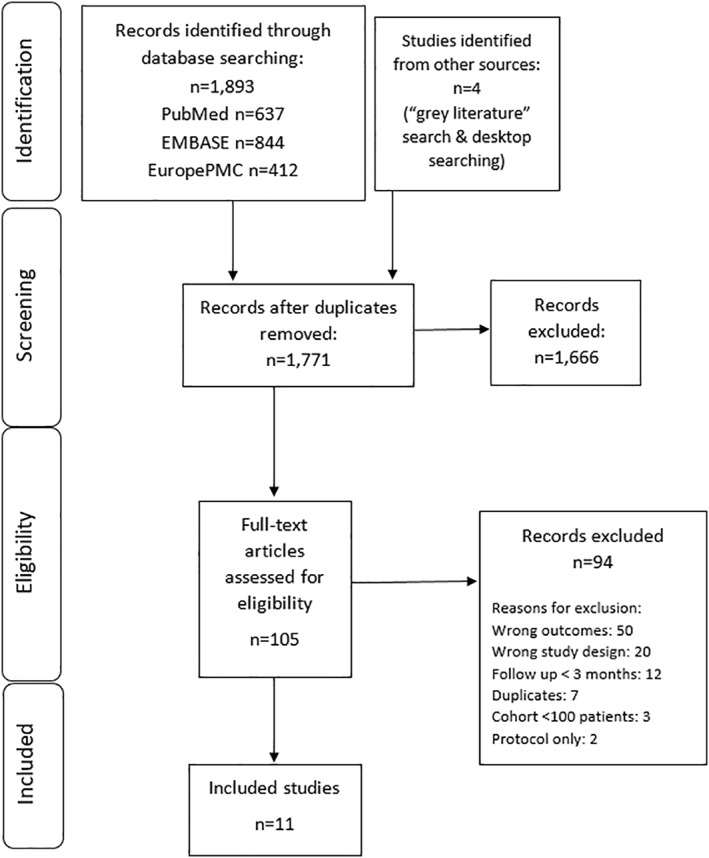
PRISMA diagram of study selection
Eleven studies were identified that met the inclusion criteria. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Five studies were conducted in the United Kingdom, 8 , 9 , 11 , 13 , 14 of which three enrolled healthcare workers 8 , 9 , 11 and two enrolled the staff and residents of elderly care homes. 13 , 14 The remaining six studies were all general population studies, conducted in Austria, 16 Denmark, 17 Israel, 12 Qatar 7 and the United States. 10 , 15 Six studies were published as preprints at the time of submission. 7 , 8 , 10 , 12 , 14 , 15 Across studies, the total number of PCR‐ or antibody‐positive participants at baseline was 615,777 (median: 8845; range: 88–378,606). The median follow‐up of individuals within studies was 131 days (4.4 months; range of medians: 54–210 days), with a maximum follow‐up of ≥300 days (10 months) in three studies. 12 , 14 , 16
Studies reported a range of primary endpoints (Table 2 and Table S2). Studies either determined evidence of prior infection based on a history of RT‐PCR confirmed infection (n = 5 studies), 10 , 12 , 15 , 16 , 17 documented antibody detection (n = 4 studies) 7 , 8 , 11 , 14 or a combination of both (n = 2 studies). 9 , 13 Three studies separately reported the relative risks of symptomatic reinfections and ‘all’ reinfections (symptomatic/asymptomatic), 8 , 11 , 15 one study reported symptomatic reinfections only 9 and the remaining studies did not differentiate between symptomatic and asymptomatic reinfections. 7 , 10 , 12 , 13 , 14 , 15 , 16 , 17 In addition to quantifying the absolute risks of SARS‐CoV‐2 reinfection, the risks compared with PCR‐negative or antibody‐negative cohorts at baseline were expressed by a number of different measures, such as relative risks, odds ratios, risk ratios and hazard ratios. Due to heterogeneity in outcome measures and populations, meta‐analysis of data were not considered appropriate. The following sections narratively report the findings of included studies by population group (general population, healthcare workers, and residents and staff of care homes).
TABLE 2.
