Abstract
Long before the nature of infection was recognized, or the significance of biofilms in delayed healing was understood, antimicrobial agents were being used in wound care. In the last 70 years, antibiotics have provided an effective means to control wound infection, but the continued emergence of antibiotic-resistant strains and the documented antibiotic tolerance of biofilms has reduced their effectiveness. A range of wound dressings containing an antimicrobial (antibiotic or non-antibiotic compound) has been developed. Whereas standardized methods for determining the efficacy of non-antibiotic antimicrobials in bacterial suspension tests were developed in the early twentieth century, standardized ways of evaluating the efficacy of antimicrobial dressings against microbial suspensions and biofilms are not available. Resistance to non-antibiotic antimicrobials and cross-resistance with antibiotics has been reported, but consensus on breakpoints is absent and surveillance is impossible. Antimicrobial stewardship is therefore in jeopardy. This review highlights these difficulties and in particular the efficacy of current non-antibiotic antimicrobials used in dressings, their efficacy, and the challenges of translating in vitro efficacy data to the efficacy of dressings in patients. This review calls for a unified approach to developing standardized methods of evaluating antimicrobial dressings that will provide an improved basis for practitioners to make informed choices in wound care.
1. Introduction
Knowledge of wound care is derived from carvings on artefacts, ancient papyri, Sanskrit documents, religious texts, scientific works and literature. The earliest evidence found on Mesopotamian clay tablets (approximately 2500 bce) describes three stages in wound care: washing the wound, preparing topical treatments (known as ‘plasters’) and bandaging.1 Ancient civilizations washed wounds with beer (Sumerians), or boiled water, vinegar or wine (Greeks) and used local materials to prepare topical remedies from plants, animal products and minerals (clay and metals), whilst leaves, grasses, wool or linen acted as bandages.2 Consideration of wound care can be dated as far back as ancient Egypt, with the Sumerians, Greeks and Romans making significant contributions.3,4 The development of the chemical industry from the nineteenth century onwards began to provide antimicrobial agents that were employed in treating and preventing infection. Initially chlorine solutions were used in cleaning hospital surfaces during the 1820s and later chlorinated lime was used to disinfect obstetricians’ hands.4 Sodium hypochlorite was first applied to wounds by Labarraque in 1825 and formulated as EUSOL (hypochlorous acid) and Dakin’s solution (sodium hypochlorite with boric acid) in 1915. Hydrogen peroxide was discovered in 1818, but not used as an antiseptic until the late nineteenth century.5
Bark and pitch seeping from oil fields are two natural products that were utilized in ancient wound treatments.2 Fractionation of wood tar and coal tar during the nineteenth century produced many phenolic compounds that became important disinfectants and antiseptics. Creosote was used as a wound dressing by Smith in 1836 and phenol was initially used on wounds in 1860 by Küchmeister.5 Importantly, carbolic acid (phenol and sodium hydroxide) was applied to compound fractures by Lister in 1865, and then used to disinfect surgical instruments and operating theatres as the basis of aseptic surgery. Antiseptic solutions were widely employed in managing wounds until the end of World War II even though Alexander Fleming had demonstrated that they were rapidly inactivated by body fluids, impaired leucocyte activity and failed to permeate all areas of an irregular wound.6 Iodine was first used for treating wounds in France by Lugol, promoted for treating wounds by Davies in 1839 and used throughout the American Civil War. However, the painful nature of iodine, its possible influence on the thyroid function and the possibility of allergic reactions, together with observations of adverse tissue effects of traditional antiseptics in animal models,7,8 further limited their appeal and use declined after this time.
Since the latter half of the twentieth century antiseptic solutions that are better tolerated and have improved delivery mechanisms have been introduced into clinical practice (Table 1). These include povidone iodine (PVP-I), cadexomer iodine, chlorhexidine digluconate (CHG), octenidine dihydrochloride (OCT) and polyhexamethylene biguanide (PHMB). Although an ancient wound remedy, the use of silver in treating wounds was relatively uncommon until silver nitrate was re-introduced in 1964, closely followed by silver sulphadiazine.9 Honey is another ancient wound antiseptic product that lost favour in British hospitals during the 1970s, but the first modern wound care device containing medical grade honey was registered in Australia in 1999 and several types of honey are now included in formularies throughout the world.
Table 1.
Events that have influenced the development of modern antimicrobial wound care
| Intervention | Date of introduction | Location | Use |
|---|---|---|---|
| Wine, vinegar, beer | antiquity | Mesopotamia, Egypt, Greece | wound cleansing |
| Honey | antiquity | Mesopotamia, Egypt, Greece, India, China | in ointments applied to various wounds |
| Metallic silver | circa 420 bce | Persia | storage of potable water |
| Mercuric chloride | Middle Ages | France and Arabic civilizations | various wounds |
| Silver nitrate | eighteenth century | Europe | treatment of ulcers |
| Iodine | 1829 | France | various wounds |
| Chlorinated water and chlorinated lime | 1820s | UK | hospital cleaning |
| 1847 | Austria | antiseptic handwashing | |
| Sodium hypochlorite | 1825 | France | various wounds |
| Creosote (wood) | 1837 | Ireland | dressing venereal ulcers, fistula and nasal septum |
| Phenol | 1860 | Germany | wound antiseptic |
| Carbolic acid | 1865 | UK | treatment of compound fractures |
| Sterile cotton/gauze | 1891 | USA | wound dressing |
| Hydrogen peroxide | 1887 | UK | wound antiseptic |
| Silver foil | 1895 | USA | surgical wound dressing (hernia) |
| Tulle gras (gauze with soft paraffin, balsam of Peru and olive oil) | 1915 | France | non-adherent wound dressing |
| EUSOL | 1915 | UK | wound antiseptic |
| Dakin’s solution | 1915 | UK | wound antiseptic |
| Chlorhexidine digluconate | 1954 | UK | antiseptic hand scrub and irrigating wounds |
| Povidone iodine | 1956 | USA | wound antiseptic |
| Cadexomer iodine | 1980s | Sweden | wound dressing |
| Silver nitrate | 1964 | UK | over-granulating wounds |
| Silver sulfadiazine | 1968 | USA | infection control in burns |
| Polihexanide | 1991 | Switzerland | antiseptic solution |
| Octenidine dihydrochloride | 1988 | Germany | antiseptic solution |
| Medical honey | 1999 | Australia | topical treatment of wounds |
| Reactive oxygen species | 2006 | Belgium and UK | enzyme alginogelsa |
Here, the term antiseptic refers to a non-antibiotic antimicrobial (see section 3).
Note that alginogels are gels rather than dressings.
The development of wound dressings was substantially influenced after the positive effect of a moist environment in promoting rapid healing was established.10 Occlusive and semi-permeable dressings have largely replaced dry gauze dressings and a wide range of wound dressing materials, which include paraffin gauze, polyurethanes, hydrocolloids, hydrogels, alginates and foams, have been developed since the 1980s. Integrating antimicrobial agents into these materials has provided a range of antimicrobial wound dressings.
Although the discovery of antibiotics provided an effective means to treat and prevent wound infection after World War II, the continued emergence of antibiotic resistance has compromised efficacy and the report of a pan-resistant strain of Klebsiella pneumoniae causing a fatal wound infection in 2016 is significant for future wound care.11 With decreased confidence in the effectiveness of antibiotics, the search for novel non-antibiotic antimicrobial strategies has become more important, and the need to prevent infection is more acute.
