Abstract
Monoclonal antibodies targeting programmed cell death 1/programmed cell death ligand 1 (PD-1/PD-L1) immune checkpoints have improved the treatments of cancers. However, not all patients equally benefit from immunotherapy. The use of cytotoxic drugs is practically inevitable to treat advanced cancers and metastases. The repertoire of cytotoxics includes 80 products that principally target nucleic acids or the microtubule network in rapidly proliferating tumor cells. Paradoxically, many of these compounds tend to become essential to promote the activity of immunotherapy and to offer a sustained therapeutic effect. We have analyzed each cytotoxic drug with respect to effect on expression and function of PD-(L)1. The major cytotoxic drugs—carboplatin, cisplatin, cytarabine, dacarbazine, docetaxel, doxorubicin, ecteinascidin, etoposide, fluorouracil, gemcitabine, irinotecan, oxaliplatin, paclitaxel and pemetrexed—all have the capacity to upregulate PD-L1 expression on cancer cells (via the generation of danger signals) and to promote antitumor immunogenicity, via activation of cytotoxic T lymphocytes, maturation of antigen-presenting cells, depletion of immunosuppressive regulatory T cells and/or expansion of myeloid-derived suppressor cells. The use of ‘immunocompatible’ cytotoxic drugs combined with anti-PD-(L)1 antibodies is a modern approach, not only for increasing the direct killing of cancer cells, but also as a strategy to minimize the activation of immunosuppressive and cancer cell prosurvival program responses.
INTRODUCTION
The therapeutic arsenal to treat cancers is regularly enriched with new small and large molecules directed against signaling factors implicated in tumorigenesis or tumor expansion. This highly diversified molecular arsenal can be divided into several classes based on the drugs’ mechanisms of action. To simplify, we can define three major classes. Cytotoxic drugs, comprising many natural products and derivatives, essentially combat highly proliferating cells. Targeted therapeutics, including numerous kinase inhibitors and monoclonal antibodies (mAbs) directed against intracellular effectors and cell surface receptors on cancer cells, permit to control signaling pathways that represent tumor drivers or key factors involved in tumor growth and dissemination. Immunotherapeutic drugs are designed to turn on/off specific immune checkpoints implicated in immune surveillance. Immunotherapy has emerged as the seventh pillar of cancer therapy alongside surgery, cytotoxic chemotherapy, targeted therapy, radiotherapy, hormonal therapy and cell therapy (Figure 1). This is a simplified view: there are many types of anticancer drugs that target one or the other of the hallmarks of cancer, and an extended repertoire of molecules, ranging from small synthetic compounds to complex polymeric particles and biotherapeutic peptides and proteins, and engineered therapeutic cells. More than 200 anticancer drugs used to treat cancers in humans have been approved over the past 50 years.
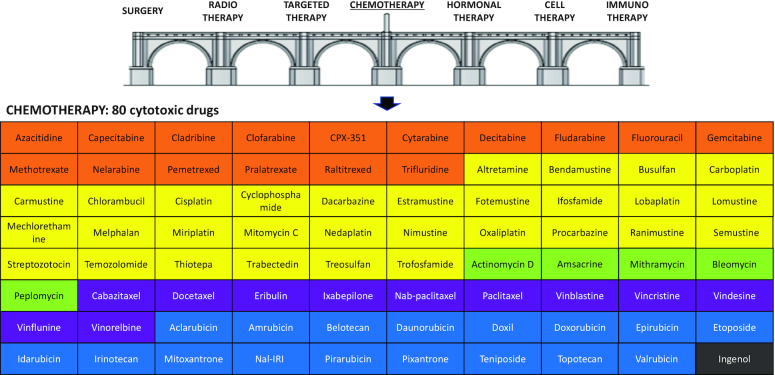
Figure 1.
The seven pillars of cancer therapy and the panel of 80 cytotoxic chemotherapeutic drugs available for the treatment of cancers. Drugs are grouped according to their mechanisms of action (antimetabolites in orange, DNA alkylators in yellow, DNA binders or cleavers in green, DNA topoisomerase inhibitors in blue and tubulin/microtubule inhibitors in purple) and by alphabetical order within each drug category.
Many cytotoxic anticancer drugs were discovered empirically in the 1950–70s, generally from natural products (anthracyclines, vinca alkaloids) or after serendipitous discoveries (1). Other cytotoxic products were developed much later (e.g. vinflunine, pixantrone) and occasionally new cytotoxic drugs and formulations continue to be developed (Figure 1). There are ∼80 approved anticancer drugs considered as cytotoxic products, generally classified according to their mechanism of action or chemical family (Table 1). A large proportion of these cytotoxics interferes with nucleic acid metabolism, inhibiting DNA/RNA synthesis, binding covalently or not to DNA, cleaving DNA or blocking DNA-manipulating enzymes such as topoisomerases to cause DNA strand breaks. Another major category of cytotoxics includes drugs that affect cell mitosis through interference with the tubulin/microtubule network. Many of these old drugs remain largely used today, notably to treat advanced cancers.
Table 1.
Cytotoxic drugs used to treat cancers and their effects on PD-(L)1
| Drug category | # | INN | Abbreviation | Brand namea | Year of first approvalb | Effect on PD-(L)1 |
|---|---|---|---|---|---|---|
| Antimetabolites | 1 | 5-Fluorouracil | 5-FU | 5-Fluorouracil | 1962 | Combo (+). Expression (+) |
| Pyrimidine analogues | 2 | Capecitabine | CAP | Xeloda | 1998 | Combo (+) |
| 3 | Trifluridine | FTD | Lonsurf | 2014 | Combo (+) | |
| 4 | Gemcitabine | GEM | Gemcitabine | 1995 | Combo (+). Expression (+) | |
| 5 | Cytarabine | Ara-C | Cytarabine | 1969 | Combo (+) | |
| 6 | Cytarabine/daunorubicin liposomal | CPX-351 | Vyxeos | 2017 | n.i. | |
| Purine analogues | 7 | Fludarabine | 2-FA | Fludara | 1991 | No effect |
| 8 | Cladribine | CLA | Leustatin | 1993 | n.i. | |
| 9 | Clofarabine | CLO | Clolar | 2005 | n.i. | |
| 10 | Nelarabine | NEL | Arranon | 2006 | n.i. | |
| Folate derivatives | 11 | Methotrexate | MTX | Methotrexate | 1954 | Combo (−) |
| 12 | Pralatrexate | PLX | Folotyn | 2009 | Combo (−) | |
| 13 | Pemetrexed | PMX | Alimta | 2004 | Combo (+) | |
| 14 | Raltitrexed | RTX | Tomudex | 1996 | n.i. | |
| DNMT inhibitors | 15 | Azacitidine | 5-AZA | Vidaza | 2004 | Combo (+). Expression (+) |
| 16 | Decitabine | DAC | Dacogen | 2006 | Combo (+). Expression (+) | |
| DNA-alkylating drugs | 17 | Cisplatin | CDDP | Cisplatin | 1978 | Combo (+). Expression (+) |
| Platinum derivatives | 18 | Oxaliplatin | OXA | Eloxatin | 1996 | Combo (+). Expression (+) |
| 19 | Carboplatin | CARB | Paraplatin | 1986 | Combo (+). Expression (+) | |
| 20 | Nedaplatin | NEDA | Aqupla | 1995 | Combo (+) | |
| 21 | Lobaplatin | LPT | Lobaplatin | 1998 | n.i. | |
| 22 | Miriplatin | MPT | Miripla | 2010 | n.i. | |
| Oxazaphosphorine derivatives | 23 | Cyclophosphamide | CPX | Cytoxan | 1957 | Combo (+) |
| 24 | Ifosfamide | IFS | Ifex | 1976 | n.i. | |
| 25 | Trofosfamide | TFF | Ixoten | 2007 | n.i. | |
| Nitrosoureas | 26 | Carmustine | BiCNU | Gliadel | 1977 | Combo (+) |
| 27 | Nimustine | ACNU | Nidran | 1981 | n.i. | |
| 28 | Fotemustine | FTM | Muphoran | 1989 | n.i. | |
| 29 | Lomustine | CCNU | CeeNU | 1976 | n.i. | |
| 30 | Semustine | SEMU | Me-CCNU | 1977 | n.i. | |
| 31 | Ranimustine | MCNU | Cymerine | 1987 | n.i. | |
| 32 | Streptozotocin | STZ | Zanosar | 1982 | Expression (+) | |
| Bis-chloroethyl-amine | 33 | Mechlorethamine | MCE | Valchlor | 1913 | n.i. |
| 34 | Bendamustine | BDM | Treanda | 2008 | n.i. | |
| 35 | Chlorambucil | CHB | Leukeran | 1956 | n.i. | |
| 36 | Melphalan | MLP | Alkeran | 1961 | Combo (+) | |
| 37 | Estramustine | EMP | Emcyt | 1980 | n.i. | |
| Alkyl sulfonates | 38 | Busulfan | BUS | Busilvex | 1954 | Expression (+) |
| 39 | Treosulfan | TREO | Trecondi | 2004 | n.i. | |
| Others | 40 | Thiotepa | TEPA | Thioplex | 1959 | n.i. |
| 41 | Temozolomide | TMZ | Temodal | 1999 | Combo (+) | |
| 42 | Mitomycin C | MMC | Mutamycin | 1956 | Combo (+) | |
| 43 | Procarbazine | PRO | Procarbazine | 1969 | n.i. | |
| 44 | Dacarbazine | DTIC | Dacarbazine | 1975 | Expression (+) | |
| 45 | Altretamine | HMM | Hexalen | 1990 | n.i. | |
| 46 | Trabectedin | TBT | Yondelis | 2007 | Combo (+). Expression (+) | |
| DNA-binding or -cleaving drugs | 47 | Bleomycin | BLM | Blenoxane | 1966 | n.i. |
| 48 | Peplomycin | PLM | Pepleo | 1981 | n.i. | |
| 49 | Mithramycin | MTM | Plicamycin | 1961 | n.i. | |
| 50 | Actinomycin D | Act-D | Cosmegen | 1964 | Expression (−) | |
| 51 | Amsacrine | Amsa | Amsidine | 1987 | n.i. | |
| Topoisomerase 1 inhibitors | 52 | Irinotecan | CPT-11, IRI | Campto | 1994 | Combo (+). Expression (+) |
| Camptothecin derivatives | 53 | Topotecan | TPT | Hycamtin | 1996 | n.i. |
| 54 | Nal-IRI | Nal-IRI | Onivyde | 2015 | Combo (+) | |
| 55 | Belotecan | BLT | Camtobell | 2004 | n.i. | |
| Topoisomerase 2 inhibitors | 56 | Etoposide (etoposide phosphate) | VP-16, ETO | Etoposide (Etopophos) | 1980 (1996) | Combo (+). Expression (+) |
| Epipodophyllotoxins | 57 | Teniposide | VM-26 | Vumon | 1967 | n.i. |
| Anthracyclines | 58 | Doxorubicin | DOX | Adriamycin | 1966 | Combo (+). Expression (+) |
| 59 | Liposomal doxorubicin | Doxil | Doxil | 1999 | Combo (+). Expression (+) | |
| 60 | Epirubicin | EPI | Pharmorubicin | 1984 | Combo (+) | |
| 61 | Idarubicin | IDA | Idamycin | 1990 | Combo (+) | |
| 62 | Daunorubicin | DAU | Cerubidin | 1967 | n.i. | |
| 63 | Pirarubicin | THP | Pinorubicin | 1988 | n.i. | |
| 64 | Amrubicin | AMR | Calsed | 2002 | n.i. | |
| 65 | Valrubicin | VAL | Valstar | 1999 | n.i. | |
| 66 | Aclarubicin | ACLA | Aclacin | 1981 | n.i. | |
| Anthraquinones | 67 | Mitoxantrone | MTX | Novantrone | 1984 | n.i. |
| 68 | Pixantrone | PXT | Pixuvri | 2012 | n.i. | |
| Tubulin inhibitors | 69 | Vinorelbine | NVB | Navelbine | 1989 | Expression (−) |
| Vinca alkaloids | 70 | Vindesine | VDS | Eldisine | 1979 | n.i. |
| 71 | Vinblastine | VBL | Velban | 1965 | n.i. | |
| 72 | Vincristine | VCR | Oncovin | 1963 | n.i. | |
| 73 | Vinflunine | VFL | Javlor | 2010 | n.i. | |
| Taxanes | 74 | Paclitaxel | PACLI | Taxol | 1993 | Combo (+) |
| 75 | Nab-paclitaxel | NABP | Abraxane | 2005 | Combo (+) | |
| 76 | Docetaxel | DOCE | Taxotere | 1995 | Combo (+) | |
| 77 | Cabazitaxel | CBZ | Jevtana | 2010 | n.i. | |
| Others | 78 | Ixabepilone | IXA | Ixempra | 2007 | n.i. |
| 79 | Eribulin mesylate | ERI | Halaven | 2010 | Combo (+) | |
| Miscellaneous | 80 | Ingenol mebutate | ING | Picato | 2012 | n.i. |
DNMT: DNA methyl transferase; INN, international nonproprietary name. aThe brand name can vary significantly from one country to another and different brand names are used for the generic compounds. bThe year of first approval was mainly collected from (1). ‘n.i.’ means no information available about effect of the indicated drug on PD-(L)1. Combo (+) indicates a positive combination potential with mAbs targeting PD-1 or PD-L1. Expression (+) or (−) refers to a drug-induced up- or downregulation of PD-1 or PD-L1.
Lack of antitumor immunity is a key element that leaves cancer cells free to multiply, disseminate and metastasize. Among the different immune checkpoints characterized over the past 10 years, the programmed cell death 1 (PD-1, also called CD279) and programmed cell death ligand 1 (PD-L1, CD274, B7-H1) axis plays an essential role in the ability of cancer cells to evade the immune system. The blockade of the PD-1/PD-L1 interaction represents an essential strategy to prevent cancer cells to escape antitumor immune responses. Six mAbs targeting PD-1 (three) or PD-L1 (three) are currently used for the treatment of specific solid tumors (Figure 2) and >15 other mAbs are in clinical development (2,3). The use of these biotherapeutic products has revolutionized the treatment of certain cancers such as melanoma, non-small- and small-cell lung cancers (NSCLCs and SCLCs), urothelial cancers and triple-negative breast cancers (TNBCs). Many cancer patients will benefit from these novel immunotherapies, with the expected success of a huge number of ongoing clinical trials. However, unfortunately, not all tumors respond to PD-(L)1 immunotherapy. The efficacy of anti-PD-(L)1 treatment is highly variable. We realize that it is not a complete overthrow of a therapeutic model, in favor of a totally new treatment system, but a more profound change that complements existing therapies. Immunotherapies alone, and in particular anti-PD-1 or anti-PD-L1 mAbs used as monotherapy, generally provide insufficient responses or nonsignificant therapeutic advantages. A major benefit is expected from mAbs combined with other therapeutic modalities and in particular with cytotoxic agents.
Figure 2.
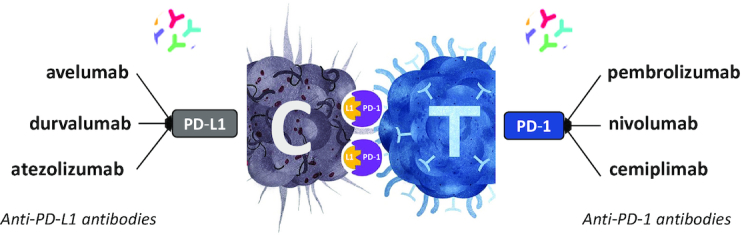
The six mAbs targeting PD-1 or PD-L1 currently approved for the treatment of cancer. Pembrolizumab (Keytruda, from MSD), nivolumab (Opdivo, from BMS) and cemiplimab (Libtayo, from Sanofi) target PD-1. Atezolizumab (Tecentriq, from Roche–Genentech), avelumab (Bavencio, from Merck KGaA–Pfizer) and durvalumab (Imfinzi, from AstraZeneca) target PD-L1. They target PD-L1 expressed on cancer cells (C) and PD-1 expressed on cytotoxic T lymphocytes (T). They are currently used to treat melanoma, metastatic NSCLC, metastatic urothelial carcinoma, advanced cutaneous squamous cell carcinoma, renal carcinoma and Hodgkin’s disease.
