Abstract
Background
HIV drug resistance (HIV-DR) is rising in sub-Saharan Africa in both ART-naive and ART-experienced patients.
Objectives
To estimate the level of acquired DR (ADR) and pre-treatment DR (PDR) across selected urban and rural sites in Southern Africa, in Mozambique.
Methods
We conducted two cross-sectional surveys among adult HIV patients (October 2017–18) assessing ADR and PDR. In the (ADR) survey, those on NNRTI-based first-line ART for ≥6 months were recruited (three sites). In the PDR survey, those ART-naive or experienced with ≥3 months of treatment interruption prior were enrolled (eight sites).
Results
Among 1113 ADR survey participants 83% were receiving tenofovir (TDF)/lamivudine (3TC)/efavirenz (EFV). The median time on ART was 4.5 years (Maputo) and 3.2 years (Tete), 8.3% (95% CI 6.2%-10.6%, Maputo) and 15.5% (Tete) had a VL ≥ 1000 copies/mL, among whom 66% and 76.4% had NNRTI+NRTI resistance, and 52.8% and 66.7% had 3TC+TDF-DR. Among those on TDF regimens, 31.1% (Maputo) and 42.2% (Tete) were still TDF susceptible, whereas 24.4% and 11.5% had TDF+zidovudine (ZDV)-DR. Among those on ZDV regimens, 25% and 54.5% had TDF+ZDV-DR. The PDR survey included 735 participants: NNRTI-PDR was 16.8% (12.0–22.6) (Maputo) and 31.2% (26.2–36.6) (Tete), with a higher proportion (≥50%) among those previously on ART affected by PDR.
Conclusions
In Mozambique, viral failure was driven by NNRTI and NRTI resistance, with NRTI DR affecting backbone options. NNRTI-PDR levels surpassed the WHO 10% ‘alert’ threshold. Replacing NNRTI first-line drugs is urgent, as is frequent viral load monitoring and resistance surveillance. Changing NRTI backbones when switching to second-line regimens may need reconsideration.
Introduction
In sub-Saharan Africa (SSA), where two-thirds of global HIV cases occur,1 the WHO’s goal of viral suppression in 90% of those on treatment is complicated by HIV drug resistance (HIV-DR). Identifying treatment failure and switching resistant cases to new regimens rarely happens quickly or enough.2
Lack of routine viral load (VL) testing, the gold standard for treatment monitoring, amplifies these problems,3–5 as does a lack of drug resistance testing (DRT) in resource-limited settings.6 The absence of accessible DRT, in turn, creates a dependence on population-level surveys to monitor HIV-DR, to inform future therapeutic recommendations.
HIV-DR is rising in SSA, occurring in patients failing treatment (often due to substandard drug compliance, or other factors) defined as acquired drug resistance (ADR), as well as in individuals who never took HIV therapy or re-initiate ART after a break [defined as pre-treatment drug resistance (PDR)].7 NNRTIs are becoming widely inactive because their low genetic barrier promotes resistance, with ADR rates of 70%–90% for efavirenz and nevirapine in patients failing treatment, and as much as 53%–88% of patients with ADR to some NRTIs.3,8,9 PDR levels are also alarming in SSA, with recent national HIV-DR surveys in eSwatini, Namibia, Uganda, South Africa, and Zimbabwe nearly all showing NNRTI PDR levels >10% (which is the WHO alert threshold for changing first-line ART regimens nationally).9–12 To address the high level of PDR (especially in countries with estimated PDR >10%) and minimize its impact on treatment outcomes, WHO issued new guidelines in 2019 recommending the use of dolutegravir as a first and second-line ART regimen with tenofovir or zidovudine as an optimized backbone.13,14 Dolutegravir is a potent integrase inhibitor with a high genetic barrier, high tolerability profile, and lower pill burden and is now recommended as a preferred first-line regimen (in combination with an NRTI backbone).14
However, many countries operate with few HIV-DR statistics. This is the case in Mozambique where Médecins Sans Frontières (MSF) collaborates with the Ministry of Health (MoH) and supports HIV-programmes and VL testing in the capital city of Maputo and in Tete, in the rural northwest of the country. After high rates of virological treatment failure were seen in 2015, MSF conducted a DR survey across rural and urban settings to better understand ADR’s contribution to treatment failure, to assess HIV-DR prevalence in ART-initiators and re-initiators, to establish resistance profiles, and to evaluate the potential implications of the new WHO recommendations in this context.15 Mozambique began its dolutegravir rollout in 2020, following WHO recommendations.
Patients and methods
Study settings, participants and sampling
ADR was assessed using a cross-sectional investigation of ≥18-year-old HIV patients on NNRTI-based first-line ART ≥6 months in MSF-supported health facilities in Maputo [Alto Maé health centre (HC)] and Tete (Changara District Hospital; Marara Centro HC). A sample of at least 460 participants from Tete (Changara, n = 354; Marara n = 106, proportionally) and 632 from Maputo was sought. In Maputo, 10 participants were randomly selected from eligible patients presenting at the health facility each day for any reason. In the Tete sites, a convenience sample was recruited by inviting every eligible patient to participate until the requisite sample size was achieved.
PDR was assessed using a cross-sectional survey across eight study sites: one in Maputo (Alto Maé HC) and seven in Tete (Cachembe, Changara Sede, Dzunga, Marara Centro, Matambo, Mazoe, Missawa). All ≥18-year-old, ART-naive HIV patients were included as well as those who had previously taken first-line ART but had stopped treatment >3 months prior to starting again. Sampling proportionate to the size of each clinic was calculated (excluding two smaller clinics with <20% of the total HIV cohort) to establish a sample size of 345 in both Maputo and Tete. Study participants were enrolled between October 2017 and October 2018.
Study procedures
ADR
Participants’ socio-demographic information was collected, a clinical evaluation was conducted, and a venous blood sample was taken for CD4 count and HIV-1 plasma RNA VL measurement at enrolment. HIV-DR genotyping was performed for those with VL ≥ 500 copies/mL (technical threshold). Patients with VL ≥ 1000 copies/mL were considered virological failures, were linked to programmatic enhanced adherence counselling (EAC) (1–2 sessions for 2–3 months) and received follow-up to reassess VL and CD4 count.
PDR
Socio-demographic information was recorded, and a venous sample collected for CD4 count measurement. Dried blood spots (DBS) were prepared for VL and DR genotyping (if VL ≥1000 copies/mL).
For both surveys, data were entered into a dedicated electronic database (REDCap, Research Electronic Data Capture, Vanderbilt, USA).16
Laboratory procedures
After venous sample collection, 25 mL of blood was immediately added to the PIMA™ CD4 cartridge for both ADR and PDR. Plasma samples for the ADR survey were prepared within 6 h of blood collection. DBS specimens for the PDR survey were prepared using 50 μL per spot and dried overnight. All human samples were stored on-site between −80°C and −20°C (for <2 months) before being shipped to the WHO regional reference laboratory (HIVResNet laboratory) at the National Institute for Communicable Diseases in Johannesburg, South Africa for VL and DRT analysis.
