Abstract
UK good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) were published in 2012 and 2015 for adult and paediatric patients, respectively. Here we update the initial good practice recommendations in a combined document based on a further review of the OPAT literature and an extensive consultation process. As with the previous good practice recommendations, these updated recommendations are intended to provide pragmatic guidance for new and established OPAT services across a range of settings and to act as a set of quality indicators for service evaluation and quality improvement.
Contents
1. Introduction
-
2. Methods
2.1 Scope and purpose
2.2 Stakeholder involvement
2.3 Literature review
2.4 Consensus process and guideline development
-
3. Recommendations
-
3.1 OPAT team and service structure
3.1.1 Formal OPAT programme
3.1.2 OPAT ‘bundles’
3.1.3 OPAT team composition
3.1.4 OPAT and telemedicine
3.1.5 OPAT in new settings
3.1.6 Evidence gaps
-
3.2 Patient selection
3.2.1 Identification of patients
3.2.2 Selection criteria
3.2.3 OPAT in hard-to-reach groups
3.2.4 Evidence gaps
-
3.3. Antimicrobial management and drug delivery
3.3.1 Continuous antimicrobial infusions
3.3.2 Infusion devices
3.3.3 Vascular access
3.3.4 Antimicrobial agents
3.3.5 Antimicrobial stewardship
3.3.6 Clostridioides difficile risk in OPAT
3.3.7 Evidence gaps
-
3.4 Monitoring of the patient during OPAT
3.4.1 General considerations
3.4.2 Laboratory test monitoring
3.4.3 Antimicrobial switches
3.4.4 Evidence gaps
-
3.5 Outcome monitoring and clinical governance
3.5.1 Outcome monitoring for quality, service development and research
3.5.2 Standard outcome measures
3.5.3 Patient experience
3.5.4 Evidence gaps
-
4. Conclusions
1. Introduction
Outpatient parenteral antimicrobial therapy (OPAT) has been shown to be safe and effective for a wide range of infections in adults and children,1–7 and is now a routine part of patient care in the UK. First described >40 years ago in the USA, it was developed in several UK teaching hospitals around 20 years ago. Since then there has been an expansion in the number of UK OPAT services, with a conservative estimate of >100 formal hospital-based services.8 The increase in OPAT services has been attributed to a number of factors, including financial pressures in the NHS, the focus on moving care out of acute hospitals, development of antimicrobial agents that can be administered once daily, weekly or as continuous infusions, advances in vascular access and infusion devices, and OPAT acceptance by patients and healthcare professionals.9,10 Furthermore, OPAT is now being actively promoted as part of the UK government’s stewardship initiatives.11
In the UK, OPAT is being delivered in an ever-increasing variety of clinical and non-clinical settings. In hospitals, OPAT services have traditionally been based in infectious diseases (ID) units and, less frequently, in specialist units such as those for patients with cystic fibrosis. Now, however, we are increasingly seeing OPAT services run by acute or general physicians with infection input from a clinical microbiologist, rather than the ‘traditional’ UK model of an ID physician undertaking both of these roles. New OPAT services may be established in acute medicine or emergency department (ED) ambulatory care units or based in the community.12,13 Notably, in a 2015 BSAC survey of UK acute medicine and ED physicians, it was reported that the majority of patients with cellulitis treated with intravenous antibiotic therapy without hospital admission were not managed within a structured OPAT service, often without daily review for intravenous-to-oral switch (R. A. Seaton, personal communication). As ambulatory care services develop, it is important that the same OPAT governance procedures are in place to ensure appropriate and safe antibiotic prescribing, particularly the involvement of a clinical microbiologist or ID consultant. In addition to increasing variation in OPAT service delivery, self-administration or carer administration is increasingly being used as a cost-efficient alternative to the infusion centre model.1,14,15
There has been an increase in the complexity and comorbidity of patients and in the complexity of the infections being managed, with a move away from predominantly short-course therapy towards prolonged treatment courses for bone and joint infections, endocarditis and other complex deep-seated infections.1,4,16,17
Over the last 5–10 years there has been increasing recognition of the important relationship between antimicrobial stewardship (AMS) and OPAT. The prudent use of antimicrobial agents is now viewed as essential to maintain the effectiveness of our antimicrobial armoury against increasing global antimicrobial resistance.18–20 OPAT has been recognized as playing an important role in AMS and is one of the five options for ‘focusing’ antimicrobial therapy when reviewing therapy after an initial empirical approach.11 Interestingly, the role of OPAT services has been extended in some areas to the supervision of complex oral antimicrobial therapies, for example weekly toxicity monitoring for patients receiving linezolid.21 However, OPAT also has disadvantages as regards AMS, in particular the potential use of agents with a broader antimicrobial spectrum than may be necessary due to the logistics of once daily versus multiple daily dosing regimens, or the unnecessary prolongation of intravenous therapy when oral antibiotics would be suitable.22 Furthermore, it is recognized that OPAT is only one element of a patient’s management and that there needs to be consideration of other aspects of care, including surgical or radiological intervention and determination of clear treatment goals, including, when appropriate, long-term suppression or palliation in incurable infection.
Since the publication of the original UK OPAT good practice recommendations (GPRs) for adults and children23,24 a number of other national OPAT guidelines and recommendations have been published.25–27 The aim of the original UK recommendations was to provide a resource for teams developing new services, as well as to provide a practical set of quality indicators for existing services. Considering the growing OPAT literature and developments in clinical practice, it felt timely to update the UK recommendations to ensure that they continue to provide appropriate guidance to OPAT services across a range of healthcare settings.
2. Methods
2.1 Scope and purpose
This update covers both adult and paediatric OPAT; specific recommendations relating to either adult or paediatric populations are highlighted. Updated GPRs are presented as a literature update following the original literature reviews, with revised recommendations for each of the five key areas used in the original GPRs. Revised or amended recommendations are depicted using italics.
2.2 Stakeholder involvement
The BSAC was the host organization. Working Party membership comprised consultants in adult and paediatric ID, medical microbiologists, antimicrobial pharmacists and clinical nurse specialists in paediatric ID and OPAT.
2.3 Literature review
The Cochrane Library issues 8 of 12 (including the Central Register of Controlled Trials), CINAHL, EMBASE, PubMed and Web of Science (Science Citation Index Expanded) databases were comprehensively searched from 1 July 2010 to 31 July 2017 (Table S1, available as Supplementary data at JAC Online). A further search, covering the period 1 July 2017 to 31 August 2018, was completed shortly before the consultation process to capture any additional papers published. Terms were searched and collated for adults and run again with search terms specific for paediatrics (Table S1).
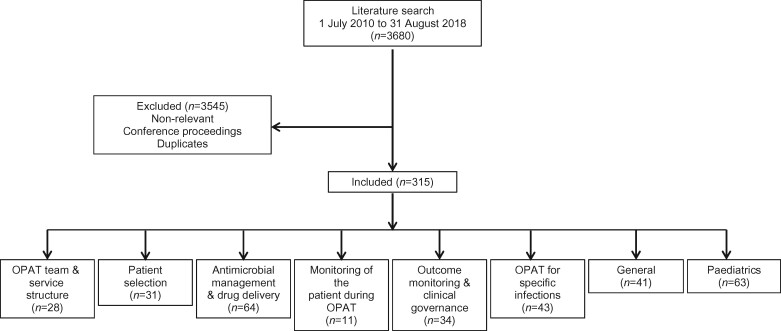
A total of 3007 references were identified from the first literature search (2463 references for adults and 544 for paediatrics) and 673 references in the second search (Figure 1). An initial screen identified non-relevant references (defined as those references with no mention of intravenous antibiotics or outpatient therapy), conference proceedings and duplicate references, which were removed from further appraisal. Detailed screening using the abstract was completed by members of the core working group. Remaining references were divided into several key areas relating to the areas of the previous GPRs (Figure 1). Where references were deemed to be relevant to all key areas, they were allocated to the ‘General’ category; references related to the use of OPAT for specific infections were allocated to the ‘OPAT for specific infections’ category, and papers relating specifically to the paediatric population were categorized into a ‘Paediatrics’ category. Once references had been divided into the appropriate key groups, full-text articles in the English language were obtained and reviewed. Where evidence on a particular recommendation was lacking in the literature this was noted. As most reviewed references described non-interventional, observational studies or case series, levels of evidence have not been included in this review.
Figure 1.
Flow diagram illustrating the process of the literature search.
2.4 Consensus process and guideline development
A core working group was established (A. L. N. C., M. G., R. A. S., S. P. and C. H.). Clinicians from across the range of professional groups involved in OPAT (nursing/medical/pharmacy) were invited to provide an appraisal of the literature identified by the searches. The evidence appraisal was reviewed in detail by the core working group at a meeting in May 2018, and subsequently by telephone and e-mail communication. Changes to the initial GPRs were made and new GPR statements were written (both indicated as italic text). The revised recommendations were circulated to all reviewers for checking sense and to ensure that there were no omissions. Following further revision, the draft updated GPRs were sent to a comprehensive list of stakeholders and uploaded to the BSAC website (www.bsac.org.uk). A formal 4 week consultation process was completed. The GPRs were revised in the light of the comments received, with nine revisions to the recommendations.
3. Recommendations
3.1 OPAT team and service structure
3.1.1 Formal OPAT programme
Recommendations relating to the OPAT team and service structure are listed in Figure 2. The previous UK GPRs stressed the importance of a formal OPAT service structure with clear clinical and managerial accountability. Many recent publications supported this view.28–32 Heintz et al.33 prospectively estimated the effect such a strategy has on patient safety and cost-effectiveness. Of 569 referrals in 536 patients enrolled into an OPAT programme, involvement of the OPAT team resulted in safety, regimen simplification or efficacy interventions for OPAT courses in 56.1%, 40.6% and 26.8%, respectively. Interestingly, OPAT team review resulted in a significant improvement in interventions related to safety (64% versus 48%) and efficacy (36% versus 21%) but not regimen simplification, compared with those not reviewed by the OPAT team. This suggests that knowledge of antimicrobials alone will not recoup all the value of OPAT team involvement. OPAT interventions may relate to a broader holistic assessment of the patient and the infection and/or more detailed assessment of social and logistical factors.33 Yan et al.34 retrospectively described a Canadian cohort of 104 patients discharged to receive intravenous antimicrobials without a formal OPAT programme. Although 56 did receive post-discharge follow-up from an ID physician, 56% returned to the ED within 60 days of discharge, while 26% required readmission; 48% of the returns were due to infection relapse or treatment failure and 23% could be attributed to OPAT-related complications.34 The implication is that with a formal OPAT programme these return visits to the ED could be avoided with careful patient selection and appropriate and timely monitoring and intervention during follow-up.
Figure 2.
OPAT team and service structure. Text in italics denotes a new recommendation or a previous recommendation that has been amended.
There was further evidence that many OPAT services worldwide lack a formal service structure. Lane et al.35 conducted a survey of US ID physicians and concluded that OPAT is frequently delivered by non-specialist OPAT teams without systems for tracking adverse events or monitoring patient outcomes. A further survey of US ID physicians in 2015 reported that only 56% of respondents were part of a formal OPAT programme.17 Respondents reported difficulties in communication between hospital physicians and community teams delivering OPAT, and variability in blood test monitoring and follow-up.
