Abstract
Background
Homocysteine assessment has been proposed as a potential predictive biomarker for the severity of COVID‐19 infection. The purpose of this review was to analyze the correlation between the prevalence of MTHFR C677 T gene polymorphism and COVID‐19 incidence and mortality worldwide.
Methods
Data regarding MTHFR C677 T gene mutation were obtained from the interrogation of the Genome Aggregation Database (genomAD), which is publicly available from the web“https://gnomad.broadinstitute.org.” COVID‐19 cases, including prevalence and mortality, were obtained from“https://www.worldometers.info/coronavirus” 27 August 2020.
Results
There is a clear trend toward the worldwide prevalence of MTHFR 677 T and COVID‐19 incidence and mortality. The prevalence of MTHFR 677 T allele in the Latino population, and the incidence and mortality for COVID‐19 was higher for this ethnic group than that reported for most other populations globally. Statistical analysis showed a relatively strong correlation between C677 T and death from coronavirus.
Conclusions
Genetic polymorphism of MTHFR C677 T may modulate the incidence and severity of COVID‐19 pandemic infection.
Keywords: C677 T mutation prevalence, COVID‐19 vulnerability, Homocysteine, MTHFR gene
1. INTRODUCTION
Since the beginning of the novel coronavirus pandemic, caused by the viral pathogen COVID‐19, the medical and scientific community faces challenges for managing patients and identifying reliable biomarkers related to disease progression to stratify patients for their risk of critical manifestations promptly. Furthermore, it is crucial to identify clinical, epidemiological, and laboratory markers for one of the leading causes of mortality, especially regarding the damage to the microvasculature, potentially modifiable by safe therapeutic interventions.
At present, there are over 119 million confirmed COVID‐19 cases with 2.6 million deaths. Looking at the worldwide epidemiologic data, there are remarkable differences among countries regarding COVID‐19 infection rates and mortality, especially comparing very high data relative to the United States of America or South America and sub‐Saharan Africa or Finland, where incidence and mortality appear remarkably low (WHO Coronavirus Disease (COVID‐19) Dashboard https://covid19.who.int/). This simple epidemiological observation represents the base for searching genetic differences among populations or ethnicities, which may explain different clinical manifestations of COVID‐19 infection). 1
In addition to serological and clinical biomarkers that are clearly correlated with a severe clinical course of COVID‐19 infection, 1 , 2 the role of Hcy as an important prognostic marker has been recently hypothesized. 3 , 4 The role of Hcy in several metabolic and inflammatory processes has already been demonstrated, and different populations with different ethnical predominances show different prevalences for MTHFR gene mutations and MTHFR activities. 5 The population frequency of C677 T homozygosity ranges from 1% or less among Blacks from Africa and the Finnish population to 20% or more among Latino and US Hispanics. Intriguingly, the world distribution of MTHFR 677 T polymorphism has a high degree of heterogeneity following a geographical gradient of south‐north and west‐east both in Europe and Americas. 6 , 7 , 8
Homocysteine has been under a lot of speculation since its discovery in 1932. 9 It is known that a high plasma level of homocysteine significantly increases the incidence of vascular damage in both small and large vessels. 9 , 10 Hyperhomocysteinemia has neurotoxic, neuroinflammatory, neurodegenerative, proatherogenic, prothrombotic, and prooxidative effects. 11 Homocysteine concentrations above the 90th percentile are associated with increased risk of degenerative and atherosclerotic processes 12 in the coronary, cerebral and peripheral circulatory system. In this regard, determining homocysteine together with other cardiovascular risk markers (Apo B, Lp (a), LDL, fibrinogen, PAI‐1) now is implemented in the clinical practice; 13 moreover, recent evidence suggests the role of homocysteine as a risk factor for thromboembolism, given its effect on platelet reactivity. 14
Homocysteine has been found to be a predictor of disease progression in 273 patients with mild COVID‐19 disease in Shanghai: 72 of the patients showed disease progression as assessed by lung CT scan. More than 40 parameters were measured in these 273 patients at admission of which only age, homocysteine and monocyte‐to‐lymphocyte ratio (MLR) were found as significant predictors of disease progression as shown by CT changes in the lung. Patients with hyperhomocysteinemia (>15.4 µmol/L) had a threefold increased risk of CT changes progression. Of the three predictor markers, only homocysteine is readily modifiable. Very recent data witnessed a predictive value of Hcy (together with age, MLR, and period from disease onset to hospital admission) for severe pneumonia on chest CT at first week from COVID‐19 patients, but did not report additional organ involvement. 4
To our knowledge, till today, no evidences have been published about the MTHFR gene polymorphisms that are implicated in a defective Hcy metabolism and COVID‐19 disease. The purpose of our research was to illustrate the relationship between the prevalence of the genetic polymorphism of MTHFR C677 T and COVID‐19 incidence and mortality rates, demonstrating that COVID‐19 incidence and mortality rates are strongly correlated to the majority of MTHFR C677 T mutation, which is linked explicitly to prothrombotic events due to altered homocysteine metabolism.