Unfortunately, bacterial resistance to antibiotics is globally increasing not only in healthcare but also in animals.12 It is recognized that the spread of antibiotic resistance in bacteria must be tackled in the most effective ways possible.13 Antibiotic stewardship combined with infection prevention comprises a collaborative, multidisciplinary approach to optimize the use of antibiotics.14,15 Optimizing the use of biocidal agents has also been proposed as an antimicrobial stewardship initiative to reduce risk of bacterial resistance and cross-resistance to antibiotics.16 As an example, reducing the use of a low concentration chlorhexidine solution (500 mg/L) for dressings on burn wounds may have increased the susceptibility of wound isolates.17
In addition to the antibiotics used in treating infection, effective wound management today relies on non-antibiotic antimicrobial agents employed in hand hygiene, the cleaning and decontamination of environmental surfaces and medical equipment, the decolonization of MDR strains from patients and healthcare practitioners, pre-operative skin disinfection and the appropriate use of antimicrobial dressings. However, this review is about non-antibiotic antimicrobials incorporated into wound dressings only. It aims to provide up-to-date information on their efficacy, their impact on emerging microbial tolerance and their efficacy against wound-associated microbial biofilms. This review also reflects on the appropriateness of test protocols used to measure efficacy and make a product claim. The review focuses on Europe but uses products available in the UK as examples as such products are also available in the European market.
2. Wounds and wound microbiology
2.1 Types of wound
Disrupting the normal anatomical structure and function of the skin, by either deliberate actions (such as surgery) or traumatically from chemical, physical, mechanical and thermal insults, results in a wound. The sustainable integrity of the skin is restored by a complex sequence of events that include control of infection, resolution of inflammation, removal of damaged tissue, angiogenesis, regeneration of functional extracellular tissue matrix, wound contraction, re-epithelialization, differentiation and remodelling. Wounds that complete this sequence in an orderly and timely manner are described as acute, but wounds that fail to do so are known as chronic wounds.18
Although non-healing wounds have been reported since the ancients Greeks, the causes of impaired healing have not been clearly established. During the last decade an insight was gained when wound chronicity was linked to the presence of microbial biofilm: light and scanning electron microscopy was used to observe biofilm in 60% of chronic wounds whereas biofilm was seen in only 6% of acute wounds.19 Biofilms have been detected in chronic leg ulcers,19–21 diabetic foot ulcers,22 pressure ulcers,19 burns,23 malignant wounds24 and surgical wounds.25 Recently, a systematic review and meta-analysis of published data from in vivo studies found the prevalence of biofilm in chronic wounds using microscopical detection methods to be 78.2%.26
2.2 Wound microbiology
Routine testing in pathology laboratories has largely relied on culture to recover potential pathogens from swabs, pus or tissue biopsies in order to determine putative identities and evaluate antibiotic susceptibilities as a guide to informed antimicrobial intervention. Standardized methodology enables international surveillance of antibiotic resistance.
Wounds often support polymicrobial communities.27Staphylococcus aureus is most frequently isolated, with Pseudomonas aeruginosa, Escherichia coli, Enterobacter cloacae, Klebsiella species, Streptococcus species, Enterococcus species and Proteus species also detected.28 Anaerobes have been underestimated;29 the most common species are Peptostreptococcus, Prevotella, Porphyromonas and Bacteroides, with Finegoldia magna and Peptoniphilus asaccharolyticus.28
In chronic wounds, culture-independent methods demonstrate the presence of more bacterial taxa than culture-dependent methods.30–32 Additionally, samples collected from diabetic patients treated with antibiotics in the previous 2 weeks prior to sampling had elevated abundance of Pseudomonas and decreased Streptococcus spp. compared with untreated patients,31 and fungal diversity increased following antibiotic administration.32
The distribution of microbial species in wounds is not uniform. Comparisons of bacterial abundance in chronic venous leg ulcers using qPCR showed that numbers of S. aureus and P. aeruginosa varied at different locations within the same ulcer.33 Next-generation DNA sequencing suggested the presence of diverse polymicrobial communities in 65 diabetic foot ulcers, but visualization with PNA-FISH and confocal laser scanning microscopy found mono-species and multi-species biofilms in the same tissue sections at locations on average 50–70 μm from the wound surface.34
Evidence of biofilm in wounds currently relies on scanning electron microscopy, epifluorescence microscopy or confocal laser scanning microscopy. These techniques are not yet available in pathology laboratories and there are no routine cultural methods to identify the presence of a biofilm in wounds. Clinical indicators suggestive of a biofilm in a wound are (i) failure of appropriate antibiotic therapies; (ii) recalcitrance to appropriate antimicrobial therapies; and (iii) persistent, delayed healing.35 As a result, a biopsy is recommended for laboratory investigation when biofilm is suspected.36
3. Application of non-antibiotic antimicrobials to wound
In this review, the term antibiotic refers to chemotherapeutic antibiotics used for topical or systemic applications. The term antimicrobial refers to both antibiotic and non-antibiotic compounds, the so-called biocidal active substances. Antiseptics refers to biocides used on intact and broken skin and on mucosa. When ‘resistance’ is mentioned in the text this often refers to antimicrobial susceptibility evaluation based on MIC determination.
3.1 Types of dressings and dressing functions
There are numerous dressings commercially available in the EU with varying availability throughout Europe. Table S1 (available as Supplementary data at JAC-AMR Online) shows dressing availability in the UK as an example. Dressings vary in their nature, composition, function, efficacy and role. The choice of the correct dressing will depend on the nature of the wound but also the healing process stage—cleansing, removal of debris, granulation, vascularization epithelialization.37 It is likely different types of dressing will be needed as the wound is progressing. Additional factors in choosing a dressing are patient preference and tolerance, site of the wound and cost. Ideally a dressing should ensure that a wound remain moist (under normal circumstances), free of exogenous materials (e.g. toxic chemicals, fibre materials), at the right temperature and pH, and free of infection.
Antimicrobial dressings are one type of dressing that may be used for a wound with signs of infection. They do not replace the use of systemic chemotherapeutic antibiotics if the infection spreads or becomes systemic but are used to control local wound infection. Antimicrobial dressings can be divided into those that release an antimicrobial into the wound and those that exert their antimicrobial activity following the bacterial adsorption from the wound into the dressing.38,39 The majority of antimicrobial dressings contain either honey or silver and their derivatives (Table S1).
3.2 Efficacy of biocides used in wound dressings
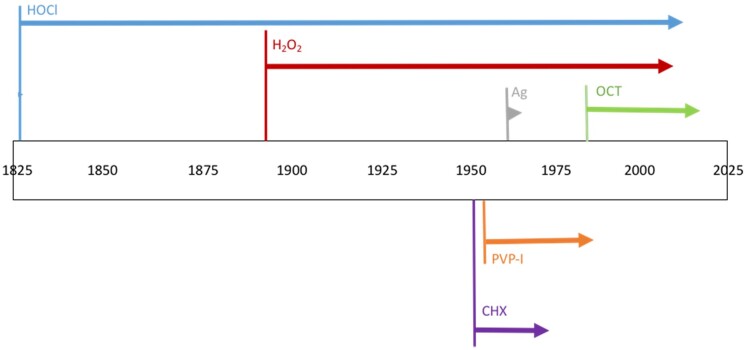
Evidence for the antimicrobial potential of wound dressings comes from laboratory tests with either the active component alone or the entire dressing, or animal models using either explants or live animals. Clinical efficacy is determined with case studies, cohort studies or randomized controlled clinical trials. Decreased biocide susceptibility has now been described for all biocides, although evidence of bacterial decreased susceptibility may have been documented sometime after the use of a biocide in practice (Figure 1).