Biomarkers are actively searched to predict tumor response, to select patient populations and to adjust treatment combinations. PD-L1 is expressed on a variety of normal and immune cell types and cancer cells. The tumor expression level of PD-L1 plays a role in the treatment response. PD-L1 expression is often associated with poor prognosis of cancer patients. However, at the same time, overexpression of tumor PD-L1 represents a favorable prognostic for response to immunotherapy. High PD-L1 expression is correlated with better response to PD-1/PD-L1 inhibition in different tumors (4). Although clinical response has been demonstrated also in patients with PD-L1-negative tumors [via the activation of PD-L1+ natural killer (NK) cells], efficacy of immunotherapy is greater in PD-L1-positive patients (5). To address this dilemma, PD-L1 expression was compared in tumor cells and cytotoxic T cells to show that PD-L1 is primarily reactive rather than constitutive in most tumors (6). Other studies have shown that PD-L1 expression on both tumor cells and tumor-infiltrating immune cells can independently attenuate anticancer immunity (7). A model of adaptive immune resistance has been proposed in which PD-L1 expression is primarily driven by cytokines [notably interferon gamma (IFN-γ)] induced by cytotoxic T cells. PD-L1 appears as a marker of an ongoing immune response to tumor and the administration of checkpoint blocker helps to tip the balance of this interaction in favor of the immune system (8). It is therefore of the utmost importance to properly understand how chemotherapy affects PD-(L)1 expression and function, to design suitable drug combinations.
With >200 anticancer drugs available for many tumor indications, the possibilities of combinations are huge (Figure 3). It is a challenge to design the best drug combinations that outperform anti-PD-L1 alone with regard to antitumor activity and effect on PD-L1 expression. Drugs that enhance PD-L1 expression on cancer cells can promote drug resistance, but the combination with an mAb targeting the PD-1/PD-L1 axis can improve tumor sensitization to the antibody treatment. In contrast, a drug that downregulates PD-L1 expression could waive tumor immunity and thus could reinforce the activity of the combined PD-(L)1 mAbs. The challenge is to find the right balance to take full advantage of the association. Consequently, numerous drug combination therapies are currently investigated for their potential to boost antitumor immunity and improve survival of cancer patients. In this context, we have analyzed the published information on the capacity of all cytotoxic drugs to modulate PD-(L)1 expression and function. About 80 cytotoxic drugs used to treat cancers were selected on the basis of their current clinical use (and to cover all the cytotoxic drug arena, with different mechanisms of action) and analyzed for their impact on the PD-(L)1 inducibility. We found PD-(L)1-related data for 38 of them; for the other 42 compounds, the effects on PD-(L)1 are not known (Table 1). The drugs, discussed according to their chemical categories and/or mechanisms of action, include antimetabolites, antibiotics, DNA-binding and -alkylating agents, topoisomerases inhibitors and microtubule poisons. We will refer neither to hormone antagonists, nor to radiotherapy and targeted therapy.
Figure 3.
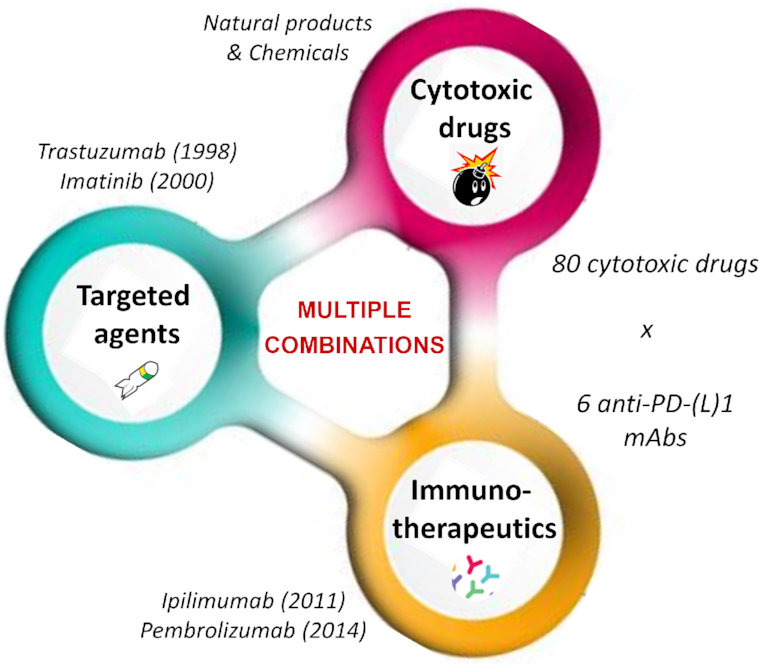
The anticancer drug combination challenge. Two successive revolutions in cancer treatment have occurred, around the 2000s with the advent of targeted therapeutics such as the kinase inhibitor imatinib (Glivec®) used to treat chronic myelocytic leukemia and the mAb trastuzumab (Herceptin®) for breast cancer, and then around the 2010s with booming of immunotherapy and the launch of mAbs such as ipilimumab (Yervoy®) and pembrolizumab (Keytruda®) targeting CTLA4 and PD-1, respectively, to treat melanoma. More than 200 anticancer drugs are available to design new combinations. The 80 cytotoxic drugs and 6 mAbs targeting PD-(L)1 offer a wide range of potential combinations.
ANTIMETABOLITES
Pyrimidine antimetabolites
5-Fluorouracil (5-FU) primarily targets thymidylate synthase, leading to defect of DNA synthesis in rapidly proliferating cells. Repeated cycle treatment with 5-FU tends to repress the antitumor immune functions and to elevate the expression of PD-L1 on tumor cells. The drug initially promotes proliferation and cytotoxicity of tumor-infiltrating CD8+ T cells after one cycle of treatment, but after repeated cycles the antitumor immune functions get impaired, with the release of immune-suppressive factors such as transforming growth factor beta and interleukin 10 (IL-10) (9). This trend could diminish the antitumor efficacy of the chemotherapy. 5-FU upregulates PD-L1 (10) and this early induction of PD-L1 expression is beneficial when combining the drug with an anti-PD-L1 antibody (Figure 4). The combined treatment of 5-FU + an anti-PD-L1 mAb displays a greater efficacy compared to 5-FU or immunotherapy alone. The overall survival was significantly improved, at least in an immune-competent colon carcinoma model in mice (9). The immune-regulatory roles of 5-FU and the related products capecitabine (oral fluoropyrimidine prodrug), trifluridine and gemcitabine (GEM, pyrimidine nucleoside) have been reported in different studies (Table 2). With no doubt, GEM can be considered as a PD-(L)1-compatible drug. The drug helps anti-PD-1 mAbs to reactivate an immune response in dormant tumor cells and to restrain tumor dissemination and recurrence. Upregulation of PD-L1 membrane expression has been observed also in murine and human pancreatic cancer cell lines, and similar effects were noted with 5-FU and paclitaxel (PACLI). In each case, the JAK2/STAT1 pathway was involved in the drug-mediated PD-L1 transcription and membrane expression, an effect that could induce a tumor immune escape (20). In fact, GEM can not only facilitate tumor infiltration with antigen-specific CD8+ T cells but also reduce the number of immunosuppressive cells, thus facilitating the eradication of tumors (21). The immune-modulating functions of GEM encourage the use of the drug at low doses, associated with an anti-PD-(L)1 and a targeted therapeutic agent (e.g. an oral CHK1 inhibitor) to treat SCLC (22).
Figure 4.
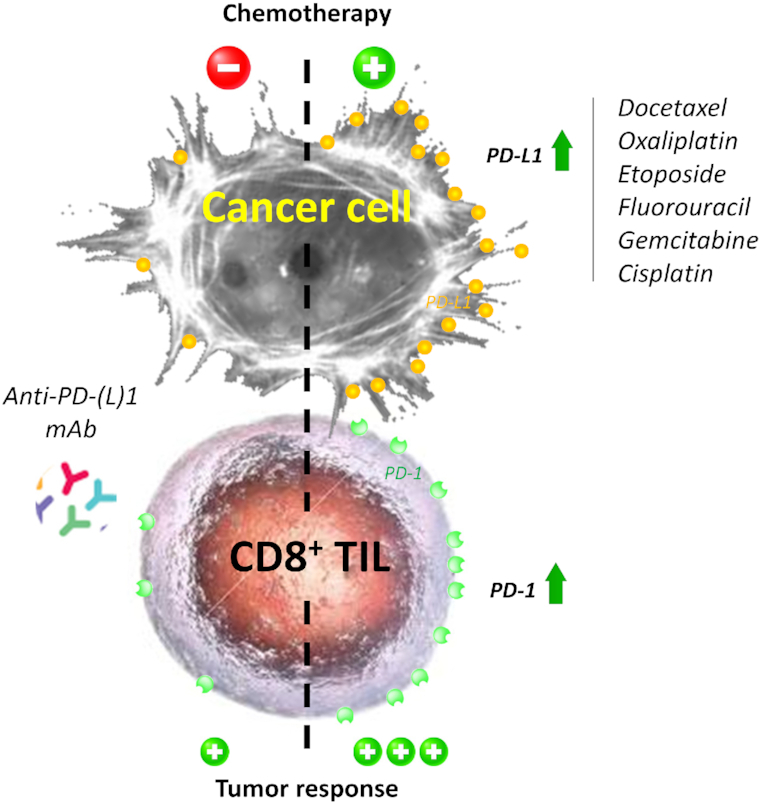
The indicated cytotoxic drugs induce an upregulation of PD-L1 at the surface of cancer cells and/or PD-1 at the surface of CD8+ tumor-infiltrating lymphocytes (TILs). Thus, they promote tumor response to antibodies targeting PD-L1 or PD-1.
Table 2.
Immune-related effects of selected pyrimidine antimetabolites
| Drug | Effects on PD-(L)1 checkpoint | References |
|---|---|---|
| 5-FU | Increases IFN-γ production by CD8+ T cells that infiltrate the tumor and reduces the number of circulating monocytes. | (11,12) |
| 5-FU plus oxaliplatin (OXA) strongly upregulates PD-1 expression on activated CD8 TILs and PD-L1 expression on tumor cells. | (13) | |
| Capecitabine | Potent antitumor activity in advanced gastric cancer when combining anti-PD-1 toripalimab with capecitabine and OXA (CAPOX regimen). | (14) |
| Trifluridine | Potent antitumor efficacy of the triple combination anti-PD-1, OXA and trifluridine. The combo trifluridine + OXA induces immunogenic cell death (ICD) and exerts a profound immunomodulatory action in eliminating type 2 tumor-associated macrophages. | (15) |
| Significant antitumor activity in combination with a PD-1 mAb in a colon cancer model, with infiltrated cytotoxic CD8+ T cells. | (16) | |
| GEM | Combination of GEM and anti-PD-1 mAbs outperformed immunotherapy alone with regard to tumor control and survival in mesothelioma. Objective clinical response with the combo, whereas patients were resistant to the monotherapy, GEM or the anti-PD-1 mAb alone. | (17) |
| In bladder cancer, GEM upregulated PD-L1 expression, alone and prominently in combination with cisplatin. | (18) | |
| GEM/anti-PD-1 combo strongly promoted expression of PD-L1 on tumor epithelium and the generation of antitumoral CD8+ T-cell responses. | (19) |
Cytarabine (cytosine arabinoside, Ara-C) is extensively used since the late 1960s to treat a variety of oncohematological diseases, in particular acute myeloid leukemia (AML), acute lymphocytic leukemia and different types of lymphomas. High-dose Ara-C is a classical induction regimen aimed at eradicating residual leukemic cells, used to treat AML patients. A recent experimental study indicated that Ara-C can be combined with an anti-PD-L1 mAb (plus the CXCR4 inhibitor plerixafor) to reinforce the antileukemic effect via the modulation of leukemic microenvironment, with the elimination of regulatory T cells (Tregs) and both monocytic and granulocytic myeloid-derived suppressor cells (MDSCs) (23). Induction chemotherapy using Ara-C with an anti-PD-(L)1 mAb has also been performed in humans. Recently, the combination of anti-PD-1 nivolumab and the chemotherapeutic drugs idarubicin (IDA) and Ara-C was tested in patients with newly diagnosed AML or high-risk myelodysplastic syndrome (MDS): 43% patients achieved a response and proceeded to allogeneic stem cell transplantation, indicating that the combination is safe and feasible (24). A mechanism has been proposed to explain the benefit of such a combination. The release of ATP molecules, with well-known immune-stimulatory effects, from chemotherapy-treated dying cells contributes to create an immune-suppressive microenvironment in AML. In AML patients treated with the drug combo daunorubicin plus Ara-C, a significant increase of PD-1-expressing Tregs with suppressive phenotype was observed (25). The combination with an anti-PD-(L)1 mAb can limit this detrimental effect of the chemotherapy.
The data on PD-(L)1 are very limited for the purine nucleoside antimetabolites (fludarabine, clofarabine, nelarabine, cladribine), probably because these compounds are toxic for T cells and can deplete Tregs. Fludarabine did not affect total or cell surface expression of PD-L1 in an NSCLC cell line (26). The bone marrow toxicity (myelosuppression) could have a negative impact on T lymphopoiesis and thus on the development of the immunotherapy, but it remains to demonstrate that the detrimental effect affects all T cells (naïve T cells are likely affected, probably not memory T cells).
Folate derivatives
Methotrexate (MTX) is a prototypical antifolate extensively used to treat cancer and certain chronic inflammatory diseases and autoimmune diseases. In particular, MTX is the cornerstone to management across a variety of rheumatic diseases, chiefly rheumatoid arthritis, because of its ability to control inflammation and pain. In oncology, high-dose MTX regimen is regularly used, to treat tumors such as primary central nervous system lymphoma (PCNSL) and acute lymphoblastic leukemia in children.
PD-1 is suggested to play a prognostic role in PCNSL because high PD-1 expression has been associated with inferior overall survival in PCNSL patients (27). These patients respond well to PD-1 blockade with nivolumab (28). Given the anti-inflammatory properties of MTX, a combination with a drug utilized to induce an immunosuppression does not appear as a judicious approach. There is no study combining MTX (or its close antifolate analogue pralatrexate) with a PD-(L)1 mAb to treat cancer. Patients must be off of all systemic immunosuppressive medications at the time of treatment with an anti-PD-1 mAb. In contrast, there are now many studies employing MTX to treat the rheumatological immune-related adverse events caused by PD-1/PD-L1 pathway inhibitors or the anti-CTLA-4 inhibitor ipilimumab (29). MTX can be used to alleviate cancer patients with polymyalgia rheumatica-type conditions and/or peripheral synovitis after treatment with a PD-1 or PD-L1 pathway inhibitor (30).
Pemetrexed (PMX) also belongs to the group of folate antimetabolites and is used to treat various cancers, chiefly pleural mesothelioma and NSCLC. The association of PMX and carboplatin (CARB) with an anti-PD-1 mAb (pembrolizumab) was the first approved chemotherapy–immunotherapy combination for the treatment of NSCLC. Like cisplatin and OXA, PMX displays a strong immunomodulatory capacity. It can increase T-cell infiltration and activation in tumors and induce ICD. It triggers a pronounced immune activation upon combination with an anti-PD-L1 mAb (31). PD-L1 expression was found to be significantly associated with better prognosis for patients with PMX-based treatment, thus providing a solid rationale to combine PMX and anti-PD(L)1 mAbs (32). Although PD-L1 expression appears as a favorable prognosis biomarker for PMX-combined anti-PD-(L)1 immunotherapy, PMX itself seems to induce a decreased expression of membrane PD-L1 on NSCLC cells and the downregulation is amplified when PMX is associated with the phosphodiesterase 5 inhibitor sildenafil (33). Two other studies found no significant change in the expression of PD-L1 on three mesothelioma cell lines exposed to PMX, whereas PD-L1 expression was significantly increased with the vinca alkaloid vinorelbine (NVB) (34,35). However, PMX clearly represents an ‘immuno-friendly’ chemotherapeutic agent that combines very well with PD-(L)1-targeted therapy. Not only PMX promotes the efficacy of the anti-PD(L)1 drug, but the immunotherapy can also restore the tumor sensitivity to PMX-containing chemotherapy (Figure 5). A case of reversal of resistance to chemotherapy following anti-PD-1 immunotherapy in metastatic lung adenocarcinoma has been reported (36). This example illustrates perfectly the importance of optimizing chemotherapy in the era of immunotherapy. Immunotherapy has reinforced the importance of the old-fashion drug PMX as a cornerstone in the management of non-squamous NSCLC (37).