Summary of included studies and primary outcome results
| First author; country; population | Participants a Follow‐up | Author reported primary outcomes |
|---|---|---|
| Abu‐Raddad 2021 7 (preprint); Qatar; General population | N = 43,044 antibody‐positive at baseline | Risk of reinfection (confirmed by WGS) b : 0.10% (95% CI: 0.08%–0.11%) Risk over time (any reinfection): Incidence rate of reinfection by month of follow‐up did not show any evidence of waning of immunity over seven months of follow‐up |
| Median f/u: 114 days (3.8 months) | ||
| Maximum f/u: 242 days (8.1 months) | ||
| Hall 2021 8 (preprint); United Kingdom; HCWs | N = 6614 antibody‐positive at baseline |
Adjusted odds ratio of reinfection comparing antibody or PCR‐positive group with negative group
|
| Median f/u: 202 days (6.7 months) | ||
| Maximum f/u: 227 days (7.6 months) | ||
| Hanrath 2020 9 United Kingdom; HCWs | N = 1038 PCR and/or antibody‐positive at baseline | Symptomatic reinfection: A positive PCR test was returned in 0/1038 (0% [95% CI: 0–0.4) of those with previous infection, compared with 290/10,137 (2.9% [95% CI: 2.6–3.2) of those without (p < 0.0001 χ2 test) |
| Median f/u: 173 days (5.8 months) | ||
| Maximum f/u: 229 days (7.6 months) | ||
| Hansen 2021 17 Denmark; General population | N = 11,068 PCR positive at baseline |
Main analysis: aRR (any reinfection): 0.20 (0.16–0.25). This represents 72 reinfections out of 1,346,920 person‐days in PCR‐positive group, compared with 16,819 new infections out of 62,151,056 person‐days in PCR‐negative group Additional cohort analysis (that includes all infection periods): aRR = 0.21 (0.18–0.25) by age group:
|
| Median f/u: 122 days (4.1 months) | ||
| Maximum f/u: 295 days (9.8 months) | ||
| Harvey 2020 10 (preprint); United States; General population | N = 378,606 PCR positive at baseline | Ratio of positive NAAT results (comparing patients who had a positive antibody test at index vs. those without) d : 2.85 (95% CI: 2.73–2.97) at 0‐30 days; 0.67 (95% CI: 0.6–0.74) at 31–60 days; 0.29 (95% CI: 0.24–0.35) at 60–90 days; 0.10 (95% CI: 0.05–0.19) at >90 days; note that NAAT positivity at <90 days is likely due to prolonged viral shedding |
| Median f/u: 54 days (1.8 months) | ||
| Maximum f/u: 92 days (3.1 months) | ||
| Jeffery‐Smith 2021 13 United Kingdom; Staff &residents at care homes | N = 88 PCR and/or antibody‐positive at baseline | Relative risk (any reinfection): 0.04 (95% CI: 0.005–0.27) This represents 1 reinfection out of 88 in seropositive group compared with 22/73 in seronegative group |
| Mean f/u: 120 days (4 months) | ||
| Maximum f/u: Unclear | ||
| Krutikov 2021 14 (preprint); United Kingdom; Staff & residents at care homes | N = 634 antibody‐positive at baseline | Relative adjusted hazard ratios (any reinfection):Residents of care home: aHR = 0.15 (0.05–0.44) e Staff of care home: aHR = 0.39 (0.19–0.82) e |
| Median f/u: 79 days (2.6 months) | ||
| Maximum f/u: 300 days (10 months) | ||
| Lumley 2021 11 United Kingdom; HCWs | N = 1265 antibody‐positive at baseline | IRR f (any reinfection): 0.12 (95% CI: 0.03–0.47; p = 0.002); 2/1265 seropositive (both asymptomatic reinfections) and N = 223/11,364 seronegative had positive PCR. Symptomatic reinfection: Incidence was 0.60 per 10,000 days at risk in seronegative HCWs; there were no symptomatic infections in seropositive HCWs Adjusted IRR g : 0.11 (95% CI: 0.03–0.44; p = 0.002) (any reinfection) |
| Median f/u: 139 days (4.6 months) | ||
| Maximum f/u: 217 days (7.2 months) | ||
| Perez 2021 12 (preprint); Israel; General population | N = 149,735 PCR positive at baseline | Overall reinfection risk: 0.1% (any reinfection between Mar 2020 and Jan 2021) This represents 154 individuals who had two positive tests at least 100 days apart out of 149,735 individuals with a record of a prior positive PCR test |
| Median f/u: 165 days (5.5 months) | ||
| Maximum f/u: Approx. 325 days h (10.8 months) | ||
| Pilz 2021 16 Austria; General population | N = 14,840 PCR positive at baseline | Odds ratio: 0.09 (95% CI: 0.07–0.13) (any reinfection) This represents 40 reinfections out of 14,840 individuals PCR positive in the first wave (0.27%) compared with 253,581 infections out of 8,885,640 (2.85%) in the remaining general population |
| Median f/u: 210 days (7 months) | ||
| Maximum f/u: 300 days (10 months) | ||
| Sheehan 2021 15 (preprint); United States; General population | N = 8845 PCR positive at baseline | Protective effectiveness (any reinfection): 78.5% (95% CI: 72.0%–83.5%) i Protective effectiveness against symptomatic infection: 83.1% (95% CI: 75.1%–88.5%) |
| Median f/u: 131 days (4.4 months) | ||
| Maximum f/u: 269 days (9 months) |
Note: ‘Any’ reinfection—all reinfections, both symptomatic and asymptomatic. Numbers rounded to two decimal points. No cases were identified on the basis of antigen testing. The longest duration of follow‐up was not stated in all studies or was provided only as an approximate estimate; when not stated, duration of follow‐up was inferred from figures or tables within the study.
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio (adjusted for week group); ARR, adjusted rate ratio; CI, confidence interval; f/u, follow‐up; HCW, healthcare worker; IRR, incidence rate ratio; NAAT, nucleic acid amplification test; WGS, whole genome sequencing.
In the baseline antibody and or PCR‐positive group (‘seropositive’ or prior positive cohort).
Based on cases with WGS confirming the first and second infections were from different viral strains (N = 16).
‘Possible’ reinfection was defined as a participant with two PCR‐positive samples ≥90 days apart with available genomic data, or an antibody‐positive participant with a new positive PCR at least 4 weeks after the first antibody‐positive result. A ‘probable’ case additionally required supportive quantitative serological data and or supportive viral genomic data from confirmatory samples.