3.1.2 OPAT ‘bundles’
Several papers reported the impact of incorporating formal processes into an OPAT service. Keller et al.36 developed what they termed an ‘infectious diseases transition service’, comprising a physician, specialist nurse and pharmacist, and evaluated its impact on the care and outcomes of 488 OPAT patients. After the implementation of the transition service, readmissions decreased from 38.1% to 27.9%. However, importantly, the authors had also included a control group of patients who had been discharged on OPAT without formal ID consultation. Readmission rates also fell in this group with no significant impact of the transition service per se. Similarly, implementation of the transition service had no significant impact on ED visits. However, the transition team care was associated with improved process-of-care outcomes, such as fewer antimicrobial therapy errors, improved laboratory test receipt and increased follow-up visits.36
Similarly, Nguyen37 described an acute infection management service designed to transition patients with infections safely from the acute hospital setting to receive OPAT. In this study, 80 patients, of whom 66% had a diagnosis of cellulitis, received the service over 13 months, generating 618 follow-up visits. The service was safe, with only two patients requiring admission, one for fever and one for reasons of transportation.
Muldoon et al.38 and Madaline et al.39 report a more comprehensive approach to OPAT using OPAT ‘bundles’, where a bundle has been defined as ‘a set of practices that together should improve outcomes’.40 Muldoon et al.38 outline a theoretical approach using a bundle comprising six components, based on the IDSA guidelines and UK GPRs: (i) patient identification/selection; (ii) ID consultation; (iii) patient/family education; (iv) care transition; (v) outpatient monitoring; and (vi) OPAT programme measures.
In addition to the recommendations described in the initial UK GPRs, four further recommendations are made: (i) patients or carers should be given information about OPAT, its benefits and risks, and the potential complications and side effects of treatment; (ii) the follow-up appointment should be handed to the patient prior to discharge from hospital; (iii) a clear plan should be made for line removal at the end of therapy; and (iv) consideration should be given to developing novel approaches to patient education,23,24 for example mobile phone applications or simple cartoon-based educational material.29
Madaline et al.39 used a very similar bundle in a pre- and post- intervention study, but without a contemporaneous control group. Those patients receiving the bundle demonstrated a lower 30 day readmission rate when compared with the previous standard of care (13% versus 26.1%) and improved monitoring of blood tests and attendance at follow-up appointments, but no significant difference in ED attendances. The theoretical paper of Halilovic et al.41 on risks associated with OPAT further breaks down the bundle described by Muldoon et al.38 into key elements, designating an OPAT team member to take responsibility for each.
3.1.3 OPAT team composition
The previous UK OPAT GPRs made a recommendation about the composition of the OPAT multidisciplinary team (Figure 2; Recommendation 1.4) and several more recent papers supported this recommendation. In a retrospective case-controlled study, Shah et al.42 investigated 99 OPAT patients, of whom 60 were assessed by an ID physician and 39 received non-ID physician care. Those assessed by ID physicians were 3.9 times more likely to adhere to monitoring guidance. The addition of an ID pharmacist to the non-ID physician care increased the adherence to monitoring from 35.9% to 100%, underlining the critical importance and additional value of having an antimicrobial pharmacist on the OPAT team.42 Shresthra et al.43 retrospectively looked at 263 potential OPAT patients referred to ID physicians over a 3 month period. In 260 of 263 episodes the authors concluded that value was added by the ID physician; antimicrobial treatment was optimized in 84%, significant patient assessment was made in 52% and an additional medical care contribution was made in 71%. In 33% of cases, an intervention was made in all three of these domains. Perhaps most critically, OPAT was deemed not necessary in 27% (60% of these patients were changed to oral antimicrobials and for 40% no antimicrobial was deemed necessary).43 Hersh et al.44 also demonstrated a 24% reduction in use of OPAT in a paediatric cohort following introduction of a process of expert review.
OPAT must be guided by the principles of AMS and should operate within an AMS programme.22 In a retrospective observational study, Hase and Hosokawa32 described OPAT use of ceftriaxone in a Japanese hospital without a formal OPAT team. A total of 268 patients received ceftriaxone with some courses curtailed due to readmission (10.8%) and death (4.5%). Disappointingly, ceftriaxone was used empirically in 92.2%, blood cultures were not performed in 62.3% and no cultures of any type were performed in 30%.32 For children managed within paediatric OPAT (pOPAT) services, there is also increasing evidence that in the absence of paediatric AMS team oversight children have higher rates of bug/drug mismatches, drug-dosing errors and readmissions, and less rigorous laboratory monitoring of drug side effects.45 Embedding paediatric AMS within OPAT services has been shown to reduce the duration of intravenous antibiotics, through earlier cessation of antibiotics or prompt intravenous-to-oral switching.44,46 This is especially relevant when children are being ambulated directly from the ED or paediatric assessment unit as part of an admission avoidance strategy.47
3.1.4 OPAT and telemedicine
Three studies where telemedicine was used in the management of patients receiving OPAT were reviewed. Bradford et al.48 reported the use of telemedicine for remote monitoring of paediatric oncology patients receiving home intravenous therapy administered by parents in comparison with administration in the home by a visiting nurse or administration in an infusion centre. They found that the telemedicine model allowed the delivery of safe care with significant cost reduction compared with the other treatment strategies.48 Greenup et al.49 used telemedicine to provide support to hospital-in-the-home (HITH) nurses when discharging patients from the service. The use of telemedicine to obtain clinical advice from a hospital-based physician allowed patients to be discharged from the service without the need for in-person consultation and had no significant impact on 28 day readmissions.49 Thirdly, Tan et al.50 reported the use of telemedicine in the management of 88 episodes of OPAT in 83 patients over a wide geographical area around Perth, Western Australia. OPAT was initiated in hospital and ongoing treatment was administered by local nursing services, supported by a once-weekly videoconference with an ID physician. Clinical outcomes were comparable to conventional OPAT and the authors estimated that >100 000 km of travel was avoided.50 Telemedicine is likely to be used increasingly in future51 and should be incorporated into OPAT programmes systematically with appropriate plans for escalation/safety-netting.
3.1.5 OPAT in new settings
OPAT in the UK has predominantly been delivered by teams based in acute hospitals. Such services tended to deliver OPAT through one or more of three models: the ‘infusion centre’ model, where patients attend an OPAT facility daily; the ‘visiting nurse’ model, where a nurse (from either primary or secondary care) delivers therapy in the patient’s home; or the ‘self-administration’ model, where the patient or a carer is taught to administer therapy with regular supervision from the OPAT service. During the early years of OPAT in the UK, OPAT services were usually run by ID units, but increasingly other specialities are setting up OPAT. In particular there are now reports in the literature of services based in acute medicine or emergency ambulatory care units. Yan et al.13 described a predominantly infection nurse-led service in a British hospital where access to medical care was via the nurse through the ED physicians. In this retrospective cohort study, 140 patients received OPAT, either returning to hospital daily (n = 94) or in their own home through a district nursing visit (n = 46). The service was safe, with a failure/complication rate of 5.7% and hospital readmission rate of 3.6%. The mean duration of OPAT was 4.4 days, with the predominant diagnosis being cellulitis.13
In addition to increasing diversity in OPAT providers in secondary care, another key development has been the establishment of OPAT based within primary care organizations and delivered in the community. Antimicrobial therapy may be initiated and carried out exclusively in the community, or alternatively may be initiated in hospital and transferred to a community-based OPAT service.10 Several papers describe such services.12,52–55 Nazarko12 outlines the advantages and potential disadvantages of developing a specialist intravenous therapy team or incorporating OPAT into the day-to-day activity of established community nurses. A dedicated intravenous therapy team would provide enhanced expertise in management of different devices and antimicrobial agents and in practical skills such as venepuncture and cannulation. Furthermore, it may be easier to train a small team to recognize clinical deterioration. Gray et al.56 used patient scenarios to investigate how HITH nurses recognize and respond to the deteriorating patient; however, service capacity may be limited by a small team, particularly over large geographical areas. The use of the larger pool of community nurses would provide greater capacity and also allow nurses to deliver more than one type of care to housebound patients at the same visit, for example wound or ulcer dressings and insulin injections. However, community nurses may be unfamiliar with intravenous therapies and require theoretical and practical training; there is also the issue of maintaining competency. Whatever the model for antimicrobial administration, as with services based in secondary care, the involvement and oversight of a full OPAT team is essential, including a lead OPAT nurse, antimicrobial pharmacist and infection specialist.55,57 It is also essential that a responsible physician is clearly identified for every patient; this may be a primary care doctor or hospital specialist. Mace et al.58 examined the impact of introducing a dedicated OPAT team to a paediatric HITH service in Australia and reported improved adherence to monitoring guidelines, reduced readmissions and fewer patients on prolonged antimicrobial therapy, demonstrating the importance of medical governance in a nurse-led service.
In establishing a community-based OPAT service, key issues to consider include workload and capacity, dose frequency, use of boluses or infusions, line insertion and care, and arrangements for prescribing and dispensing antimicrobials, for clinical reviews and for escalation if complications occur. Several authors reported the use of ‘OPAT kits’ made up by local pharmacy manufacturing units and containing all medications, diluents and consumables required for a week, tailored to each patient and type of vascular access device.10,12,52,57 Barker and Lyden-Rodgers55 described an audit of 26 patients receiving intravenous antibiotics in their homes and concluded that cost savings in nursing time may be possible if single as opposed to paired visiting community nurses deliver bolus doses in comparison with infusions. Although community intravenous therapy services may deliver a range of parenteral agents, such as bisphosphonates, iron, chemotherapy or blood products, involvement of an infection specialist and antimicrobial pharmacist in the care of patients receiving intravenous antibiotics is essential in ensuring that AMS is prioritized. Outcome monitoring in general may be more challenging in the community context where patients are under different primary care physicians and teams of nurses, and also needs to be considered when establishing a community-based service.
3.1.6 Evidence gaps
One key evidence gap relates to the appropriate time commitment for OPAT team members. Financial pressures within healthcare systems may have an impact on the number of staff being employed to deliver OPAT; data on the appropriate ratio of patients to OPAT specialist nurses in particular would be helpful to justify the funding required to deliver an OPAT service. Review of the UK BSAC OPAT National Outcomes Registry System (NORS) (http://opatregistry.com) in 2018 suggested that for established hospital-based infection-led OPAT services, the average establishment per 100 episodes per year was 1.5 whole-time equivalents (WTE) for nursing, 0.3 WTE for medical staff, and 0.25 WTE for specialist pharmacists (M. Gilchrist, personal communication). There are currently no data available for community-based OPAT services.
3.2 Patient selection
3.2.1 Identification of patients
Recommendations relating to patient selection for OPAT are listed in Figure 3. In the past, identification of suitable patients for OPAT was often through direct referral from inpatient teams or ad hoc referrals from other infection specialists. With increasing experience of OPAT and demonstration of its safety and patient focus, there is a realization that we must move from a passive or ‘opportunistic’ approach to identifying suitable patients to a more active approach. Examples include participation in multidisciplinary clinical meetings in key specialties such as the diabetic foot service or orthopaedics. O’Hanlon et al.52 describe 14 patients with diabetic foot infections, suggesting that nurses with OPAT training can be useful case finders for patients who may be suitable for OPAT within the diabetic clinic.
Figure 3.
Patient selection. Text in italics denotes a new recommendation or a previous recommendation that has been amended.