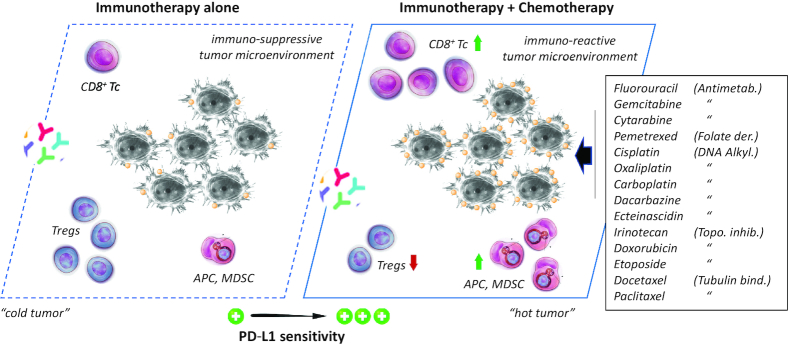
Figure 5.
The combination of immunotherapy and chemotherapy, with the indicated cytotoxic drugs, increases tumor sensitivity to PD-L1-targeted mAbs. Chemotherapy induces upregulation of PD-L1 on cancer cells, facilitates infiltration of CD8+ T cells and NK cells, increases maturation of antigen-presenting cells (APCs) including dendritic cells (DCs) and tumor macrophages, and in some cases promotes activity of MDSCs. Via this mechanism, the drugs restore an immune-reactive tumor microenvironment and significantly promote the sensitivity of the tumor to PD-L1-targeted mAbs.
DNA methyl transferase inhibitors
The two hypomethylating agents azacitidine (5-AZA) and decitabine (DAC) are extensively used in the treatment of patients with MDS, chronic myelomonocytic leukemia or AML in patients who are not eligible for intensive chemotherapy (38). These azanucleosides are also gaining interest as priming agents in the treatment of solid tumors. At low doses, these drugs induce DNA hypomethylation by inhibiting DNMTs, causing reactivation of silenced genes and affecting the processes of cell differentiation and tumor suppression (39). In addition, these drugs exert immunomodulatory activities. They were found to induce an upregulation of PD-1, PD-L1 and PD-L2 (and CTLA4) in patients with MDS and to induce a partial demethylation of PD-1 in leukemia cell lines and human samples (40). Other studies with 5-AZA, DAC and the second-generation prodrug guadecitabine (Table 3) have clearly established that these DNA hypomethylating agents combine well with PD-(L)1-targeting mAbs and may be useful to convert immunotherapy-refractory tumors to immunotherapy-responsive tumors.
Table 3.
Effects of DNMT inhibitors on the PD-(L)1 checkpoint
| Drug | Effects on PD-(L)1 checkpoint | References |
|---|---|---|
| DAC | DAC increases PD-L1 expression in melanoma and malignant pleural mesothelioma cell lines. | (41,42) |
| The mechanism leading to PD-L1 expression involves a DAC-induced hypomethylation of interferon-related genes IRF1/7 to restore PD-L1 level. | (43) | |
| A combination of DAC plus anti-PD-1 camrelizumab gave a better complete remission rate than camrelizumab alone in patients with relapsed/refractory Hodgkin’s lymphoma. | (44) | |
| Low-dose DAC enhances PD-1 blockade in colorectal cancer by remodulating the tumor microenvironment. | (45) | |
| 5-AZA | Synergistic combination of 5-AZA plus a PD-(L)1 inhibitor in hematological malignancies. | (46) |
| 5-AZA upregulates PD-1, PD-L1 and PD-L2 transcripts and protein in patients with AML/MDS; upregulation associated with drug resistance. | (47,48) | |
| The combination of 5-AZA and durvalumab (anti-PD-L1) provided no significant advantages compared to 5-AZA alone in patients with AML or high-risk MDS. | (49) | |
| The combination of 5-AZA and pembrolizumab (anti-PD-1) is safe, feasible and well tolerated by AML patients. | (50) | |
| Guadecitabine (SGI-110) | Guadecitabine (plus ipilimumab) exhibits significant antitumor and immunomodulatory activity in advanced melanoma, increasing the number of CD8+, PD-1+ T cells in tumor. | (51) |
| SGI-110 negatively regulates inhibitory accessory cells in the tumor microenvironment by decreasing PD-1-expressing T cells. | (52) | |
| In a mouse model of breast cancer, guadecitabine potentiated T-cell recruitment and enhanced antitumor immunity. | (53) |
DNA-ALKYLATING DRUGS
Platinum derivatives
The classical platinum derivatives cisplatin, OXA and CARB remain largely used in cancer chemotherapy today. These DNA-alkylating drugs robustly reduce cancer cell proliferation and induce cancer cell death. They are known also to stimulate antitumor immunity, via different mechanisms and pathways, including (i) an increased tumor infiltration of CD8+ T cells; (ii) a drug-induced maturation of APCs that enhances antigen presentation; (iii) a downregulation of Tregs; and (iv) a decrease of MDSCs at the tumor sites (Figure 5). These effects tend to sensitize the tumor to immune checkpoint blockade, in particular to PD-1/PD-L1 therapy. Indeed, cisplatin was found to upregulate PD-L1 expression both in vitro and in vivo (54). Recently, in bladder cancer cell lines, cisplatin was shown to induce PD-L1 (but not PD-L2) expression through a mechanism implicating the ERK1/2 and AP-1 signal transduction pathways (55). The same observation was made previously with H22 hepatoma cells; cisplatin-induced PD-L1 expression is dependent on Erk1/2 phosphorylation (56). OXA was found also to induce ICD in tumor tissues, to enhance T-cell infiltration and activation of DCs. This platinum drug increases both mRNA and protein levels of PD-L1 in tumor cells. In cancer cells, PD-L1 can associate with the catalytic subunit of DNA-dependent protein kinase and this association promotes the activation of ERK or p38 MAPK, a mechanism implicated in the development of chemoresistance. Targeting with an anti-PD-L1 mAb permits to resensitize the cancer cells to chemotherapy (57). In other words, chemoresistance induces PD-L1 expression, which in turn augments the tumor sensitivity to an anti-PD-L1 mAb (58). Other studies with OXA as well as the related drugs CARB and nedaplatin have confirmed that a platinum-based drug and a PD-(L)1 mAb form a mutually helpful tandem working in concert to augment the antitumor activity (Table 4).
Table 4.
Effects of DNA-alkylating platinum drugs on the PD-(L)1 checkpoint
| Drug | Effects on PD-(L)1 checkpoint | References |
|---|---|---|
| OXA | Combination of OXA plus an anti-PD-L1 mAb leads to an efficient inhibition of tumor growth in vivo, more potent than the mAb or drug alone (Lewis lung carcinoma and CT26 colon cancer models). | (59,60) |
| Cisplatin and OXA induce cell surface expression of PD-L1 in head and neck cancer cell lines. Efficient combination with an anti-PD-L1 mAb. | (61) | |
| Experimental studies: additive or synergistic effect of a platinum drug combined with an anti-PD-(L)1 mAb. Effect associated with an early and sustainable enhancement of PD-L1 expression. | (54,62,63) | |
| Clinical studies: decrease in PD-L1 expression in patients with lung cancer who received cisplatin–GEM combination. | (64) | |
| CARB | CARB combines well with an anti-PD-L1 mAb to increase antitumor effector CD4+, CD8+ T cells. Decreases immunosuppressive Tregs and myeloid suppressor cells for the treatment of ovarian cancer. | (65) |
| PD-L1 plays a crucial role in regulating the resistance mechanism to CARB. | (66) | |
| CARB significantly increases cell surface expression of PD-L1 in an ovarian cancer cell line. | (67) | |
| The combination of anti-PD-L1 atezolizumab plus CARB/nab-paclitaxel is more efficient than chemotherapy alone for the treatment of metastatic non-squamous NSCLC. | (68) | |
| Nedaplatin | The PACLI–nedaplatin combo can facilitate the migration of peripheral T cells into the chronically inflamed tumor microenvironment. | (69) |
Oxazaphosphorine derivatives
Cyclophosphamide (CPX) is the leading product in the class of oxazaphosphorine DNA-alkylating agents. It is a prodrug metabolized in the liver by CYP450 enzymes to generate the active metabolites that bind to DNA to form interstrand and intrastrand cross-links lethal for the cells. CPX remains a key element of the regimen R-CHOP [rituximab combined with CPX, doxorubicin (DOX), vincristine and prednisone] or EPOCH-R [etoposide (ETO), prednisone, vincristine, CPX and DOX plus rituximab] used to treat lymphoma.
The therapeutic efficacy of CPX is due, in part, to its ability to stimulate antitumor immune responses, via the implication of the gut microbiota (70). Indeed, CPX is known to induce immunologic cell death and as such it combines well with antibodies and small molecules targeting the PD-1/PD-L1 immune checkpoint (71). It is an efficient cytotoxic drug used to reverse immune evasion by virtue of an active suppression of Treg function, but does not affect effector T cells (72). CPX can not only reduce circulating Tregs and B cells but also increase circulating MDSCs (12). It is a paradox: CTX exhibits marked immune-stimulatory effects and at the same time it can induce some specific suppressor cells that inhibit immune responses (73). Based on this unique capacity of CTX to re-engage T lymphocytes into the effector program (74), different studies now utilize long-term, low-dose (metronomic) oral CTX to transiently deplete Treg cell populations and thus try to (re-)activate tumor-specific immunity (75). A marked synergy has been observed when using CPX and an anti-PD-(L)1 mAb (76). Conversely, an overactivated PD-1/PD-L1 axis is associated with chemotherapeutic resistance of lymphoma cells to the CTX-containing CHOP regimen (77,78). A case study has reported the combined use of the related products ifosfamide and adriamycin to treat a patient with pleural and pancreatic tail metastases. The efficacy was very limited but interestingly PD-L1 expression was detected in the metastasis but not in the primary tumor (a locally invading dermatofibrosarcoma). PD-L1 expression was probably induced by the fibrosarcomatous transformation of the initial tumor, contributing to the metastasis through escape from immune surveillance (79).
Nitrosoureas
Chloroethyl-nitrosoureas are among the oldest DNA-alkylating drugs used to treat cancer. The group includes several drugs, more or less used today in different countries, such as carmustine, nimustine (ACNU), fotemustine, lomustine, ranimustine and semustine (Table 1). In an experimental study combining carmustine [bis-chloroethyl-nitrosourea (BCNU)] with an anti-PD-1 mAb, no synergy and no difference in overall survival compared to anti-PD-1 alone was observed in a mouse model of glioblastoma. However, when BCNU was used in a form allowing local delivery (using BCNU-eluting polymers, as opposed to systemic chemotherapy), the drug could potentiate the antitumor immune response mediated by an anti-PD-1 mAb (80). The BCNU + anti-PD-1 mAb provided a survival benefit as well as increased tumor-infiltrating immune cells and memory cells. The order and method of delivery of the chemotherapeutic agent can thus be important to design the best chemotherapy–immunotherapy combination regimen. The structurally related DNA-alkylating drug ACNU can reduce the production of CCL22 chemokine in tumor-associated macrophages and decreases the expression of PD-L1 on macrophages, although its effects were less pronounced than those observed with dacarbazine (DTIC) or vincristine (81).
We found no specific information about the effects of the DNA-alkylating bacterial natural product streptozotocin (a nitrosourea) on PD-(L)1 expression and activity in cancer. However, in a model of high-dose streptozotocin-induced diabetes in mice bearing a 4T1 murine breast cancer, an increased percentage of PD-1+ NK cells was observed, including in the primary tumor (82). Streptozotocin seems to upregulate both PD-1 and PD-L1 expression, at least in a streptozotocin-induced model of diabetes (83).
Bis-chloroethyl-amines and alkyl sulfonates
The activity of the DNA-alkylating drug melphalan, used to treat myeloma, is directly impacted by PD-L1. PD-1/PD-L1 interaction on myeloma cells inhibits tumor-specific CTLs but also induces cell resistance to melphalan through the PI3K/AKT signaling pathway. Indeed, the PD-1/PD-L1 binding suppresses drug-induced apoptosis through upregulation of the antiapoptotic response via activation of the PI3K/AKT pathway (84). The blockade of the PD-1/PD-L1 interaction could thus prevent the occurrence of the resistance mechanism. A melphalan-containing therapy (including an oncolytic vaccinia virus) was found to augment the efficacy of PD-1 blockade (85). A benefit can thus be expected when combining melphalan and anti-PD-(L)1 therapy. However, like CPX and DOX, melphalan can induce and drive the expansion of inflammatory monocytic myeloid suppressor cells that inhibit immune responses (86). Melphalan is used essentially as a conditioning agent for autologous hemopoietic cell transplantation in patients with multiple myeloma. Its efficacy is reinforced when it is combined with the bifunctional DNA-alkylating drug busulfan. In an experimental study in mice transplanted with allogeneic bone marrow cells (model of acute graft-versus-host disease), the alkyl sulfonate busulfan was also found to enhance the expression of PD-L1 in the target organs (87).
Other DNA-alkylating drugs
The effect of DTIC on immune checkpoint expression and function is not well documented, but the drug can increase CD8+ T-cell infiltration and expression of PD-L1 in melanoma. A case study has shown that DTIC can promote PD-L1 expression but not PD-1 (88). In contrast, in an experimental study DTIC significantly decreased both the PD-L1 mRNA expression in M2 macrophages and the production of chemokine CCL22 in a mouse melanoma model, leading to abrogation of the suppressive function of T-cell proliferation (81). CCL22 is a ligand of the major chemokine receptor CCR4 expressed by Treg cells and Th17 cells. Gliomas augment immunosuppression by recruitment of Tregs into the tumor microenvironment, via selective chemokines such as CCL22. It is possible to modulate this interaction with DNA-alkylating drugs like temozolomide (TMZ) and carmustine that reduce production of CCL22, to promote the action of immune-therapeutic agents (89).
TMZ is a long-established DNA-alkylating drug used to treat various cancers, notably glioblastoma multiforme (GBM). The treatment of glioblastoma cells with IFN-γ to upregulate PD-L1 expression can be reduced upon addition of TMZ via a mechanism implicating activation of the JAK/STAT pathway (90). Therefore, it has been suggested that it is the TMZ-induced downregulation of the target PD-L1 in primary GBM cells that diminishes the efficacy of anti-PD-1/PD-L1 mAb inhibitors (90). However, the situation is not so clear because another recent study reported that an abundant expression of membranous PD-L1 increased upon treatment of GBM cells with TMZ. In this study, TMZ promoted GBM cells’ immune escape (91). Preclinical data gave very encouraging data using PD-L1 blockade in mice with brain tumors (92), but the clinical translation of these data has been somewhat disappointing. Today, the clinical efficacy of the PD-1/PD-L1 checkpoint blockade in human glioblastoma is controversial. Despite the immunogenicity of glioblastoma, immune-mediated eradication of these tumors remains deficient (93).
Little PD-(L)1-related information is available about the old DNA-alkylating drug mitomycin C (MMC), which is still used to treat some cancers. In an orthotopic bladder cancer mouse model, the intravesical treatment with MMC affected the composition of immune-related cells in the tumor microenvironment, inducing in particular a significant reduction in Treg expression. The effect of MMC was comparable to that of adriamycin, suggesting that these two drugs exhibit a similar capacity to reverse the immune suppression caused by cancer cells. In parallel, an elevated level of cytokines (IL-4, IL-17A, G-CSF) was observed in the serum, but they showed no significant effect of PD-L1 tumor expression (94).
Trabectedin (TBT, ET-743) is another ‘modern’ DNA-alkylating drug used in combination with pegylated liposomal DOX to treat patients with relapsed platinum-sensitive ovarian cancer. It is also approved for the treatment of soft tissue sarcoma. This marine-derived anticancer agent inhibits proliferation of cancer cells and modulates the tumor microenvironment via the selective depletion of protumoral monocytes, such as tumor-associated macrophages and MDSCs. An anti-PD-1 mAb was found to produce a synergistic antitumor efficacy when combined with TBT in an ovarian cancer model, through the induction of a systemic tumor-specific immunity with the cytotoxicity intervention of effector CD8+ (+/− CD4+) T cells (95). Treatment with TBT induced a pronounced PD-L1 expression within tumors in vivo (but not in vitro), via the upregulated expression of IFN-γ. The drug provoked a significant accumulation of IFN-γ-producing CD4+ and CD8+ effector T cells, while concomitantly decreasing the number of immunosuppressive Tregs and MDSCs (95). A similar study using immunocompetent models of osteosarcoma also revealed that the combination with a PD-1-blocking antibody significantly increased TBT efficacy in controlling osteosarcoma progression, via an enhancement of the number of tumor-infiltrating T lymphocytes in the tumor microenvironment (96). Another recent study has confirmed that TBT effectively depletes MDSCs and tumor-associated macrophages and increases memory T cells in xenograft and immunocompetent mouse models of chronic lymphocytic leukemia (97). Clearly, this DNA-alkylating agent functions as an immune-modulatory drug, perfectly suited for combination with anti-PD(L)1 mAbs.