NAAT used as proxy; includes all symptomatic reinfections and prolonged viral shedding, comparing patients who had a positive antibody test at index versus those with a negative antibody.
Multivariate analysis of risk of PCR‐positive infection by baseline antibody status, stratified by LTCF and adjusted for sex and age.
IRR is the relative incidence of subsequent positive SARS‐CoV‐2 PCR tests and symptomatic infections comparing antibody‐positive and antibody‐negative groups at baseline.
gAfter adjustment for age, gender and month of testing or calendar time as a continuous variable.
The midpoint of a range of follow‐up dates was taken (300–349 days).
Authors report effectiveness with the following calculation: 1−([56/8845]/[4163/141480]).
3.1. General population studies
3.1.1. Austria
In the study by Pilz et al., 16 national SARS‐CoV‐2 infection data from the Austrian epidemiological reporting system were used to investigate potential reinfection events, with a maximum follow‐up of 10 months. The primary outcome was the odds of PCR positivity in individuals who recovered from a confirmed SARS‐CoV‐2 infection during the first wave (22 February to 30 April 2020) compared with the odds of first infections in the remainder of the general population during the second wave (1 September to 30 November 2020). In total, 40 possible reinfections were recorded out of 14,840 individuals with a history of prior infection during the first wave (0.27%), compared with 253,581 infections out of 8,885,640 individuals of the remaining general population (2.85%). This translated into an odds ratio of 0.09 (95% CI: 0.07–0.13).
3.1.2. Denmark
In the study by Hansen et al., 17 individual‐level data were collected on patients who had been tested in Denmark in 2020 from the Danish Microbiology Database, with a maximum follow‐up of 9.8 months. Infection rates were analysed during the second wave of the COVID‐19 epidemic, from 1 September 2020 to 31 December 2020, comparing PCR‐positive individuals with PCR‐negative individuals during the first wave (March to May 2020). During the first wave (prior to June 2020), 533,381 people were tested, of whom 11,727 (2.2%) were PCR positive. Of these, 525,339 were eligible for follow‐up in the second wave, of whom 11,068 (2.11%) had tested positive during the first wave. Among eligible PCR‐positive individuals from the first wave, 72 (0.65%, 95% CI: 0.51%–0.82%) tested positive again during the second wave compared with 16,819 of 514,271 (3.27%, 95% CI: 3.22%–3.32%) who tested negative during the first wave. After adjusting for sex, age group and test frequency, the adjusted RR (aRR) of reinfection was 0.20 (95% CI: 0.16–0.25). Protection against repeat infection was estimated at 80.5% (95% CI: 75.4–84.5). In an alternative analysis, aRR by age category was reported. In individuals aged 65 years or more, the aRR was 0.53 (0.37–0.75), compared with 0.17, 0.20 and 0.19 in individuals aged 0–34 years, 35–49 years and 50–64 years, respectively.
3.1.3. Israel
In the study by Perez et al., 12 published as a preprint, preliminary reinfection rates within the members of a large healthcare provider (Maccabi Healthcare Services) in Israel were reported, with a maximum follow‐up of over 10 months. A total of 149,735 individuals had a recorded positive PCR test between March 2020 and January 2021. Among them, 154 members had two positive PCR tests at least 100 days apart and were included in this study. The reinfection rate was estimated at approximately 0.1%. In this cohort, 73 individuals (47.4%) had symptoms at both PCR‐positive events.
3.1.4. Qatar
In the study by Abu‐Raddad et al., published as a preprint, 43,044 anti‐SARS‐CoV‐2 nucleocapsid antibody‐positive participants were followed for up to 8 months for evidence of reinfection. 7 This retrospective cohort was identified from a database that covers all serological testing for SARS‐CoV‐2 conducted in Qatar.
There was evidence of a decreasing trend in the incidence rate of reinfection with each additional month of follow‐up from the first month (incidence rate: 0.97 per 10,000; 52 cases per 167,149 person‐weeks) to the sixth month (zero cases per 19,148 person‐weeks) (Mantel‐Haenszel trend analysis p‐value: <0.001), noting that early reinfection cases (i.e., within 3 months) were likely due to persistent viral shedding following the primary infection. There was an increase at ≥7 months; however, this was based on only one case of reinfection (out of 3094 person‐weeks). Applying a confirmation rate obtained through viral genome sequencing in a subset of patients with supporting clinical evidence for reinfection, the risk of documented reinfection was 0.1% (95% CI: 0.08%–0.11%).
These reinfections were compared to a cohort of 149,923 antibody‐negative individuals followed for a median of 17 weeks (range: 0–45.6 weeks). Risk of infection was estimated at 2.15% (95% CI: 2.08%–2.22%). The efficacy of natural infection in protecting against reinfection was estimated at 95.2% (95% CI: 94.1%–96.0%).
3.1.5. United States
Two US studies were identified, both published as preprints. In the first, a retrospective database analysis of electronic health records was used to determine the risk of nucleic acid amplification technology (NAAT) test positivity, a proxy for reinfection, over a maximum follow‐up of 3.1 months (Harvey et al. 10 ). Of 3,257,478 unique patients with an index antibody test, 378,606 (11.6%) had a positive antibody result at baseline. The ratio of positive NAAT test results among patients who had a positive antibody test at index versus those with a negative antibody test at index declined from 2.85 (95% CI: 2.73–2.97) at 0–30 days; to 0.67 (95% CI: 0.6–0.74) at 31–60 days; to 0.29 (95% CI: 0.24–0.35) at 60–90 days and to 0.10 (95% CI: 0.05–0.19) at >90 days.