Patients may also be identified as potentially suitable for OPAT actively through infection initiatives such as bacteraemia reviews and antimicrobial ward rounds. One study demonstrated an increase in the numbers of patients receiving OPAT through the use of a bacteraemia database to identify patients who may be suitable for OPAT once stable.59 Dryden et al.60 describe infection team review of inpatients receiving antimicrobial therapy in six UK hospitals. Of 89 patients who were suitable for discharge, 55 were suitable for oral outpatient treatment, 24 had their antibiotics stopped and 10 would have required OPAT. This study demonstrated the value of an infection team in identifying patients appropriate for OPAT, but more significantly in recommending the use of oral agents over intravenous therapy where clinically appropriate. Conant et al.61 also reiterated the importance of involvement of an infection specialist in optimizing antimicrobial therapy, ensuring appropriateness of patients for OPAT and contributing to AMS.
3.2.2 Selection criteria
As with the previous GPRs, many papers concluded that careful patient selection was critical to improving outcomes and reducing risks surrounding OPAT. Selection involves consideration of patient-specific criteria, such as ability to understand and consent to OPAT, likelihood of compliance, appropriate home circumstances, ability to attend for OPAT, support from family members and safety of visiting healthcare staff. Having no primary care provider has also been found to be a risk factor for OPAT complications.62,63
Infection-related criteria are also important in patient selection, for example the site and severity of infection, presence of complications of infection, prior duration of antimicrobial therapy, initial response to treatment and availability of oral antibiotic options.64,65 Underwood et al.66 reported that of 781 patients referred to their OPAT service, 31% were assessed as not requiring intravenous therapy following review of their proposed management plan.
There is also increasing evidence that patient selection should move beyond application of rigid criteria but should take into consideration additional factors that may influence the likelihood of OPAT failure or complications. Schmidt et al.67 found that the risk of unplanned hospitalization in OPAT patients was increased in older patients with more comorbidities: for each additional point in the Charlson comorbidity index the risk of unplanned readmission increased by 5%. They also found that the risk of unplanned hospitalization varied depending on the type of facility in which patients were receiving OPAT. It is not clear whether this relates to patient factors determining the need for a specific OPAT facility or to factors relating to the facility itself, for example expertise of staff or robustness of arrangements for monitoring blood tests.68 Whatever the reason, Schmidt et al.67 suggested that the Charlson comorbidity index may be useful both in patient selection and in determining the most appropriate site or model of OPAT. Duncan et al.69 retrospectively reviewed 80 episodes of OPAT for infective endocarditis and found that on multivariate analysis cardiac or renal failure were independently associated with OPAT failure. Similarly, Seaton et al.70 found that the presence of diabetes or vascular disease was a predictor of poorer outcomes in 963 patients with skin and soft tissue infection managed via OPAT. However, Allison et al.71 found an association between 30 day readmissions and four factors—increasing age, use of aminoglycosides, presence of antibiotic-resistant organisms and the number of prior hospitalizations in the preceding year—but found no impact of comorbidities. These findings may have been due to differences in population sizes or in the screening protocol for acceptance for OPAT and demonstrate the difficulty in comparing studies and also the need for further work to look at predictors of poor outcomes in OPAT.
In assessing the appropriateness of a patient for OPAT there may also be considerations relating to their longitudinal progress. As an example, a recent review of the paediatric cystic fibrosis literature confirmed that no randomized controlled trials have been conducted comparing inpatient versus OPAT management of children with cystic fibrosis.2 Cohort studies have yielded conflicting results in terms of patient/parent satisfaction and clinical outcomes.72–74 It is difficult therefore to offer clear recommendations about the safety and effectiveness of pOPAT in children with cystic fibrosis, and the authors suggest a more holistic view of the likely benefits to the child/young person/family regarding the potential impact of pOPAT on their long-term respiratory function.
3.2.3 OPAT in hard-to-reach groups
OPAT was offered to patients with mental health diagnoses, people who inject drugs (PWIDs) and homeless patients by a few centres. Ho et al.75 describe successful treatment of 29 PWIDs at their centre in Singapore; however, they used strict selection criteria and standardized measures to prevent and detect line misuse, including tamper-detectable line dressings. Beieler et al.76 used OPAT successfully for treatment of infections in homeless people, most of whom were PWIDs; however, OPAT was delivered in a medical respite facility with close supervision, an overnight curfew and access to substance misuse services and opioid replacement therapy. The importance of careful patient selection was highlighted by several authors;77–79 Camsari and Libertin77 concluded that they would offer OPAT to PWIDs only if they had been abstinent for >12 months. Buehrle et al.80 stressed the importance of a comprehensive support package; in their study only 39% of PWIDs completed OPAT successfully due to a combination of clinical and social factors. They, and others, have concluded that recent or ongoing injection drug use may be considered a contraindication to OPAT and that there was a need for further research into the reasons for the high rates of OPAT failure, the effectiveness of oral therapy as an alternative to OPAT, and the benefit of residential addiction treatment alongside OPAT.80,81 Hernandez et al.82 also identified social factors, such as missed appointments or loss of temporary accommodation, as important in success or failure of OPAT in their retrospective study of 43 homeless people, 33 (77%) of whom completed treatment successfully. Longer-acting antimicrobial agents may also be of benefit in PWIDs and other hard-to-reach groups to reduce the reliance on patient compliance with daily therapy and to avoid long line placement.83–86
3.2.4 Evidence gaps
As noted above, more prospective research is required to enable us to predict more accurately which patients are most likely to have a successful, or unsuccessful, outcome of their OPAT episode. There is also a paucity of paediatric data on patient selection and risk stratification. Although there is always a need for clinical judgement, it would be helpful to develop evidence-based algorithms to support patient selection in future.
The previous GPRs included a recommendation on risk assessment for venous thromboembolism (VTE) in patients undergoing OPAT following an inpatient stay. The updated literature review identified one paper on the risk of VTE with OPAT.87 This was a retrospective review of 780 OPAT episodes over a 3 year period; no patients received VTE prophylaxis. Two patients developed deep vein thrombosis within 90 days of OPAT, giving a VTE incidence of 0.26%. The authors concluded that patients commencing OPAT should not be assessed routinely for VTE prophylaxis using an inpatient algorithm. Although these data are reassuring, patients on OPAT do have significant infections and are therefore at increased risk of VTE compared with the general population88 and it would be useful to have further prospective data on the risk of VTE and the optimal assessment strategy for prophylaxis.
3.3 Antimicrobial management and drug delivery
3.3.1 Continuous antimicrobial infusions
Recommendations relating to antimicrobial management and drug delivery in OPAT are listed in Figure 4. Four papers presented data on the use of continuous infusions of amoxicillin, meropenem, vancomycin and clindamycin, respectively, presenting data on clinical outcomes and drug stability.89–92 All reported relative success; however, there were questions over antibiotic degradation and the potential need for therapeutic drug monitoring. Voumard et al.93 also reported that OPAT using elastomeric pumps for the continuous administration of four antibiotics (flucloxacillin, cefepime, vancomycin and piperacillin/tazobactam) was efficacious and safe. Drug concentration measurements, considered a proxy for efficacy, confirmed adequate circulating antibiotic exposures consistent with the observed high rate of therapeutic success.
Figure 4.
Antimicrobial management and drug delivery. Text in italics denotes a new recommendation or a previous recommendation that has been amended.
Within the wider OPAT arena concerns have been expressed about the lack of antimicrobial stability data, particularly within elastomeric devices.22 The UK BSAC OPAT initiative conducted a separate literature review into antimicrobial stability within elastomeric devices.94 It found no published studies that comply with UK national standards for stability testing.95 As a result of this work an antimicrobial stability testing work stream was created within the UK BSAC OPAT initiative. Two stability studies have been published to date. Flucloxacillin was demonstrated to be chemically stable when reconstituted with 5% citrate buffer for up to 14 days of storage and for an additional 24 h at ‘body worn’ temperature. Flucloxacillin is therefore suitable for extended infusion via an elastomeric device within an OPAT setting.96 In contrast, meropenem showed significant degradation with or without buffering and so is not suitable for continuous infusions in the OPAT setting.97 BSAC drug stability testing studies are open-access and designed to allow OPAT services to use these agents where the clinical need exists (http://www.bsac.org.uk/drug-stability-testing-programme/).
3.3.2 Infusion devices
More generally, some papers explored the use of new devices. Oliver98 reviewed benefits and disadvantages of elastomeric devices and described experience with one type of elastomeric device, with very positive nurse evaluations. Saillen et al.14 reported high levels of patient satisfaction with elastomeric devices, particularly from patients who were self-administering via an elastomeric device, as opposed to those who had devices changed by visiting nurses or at the OPAT unit. Hobbs et al.99 present the protocol for a study to evaluate patient and nurse satisfaction with electronic and elastomeric portable infusion pumps used at home (CHID study), which may be useful in guiding their further use.
In contrast, Pandya et al.100 reported 14 adverse events in 10 of 31 patients receiving intravenous antibiotics by elastomeric device infusion. Five adverse events were related to the infusion device, including device failure and leakage. These adverse events resulted in additional telephone calls and nurse visits. This reinforces the need for a robust OPAT service structure with escalation pathways, including flexibility to provide specialist patient input when needed, rather than defaulting to the ED, where staff may be unfamiliar with the device.101
3.3.3 Vascular access
The literature search identified only one paper relating to vascular access in adults. Bedford and Waterhouse102 described the development of an out-of-hospital nurse-led service for insertion of peripherally inserted central cannulae (PICCs) using ECG-guided central line tip placement. This new technology removes the need for radiological or fluoroscopic confirmation of the correct position of the PICC tip within the lower third of the superior vena cava, and therefore the need for patients to attend hospital for their procedure. The authors described the process for doing this, including patient consent and safety measures, and reported 55 successful community-based insertions without complications. The ability to insert long-term venous access in a community setting will facilitate the expansion of community-based OPAT, as described in the service structure section above.
Four papers reviewed line complications in adults.66,103–105 Barr et al.103 retrospectively analysed line infections and other line events in 854 patients with midline catheters, PICCs or tunnelled central venous catheters. Incidence of line-related complication was 3.6 per 1000 intravenous catheter days, the majority of which were not infection related. Incidence of line infection was 2.3% (0.53 per 1000 intravenous catheter days) and on multivariate analysis was associated with duration of intravenous line placement with a 1.2% odds increase per additional day of intravenous therapy. Line type (midline versus central line) was not independently associated with risk of infection. Incidence of other line events (including phlebitis, leakage, extravasation, occlusion or unplanned removal) was 14.6%. As with previous studies, patient self-administration of OPAT was not associated with an increased risk of line complications, although use of multiple daily dosing of flucloxacillin and use of a midline versus a tunnelled central line were.103 Shrestha et al.105 reported line complications in 9% of OPAT courses, most frequently line occlusion, with increasing risk of complications with duration of OPAT. Line infection occurred in <1% of OPAT courses overall. Lam et al.104 reported that prolonged OPAT, use of double lumens and administration of benzylpenicillin and cloxacillin appeared to increase the risk of PICC occlusion, and suggested that these factors be considered by OPAT teams when choosing lines and therapeutic agents. Finally, Underwood et al.66 described 544 OPAT episodes, 5.9% of which were complicated by line-related complications (5.7 per 1000 intravenous catheter days). Most complications were non-infectious. In contrast to other published studies, the authors noted that self-administered antimicrobials were more likely to be associated with vascular device-related complications. As in other studies, non-radiologically inserted midline catheters were associated with higher rates of complications.