DNA-BINDING OR -CLEAVING DRUGS
Bleomycin (BLM) is a redox-active drug that forms complexes with iron and promotes DNA double-strand breaks, via the generation of oxygen radicals. However, the induction of pulmonary fibrosis upon treatment with BLM has strongly limited its use in oncology. Recently, it was reported that PD-1+ CD4+ T cells play a role in pulmonary fibrosis. PD-1 is upregulated on idiopathic pulmonary fibrosis lymphocytes. The administration of BLM to PD-1-null mice or the use of antibody against PD-L1 demonstrated significantly reduced fibrosis compared to controls (98). Human mesenchymal stem cells, which have immunomodulatory capacity, can alleviate pulmonary fibrosis and improve lung function by suppressing BLM-induced human T-cell infiltration. This effect is mediated by the PD-(L)1 pathway (99). Other studies also suggested that the targeting of this pathway can be beneficial in the treatment of pulmonary fibrosis. The related drug peplomycin (PLM) has been shown to decrease immunosuppressive cells and increase cytotoxic T lymphocytes at the tumor sites (100).
TOPOISOMERASE INHIBITORS
Topoisomerase 1 inhibitors (camptothecins)
The topoisomerase 1 inhibitor irinotecan (IRI, also known as CPT-11) is an essential anticancer drug used to treat a variety of solid tumors. This camptothecin derivative stabilizes topoisomerase 1–DNA covalent complexes, leading to DNA strand breaks and cytotoxic damages in cells (101). This drug combines well also with immunotherapy. IRI exerts three major effects on the tumor immune microenvironment: (i) a direct cytotoxic effect on tumor cells; (ii) a modulation of the microenvironment via a reduction in the abundance of some Tregs and MDSCs, leading to production of IFN-γ by tumor-specific CD8 T cells; and (iii) an increase in the expression of both major histocompatibility complex (MHC) class I and PD-L1 on tumor cells and tumor-infiltrating immune cells (102) (Figure 5). These immune-modulating functions lead to a supra-additive effect when IRI was administered with anti-PD-L1-blocking antibodies (103). The upregulation of PD-1/PD-L1 expression by an IRI-containing chemotherapy regimen could be a negative feedback mechanism, but it represents also an indirect sign of chemotherapy-induced antitumor immune response with a favorable outcome for an association with an anti-PD-(L)1 mAb. These properties can be very useful to sensitize tumors to immunotherapy. For example, it is known that SCLC is more immunodeficient than NSCLC and the potential immune escape mechanisms in SCLC likely involve the low expression of PD-L1 and the downregulation of MHC molecules and regulatory chemokines (104). IRI could well improve the clinical efficacy of immunotherapy. The parent natural product camptothecin also induces expression of PD-L1 and immunomodulatory cytokines (105).
The liposomal form of IRI, called Nal-IRI, has received worldwide approval in 2015 in combination with fluorouracil, for the treatment of metastatic pancreatic adenocarcinoma (101). Nal-IRI has been shown also to enhance T-cell-mediated cytotoxicity toward tumor cells in vivo; as such, it provided a significantly enhanced antitumor activity when it was combined with an anti-PD-1 mAb. This combination treatment led to an increased infiltration of functionally cytotoxic CD8+ T cells in the tumor (106). Similarly, an augmented expression of MHC class I in tumor cells and enhanced tumor recognition by T cells have been observed with the antibody–drug conjugate (ADC) trastuzumab deruxtecan (DS-8201a), which combines an HER2-targeting antibody with a camptothecin derivative. Through its topoisomerase 1-targeted payload, this ADC enhances antitumor immunity and upregulates PD-L1 expression. As a result, the combination therapy of DS-8201a and anti-PD-1 antibody was more effective than either monotherapy (107). Collectively, the data suggest that topoisomerase 1 inhibitors have a capacity to remodel the tumor microenvironment to allow for more pervasive cytotoxicity by effector T cells (Figure 5). They represent excellent candidates for combination with immunotherapy.
Topoisomerase 2 inhibitors
Epipodophyllotoxins
ETO (also known as VP-16) remains extensively used in polychemotherapy protocols for the treatment of various types of cancer and metastatic diseases. ETO is a major topoisomerase 2 poison, promoting DNA double-stranded breaks in cells, and these DNA damage signals strongly promote PD-L1 upregulation in cancer cells. The DNA double-stranded break repair machinery is involved in PD-L1 expression, notably through an upregulation of ATM/ATR/Chk1 kinases (108). It is therefore not surprising to observe a potent synergistic action between ETO and a PD-(L)1 checkpoint inhibitor. The efficacy of the drug pair platinum plus ETO, which is the standard of care of advanced SCLC, can be significantly enhanced with the concomitant use of the anti-PD-L1 mAb atezolizumab. Here again, the inhibition of the immune checkpoint has contributed to a net improvement in progression-free survival and overall survival (109,110). Atezolizumab alone, as a monotherapy, has failed to show efficacy in relapsed SCLC patients (111), whereas the addition of atezolizumab to chemotherapy (in this case, CARB + ETO) in the first-line treatment of extensive-stage SCLC was found to prolong significantly the survival compared to chemotherapy alone (112).
This epipodophyllotoxin derivative has the capacity to downregulate PD-L1 expression in different populations of mesenchymal-like TNBC cells, including cancer stem-like cells (CSCs) to which it induces an epithelial-like status. The mesenchymal–epithelial transition induced by ETO is accompanied with a diminished PD-L1 expression on CSCs, thereby impairing the capacity of these cells to generate tumors. In a syngeneic mouse model, the effect of ETO was accompanied with an increased number of tumor-infiltrating cytotoxic CD8+ T cells. ETO exerts profound immune-modulatory effects (113). The capacity of ETO to exert its antitumor immunity through a drug-induced reversal of the epithelial–mesenchymal transition leading to a downregulation of PD-L1 in cancer cells is unique. ETO and atezolizumab cooperate to profoundly suppress PD-L1 expression. The immune-modulatory effects of ETO also explain its synergistic action with other immune checkpoints, such as ipilimumab that targets cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (114). However, in at least two other studies, ETO was found to induce PD-L1 surface expression. ETO promoted PD-L1 surface expression in human breast cancer cells, inhibited cell proliferation and promoted PD-L1-mediated apoptosis of tumor-reactive cytotoxic T cells (115). This effect may ultimately lead to immune resistance. More recently, at a subtoxic low concentration ETO was found to massively upregulate both PD-L1 gene and protein expression in bone marrow stromal cell lines, via a mechanism implicating the secretion of granulocyte macrophage colony-stimulating factor (GM-CSF), which in turn activates the ERK pathway and then PD-L1 upregulation on stromal cells (116). This is entirely consistent with recent observations showing that supplementary GM-CSF to neoadjuvant GEM–cisplatin chemotherapy plus PD-L1 blockade enhances the antitumor effect (18). ETO can be usefully combined with mAbs targeting PD-1 or PD-L1. Immunotherapy may well renew interest in this old natural product. Interestingly, the related product teniposide (VM-26) occasionally used to treat cancers was found recently to induce tumor cell DNA damage and innate immune signaling, including NF-κB activation and STING-dependent type I interferon signaling, both of which contribute to the activation of DCs and subsequent T cells. Teniposide effectively potentiates the antitumor efficacy of anti-PD-1 on different tumor models (117).
Anthracycline antibiotics
DOX (also called adriamycin) was introduced in the clinical practice to treat cancers in the 1960s and ∼60 years later this bacterial anthracycline derivative remains indispensable in cancer therapy. DOX is a pivotal drug to treat solid tumors and hematological cancers. DOX not only exerts cytotoxic effects on proliferating cells but also uses the immune system by activating CD8+ T-cell responses to kill cancer cells (118). It is largely combined with immune-active therapies, in particular with the anti-PD-(L)1 mAbs pembrolizumab and atezolizumab, to treat various cancers. A recent clinical investigation described a major upregulation of immune-related genes involved in PD-(L)1 and T-cell cytotoxicity pathways, after induction treatment with DOX (and cisplatin) in patients with TNBC (119). DOX induces a transcriptional activation of PD-L1 mRNA and the ensuing PD-L1 protein translation that leads to a marked increase in the percentage of PD-L1+ breast cancer cells (120). The same effect was observed with CARB, GEM and PACLI (121).
The capacity of DOX to upregulate cell surface expression of PD-L1 in cancer cells has been well described, although there are also studies indicating a drug-induced downregulation (Table 5). DOX affects PD-(L)1 activity and the reverse is also true. Activation of the PD-1/PD-L1 axis leads to tumor cell resistance to DOX (and docetaxel) in a panel of PD-L1-expressing human and mouse breast and prostate cancer cell lines. The PD-1 axis promotes resistance to DOX and the blockade of either PD-L1 or PD-1 (or by silencing the PD-L1 gene) prevents the development of chemoresistance and progression to metastatic disease (128). Interaction of PD-L1 with PD-1 induced phosphorylation of the signaling molecules AKT and ERK, resulting in the activation of PI3K/AKT and MAPK/ERK pathways and increased expression of MDR1 [P-glycoprotein (P-gp), ABCB1] in cancer cells (129). Because MDR1 is a transporter of many cytotoxic drugs, inhibition of PD-1/PD-L1 can increase the efficacy of different types of chemotherapeutic agents by MDR1/P-gp expression in cancer cells. The fact that PD-1 blockade improves the antitumor efficiency of DOX provides a strong rationale to combine the two drugs and to design nanostructured particles associated with the two products (130).
Table 5.
Effects of anthracycline drugs on the PD-(L)1 checkpoint
| Drug | Effects on PD-(L)1 checkpoint | References |
|---|---|---|
| DOX | DOX promotes expression of PD-L1 in osteosarcoma cell lines and clinical tissue samples. DOX inhibits proliferation of CD8+ T lymphocytes and their enhanced apoptosis. The use of an anti-PD-L1 antibody reversed the effect. | (122) |
| DOX-induced upregulation of PD-L1 observed in bone marrow stromal cells in mice and in lymphoma patients, leading to T-cell exhaustion and impairment of T-cell functions. | (116) | |
| Downregulation of cell surface expression of PD-L1 in vitro and in vivo after DOX treatment. Cellular redistribution. | (123) | |
| Doxil (pegylated liposomal DOX) | Doxil synergizes with anti-PD-1 mAbs, decreasing the percentage of tumor-infiltrating Tregs. In combination with anti-PD-L1, Doxil increases the percentage of tumor-infiltrating CD8+ T cells, in a tumor model. | (124) |
| Epirubicin (EPI) | EPI upregulates PD-L1 expression in subtypes of TNBC cell lines and patient samples. | (125) |
| EPI decreases PD-L1 expression in the TNBC cell line MDA-MB-231 that strongly expresses PD-L1. | (126) | |
| The reduction of the expression of PD-L1 in hepatocellular carcinoma cells upon treatment with the cyclooxygenase-2 inhibitor celecoxib augments the therapeutic efficacy of EPI. | (127) | |
| IDA | IDA can be combined with anti-PD-1 nivolumab to treat patients with newly diagnosed AML or high-risk MDS. | (24) |
TUBULIN INHIBITORS
Tubulin inhibitors such as the taxanes and vinca alkaloids are key components of chemotherapy regimens used in advanced cancer (such as advanced NSCLC and TNBC) and with no doubt these drugs will continue to play important roles in the treatment of aggressive tumors for years to come. Vinca alkaloids suppress microtubule dynamics, resulting in mitotic block and apoptosis. The main vinca is arguably NVB, used for many years in the treatment of advanced NSCLCs and breast cancers, for example. Metronomic oral NVB is an active and well-tolerated chemotherapy still recommended in frail patients with metastatic NSCLC. The drug has the capacity to downregulate PD-L1 expression in lung cancer cell lines (131) and it was also shown to reduce circulating Tregs and circulating NK cells (12). There is no specific PD(L)1-related information about the other vinca drugs vinblastine, vindesine and vincristine, but it is interesting to note that the chemosensitivity of a DLBCL cell line to the vincristine-containing CHOP regimen (CPX, hydroxydaunorubicin/adriamycin, oncovin/vincristine and prednisone) was found to be dependent on the activation of the PD-1/PD-L1 axis. An overactivation of the PD-1/PD-L1 axis, by a pretreatment with recombinant rPD-1, decreased the cytotoxic effects of CHOP regimen on DLBCL cell lines. The attenuation effect was abolished when using jointly rPD-1 and an siRNA against PD-L1. The effect was attributed to CHOP-induced reduction of the phosphorylated PI3K and Akt1, which can be prevented by rPD-1 but aggravated by PD-L1 knockdown. The active status of PD-1/PD-L1 axis is thus a key element that seems to control the sensitivity of DLBCL cells to the conventional CHOP chemotherapeutic regimen (132).
The taxanes docetaxel and PACLI, as well as nab-paclitaxel (albumin-bound PACLI), are widely used as chemotherapy agents, notably to treat advanced NSCLC. However, anti-PD-1 nivolumab is a better therapy for advanced NSCLC in terms of both antitumor efficacy and safety than docetaxel-based chemotherapy. Several studies have shown that anti-PD-1/PD-L1 therapy results in longer overall survival than docetaxel, regardless of PD-L1 expression in patients with advanced NSCLC (133,134). Nevertheless, there are good reasons to combine a taxane with an mAb targeting the PD-(L)1 pathway due to the complementary effects (Table 6). Several beneficial combinations have been reported, such as anti-PD-L1 atezolizumab plus nab-paclitaxel, as first-line therapy for non-squamous NSCLC (68). The data in Table 6 underline the immune-related activity of the taxanes and the microtubule-depolymerizing drug eribulin mesylate used to treat advanced or metastatic breast cancer patients. These different observations strengthen the evidence for an underappreciated role of tubulin/microtubule inhibitors in the expression and function of immune checkpoints and their role in the drug anticancer effects. It will be essential to clarify the immune-modulatory effects of these taxanes, and specifically their influence on the PD-(L)1 pathway.
Table 6.
Immune-related effects of selected tubulin inhibitors
| Drug | Effects on PD-(L)1 checkpoint | References |
|---|---|---|
| PACLI | Promotes tumor antigen presentation through upregulation of tumor antigens or MHC class I molecules. | (135) |
| Increases cell surface PD-L1 protein expression in human ovarian cancer cell lines. | (67) | |
| Antitumor immune activation when combined with PD-L1 blockade. Increases the proportion of tumor-infiltrating effector and cytotoxic CD8+ T cells in a model of TNBC. Upregulation of PD-L1 on tumor-associated macrophages. | (136) | |
| Increases PD-L1 levels via NF-κB signaling, facilitating the accumulation of CD8+ T cells in the tumor site, leading to immune reactivation. The anticancer effect results from a PACLI-increased population of CD8+ and CD4+ TILs, and a decreasing population of PD-1+ TILs at the tumor site. | (137) | |
| Docetaxel | The combination of docetaxel, platinum and fluorouracil increases PD-L1 expression in patients with advanced head and neck squamous cell carcinoma. Enhances PD-L1 positivity on tumor-infiltrating immune cells and the density of CD8+ lymphocytes. | (138) |
| Downregulation of PD-1 expression in T lymphocytes, via an activation of the STAT3 signaling pathway. | (139) | |
| NE-DHA-SBT-1214 (nanoemulsion of a taxoid prodrug) | Increases PD-L1 expression in Panc02 pancreatic tumor cells. The combination with an anti-PD-L1 enhanced CD8+ T-cell infiltration and promoted the therapeutic effect. | (140) |
| Eribulin | Decreased expression of PD-1 and PD-L1 in five responders and increased expression of CD8 in four out of five responders. No expression change in the nonresponder patients. | (141) |
Recent studies confirm that chemotherapeutic drugs capable of microtubule destabilization can have direct effects on DC function, in particular a potent induction of DC maturation that contributes to promote the antitumor immunity (142). At this stage, it is worth to mention the ADC brentuximab vedotin, which combines an anti-CD30 antibody with the highly potent cytotoxic drug dolastatin targeting microtubules. In addition to the direct cytotoxic effect on tumor cells, the released dolastatin entities function as potent inducers of phenotypic and functional DC maturation, promoting antigen uptake and migration of tumor-resident DCs to the tumor-draining lymph nodes. A therapeutic synergy has been observed when combining dolastatin with blockade of the PD-1/PD-L1 signaling (143).