2. MATERIALS AND METHODS
Data regarding MTHFR status, regarding MTHFR C677 T gene mutation, were obtained from the interrogation of the Genome Aggregation Database (genomAD), which is publicly available from the web“https://gnomad.broadinstitute.org.” COVID‐19 cases, including prevalence and mortality, were obtained from“https://www.worldometers.info/coronavirus” 27 August 2020, before that COVID‐19 viral variants became predominant and that massive vaccination programs began.
MedCalc Statistical Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014) was used to perform statistical analysis. The correlation analysis was used to highlight if there is a possible association between the two variables. We speak of a positive correlation between two variables if when the first variable increases, the second also increases accordingly.
The P‐value is the probability that the current result to be found if the correlation coefficient were, in fact, zero (null hypothesis). The correlation coefficient is defined as statistically significant if this probability is lower than the conventional 5% (p <0.05).
Correlations between the measured variables in each group were analyzed using Pearson's correlation method. All statistical tests were performed at a significance level (α) of 0.05.
3. RESULTS
The analysis examined their associations for the allele frequency with cases of coronavirus COVID‐19 death and incidence for the polymorphism. Subgroup analyses were stratified by ethnicity. Officially reported COVID‐19 cases and the number of COVID‐19‐related deaths were stratified according to the ethnic groups (Table 1).
TABLE 1.
The frequency of MTHFR C677 T alleles and mortality rate of COVID‐19 in some populations
| 677C>T p. Ala222Val rs1801133 | Coronavirus | |||||||
|---|---|---|---|---|---|---|---|---|
| Population | Allele count | Allele Number | Number of Homozygotes | Allele Frequency | Total Cases | Total Deaths | Tot Cases /1 M pop | Deaths /1 M pop |
| Latino | 17788 | 35440 | 4654 | 0,5019 | 2899134 | 105354 | 6729 | 245 |
| European (non‐Finnish) | 43635 | 129108 | 7511 | 0,338 | 2574301 | 196444 | 2136 | 204 |
| East Asian | 5790 | 19944 | 929 | 0,2903 | 119264 | 5912 | 73 | 4 |
| European (Finnish) | 5799 | 25116 | 694 | 0,2309 | 7295 | 329 | 1317 | 59 |
| South Asian | 4443 | 30616 | 389 | 0,1451 | 2888739 | 65492 | 974 | 22 |
| African | 2714 | 24970 | 158 | 0,1087 | 599849 | 13315 | 447 | 10 |
A correlation between MTHFR 677 T prevalence and COVID‐19 incidence and mortality rates can be observed if data were stratified for different ethnic groups, demonstrating the presence of a gradient with South‐North and East‐West directions worldwide (Figures 1, 2, 3, 4). The frequency of MTHFR C677 T allele in the Latino population (50%) was higher than that reported for most of the other populations in the world, similarly coronavirus death correlation with the frequency of this allele in most of the different populations was lower (Finnish, African sub‐Saharan, etc.). Correlation analysis showed a relatively strong correlation between C677 T and death coronavirus with 85%, p=0.03.
FIGURE 1.