VL testing
ADR
HIV VL (plasma) was quantified by automated real-time PCR using Cobas Ampliprep/Cobas Taqman HIV-1 v2.0 (Roche Diagnostic System Branchburg, NL, USA; quantification range: 20–10 000 000 HIV RNA copies/mL; values <20 copies/mL reported as ‘target not detected’). Virological failure (VF) was defined as plasma VL ≥1000 copies/mL at inclusion. Confirmed virological failure (CVF) was defined as plasma VL > 1000 copies/mL on two consecutive measurements within 2–3 months with adherence support in between.
PDR
VL was assessed on DBS samples using Cobas Ampliprep/Cobas Taqman HIV-1 v2.0. Samples with VL <1000 copies/mL were re-tested on Abbott Real-Time™ HIV-1 assay (Abbott Molecular Inc., Des Plaines, IL) and recategorized when needed for DRT.
HIV-DR genotyping
Resistance genotyping was conducted on plasma (ADR) or DBS (PDR) specimens for participants with a VL ≥1000 copies/mL using an in-house validated assay of the pol open reading frame region (up to codon 335).17 Interpretation of DR mutations (DRMs) and DR level used the Stanford HIV-1 Drug Resistance Database v 8.4. Low, intermediate, or high-level resistance were all considered ‘resistant’ (penalty score ≥15). Resistance prevalence was calculated in participants with VL ≥ 1000 copies/mL and DRMs were reported for this subgroup. ‘Any HIV-DR’ was defined as resistance to any NRTI, to nevirapine/efavirenz, or to protease inhibitors atazanavir/ritonavir, lopinavir/ritonavir, or darunavir/ritonavir. ‘NRTI-DR’ or ‘NNRTI-DR’ was defined as resistance to any NRTI or NNRTI molecule, respectively. Dual-class resistance was defined as NRTI-DR plus NNRTI-DR. PDR was defined as resistance to efavirenz and/or nevirapine considering penalty scores ≥15. For PDR, highly related sequences (<0.5% genetic distance) were excluded from analysis per WHO recommendations.18
Statistical analysis
Descriptive analyses included medians with IQR or counts with proportions. Proportions are provided with 95% CI for the Maputo site (random sampling strategy) though not for Tete (exhaustive sampling strategy). Proportions were compared using Pearson chi-squared testing. Univariate and multivariate analyses were performed to estimate odds ratios (ORs) with 95% CIs to identify factors associated with PDR. Variables associated with a P < 0.2 in univariate analyses were included in multivariate models. A backwards stepwise selection process using ORs was used to keep only those with P values <0.05. Age and sex were retained in the final model. Analyses were performed using STATA v.14 (STATA Corp., USA).
Ethics
The study was approved by the National Ethics Review Board (ERB) of Mozambique (Comité Nacional de Bioética para a Saùde-CNBS) and MSF-ERB. Participants provided written informed consent.
Results
ADR survey
Baseline characteristics and VL assessments
A total of 1113 participants were included in Maputo (n = 642) and Tete (n = 471: 365 in Changara sede; 106 in Marara Centro). The median time on ART was 4.5 (IQR: 2.4–6.9) and 3.2 years (IQR: 1.6–5.6) respectively, and all participants were clinically stable (Table 1). In both sites, most (>82%) were receiving a tenofovir-based first-line regimen (Table 1).
Table 1.
ADR Survey: study participant characteristics
| Maputo (N = 642) |
Tete (N = 471) |
|||||
|---|---|---|---|---|---|---|
| N | % or median | 95% CI or IQR | N | % or median | IQR | |
| Gender | ||||||
| Female | 435 | 67.8 | 63.9–71.3 | 319 | 67.7 | |
| Male | 207 | 32.2 | 28.6–30.0 | 152 | 32.4 | |
| Age, years, median [IQR] | 44 | [37–52] | 38 | [31–46] | ||
| 18–35 | 136 | 21.2 | 18.1–24.5 | 198 | 42.0 | |
| 36–49 | 297 | 46.2 | 42.3–50.2 | 190 | 40.3 | |
| ≥50 | 209 | 32.6 | 28.9–36.3 | 83 | 17.6 | |
| CD4 count, cells/mm3, median [IQR] | 492 | [476–510] | 529 | [365–703] | ||
| <200 | 44 | 6.9 | 5.0–9.0 | 38 | 8.0 | |
| 200–350 | 133 | 20.7 | 17.6–24.0 | 72 | 15.3 | |
| ≥350 | 465 | 72.4 | 68.8–75.8 | 361 | 76.7 | |
| WHO stage | ||||||
| Stage 1 | 631 | 98.2 | 96.9–99.1 | 452 | 96.0 | |
| Stage 2 | 11 | 1.8 | 0.8–3.0 | 7 | 1.4 | |
| Stage 3 | – | – | – | 12 | 2.6 | |
| Time on ART, years, median [IQR] | 4.5 | [2.4–6.9] | 3.2 | [1.6–5.6] | ||
| 6–24 months | 134 | 20.8 | 17.8–24.2 | 147 | 31.1 | |
| ≥25 months | 508 | 79.2 | 75.7–82.2 | 324 | 68.9 | |
| ART regimen at ART initiation | ||||||
| TDF-containing regimens | 293 | 45.6 | 297 | 63.0 | ||
| ZDV-containing regimens | 307 | 47.8 | 173 | 36.8 | ||
| Other/Not available | 41 | 6.6 | 1 | 0.2 | ||
| Current ART regimen | ||||||
| TDF/(3TC or ABC)/EFV | 529 | 82.4 | 79.2–85.3 | 400 | 84.9 | |
| ZDV/3TC/(EFV or NVP) | 113 | 17.6 | 14.4–20.4 | 71 | 15.1 | |
TDF, tenofovir; 3TC, lamivudine; ABC, abacavir; EFV, efavirenz; ZDV, zidovudine; NVP, nevirapine.
Large majorities were virally suppressed (91.7%, Maputo; 84.7%, Tete) (Table 2). Among the 53 (Maputo) and 72 (Tete) participants with a VF result, 16 (30.1%) and 15 (20.8%) did not have a repeat VL test: 18 were switched directly to a second-line regimen (CD4 < 100 μL/mm3 or two recent VL results ≥1000 copies/mL), 10 were lost-to follow-up or refused, and 3 died. For those who received follow-up VL tests (n = 37 Maputo, n = 57 Tete), they occurred after 4.4 (IQR: 3.9–4.8) and 4.8 (IQR: 4.3–5.5) months in Maputo and Tete, respectively. Virological failure was confirmed by a second VL result ≥1000 copies/mL for 67.6% in Maputo and 77.2% of participants in Tete (Table 2). Characteristics of patients with virological failure are described in Table 3.
Table 2.