Collectively, our analysis clearly underlines that PD-(L)1 expression is highly sensitive to different types of microtubule inhibitors. These drugs can improve outcomes of cancer immunotherapy, in addition to their direct well-established cytotoxic effects via targeting of the microtubule network. Therefore, they represent good candidate for combination with immunotherapy directed against T-cell inhibitory receptors, such as PD-1 and CTLA-4. Consequently, the design of new microtubule-targeting agents now integrates this immune-modulatory capacity (144).
OTHER CYTOTOXIC DRUGS
A few other cytotoxic compounds approved for the treatment of cancers can be cited, such as the ellipticine derivative elliptinium (Celliptium) and bisantrene, but they are no longer used today. The only other drug that merits mention is ingenol mebutate, which is approved by the Food and Drug Administration for the topical treatment of actinic keratoses. Although there is no specific information on ingenol and PD-(L)1, the drug encapsulated for a systemic use has the capacity to accelerate the expansion of CD4+ and CD8+ T cells and to deplete Tregs (145). Applied topically, ingenol induces profound epidermal cell death, along with a strong infiltrate of CD4+ and CD8+ T cells, neutrophils and macrophages (146).
DISCUSSION
Cytotoxic drugs have been used since the mid-20th century in cancer chemotherapy. They generally target nucleic acids, essentially double-stranded DNA, or the microtubule network, generally with little cell selectivity. In most cases, DNA and microtubules in normal and cancer cells are damaged unselectively by these drugs, although in some cases they can exert tumor-specific killing effects because of downregulation of some DNA repair activities in cancer cells (147). Although most chemotherapeutic agents have detrimental effects on immune homeostasis—causing lymphodepletion notably—they can be extremely useful to augment antitumor immunogenicity and thus sensitize cancer cells to immunotherapy. These drugs can favor the activation and functionality of cytotoxic T lymphocytes and NK cells; they can promote the maturation and activity of DCs; they can induce a depletion of immunosuppressive Tregs; and they can regulate the expression and function of immune checkpoints (Figure 5). In particular, as shown here, cytotoxic drugs can exert a marked effect on the PD-1/PD-L1 pathway and this effect has a direct consequence on the design and efficacy of the immunotherapy. It is also important for the design of combined chemo-/immunotherapy and for the choice of chemotherapy before or after an immunotherapy. The therapeutic decision to combine a PD-(L)1-based immunotherapy with such cytotoxic drug is extremely important to obtain the best clinical response. Paradoxically, given the oldness of the cytotoxic agents, the choice of the cytotoxic regimen to associate with immunotherapy is a key factor today in modern oncology. A given cytotoxic drug or regimen must be chosen not only based on its intrinsic capacity to directly kill cancer and to inhibit the propagation of cancer cells but also based on the propensity to modulate the activity of immune-active cells and to sustain the activity of immunotherapy. More and more, chemotherapy will be also considered as an immunomodulatory therapy. It is therefore not surprising to see hundreds of clinical trials dedicated to the development of novel PD-(L)1-based combination treatment to improve patient outcomes (148).
Immunotherapies targeting the PD-1/PD-L1 checkpoint with mAbs have demonstrated improved survival compared with chemotherapy in several major indications, such as melanoma, renal cancer and TNBC. In melanoma, the clinical response to immunotherapy is impressive; it is not the case in glioblastoma, probably due to an immunosuppressive tumor microenvironment or a limited immuno-infiltration (the notion of non-immunocompetent ‘cold’ tumor). PD(L)1-targeted immunotherapy has fundamentally changed the treatment landscape for patients with lung cancer. However, they are still only effective in a relatively small subset of patients; most patients do not achieve durable long-term responses. The combination of immunotherapy and chemotherapy, as it is the case for the first-line treatment of SCLC for example, is essential. The challenge is to select a chemotherapy susceptible to promote the activity of the combined immuno-active bioproducts, despite the potential immunosuppressive effects of the chemotherapeutic drugs. One caveat of chemotherapy–immunotherapy combinations is the potential for lymphodepletion by chemotherapy. However, it is plausible that systemic chemotherapy exhibits negative immunologic effects, but locally the chemotherapy can potentiate an antitumor immune response. It has been shown that anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in glioblastoma (93).
We have analyzed the available literature for 80 cytotoxic drugs. For half of them, little or no information is available about their effect on PD-(L)1 expression and function (Table 1). A systematic comparative analysis of all these drugs would be useful to guide the design of new drug combinations. Nevertheless, data are available for the different families of cytotoxics, which all tend to upregulate PD-(L)1 expression in cancer cells, across a wide range of malignancies. This is particularly clear for the major, most frequently used, 14 cytotoxic drugs, including IRI, DOX, OXA and PACLI (Figure 5). They all induce a significant upregulation of PD-L1 expressions on cancer cells, but also in bone marrow stromal cells. Their capacity to induce PD-L1 has rarely been compared. Yang et al. found that DOX and ETO were much more potent at inducing PD-L1 gene and protein expression than cisplatin, OXA, vincristine and Ara-C in bone marrow stromal cell lines (116).
The increase of PD-L1 expression in cancer cells upon treatments with these drugs or drug combinations (associating up to seven cytotoxic products such as the BEACOPP regimen) has two major consequences. On the one hand, it contributes to chemoresistance, via an increased activation of ERK in cancer cells overexpressing PD-L1 (57). The upregulation of PD-L1 contributes to the suppression of T-cell function and immune escape. This represents the paradoxical prosurvival effect of chemotherapy that counteracts the primary cytotoxic activity. On the other hand, it sensitizes the cancer cells to anti-PD-(L)1 therapy, transforming a ‘cold’ immune environment into a ‘hot’ microenvironment with an inflammatory profile (149,150). Cytotoxic chemotherapy (and radiotherapy) can thus help to render poorly immunogenic tumors more sensitive to immunotherapy (Figure 5). Chemoradiation, i.e. the combination of chemotherapy and radiotherapy, tends to further upregulate PD-L1 expression in some tumor cells such as melanoma and glioblastoma cells, at least when using the cytotoxic drugs TMZ and DTIC (151). In fact, the different therapeutics considered as genomic destabilizers (i.e. radiations, chemotherapy, epigenetic modifiers) induce upregulation of immune inhibitory ligands on cancer cells, including drug-resistant cancer cells (152). We have focused our analysis on chemotherapy, but radiotherapy can also upregulate PD-L1 and provides useful combination with PD-(L)1-targeted mAbs (153). Ionizing radiation modulates the immune response and synergizes with immunotherapies. Radio-immunotherapy is now exploited to convert immunologically cold into immune-infiltrated hot environments, leading to higher treatment response rates and improved survival (154). Radiation and chemotherapy both represent the immunostimulatory process that causes ICD, inflammatory reactions and recruitment of T cells to the tumor microenvironment. Of course, there are also a huge number of combinations of immunotherapy and targeted therapy in multiple cancer indications.
Most cytotoxic drugs, notably those that induce DNA damages or perturb the microtubule network and mitosis, promote an immune response and an upregulation of PD-L1, independently of the type of DNA or tubulin damage. In fact, these drugs activate a similar sequence of events (Figure 6). Chemotherapeutic drugs induce cancer cell death via a direct action on cancer cells (promoting different types of cell death) and via ICD, which drives the release of damage-associated molecular pattern molecules (DAMPs). DAMPs stimulate the immune system to initiate the immune response via the activation of APCs and in particular DCs functionally maturated by NK cells. A variety of DAMPs have been identified (such as calreticulin, HMGB1, HSP70, ATP and others). Cytotoxics vary in their propensity to emit DAMPs and to trigger ICD (155). The messengers, notably the released cytosolic DNA fragments, are sensed via cyclic GMP-AMP synthase (cGAS) that activates STING (stimulator of interferon genes) and then induces transcription of type I interferon genes via a cascade of effectors (Figure 6). DNA damages induced by drugs (or radiations) can cause leakage into the cytosol of DNA fragments that are sensed by the STING pathway leading to the activation of innate and adaptive immune responses (156,157). Activation of the cGAS/STING pathway also promotes transcription of different chemokines (such as CXCL10 and CCL5) and maturation of DCs, leading to the stimulation of CD4+ and CD8+ lymphocyte migration in tumor environment, to trigger the antitumor immune response (158). PD-L1 upregulation by radiotherapy also implicates the DNA damage signaling pathway, the cGAS–STING pathway and IFN-γ signaling (159), although in some cases STING can also be activated in response to DNA damages independently of cGAS. A recent study indicated that antimitotic drugs, like PACLI, also trigger cGAS/STING-dependent apoptotic effects and propagate apoptotic priming across cancer cells through cytosolic DNA sensing pathway-dependent extracellular signals (160). Most cytotoxic drugs are considered as ICD inducers and possess an immune-regulatory function via this mechanism (161,162). Importantly, the drug-induced activation of the cGAS/STING pathway is connected to the enhanced expression of PD-L1 on cancer cells. Indeed, a drug-induced DNA damage-activated expression of PD-L1 in a STING-dependent manner has been reported in breast cancer (163). A link between STING activation and PD-L1 upregulation has been established in different studies (164–166). Clearly drug- or radiotherapy-induced DNA damages are implicated in the regulation of PD-L1 expression, via the cGAS/STING-dependent pathway (as well as the ATM/ATR/Chk1 kinase activity pathway) (167). DNA damage response proteins, such as PARP and Chk1, also contribute to increase expression of PD-L1 (168).
Figure 6.
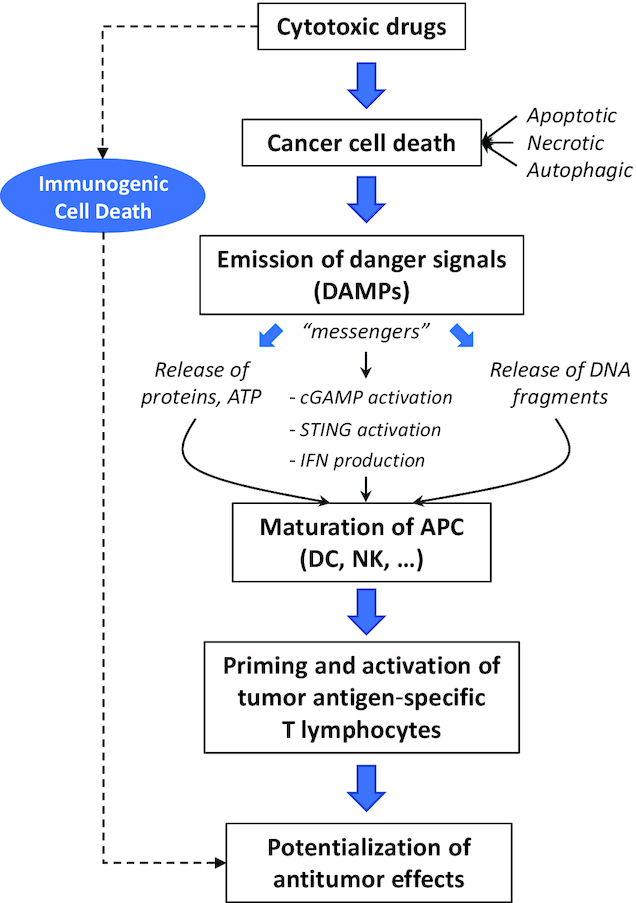
Chemotherapy-induced cancer cell death proceeds via a direct action of cytotoxic drugs on cancer cells (inducing different types of cell death) and via ICD. ICD drives the release of DAMPs that potentiate drug activity. DAMPs stimulate the immune system via the activation of APCs and in particular DCs functionally maturated by NK cells. A variety of DAMPs have been identified (calreticulin, HMGB1, HSP70, ATP and others) depending on the drug mechanism of action (155). DAMP messengers, notably the released cytosolic DNA fragments issued from tumor cells, are sensed via cGAS that activates STING and then induces transcription of type I interferon genes. Activation of the cGAS/STING pathway also promotes transcription of different chemokines (such as CXCL10 and CCL5) that stimulate CD4+ and CD8+ lymphocyte migration in tumor environment, to trigger the antitumor immune response. As such, ICD reinforces the antitumor action of the cytotoxic drugs.
As illustrated in Figure 5, chemotherapy can augment antitumor immunogenicity through different complementary pathways: (i) via the activation and functionality of cytotoxic T lymphocytes and NK cells; (ii) via an enhancement of the activity and maturation of DCs; (iii) via a depletion of immunosuppressive Tregs; and (iv) via a drug-induced expansion of MDSCs via inflammatory mediators, which then suppress T-cell activation. This MDSC-dependent T-cell suppressive activity is dependent on PD-1. Notably, CPX, DOX and melphalan can induce such immunosuppressive monocytic myeloid cells that contribute to tumor evasion and relapse (73,86). The combination with a PD-(L)1 blocker can abrogate the drug-induced immunosuppression driven by these MDSCs. For this reason, there is also a good rationale to combine conventional chemotherapy and PD-(L)1-based immunotherapy. The upregulation of PD-L1 induced by chemotherapeutic drugs is a central event in these processes that strongly contribute to sensitize cancer cells to PD-L1-targeted immunotherapy. It is worth noting that the effect of chemotherapy on MDSCs seems to vary significantly from one drug to another: CPX, DOX, melphalan and PACLI activate MDSCs, whereas OXA, ecteinascidin and IRI neutralize MDSCs (12,73,102,169).
It is not surprising to observe that most cytotoxic drugs targeting DNA, either DNA-alkylating drugs or those inducing indirect DNA damages, combine well with anti-PD-(L)1 therapy, independently of their effect on PD-L1 expression in cancer cells. DNA damages can play a significant role in the immune activation. In particular, oxidative DNA damages upregulate PD-L1 expression in cancer cells, via the DAMP/STING mechanism mentioned earlier (170). Immune activation by DNA damage can even predict response to chemotherapy in some cancers, such as esophageal adenocarcinoma. Moreover, PD-L1 expression is positively related to γH2AX expression (a sensor of double-stranded DNA damages) in some cancers such as lung squamous cell carcinoma (171).
This information is also important to consider in cases of cytotoxic chemotherapy after PD-(L)1-based immunotherapy. In general, a new treatment is given to progressive patients who show a suboptimal response to anti-PD-1 therapy and in some cases cytotoxic chemotherapy is the only alternative treatment available. Interestingly, it has been observed that these patients can experience new objective response after failure to respond to anti-PD-1 monotherapy when the chemotherapy is re-introduced. This is the case for patients with relapsed and refractory Hodgkin’s lymphoma for whom the anti-PD-1 therapy, although not sufficiently potent, seems to resensitize the patient response to chemotherapy (172). Similarly, it was observed that anti-PD-1/PD-L1 inhibitors could make advanced NSCLC tumors more vulnerable to subsequent chemotherapy (173). There are converging data supporting the fact that chemotherapy received after anti-PD(L)1 therapy has regained activity. In gastrointestinal cancers, it has also been postulated that the sequencing of immune checkpoint inhibitors prior to chemotherapy can lead to an immunomodulatory effect with potential improvement in response rates (174). Therefore, not only immunotherapy can re-educate immunosuppressive cells, but it can also resensitize cancer cells to chemotherapy.
In summary, cytotoxic drugs represent useful combination partners for PD-(L)1 immune checkpoint inhibitors and the design of novel drug combinations is essential to augment the success of combined chemo-/immunotherapy. Knowledge gained in this area will aid in the design of more efficient treatments. Given the large diversity of cytotoxic drugs and targeted small molecules, the possibilities of combinations are extremely diversified, as attested by the vast number of ongoing combination trials. We are currently witnessing a revolution in cancer therapy with the advent of immunotherapy targeting PD-(L)1 and other immune checkpoints. To gain the best benefit of these new immuno-drugs, we need to usher in a new conception of the role of cytotoxic chemotherapy in treatment: away from purely cytotoxic molecules that target highly proliferating cells and toward immunomodulators that concur to cripple cancer cells durably. In the near future, it is likely that cytotoxic drugs will continue to play a major role in anticancer therapies, in particular to augment the vulnerability of cancer cells to PD-(L)1 blockade.