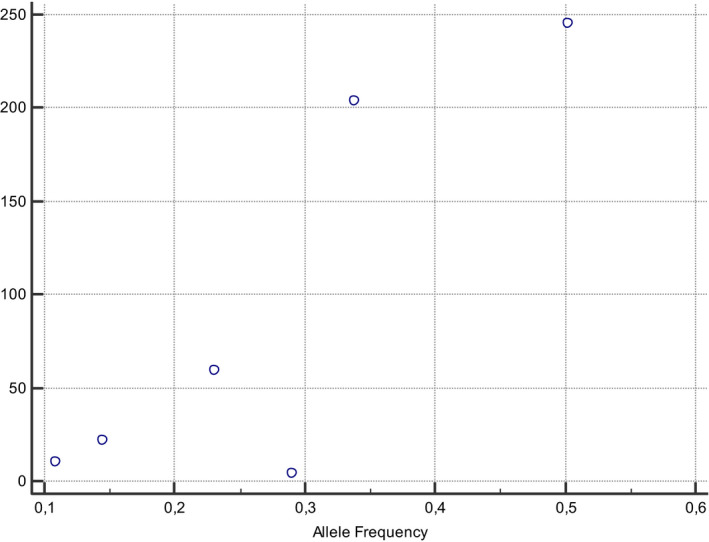
The relationship between allele frequencies of 677C>T and coronavirus deaths/1 M pop was represented graphically by a scatter diagram
FIGURE 2.
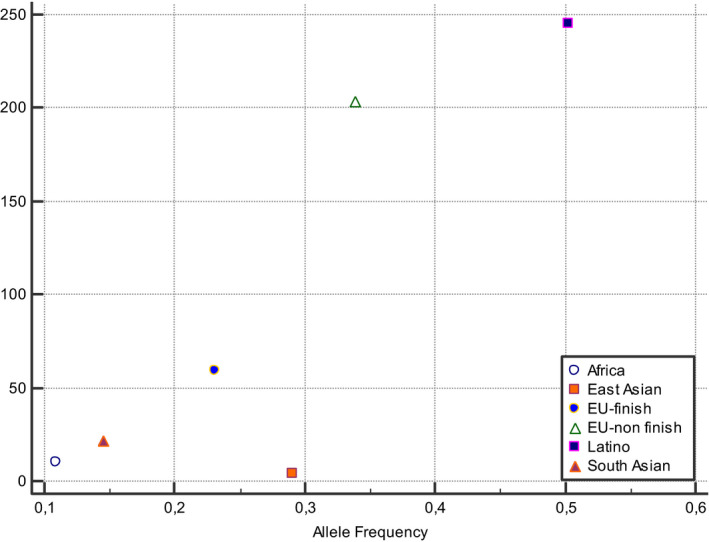
The relationship between allele frequencies of 677C>T and coronavirus deaths/1 M pop was represented graphically by a scatter diagram, stratified by ethnicity
FIGURE 3.
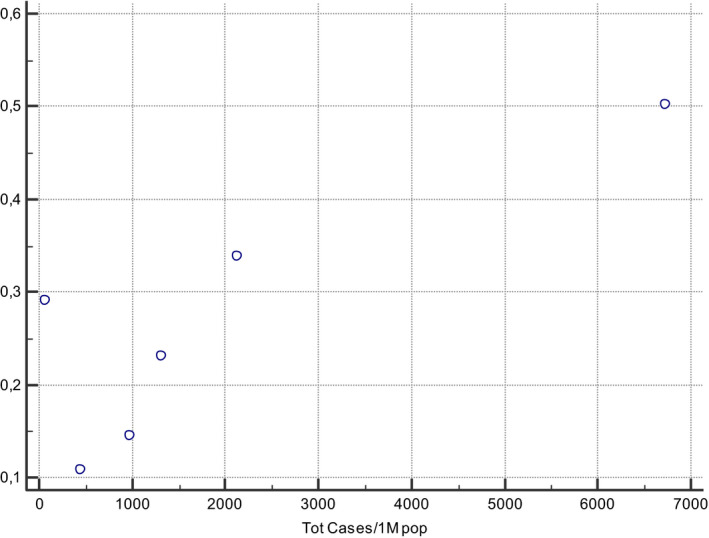
The relationship between allele frequencies of C677 T and coronavirus total cases/1 M pop was represented graphically by a scatter diagram
FIGURE 4.