In pOPAT the data suggest that PICC line complications are less common than previously reported.106,107 Recent studies have described an 8%–15% complication rate for PICC lines used for the administration of intravenous antibiotics to pOPAT patients; infections are responsible for <25% of these adverse events.7,108,109 More data are required about the rate of adverse events associated with midline catheters before they can be routinely recommended for use within pOPAT services. A complication rate of 43% has been described in one small study.110 A dedicated paediatric intravenous line service may help to provide safer and more patient-centred intravenous access.111 Long-term vascular access, such as totally implantable venous access devices (TIVADs), may be indicated in some patient groups.112,113
3.3.4 Antimicrobial agents
Several papers identified new and existing antimicrobial agents being used within the OPAT setting. One of the key areas of growth that represents a step change in antimicrobial therapy via OPAT has been the development of long-acting semi-synthetic glycopeptides, such as oritavancin83,86,114 and dalbavancin.85,115–117 These agents may be particularly useful for patients who may not otherwise be suitable for OPAT, for example where there are concerns about compliance issues or line misuse. They could also be useful in OPAT services with limited capacity due to their infrequent administration and impact on nurse workload. In using these longer-acting agents the challenge for OPAT services will be to develop clear clinical pathways and individual management plans, ensuring adequate oversight of clinical progress.84 Televancin,118 tedezolid119 and echinocandins120 are other new agents that may be used increasingly in the future.
There has been a growth in the literature surrounding more traditional antimicrobial therapies within OPAT, mainly around dosing and adverse events. Experience has grown with daptomycin,121–124 teicoplanin125,126 and ertapenem.127–129 Adverse events have been reported with ertapenem and tigecycline,128–130 adding to the literature around ensuring there is close monitoring and follow-up of OPAT patients.
3.3.5 Antimicrobial stewardship
The OPAT team is integral to the development of the antimicrobial plan and judgement is required regarding the use of broader-spectrum once-daily agents to facilitate OPAT where a narrower-spectrum agent with multiple daily doses would be used in an inpatient setting.22 The use of continuous infusion pumps and elastomeric devices may provide a solution to this, as long as robust stability data exist. Where multiple daily doses are being given it is important that the pharmacodynamics of the antimicrobial agent are not compromised by suboptimal timing of doses through logistical constraints of the OPAT service. New long-acting agents may be advantageous in terms of logistics of administration but must be integrated into the OPAT service in a systematic way, as described earlier.
Optimal AMS also includes timely switch from intravenous to oral antibiotics (Table 1) and this should be considered both at the point of referral to OPAT as well as during a course of OPAT. General principles that should be considered when deciding whether the switch from intravenous to oral antibiotics is suitable include assessing the clinical condition, ability to absorb oral antibiotics, and availability of an appropriate oral choice. For children, dosing frequency and taste of oral suspensions should also be considered. McMullan et al.139 reviewed current evidence on duration of intravenous antimicrobials and optimal timing of intravenous-to-oral switch in children. Availability of OPAT may paradoxically result in excessively long intravenous antimicrobial courses unless subject to AMS;2,58,140 comparisons of antibiotic durations for specific pathologies between OPAT centres may provide useful information to guide clinical practice where evidence about the timing of intravenous-to-oral switch is lacking.141 The antimicrobial pharmacist plays an important role in assessing the pharmacokinetic/pharmacodynamic applicability of oral agents, potential drug–drug and drug–host interactions, antibiotic compliance, potential adverse events and monitoring needs, and how these are best addressed in an out-of-hospital setting. Patients should be counselled when antimicrobials are initiated, switched to another intravenous agent and stepped down to oral therapy.
Table 1.
Evidence for oral versus intravenous antimicrobial therapy in selected infections
| Infection type (population) | Evidence |
|---|---|
| Bone and joint infections (adults)131 | Multicentre UK-wide randomized study of oral versus intravenous antibiotic treatment for bone and joint infections (OVIVA). In a heterogeneous group of patients with device-related and non-device-related bone and joint infection who had received <7 days of initial intravenous therapy, randomization to carefully selected oral antibiotic therapy was found to be non-inferior to continuation of intravenous therapy, with 86% success observed in both groups at 1 year. In addition, significantly lower rates of line-related complications and lower treatment costs were observed in the oral treatment group. |
| Bone and joint infections (children)132,133 | Increasing evidence that pOPAT is only indicated for a minority of children with bone and joint infections. The majority of patients should be managed with an early intravenous-to-oral switch. |
| Endocarditis134 | Clinically improved patients with endocarditis were randomized to early intravenous-to-oral switch or standard therapy with exclusively intravenous antibiotics. Early transition to oral therapy was found to be non-inferior to intravenous therapy. This study population would be typical of the group usually managed via OPAT; therefore, appropriate oral therapy may be a suitable alternative to OPAT for selected low-risk patients. |
| Intra-abdominal infection135 | Oral antibiotics had equivalent outcomes and incurred lower costs than intravenous antibiotics following appendicectomy. |
| Lower urinary tract infections (adults)136 | Non-inferiority of oral fosfomycin compared with intravenous ertapenem for the treatment of lower urinary tract infections caused by ESBL-producing Enterobacteriaceae. |
| Pyelonephritis (children)137 | No difference between oral antibiotics (10–14 days) and intravenous antibiotics (3 days) followed by oral antibiotics (10 days) with respect to duration of fever or subsequent renal damage. |
| Pleural empyema (children)138 | Discharge on intravenous antibiotics offers no benefit over discharging children with empyema on oral antibiotics. |
3.3.6 Clostridioides difficile risk in OPAT
The use of broad-spectrum agents such as ceftriaxone or ertapenem raises concern about the risk of Clostridioides difficile-associated diarrhoea (CDAD). The literature review identified several studies that examined the risk of CDAD with OPAT in adults. Aberdein and Chapman142 cross-referenced OPAT and hospital microbiology databases to identify patients who had had both OPAT and CDAD over a 5 year period. Among 1514 patient episodes and 16 750 OPAT days, 13 patients developed CDAD between 2 days of commencing and 84 days of ceasing OPAT. All but one patient had risk factors other than OPAT for CDAD, including prior hospitalization and oral antibiotics from their GP. The rate of ‘definitely OPAT-attributable CDAD’ was equivalent to six cases per 100 000 OPAT days. The comparable rate for hospital inpatients nationally at that time was 54 per 100 000 bed days.142 Duncan et al.143 reviewed use of ceftriaxone for OPAT and concluded that CDAD occurred in ∼0.1% of OPAT episodes. A study in the USA reported five cases of CDAD in a cohort of 681 patients, an incidence of <1 per 1000 patient-days.144 All five patients had had prior hospitalization and four were on concomitant acid-suppressive therapy.
3.3.7 Evidence gaps
As noted above, there is a need for further data on antimicrobial stability over prolonged periods in elastomeric devices or infusion pumps, and the Drug Stability Testing work stream of the BSAC OPAT UK Project will add to existing knowledge in this area. The updated literature search provided no new data on the safety of administering the first dose of antibiotic in the home setting. However, with the growth of OPAT services based entirely in the community there is increasing evidence of the safety of this approach, as long as the nurse administering therapy is trained and equipped to manage adverse reactions, including anaphylaxis.
3.4 Monitoring of the patient during OPAT
3.4.1 General considerations
Recommendations relating to monitoring patients during OPAT are listed in Figure 5. There were only a small number of new papers (n = 11) relating specifically to monitoring patients during OPAT, although papers from other sections also provided useful new data for this domain. The risks associated with OPAT are well described:23,24 overall at least 25% of patients on OPAT will develop complications of therapy, ranging from mild adverse reactions to life-threatening line infections. A high proportion of patients experience risks associated specifically with prescription of their antimicrobial agents, including potential drug interactions, issues with therapeutic drug monitoring and the need for dose changes associated with changes in renal function, and the importance of a multidisciplinary approach to monitoring, with the inclusion of a pharmacist, has been emphasized.42,145
Figure 5.
Monitoring of the patient during OPAT. Text in italics denotes a new recommendation or a previous recommendation that has been amended.
The previous GPRs stated that patients with skin and soft tissue infections should be reviewed daily to ensure that they are switched from intravenous to oral antibiotics as soon as this is clinically appropriate. This remains an important recommendation. Kameshwar et al.140 undertook a health-economic study of patients managed via HITH in Australia. They found that patients managed through HITH had a longer median duration of intravenous therapy than equivalent inpatients (7.5 versus 5.8 days, respectively) and that this difference offset any financial savings associated with home therapy. They speculated that the longer duration of antimicrobial therapy in HITH could have arisen due to less frequent clinical reviews and also the possibility that clinicians in hospitals were under greater pressure to free hospital beds while HITH clinicians may have adopted a more risk-averse approach.140
Paediatric data show that significant adverse events due to antimicrobials are infrequent in pOPAT. Readmissions due to drug side effects occurred in only 0%–2.3% of patients described in two recent pOPAT cohorts.7,110 However, a retrospective case series of children managed between 2008 and 2015 describes a 13.5% readmission rate due to antimicrobial side effects.108 Oxacillin was associated with significantly higher rates of adverse drug events (transaminitis, fever and rash) compared with ceftriaxone. High rates of adverse drug events have also been described with piperacillin/tazobactam (fever, transaminitis, neutropenia and rising inflammatory markers), with 26% of children readmitted due to drug side effects in that cohort. Adverse events occurred after a minimum of 14 days of treatment in 93% of cases.146
3.4.2 Laboratory test monitoring
Lack of availability of recommended laboratory tests has been shown to be an independent risk factor for increased readmission rates for OPAT patients.68 Keller et al.147 prospectively analysed adverse drug events in a cohort of 339 patients discharged to OPAT from two academic centres; 18% developed an adverse drug event, and such events were more likely to occur within the first 14 days of treatment. However, other groups reported increasing risk of adverse events with increasing duration of intravenous antimicrobial therapy. Briggs et al.148 reported late-onset reactions to β-lactam antibiotics: 11 out of 163 patients developed symptoms such as fever, rash or abdominal pain during drug administration, or laboratory abnormalities including thrombocytopenia, leucopenia or abnormal liver function tests, with a median duration of therapy of 25 days prior to development of the adverse event. Severe neutropenia is a late complication of ceftriaxone, usually occurring after 28 days of therapy.149 Weekly monitoring of full blood count may also detect developing eosinophilia, which is a predictor of hypersensitivity reactions: Blumenthal et al.150 identified eosinophilia in 210 of 824 (25.5%) patients receiving OPAT, with a median time to eosinophilia of 15 days (IQR 8–22 days).
The previous GPRs recommended weekly blood-test monitoring for short-term OPAT patients but did allow a reduction in frequency of monitoring to twice monthly for longer-term stable patients. However, given evidence of increasing adverse events with treatment duration and the importance of early detection of these adverse events, the new recommendation is that all OPAT patients have blood test monitoring at least weekly regardless of treatment duration.