Contributor Information
Christian Bailly, OncoWitan, Wasquehal, 59290 Lille, France.
Xavier Thuru, Centre de Recherche Jean-Pierre Aubert, INSERM, University of Lille, UMR-S 1172, CHU Lille, 59045 Lille, France.
Bruno Quesnel, Centre de Recherche Jean-Pierre Aubert, INSERM, University of Lille, UMR-S 1172, CHU Lille, 59045 Lille, France.
FUNDING
No funding.
Conflict of interest statement. The authors declare no conflict of interest. OncoWitan provides consulting services for pharmacology research and development projects. There is no financial interest that could be affected by the article.
REFERENCES
- 1. Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016; 79:629–661. [DOI] [PubMed] [Google Scholar]
- 2. Akinleye A., Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 2019; 12:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiao P., Geng Q., Jin P., Su G., Teng H., Dong J., Yan B. Small molecules as PD-1/PD-L1 pathway modulators for cancer immunotherapy. Curr. Pharm. Des. 2018; 24:4911–4920. [DOI] [PubMed] [Google Scholar]
- 4. Liu X., Guo C.Y., Tou F.F., Wen X.M., Kuang Y.K., Zhu Q., Hu H. Association of PD-L1 expression status with the efficacy of PD-1/PD-L1 inhibitors and overall survival in solid tumours: a systematic review and meta-analysis. Int. J. Cancer. 2019; doi:10.1002/ijc.32744. [DOI] [PubMed] [Google Scholar]
- 5. Roviello G., Corona S.P., Nesi G., Mini E. Results from a meta-analysis of immune checkpoint inhibitors in first-line renal cancer patients: does PD-L1 matter?. Ther. Adv. Med. Oncol. 2019; 11:doi:10.1177/1758835919861905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hao D., Wang G., Yang W., Gong J., Li X., Xiao M., He L., Wang L., Li X., Di L. Reactive versus constitutive: reconcile the controversial results about the prognostic value of PD-L1 expression in cancer. Int. J. Biol. Sci. 2019; 15:1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kowanetz M., Zou W., Gettinger S.N., Koeppen H., Kockx M., Schmid P., Kadel E.E. 3rd, Wistuba I., Chaft J., Rizvi N.A.et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc. Natl. Acad. Sci. U.S.A. 2018; 115:E10119–E10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berry S., Taube J.M. Innate vs. adaptive: PD-L1-mediated immune resistance by melanoma. Oncoimmunology. 2015; 4:e1029704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu Y., Deng Z., Wang H., Ma W., Zhou C., Zhang S. Repeated cycles of 5-fluorouracil chemotherapy impaired anti-tumor functions of cytotoxic T cells in a CT26 tumor-bearing mouse model. BMC Immunol. 2016; 17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Der Kraak L., Goel G., Ramanan K., Kaltenmeier C., Zhang L., Normolle D.P., Freeman G.J., Tang D., Nason K.S., Davison J.M.et al. 5-Fluorouracil upregulates cell surface B7-H1 (PD-L1) expression in gastrointestinal cancers. J. Immunother. Cancer. 2016; 4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vincent J., Mignot G., Chalmin F., Ladoire S., Bruchard M., Chevriaux A., Martin F., Apetoh L., Rébé C., Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010; 70:3052–3061. [DOI] [PubMed] [Google Scholar]
- 12. Orecchioni S., Talarico G., Labanca V., Calleri A., Mancuso P., Bertolini F. Vinorelbine, cyclophosphamide and 5-FU effects on the circulating and intratumoural landscape of immune cells improve anti-PD-L1 efficacy in preclinical models of breast cancer and lymphoma. Br. J. Cancer. 2018; 118:1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dosset M., Vargas T.R., Lagrange A., Boidot R., Végran F., Roussey A., Chalmin F., Dondaine L., Paul C., Lauret Marie-Joseph E.et al. PD-1/PD-L1 pathway: an adaptive immune resistance mechanism to immunogenic chemotherapy in colorectal cancer. Oncoimmunology. 2018; 7:e1433981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang F., Wei X.L., Wang F.H., Xu N., Shen L., Dai G.H., Yuan X.L., Chen Y., Yang S.J., Shi J.H.et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann. Oncol. 2019; 30:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Limagne E., Thibaudin M., Nuttin L., Spill A., Dérangère V., Fumet J.D., Amellal N., Peranzoni E., Cattan V., Ghiringhelli F. Trifluridine/tipiracil plus oxaliplatin improves PD-1 blockade in colorectal cancer by inducing immunogenic cell death and depleting macrophages. Cancer Immunol. Res. 2019; 7:1958–1969. [DOI] [PubMed] [Google Scholar]
- 16. Suzuki N., Tsukihara H., Nakagawa F., Kobunai T., Takechi T. Synergistic anticancer activity of a novel oral chemotherapeutic agent containing trifluridine and tipiracil in combination with anti-PD-1 blockade in microsatellite stable-type murine colorectal cancer cells. Am. J. Cancer Res. 2017; 7:2032–2040. [PMC free article] [PubMed] [Google Scholar]
- 17. Tallón de Lara P., Cecconi V., Hiltbrunner S., Yagita H., Friess M., Bode B., Opitz I., Vrugt B., Weder W., Stolzmann P.et al. Gemcitabine synergizes with immune checkpoint inhibitors and overcomes resistance in a preclinical model and mesothelioma patients. Clin. Cancer Res. 2018; 24:6345–6354. [DOI] [PubMed] [Google Scholar]
- 18. Miyake M., Hori S., Ohnishi S., Toritsuka M., Fujii T., Shimizu T., Owari T., Morizawa Y., Gotoh D., Itami Y.et al. Supplementary granulocyte macrophage colony-stimulating factor to chemotherapy and programmed death-ligand 1 blockade decreases local recurrence after surgery in bladder cancer. Cancer Sci. 2019; 110:3315–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brooks J., Fleischmann-Mundt B., Woller N., Niemann J., Ribback S., Peters K., Demir I.E., Armbrecht N., Ceyhan G.O., Manns M.P.et al. Perioperative, spatiotemporally coordinated activation of T and NK cells prevents recurrence of pancreatic cancer. Cancer Res. 2018; 78:475–488. [DOI] [PubMed] [Google Scholar]
- 20. Doi T., Ishikawa T., Okayama T., Oka K., Mizushima K., Yasuda T., Sakamoto N., Katada K., Kamada K., Uchiyama K.et al. The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines. Oncol. Rep. 2017; 37:1545–1554. [DOI] [PubMed] [Google Scholar]
- 21. Chang L.S., Yan W.L., Chang Y.W., Yeh Y.C., Chen H.W., Leng C.H., Liu S.J. Gemcitabine enhances antitumor efficacy of recombinant lipoimmunogen-based immunotherapy. Oncoimmunology. 2015; 5:e1095433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sen T., Della Corte C.M., Milutinovic S., Cardnell R.J., Diao L., Ramkumar K., Gay C.M., Stewart C.A., Fan Y., Shen L.et al. Combination treatment of the oral CHK1 inhibitor, SRA737 and low dose gemcitabine, enhances the effect of PD-L1 blockade by modulating the immune microenvironment in small cell lung cancer. J. Thorac. Oncol. 2019; 14:2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hwang H.S., Han A.R., Lee J.Y., Park G.S., Min W.S., Kim H.J. Enhanced anti-leukemic effects through induction of immunomodulating microenvironment by blocking CXCR4 and PD-L1 in an AML mouse model. Immunol. Invest. 2019; 48:96–105. [DOI] [PubMed] [Google Scholar]
- 24. Ravandi F., Assi R., Daver N., Benton C.B., Kadia T., Thompson P.A., Borthakur G., Alvarado Y., Jabbour E.J., Konopleva M.et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a single-arm, phase 2 study. Lancet Haematol. 2019; 6:e480–e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lecciso M., Ocadlikova D., Sangaletti S., Trabanelli S., De Marchi E., Orioli E., Pegoraro A., Portararo P., Jandus C., Bontadini A.et al. ATP release from chemotherapy-treated dying leukemia cells elicits an immune suppressive effect by increasing regulatory T cells and tolerogenic dendritic cells. Front. Immunol. 2017; 8:1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ikeda S., Okamoto T., Okano S., Umemoto Y., Tagawa T., Morodomi Y., Kohno M., Shimamatsu S., Kitahara H., Suzuki Y., Fujishita T., Maehara Y. PD-L1 is upregulated by simultaneous amplification of the PD-L1 and JAK2 genes in non-small cell lung cancer. J. Thorac. Oncol. 2016; 11:62–71. [DOI] [PubMed] [Google Scholar]
- 27. Cho H., Kim S.H., Kim S.J., Chang J.H., Yang W.I., Suh C.O., Kim Y.R., Jang J.E., Cheong J.W., Min Y.H., Kim J.S. Programmed cell death 1 expression is associated with inferior survival in patients with primary central nervous system lymphoma. Oncotarget. 2017; 8:87317–87328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nayak L., Iwamoto F.M., LaCasce A., Mukundan S., Roemer M.G.M., Chapuy B., Armand P., Rodig S.J., Shipp M.A. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017; 129:3071–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cappelli L.C., Gutierrez A.K., Baer A.N., Albayda J., Manno R.L., Haque U., Lipson E.J., Bleich K.B., Shah A.A., Naidoo J.et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann. Rheum. Dis. 2017; 76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuswanto W.F., MacFarlane L.A., Gedmintas L., Mulloy A., Choueiri T.K., Bermas B.L. Rheumatologic symptoms in oncologic patients on PD-1 inhibitors. Semin. Arthritis Rheum. 2018; 47:907–910. [DOI] [PubMed] [Google Scholar]
- 31. Schaer D.A., Geeganage S., Amaladas N., Lu Z.H., Rasmussen E.R., Sonyi A., Chin D., Capen A., Li Y., Meyer C.M.et al. The folate pathway inhibitor pemetrexed pleiotropically enhances effects of cancer immunotherapy. Clin. Cancer Res. 2019; 25:7175–7188. [DOI] [PubMed] [Google Scholar]
- 32. Zhang P., Bao Z., Xu L., Zhou J., Lu G., Yao Y., Liu R., Gao Q., Shen Y., Zhou J. PD-L1 expression indicates favorable prognosis for advanced lung adenocarcinoma patients treated with pemetrexed. Oncotarget. 2017; 8:66293–66304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Booth L., Roberts J.L., Poklepovic A., Dent P. [Pemetrexed + sildenafil], via autophagy-dependent HDAC downregulation, enhances the immunotherapy response of NSCLC cells. Cancer Biol. Ther. 2017; 18:705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Terra S.B.S.P., Mansfield A.S., Dong H., Peikert T., Roden A.C. Temporal and spatial heterogeneity of programmed cell death 1-ligand 1 expression in malignant mesothelioma. Oncoimmunology. 2017; 6:e1356146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marcq E., Audenaerde J.R.V., Waele J., Jacobs J., Loenhout J.V., Cavents G., Pauwels P., Meerbeeck J.P.V., Smits E.L. Building a bridge between chemotherapy and immunotherapy in malignant pleural mesothelioma: investigating the effect of chemotherapy on immune checkpoint expression. Int. J. Mol. Sci. 2019; 20:E4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu X., Liu H., Luo S. Reversal of resistance to chemotherapy following anti-programmed cell death-1 immunotherapy in metastatic lung adenocarcinoma: a case report. Medicine. 2018; 97:e13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rossi G., Alama A., Genova C., Rijavec E., Tagliamento M., Biello F., Coco S., Dal Bello M.G., Boccardo S., Grossi F. The evolving role of pemetrexed disodium for the treatment of non-small cell lung cancer. Expert Opin. Pharmacother. 2018; 19:1969–1976. [DOI] [PubMed] [Google Scholar]
- 38. Santini V., Ossenkoppele G.J. Hypomethylating agents in the treatment of acute myeloid leukemia: a guide to optimal use. Crit. Rev. Oncol. Hematol. 2019; 140:1–7. [DOI] [PubMed] [Google Scholar]
- 39. Agrawal K., Das V., Vyas P., Hajdúch M. Nucleosidic DNA demethylating epigenetic drugs: a comprehensive review from discovery to clinic. Pharmacol. Ther. 2018; 188:45–79. [DOI] [PubMed] [Google Scholar]
- 40. Yang H., Bueso-Ramos C., DiNardo C., Estecio M.R., Davanlou M., Geng Q.R., Fang Z., Nguyen M., Pierce S., Wei Y.et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014; 28:1280–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chatterjee A., Rodger E.J., Ahn A., Stockwell P.A., Parry M., Motwani J., Gallagher S.J., Shklovskaya E., Tiffen J., Eccles M.R., Hersey P. Marked global DNA hypomethylation is associated with constitutive PD-L1 expression in melanoma. iScience. 2018; 4:312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bensaid D., Blondy T., Deshayes S., Dehame V., Bertrand P., Grégoire M., Errami M., Blanquart C. Assessment of new HDAC inhibitors for immunotherapy of malignant pleural mesothelioma. Clin. Epigenetics. 2018; 10:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lai Q., Wang H., Li A., Xu Y., Tang L., Chen Q., Zhang C., Gao Y., Song J., Du Z. Decitibine improve the efficiency of anti-PD-1 therapy via activating the response to IFN/PD-L1 signal of lung cancer cells. Oncogene. 2018; 37:2302–2312. [DOI] [PubMed] [Google Scholar]
- 44. Nie J., Wang C., Liu Y., Yang Q., Mei Q., Dong L., Li X., Liu J., Ku W., Zhang Y.et al. Addition of low-dose decitabine to anti-PD-1 antibody camrelizumab in relapsed/refractory classical Hodgkin lymphoma. J. Clin. Oncol. 2019; 37:1479–1489. [DOI] [PubMed] [Google Scholar]
- 45. Yu G., Wu Y., Wang W., Xu J., Lv X., Cao X., Wan T. Low-dose decitabine enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by re-modulating the tumor microenvironment. Cell. Mol. Immunol. 2019; 16:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jelinek T., Mihalyova J., Kascak M., Duras J., Hajek R. PD-1/PD-L1 inhibitors in haematological malignancies: update 2017. Immunology. 2017; 152:357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ørskov A.D., Treppendahl M.B., Skovbo A., Holm M.S., Friis L.S., Hokland M., Grønbæk K. Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS/AML patients: a rationale for combined targeting of PD-1 and DNA methylation. Oncotarget. 2015; 6:9612–9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boddu P., Kantarjian H., Garcia-Manero G., Allison J., Sharma P., Daver N. The emerging role of immune checkpoint based approaches in AML and MDS. Leuk. Lymphoma. 2018; 59:790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zeidan A.M., Cavenagh J., Voso M.T., Taussig D., Tormo M., Boss I., Copeland W.B., Gray V.E., Previtali A., O’Connor T.et al. Efficacy and safety of azacitidine (AZA) in combination with the anti-PD-L1 durvalumab (durva) for the front-line treatment of older patients (pts) with acute myeloid leukemia (AML) who are unfit for intensive chemotherapy (IC) and patients with higher-risk myelodysplastic syndromes (HR-MDS): results from a large, international, randomized phase 2 study. 2019; ASH Annual Meeting, Session 616, Abstract 829.
- 50. Gojo I., Stuart R.K., Webster J., Blackford A., Varela J.C., Morrow J., DeZern A.E., Foster M.C., Levis M.J., Coombs C.C.et al. Multi-center phase 2 study of pembroluzimab (Pembro) and azacitidine (AZA) in patients with relapsed/refractory acute myeloid leukemia (AML) and in newly diagnosed (≥65 years) AML patients. 2019; ASH Annual Meeting, Session 316, Abstract 832.