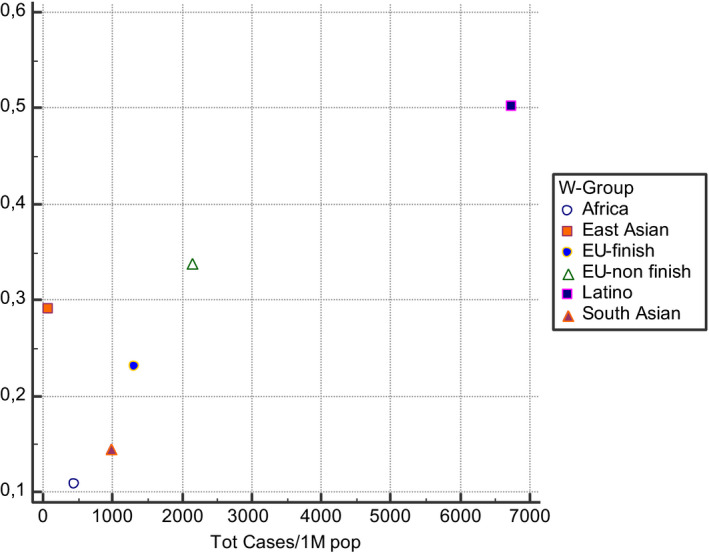
The relationship between allele frequencies of C677 T and coronavirus cases/1 M pop was represented graphically by a scatter diagram, stratified by ethnicity
The correlation between the allele frequency and coronavirus mortality was found very high (85%).
4. DISCUSSION
The relationship between the prevalence of the genetic polymorphism of MTHFR C677 T and COVID‐19 incidence and mortality rates seem to be intriguing. It may be a useful biomarker to stratify COVID‐19 infection severity and can be used for preventive medical treatments and supplementations.
The correlation between Hcy level and COVID‐19 severity of infection has been recently demonstrated 3 , 4 and is currently under our evaluation in a cohort of 200 patients affected by COVID‐19 infection which confirms the significant correlation between blood Hcy >16 µmol/L and severe prognosis or mortality (unpublished data).
Several studies have clarified the pathogenic correlation between COVID‐19 infection and Hcy metabolism. Recently, it has been described the novel regulatory mechanisms directly involved with Hcy that activates the angiotensin II type receptor. 15 , 16 Ferroptosis, a newly identified form of regulated cell death, is characterized by iron and lipid reactive oxygen species (ROS) accumulation and smaller mitochondria with condensed mitochondrial membrane densities. Still, it does not share morphological, biochemical, or genetic similarities with other forms of regulated cell death, such as apoptosis. 17 Increasing evidence suggests that ferroptosis dysfunction is positively related to various human diseases, including tumorigenesis. 18 Ferroptosis was found to be linked to neurological disturbances, including cognitive impairment, 19 ageusia, and anosmia (taste and smell loss) 20 , 21 that are common manifestations of COVID‐19 disease.
The SARS‐CoV‐2 may involve the transfer of methyl group for viral RNA capping from the host cell synthesized S‐adenosylmethionine (SAM). The latter is converted into S‐adenosylhomocysteine (SAH), which further in the presence of SAH hydrolase (SAHH) removes adenosine and produces an intermediate product called “homocysteine,” which is recycled by remethylation and trans‐sulphuration pathway in the human body. 22
Regarding the genetic background of Hcy metabolisms, the enzyme 5,10‐methylenetetrahydrofolate reductase (MTHFR) is involved in the folate metabolism. The MTHFR converts 5,10‐methylenetetrahydrofolate to 5‐methyltetrahydrofolate, which produces methyl donors to convert homocysteine to methionine. 23
Based on this evidence, a correlation between some common polymorphism of the MTHFR gene was sought. The most frequent polymorphism in this gene, present on chromosome 1 (1p36.3), is the thermolabile C677 T and the A129C alleles. The frequencies of these alleles vary considerably from population to population. The most significant contribution is present in the US Hispanic and Italian people. The population frequency of C677 T homozygosity ranges from 1% or less among Blacks from Africa and the United States to 20%. C677 T homozygosity in infants is associated with a moderately increased risk for spina bifida (pooled odds ratio =1.8; 95% confidence interval: 1.4, 2.2).