ADR Survey: plasma VL at inclusion and during follow-up visits
| Maputo (N = 642) |
Tete (N = 471) |
||||
|---|---|---|---|---|---|
| Characteristic | N | % | 95% CI | N | % |
| HIV RNA (copies/mL) at study inclusion | |||||
| VL < 1000 | 589 | 91.7 | 89.3–93.7 | 398 | 84.5 |
| Target not detected | 141 | 22.0 | 18.8–25.4 | 271 | 57.7 |
| <20–1000 | 448 | 69.8 | 66.0–73.3 | 127 | 27.0 |
| VL ≥ 1000 | 53 | 8.3 | 6.2–10.6 | 72 | 15.5 |
| 1000–10 000 | 14 | 2.2 | 1.2–3.6 | 23 | 31.9 |
| 10 000–100 000 | 29 | 4.5 | 3.0–6.4 | 34 | 37.2 |
| ≥100 000 | 10 | 1.5 | 0.7–2.8 | 15 | 20.8 |
|
| |||||
| HIV RNA (copies/mL) at follow-up visit | Maputo (N = 37) | Tete (N = 57) | |||
|
| |||||
| VL < 1000 | 12 | 32.4 | 18.0–49.7 | 13 | 22.8 |
| Target not detected | 1 | 2.7 | 0.07–14.2 | 1 | 1.8 |
| <20–1000 | 11 | 29.7 | 15.9 -47.0 | 12 | 21.0 |
| VL ≥ 1000 | 25 | 67.6 | 50.2–81.9 | 44 | 77.2 |
| 1000–10 000 | 7 | 18.9 | 8.0–35.1 | 17 | 29.8 |
| 10 000–100 000 | 13 | 35.2 | 20.2–52.5 | 19 | 33.4 |
| ≥100 000 | 5 | 13.5 | 4.5–28.8 | 8 | 14.0 |
Table 3.
ADR survey: characteristics of patients with virological failure
| Characteristics of patients with virological failure | Maputo VL ≥ 1000 copies/mL |
Tete VL ≥ 1000 copies/mL |
||||||
|---|---|---|---|---|---|---|---|---|
| N a | n b | %c | P valued | N | n | % | P value | |
| Total (N) | 642 | 53 | 8.2 | 471 | 72 | 15.3 | ||
| Sex | <0.97 | 0.30 | ||||||
| Male | 207 | 17 | 8.2 | 152 | 27 | 17.8 | ||
| Female | 435 | 36 | 8.2 | 319 | 45 | 14.1 | ||
| Age, years | <0.05 | 0.17 | ||||||
| 18–35 | 136 | 19 | 198 | 29 | 14.6 | |||
| 36–49 | 297 | 20 | 190 | 35 | 18.4 | |||
| ≥50 | 209 | 14 | 83 | 8 | 9.6 | |||
| Time on ART | 0.74 | 0.49 | ||||||
| 6–24 months | 134 | 12 | 8.9 | 147 | 20 | 13.6 | ||
| ≥25 months | 508 | 41 | 8.0 | 324 | 52 | 16.0 | ||
| CD4 count (cells/mm3) | <0.05 | <0.05 | ||||||
| <200 | 44 | 16 | 3.7 | 38 | 20 | 52.6 | ||
| 200–350 | 133 | 21 | 9.1 | 72 | 17 | 23.6 | ||
| ≥350 | 465 | 16 | 13.0 | 361 | 35 | 9.7 | ||
| CD4 count, median [IQR] | 242 [157–397] | 343 [186–490] | ||||||
| Previous ZDV exposure | 0.32 | 0.34 | ||||||
| Yes | 359 | 29 | 8.0 | 173 | 30 | 17.3 | ||
| No | 283 | 24 | 8.5 | 298 | 42 | 14.0 | ||
Total number of participants.
Number with VL ≥ 1000 copies/mL.
Percentage [n/N].
Pearson Chi-Square Test.
ADR results
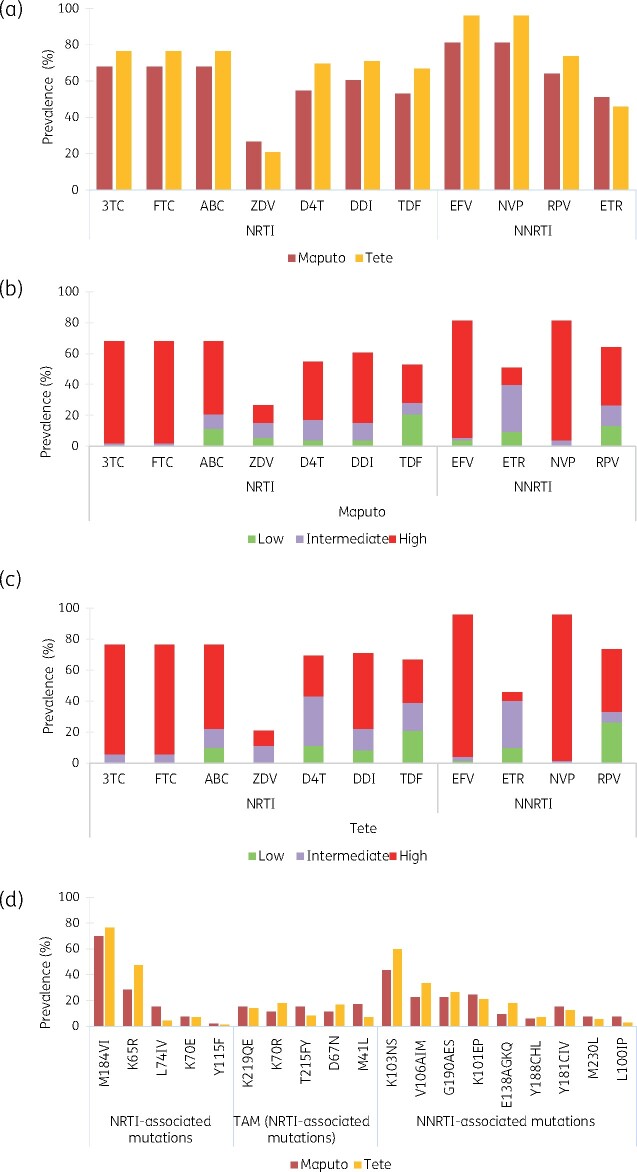
One-hundred and forty-one samples were submitted for DRT, of which 138 were successfully amplified (3 of 16 with VL 500–1000 copies/mL failed amplification). Nearly all were HIV-1 subtype C (91.4% in Maputo, 100% in Tete). A few were subtype A (1.7%), B (1.7%), D (3.5%) or G (1.7%) in Maputo. Overall HIV-DR prevalence was 81.1% (95% CI 68.0%–90.5%) in Maputo and 95.8% in Tete. Dual class resistance (NNRTI+NRTI) was detected in 66% of Maputo and 76.4% of Tete samples (Table 4). The majority of those with NRTI resistance had resistance to lamivudine/emtricitabine and abacavir [68% (Maputo) and 76% (Tete)] (Figure 1a–c). A high proportion of participants with viral failure had dual resistance to the common NRTI drugs (tenofovir and lamivudine) (Table 5).
Table 4.