In the second, 150,325 patients were followed for a maximum of 10 months (Sheehan et al. 15 ). In total, 56 reinfections were identified from the positive cohort of 8845 individuals, compared with 4163 infections from the negative cohort of 141,480 individuals. The protective effectiveness of prior infection against reinfection was estimated at 78.5% (95% CI: 72.0–83.5) and 83.1% (95% CI: 75.1–88.5) against symptomatic reinfection.
3.2. Healthcare workers
Three UK studies were identified that exclusively enrolled healthcare workers. In the first study, published as a preprint, 20,787 hospital staff were followed, of whom 32% (n = 6614) were assigned to the positive cohort (antibody or PCR positive) and 68% (n = 14,173) to the negative cohort (antibody negative, not previously known to be PCR or antibody positive) (Hall et al. 8 ). In total, 1,339,078 days of follow‐up data were analysed from the baseline positive cohort (maximum follow‐up of 7.6 months). In total, 44 reinfections (2 probable and 42 possible) were detected in the baseline positive cohort (15 of which were symptomatic), compared with 318 new PCR‐positive infections (249 of which were symptomatic) and 94 antibody seroconversions in the negative cohort. The adjusted odds ratio (aOR) was 0.17 for all reinfections (‘possible’ or ‘probable’; 95% CI: 0.13–0.24). Restricting reinfections to probable reinfections only, participants in the positive cohort had a 99% lower odds of probable reinfection (aOR of 0.01, 95% CI: 0.00–0.03). Restricting reinfections to those who were symptomatic, investigators estimated that participants in the positive cohort had an aOR of 0.08 (95% CI 0.05–0.13).
In the second study, 1038 healthcare workers with evidence of previous infection (PCR and or antibody positive) and 10,137 without (negative antibody and PCR) were followed for a maximum of 7.6 months (Hanrath et al. 9 ). A positive PCR test was returned in 0% (0/1038 [95% CI: 0%–0.4%]) of those with previous infection, compared to 2.9% (290/10,137 [95% CI: 2.6–3.2]) of those without (p < 0.0001, χ2 test).
In the third study, 12,541 UK healthcare workers were followed for up to 31 weeks to compare the incidence of SARS‐CoV‐2 infection in seropositive (N = 1265, including 88 who seroconverted during follow‐up) versus seronegative (N = 11,364) groups at baseline (Lumley et al. 11 ). A total of 223 anti‐spike seronegative healthcare workers had a positive PCR test, 100 during screening while they were asymptomatic and 123 while symptomatic, whereas two anti‐spike seropositive healthcare workers had a positive PCR test; both workers were asymptomatic when tested. Incidence varied by calendar time, reflecting the first (March through April) and second (October and November) waves of the pandemic in the United Kingdom and was consistently higher in seronegative healthcare workers. After adjustment for age, gender and month of testing or calendar time as a continuous variable, the incidence rate ratio in seropositive workers was 0.11 (95% CI: 0.03–0.44) compared with those who were seronegative at baseline.
3.3. Residents and staff of elderly care homes
Two studies were identified that enrolled both residents and staff at UK care homes. 13 , 14
In the first study (Jeffery‐Smith et al. 13 ), the risk of reinfection according to antibody seropositivity was investigated following outbreaks in two London care homes 13 , 18 over 4 months. The median age of residents was 84 and 85 in each care home.
In total, 88 individuals with evidence of prior infection were investigated for evidence of reinfection (antibody positive N = 87; PCR positive N = 1). The reinfection rate in this cohort was 1/88 (1.1%), and this reinfection event was observed in a staff member. By comparison, infection risk in the seronegative cohort was 30.1% (22/73, including four people diagnosed by seroconversion). The RR was estimated at 0.038 (95% CI: 0.005–0.273). The protection against reinfection after four months in seropositive group was estimated at 96.2% (95% CI: 72.7%–99.5%).
In the second study, published as a preprint, staff and residents in 100 long‐term care facilities (LTCFs) in England were followed between October 2020 and February 2021 (Krutikov et al. 14 ). In total, 2111 individuals were enrolled (682 residents and 1429 staff). The median age of residents was 86 years (IQR: 79–91) and 47 years for staff (IQR range: 34–56). Blood sampling was offered to all participants at three time points separated by 6–8 weeks intervals in June, August and October 2020. Samples were tested for IgG antibodies to nucleocapsid and spike protein. PCR testing for SARS‐CoV‐2 was undertaken weekly in staff and monthly in residents. The primary analysis estimated the adjusted hazard ratio (aHR) of a PCR‐positive test by baseline antibody status (Cox regression adjusted for age and gender, and stratified by LTCF).