The variant C677 T (c.677C> T) leads to a missense mutation in which an alanine is replaced by a valine (p. Ala222Val ‐rs1801133). Homozygous carriers of this variant show higher homocysteine levels than normal subjects, wt for the variant. Heterozygotes show a moderate increase, intermediate between homozygotes and wt. 5
The population frequency of the C677 T allele showed regional and ethnic variations (Table 1, Figures 1, 2, 3, 4). For example, the allele frequency was high in Italy and among Hispanics living in California and was low among US Blacks and in some areas of sub‐Saharan Africa. The frequency of C677 T homozygosity showed similar variability. 24 Amongst the European, the homozygous allele was found highest in the Italians and lowest in the Germans. 25 , 26 , 27 The prevalence of the homozygous TT genotype was 10–12% in Europe's several areas (for example, Spain, France, and Hungary). In Britain, the percentage of homozygosity in the population was approximately 13%. However, the prevalence appeared to be lower (4% and 6%, respectively) in Finland, Helsinki, and the northern Netherlands. In contrast, in some southern European areas, it was much higher (26% and 20% in Campania and Sicily, respectively). In the Americas, the frequency of the homozygous TT genotype was more elevated in Mexico (32%), intermediate in Atlanta (11% among whites), and somewhat lower in Alberta (6%). For the Blacks living in America and Brazil, the frequency of homozygosity was very low (1 or 2% only). 28 , 29 , 30
In Australia, TT prevalence was 7.5% among whites. Among the Whites not within Europe, the homozygous mutation percentages ranged from 10 to 14% in countries like Canada, America, Brazil, and Australia. 31 , 32 , 33 Zero rate was found on the homozygosity of the sub‐Saharan African population.
The several fold variations in the prevalence of the TT homozygous genotype across the study areas were also consistent, in some areas, with the presence of geographical gradients. In Europe, for example, the prevalence of the TT genotype increased in a roughly southerly direction, from low values in the north (4–7% in Finland, Helsinki, northern Netherlands, and Russia), to intermediate values (8–10%) in France and Hungary, to higher values in southern Europe (12–15% in Spain and northern Italy), peaking in south Italy (20–26% in Campania and Sicily). In North America, TT homozygotes’ frequency increased from western Canada (Alberta) to the southeastern United States (Atlanta) and peaked in Mexico. 6
The comparison of the prevalence of homozygous MTHFR‐677 mutation and the incidence and mortality by COVID‐19 showed a high degree of correlation, especially regarding South‐North and East‐West gradients, as well as for Americans, Europeans, and Asians populations. This data could be traced back to ancestral migratory phenomena related to population genetic or to a reduced dietary intake of B9 (folic acid) and other B‐vitamins that could characterize different dietary regimens among different cultures. 34
The genetic data related to MTHFR status coupled with Hcy dosage could represent important information for the assessment and stratification of COVID‐19 patients. In association with other epidemiologic, hematologic, biochemical and functional parameters, these data could be useful to define better population‐based risk strategies to fight COVID‐19 infection and lethality. It has been demonstrated that the presence of MTHFR T677 may result in the need for a higher folate and B‐vitamins intake to maintain normal Hcy levels; therefore, a preventive therapeutic integration of Folic acid and B‐vitamins could result in the reduction of prevalence and mortality for COVID‐19 viral infection. 34 , 35 , 36 , 37 , 38
5. CONCLUSION
Genetic polymorphism of MTHFR C677 T may modulate the risk of COVID‐19 incidence and severity.
Population data on correlation between the C677 T variant and COVID‐19 incidence and mortality would be very useful in regional and national management strategy and might help the population and public health geneticists to assess the potential impact of preventive measures based on environmental modifications. Some adverse biochemical effects of the thermolabile enzyme coded by the T allele, such as the increase in total plasma homocysteine, appear to be reversible by increasing the consumption of the vitamin B and folic acid.
DATA AVAILABILITY STATEMENT
The data presented in this study are available on request from the corresponding author.