ADR survey: prevalence of HIV DR by drug class
| Maputo (N = 5) |
Tete (N = 8) |
||||
|---|---|---|---|---|---|
| Characteristic | N | % | 95% CI | N | % |
| VL from 500–999 copies/mL | |||||
| NRTI DR | 2 | 40 | 5.2–85.0 | 6 | 75.0 |
| NNRTI DR | 2 | 40 | 5.2–85.0 | 8 | 100 |
| NRTI and NNRTI DR | 2 | 40 | 5.2–85.0 | 8 | 100 |
| PI DR | 0 | 0 | 0.0–50.0 | 0 | 0 |
| No DR | 3 | 60.0 | 14.6–94.7 | 0 | 0 |
| Failed DRT amplification | 2 | 40.0 | 1 | 12.5 | |
|
| |||||
| VL ≥ 1000 copies/mL | Maputo (N = 53) | Tete (N = 72) | |||
|
| |||||
| Any HIVDR | 43 | 81.1 | 68.0–90.5 | 69 | 95.8 |
| NRTI DR | 37 | 69.8 | 55.6–81.6 | 55 | 76.4 |
| NNRTI DR | 43 | 81.1 | 68.0–90.5 | 69 | 95.8 |
| NRTI and NNRTI DR | 35 | 66.0 | 51.7–78.4 | 55 | 76.4 |
| PI DR | 0 | 0 | 0.0–6.0 | 0 | 0 |
| No DR | 10 | 18.9 | 9.4–31.2 | 3 | 4.2 |
Figure 1.
ADR survey: prevalence of HIVDR and DRMs in Maputo and Tete. HIVDR prevalence by ARV (a) was estimated from RT sequencing using Stanford HIV db v.8.4. Drug resistance levels reported for both study sites (b, c) were classified according to Stanford score (SS): Low-level resistance (SS: 15–29), Intermediate level resistance (SS: 30–59) and High-level resistance (SS ≥ 60). Frequency of DRMs (d) is reported by drug class. NRTIs: 3TC, lamivudine; FTC, emtricitabine; ABC, abacavir; ZDV, zidovudine; D4T, stavudine; DDI, didanosine; TDF, tenofovir. NNRTIs: EFV, efavirenz; NVP, nevirapine; RPV, rilpivirine; ETR, etravirine.
Table 5.
ADR survey: prevalence of main NRTI backbone resistance
| Maputo (N = 53) |
Tete (N = 72) |
||||
|---|---|---|---|---|---|
| Characteristic | N | % | 95% CI | N | % |
| NRTI backbone resistance in VL ≥ 1000 copies/mL | |||||
| TDFR only (ZDVS) | 15 | 28.3 | 16.8–42.3 | 35 | 48.6 |
| ZDVR only (TDFS) | 4 | 7.5 | 2.0–18.2 | 7 | 9.7 |
| TDFR | 28 | 52.8 | 38.6–66.7 | 48 | 66.7 |
| ZDVR | 13 | 24.5 | 13.7–38.3 | 15 | 20.8 |
| (TDF+ZDV)R | 13 | 24.5 | 13.7–38.3 | 13 | 18.0 |
| TDFR +ZDVS | 15 | 28.3 | 16.8–42.3 | 35 | 48.6 |
| ZDVR +TDFS | 0 | 0 | – | 2 | 2.7 |
| (TDF+ZDV)R | 13 | 24.5 | 13.7–38.3 | 13 | 18.0 |
| K65R+M184V | 14 | 26.4 | 15.2–40.3 | 25 | 34.7 |
|
| |||||
| Amongst individuals with TDF regimen | Maputo (N = 45) | Tete (N = 61) | |||
|
| |||||
| TDFR | 26 | 57.8 | 42.1–72.3 | 42 | 68.9 |
| TDFS | 19 | 42.2 | 27.6–57.8 | 19 | 31.1 |
| ZDVR | 12 | 26.7 | 14.6–41.9 | 9 | 14.8 |
| (TDF+ZDV)R | 11 | 24.4 | 12.9–39.5 | 7 | 11.5 |
| TDFR+ZDVS | 15 | 33.3 | 20.0–48.9 | 35 | 57.4 |
|
| |||||
| Amongst individuals with ZDV regimen | Maputo (N = 8) | Tete (N = 11) | |||
|
| |||||
| ZDVR | 2 | 25.0 | 3.2–65.0 | 6 | 54.5 |
| ZDVS | 6 | 75.0 | 34.9–96.8 | 5 | 45.5 |
| TDFR | 2 | 25.0 | 3.2–65.0 | 6 | 54.5 |
| (ZDV+TDF)R | 2 | 25.0 | 3.2–65.0 | 6 | 54.5 |
| ZDVR+TDFS | 0 | 0.0 | – | 0 | 0.0 |
=Resistant (Penalty score ≥15), S=Susceptible (Penalty score < 15).
For NNRTI drugs, efavirenz and nevirapine resistance ranged from 81% (Maputo) to 96% (Tete) (Figure 1a–c). The most frequent NRTI-DRM (68.8%) was M184V/I (conferring high level DR to lamivudine/emtricitabine), followed by K65R (35.2%; reducing susceptibility to tenofovir, abacavir, and didanosine) (Figure 1d). Among those failing tenofovir-based regimens, more than half (Maputo: 57.8%; Tete: 68.9%) had tenofovir-DR (Table 5) which was driven by K65R in one-third (33.3%) in Maputo and in more than two-thirds (69%) in Tete. Zidovudine-DR among viral failures on tenofovir-based regimens was 26.7% (Maputo) and 14.8% (Tete), and 24.4% (Maputo) and 11.5% (Tete) had tenofovir/zidovudine dual-DR. About half of viral failures on tenofovir regimens in Maputo (51.1%) and 31.1% in Tete were pre-exposed to zidovudine. Among these, 39.1% (in Maputo) and 26.3% (in Tete) had zidovudine-DR. Among viral failures on zidovudine regimens, 25.0% and 54.5% had tenofovir+zidovudine dual-DR in Maputo and Tete, respectively.
PDR survey
Characteristics of the study population
A total of 735 participants (53.3% in Tete) were included and analysed after exclusions (n = 19) based on phylogenetic analysis that revealed clustering (<0.5 genetic distance). Self-reported prior antiretroviral (ARV) exposure in Maputo (5.2%) was lower than in Tete (19.1%) (P < 0.01). The most common exposure was ART interruption ≥3 months [self-reported by 72% (Maputo) and 100% (Tete) of those with prior ARV-exposure]. The median treatment interruption was 11 months (IQR = 8–24) in Maputo and 15 months (IQR = 5–38) in Tete. Study population characteristics are depicted in Table 6.
Table 6.