IgG antibodies to nucleocapsid were detected at baseline in 226 residents (33%) and 408 staff (29%). Staff and residents contributed 3749 and 1809 months of follow‐up time, respectively. There were 93 PCR‐positive tests in seronegative residents (0.054 per month at risk) compared with four in seropositive residents (0.007 per month at risk). There were 111 PCR‐positive tests in seronegative staff (0.042 per month at risk) compared with 10 in seropositive staff (0.009 per month at risk). Controlling for the potential confounding effect of individual LTCFs, the relative aHRs for PCR‐positive infection were 0.15 (95% CI: 0.05–0.44) and 0.39 (95% CI: 0.19–0.82) comparing seropositive versus seronegative residents and staff, respectively. Study authors concluded that the presence of IgG antibodies to nucleocapsid was associated with substantially reduced risk of reinfection in staff and residents for up to 10 months after primary infection, assuming that the earliest infections occurred in March 2020.
3.4. Quality of included studies
The NIH quality assessment tools was used for appraisal of observational cohort studies. 6 Ten studies were considered of ‘good’ or ‘fair’ methodological quality (Table S3), with one study 10 that used a proxy measure for outcomes (NAAT test positivity) considered to be of poor quality.
Each of the 10 studies of ‘good’ (n = 4) or ‘fair’ (n = 6) methodological quality was considered large enough to adequately capture reinfection events in their respective populations. A number of studies was downgraded due to lack of controlling for confounders (n = 7 studies). In these studies, potential confounding variables were either not assessed or not measured appropriately, or the statistical analysis was not adequately described. As all studies were observational in nature, they cannot be used to demonstrate causality. Therefore, only associations between prior infection and reinfection risk can be measured. While estimates of the effectiveness of natural infection to prevent reinfection were reported in a number of studies, such measures cannot be reliably estimated on the basis of these data.
Six studies are currently published as preprints, 7 , 8 , 10 , 12 , 14 , 15 so have not yet been formally peer‐reviewed, raising additional concerns about overall quality and the potential for results to change prior to formal publication.
4. DISCUSSION
4.1. Summary of findings
Eleven cohort studies estimated the risk or relative risk of SARS‐CoV‐2 reinfection in individuals who were either antibody‐positive or who had a history of PCR‐confirmed Covid‐19 at baseline, compared with those who did not, for up to 10 months. Across studies, the total number of PCR‐ or antibody‐positive participants at baseline was 615,777, with a maximum follow‐up of over 10 months in three studies. Reinfection was a rare event (median PCR‐confirmed reinfection rate: 0.27%, range: 0%–1.1%), with no study reporting an increase in the risk of reinfection over time.
Of the six general population studies, only one estimated the population‐level risk of reinfection based on whole genome sequencing in a subset of patients with supporting evidence of reinfection. 7 The estimated risk was low (0.1% [95% CI: 0.08%–0.11%]) in this large cohort of 43,044 anti‐SARS‐CoV‐2 nucleocapsid antibody‐positive participants. Importantly, the incidence rate of reinfection by month did not show any evidence of waning of immunity over the seven months of follow‐up. The remaining population‐based studies (conducted in Austria, Denmark, Israel and the United States) also reported low absolute and relative risks of reinfection, and none reported an increased risk over time.
Only one study reported the relative risk of reinfection by age category, allowing comparisons across groups. In individuals aged 65 years or more, the aRR was 0.53 (0.37–0.75), compared with 0.17, 0.20 and 0.19 in individuals aged 0–34 years, 35–49 years and 50–64 years, respectively. 17 The lower protection in the over‐65s group may be attributable to immunosenescence; however, little is known about this phenomenon in the context of COVID‐19.
Two UK studies reported lower risks of reinfection in elderly individuals. Both studies enrolled residents of care homes (median age ≥84 years), a group that has been disproportionately affected by the COVID‐19 pandemic, with high rates of infection and deaths among frail, elderly residents. In the first study, the relative risk of reinfection in staff and residents of two London care homes was very low (RR = 0.038; 95% CI: 0.005–0.273), and the protection against reinfection after four months in seropositive group was estimated at 96.2% (95% CI: 72.7%–99.5%). 13 This relative risk was based on a single reinfection event in a seropositive staff member, indicating the relative risk in the elderly resident cohort is even lower. The second study reported higher relative rates of reinfection 14 in a sample of staff and residents (N = 2111) across 100 LTCFs in England. The study, conducted between October 2020 and February 2021, coincided with a period of high community prevalence of SARS‐CoV‐2 in the United Kingdom, associated with the rapid emergence of the B.1.1.7 variant. 19 The estimated aHR for reinfection was 0.15 (95% CI: 0.05–0.44) in residents and 0.39 (95% CI: 0.19–0.82) in staff. The higher relative rates of infection compared with the earlier UK study raises concerns regarding the impact of new variants on the protective immunity of natural infection. Nonetheless, only four cases of possible reinfection were identified in residents, and although all cases reported symptoms, none required hospital treatment. Taking into consideration that most residents were likely first infected during the first wave (up to 6 months prior), the risk of reinfection was substantially reduced in residents even in the context of high community transmission of the B.1.1.7 variant.