Figure 1.
Biocide deployment and time for decreases in susceptibility to be documented. Each arrow’s length represents the time between clinical use and reported bacterial non-susceptibility.
Epidemiological resistance is defined as an MIC above a cut-off value [where unimodal MIC or MBC/minimal fungicidal concentration (MFC) distributions were shown, epidemiological cut-offs were determined as concentrations representing ≥99.9% of the bacterial population (MIC99.9, MBC99.9 or MFC99.9)].40 An isolate is defined as clinically resistant when it is not inactivated by an in-use concentration of a biocide, or a biocide concentration that inactivates other strains of that organism, suggesting a high likelihood of therapeutic failure even when there is increased exposure.41 The term ‘tolerance’ describes any elevated MIC above those typical for a species.
CHG
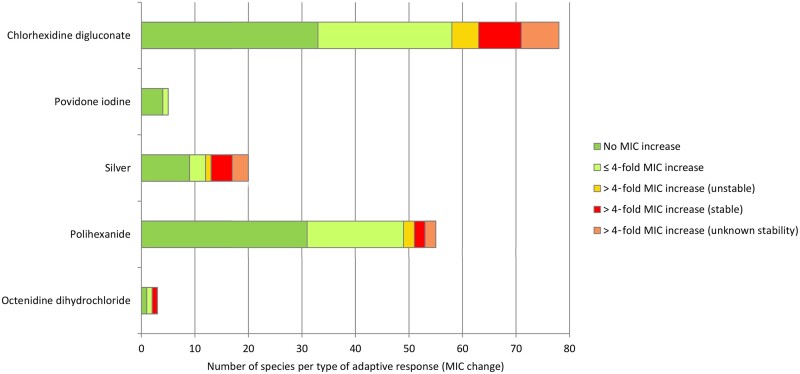
CHG is a cationic biguanide and available as a solution for wound cleansing (e.g. at 50 mg/L) or as an impregnated wound dressing.42 CHG (500 mg/L; 5–15 min exposure) has been shown to be bactericidal in vitro against a wide range of pathogens.43–46 The cut-off values to determine CHG resistance proposed by Morrissey and colleagues40 varies between 8000 and 32000 mg/L depending on bacterial species. Bacterial exposure to CHG has led to >4-fold increase in MIC in vitro (Table 2),47–54 although such decreases in susceptibility may be unstable.41,48,55 Of note is of the possible cross-resistance to antibiotics in isolates with high CHG MIC (Table 2).56–58 Most isolates have so far only shown a weak or no adaptive response to CHG (Figure 2).
Table 2.
Decreased bacterial susceptibility to biocides used in wound dressings
| Examples of bacterial adaptation following exposure to biocides | Mechanisms | Cross-tolerance to antimicrobial agents | References |
|---|---|---|---|
| CHG | |||
|
|
|
50 |
|
56–58 | ||
| PVP-I | |||
|
|
|
66–72 |
| Silver/silver nanoparticles | |||
|
|
|
79 , 83 , 84 , 86 , 88–94 |
| Polihexanide | |||
|
|
|
47 , 48 |
| OCT | |||
|
|
|
112 |
Figure 2.
Number of species with no, ≤4-fold) or >4-fold MIC increase after low-level exposure to non-antibiotic antimicrobials used in wound dressings; (adapted from Kampf).225
The expression of efflux pumps such as the qacA/B gene is a well-documented mechanism resulting in elevated CHG MIC (Table 2).59,60 MRSA strains carrying qacA/B have been reported to have a CHG MIC of 256 mg/L in the presence of 3% BSA.61 The presence of smr (qacC), another efflux pump, was associated with a phenotypically reduced susceptibility to CHG in 88 MRSA isolates, leading to MBCs of 5, 10 and 20 mg/L in 15%, 28% and 50% of isolates, respectively.62 In a Klebsiella oxytoca isolate from a diabetic foot ulcer, the presence of qacE was associated with a reduced susceptibility to CHG (MIC of 30 mg/L).63
Iodophors
Iodophors (PVP-I and cadexomer iodine) facilitate the gradual release of elemental iodine when integrated into wound dressings.64 Typically, 10% PVP-I ointment is impregnated onto a viscose dressing and 0.9% iodine as cadexomer iodine is formulated as a paste, ointment or powder in dressings. Information on bacterial adaptation to PVP-I is limited,65 and data from different studies pre- and post-PVP-I exposure showed a wide MIC range in different bacterial species (Table 2).66–72 All isolates have so far only shown a weak or no adaptive response to PVP-I (Figure 2). Cross-tolerance to other biocides or antibiotics has not been observed.65,73,74
Silver and silver nanoparticles
Silver compounds ionize in the presence of water, bodily fluids and other exudates and antimicrobial action is dependent upon the bioavailability of the silver ion (Ag+).75 There have been many studies on the efficacy of silver ions and silver nanoparticles (AgNPs) against diverse bacterial pathogens.76,77 AgNPs has been reported to have a better activity than Ag+,77 and their efficacy seem to be size dependent suggesting that AgNPs with a diameter of 1–10 nm can have a direct interaction with the bacteria.78
The cut-off value for determining silver resistance in wound bacterial isolates varies from 27 to 512 mg/L in the literature, although resistance is often undefined or poorly evaluated.79–82 Bacterial exposure to Ag+/AgNP has led to significant changes (>16-fold) in MIC, with values reaching >1000 mg/L in E. coli and E. cloacae.83,84 The use of MIC as an indicator of efficacy is controversial, however, as it does not necessarily reflect the concentration of a biocide that can be attained in practice.41,85
Bacterial decreased susceptibility to Ag+/AgNP has been linked to silver resistance genes encoding for a silver binding protein (silE), efflux pump (silA and silP) and a membrane sensor kinase (silS), as well as other efflux pumps (Table 2).79,86–93 The effect of exposure to sublethal silver concentrations depends mainly on the presence or absence of sil genes.81,84,94–97 Upregulation of efflux pumps as well as upregulation of metal oxidoreductases has also been described as a mechanism of silver decreased susceptibility.98 Silver may contribute to the promotion of antibiotic resistance through co-selection, which occurs when resistance genes to both antibiotics and silver are co-located together in the same plasmid leading to the co-selection of the mobile genetic elements that they carry (Table 2).99 The majority of isolates have so far only shown a weak or no adaptive response to silver (Figure 2).