PDR Survey: study participant characteristics
| Characteristic | Maputo (N = 343) |
Tete (N = 392) |
||||
|---|---|---|---|---|---|---|
| N | % or median | 95% CI or [IQR] | N | % or median | 95% CI or [IQR] | |
| Sex | ||||||
| Female | 196 | 57.1 | 51.7–62.4 | 217 | 55.4 | 50.3–60.3 |
| Male | 147 | 42.9 | 37.6–48.3 | 175 | 44.6 | 40.2–47.5 |
| Age (years), median [IQR] | 36 | [30–43] | 31 | [25–39] | ||
| 18–35 | 160 | 46.6 | 41.2–52.0 | 251 | 64.0 | 59.0–68.7 |
| 36–49 | 144 | 42.0 | 36.7–47.4 | 115 | 29.3 | 24.8–34.1 |
| ≥50 | 39 | 11.4 | 8.2–15.2 | 26 | 6.7 | 4.3–9.6 |
| CD4 count (cells/mm3), median [IQR] | 298 | [144–463] | 382 | [259–537] | ||
| <200 | 120 | 35 | 30.0–40.3 | 70 | 17.9 | 14.2–22.0 |
| 200–350 | 79 | 23 | 18.7–27.9 | 100 | 25.5 | 21.2–30.1 |
| ≥350 | 144 | 42 | 36.7–47.4 | 222 | 56.6 | 51.6–61.6 |
| Reported prior ARV exposure | ||||||
| Yes | 18 | 5.2 | 3.1–8.1 | 75 | 19.1 | 15.4–23.4 |
| No | 325 | 94.8 | 91.8–96.8 | 317 | 80.9 | 76.6–84.6 |
| Type of ARV exposure | ||||||
| ART interruption ≥3 months | 13 | 72.2 | 46.5–90.3 | 75 | 100 | 95.2 -100 |
| PMTCT | 5 | 27.8 | 9.7–53.5 | 10 | 13.3 | 6.6–23.2 |
| PEP | – | – | – | 1 | 1.3 | 0.0–7.2 |
| DBS HIV RNA copies/mL | ||||||
| Target not detected | 40 | 11.7 | 8.4–15.5 | 53 | 13.5 | 10.3–17.3 |
| 20–500 | 20 | 5.8 | 3.6–8.8 | 15 | 3.9 | 2.1–6.2 |
| 500–1000 | 3 | 0.8 | 0.2–2.5 | 1 | 0.2 | 0.06–1.4 |
| 1000–10 000 | 171 | 49.9 | 44.4–55.2 | 73 | 18.7 | 14.9–22.8 |
| 10 000–100 000 | 91 | 26.6 | 21.9–31.5 | 136 | 34.7 | 30.0–39.6 |
| ≥100 000 | 18 | 5.2 | 3.1–8.1 | 114 | 29.0 | 24.6–33.8 |
PMTCT, prevention of mother-to-child transmission, PEP, post-exposure prophylaxis; DBS, dried blood spot.
All DBS samples (82%) with VL ≥ 1000 copies/mL underwent DRT, amplification failed for 25.7% (Maputo) and 1.9% (Tete). DRT was available for 39.6% in Maputo and 60.4% in Tete. In Tete all HIV subtypes were C whereas in Maputo most were subtype C (97.1%) except for a few with subtypes A (1.9%), B (0.5%), or G (0.5%).
Drug resistance in PDR
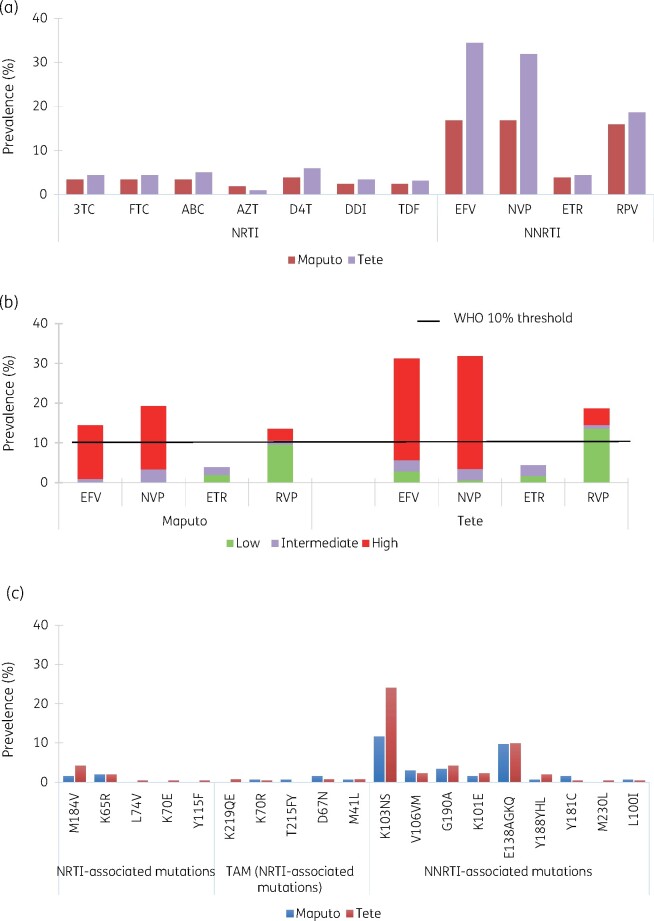
Prevalence of any HIV-DR was 18.7% and 31.8% in Maputo and Tete, respectively (Table 7). PDR (NNRTI-DR) was 16.8% in Maputo and 31.2% in Tete (Table 7), mostly due to intermediate or high-level efavirenz and nevirapine-DR (Figure 2). PDR was more frequent among ARV pre-exposed participants (55%) than in the ARV-naive (20.9%) (P < 0.01). NRTI-DR was 4.8% (95% CI 2.3%–8.7%) in Maputo and 5.7% (95% CI 3.4%–8.8%) in Tete, and dual class resistance (NRTI+NNRTI) was present in 3.4% (95% CI 1.3%–6.8%) and 5.4%(95% CI 3.1%–8.4%) individuals, respectively. Only two participants presented with PI resistance (none of them pre-exposed). The most common NNRTI mutation among PDR survey participants, regardless of previous ARV-exposure, was K103N/S (19.2%) (Figure 2). Sixteen participants (3%) had M184V/I and seven had K65R (1.3%), few had thymidine analogue mutations (TAMs) detected (D67N, K219QE, K70R, or T215) (previous exposure to stavudine/zidovudine in Maputo was 1.07%, 95% CI 0.2%–3% and 0.09%, 95% CI 0.2%–2.7% in Tete). In univariate analysis, previous exposure to ARV, the duration of ARV interruption, and participants from Tete were associated with increased PDR risk (P < 0.2) (Table 7). In multivariate analysis, previous ARV exposure (OR = 3.3, P = 0.0, 95% CI 2.0–5.5) and participants from Tete (OR = 2.5, P = 0.00, 95% CI 1.6–3.9) were associated with higher PDR risk (Table 8).
Table 7.