Three UK studies estimated the relative risk of reinfection specifically among healthcare workers. 8 , 9 , 11 The first study detected zero symptomatic infections in 1038 healthcare workers with evidence of a prior infection, compared with 290 in 10,137 without evidence of prior infection (p < 0.0001). 9 The second study detected two asymptomatic infections (and no symptomatic infections) out of 1265 seropositive individuals, compared with 223 infections (100 during screening while they were asymptomatic and 123 while symptomatic) out of 11,364 seronegative individuals. 11 After adjustment for age, gender and month of testing or calendar time, the incidence rate ratio in seropositive healthcare workers was 0.11 (95% CI: 0.03–0.44). The third study reported 44 reinfections in the baseline positive cohort of 6614 individuals (15 of which were symptomatic), compared with 318 new PCR‐positive infections (249 of which were symptomatic) and 94 antibody seroconversions in the negative cohort of 14,173 individuals. 8 The aOR was 0.17 for all reinfections (95% CI: 0.13–0.24), and restricting reinfections to those who were symptomatic, the aOR was 0.08 (95% CI 0.05–0.13). This pattern of a lower relative risk of symptomatic reinfections in healthcare workers, compared with ‘any’ reinfection (symptomatic and asymptomatic), was also observed in the study by Sheehan et al. in general populations. 15 This finding suggests that not only is the risk of reinfection following natural infection low, when it does occur, it may represent a less severe form of disease.
4.2. Strengths and limitations
To our knowledge, this is the first systematic review to quantify the risk of SARS‐CoV‐2 reinfection over time. All studies were considered large enough to adequately capture reinfection events in their respective populations. Results across studies consistently demonstrated a substantially lower risk of reinfection in previously infected individuals without a waning of the protective response over time. However, despite these strengths, there are a number of limitations associated with this review.
First, as the studies are observational in nature, the prevention of reinfection cannot be causally confirmed, although longitudinal associations can be estimated. Additional concerns relating to observational studies include the greater potential for bias. It is possible that antibody test results affected individual behaviour. Individuals with evidence of prior infection may have believed that they possessed immunity to SARS‐CoV‐2, resulting in a reduction in health‐seeking behaviour and testing (outcome ascertainment bias). Conversely, these individuals may have increased their engagement in social behaviour, placing them at greater risk for infection. The overall direction of bias (whether over‐ or under‐estimating reinfection) cannot be determined.
Second, studies included in this review could not determine whether past seroconversion, or current antibody levels, determine protection from infection. Furthermore, none could define which characteristics are associated with reinfection. For example, there is evidence to suggest immune responses are weaker following asymptomatic SARS‐CoV‐2 infections 20 and in immunocompromised patients, 21 which may increase susceptibility to repeat infection. Mucosal immunity and neutralising antibodies present in respiratory secretions may be more important for sterilising immunity than circulating IgG levels. The role of T‐cell immunity was not assessed in any study; therefore, it is not possible to determine whether protection from reinfection is conferred through the measured antibodies or T‐cell immunity. Future longitudinal serological cohorts may be able to determine protective correlates of immunity.
Third, only two studies undertook genomic sequencing of reinfected cases; consequently, the results of nine studies are only based on potential reinfections. The effect of this, however, is to overestimate the number of reinfections, thereby affirming the conclusion that reinfection is rare.
Fourth, due to the nature of a number of retrospective database analyses included in this review, many studies could not correlate symptomatic infections with protection against repeat infection or evaluate disease progression comparing first and second infections. This was true for studies that accessed large databases in Austria, 16 Denmark 17 and the United States. 10
Finally, this review included a number of studies that were published as preprints (n = 6 studies 7 , 8 , 10 , 12 , 14 , 15 ). While preprints have been pivotal to guide policy and practice throughout this pandemic, these studies have not yet been formally peer‐reviewed raising concerns over the quality and accuracy of presented data.
4.3. Generalisability of findings
There are a number of issues relating to the applicability and generalisability of the presented results. First, all but two studies preceded the widespread identification and spread of a number of new viral strains of international concern (e.g., variant 202012/01 [also known as 501Y.V1/B.1.1.7] from the United Kingdom and 501Y.V2 [B.1.351] from South Africa, both identified in December 2020 22 ). In the first study that extended beyond December 2020, reinfection events between March 2020 and January 2021 in Israel were recorded. 12 A higher number of reinfections was recorded in January 2021 compared with previous months. However, genomic sequencing was not reported and statistical analysis of the recorded data (e.g., controlling for confounders and significance testing) was not undertaken. In the second study, elderly care home staff and residents in the United Kingdom were followed between October 2020 and February 2021. 14 Sequencing data were not available for suspected reinfections, and study authors did not investigate the potential impact of new variants on the risk of reinfection. Nonetheless, the risk of reinfection was substantially reduced in elderly residents, most of whom were first infected up to 6 months previously. While these findings are reassuring, further research is needed on the role of natural immunity in populations that are experiencing the emergence and spread of new variants of concern.
Second, all presented data relate to unvaccinated cohorts as they preceded vaccine roll‐out in 10 studies, and in the only study that was conducted during vaccine roll‐out, all vaccinated individuals were excluded once 12 days had passed since their vaccination. 14 The applicability of the data to vaccinated populations is therefore unknown.