3.4.3 Antimicrobial switches
Lee et al.151 reviewed outcomes of OPAT with β-lactam antibiotics. In 400 OPAT courses, antibiotic switches were required in 50 episodes, of which 37 were accomplished without readmission. The authors stressed the importance of close monitoring and the involvement of the infection specialist in optimizing antimicrobial therapy to minimize patient morbidity and the need for readmission.151
3.4.4 Evidence gaps
Further information is required regarding the efficacy and potential adverse reaction profile of prolonged use of the newer long-acting semi-synthetic glycopeptides and optimal monitoring strategy. This is also true for other new agents. Another interesting area relates to patient involvement in monitoring, both in terms of awareness and early reporting of symptoms and also patient access to results through new web-based systems such as those used in diabetes or renal medicine. One key aim of OPAT is personalized, person-centred care, and involvement in monitoring their therapy may be useful in promoting patient engagement in their care and also contributing to earlier detection of adverse reactions.
3.5 Outcome monitoring and clinical governance
3.5.1 Outcome monitoring for quality, service development and research
Recommendations relating to outcome monitoring and clinical governance associated with OPAT are listed in Figure 6. Regular reviews of the OPAT service are essential to review the activity of the service and to benchmark it against national guidelines and GPRs, and other OPAT centres and registries. This is only possible if data are collected prospectively at the level of the individual patient, using an electronic database or online outcome registry. In addition to clinical outcomes such as response to treatment and adverse events, it is also important to collect data on the OPAT episode, for example patient demographics, antimicrobial agent(s) used, duration of treatment, method of OPAT used, type of line and infusion device. It may also be useful to record other data for the purpose of local service development, for example the number of episodes and OPAT days, type of transport used by OPAT patients, time taken for antimicrobial administration and other interventions performed during OPAT attendance (e.g. podiatry review, dressing changes on ulcers and monitoring of anticoagulation).
Figure 6.
Outcome monitoring and clinical governance. Text in italics denotes a new recommendation or a previous recommendation that has been amended.
The importance of effective AMS is now well recognized and a key UK government and NHS priority.11,60 Gilchrist and Seaton22 reviewed AMS as it applies to OPAT and proposed an OPAT AMS checklist, comprising checks relating to the individual patient, OPAT antimicrobial use, staffing and links to organizational governance procedures and policies. Again, there is a need for prospective data collection to quality-assure OPAT services from an AMS perspective.
Finally, collection of rich prospective patient data provides the opportunity to study factors influencing patient outcomes such as those described earlier (section 3.2, Patient selection). It is clear that we do not fully understand predictors of success or failure of OPAT and this must be a priority for further prospective research.
3.5.2 Standard outcome measures
The previous OPAT GPRs suggested that it would be useful to develop standardized outcome measures for OPAT. These outcome criteria were divided into patient infection and OPAT service-related outcomes and have been used by UK OPAT centres and the BSAC OPAT NORS to drive improvement and allow benchmarking exercises. However, there remains a lack of standardization and clarity as to which outcomes are measured. For example, a patient may be switched from one antimicrobial to another due to a recognized side effect. This is managed as part of the OPAT management plan, resulting in successful treatment. Under the previous outcome recommendations this would be classed as partial success. The authors also recognized that patient outcomes are very much dependent on the individual treatment aim. This is particularly relevant where short- or longer-term control of the infection is the only realistic outcome of therapy. The NORS outcome measures include ‘death’ as both a patient infection ‘failure’ and an OPAT ‘failure’, which may not be appropriate where the aim of OPAT for that individual is palliation or long-term suppression. The authors therefore concluded it would be helpful to include outcomes for those clinical episodes where cure is not achievable.
Here we propose new treatment aims to be considered at the outset of an OPAT treatment course and updated OPAT outcomes (Table 2). In addition, there is increasing literature to suggest that OPAT adverse events should be reviewed in line with local organizational AMS programmes, for example healthcare-associated infections such as CDAD and bloodstream infections. In addition to patient outcomes, as in the last GPRs, it is also recommended that OPAT teams monitor specific adverse outcomes (see Recommendation 5.2).
Table 2.
Proposed treatment aims and OPAT service outcomes
| Description | |
|---|---|
| Treatment aim | |
| cure | To complete an agreed OPAT duration of therapy on either intravenous and/or complicated oral antimicrobialsa with no requirement for long-term antimicrobial therapy. |
| improvement | To complete an agreed OPAT duration of therapy on either intravenous and/or complicated oral antimicrobials (a) as part of an agreed surgical infection management plan with further surgery planned or (b) where there is a requirement for subsequent long-term or an extended course of oral suppressive antimicrobial therapy, or (c) where potentially infective prosthetic material is still in situ. |
| palliation | To undertake a course of OPAT on either intravenous and/or complicated oral antimicrobials where there are agreed ceilings of care due to comorbidities, with death being the likely outcome. |
| OPAT outcome | |
| treatment aim attained—uncomplicated | Completed OPAT therapy as per treatment aim with:
|
| treatment aim attained—complicated | Completed OPAT therapy as per treatment aim but with one or more of the following:
|
| treatment aim not attained |
|
| indeterminate | Readmission for ≥24 h due to unrelated event. |
| death | Death due to any cause, except palliation. |
Complicated oral antimicrobials refers to oral regimens that require specific monitoring or are associated with particular risk of toxicity.
3.5.3 Patient experience
A major advantage of OPAT has always been the opportunity to tailor treatment regimens to individual patients, taking into consideration their circumstances and preferences. Although many studies report patient experience surveys, there has been a lack of in-depth qualitative analysis of the experiences and views of OPAT patients and family members, until two recent publications.152,153 Castor et al.152 undertook 37 qualitative interviews with members of 12 families of children receiving home therapies. They identified three essential themes—(i) strengthening family life; (ii) promoting health; and (iii) creating alliances—and stressed the importance of developing good relationships between family, home care service and hospital, and of paying close attention to the needs of each family member to ensure a positive experience for all.152 Twiddy et al.153 also undertook semi-structured interviews with 28 adult OPAT patients, as well as a focus group of 4 patients. They identified two key themes on qualitative analysis. The first comprised functional aspects of care, including the subthemes of ‘being at home but not well’, ‘convenience and flexibility’, ‘location of care’ and ‘is it safe?’ The second theme was relational aspects of care; one important element of this was a desire amongst patients for clear communication with staff who knew them to give patients confidence to collaborate in their own care. The authors then used a discrete choice experiment to identify overall patient preferences regarding OPAT. Although the most favoured model of OPAT was the model of the visiting nurse administering therapy at home, there was sufficient heterogeneity for the authors to conclude that services should ideally offer a range of OPAT delivery models.153
3.5.4 Evidence gaps
Although there are some recent publications focusing on patients’ experiences and perspectives of OPAT, there remains a need for further research in this area, particularly relating to patient self-administration using elastomeric devices and portable infusion pumps. Further quality-of-life studies comparing OPAT with hospitalization may support improved patient selection for OPAT and contribute to optimizing the patient experience of OPAT.
4. Conclusions
OPAT is likely to continue to grow in the UK and internationally, driven by a large body of evidence that it is clinically and cost effective, and preferred by patients. The literature review for this update has illustrated the increasing diversity of OPAT services, including an expansion of services based in the community and in acute ambulatory care units.
As with the previous GPRs, further studies have demonstrated the critical importance of a formal OPAT service with a dedicated OPAT team and clear links to organizational governance structures. Complications occur while patients are receiving OPAT and processes must be in place to ensure timely and accurate management. The evidence supports improved outcomes for patients when clinicians with expertise in OPAT have continuing involvement in their care. Furthermore, AMS is now a high priority for healthcare organizations and OPAT has a clear role to play in optimizing this. Unsurprisingly, the literature review included consideration of stewardship in every section of this update. One key consideration is the use of oral therapy in preference to intravenous where appropriate, and OPAT teams may contribute to safe administration of such agents as an extension of their role beyond parenteral therapies.
This update includes some changes from the previous GPRs. Firstly, this update combines both adult and paediatric OPAT in recognition that the principles of safe and effective OPAT are the same in these two groups. When considering the OPAT team, there is a novel concept of the OPAT ‘practitioner’ with a blurring of the professional boundaries between members of the team and a recognition that competence in different aspects of delivering OPAT is not restricted by job title. In patient selection, the literature review highlighted a move away from using rigid selection criteria relating to infection parameters and social factors to a more individualized approach incorporating consideration of comorbidities and recognition that different patient groups may be better suited, or less suited, to specific antibiotics and/or specific delivery models.
With the increasing use of continuous infusion devices there is a need for robust data on the stability of antimicrobial agents, particularly in the ‘real-life’ situation where the device may be maintained near body temperature for prolonged periods. In this update of the GPRs the required standard for stability testing is set deliberately at a high level—that of the BSAC Drug Stability Testing Programme—and we do need to work towards obtaining this level of robust data for a wider range of antimicrobial agents.
In terms of monitoring during OPAT, the requirement for weekly blood tests in patients on prolonged OPAT courses is an evidence-based change from the previous recommendations. Finally, there is the recognition that OPAT may be used in situations where the anticipated outcome is not cure of infection, particularly with increasing use of prosthetic devices in orthopaedics or vascular surgery and use of OPAT for suppression of infection or palliation.
The initial GPRs were intended to serve as a practical resource to help teams to develop or review their OPAT services. This update retains the same practical format and will also serve as a useful summary of the literature relating to OPAT since the publication of the previous recommendations. Additional resources for OPAT services are available at http://www.e-opat.com/; educational resources are available at https://www.futurelearn.com/courses/outpatient-patenteral-antimicrobial-therapy.
Supplementary Material
Acknowledgements
We thank Dr Vittoria Lutje for completing the literature searches and to colleagues who responded to the consultation and provided feedback about the recommendations. We also acknowledge the contribution of the authors of the original adult and paediatric GPRs:23,24 Ed Abrahamson, Mike A. Cooper, Stephen Goldring, Vicky Goodall, Matt Laundy, Dilip Nathwani, Corienne Reed, Frances Sanderson and Hayley Wickens. We thank the BSAC Secretariat for their help coordinating the consultation process.
Funding
The OPAT GPRs were produced by a working group on behalf of the BSAC. BSAC provided administrative and logistic support for the working group’s activities but had no involvement in the content of the recommendations.
Transparency declarations
A. G. has received a speaker’s fee from SBK Healthcare. The remaining authors have none to declare.
Peer review: This article was not sent for external peer review owing to the peer review provided by the external consultation process.