PDR Survey: HIVDR prevalence by drug class
| Maputo site (N = 208) |
Tete site (N = 317) |
|||||
|---|---|---|---|---|---|---|
| N | % | 95% CI | N | % | 95% CI | |
| All | ||||||
| Any HIVDR | 39 | 18.7 | 13.7–24.7 | 101 | 31.8 | 26.8–37.3 |
| NNRTI DR (PDR) | 35 | 16.8 | 12.0–22.6 | 99 | 31.2 | 26.2–36.6 |
| NRTI DR | 10 | 4.8 | 2.3–8.7 | 18 | 5.7 | 3.4–8.8 |
| PI DR | 1 | 0.5 | 0.1–2.6 | 1 | 0.3 | 0.0–1.7 |
|
| ||||||
| Pre-exposed | Maputo site (N = 12) | Tete site (N = 59) | ||||
|
| ||||||
| Any HIVDR | 6 | 50.0 | 21.0–78.9 | 33 | 55.9 | 42.4–68.8 |
| NNRTI DR (PDR) | 6 | 50.0 | 21.0–78.9 | 33 | 55.9 | 42.4–68.8 |
| NRTI DR | 1 | 8.3 | 0.3–38.5 | 7 | 11.3 | 4.9–22.9 |
| PI DR | 0 | 0 | – | 0 | 0 | – |
|
| ||||||
| ART-naive | Maputo site (N = 196) | Tete site (N = 258) | ||||
|
| ||||||
| Any HIVDR | 33 | 16.8 | 11.9–22.8 | 68 | 26.4 | 42.4–68.8 |
| NNRTI DR (PDR) | 29 | 14.8 | 10.1–20.6 | 66 | 25.6 | 42.4–68.8 |
| NRTI DR | 9 | 4.6 | 2.1–8.5 | 11 | 4.3 | 2.1–7.5 |
| PI DR | 1 | 0.5 | 0.0–2.8 | 1 | 0.4 | 0.0–2.1 |
|
| ||||||
| Female | Maputo site (N = 115) | Tete site (N = 172) | ||||
|
| ||||||
| Any HIVDR | 19 | 16.5 | 10.3–24.6 | 64 | 37.2 | 29.9–44.9 |
| NNRTI DR (PDR) | 17 | 14.8 | 8.9–22.6 | 61 | 35.5 | 28.3–43.1 |
| NRTI DR | 5 | 4.3 | 1.4–9.9 | 11 | 6.4 | 3.2–11.2 |
| PI | – | – | – | 1 | 0.6 | 0.0–3.2 |
|
| ||||||
| Male | Maputo site (N = 93) | Tete site (N = 145) | ||||
|
| ||||||
| Any HIVDR | 20 | 21.5 | 13.7–31.2 | 39 | 26.9 | 19.9–34.9 |
| NNRTI DR (PDR) | 18 | 19.4 | 11.9–28.9 | 38 | 26.2 | 19.3–34.2 |
| NRTI DR | 5 | 5.4 | 1.8–12.1 | 7 | 4.8 | 2.0–9.7 |
| PI DR | 1 | 1.1 | 0.0–5.8 | – | – | – |
Figure 2.
PDR Survey: prevalence of drug resistance and DRMs in Maputo and Tete. HIVDR prevalence by ARV were estimated from RT sequencing using Stanford HIV db v.8.4. Drug resistance levels reported for both study sites (a). The levels of resistance were classified according to Stanford score and reported for NNRTIs. Frequency of DRMs is reported by drug (b) and drug-class (c). EFV, efavirenz; NVP, nevirapine; RPV, rilpivirine; ETR, etravirine.
Table 8.
PDR survey: univariate and multivariate analysis of factors associated with PDR
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Characteristic | OR | P value | 95% CI | OR | P value | 95% CI |
| Sex | ||||||
| Men | 1 | |||||
| Women | 1.10 | 0.60 | 0.75–1.61 | 1.17 | 0.43 | 0.78–1.75 |
| Age | ||||||
| <35 years | 1 | |||||
| ≥35 years | 0.89 | 0.61 | 0.56–1.39 | 1.10 | 0.67 | 0.68–1.78 |
| CD4 count | ||||||
| <350 cells/mm3 | 1 | |||||
| ≥350 cells/mm3 | 0.98 | 0.94 | 0.67–1.43 | |||
| Previous exposure to ARV | ||||||
| No | 1 | |||||
| Yes | 4.15 | 0.00 | 2.60–6.62 | 3.30 | 0.00 | 2.04–5.35 |
| Length of ARV interruption | ||||||
| Missing | 1 | |||||
| 3–6 months | 3.90 | 0.00 | 2.03–7.49 | |||
| >7 months | 4.82 | 0.00 | 2.59–8.95 | |||
| Risky health exposure | ||||||
| No | 1 | |||||
| Yes | 1.70 | 0.32 | 0.59–4.93 | |||
| Site | ||||||
| Maputo | 1 | |||||
| Tete | 2.97 | 0.00 | 1.95–4.51 | 2.52 | 0.00 | 1.63–3.91 |
Discussion
Our findings highlight that HIV drug resistance is one of the factors complicating Mozambique’s ability to control its HIV epidemic. Prior to our study, the limited HIV-DR data available were, to our knowledge, mostly from Maputo and lacked information about those on tenofovir-based first-line drugs. In these sites (where MSF introduced VL monitoring in 2013), high rates of viral suppression were found among those on first-line regimens.15 However, among virological failures, alarmingly high levels of ADR (NRTI and NNRTI-DR) were also detected, suggesting extended exposure to sub-optimal ARV for some, and urgently demanding better DR and VL monitoring across urban and rural settings. In Mozambique, despite improvements, VL coverage is still unsatisfactory (65% nationally in 2020).19 Receiving VL results also remains an important challenge, with delays >7 months reported in studies and low levels of retention in care and adherence to ART.20–22 This further emphasizes the importance of frequent VL monitoring and more robust (dolutegravir-based) regimen use to replace NNRTIs that, given their low genetic barrier to HIV-DR, exacerbate the structural gaps in HIV care. These findings add intensity to alarm bells about drug resistance that have repeatedly been sounded across southern Africa for more than 10 years.6,23 They also amplify previous warnings by providing comprehensive adult ADR data on tenofovir/lamivudine/efavirenz, until recently considered the ‘standard’ first-line choice in Mozambique and many other LRS countries.
Encouraging viral suppression rates were achieved in both sites (Maputo, 91.7%; Tete, 84.7%). Nevertheless, this heartening finding is tempered by the discouraging fact that more than two-thirds of all virological failures (Maputo, 66.7%; Tete, 76.4%) had dual-class DR. Even after enhanced adherence counselling, nearly two-thirds of virological failures did not re-suppress (Table 2), consistent with the high ADR rate. Given the lack of access to routine DRT, and the delayed reporting of VL test results, these findings urge clinicians to switch those failing NNRTI-based regimens quickly, particularly if CD4 levels are low.
Further, the most frequent DR mutations (K65R; M184V) affect critical NRTI drugs (tenofovir and lamivudine). TAMs (M41L, K70R, K219QE) were also still circulating despite the declining use of stavudine and zidovudine, with nearly 50% of participants in Maputo and 37% in Tete with previous stavudine/zidovudine exposure (Table 1). These findings point to a future with decreased susceptibility to all approved NRTIs for many patients. The low proportion of TAMs seen in the PDR survey could be attributed to only a few patients (<1%) reporting prior exposure to thymidine analogues (stavudine/zidovudine).