One preprint study (Lumley et al., 2021 23 ), identified after our database search, reported reinfection rates among healthcare workers according to vaccination status and in relation to the B.1.1.7 variant. This study updates the 2020 study included in this review by the same authors 11 and presents data up to 28 February 2021. At this time point, 1456 of 13,109 participating healthcare workers had received two vaccine doses (Pfizer‐BioNTech or Oxford‐AstraZeneca). Compared to unvaccinated seronegative healthcare workers, natural immunity and two vaccination doses provided similar protection against symptomatic infection: no healthcare worker who had received two vaccine doses had a symptomatic infection, and incidence was 98% lower in seropositive healthcare workers (adjusted incidence rate ratio 0.02, 95% CI: <0.01–0.18). Two vaccine doses or seropositivity reduced the incidence of any PCR‐positive result with or without symptoms by 90% (0.10, 95% CI: 0.02–0.38) and 85% (0.15 95% CI: 0.08–0.26) respectively. There was no evidence of differences in immunity induced by natural infection and vaccination for infections with the B.1.1.7 variant. These data suggest that both natural infection and vaccination both provide robust protection against SARS‐CoV‐2 infection, including against the B.1.1.7 variant. Future studies are expected to expand our understanding of the differences between natural and vaccine‐acquired immunity and the impact of new variants.
Third, there is much uncertainty in relation to the risk of reinfection in younger and older age groups. Inconsistent data were identified relating to elderly populations, with one study reporting higher rates of reinfection compared with younger age groups 17 and two reporting low rates of reinfection in elderly residents of care homes (although these two studies did not compare risk across age groups). 13 , 14
4.4. Research in context and policy implications
This review was expected to inform a range of policy questions relating to the duration of protective immunity following infection with SARS‐CoV‐2, such as:
How long can asymptomatic individuals who have recovered from a prior SARS‐CoV‐2 infection be exempted from restriction of movement policies if they become a close contact of a confirmed COVID‐19 case?
How long can asymptomatic individuals who have recovered from a prior SARS‐CoV‐2 infection be exempted from serial testing programmes?
How long can asymptomatic patients who have recovered from a prior SARS‐CoV‐2 infection be exempted from the requirement for testing prior to scheduled admission to hospital?
This review identified a large body of evidence that indicates the duration of presumptive protective immunity may last for at up to 10 months post‐infection. However, given the uncertainty that exists relating to reinfection potential with emerging variants, any policy changes may not be applicable to possible exposure to emerging immune escape variants of concern. In addition, policies should be kept under review and informed by the international evidence and national surveillance data. In light of the findings of this review, policy was updated in Ireland to extend the period of presumptive immunity from 3 months to 6 months; therefore, a person who is an asymptomatic contact of a case and has had a positive test result within the previous 6 months is exempt from restriction of movements and serial testing. A period of 6 months was selected over 10 months due to the ongoing uncertainties relating to new variants.
Increasingly, reinfection cases are being investigated on a country level and are reported on websites of national public health agencies (e.g., Czechia now report a national reinfection rate of 0.1%, or 1400 cases out of 1,225,000 infections 24 ). Future longitudinal studies should focus on the following issues that were not addressed in the aforementioned studies, including:
The durability of immunity beyond 10 months
Immune correlates of protection
Protective immunity in populations with comorbidities and the immunocompromised
The impact of new variants on protective immunity
5. CONCLUSIONS
Eleven large cohort studies were identified that estimated the risk of SARS‐CoV‐2 reinfection over time, including three that enrolled healthcare workers and two that enrolled elderly care home residents. All studies reported low relative SARS‐CoV‐2 reinfection rates in individuals with prior evidence of infection, compared with those without, for up to 10 months. The relative risk of reinfection was low across studies, although there was some inconsistent evidence of a higher risk in older populations compared with younger populations. A limitation of this review was the uncertainty regarding the applicability of data to new variants of concern and to vaccinated populations.
CONFLICT OF INTEREST
No conflict of interest declared.
AUTHOR CONTRIBUTIONS
Eamon O Murchu: Investigation, formal analysis and writing‐original draft. Paula Byrne: Investigation and writing‐original draft. Paul G. Carty: Investigation and writing‐original draft. Cillian De Gascun: Writing‐reviewing and editing. Mary /Keogan: Writing‐reviewing and editing. Michelle O’Neill: Supervision, writing‐reviewing and editing. Patricia Harrington: Supervision, writing‐reviewing and editing. Máirín Ryan: Supervision, writing‐reviewing and editing. All authors attest they meet the ICMJE criteria for authorship.
Supporting information
Supplementary Material 1
ACKNOWLEDGEMENTS
The authors would like to thank our executive assistant Debra Spillane (HIQA) and librarians from the Health Service Executive (HSE) for their valued input and support. This research was funded in part by the Health Research Board under grant no. HRB‐CICER‐2016‐1871.
O Murchu E, Byrne P, Carty PG, et al. Quantifying the risk of SARS‐CoV‐2 reinfection over time. Rev Med Virol. 2022;32(1):e2260. 10.1002/rmv.2260
Patricia Harrington and Máirín Ryan are co‐senior authors.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. O Murchu E, Byrne P, Walsh KA, et al. Immune response following infection with SARS‐CoV‐2 and other coronaviruses: a rapid review. Rev Med Virol. 2021;31(2):e2162. 10.1002/rmv.2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johns Hopkins University & Medicine . COVID‐19 Dashboard 2020. https://coronavirus.jhu.edu/map.html. Accessed February 6, 2021.