References
- 1. Barr DA, Semple L, Seaton RA.. Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital-based practice: a retrospective cohort study describing experience and evolution over 10 years. Int J Antimicrob Agents 2012; 39: 407–13. [DOI] [PubMed] [Google Scholar]
- 2. Bryant PA, Katz NT.. Inpatient versus outpatient parenteral antibiotic therapy at home for acute infections in children: a systematic review. Lancet Infect Dis 2018; 18: e45–54. [DOI] [PubMed] [Google Scholar]
- 3. Chapman AL. Outpatient parenteral antimicrobial therapy. BMJ 2013; 346: f1585. [DOI] [PubMed] [Google Scholar]
- 4. Durojaiye OC, Bell H, Andrews D. et al. Clinical efficacy, cost analysis and patient acceptability of outpatient parenteral antibiotic therapy (OPAT): a decade of Sheffield (UK) OPAT service. Int J Antimicrob Agents 2018; 51: 26–32. [DOI] [PubMed] [Google Scholar]
- 5. MacKenzie M, Rae N, Nathwani D.. Outcomes from global adult outpatient parenteral antimicrobial therapy programmes: a review of the last decade. Int J Antimicrob Agents 2014; 43: 7–16. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell ED, Czoski Murray C, Meads D. et al. Clinical and cost-effectiveness, safety and acceptability of community intravenous antibiotic service models: CIVAS systematic review. BMJ Open 2017; 7: e013560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel S, Burzio V, Green H. et al. The impact of pediatric outpatient parenteral antibiotic therapy implementation at a tertiary children's hospital in the United Kingdom. Pediatr Infect Dis J 2018; 37: e292–7. [DOI] [PubMed] [Google Scholar]
- 8. Durojaiye OC, Cartwright K, Ntziora F.. Outpatient parenteral antimicrobial therapy (OPAT) in the UK: a cross-sectional survey of acute hospital trusts and health boards. Diagn Microb Infect Dis 2018; 93: 58–62. [DOI] [PubMed] [Google Scholar]
- 9. Chapman ALN. Outpatient parenteral antimicrobial therapy in a changing NHS: challenges and opportunities. Clin Med 2013; 13: 322–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nazarko L. Avoiding admission and facilitating early discharge through OPAT. Br J Nurs 2014; 23: S30–6. [DOI] [PubMed] [Google Scholar]
- 11. Ashiru-Oredope D, Sharland M, Charani E. et al. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart–Then Focus. J Antimicrob Chemother 2012; 67 Suppl 1: i51–63. [DOI] [PubMed] [Google Scholar]
- 12. Nazarko L. Outpatient parenteral antimicrobial therapy: its delivery in the community. Br J Community Nurs 2013; 18: 163–7. [DOI] [PubMed] [Google Scholar]
- 13. Yan YM, Singh M, Tonks K. et al. Delivering outpatient antibiotic therapy (OPAT) in an acute medical unit. Acute Med 2011; 10: 22–5. [PubMed] [Google Scholar]
- 14. Saillen L, Arensdorff L, Moulin E. et al. Patient satisfaction in an outpatient parenteral antimicrobial therapy (OPAT) unit practising predominantly self-administration of antibiotics with elastomeric pumps. Eur J Clin Microbiol Infect Dis 2017; 36: 1387–92. [DOI] [PubMed] [Google Scholar]
- 15. Subedi S, Looke DF, McDougall DA. et al. Supervised self-administration of outpatient parenteral antibiotic therapy: a report from a large tertiary hospital in Australia. Int J Infect Dis 2015; 30: 161–5. [DOI] [PubMed] [Google Scholar]
- 16. Miron-Rubio M, Gonzalez-Ramallo V, Estrada-Cuxart O. et al. Intravenous antimicrobial therapy in the hospital-at-home setting: data from the Spanish Outpatient Parenteral Antimicrobial Therapy Registry. Future Microbiol 2016; 11: 375–90. [DOI] [PubMed] [Google Scholar]
- 17. Muldoon EG, Switkowski K, Tice A. et al. A national survey of infectious disease practitioners on their use of outpatient parenteral antimicrobial therapy (OPAT). Infect Dis (Lond) 2015; 47: 39–45. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence. Antimicrobial Stewardship: Systems and Processes for Effective Antimicrobial Medicine Use. 2015. https://www.nice.org.uk/guidance/ng15.
- 19.WHO. Global Action Plan on Antimicrobial Resistance. 2015. http://www.who.int/antimicrobial-resistance/global-action-plan/en/.
- 20. O'Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf.
- 21. Seaton RA, Ritchie ND, Robb F. et al. From ‘OPAT’ to ‘COpAT’: implications of the OVIVA study for ambulatory management of bone and joint infection. J Antimicrob Chemother 2019; doi:10.1093/jac/dkz122. [DOI] [PubMed] [Google Scholar]
- 22. Gilchrist M, Seaton RA.. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother 2015; 70: 965–70. [DOI] [PubMed] [Google Scholar]
- 23. Chapman AL, Seaton RA, Cooper MA. et al. Good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: a consensus statement. J Antimicrob Chemother 2012; 67: 1053–62. [DOI] [PubMed] [Google Scholar]
- 24. Patel S, Abrahamson E, Goldring S. et al. Good practice recommendations for paediatric outpatient parenteral antibiotic therapy (p-OPAT) in the UK: a consensus statement. J Antimicrob Chemother 2015; 70: 360–73. [DOI] [PubMed] [Google Scholar]
- 25. Candel FJ, Julian-Jimenez A, Gonzalez-Del Castillo J.. Current status in outpatient parenteral antimicrobial therapy: a practical view. Rev Esp Quimioter 2016; 29: 55–68. [PubMed] [Google Scholar]
- 26. Norris AH, Shrestha NK, Allison GM. et al. 2018 IDSA clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis 2018; 68: e1–35. [DOI] [PubMed] [Google Scholar]
- 27. Oliveira PR, Carvalho VC, Cimerman S. et al. Recommendations for outpatient parenteral antimicrobial therapy in Brazil. Braz J Infect Dis 2017; 21: 648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baker JR, Riske B, Voutsis M. et al. Insurance, home therapy, and prophylaxis in U.S. youth with severe hemophilia. Am J Prev Med 2011; 41: S338–45. [DOI] [PubMed] [Google Scholar]
- 29. Bhavan KP, Brown LS, Haley RW.. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med 2015; 12: e1001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chung EK, Beeler CB, Muloma EW. et al. Development and implementation of a pharmacist-managed outpatient parenteral antimicrobial therapy program. Am J Health Syst Pharm 2016; 73: e24–33. [DOI] [PubMed] [Google Scholar]
- 31. Fisher D, Michaels J, Hase R. et al. Outpatient parenteral antibiotic therapy (OPAT) in Asia: missing an opportunity. J Antimicrob Chemother 2017; 72: 1221–6. [DOI] [PubMed] [Google Scholar]
- 32. Hase R, Hosokawa N.. Patterns of outpatient ceftriaxone use in a Japanese general hospital: an increased need for development of outpatient parenteral antimicrobial therapy programs. Infect Dis (Lond) 2015; 47: 668–71. [DOI] [PubMed] [Google Scholar]
- 33. Heintz BH, Halilovic J, Christensen CL.. Impact of a multidisciplinary team review of potential outpatient parenteral antimicrobial therapy prior to discharge from an academic medical center. Ann Pharmacother 2011; 45: 1329–37. [DOI] [PubMed] [Google Scholar]
- 34. Yan M, Elligsen M, Simor AE. et al. Patient characteristics and outcomes of outpatient parenteral antimicrobial therapy: a retrospective study. Can J Infect Dis Med Microbiol 2016; 2016: 8435257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lane MA, Marschall J, Beekmann SE. et al. Outpatient parenteral antimicrobial therapy practices among adult infectious disease physicians. Infect Control Hosp Epidemiol 2014; 35: 839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keller SC, Ciuffetelli D, Bilker W. et al. The impact of an infectious diseases transition service on the care of outpatients on parenteral antimicrobial therapy. J Pharm Technol 2013; 29: 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nguyen HH. Hospitalist to home: outpatient parenteral antimicrobial therapy at an academic center. Clin Infect Dis 2010; 51 Suppl 2: S220–3. [DOI] [PubMed] [Google Scholar]
- 38. Muldoon EG, Snydman DR, Penland EC. et al. Are we ready for an outpatient parenteral antimicrobial therapy bundle? A critical appraisal of the evidence. Clin Infect Dis 2013; 57: 419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Madaline T, Nori P, Mowrey W. et al. Bundle in the Bronx: impact of a transition-of-care outpatient parenteral antibiotic therapy bundle on all-cause 30-day hospital readmissions. Open Forum Infect Dis 2017; 4: ofx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marwick C, Davey P.. Care bundles: the holy grail of infectious risk management in hospital? Curr Opin Infect Dis 2009; 22: 364–9. [DOI] [PubMed] [Google Scholar]
- 41. Halilovic J, Christensen CL, Nguyen HH.. Managing an outpatient parenteral antibiotic therapy team: challenges and solutions. Ther Clin Risk Manag 2014; 10: 459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shah PJ, Bergman SJ, Graham DR. et al. Monitoring of outpatient parenteral antimicrobial therapy and implementation of clinical pharmacy services at a community hospital infusion unit. J Pharm Pract 2015; 28: 462–8. [DOI] [PubMed] [Google Scholar]
- 43. Shrestha NK, Bhaskaran A, Scalera NM. et al. Contribution of infectious disease consultation toward the care of inpatients being considered for community-based parenteral anti-infective therapy. J Hosp Med 2012; 7: 365–9. [DOI] [PubMed] [Google Scholar]
- 44. Hersh AL, Olson J, Stockmann C. et al. Impact of antimicrobial stewardship for pediatric outpatient parenteral antibiotic therapy. J Pediatr Infect Dis Soc 2018; 7: e34–6. [DOI] [PubMed] [Google Scholar]
- 45. Akar A, Singh N, Hyun DY.. Appropriateness and safety of outpatient parenteral antimicrobial therapy in children: opportunities for pediatric antimicrobial stewardship. Clin Pediatr (Phila) 2014; 53: 1000–3. [DOI] [PubMed] [Google Scholar]
- 46. Knackstedt ED, Stockmann C, Davis CR. et al. Outpatient parenteral antimicrobial therapy in pediatrics: an opportunity to expand antimicrobial stewardship. Infect Control Hosp Epidemiol 2015; 36: 222–4. [DOI] [PubMed] [Google Scholar]
- 47. Xu M, Doan Q.. Outpatient parenteral antimicrobial therapy and judicious use of pediatric emergency resources. Pediatr Emerg Care 2017; doi:10.1097/pec.0000000000001215. [DOI] [PubMed] [Google Scholar]
- 48. Bradford N, Armfield NR, Young J. et al. Internet video to support intravenous medication administration in the home: a cost minimisation study. J Telemed Telecare 2013; 19: 367–71. [DOI] [PubMed] [Google Scholar]