Polihexanide (PHMB)
PHMB is a cationic biguanide polymer. Preparations of PHMB are polydisperse mixtures of polymeric biguanides, with a weighted average number of 12 repeating hexamethylene biguanide units. The heterogeneity of the molecule is increased further by the presence of either amine, or cyanoguanidine or guanidine end-groups in any combination at the terminal positions of each chain.100
At concentrations of 200 mg/L and above, PHMB has been shown to be bactericidal (>5 log10 reduction in viability) within 1 h, although efficacy will decrease with lower contact time.101–106
Increases in MIC following PHMB exposure have been reported in a number of bacterial species.47,48,107,108 A stable increase in MIC has been described in Enterococcus faecalis (8-fold) and S. aureus (6-fold) but the majority of isolates have so far only shown a weak (<4-fold increase in MIC) or no adaptive response to PHMB (Figure 2).47,48
OCT
OCT is a cationic biocide and available in a gel for dressing wounds. OCT (500–1000 mg/L), often in combination with 2% phenoxyethanol, has a broad bactericidal activity in 1 min in suspension tests.44,109–111
Only few published data on the adaptive potential to OCT exist (Figure 2). Low-level exposure to OCT has resulted in stable 32-fold increases in MIC in P. aeruginosa.112 No specific resistance mechanisms or resistance genes associated with a reduced susceptibility to OCT have been described so far, although MFS efflux pump expression has been shown to be elevated (70-fold) in K. pneumoniae after low-level exposure to OCT.113
Honey
Honey is produced by honeybees foraging on blossoms and secretions from plants and insects. Being a natural product, the chemical composition of honey is variable and depends on its biological source and post-harvesting conditions. Honey destined for modern wound care products is known as medical grade honey because it is produced under hygienic conditions from relatively remote regions and is traceable and conforms to the regulatory requirements in specific countries such as Australia, Canada, USA and UK, as well as the EU. It is normally tested for antibacterial activity and contaminants, such as pesticides and antibiotics, and is incorporated into devices sterilized by gamma irradiation.114
Unlike antiseptics, the antibacterial properties of honey are derived from multiple factors. These include high sugar content, low water content, acidity, ability to produce hydrogen peroxide on dilution, insect-derived antimicrobial peptides, phytochemicals and methylglyoxal. Yet the relative contributions of these factors vary between different honeys.115 Antimicrobial components in manuka honey have not been fully characterized.116,117 One key inhibitor is methylglyoxal, of which levels vary for different batches of honey. Evaluating the antimicrobial efficacy of methylglyoxal from published reports may be misleading since its concentration may not be stated on wound devices or for honey samples utilized in laboratory studies. However, levels of antibacterial activity can be assured during the manufacture of devices by blending differing honey samples to achieve a specific endpoint.
The broad spectrum of antimicrobial activity of honey is well documented, with much information on manuka honey.118,119 Repeated subculture of bacterial suspensions in sublethal concentrations of manuka honey demonstrated that decreased susceptibility to manuka honey was transient and resistance did not arise.120,121
3.3 Antibiofilm activity
The importance and occurrence of microbial biofilms in a wound has been detailed above. The efficacy of an antimicrobial dressing should ideally be conducted against bacteria in biofilms. Most of the efficacy data of biocides relevant to dressings comes, however, from the study of planktonic bacteria. Recognizing the importance of microbial biofilms, some studies have investigated the efficacy of biocidal active substances against bacteria in biofilms and their impact on the development and mass reduction of existing biofilms.
CHG
There are conflicting accounts on the efficacy of CHG (500 mg/L) against single-species biofilms. While some studies showed that CHG (500 mg/L) exhibited >4 log10 reduction against bacteria in single species biofilms with a 5 min exposure time,122–124 others were unable to establish any activity (Table 3).125,126 The efficacy of CHG against polymicrobial biofilms seems limited.127–131 Biofilm maturity and bacterial species in polymicrobial communities play a role in decreasing CHG efficacy.68,134–139
Table 3.
Antimicrobial efficacy of biocides used in wound dressings against biofilms
| Examples of efficacy against bacteria in biofilm | Additional effect on biofilm | References |
|---|---|---|
| CHG | ||
|
|
122–126 , 133 |
| Povidone iodine/cadexomer | ||
|
|
68 , 135 , 140–144 |
| Silver/silver nanoparticles | ||
|
|
76 , 146–152 |
| OCT | ||
|
|
110 , 143 , 157–164 |
| Honey | ||
|
|
116 , 165–168 |
PVP-I
PVP-I (1%) was shown to be efficacious (≥5.0 log10 reduction) in single-species biofilms, but its efficacy against mixed-species biofilms is more limited even with long exposure times (Table 3).68,140–142 Additional reported effect was PVP-I ability to reduce biofilm formation in E. faecalis and S. aureus.135 Moderate or even complete biofilm reduction by PVP-I was reported with S. aureus and P. aeruginosa (Table 3).143,144
Silver
The effect of the silver in silver-containing wound dressings against bacteria in biofilms depends on the type of dressing material and structure.145 Several studies reported a low efficacy of Ag+/AgNP against bacteria in biofilms (Table 3). 76,146–152 Silver alone might require a concentration of at least 0.1 mg/L to inhibit polymicrobial biofilm formation at >50% within 24 h.153 A comparison of seven different types of silver-coated dressing showed that there is a large variation in their ability to prevent biofilm formation of P. aeruginosa and Acinetobacter baumannii over 72 h.154
High biofilm biomass amount, high thickness, low surface-to-volume ratio and low roughness coefficient have been shown to compromise biocide efficacy.147 The combination of ionic silver with a metal chelating agent and a surfactant substantially improved the antimicrobial efficacy of ionic silver against biofilm pathogens (MRSA and P. aeruginosa) in a simulated wound biofilm model.155 Similarly, increased efficacy against S. aureus biofilm was reported with the combination of silver, EDTA and benzethonium chloride.156
PHMB
PHMB 0.02% and 0.04% has been shown to have low efficacy (<2 log10 reduction) against bacteria in biofilms.150
OCT
OCT (1000 mg/L) has been shown to produce >6 log10 reduction in bacteria (Actinomyces viscosus, P. aeruginosa and S. aureus) embedded in a biofilm, although such activity was dependent on species and whether the biofilm was polymicrobial or not (Table 3).110,157–164
Honey
Honey has been demonstrated to inhibit the formation of biofilms, as well as disrupting established biofilms of wound pathogens such as Staphylococcus spp., Streptococcus pyogenes, P. aeruginosa, Proteus mirabilis, E. cloacae and A. baumannii.116,165–168 These studies utilized single-species biofilms grown in microtitre plates and the range of minimum biofilm inhibitory concentrations (MBICs) recorded was 120 000–500 000 mg/L, which is less than the quantity of honey normally contained within wound dressings. However, honey is diluted by wound exudate in practice and the concentration of honey achievable within a honey-treated wound over time has not been evaluated. Bioengineered honey was found to be more effective at preventing biofilm formation than two medical grade honeys and five antimicrobial dressings.168
One study investigated the inhibition of wound pathogens by a manuka honey-impregnated dressing using a modified AATCC-TM100 test. Compared with control dressings without honey, >5 log10 reductions after 24 h were reported for S. aureus, K. pneumoniae, P. aeruginosa, E. cloacae, A. baumannii, P. mirabilis and Candida albicans.169 Another study using a chronic wound model showed that most of the commercial wound care products (only one medical grade honey) tested showed limited effects on mature biofilms.170
Bacterial adaptation to honey has been reported in one study, in which P. aeruginosa clinical isolates produced biofilms of increased biomass compared following honey exposure (Table 3).165
The interpretation of biocidal active substances activity against bacteria in biofilms in the wound environment is difficult to ascertain at this time. There are many biofilm models used to measure biocide efficacy (see section 4.3) and as such reported efficacy of a specific biocide varies in the literature (Table 3). Evidence—or lack of evidence—of CHG or PVP-I bactericidal efficacy against bacteria in biofilms depends on the study,122–132,147–152 whilst information on antibiofilm activity of PHMB is scarce.150 Silver and AgNP efficacy depend very much on the presence of organic materials.145,153,154 More information is available about honey, which was shown to have some bactericidal efficacy against bacteria in biofilms in a variety of test models, in diverse studies.116,165–169
3.4. Guidelines on using antimicrobial interventions in wound care
Non-antibiotic antimicrobial interventions play an important role in wound care. For the management of infection in diabetic foot ulcers, pressure ulcers and chronic wounds guidelines for diagnosis and treatment are available.35,171,172 For wound applications, the importance of balancing antimicrobial effectiveness with cytotoxicity,173 and the need to review an unsuccessful intervention after 2 weeks, is recognized.174 However, evidence of clinical efficacy is weak.175–180
Increased tolerance of biofilms to antimicrobials and their involvement in recurring infection has prompted the development of antibiofilm strategies. The benefits of wound debridement followed immediately by antibiotic therapy have been demonstrated181,182 and topical antiseptics have been suggested,35 despite the lack of standardized tests to evaluate antibiofilm effectiveness. Evidence of clinical efficacy of antibiofilm interventions is limited to date. Using culture-independent methodology and microscopic investigation, cadexomer iodine reduced microbial load in chronic non-healing diabetic foot ulcers containing biofilm.183 Similarly, the effect of duration of treatment of cadexomer iodine for diabetic foot ulcers containing biofilm on microbial load and wound healing rates were investigated.184 Further studies of this nature are needed to inform clinical guidance.