- 3. HIQA. Health Information and Quality Authority . Protocol for evidence synthesis support—COVID‐19 2020. http://www.hiqa.ie/sites/default/files/2020‐05/Protocol‐for‐HIQA‐COVID‐19‐evidence‐synthesis‐support_1‐6.pdf
- 4.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane; 2021. Available from www.training.cochrane.org/handbook
- 5. World Health Organization (WHO). Criteria for Releasing COVID‐19 Patients from Isolation: Scientific Brief. 2020. https://www.who.int/news‐room/commentaries/detail/criteria‐for‐releasing‐covid‐19‐patients‐from‐isolation. Accessed September 22, 2020. [Google Scholar]
- 6. National Heart Lung and Blood Institute (NIH) . Study quality assessment tools. Available at: https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools
- 7. Abu‐Raddad L, Chemaitelly H, Coyle P, et al. SARS‐CoV‐2 reinfection in a cohort of 43,000 antibody‐positive individuals followed for up to 35 weeks. medRxiv; 2021. 10.1101/2021.01.15.21249731 [DOI]
- 8. Hall V, Foulkes S, Charlett A, et al. Do antibody positive healthcare workers have lower SARS‐CoV‐2 infection rates than antibody negative healthcare workers? Large multi‐centre prospective cohort study (the SIREN study), England: June to November 2020. medRxiv; 2021. 10.1101/2021.01.13.21249642 [DOI]
- 9. Hanrath AT, Payne BAI, Duncan CJA. Prior SARS‐CoV‐2 infection is associated with protection against symptomatic reinfection. J Infect. 2020;82(4):e29‐e30. 10.1016/j.jinf.2020.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harvey R, Rassen J, Kabelac C, et al. Real‐world data suggest antibody positivity to SARS‐CoV‐2 is associated with a decreased risk of future infection. medRxiv; 2020. 10.1101/2020.12.18.20248336 [DOI]
- 11. Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS‐CoV‐2 infection in health care workers. N. Engl J Med. 2020;384(6):533‐540. 10.1056/nejmoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perez G, Banon T, Gazit S, et al. A 1 to 1000 SARS‐CoV‐2 reinfection proportion in members of a large healthcare provider in Israel: a preliminary report. medRxiv; 2021. 10.1101/2021.03.06.21253051 [DOI]
- 13. Jeffery‐Smith A, Iyanger N, Williams SV, et al. Antibodies to SARS‐CoV‐2 protect against re‐infection during outbreaks in care homes, September and October 2020. Euro Surveill. 2021;26(5):2100092. 10.2807/1560-7917.es.2021.26.5.2100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krutikov M, Palmer T, Tut G, et al. Incidence of SARS‐CoV‐2 infection according to baseline antibody status in staff and residents of 100 long term care facilities (VIVALDI study). medRxiv; 2021. 10.1101/2021.03.08.21253110 [DOI] [PMC free article] [PubMed]
- 15. Sheehan M, Reddy A, Rothberg M. Reinfection rates among patients who previously tested positive for COVID‐19: a retrospective cohort study. medRxiv; 2021. 10.1101/2021.02.14.21251715 [DOI] [PMC free article] [PubMed]
- 16. Pilz S, Chakeri A, Ioannidis JP, et al. SARS‐CoV‐2 re‐infection risk in Austria. Eur J Clin Invest. 2021;51(4):e13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS‐CoV‐2 among 4 million PCR‐tested individuals in Denmark in 2020: a population‐level observational study. Lancet. 2021;397(10280):1204‐1212. 10.1016/s0140-6736(21)00575-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ladhani SN, Jeffery‐Smith A, Patel M, et al. High prevalence of SARS‐CoV‐2 antibodies in care homes affected by COVID‐19: prospective cohort study, England. EClinicalMedicine. 2020;28:100597. 10.1016/j.eclinm.2020.100597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Public Health England (PHE) . Investigation of novel SARS‐CoV‐2 variant Variant of concern 202012/01 technical briefing 4. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment‐data/file/952490/Variant‐of‐Concern‐VOC‐20201201‐Technical_Briefing_4_England.pdf
- 20. Long Q‐X, Tang X‐J, Shi Q‐L, et al. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med. 2020;26(8):1200‐1204. 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 21. Hunsinger DHP, Kutti Sridharan DG, Rokkam DVRP, Fantry DLE. COVID‐19 reinfection in an immunosuppressed patient without an antibody response. Am J Med Sci. 2021;S0002‐9629(0021):00050‐00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. European Centre for Disease Prevention and Control (ECDC) . Risk related to the spread of new SARS‐CoV‐2 variants of concern in the EU/EEA—first update. https://www.ecdc.europa.eu/sites/default/files/documents/COVID‐19‐risk‐related‐to‐spread‐of‐new‐SARS‐CoV‐2‐variants‐EU‐EEA‐first‐update.pdf
- 23. Lumley SF, Rodger G, Constantinides B, et al. An Observational Cohort Study on the Incidence of SARS‐CoV‐2 Infection and B.1.1.7 Variant Infection in Healthcare Workers by Antibody and Vaccination Status. medRxiv.; 2021. 10.1101/2021.03.09.21253218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. State Institute of Public Health (SZU) . The number of cases of covid‐19 reinfections in the Czech Republic has increased 2021. http://www.szu.cz/tema/prevence/pocet‐pripadu‐reinfekci‐covid‐19‐v‐cr‐vzrostl
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