REFERENCES
- 1. van der Made CI, Simons A, Schuurs‐Hoeijmakers J, et al. Presence of genetic variants among young men with severe COVID‐19. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID‐19 disease progression. Crit Rev Clin Lab Sci. 2020;57(6):389‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ponti G, Ruini C, Tomasi A. Homocysteine as a potential predictor of cardiovascular risk in patients with COVID‐19. Med. Hypotheses. 2020;143:109859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Z, Shi J, He Z, et al. Predictors for imaging progression on chest CT from coronavirus disease 2019 (COVID‐19) patients. Aging (Albany NY). 2020;12(7):6037‐6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liew S‐C, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur J Med Genet. 2015;58(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 6. Wilcken B, Bamforth F, Li Z, et al. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. J. Med. Genet. 2003;40(8):619‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yafei W, Lijun P, Jinfeng W, Xiaoying Z. Is the prevalence of MTHFR C677T polymorphism associated with ultraviolet radiation in Eurasia? J Hum Genet. 2012;57(12):780‐786. [DOI] [PubMed] [Google Scholar]
- 8. Yadav U, Kumar P, Gupta S, Rai V. Distribution of MTHFR C677T gene polymorphism in healthy North Indian population and an updated meta‐analysis. Indian J Clin Biochem. 2017;32(4):399‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butz L, du Vigneaud V. The formation of a homologue of cystine by the decomposition of methionine with sulfuric acid. J Biolog Chem. 1932;99:135‐142. [Google Scholar]
- 10. Balint B, Jepchumba VK, Guéant J‐L, Guéant‐Rodriguez R‐M. Mechanisms of homocysteine‐induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie. 2020;173:100‐106. [DOI] [PubMed] [Google Scholar]
- 11. Zoccolella S, Martino D, Defazio G, Lamberti P, Livrea P. Hyperhomocysteinemia in movement disorders: current evidence and hypotheses. Curr Vasc Pharmacol. 2006;4(3):237‐243. [DOI] [PubMed] [Google Scholar]
- 12. Graham IM, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk factor for vascular disease. The European concerted action project. JAMA. 1997;277(22):1775‐1781. [DOI] [PubMed] [Google Scholar]
- 13. Durand P, Prost M, Loreau N, Lussier‐Cacan S, Blache D. Impaired homocysteine metabolism and atherothrombotic disease. Lab Invest. 2001;81(5):645‐672. [DOI] [PubMed] [Google Scholar]
- 14. Verdoia M, Rolla R, Negro F, et al. Homocysteine levels and platelet reactivity in coronary artery disease patients treated with ticagrelor. Nutr Metab Cardiovasc Dis. 2020;30(2):292‐299. [DOI] [PubMed] [Google Scholar]
- 15. Cao JI, Chen X, Jiang LI, et al. DJ‐1 suppresses ferroptosis through preserving the activity of S‐adenosyl homocysteine hydrolase. Nat Commun. 2020;11(1):1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li T, Yu B, Liu Z, et al. Homocysteine directly interacts and activates the angiotensin II type I receptor to aggravate vascular injury. Nat Commun. 2018;9(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dixon S, Lemberg K, Lamprecht M, et al. Ferroptosis: an iron‐dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu B, Chen XB, Ying MD, He QJ, Cao J, Yang B. The role of ferroptosis in cancer development and treatment response. Front Pharmacol. 2017;8:992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun Y, He L, Wang T, et al. Activation of p62‐Keap1‐Nrf2 pathway protects 6‐hydroxydopamine‐induced ferroptosis in dopaminergic cells. Mole Neurobiol. 2020. [DOI] [PubMed] [Google Scholar]
- 20. Osaki T, Ohshima M, Tomita Y, Matsugi N, Nomura Y. Clinical and physiological investigations in patients with taste abnormality. J Oral Pathol Med. 1996;25(1):38‐43. [DOI] [PubMed] [Google Scholar]
- 21. Dinc ME, Dalgic A, Ulusoy S, Dizdar D, Develioglu O, Topak M. Does iron deficiency anemia affect olfactory function? Acta Otolaryngol. 2016;136(7):754‐757. [DOI] [PubMed] [Google Scholar]