Mozambique is currently rolling out new dolutegravir-based first-line regimens, replacing NNRTIs with dolutegravir in a fixed-dose combination with tenofovir + lamivudine (TLD). However, in our study, tenofovir/lamivudine dual resistance affected more than half of those with suspected treatment failure, meaning that if this TLD-transition occurs in viraemic patients without assessing their VL, half of those in Maputo (52.8%) and two-thirds in rural Tete (66.7%) would be treated with a functional monotherapy (Table 5).
Our findings raise important questions about the value of systematically substituting tenofovir with zidovudine when switching from standard tenofovir-based first-line to any second-line regimen. One quarter of Maputo’s viral failures on zidovudine or tenofovir-based regimens had triple drug resistance to lamivudine, zidovudine and tenofovir. In Tete, triple resistance affected more than half of failures on zidovudine (Table 5). Yet large numbers of viral failure patients were also still tenofovir-susceptible (nearly one-third in Tete: 31%, and nearly one-half in Maputo: 42%), meaning that more than half of viral failures on a tenofovir regimen would have been either unnecessarily switched to zidovudine or were already zidovudine resistant. Switching these patients to zidovudine would also expose these patients to other harms related to the drug’s additional safety and adherence issues.
While these findings suggest that a blanket substitution of tenofovir with zidovudine is not appropriate, more evidence is needed on whether tenofovir recycling can be effective in the presence of tenofovir-DR. While encouraging virological outcomes occurred for those on protease inhibitor-based second-line regimens (despite NRTI-backbone resistance), clinical evidence of TLD effectiveness in viraemic patients and dual resistance to tenofovir/lamivudine is still pending.24 The few viral failures in our cohort who were on zidovudine-based regimens may have benefitted from substituting zidovudine with tenofovir when balancing resistance and tolerability (all of those with zidovudine-DR also had tenofovir-DR). Like Mozambique, many sub-Saharan African countries are currently phasing out efavirenz and introducing dolutegravir-based first-line regimens. A general challenge to this transition is ensuring that patients are virally suppressed, as VL scale-up in most settings is incomplete and returning VL results may take time. Given a high proportion of resistance among virological failures, making VL testing and HIV-DR surveillance routine is urgent to prevent the new first-line TLD regimen from becoming similarly obsolete.
Similar to other investigations, we also found that in patients with VL 500–1000 copies/mL, DR mutations were frequent (40% Maputo; 100% Tete). This confirms the existence of DR in patients with low HIV replication levels and poses a significantly higher risk of virological failure.25,26 In Mozambique, as in most settings, VL testing is conducted using DBS-samples with technical limitations when detecting low levels of virus. Despite our small numbers, our results urge the reconsideration of viral thresholds and the benefits of plasma-based VL testing (possibly supported by new point-of-care VL technologies).
PDR surveys allow the critical analysis of first-line ART regimen efficacy. They guide public health actions and response. We are encouraged that a national PDR survey is currently being conducted in Mozambique. In the meantime, our findings can inform policymakers. The high PDR levels found here in both urban (16.8%) and rural (31.2%) areas are alarming and significantly surpass the 10% WHO ‘alert’ threshold above which countries should take urgent action to preserve first-line ART effectiveness.13 Our findings provide further detail in the emerging HIV-DR picture in the country and highlight the serious challenge that Mozambique faces. Previous surveys of women using antenatal care services estimated NNRTI-DR levels between 5%–14%, and a nationally representative survey of infants reported a PDR prevalence >50%.27–29 Other countries with available PDR data above the WHO alert threshold are eSwatini (10.5% PDR), Namibia (13.8%), Uganda (15.4%), and South Africa (23.6%).7 High-level NNRTI resistance, especially to efavirenz, remains a challenge in the dolutegravir era where tenofovir/lamivudine/efavirenz is the alternative first-line regimen for pregnant women and women of childbearing age (exposure to dolutegravir at conception may be associated with neural tube defects).14 In Mozambique and countries with similar NNRTI-DR data, a woman-centred approach should enable women to make informed decisions about dolutegravir- or efavirenz-based drug regimens and should clarify the risks and benefits of each.
In Mozambique, the initiation of failing NNRT-based first-line regimens must stop and universal access to dolutegravir must occur as quickly as possible.
In our study, 5.2% of PDR-survey participants in Maputo and 19.1% in Tete had previous ARV exposure. In multivariate models, ART pre-exposure was associated with increased PDR risk (OR = 3.30, P = 0.00, 95% CI 2.04–5.35) in line with other recent research on the topic.11 Yet in contrast to other studies, we did not identify a significantly increased PDR risk among women (OR = 1.17, 95% CI 0.78–1.75).7 The most prevalent PDR mutation was K103NS (19.2%) which, importantly, reduces nevirapine and efavirenz susceptibility.30 Among NRTI-DR mutations, a small amount of M184VI occurred (3%), though this mutation is not commonly transmissible since it reduces viral fitness and its presence in our sample may partially be explained by unreported previous ARV exposure. Finally, although little is still known about PDR’s effects on dolutegravir-based first-line therapy in Mozambique, a recent South African study associated NNRTI-PDR with reduced dolutegravir-based regimen efficacy, findings that may have serious repercussions for first-line regimen choice and treatment monitoring.31 Once again, improved access to VL monitoring is a critical need. This study presents some limitations. In the ADR survey, Tete participants were recruited by convenience sampling. Results from Alto Maé, Maputo are not generalizable to other HCs given the tight connection to Alto Maé Reference Centre (CRAM), where suspected treatment failures are referred. In the PDR survey, self-reported ARV pre-exposure was not clinically verified, may therefore be under-estimated, and may have differences in self-reporting bias in the two sites. Overall PDR prevalence is probably underestimated because samples from virally suppressed participants were not eligible for DRT. Finally, DBS sensitivity limitations may have caused some misclassification.
Conclusions
This study fills important HIV-DR information gaps in Mozambique by providing recent PDR and ADR data for first-line treatment in two distinct settings. Our findings underline the importance of dolutegravir-based first-line regimens in Mozambique and stress the need for their immediate scale-up to all patients initiating ART and those with prior exposure to HIV therapies. High levels of NRTI-resistance urge caution when transitioning patients to new regimens without the guidance of VL-results, since the risk of providing an ineffective regimen is high. Continuous VL monitoring and routine HIV resistance surveillance will remain of pivotal importance to safeguard current treatment options for Mozambicans.
Acknowledgements
We acknowledge the support of the people and institutions without whom the study would not have been possible: the study teams and MoH clinical staff in the health facilities in Maputo and Tete in Mozambique, the Ministry of Health of Mozambique (the Direcçao Nacional de Saude Publique of Maputo, the Direcçao Provincial de Saude of Tete and the National HIV/AIDS program) for their collaboration. We are thankful to the National Infectious Control Diseases laboratory in Johannesburg (Dr M. Gillian Hunt), Jihane Ben-Farhat for data analysis support, Janet Ousley for manuscript editing, Ruggero Giuliani, David Maman, Denis-Luc Ardiet, Silvia Bertagnolio and Ana Torrens for technical discussions helping to organize and implement this work. Jeanne Haidar for her guidance in the ethical-administrative aspects of the research. Finally, we thank our patients for participating in the study and trying to help others with the information they have shared.