4. Measuring the activity of biocidal products/medical devices for wounds
4.1 Factors affecting antimicrobial efficacy
There are many factors affecting the efficacy of biocides.41 These have been well described for most of the active compounds found in antimicrobial dressings. Factors affecting efficacy can be separated into those depending upon the formulation/product, those depending on product usage and those depending on the target microorganisms.41 There are many different types of antimicrobial dressing used for a wide range of applications (Table S1). When considering antimicrobial dressings, biocides can be either an inherent part of the dressing material and not released, or the biocide diffuses from the materials into the wound, regardless of the dressing application. Either way, the available biocide concentration is paramount for activity.85 The impact of organic load (mainly proteinaceous in nature) in the wound or in the exudate, on antimicrobial activity, is an important factor to be considered. Additional factors contributing to a reduction of an effective concentration would be biocide adsorption to surfaces and precipitation. In the case of silver, it has been reported that the maximum attainable concentration of silver in a wound is likely to be around 1 mg/L.185 Above this concentration, it is expected that silver ions would complex with anions forming an ineffective insoluble silver salt.186 Incompatibility of the biocides with materials and excipients may also contribute to a decrease in antimicrobial efficacy. Chlorhexidine, for example, precipitates at concentrations above 0.5% w/v in the presence of inorganic acids and many salts (benzoates, bicarbonates, borates, carbonates, chlorides, citrates, iodides, nitrates, phosphates and sulphates), and incompatibilities have been reported with viscous materials such as sodium alginate, sodium carboxymethylcellulose, starch, tragacanth and hydrogel poly(2-hydroxyethyl methacrylate).187 Skin pH, which is usually around 5188 would also impact somewhat on biocidal efficacy; for example, silver efficacy will increase with alkaline pH. The pH attained in a wound is likely to be different, while microbial growth would also affect pH. Two factors of perhaps less importance are temperature and contact time. Wound temperature is unlikely to decrease dramatically (i.e. by >10°C), while dressings are usually in place for a long period of time (>24 h).
Bacterial susceptibility of different pathogens to specific biocides has been well established with most but not all biocides used in antimicrobial dressings;41 whilst information on silver, CHG, PHMB and PVP-I is available, information with OCT is scarce. Furthermore, a wound is likely to be polymicrobial in nature and the efficacy of a biocide will be reduced against biofilms.41
4.2 Measuring the antimicrobial activity of antimicrobial dressings
The bactericidal efficacy of biocides used in biocidal products is usually measured using defined standard efficacy tests reflecting specific applications. Until recently, in Europe, the efficacy of the biocide formulation alone was tested rather than the finished product.189 It is however clear that measuring the MIC of a biocide is not appropriate.41,85
With the many types of antimicrobial dressings available (Table S1), and the absence of specific standard tests, the main question is how the antimicrobial activity of the dressing should be measured. The efficacy of antimicrobial dressings has been tested in vitro during product development (Table 4), and in vivo using diverse animal models (Table 5).
Table 4.
In vitro protocols used for testing the activity of new dressings
| Antimicrobial | Protocol | Bacterial target | Reference |
|---|---|---|---|
| Chlorhexidine | |||
| chlorhexidine | ASTM E2647-13 | A. baumannii, Enterobacter aerogenes, E. faecalis, E. coli, K. pneumoniae, P. aeruginosa, S. marcescens, S. aureus | 204 |
| Non-standard test | 190 | ||
| CLSI disc diffusion | S. aureus | 204 | |
| CLSI disc diffusion | E. coli (ATCC 25922), A. baumannii (ATCC 19606), P. aeruginosa (ATCC 27853), B. subtilis (ATCC 6633), S. aureus (ATCC 25923), and S. aureus (MRSA) | 190 | |
| Non-standard. Immersing dressing in solution, adding bacterial inoculum for 16 h at 37°C, removing dressing and recovering bacteria from the dressing |
S. aureus (EMRSA-15 and MSSA), P. aeruginosa (ATCC9027 and PA14), K. pneumoniae (ATCC10031), A. baumannii (121J6), E. coli (NCTC10418) and S. epidermidis, C. difficile |
198 | |
| CHG-containing dressing | Zone of inhibition on seeded agar + dressing in broth for up to 24 h at 35°C | S. aureus, B. subtilis, E. coli, P. aeruginosa. | 191 |
| Iodine | |||
| cadexomer iodine | Porcine ex vivo | P. aeruginosa (biofilm) | 207 |
| cadexomer iodine dressing | Shake flask assay: inoculum in the presence of dressing for 1–6 h at 37°C + use of neutralizer | P. aeruginosa ATCC 27312 and ATCC 15442, S. aureus ATCC 6538 | 199 |
| cadexomer iodine | Porcine ex vivo | P. aeruginosa (biofilm) | 207 |
| Silver | |||
| silver sulfadiazine | S. aureus, P. aeruginosa | 205 | |
| silver sulfadiazine 1% | Non-standard ex vivo test on human skin | P. aeruginosa | 208 |
| silver sulfadiazine/ silver nitrate | Zone of inhibition on seeded agar | S. aureus | 192 |
| AgNPs | Zone of inhibition on seeded agar | S. aureus ATCC25923 | 211 |
| silver-based dressings | Bacteria inoculated on hydrogels and recovered after 1 h at 37°C with 90% relative humidity | E. coli 8379, S. aureus 29213, K. pneumoniae 13883, A. baumannii 19606, MRSA USA300, P. aeruginosa PAO1 + carbapenem-resistant, P. aeruginosa, carbapenem-resistant A. baumannii | 218 |
| nano-composite alginate gel discs containing AgNPs | Coated discs in inoculate broth for 24 h at 37°C | S. aureus (ATCC 6538) and MRSA (ATCC 43300), A. baumannii (ATCC 19606) + 13 carbapenem- resistant strains, E. coli (ATCC 10536) and P. aeruginosa (ATCC 9027) + 1 wound isolate | 200 |
| 200 ppm AgNPs | CLSI disc diffusion | E. coli (ATCC 25922), A. baumannii (ATCC 19606), P. aeruginosa (ATCC 27853), B. subtilis (ATCC 6633), S. aureus (ATCC 25923), and S. aureus (MRSA) | 190 |
| calcium alginate–nanocrystalline silver | Porcine ex vivo | P. aeruginosa (biofilm) | 207 |
| cotton gauze–silver sulphate | Porcine ex vivo | P. aeruginosa (biofilm) | 207 |
| hydrocolloid–silver | Porcine ex vivo | P. aeruginosa (biofilm) | 207 |
| polyacrylate–silver chloride | Porcine ex vivo | P. aeruginosa (biofilm) | 207 |