- 22. Singh Y, Gupta G, Satija S, Negi P, Chellappan DK, Dua K. RAAS blockers in hypertension posing a higher risk toward the COVID‐19. Dermatol Ther. 2020:e13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goyette P, Rosenblatt D, Rozen R. Homocystinuria (methylenetetrahydrofolate reductase deficiency) and mutation of factor V gene. J Inherit Metab Dis. 1998;21(6):690‐691. [DOI] [PubMed] [Google Scholar]
- 24. Botto LD, Yang Q. 5,10‐Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000;151(9):862‐877. [DOI] [PubMed] [Google Scholar]
- 25. Adams M, Smith PD, Martin D, Thompson JR, Lodwick D, Samani NJ. Genetic analysis of thermolabile methylenetetrahydrofolate reductase as a risk factor for myocardial infarction. QJM. 1996;89(6):437‐444. [DOI] [PubMed] [Google Scholar]
- 26. Bowen DJ, Bowley S, John M, Collins PW. Factor V leiden (G1691A), the prothrombin 3’‐untranslated region variant (G20210A) and thermolabile methylenetetrahydrofolate reductase (C677T): a single genetic test genotypes all three loci–determination of frequencies in the S. Wales population of the UK. Thromb Haemost. 1998;79(5):949‐954. [PubMed] [Google Scholar]
- 27. Markus HS, Ali N, Swaminathan R, Sankaralingam A, Molloy J, Powell J. A common polymorphism in the methylenetetrahydrofolate reductase gene, homocysteine, and ischemic cerebrovascular disease. Stroke. 1997;28(9):1739‐1743. [DOI] [PubMed] [Google Scholar]
- 28. Arruda VR, Siqueira LH, Gonçalves MS, et al. Prevalence of the mutation C677 –> T in the methylene tetrahydrofolate reductase gene among distinct ethnic groups in Brazil. Am J Med Genet. 1998;78(4):332‐335. [DOI] [PubMed] [Google Scholar]
- 29. Dilley A, Austin H, Hooper WC, et al. Relation of three genetic traits to venous thrombosis in an African‐American population. Am J Epidemiol. 1998;147(1):30‐35. [DOI] [PubMed] [Google Scholar]
- 30. Stevenson RE, Schwartz CE, Du YZ, Adams MJ. Differences in methylenetetrahydrofolate reductase genotype frequencies, between Whites and Blacks. Am J Hum Genet. 1997;60(1):229‐230. [PMC free article] [PubMed] [Google Scholar]
- 31. Chen J, Giovannucci E, Kelsey K, et al. A methylenetetrahydrofolate reductase polymorphism and the risk of colorectal cancer. Cancer Res. 1996;56(21):4862‐4864. [PubMed] [Google Scholar]
- 32. Ma J, Stampfer MJ, Hennekens CH, et al. Methylenetetrahydrofolate reductase polymorphism, plasma folate, homocysteine, and risk of myocardial infarction in US physicians. Circulation. 1996;94(10):2410‐2416. [DOI] [PubMed] [Google Scholar]
- 33. Wilcken DE, Wang XL, Sim AS, McCredie RM. Distribution in healthy and coronary populations of the methylenetetrahydrofolate reductase (MTHFR) C677T mutation. Arterioscler Thromb Vasc Biol. 1996;16(7):878‐882. [DOI] [PubMed] [Google Scholar]
- 34. Ibrahimagic O, Smajlovic D, Dostovic Z, et al. Hyperhomocysteinemia and its treatment in patients with Parkinson's disease. Mater Sociomed. 2016;28(4):303‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rozen R. Genetic predisposition to hyperhomocysteinemia: deficiency of methylenetetrahydrofolate reductase (MTHFR). Thromb Haemost. 1997;78(1):523‐526. [PubMed] [Google Scholar]
- 36. Pepe G, Camacho Vanegas O, Giusti B, et al. Heterogeneity in world distribution of the thermolabile C677T mutation in 5,10‐methylenetetrahydrofolate reductase. Am J Hum Genet. 1998;63(3):917‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi M, Caprau D, Romitti P, Christensen K, Murray JC. Genotype frequencies and linkage disequilibrium in the CEPH human diversity panel for variants in folate pathway genesMTHFR,MTHFD,MTRR,RFC1, andGCP2. Birth Defects Res Part A Clin Mol Teratol. 2003;67(8):545‐549. [DOI] [PubMed] [Google Scholar]
- 38. Belcastro V, Pierguidi L, Castrioto A, et al. Hyperhomocysteinemia recurrence in levodopa‐treated Parkinson’s disease patients. Eur J Neurol. 2010;17(5):661‐665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.