- 51. Di Giacomo A.M., Covre A., Finotello F., Rieder D., Danielli R., Sigalotti L., Giannarelli D., Petiprez F., Lacroix L., Valente M.et al. Guadecitabine plus ipilimumab in unresectable melanoma: the NIBIT-M4 clinical trial. Clin. Cancer Res. 2019; 25:7351–7362. [DOI] [PubMed] [Google Scholar]
- 52. Nahas M.R., Stroopinsky D., Rosenblatt J., Cole L., Pyzer A.R., Anastasiadou E., Sergeeva A., Ephraim A., Washington A., Orr S.et al. Hypomethylating agent alters the immune microenvironment in acute myeloid leukaemia (AML) and enhances the immunogenicity of a dendritic cell/AML vaccine. Br. J. Haematol. 2019; 185:679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luo N., Sugiura A., Balko J.M. Therapeutic potential of DNA methyltransferase inhibitors with immune checkpoint inhibitor therapy in breast cancer. Cell Stress. 2018; 2:69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Grabosch S., Bulatovic M., Zeng F., Ma T., Zhang L., Ross M., Brozick J., Fang Y., Tseng G., Kim E., Gambotto A., Elishaev E.P., Edwards R., Vlad A.M. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene. 2019; 38:2380–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsai T.F., Lin J.F., Lin Y.C., Chou K.Y., Chen H.E., Ho C.Y., Chen P.C., Hwang T.I. Cisplatin contributes to programmed death-ligand 1 expression in bladder cancer through ERK1/2-AP-1 signaling pathway. Biosci. Rep. 2019; 39:doi:10.1042/BSR20190362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Qin X., Liu C., Zhou Y., Wang G. Cisplatin induces programmed death-1-ligand 1(PD-L1) over-expression in hepatoma H22 cells via Erk/MAPK signaling pathway. Cell. Mol. Biol. 2010; 56:1366–1372. [PubMed] [Google Scholar]
- 57. Wu X., Li Y., Liu X., Chen C., Harrington S.M., Cao S., Xie T., Pham T., Mansfield A.S., Yan Y., Kwon E.D., Wang L., Ling K., Dong H. Targeting B7-H1 (PD-L1) sensitizes cancer cells to chemotherapy. Heliyon. 2018; 4:e01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wangpaichitr M., Kandemir H., Li Y.Y., Wu C., Nguyen D., Feun L.G., Kuo M.T., Savaraj N. Relationship of metabolic alterations and PD-L1 expression in cisplatin resistant lung cancer. Cell Dev. Biol. 2017; 6:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sun F., Cui L., Li T., Chen S., Song J., Li D. Oxaliplatin induces immunogenic cells death and enhances therapeutic efficacy of checkpoint inhibitor in a model of murine lung carcinoma. J. Recept. Signal Transduct. Res. 2019; 39:208–214. [DOI] [PubMed] [Google Scholar]
- 60. Golchin S., Alimohammadi R., Rostami Nejad M., Jalali S.A. Synergistic antitumor effect of anti-PD-L1 combined with oxaliplatin on a mouse tumor model. J. Cell. Physiol. 2019; 234:19866–19874. [DOI] [PubMed] [Google Scholar]
- 61. Park S.J., Ye W., Xiao R., Silvin C., Padget M., Hodge J.W., Van Waes C., Schmitt N.C. Cisplatin and oxaliplatin induce similar immunogenic changes in preclinical models of head and neck cancer. Oral Oncol. 2019; 95:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tran L., Allen C.T., Xiao R., Moore E., Davis R., Park S.J., Spielbauer K., Van Waes C., Schmitt N.C. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol. Res. 2017; 5:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fournel L., Wu Z., Stadler N., Damotte D., Lococo F., Boulle G., Ségal-Bendirdjian E., Bobbio A., Icard P., Trédaniel J., Alifano M., Forgez P. Cisplatin increases PD-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett. 2019; 464:5–14. [DOI] [PubMed] [Google Scholar]
- 64. Rojkó L., Reiniger L., Téglási V., Fábián K., Pipek O., Vágvölgyi A., Agócs L., Fillinger J., Kajdácsi Z., Tímár J.et al. Chemotherapy treatment is associated with altered PD-L1 expression in lung cancer patients. J. Cancer Res. Clin. Oncol. 2018; 144:1219–1226. [DOI] [PubMed] [Google Scholar]
- 65. Zhu X., Xu J., Cai H., Lang J. Carboplatin and programmed death-ligand 1 blockade synergistically produce a similar antitumor effect to carboplatin alone in murine ID8 ovarian cancer model. J. Obstet. Gynaecol. Res. 2018; 44:303–311. [DOI] [PubMed] [Google Scholar]
- 66. Zarogoulidis P., Petanidis S., Domvri K., Kioseoglou E., Anestakis D., Freitag L., Zarogoulidis K., Hohenforst-Schmidt W., Eberhardt W. Autophagy inhibition upregulates CD4+ tumor infiltrating lymphocyte expression via miR-155 regulation and TRAIL activation. Mol. Oncol. 2016; 10:1516–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wahba J., Natoli M., Whilding L.M., Parente-Pereira A.C., Jung Y., Zona S., Lam E.W., Smith J.R., Maher J., Ghaem-Maghami S. Chemotherapy-induced apoptosis, autophagy and cell cycle arrest are key drivers of synergy in chemo-immunotherapy of epithelial ovarian cancer. Cancer Immunol. Immunother. 2018; 67:1753–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. West H., McCleod M., Hussein M., Morabito A., Rittmeyer A., Conter H.J., Kopp H.G., Daniel D., McCune S., Mekhail T.et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019; 20:924–937. [DOI] [PubMed] [Google Scholar]
- 69. Zhang C., Palashati H., Tan Q., Ku W., Miao Y., Xiong H., Lu Z. Immediate and substantial evolution of T-cell repertoire in peripheral blood and tumor microenvironment of patients with esophageal squamous cell carcinoma treated with preoperative chemotherapy. Carcinogenesis. 2018; 39:1389–1398. [DOI] [PubMed] [Google Scholar]
- 70. Alexander J.L., Wilson I.D., Teare J., Marchesi J.R., Nicholson J.K., Kinross J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017; 14:356–365. [DOI] [PubMed] [Google Scholar]
- 71. Sasikumar P.G., Ramachandra R.K., Adurthi S., Dhudashiya A.A., Vadlamani S., Vemula K., Vunnum S., Satyam L.K., Samiulla D.S., Subbarao K.et al. A rationally designed peptide antagonist of the PD-1 signaling pathway as an immunomodulatory agent for cancer therapy. Mol. Cancer Ther. 2019; 18:1081–1091. [DOI] [PubMed] [Google Scholar]
- 72. Ghiringhelli F., Menard C., Puig P.E., Ladoire S., Roux S., Martin F., Solary E., Le Cesne A., Zitvogel L., Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007; 56:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ding Z.C., Lu X., Yu M., Lemos H., Huang L., Chandler P., Liu K., Walters M., Krasinski A., Mack M.et al. Immunosuppressive myeloid cells induced by chemotherapy attenuate antitumor CD4+ T-cell responses through the PD-1–PD-L1 axis. Cancer Res. 2014; 74:3441–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hanoteau A., Henin C., Svec D., Bisilliat Donnet C., Denanglaire S., Colau D., Romero P., Leo O., Van den Eynde B., Moser M. Cyclophosphamide treatment regulates the balance of functional/exhausted tumor-specific CD8+ T cells. Oncoimmunology. 2017; 6:e1318234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Merlano M.C., Merlotti A.M., Licitra L., Denaro N., Fea E., Galizia D., Di Maio M., Fruttero C., Curcio P., Vecchio S.et al. Activation of immune responses in patients with relapsed-metastatic head and neck cancer (CONFRONT phase I–II trial): multimodality immunotherapy with avelumab, short-course radiotherapy, and cyclophosphamide. Clin. Transl. Radiat. Oncol. 2018; 12:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Weir G.M., Hrytsenko O., Quinton T., Berinstein N.L., Stanford M.M., Mansour M. Anti-PD-1 increases the clonality and activity of tumor infiltrating antigen specific T cells induced by a potent immune therapy consisting of vaccine and metronomic cyclophosphamide. J. Immunother. Cancer. 2016; 4:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu J., Quan L., Zhang C., Liu A., Tong D., Wang J. Over-activated PD-1/PD-L1 axis facilitates the chemoresistance of diffuse large B-cell lymphoma cells to the CHOP regimen. Oncol. Lett. 2018; 15:3321–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Suzuki R. NK/T cell lymphoma: updates in therapy. Curr. Hematol. Malig. Rep. 2018; 13:7–12. [DOI] [PubMed] [Google Scholar]
- 79. Tsuchihashi K., Kusaba H., Yamada Y., Okumura Y., Shimokawa H., Komoda M., Uchino K., Yoshihiro T., Tsuruta N., Hanamura F.et al. Programmed death-ligand 1 expression is associated with fibrosarcomatous transformation of dermatofibrosarcoma protuberans. Mol. Clin. Oncol. 2017; 6:665–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mathios D., Kim J.E., Mangraviti A., Phallen J., Park C.K., Jackson C.M., Garzon-Muvdi T., Kim E., Theodros D., Polanczyk M.et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci. Transl. Med. 2016; 8:370ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fujimura T., Kakizaki A., Kambayashi Y., Sato Y., Tanita K., Lyu C., Furudate S., Aiba S. Cytotoxic antimelanoma drugs suppress the activation of M2 macrophages. Exp. Dermatol. 2018; 27:64–70. [DOI] [PubMed] [Google Scholar]
- 82. Gajovic N., Jurisevic M., Pantic J., Radosavljevic G., Arsenijevic N., Lukic M.L., Jovanovic I. Attenuation of NK cells facilitates mammary tumor growth in streptozotocin-induced diabetes in mice. Endocr. Relat. Cancer. 2018; 25:493–507. [DOI] [PubMed] [Google Scholar]
- 83. Li T., Ma R., Zhu J.Y., Wang F.S., Huang L., Leng X.S. PD-1/PD-L1 costimulatory pathway-induced mouse islet transplantation immune tolerance. Transplant. Proc. 2015; 47:165–170. [DOI] [PubMed] [Google Scholar]
- 84. Ishibashi M., Tamura H., Sunakawa M., Kondo-Onodera A., Okuyama N., Hamada Y., Moriya K., Choi I., Tamada K., Inokuchi K. Myeloma drug resistance induced by binding of myeloma B7-H1 (PD-L1) to PD-1. Cancer Immunol. Res. 2016; 4:779–788. [DOI] [PubMed] [Google Scholar]
- 85. Smith H.G., Mansfield D., Roulstone V., Kyula-Currie J.N., McLaughlin M., Patel R.R., Bergerhoff K.F., Paget J.T., Dillon M.T., Khan A.et al. PD-1 blockade following isolated limb perfusion with vaccinia virus prevents local and distant relapse of soft-tissue sarcoma. Clin. Cancer Res. 2019; 25:3443–3454. [DOI] [PubMed] [Google Scholar]
- 86. Ding Z.C., Munn D.H., Zhou G. Chemotherapy-induced myeloid suppressor cells and antitumor immunity: the Janus face of chemotherapy in immunomodulation. Oncoimmunology. 2014; 3:e954471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Al-Chaqmaqchi H., Sadeghi B., Abedi-Valugerdi M., Al-Hashmi S., Fares M., Kuiper R., Lundahl J., Hassan M., Moshfegh A. The role of programmed cell death ligand-1 (PD-L1/CD274) in the development of graft versus host disease. PLoS One. 2013; 8:e60367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Funazumi M., Namiki T., Arima Y., Kato K., Nojima K., Tanaka K., Miura K., Yokozeki H. Increased infiltration of CD8+ T cells by dacarbazine in a patient with mucosal penile melanoma refractory to nivolumab. Ann. Dermatol. 2016; 28:486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jordan J.T., Sun W., Hussain S.F., DeAngulo G., Prabhu S.S., Heimberger A.B. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol. Immunother. 2008; 57:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Heynckes S., Daka K., Franco P., Gaebelein A., Frenking J.H., Doria-Medina R., Mader I., Delev D., Schnell O., Heiland D.H. Crosslink between temozolomide and PD-L1 immune-checkpoint inhibition in glioblastoma multiforme. BMC Cancer. 2019; 19:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang S., Yao F., Lu X., Li Q., Su Z., Lee J.H., Wang C., Du L. Temozolomide promotes immune escape of GBM cells via upregulating PD-L1. Am. J. Cancer Res. 2019; 9:1161–1171. [PMC free article] [PubMed] [Google Scholar]
- 92. Dai B., Qi N., Li J., Zhang G. Temozolomide combined with PD-1 antibody therapy for mouse orthotopic glioma model. Biochem. Biophys. Res. Commun. 2018; 501:871–876. [DOI] [PubMed] [Google Scholar]
- 93. Wang X., Guo G., Guan H., Yu Y., Lu J., Yu J. Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. J. Exp. Clin. Cancer Res. 2019; 38:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hori S., Miyake M., Tatsumi Y., Onishi S., Morizawa Y., Nakai Y., Tanaka N., Fujimoto K. Topical and systemic immunoreaction triggered by intravesical chemotherapy in an N-butyl-N-(4-hydroxybutyl) nitrosamine induced bladder cancer mouse model. PLoS One. 2017; 12:e0175494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Guo Z., Wang H., Meng F., Li J., Zhang S. Combined trabectedin and anti-PD1 antibody produces a synergistic antitumor effect in a murine model of ovarian cancer. J. Transl. Med. 2015; 13:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ratti C., Botti L., Cancila V., Galvan S., Torselli I., Garofalo C., Manara M.C., Bongiovanni L., Valenti C.F., Burocchi A.et al. Trabectedin overrides osteosarcoma differentiative block and reprograms the tumor immune environment enabling effective combination with immune checkpoint inhibitors. Clin. Cancer Res. 2017; 23:5149–5161. [DOI] [PubMed] [Google Scholar]
- 97. Banerjee P., Zhang R., Ivan C., Galletti G., Clise-Dwyer K., Barbaglio F., Scarfò L., Aracil M., Klein C., Wierda W.et al. Trabectedin reveals a strategy of immunomodulation in chronic lymphocytic leukemia. Cancer Immunol. Res. 2019; 7:2036–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Celada L.J., Kropski J.A., Herazo-Maya J.D., Luo W., Creecy A., Abad A.T., Chioma O.S., Lee G., Hassell N.E., Shaginurova G.I.et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci. Transl. Med. 2018; 10:8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ni K., Liu M., Zheng J., Wen L., Chen Q., Xiang Z., Lam K.T., Liu Y., Chan G.C., Lau Y.L., Tu W. PD-1/PD-L1 pathway mediates the alleviation of pulmonary fibrosis by human mesenchymal stem cells in humanized mice. Am. J. Respir. Cell. Mol. Biol. 2018; 58:684–695. [DOI] [PubMed] [Google Scholar]
- 100. Fujimura T., Kambayashi Y., Furudate S., Kakizaki A., Haga T., Hashimoto A., Aiba S. Immunomodulatory effects of peplomycin on immunosuppressive and cytotoxic cells in the lesional skin of cutaneous squamous cell carcinoma. Dermatology. 2015; 230:250–255. [DOI] [PubMed] [Google Scholar]
- 101. Bailly C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019; 148:104398. [DOI] [PubMed] [Google Scholar]
- 102. Koyama S., Nagatomo I., Kijima T., Kumanogoh A. Selecting suitable chemotherapies for PD-1/PD-L1 blockade to optimize the tumor immune microenvironment. Oncotarget. 2018; 9:32552–32553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Iwai T., Sugimoto M., Wakita D., Yorozu K., Kurasawa M., Yamamoto K. Topoisomerase I inhibitor, irinotecan, depletes regulatory T cells and up-regulates MHC class I and PD-L1 expression, resulting in a supra-additive antitumor effect when combined with anti-PD-L1 antibodies. Oncotarget. 2018; 9:31411–31421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tian Y., Zhai X., Han A., Zhu H., Yu J. Potential immune escape mechanisms underlying the distinct clinical outcome of immune checkpoint blockades in small cell lung cancer. J. Hematol. Oncol. 2019; 12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bedi D., Henderson H.J., Manne U., Samuel T. Camptothecin induces PD-L1 and immunomodulatory cytokines in colon cancer cells. Medicines. 2019; 6:E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. McKenzie J.A., Mbofung R.M., Malu S., Zhang M., Ashkin E., Devi S., Williams L., Tieu T., Peng W., Pradeep S.et al. The effect of topoisomerase i inhibitors on the efficacy of T-cell-based cancer immunotherapy. J. Natl. Cancer Inst. 2018; 110:777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Iwata T.N., Ishii C., Ishida S., Ogitani Y., Wada T., Agatsuma T. A HER2-targeting antibody–drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol. Cancer Ther. 2018; 17:1494–1503. [DOI] [PubMed] [Google Scholar]