| silver dressings | Prevention of sedimentation biofilm formation measured by crystal violet—not quantitative—1 cm2 dressing added to bacterial suspension—biofilm formation measured by crystal violet | P. aeruginosa, S. aureus, E. coli, A. baumannii | 168 |
| keratin biomaterial containing AgNPs | Lysogeny broth solid plates and shake-flask method. Non-standard | E. coli C600, S. aureus RN4220, B. subtilis YB886 | 193 |
| silver nanocoating | Non-standard. Immersing dressing in solution, adding bacterial inoculum for 16 h at 37°C, removing dressing and recovering bacteria from the dressing | S. aureus ATCC 25923 and P. aeruginosa ATCC27853 | 206 |
| silver-containing crosslinked poly (acrylic acid) fibres | Zone inhibition—non-standard | MRSA USA 300 | 192 |
| various commercially available silver dressings | Shake flask assay: inoculum in the presence of dressing for 1–6 h at 37°C + use of neutralizer | P. aeruginosa ATCC 27312 and ATCC 15442, S. aureus ATCC 6538 | 201 |
| silver-containing dressing | Zone of inhibition on seeded agar + dressing in broth for up to 24 h at 35°C | S. aureus, B. subtilis, E. coli, P. aeruginosa. | 191 |
| antimicrobial polyur ethane foam dressing containing silver | Porcine ex vivo (loin roast) | S. aureus (DSM 20231) | 209 |
| commercially available silver-containing dressings | CLSI disc diffusion assay + zone of inhibition on seeded agar (some selective agar was used) | S. aureus (PCM 2051), S. epidermidis (PCM 2118), P. aeruginosa (ATCC 27853), E. coli (K12) | 194 |
| PHMB | |||
| PHMB | CLSI disc diffusion | E. coli (ATCC 25922), A. baumannii (ATCC 19606), P. aeruginosa (ATCC 27853), B. subtilis (ATCC 6633), S. aureus (ATCC 25923) and S. aureus (MRSA) | 190 |
| cotton gauze PHMB | Porcine ex vivo | P. aeruginosa (biofilm) | 207 |
| PHMB | Porcine ex vivo | P. aeruginosa (biofilm) | 190 |
| antimicrobial gauze dressing containing polihexanide | Porcine ex vivo (loin roast) | S. aureus (DSM 20231) | 209 |
| OCT | |||
| OCT | Non-standard broth dilution | S. aureus | 202 |
| Direct contact test (according to JIS L 1902:2002) | S. aureus | 202 | |
| non-antimicrobial poly urethane foam dressing intermittently irrigated with octenidine | Porcine ex vivo (loin roast) | S. aureus (DSM 20231) | 209 |
| Honey | |||
| L-Mesitran Soft | Non-standard ex vivo test on human skin | P. aeruginosa | 208 |
| iodine, calcium alginate Leptospermum honey | Porcine ex vivo | P. aeruginosa (biofilm) | 207 |
| Leptospermum honey | Porcine ex vivo | P. aeruginosa (biofilm) | 207 |
| 3 medical-grade honeys: Surgihoney RO, Activon manuka honey and Medihoney manuka honey | Prevention of sedimentation biofilm formation measured by crystal violet—not quantitative—diluted concentration of honey used | P. aeruginosa, S. aureus, E. coli, A. baumannii | 168 |
| honey-based dressings | Prevention of sedimentation biofilm formation measured by crystal violet—not quantitative—1 cm2 dressing added to bacterial suspension—biofilm formation measured by crystal violet | P. aeruginosa, S. aureus, E. coli, A. baumannii | 168 |
| chestnut honey- impregnated CMC hydrogel | Zone of inhibition on seeded agar | E. coli and S. aureus | 195 |
| honey-loaded nanofibre membrane | Non-standard broth evaluation by OD in the presence of material | E. coli | 201 |
| honey-loaded nanofibre membrane | Biofilm formation evaluated by crystal violet in presence of materials—non-standard and non-quantitative | E. coli | 201 |
| nano-composite alginate gel discs containing honey | Coated discs in inoculate broth for 24 h at 37°C | S. aureus (ATCC 6538) and MRSA (ATCC 43300), A. baumannii (ATCC 19606) + 13, carbapenem- resistant strains, E. coli (ATCC 10536) and P. aeruginosa (ATCC 9027) + 1 wound isolate | 200 |
| commercially available manuka honey-containing dressings | CLSI disc diffusion assay + zone of inhibition on seeded agar | S. aureus (PCM 2051), S. epidermidis (PCM 2118), P. aeruginosa (ATCC 27853), E. coli (K12) | 194 |
CMC, carboxymethyl cellulose.
Table 5.
In vivo protocols used for testing the activity of new dressings
| Antimicrobial | Model | Bacterial target | Study aim | Reference |
|---|---|---|---|---|
| Chlorhexidine | ||||
| CHG | pig | MRSA | bacterial recovery after application of CHG dressing <1.7 log10 cfu/g tissue after 3 days compared with 4.2 log10 cfu/g tissue with the placebo and 3.2 log10 cfu/g tissue with the gauze | 215 |
| mice | — | wound healing | 198 | |
| 0.5% CHX | rat | P. aeruginosa | wound healing | 212 |
| 0.5% CHX | rat | A. baumannii | systemic infection, and bacterial recovery | 216 |
| CHG/chitosan | mice | — | wound healing | 196 |
| Iodine | ||||
| PVI antiseptic | rat | P. aeruginosa | systemic infection, and bacterial recovery | 220 |
| PVI 3% in polyurethane foam dressing | rat | — | wound healing | 213 |
| cadexomer iodine | pig | P. aeruginosa | bacterial recovery | 207 |
| Silver | ||||
| silver sulfadiazine 1% | rat | P. aeruginosa | wound healing | 212 |
| silver-coated dressing | rat | P. aeruginosa | wound healing | 212 |
| calcium alginate– nanocrystalline silver | pig | P. aeruginosa | bacterial recovery | 207 |
| cotton gauze–silver sulphate | pig | P. aeruginosa | bacterial recovery | 207 |
| hydrocolloid–silver | pig | P. aeruginosa | bacterial recovery | 207 |
| polyacrylate–silver chloride | pig | P. aeruginosa | bacterial recovery | 207 |
| ActicoatTM | rat | A. baumannii | systemic infection, and bacterial recovery | 216 |
| silver sulfadiazine 1% | rat | A. baumannii | systemic infection, and bacterial recovery | 216 |
| silver sulfadiazine | rat | — | wound healing | 205 |
| silver sulfadiazine/ silver nitrate | rat | — | wound healing—skin prepared with PVI and ethanol | 192 |
| AgNPs | rat | S. aureus | bacterial recovery and wound healing | 211 |
| AgNPs/silver sulfadiazine | rat | — | wound healing | 200 |
| silver-based dressings | mice | MRSA, carbapenem-resistant P. aeruginosa, carbapenem-resistant A. baumannii | bacterial recovery and wound healing | 218 |
| keratin biomaterial containing AgNPs | mice | — | wound healing | 193 |
| polihexanide antiseptic | rat | P. aeruginosa | systemic infection, and bacterial recovery | 220 |
| OCT | ||||
| OCT | rat | P. aeruginosa | systemic infection, and bacterial recovery | 220 |
| Honey | ||||
| calcium alginate Leptospermum honey | pig | P. aeruginosa | bacterial recovery | 207 |
| Leptospermum honey | pig | P. aeruginosa | bacterial recovery | 207 |
| Melipona scutellaris honey | rat | MRSA ATTC43300 | wound healing and bacterial recovery | 217 |
| chestnut honey-impregnated CMC hydrogel | mice | — | wound healing | 195 |
| Medihoney medical grade honey | rat | — | wound healing | 218 |
CHX, chlorhexidine acetate; CMC, carboxymethyl cellulose.