- 49. Greenup EP, McCusker M, Potts BA. et al. The efficacy of telemedicine-supported discharge within an in home model of care. Telemedicine J E Health 2017; 23: 763–5. [DOI] [PubMed] [Google Scholar]
- 50. Tan SJ, Ingram PR, Rothnie AJ. et al. Successful outpatient parenteral antibiotic therapy delivery via telemedicine. J Antimicrob Chemother 2017; 72: 2898–901. [DOI] [PubMed] [Google Scholar]
- 51. Eron L. Telemedicine: the future of outpatient therapy? Clin Infect Dis 2010; 51 Suppl 2: S224–30. [DOI] [PubMed] [Google Scholar]
- 52. O'Hanlon S, McGrail P, Hodgkins P.. Community intravenous therapy provision. Nurs Stand 2017; 31: 45–53. [DOI] [PubMed] [Google Scholar]
- 53. Regan K, Morgan J.. Implementing a nurse-led community intravenous antibiotic service. Primary Health Care 2015; 25: 18–24. [Google Scholar]
- 54. Romoren M, Gjelstad S, Lindbaek M.. A structured training program for health workers in intravenous treatment with fluids and antibiotics in nursing homes: a modified stepped-wedge cluster-randomised trial to reduce hospital admissions. PLoS One 2017; 12: e0182619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barker A, Lyden-Rodgers M.. IV antibiotic therapy in the community: clinically effective and cost effective. Br J Nurs 2016; 25: S4–8. [DOI] [PubMed] [Google Scholar]
- 56. Gray E, Currey J, Considine J.. Hospital in the Home nurses' recognition and response to clinical deterioration. J Clin Nursing 2018; 27: 2152–60. [DOI] [PubMed] [Google Scholar]
- 57. Davis G, Woodhead J.. Setting up a unique outpatient parenteral antimicrobial therapy (OPAT) service. J Community Nurs 2016; 30: 61–5. [Google Scholar]
- 58. Mace AO, McLeod C, Yeoh DK. et al. Dedicated paediatric outpatient parenteral antimicrobial therapy medical support: a pre-post observational study. Arch Dis Child 2018; 103: 165–9. [DOI] [PubMed] [Google Scholar]
- 59. Melzer M, Macpherson L, Welch C.. The utility of a blood culture database to identify patients suitable for outpatient parenteral antibiotic treatment. Postgrad Med J 2017; 93: 382–8. [DOI] [PubMed] [Google Scholar]
- 60. Dryden M, Saeed K, Townsend R. et al. Antibiotic stewardship and early discharge from hospital: impact of a structured approach to antimicrobial management. J Antimicrob Chemother 2012; 67: 2289–96. [DOI] [PubMed] [Google Scholar]
- 61. Conant MM, Erdman SM, Osterholzer D.. Mandatory infectious diseases approval of outpatient parenteral antimicrobial therapy (OPAT): clinical and economic outcomes of averted cases. J Antimicrob Chemother 2014; 69: 1695–700. [DOI] [PubMed] [Google Scholar]
- 62. Felder KK, Marshall LM, Vaz LE. et al. Risk factors for complications during outpatient parenteral antimicrobial therapy for adult orthopedic and neurosurgical infections. South Med J 2016; 109: 53–60. [DOI] [PubMed] [Google Scholar]
- 63. Means L, Bleasdale S, Sikka M. et al. Predictors of hospital readmission in patients receiving outpatient parenteral antimicrobial therapy. Pharmacotherapy 2016; 36: 934–9. [DOI] [PubMed] [Google Scholar]
- 64. Lillie PJ, Andrews D, Eaves K. et al. Baseline factors predicting the duration of intravenous antibiotic therapy for cellulitis in an outpatient setting. Eur J Clin Microbiol Infect Dis 2010; 29: 347–9. [DOI] [PubMed] [Google Scholar]
- 65. Quirke M, Curran EM, O'Kelly P. et al. Risk factors for amendment in type, duration and setting of prescribed outpatient parenteral antimicrobial therapy (OPAT) for adult patients with cellulitis: a retrospective cohort study and CART analysis. Postgrad Med J 2018; 94: 25–31. [DOI] [PubMed] [Google Scholar]
- 66. Underwood J, Marks M, Collins S. et al. Intravenous catheter-related adverse events exceed drug-related adverse events in outpatient parenteral antimicrobial therapy. J Antimicrob Chemother 2018; 74: 787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schmidt M, Hearn B, Gabriel M. et al. Predictors of unplanned hospitalization in patients receiving outpatient parenteral antimicrobial therapy across a large integrated healthcare network. Open Forum Infect Dis 2017; 4: ofx086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huck D, Ginsberg JP, Gordon SM. et al. Association of laboratory test result availability and rehospitalizations in an outpatient parenteral antimicrobial therapy programme. J Antimicrob Chemother 2014; 69: 228–33. [DOI] [PubMed] [Google Scholar]
- 69. Duncan CJ, Barr DA, Seaton RA.. OPAT outcomes in endocarditis. BMJ 2013; 346: f2484. [DOI] [PubMed] [Google Scholar]
- 70. Seaton RA, Sharp E, Bezlyak V. et al. Factors associated with outcome and duration of therapy in outpatient parenteral antibiotic therapy (OPAT) patients with skin and soft-tissue infections. Int J Antimicrob Agents 2011; 38: 243–8. [DOI] [PubMed] [Google Scholar]
- 71. Allison GM, Muldoon EG, Kent DM. et al. Prediction model for 30-day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis 2014; 58: 812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Collaco JM, Green DM, Cutting GR. et al. Location and duration of treatment of cystic fibrosis respiratory exacerbations do not affect outcomes. Am J Respir Crit Care Med 2010; 182: 1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Proesmans M, Heyns L, Moons P. et al. Real life evaluation of intravenous antibiotic treatment in a paediatric cystic fibrosis centre: outcome of home therapy is not inferior. Respir Med 2009; 103: 244–50. [DOI] [PubMed] [Google Scholar]
- 74. Termoz A, Touzet S, Bourdy S. et al. Effectiveness of home treatment for patients with cystic fibrosis: the intravenous administration of antibiotics to treat respiratory infections. Pediatr Pulmonol 2008; 43: 908–15. [DOI] [PubMed] [Google Scholar]
- 75. Ho J, Archuleta S, Sulaiman Z. et al. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother 2010; 65: 2641–4. [DOI] [PubMed] [Google Scholar]
- 76. Beieler AM, Dellit TH, Chan JD. et al. Successful implementation of outpatient parenteral antimicrobial therapy at a medical respite facility for homeless patients. J Hosp Med 2016; 11: 531–5. [DOI] [PubMed] [Google Scholar]
- 77. Camsari UM, Libertin CR.. Small-town America's despair: infected substance users needing outpatient parenteral therapy and risk stratification. Cureus 2017; 9: e1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. D'Couto HT, Robbins GK, Ard KL. et al. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis 2018; 5: ofy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dobson PM, Loewenthal MR, Schneider K. et al. Comparing injecting drug users with others receiving outpatient parenteral antibiotic therapy. Open Forum Infect Dis 2017; 4: ofx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Buehrle DJ, Shields RK, Shah N. et al. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis 2017; 4: ofx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Suzuki J, Johnson J, Montgomery M. et al. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis 2018; 5: ofy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hernandez W, Price C, Knepper B. et al. Oral parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs 2016; 39: 81–5. [DOI] [PubMed] [Google Scholar]
- 83. Anastasio PJ, Wolthoff P, Galli A. et al. Single-dose oritavancin compared to standard of care IV antibiotics for acute bacterial skin and skin structure infection in the outpatient setting: a retrospective real-world study. Infect Dis Ther 2017; 6: 115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bosso JA, Casapao AM, Edwards J. et al. Clinical pathway for moderate to severe acute bacterial skin and skin structure infections from a US perspective: a roundtable discussion. Hosp Pract (1995) 2016; 44: 183–9. [DOI] [PubMed] [Google Scholar]
- 85. Cho JC, Estrada SJ, Beltran AJ. et al. Treatment of methicillin-sensitive Staphylococcus aureus bacteremia secondary to septic phlebitis using dalbavancin. J Clin Pharm Ther 2015; doi:10.1111/jcpt.12306. [DOI] [PubMed] [Google Scholar]
- 86. Deck DH, Jordan JM, Holland TL. et al. Single-dose oritavancin treatment of acute bacterial skin and skin structure infections: SOLO trial efficacy by Eron severity and management setting. Infect Dis Ther 2016; doi:10.1007/s40121-016-0119-9: 353-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Barr DA, Irvine S, Ritchie ND. et al. Risk of venous thromboembolism in patients treated for bacterial infection in the community with outpatient parenteral antimicrobial therapy. QJM 2014; 107: 207–11. [DOI] [PubMed] [Google Scholar]
- 88. Schmidt M, Horvath-Puho E, Thomsen RW. et al. Acute infections and venous thromboembolism. J Intern Med 2012; 271: 608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Arensdorff L, Boillat-Blanco N, Decosterd L. et al. Adequate plasma drug concentrations suggest that amoxicillin can be administered by continuous infusion using elastomeric pumps. J Antimicrob Chemother 2017; 72: 2613–15. [DOI] [PubMed] [Google Scholar]
- 90. Manning L, Wright C, Ingram PR. et al. Continuous infusions of meropenem in ambulatory care: clinical efficacy, safety and stability. PLoS One 2014; 9: e102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Verrall AJ, Llorin R, Tam VH. et al. Efficacy of continuous infusion of vancomycin for the outpatient treatment of methicillin-resistant Staphylococcus aureus infections. J Antimicrob Chemother 2012; 67: 2970–3. [DOI] [PubMed] [Google Scholar]
- 92. Zeller V, Dzeing-Ella A, Kitzis MD. et al. Continuous clindamycin infusion, an innovative approach to treating bone and joint infections. Antimicrob Agents Chemother 2010; 54: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Voumard R, Gardiol C, Andre P. et al. Efficacy and safety of continuous infusions with elastomeric pumps for outpatient parenteral antimicrobial therapy (OPAT): an observational study. J Antimicrob Chemother 2018; 73: 2540–5. [DOI] [PubMed] [Google Scholar]
- 94. Jenkins A, Hills T, Santillo M. et al. A systematic literature review of antimicrobial stability data in elastomeric devices. Eur J Hosp Pharm 2017; 24: A116–7. [Google Scholar]
- 95.NHSPQA Committee. A Standard Protocol for Deriving and Assessment of Stability. Part 1 – Aseptic Preparations (Small Molecules).2017. https://www.sps.nhs.uk/wp-content/uploads/2017/06/Stability-part-1-small-molecules-v4-April-17.pdf.