- 108. Sato H., Niimi A., Yasuhara T., Permata T.B.M., Hagiwara Y., Isono M., Nuryadi E., Sekine R., Oike T., Kakoti S.et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017; 8:1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schulze A.B., Evers G., Kerkhoff A., Mohr M., Schliemann C., Berdel W.E., Schmidt L.H. Future options of molecular-targeted therapy in small cell lung cancer. Cancers. 2019; 11:E690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Armstrong S.A., Liu S.V. Immune checkpoint inhibitors in small cell lung cancer: a partially realized potential. Adv. Ther. 2019; 36:1826–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pujol J.L., Greillier L., Audigier-Valette C., Moro-Sibilot D., Uwer L., Hureaux J., Guisier F., Carmier D., Madelaine J., Otto J.et al. A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. J. Thorac. Oncol. 2019; 14:903–913. [DOI] [PubMed] [Google Scholar]
- 112. Horn L., Mansfield A.S., Szczęsna A., Havel L., Krzakowski M., Hochmair M.J., Huemer F., Losonczy G., Johnson M.L., Nishio M.et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018; 379:2220–2229. [DOI] [PubMed] [Google Scholar]
- 113. Hsu J.M., Xia W., Hsu Y.H., Chan L.C., Yu W.H., Cha J.H., Chen C.T., Liao H.W., Kuo C.W., Khoo K.H.et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat. Commun. 2018; 9:1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Arriola E., Wheater M., Galea I., Cross N., Maishman T., Hamid D., Stanton L., Cave J., Geldart T., Mulatero C.et al. Outcome and biomarker analysis from a multicenter phase 2 study of ipilimumab in combination with carboplatin and etoposide as first-line therapy for extensive-stage SCLC. J. Thorac. Oncol. 2016; 11:1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang P., Su D.M., Liang M., Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol. Immunol. 2008; 45:1470–1476. [DOI] [PubMed] [Google Scholar]
- 116. Yang M., Liu P., Wang K., Glorieux C., Hu Y., Wen S., Jiang W., Huang P. Chemotherapy induces tumor immune evasion by upregulation of programmed cell death ligand 1 expression in bone marrow stromal cells. Mol. Oncol. 2017; 11:358–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wang Z., Chen J., Hu J., Zhang H., Xu F., He W., Wang X., Li M., Lu W., Zeng G.et al. cGAS/STING axis mediates a topoisomerase II inhibitor-induced tumor immunogenicity. J. Clin. Invest. 2019; 130:4850–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Stagg J., Allard B. Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther. Adv. Med. Oncol. 2013; 5:169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Voorwerk L., Slagter M., Horlings H.M., Sikorska K., van de Vijver K.K., de Maaker M., Nederlof I., Kluin R.J.C., Warren S., Ong S.et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat. Med. 2019; 25:920–928. [DOI] [PubMed] [Google Scholar]
- 120. Gilad Y., Eliaz Y., Yu Y., Han S.J., O’Malley B.W., Lonard D.M. Drug-induced PD-L1 expression and cell stress response in breast cancer cells can be balanced by drug combination. Sci. Rep. 2019; 9:15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Samanta D., Park Y., Ni X., Li H., Zahnow C.A., Gabrielson E., Pan F., Semenza G.L. Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 2018; 115:E1239–E1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang J., Hu C., Wang J., Shen Y., Bao Q., He F., Wang H., Gong L., Liu Z., Hu F.et al. Checkpoint blockade in combination with doxorubicin augments tumor cell apoptosis in osteosarcoma. J. Immunother. 2019; 42:321–330. [DOI] [PubMed] [Google Scholar]
- 123. Ghebeh H., Lehe C., Barhoush E., Al-Romaih K., Tulbah A., Al-Alwan M., Hendrayani S.F., Manogaran P., Alaiya A., Al-Tweigeri T.et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010; 12:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rios-Doria J., Durham N., Wetzel L., Rothstein R., Chesebrough J., Holoweckyj N., Zhao W., Leow C.C., Hollingsworth R. Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia. 2015; 17:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yoon H.K., Kim T.H., Park S., Jung H., Quan X., Park S.J., Han J., Lee A. Effect of anthracycline and taxane on the expression of programmed cell death ligand-1 and galectin-9 in triple-negative breast cancer. Pathol. Res. Pract. 2018; 214:1626–1631. [DOI] [PubMed] [Google Scholar]
- 126. Rom-Jurek E.M., Kirchhammer N., Ugocsai P., Ortmann O., Wege A.K., Brockhoff G. Regulation of programmed death ligand 1 (PD-L1) expression in breast cancer cell lines in vitro and in immunodeficient and humanized tumor mice. Int. J. Mol. Sci. 2018; 19:E563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chu T.H., Chan H.H., Hu T.H., Wang E.M., Ma Y.L., Huang S.C., Wu J.C., Chang Y.C., Weng W.T., Wen Z.H.et al. Celecoxib enhances the therapeutic efficacy of epirubicin for Novikoff hepatoma in rats. Cancer Med. 2018; 7:2567–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Black M., Barsoum I.B., Truesdell P., Cotechini T., Macdonald-Goodfellow S.K., Petroff M., Siemens D.R., Koti M., Craig A.W., Graham C.H. Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget. 2016; 7:10557–10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Liu S., Chen S., Yuan W., Wang H., Chen K., Li D., Li D. PD-1/PD-L1 interaction up-regulates MDR1/P-gp expression in breast cancer cells via PI3K/AKT and MAPK/ERK pathways. Oncotarget. 2017; 8:99901–99912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Emami F., Banstola A., Vatanara A., Lee S., Kim J.O., Jeong J.H., Yook S. Doxorubicin and anti-PD-L1 antibody conjugated gold nanoparticles for colorectal cancer photochemotherapy. Mol. Pharm. 2019; 16:1184–1199. [DOI] [PubMed] [Google Scholar]
- 131. Suda K., Rozeboom L., Rivard C.J., Yu H., Ellison K., Melnick M.A.C., Hinz T.K., Chan D., Heasley L.E., Politi K., Mitsudomi T., Hirsch F.R. Therapy-induced E-cadherin downregulation alters expression of programmed death ligand-1 in lung cancer cells. Lung Cancer. 2017; 109:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Resch I., Shariat S.F., Gust K.M. PD-1 and PD-L1 inhibitors after platinum-based chemotherapy or in first-line therapy in cisplatin-ineligible patients: dramatic improvement of prognosis and overall survival after decades of hopelessness in patients with metastatic urothelial cancer. Memo. 2018; 11:43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E.et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015; 373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Xu Z., Yi F., Yu D., Xu J., Wei Y., Zhang W. Nivolumab provides improved effectiveness and safety compared with docetaxel as a second-line treatment for advanced non-small cell lung cancer: a systematic review and meta-analysis. Cancer Med. 2019; 8:629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wan S., Pestka S., Jubin R.G., Lyu Y.L., Tsai Y.C., Liu L.F. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS One. 2012; 7:e32542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Roux C., Jafari S.M., Shinde R., Duncan G., Cescon D.W., Silvester J., Chu M.F., Hodgson K., Berger T., Wakeham A.et al. Reactive oxygen species modulate macrophage immunosuppressive phenotype through the up-regulation of PD-L1. Proc. Natl. Acad. Sci. U.S.A. 2019; 116:4326–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Peng J., Hamanishi J., Matsumura N., Abiko K., Murat K., Baba T., Yamaguchi K., Horikawa N., Hosoe Y., Murphy S.K.et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015; 75:5034–5045. [DOI] [PubMed] [Google Scholar]
- 138. Leduc C., Adam J., Louvet E., Sourisseau T., Dorvault N., Bernard M., Maingot E., Faivre L., Cassin-Kuo M.S., Boissier E., Dessoliers M.C.et al. TPF induction chemotherapy increases PD-L1 expression in tumour cells and immune cells in head and neck squamous cell carcinoma. ESMO Open. 2018; 3:e000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang C., Li F., Li J., Xu Y., Wang L., Zhang Y. Docetaxel down-regulates PD-1 expression via STAT3 in T lymphocytes. Clin. Lung Cancer. 2018; 19:e675–e683. [DOI] [PubMed] [Google Scholar]
- 140. Ahmad G., Mackenzie G.G., Egan J., Amiji M.M. DHA-SBT-1214 taxoid nanoemulsion and anti-PD-L1 antibody combination therapy enhances anti-tumor efficacy in a syngeneic pancreatic adenocarcinoma model. Mol. Cancer Ther. 2019; 18:1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Goto W., Kashiwagi S., Asano Y., Takada K., Morisaki T., Fujita H., Takashima T., Ohsawa M., Hirakawa K., Ohira M. Eribulin promotes antitumor immune responses in patients with locally advanced or metastatic breast cancer. Anticancer Res. 2018; 38:2929–2938. [DOI] [PubMed] [Google Scholar]
- 142. Kashyap A.S., Fernandez-Rodriguez L., Zhao Y., Monaco G., Trefny M.P., Yoshida N., Martin K., Sharma A., Olieric N., Shah P.et al. GEF-H1 signaling upon microtubule destabilization is required for dendritic cell activation and specific anti-tumor responses. Cell Rep. 2019; 28:3367–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Müller P., Martin K., Theurich S., Schreiner J., Savic S., Terszowski G., Lardinois D., Heinzelmann-Schwarz V.A., Schlaak M., Kvasnicka H.M.et al. Microtubule-depolymerizing agents used in antibody–drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol. Res. 2014; 2:741–755. [DOI] [PubMed] [Google Scholar]
- 144. Sato-Kaneko F., Wang X., Yao S., Hosoya T., Lao F.S., Messer K., Pu M., Shukla N.M., Cottam H.B., Chan M.et al. Discovery of a novel microtubule targeting agent as an adjuvant for cancer immunotherapy. Biomed. Res. Int. 2018; 2018:8091283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Yu M., Zhao M., Yu R., Chu S., Xu J., Xia M., Wang C. Nanotechnology-mediated immunochemotherapy with ingenol-3-mebutate for systematic anti-tumor effects. J. Control. Release. 2019; 304:242–258. [DOI] [PubMed] [Google Scholar]
- 146. Emmert S., Haenssle H.A., Zibert J.R., Schön M., Hald A., Hansen M.H., Litman T., Schön M.P. Tumor-preferential induction of immune responses and epidermal cell death in actinic keratoses by ingenol mebutate. PLoS One. 2016; 11:e0160096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kaina B., Christmann M. DNA repair in personalized brain cancer therapy with temozolomide and nitrosoureas. DNA Repair. 2019; 78:128–141. [DOI] [PubMed] [Google Scholar]
- 148. Paschen A., Schadendorf D. The era of checkpoint inhibition: lessons learned from melanoma. Recent Results Cancer Res. 2020; 214:169–187. [DOI] [PubMed] [Google Scholar]
- 149. Haanen J.B.A.G. Converting cold into hot tumors by combining immunotherapies. Cell. 2017; 170:1055–1056. [DOI] [PubMed] [Google Scholar]
- 150. van den Bulk J., Verdegaal E.M., de Miranda N.F. Cancer immunotherapy: broadening the scope of targetable tumours. Open Biol. 2018; 8:180037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Derer A., Spiljar M., Bäumler M., Hecht M., Fietkau R., Frey B., Gaipl U.S. Chemoradiation increases PD-L1 expression in certain melanoma and glioblastoma cells. Front. Immunol. 2016; 7:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chacon J.A., Schutsky K., Powell D.J. The impact of chemotherapy, radiation and epigenetic modifiers in cancer cell expression of immune inhibitory and stimulatory molecules and anti-tumor efficacy. Vaccines. 2016; 4:E43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Eckert F., Zwirner K., Boeke S., Thorwarth D., Zips D., Huber S.M. Rationale for combining radiotherapy and immune checkpoint inhibition for patients with hypoxic tumors. Front. Immunol. 2019; 10:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sevenich L. Turning “cold” into “hot” tumors—opportunities and challenges for radio-immunotherapy against primary and metastatic brain cancers. Front. Oncol. 2019; 9:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rivera Vargas T., Apetoh L. Danger signals: chemotherapy enhancers?. Immunol. Rev. 2017; 280:175–193. [DOI] [PubMed] [Google Scholar]
- 156. Li T., Chen Z.J. The cGAS–cGAMP–STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018; 215:1287–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Medler T., Patel J.M., Alice A., Baird J.R., Hu H.M., Gough M.J. Activating the nucleic acid-sensing machinery for anticancer immunity. Int. Rev. Cell. Mol. Biol. 2019; 344:173–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Portella L., Scala S. Ionizing radiation effects on the tumor microenvironment. Semin. Oncol. 2019; 46:254–260. [DOI] [PubMed] [Google Scholar]
- 159. Sato H., Okonogi N., Yoshimoto Y., Tamaki T., Suzuki Y. [Radiotherapy and PD-L1 expression]. Gan To Kagaku Ryoho. 2019; 46:845–849. [PubMed] [Google Scholar]
- 160. Lohard S., Bourgeois N., Maillet L., Gautier F., Fétiveau A., Lasla H., Nguyen F., Vuillier C., Dumont A., Moreau-Aubry A.et al. STING-dependent paracriny shapes apoptotic priming of breast tumors in response to anti-mitotic treatment. Nat. Commun. 2020; 11:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zhou J., Wang G., Chen Y., Wang H., Hua Y., Cai Z. Immunogenic cell death in cancer therapy: present and emerging inducers. J. Cell. Mol. Med. 2019; 23:4854–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Opzoomer J.W., Sosnowska D., Anstee J.E., Spicer J.F., Arnold J.N. Cytotoxic chemotherapy as an immune stimulus: a molecular perspective on turning up the immunological heat on cancer. Front. Immunol. 2019; 10:1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Parkes E.E., Walker S.M., Taggart L.E., McCabe N., Knight L.A., Wilkinson R., McCloskey K.D., Buckley N.E., Savage K.I., Salto-Tellez M.et al. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J. Natl. Cancer Inst. 2017; 109:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Gutiontov S.I., Weichselbaum R.R. STING (or SRC) like an ICB: priming the immune response in pancreatic cancer. Cancer Res. 2019; 79:3815–3817. [DOI] [PubMed] [Google Scholar]
- 165. Cai H., Yan L., Liu N., Xu M., Cai H. IFI16 promotes cervical cancer progression by upregulating PD-L1 in immunomicroenvironment through STING–TBK1–NF-kB pathway. Biomed. Pharmacother. 2019; 123:109790. [DOI] [PubMed] [Google Scholar]
- 166. Grabosch S., Bulatovic M., Zeng F., Ma T., Zhang L., Ross M., Brozick J., Fang Y., Tseng G., Kim E.et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene. 2019; 38:2380–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Shevtsov M., Sato H., Multhoff G., Shibata A. Novel approaches to improve the efficacy of immuno-radiotherapy. Front. Oncol. 2019; 9:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Sen T., Rodriguez B.L., Chen L., Corte C.M.D., Morikawa N., Fujimoto J., Cristea S., Nguyen T., Diao L., Li L.et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019; 9:646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kim N.R., Kim Y.J. Oxaliplatin regulates myeloid-derived suppressor cell-mediated immunosuppression via downregulation of nuclear factor-κB signaling. Cancer Med. 2019; 8:276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Permata T.B.M., Hagiwara Y., Sato H., Yasuhara T., Oike T., Gondhowiardjo S., Held K.D., Nakano T., Shibata A. Base excision repair regulates PD-L1 expression in cancer cells. Oncogene. 2019; 38:4452–4466. [DOI] [PubMed] [Google Scholar]
- 171. Osoegawa A., Hiraishi H., Hashimoto T., Takumi Y., Abe M., Takeuchi H., Miyawaki M., Okamoto T., Sugio K. The positive relationship between γH2AX and PD-L1 expression in lung squamous cell carcinoma. In Vivo. 2018; 32:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Rossi C., Gilhodes J., Maerevoet M., Herbaux C., Morschhauser F., Brice P., Garciaz S., Borel C., Ysebaert L., Obéric L.et al. Efficacy of chemotherapy or chemo-anti-PD-1 combination after failed anti-PD-1 therapy for relapsed and refractory Hodgkin lymphoma: a series from Lysa centers. Am. J. Hematol. 2018; doi:10.1002/ajh.25154. [DOI] [PubMed] [Google Scholar]
- 173. Park S.E., Lee S.H., Ahn J.S., Ahn M.J., Park K., Sun J.M. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J. Thorac. Oncol. 2018; 13:106–111. [DOI] [PubMed] [Google Scholar]
- 174. Alsuwaigh R., Lee J., Chan G., Chee C.E., Choo S.P. Response to targeted therapy or chemotherapy following immunotherapy in patients with gastrointestinal cancers: a case series. J. Immunother. Cancer. 2019; 7:162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]