The most common in vitro tests performed are based on measuring zone of inhibition of the antimicrobial dressing on seeded agar plates190–197 and the addition of antimicrobial dressing in an inoculated broth that can be sampled for bacterial survival over a period of time,191,193,194,198–202 or a combination of both (Table 4). At best, these tests provide preliminary information that the biocide can diffuse from the dressing material and show some activity against a target bacterium. The lack of a neutralization step to quench the activity of the biocide means that, at best, only a bacteriostatic activity of the biocide can be established, and as such these tests should not be used to make a claim on the efficacy of the antimicrobial dressing. Very few studies have used a standard test designed to measure the activity of an antimicrobial textile such as ASTM100:12 (Antibacterial Finishes on Textile Materials).203 The use of standardized tests allows a better comparison of results between studies than the use of non-standard ad hoc tests, which are most commonly used (Table 4).204–206
Ex vivo testing using excised animal or human skin as a substrate, or artificially damaged (e.g. puncture, burn) excised skin, provides a more accurate test protocol better representing the in vivo conditions of a wound.207–210 A number of studies have opted to use animal models: pigs, rats, mice or rabbits (Table 5). Many of these studies did not investigate the impact of bacterial infection of the wound, but the effect of the antimicrobial dressing on wound healing.193,195,196,198,205,211–214 A smaller number of in vivo studies inoculated the wound with a pathogen and investigated both bacterial survival and wound healing following the application of the dressing, providing useful information on the impact of the dressing (Table 5).200,211,215–218 One practical issue associated with in vivo protocols is the application of PVP-I or other post-operative biocides on the wound prior to the application of the antimicrobial dressing. Such practice, although ethically necessary, will impact on measuring the antimicrobial efficacy of the dressing alone. It is however apparent that even if the in vitro model is sophisticated enough to better represent conditions found in vivo, the antimicrobial dressing efficacy in patients might not be as effective.210
4.3 Measuring the antimicrobial activity of antimicrobial dressings against biofilms
If measuring the activity of antimicrobial dressings against a specific pathogen is already complex, the evaluation of their efficacy against biofilms is even more so. There are many biofilm protocols and a great divergence in opinions about their use and reproducibility. The majority of biofilm protocols use a single-species biofilm162,170 instead of a more complex biofilm that might represent better the polymicrobial nature of an infected chronic wound.141,219 Owing to the importance of the presence of a biofilm in an infected wound,210 a number of studies have looked at the impact of an antimicrobial dressing against the formation of biofilm rather the control of an established biofilm.168 These studies made use of a staining protocol that establishes biofilm biomass rather than viable bacterial count but claimed, perhaps inappropriately, antibiofilm activity of the tested dressing.168,201 A number of studies reported on forming single-species or complex bacterial biofilms on a substratum that was then exposed to an antimicrobial dressing for a set period of time and test conditions (temperature, pH, humidity).141,162,170,219–221 These protocols differ in their complexity and biofilm formation, using a range of methods such a CDC reactor,219,221 constant depth fermenter,140 colony-drip flow reactor200 or others.162,170 More advanced protocols that are trying to better mimic a wound biofilm have been reported using skin as a substratum.207,210 Since there are no standard tests to evaluate the efficacy of antimicrobial dressings against biofilms, the merit and relevance of each study for a particular type of wound, and their claims, need to be assessed carefully. The correlation of biofilm-based studies with the efficacy of antimicrobial dressings in practice remains to be determined.
5. Antimicrobial stewardship
To date limited advice on the application of the principles of antimicrobial stewardship of non-antibiotic antimicrobials pertinent to wounds is available,222,223 and guidance has largely centred on reducing the use of antibiotics for managing infections.15 One position paper15 recommended that only clinically infected wounds be treated with antibiotics and that infected wounds should be cultured by tissue biopsy. It proposed that short-term topical antiseptic therapy could be considered in wounds of uncertain infection status, and also as a supplement to antibiotics in infected wounds. It identified the need for clinical studies to test the efficacy of various non-antibiotic antimicrobials in treating colonized and infected wounds to determine whether antibiotic therapy could be reduced.15 An online course on this topic, ‘Antimicrobial Stewardship in Wound Management’, was introduced by FutureLearn in October 2019 and attracted over 8000 participants within 12 months. The potential of alternative antimicrobial strategies to minimize antibiotic usage has also been described.224
When applying an antimicrobial dressing to a wound, a clinical benefit should be expected. It should preferably contain an antimicrobial agent with a low adaptive response, together with the potential to prevent biofilm formation and to inhibit established polymicrobial biofilms. The duration of dressing treatment should be a short as possible and, in the case of treatment failure, it may be necessary to determine the MIC of the dominant pathogen to investigate tolerance to the non-antibiotic antimicrobial being used and direct change to another biocide.
6. Conclusions
Optimal management of wounds depends on avoiding the use of antimicrobial therapies when they are not indicated and prescribing appropriate antimicrobial interventions when they are indicated in order to minimize the risk of adverse effects for the patient and community. Therefore, the development of standardized methods to evaluate the effectiveness of antimicrobial dressings against both planktonic bacteria and biofilms in vitro, and to determine the susceptibility of microbial communities associated with wounds, would provide a stronger basis for informed choice for practitioners. However, the diversity of wound dressings and their applications, and the absence of standard tests to measure the efficacy of the antimicrobial dressing—as a product and not simply the active antimicrobial component—means that there is uncertainty as to the antimicrobial efficacy of such dressings. The use of basic in vitro diffusion tests relying, for example, on the size of zone of inhibition caused by the dressing is certainly not appropriate to be reported in publication. The more stringent and versatile ex vivo tests would provide more reliable information on the potential efficacy of the dressing to be tested in vivo. Overall, a better consensus on test protocols and reporting is needed to ensure claim validity and optimize non-antibiotic antimicrobial stewardship for wounds.
Supplementary Material
Acknowledgements
We would like to thank Niels Fibæk Bertel (EWMA) for his constructive comments on the manuscript.
Transparency declarations
This position paper was jointly initiated and developed by BSAC and the European Wound Management Association (EWMA). Neither BSAC nor EWMA, nor any other organizations or companies, had a decision-making role in this project. The article was subjected to JAC-AMR’s usual peer review process. EWMA has received general operating support from BBRAUN, Coloplast, Convatec, Essity, Flen Health, MolecuLight, Mölnlycke and Smith & Nephew for development and promotion of antimicrobial stewardship in wound management. Rose Cooper has received honoraria for presentations from Flen Health and Integra Lifesciences Services (France). Günter Kampf has received personal fees from Dr. Schumacher GmbH, Germany, for presentation and consultation. Jean-Yves Maillard is the Director of Biocide Consult Ltd.
Supplementary data
Tables S1 is available as Supplementary data at JAC-AMR Online.