- 96. Allwood MC, Stonkute D, Wallace A. et al. Assessment of the stability of citrate-buffered flucloxacillin for injection when stored in two commercially available ambulatory elastomeric devices: INfusor LV (Baxter) and Accufuser (Woo Young Medical): a study compliant with the NHS Yellow Cover Document (YCD) requirements. Eur J Hosp Pharm 2018; doi:10.1136/ejhpharm-2018-001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jamieson CA, Allwood MC, Wallace A. et al. Investigation of meropenem stability after reconstitution: the influence of buffering and challenges to meet the NHS Yellow Cover Document (YCD) compliance for continuous infusions (CI) in an outpatient setting. Eur J Hosp Pharm 2018; doi:10.1136/ejhpharm-2018-001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Oliver G. Optimising patient safety when using elastomeric pumps to administer outpatient parenteral antibiotic therapy. Br J Nurs 2016; 25: S22–7. [DOI] [PubMed] [Google Scholar]
- 99. Hobbs JG, Ryan MK, Ritchie B. et al. Protocol for a randomised crossover trial to evaluate patient and nurse satisfaction with electronic and elastomeric portable infusion pumps for the continuous administration of antibiotic therapy in the home: the Comparing Home Infusion Devices (CHID) study. BMJ Open 2017; 7: e016763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pandya KH, Eaton V, Kowalski S. et al. Safety of continuous antibiotic infusions administered through an Australian hospital in the home service: a pilot study. J Pharm Pract 2017; 47: 333–9. [Google Scholar]
- 101. Grissinger M. Ambulatory pump safety: managing home infusion patients admitted to the emergency department and hospital. P T 2018; 43: 450–1. [PMC free article] [PubMed] [Google Scholar]
- 102. Bedford E, Waterhouse D.. Service development of a nurse-led community-based PICC insertion service. Br J Nurs 2017; 26: S22–7. [DOI] [PubMed] [Google Scholar]
- 103. Barr DA, Semple L, Seaton RA.. Self-administration of outpatient parenteral antibiotic therapy and risk of catheter-related adverse events: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2012; 31: 2611–9. [DOI] [PubMed] [Google Scholar]
- 104. Lam PW, Graham C, Leis JA. et al. Predictors of peripherally inserted central catheter occlusion in the outpatient parenteral antimicrobial therapy setting. Antimicrob Agents Chemother 2018; 62: e00900-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shrestha NK, Shrestha J, Everett A. et al. Vascular access complications during outpatient parenteral antimicrobial therapy at home: a retrospective cohort study. J Antimicrob Chemother 2016; 71: 506–12. [DOI] [PubMed] [Google Scholar]
- 106. Hussain S, Gomez MM, Wludyka P. et al. Survival times and complications of catheters used for outpatient parenteral antibiotic therapy in children. Clin Pediatr 2007; 46: 247–51. [DOI] [PubMed] [Google Scholar]
- 107. Van Winkle P, Wiffen T, Liu IL.. Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. Paediatr Infect Dis J 2008; 27: 1069–72. [DOI] [PubMed] [Google Scholar]
- 108. Fernandes P, Milliren C, Mahoney-West HM. et al. Safety of outpatient parenteral antimicrobial therapy in children. Pediatr Infect Dis J 2018; 37: 157–63. [DOI] [PubMed] [Google Scholar]
- 109. Kovacich A, Tamma PD, Advani S. et al. Peripherally inserted central venous catheter complications in children receiving outpatient parenteral antibiotic therapy (OPAT). Infect Control Hosp Epidemiol 2016; 37: 420–4. [DOI] [PubMed] [Google Scholar]
- 110. Hodgson KA, Huynh J, Ibrahim LF. et al. The use, appropriateness and outcomes of outpatient parenteral antimicrobial therapy. Arch Dis Child 2016; 101: 886–93. [DOI] [PubMed] [Google Scholar]
- 111. Carey B. Promoting paediatric IV access. Br J Nurs 2017; 26: S40. [DOI] [PubMed] [Google Scholar]
- 112. Royle TJ, Davies RE, Gannon MX.. Totally implantable venous access devices - 20 years' experience of implantation in cystic fibrosis patients. Ann R Coll Surg Engl 2008; 90: 679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rodgers HC, Liddle K, Nixon SJ. et al. Totally implantable venous access devices in cystic fibrosis: complications and patients' opinions. Eur Respir J 1998; 12: 217–20. [DOI] [PubMed] [Google Scholar]
- 114. Lodise TP, Redell M, Armstrong SO. et al. Efficacy and safety of oritavancin relative to vancomycin for patients with acute bacterial skin and skin structure infections (ABSSSIs) in the outpatient setting: results from the solo clinical trials. Open Forum Infect Dis 2017; 4: ofw274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Almangour TA, Fletcher V, Alessa M. et al. Multiple weekly dalbavancin dosing for the treatment of native vertebral osteomyelitis caused by methicillin-resistant Staphylococcus aureus: a case report. Am J Case Rep 2017; 18: 1315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tobudic S, Forstner C, Burgmann H. et al. Dalbavancin as primary and sequential treatment for gram-positive infective endocarditis: 2-year experience at the General Hospital of Vienna. Clin Infect Dis 2018; 67: 795–8. [DOI] [PubMed] [Google Scholar]
- 117. Rappo U, Puttagunta S, Shevchenko V. et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis 2018; 6: ofy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schroeder CP, Van Anglen LJ, Dretler RH. et al. Outpatient treatment of osteomyelitis with telavancin. Int J Antimicrob Agents 2017; 50: 93–6. [DOI] [PubMed] [Google Scholar]
- 119. Moran GJ, Fang E, Corey GR. et al. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2014; 14: 696–705. [DOI] [PubMed] [Google Scholar]
- 120. Chang CC, Slavin MA, Chen SC.. New developments and directions in the clinical application of the echinocandins. Arch Toxicol 2017; 91: 1613–21. [DOI] [PubMed] [Google Scholar]
- 121. Licitra CM, Crespo A, Licitra D. et al. Daptomycin for the treatment of osteomyelitis and prosthetic joint infection: retrospective analysis of efficacy and safety in an outpatient infusion center. Internet J Infect Dis 2011; 9: 11566. [Google Scholar]
- 122. Gonzalez-Ruiz A, Seaton RA, Hamed K.. Daptomycin: an evidence-based review of its role in the treatment of Gram-positive infections. Infect Drug Resist 2016; 9: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Schrank GM, Wright SB, Branch-Elliman W. et al. A retrospective analysis of adverse events among patients receiving daptomycin versus vancomycin during outpatient parenteral antimicrobial therapy. Infect Control Hosp Epidemiol 2018; 39: 1–8. [DOI] [PubMed] [Google Scholar]
- 124. Seaton RA, Gonzalez-Ramallo VJ, Prisco V. et al. Daptomycin for outpatient parenteral antibiotic therapy: a European registry experience. Int J Antimicrob Agents 2013; 41: 468–72. [DOI] [PubMed] [Google Scholar]
- 125. Matthews PC, Chue AL, Wyllie D. et al. Increased teicoplanin doses are associated with improved serum levels but not drug toxicity. J Infect 2014; 68: 43–9. [DOI] [PubMed] [Google Scholar]
- 126. Allen J, Adams K, Thompson F. et al. Long-term, once-weekly outpatient teicoplanin use for suppression of chronic prosthetic joint infection. Int J Antimicrob Agents 2013; 41: 200–1. [DOI] [PubMed] [Google Scholar]
- 127. Qureshi ZA, Syed A, Doi Y.. Safety and efficacy of long-term outpatient ertapenem therapy. Antimicrob Agents Chemother 2014; 58: 3437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sanroma P, Munoz P, Miron-Rubio M. et al. Effectiveness and safety of ertapenem used in hospital-at-home units: data from Spanish Outpatient Parenteral Antimicrobial Therapy Registry. Future Microbiol 2018; 13: 1363–73. [DOI] [PubMed] [Google Scholar]
- 129. Veillette JJ, Van Epps P.. Ertapenem-induced hallucinations and delirium in an elderly patient. Consult Pharm 2016; 31: 207–14. [DOI] [PubMed] [Google Scholar]
- 130. Ingram PR, Rawlins MD, Murray RJ. et al. Tigecycline use in the outpatient parenteral antibiotic therapy setting. Eur J Clin Microbiol Infect Dis 2016; 35: 1673–7. [DOI] [PubMed] [Google Scholar]
- 131. Li HK, Rombach I, Zambellas R. et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380: 425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Keren R, Shah SS, Srivastava R. et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr 2015; 169: 120–8. [DOI] [PubMed] [Google Scholar]
- 133. Peltola H, Paakkonen M.. Acute osteomyelitis in children. N Engl J Med 2014; 370: 352–60. [DOI] [PubMed] [Google Scholar]
- 134. Iversen K, Ihlemann N, Gill SU. et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 2018; 380:415–24. [DOI] [PubMed] [Google Scholar]
- 135. Arnold MR, Wormer BA, Kao AM. et al. Home intravenous versus oral antibiotics following appendectomy for perforated appendicitis in children: a randomized controlled trial. Pediatric Surgery Int 2018; 34: 1257–68. [DOI] [PubMed] [Google Scholar]
- 136. Veve MP, Wagner JL, Kenney RM. et al. Comparison of fosfomycin to ertapenem for outpatient or step-down therapy of extended-spectrum β-lactamase urinary tract infections. Int J Antimicrob Agents 2016; 48: 56–60. [DOI] [PubMed] [Google Scholar]
- 137. Hodson EM, Willis NS, Craig JC.. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev 2007; issue 7: CD003772. [DOI] [PubMed] [Google Scholar]
- 138. Stockmann C, Ampofo K, Pavia AT. et al. Comparative effectiveness of oral versus outpatient parenteral antibiotic therapy for empyema. Hosp Pediatr 2015; 5: 605–12. [DOI] [PubMed] [Google Scholar]
- 139. McMullan BJ, Andresen D, Blyth CC. et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis 2016; 16: e139–52. [DOI] [PubMed] [Google Scholar]
- 140. Kameshwar K, Karahalios A, Janus E. et al. False economies in home-based parenteral antibiotic treatment: a health-economic case study of management of lower-limb cellulitis in Australia. J Antimicrob Chemother 2016; 71: 830–5. [DOI] [PubMed] [Google Scholar]
- 141. McPherson C, Lee BR, Terrill C. et al. Characteristics of pediatric antimicrobial stewardship programs: current status of the Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS) Collaborative. Antibiotics (Basel) 2018; doi:10.3390/antibiotics7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Aberdein J, Chapman AL.. Clostridium difficile infection following outpatient parenteral antimicrobial therapy. J Hosp Infect 2015; 90: 171–2. [DOI] [PubMed] [Google Scholar]
- 143. Duncan CJ, Barr DA, Seaton RA.. Outpatient parenteral antimicrobial therapy with ceftriaxone, a review. Int J Clin Pharm 2012; 34: 410–7. [DOI] [PubMed] [Google Scholar]
- 144. Wong KK, Fraser TG, Shrestha NK. et al. Low incidence of Clostridium difficile infection (CDI) in patients treated with outpatient parenteral antimicrobial therapy (OPAT). Infect Control Hosp Epidemiol 2015; 36: 110–2. [DOI] [PubMed] [Google Scholar]
- 145. Hale CM, Steele JM, Seabury RW. et al. Characterization of drug-related problems occurring in patients receiving outpatient antimicrobial therapy. J Pharm Pract 2017; 30: 600–5. [DOI] [PubMed] [Google Scholar]
- 146. Yusef D, Gonzalez BE, Foster CB. et al. Piperacillin-tazobactam-induced adverse drug events in pediatric patients on outpatient parenteral antimicrobial therapy. Pediatr Infect Dis J 2017; 36: 50–2. [DOI] [PubMed] [Google Scholar]
- 147. Keller SC, Williams D, Gavgani M. et al. Rates of and risk factors for adverse drug events in outpatient parenteral antimicrobial therapy. Clin Infect Dis 2018; 66: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Briggs S, Smith S, Ritchie S. et al. Late-onset bolus intravenous β-lactam antibiotic adverse reaction: short-lived symptoms during drug administration and associated laboratory abnormalities. Scand J Infect Dis 2014; 46: 14–20. [DOI] [PubMed] [Google Scholar]
- 149. Duncan CJ, Evans TJ, Seaton RA.. Ceftriaxone-related agranulocytosis during outpatient parenteral antibiotic therapy. J Antimicrob Chemother 2010; 65: 2483–4. [DOI] [PubMed] [Google Scholar]
- 150. Blumenthal KG, Youngster I, Rabideau DJ. et al. Peripheral blood eosinophilia and hypersensitivity reactions among patients receiving outpatient parenteral antibiotics. J Allergy Clin Immunol 2015; 136: 1288–94.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lee B, Tam I, Weigel BT. et al. Comparative outcomes of β-lactam antibiotics in outpatient parenteral antibiotic therapy: treatment success, readmissions and antibiotic switches. J Antimicrob Chemother 2015; 70: 2389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Castor C, Landgren K, Hansson H. et al. A possibility for strengthening family life and health: family members’ lived experience when a sick child receives home care in Sweden. Health Soc Care Community 2018; 26: 224–31. [DOI] [PubMed] [Google Scholar]
- 153. Twiddy M, Czoski Murray CJ, Mason SJ. et al. A qualitative study of patients' feedback about Outpatient Parenteral Antimicrobial Therapy (OPAT) services in Northern England: implications for service improvement. BMJ Open 2018; 8: e019099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.