Funding
This work was entirely funded by the non-governmental organization Médecins Sans Frontières.
Transparency declarations
None to declare.
Author contributions
V.C., L.M., I.C., T.E. and B.S. designed the research study. V.C. supervised the research, I.A.P.T., S.F. and D.V. performed the research. V.C. analyzed the data and wrote the first draft of the paper. B.S. contributed to the writing of the paper. All authors contributed to the critical review of the manuscript.
References
- 1. UNAIDS. Global HIV & AIDS statistics — 2020. fact sheet. https://www.unaids.org/en/resources/fact-sheet.
- 2. Shroufi A, Van Cutsem G, Cambiano V. et al. Simplifying switch to second-line antiretroviral therapy in sub Saharan Africa: predicted effect of using a single viral load to define efavirenz-based first-line failure. AIDS 2019; 33: 1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boender TS, Kityo CM, Boerma RS. et al. Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J Antimicrob Chemother 2016; 71: 2918–27. [DOI] [PubMed] [Google Scholar]
- 4. Roberts T, Cohn J, Bonner K. et al. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis 2016; 62: 1043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ehrenkranz PD, Baptiste SL, Bygrave H. et al. The missed potential of CD4 and viral load testing to improve clinical outcomes for people living with HIV in lower-resource settings. PLoS Med 2019; 16: e1002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inzaule SC, Ondoa P, Peter T. et al. Affordable HIV drug-resistance testing for monitoring of antiretroviral therapy in sub-Saharan Africa. Lancet Infect Dis 2016; 16: e267–75. [DOI] [PubMed] [Google Scholar]
- 7. WHO/CDS/HIV/19.21. HIV Drug Resistance Report 2019. Geneva: World Health Organization, 2019. http://www.who.int/hiv/pub/drugresistance/hivdr-report-2019/en/. [Google Scholar]
- 8. De Luca A, Sidumo ZJ, Zanelli G. et al. Accumulation of HIV-1 drug resistance in patients on a standard thymidine analogue-based first line antiretroviral therapy after virological failure: implications for the activity of next-line regimens from a longitudinal study in Mozambique. BMC Infect Dis 2017; 17: 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization, Regional Office for Africa. Preventing and Responding to HIV Drug Resistance in the African Region: Regional action plan 2019-2023. 2019. https://www.afro.who.int/publications/preventing-and-responding-hiv-drug-resistance-african-region-regional-action-plan-2019.
- 10. World Health Organization. HIV Drug Resistance Report 2017. 2017. http://apps.who.int/iris/bitstream/10665/255896/1/9789241512831-eng.
- 11. Gupta RK, Gregson J, Parkin N. et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18: 346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a Public Health Approach Second Edition. 2016. https://apps.who.int/iris/handle/10665/208825. [PubMed]
- 13. World Health Organisation. Guidelines on the Public Health Response to Pretreatment HIV Drug Resistance, July 2017. 2017. http://www.who.int/hiv/pub/guidelines/hivdr-guidelines-2017/en/.
- 14. World Health Organisation. Update of Recommendations on First- and Second-Line Antiretroviral Regimens, Policy Brief. 2019. https://apps.who.int/iris/handle/10665/325892.
- 15. Médecins sans F. Making Viral Load Routine: Part 2 - Laboratory Report | MSF SAMU. https://samumsf.org/en/resources/hiv/viral-load-monitoring/making-viral-load-routine-part-2-laboratory-report.
- 16. Harris PA, Taylor R, Thielke R. et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Z, Wagar N, DeVos JR. et al. Optimization of a low cost and broadly sensitive genotyping assay for HIV-1 drug resistance surveillance and monitoring in resource-limited settings. PLoS One 2011; 6: e28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organisation. WHO/HIVResNet HIV Drug Resistance Laboratory Operational Framework. 2017. http://www.who.int/hiv/pub/drugresistance/hivdr-laboratory-framework-2017/en/.
- 19. PEPFAR. Mozambique Country Operational Plan COP 2020. 2020. https://www.state.gov/wp-content/uploads/2020/07/COP-2020-Mozambique-SDS-FINAL.pdf.
- 20. PEPFAR. Mozambique Country Operational Plan 2019 Strategic Direction Summary. 2019. https://mz.usembassy.gov/wp-content/uploads/sites/182/COP19_SDS_-FINAL_2019-07-28.pdf.
- 21. Pulido Tarquino IA, Venables E, de Amaral Fidelis JM. et al. “I take my pills every day, but then it goes up, goes down. I don’t know what’s going on”: Perceptions of HIV virological failure in a rural context in Mozambique. A qualitative research study. PLoS One 2019; 14: e0218364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lafort Y, Couto A, Sunderbrink U. et al. Validity of reported retention in antiretroviral therapy after roll-out to peripheral facilities in Mozambique: Results of a retrospective national cohort analysis. PLoS One 2018; 13: e0198916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sendagire H, Easterbrook PJ, Nankya I. et al. The challenge of HIV-1 antiretroviral resistance in Africa in the era of HAART. AIDS Rev 2009; 11: 59–70. [PMC free article] [PubMed] [Google Scholar]
- 24. Vitoria M, Hill A, Ford N. et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: what are the issues? Aids 2018; 32: 1551–61. [DOI] [PubMed] [Google Scholar]
- 25. Bernal E, Gómez JM, Jarrín I. et al. Low-level viremia is associated with clinical progression in HIV-Infected patients receiving antiretroviral treatment. J Acquir Immune Defic Syndr 2018; 78: 329–37. [DOI] [PubMed] [Google Scholar]
- 26. Swenson LC, Min JE, Woods CK. et al. HIV drug resistance detected during low-level viraemia is associated with subsequent virologic failure. AIDS 2014; 28: 1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bila DCA, Young P, Merks H. et al. Evolution of primary HIV drug resistance in a subtype C dominated epidemic in mozambique. PLoS One 2013; 8: e68213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jordan MR, Penazzato M, Cournil A. et al. Human immunodeficiency virus (HIV) drug resistance in African infants and young children newly diagnosed with HIV: a multicountry analysis. Clin Infect Dis 2017; 65: 2018–25. [DOI] [PubMed] [Google Scholar]
- 29. Rupérez M, Noguera-Julian M, González R. et al. HIV drug resistance patterns in pregnant women using next generation sequence in Mozambique. PLoS One 2018; 13: e0196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. HIV Drug Resistance Database. NNRTI Resistance Notes. https://hivdb.stanford.edu/dr-summary/resistance-notes/NNRTI/.
- 31. Siedner MJ, Moorhouse MA, Simmons B. et al. Reduced efficacy of HIV-1 integrase inhibitors in patients with drug resistance mutations in reverse transcriptase. Nature Comm 2020; 11: 5922. [DOI] [PMC free article] [PubMed] [Google Scholar]