References
- 1. Shah JB. The history of wound care. J Am Col Certif Wound Spec 2011; 3: 65–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forrest RD. Early history of wound treatment. J R Soc Med 1982; 75: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broughton G, Janis JE, Attinger CE.. A brief history of wound care. Plast Reconstr Surg 2006; 117 Suppl 7: 6S–11S. [DOI] [PubMed] [Google Scholar]
- 4. Forrest RD. Development of wound therapy from Dark Ages to the present. J R Soc Med 1982; 75: 268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hugo WB. A brief history of heat and chemical preservation and disinfection. J Appl Bacteriol 1991; 71: 9–18. [PubMed] [Google Scholar]
- 6. Fleming A. The action of chemical and physiological antiseptics in a septic wound. Br J Surg 1919; 7: 99. [Google Scholar]
- 7. Brennan SS, Leaper DL.. The effect of antiseptics on the healing wound: a study using the rabbit ear chamber. Br J Surg 1985; 7: 780–2. [DOI] [PubMed] [Google Scholar]
- 8. Lineaweaver W, Howard R, Soucy D. et al. Topical antimicrobial toxicity. Arch Surg 1985; 120: 267–70. [DOI] [PubMed] [Google Scholar]
- 9. Fox CL. Silver sulfadiazine - a new topical therapy for Pseudomonas in burns. Arch Surg 1968; 96: 184–8. [DOI] [PubMed] [Google Scholar]
- 10. Winter G. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962; 193: 293–4. [DOI] [PubMed] [Google Scholar]
- 11. Chen L, Todd R, Kiehlbauch J. et al. Notes from the field: pan-resistant new Delhi metallo-β-lactamase producing Klebsiella pneumoniae – Washoe County, Nevada, 2016. MMWR Morb Mortal Wkly Rep 2017; 66: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Boeckel TP, Pires J, Silvester R. et al. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 2019; 365: eaaw1944. [DOI] [PubMed] [Google Scholar]
- 13. Bush K, Courvalin P, Dantas G. et al. Tackling antibiotic resistance. Nat Rev Microbiol 2011; 9: 894–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goff DA, Kullar R, Goldstein EJC. et al. A global call from five countries to collaborate in antibiotic stewardship: united we succeed, divided we might fail. Lancet Infect Dis 2017; 17: e56–e63. [DOI] [PubMed] [Google Scholar]
- 15. Lipsky B, Dryden M, Gottrup F. et al. Antimicrobial stewardship in wound care: a position paper from the British Society for Antimicrobial Chemotherapy and European Wound Management Association. J Antimicrob Chemother 2016; 71: 3026–35. [DOI] [PubMed] [Google Scholar]
- 16. Kampf G. Challenging biocide tolerance with antiseptic stewardship. J Hosp Infect 2018; 100: e37–9. [DOI] [PubMed] [Google Scholar]
- 17. Lindford A, Kiuru V, Anttila VJ. et al. Successful eradication of multidrug resistant Acinetobacter in the Helsinki Burn Centre. J Burn Care Res 2015; 36: 595–601. [DOI] [PubMed] [Google Scholar]
- 18. Lazarus GS, Cooper DM, Knighton DR. et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994; 130: 489–93. [PubMed] [Google Scholar]
- 19. James GA, Swogger E, Wolcott R. et al. Biofilms in chronic wounds. Wound Repair Regen 2008; 16: 37–44. [DOI] [PubMed] [Google Scholar]
- 20. Bjarnsholt T, Kirketerp-Møller K, Jensen PØ. et al. Why chronic wounds will not heal: a novel hypothesis. Wounds Repair Regen 2008; 16: 2–10. [DOI] [PubMed] [Google Scholar]
- 21. Davis SC, Ricotti C, Cazzaniga A. et al. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen 2008; 16: 23–9. [DOI] [PubMed] [Google Scholar]
- 22. Neut D, Tijdens-Creusen EJ, Bulstra SK. et al. Biofilms in chronic diabetic foot ulcers-a study of 2 cases. Acta Orthop 2011; 83: 383–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kennedy P, Brammah S, Wills E.. Burns, biofilm and a new appraisal of burn wound sepsis. Burns 2010; 36: 49–56. [DOI] [PubMed] [Google Scholar]
- 24. Fromantin I, Seyer D, Watson S. et al. Bacterial flora and biofilms of malignant wounds associated with breast cancers. J Clin Microbiol 2013; 51: 3368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kathju S, Nistico L, Hall-Stoodley L. et al. Chronic surgical site infection due to suture-associated polymicrobial biofilm. Surg Infect (Larchmt) 2009; 10: 457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malone M, Bjarnsholt T, McBain A. et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published studies. J Wound Care 2017; 26: 20–5. [DOI] [PubMed] [Google Scholar]
- 27. Bowler PG, Deurden BI, Armstrong DG.. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001; 14: 244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howell-Jones RS, Wilson MJ, Hill KE. et al. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J Antimicrob Chemother 2005; 55: 143–9. [DOI] [PubMed] [Google Scholar]
- 29. Bowler PG, Davies BJ.. The microbiology of infected and noninfected leg ulcers. Int J Dermatol 1999; 38: 573–8. [DOI] [PubMed] [Google Scholar]
- 30. Wolcott RD, Hanson JD, Rees EJ. et al. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen 2016; 24: 163–74. [DOI] [PubMed] [Google Scholar]
- 31. Price LB, Liu CM, Melendez JH. et al. Community analysis of chronic wound bacteria using 16S rRNA gene-based pyrosequencing: impact of diabetes and antibiotics on chronic wound microbiota. PLoS One 2009; 4: e6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalan L, Loesche M, Hodkinson BP. et al. Redefining the chronic-wound microbiome: fungal communities are prevalent, dynamic, and associated with delayed healing. mBio 2016; 7: e01058–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomsen TR, Aasholm MS, Rudkjøbing VB. et al. The bacteriology of chronic venous leg ulcers examined by culture-independent molecular methods. Wound Repair Regen 2010; 18: 38–49. [DOI] [PubMed] [Google Scholar]
- 34. Johani K, Malone M, Jensen S. et al. Microscopy visualisation confirms multi-species biofilms are ubiquitous in diabetic foot ulcers. Int Wound J 2017; 14: 1160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schultz G, Bjarnsholt T, James GA. et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen 2017; 25: 744–57. [DOI] [PubMed] [Google Scholar]
- 36. Høiby N, Bjarnsholt T, Moser C. et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 2015; 21 Suppl 1: S1–25. [DOI] [PubMed] [Google Scholar]
- 37. Morris C. Wound management and dressing selection. Wounds Essential 2006; 1: 178–83. [Google Scholar]
- 38. Dabiri G, Damstetter E, Phillips T.. Choosing a wound dressing based on common wound characteristics. Adv Wound Care (New Rochelle) 2016; 5: 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Landriscina A, Rosen J, Friedmen AJ.. Systematic approach to wound dressings. J Drugs Dermatol 2015; 14: 740–4. [PubMed] [Google Scholar]
- 40. Morrissey I, Oggioni MR, Knight D. et al. Evaluation of epidemiological cut-off values indicates that biocide resistant subpopulations are uncommon in natural isolates of clinically-relevant microorganisms. PLoS One 2014; 9: e86669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maillard J-Y, Bloomfield S, Rosado Coelho J. et al. Does microbicide use in consumer products promote antimicrobial resistance? A critical review and recommendations for a cohesive approach to risk assessment. Microb Drug Resist 2013; 19: 344–54. [DOI] [PubMed] [Google Scholar]
- 42. Narui K, Takano M, Noguchi N. et al. Susceptibilities of methicillin-resistant Staphylococcus aureus isolates to seven biocides. Biol Pharm Bull 2007; 30: 585–7. [DOI] [PubMed] [Google Scholar]
- 43. Koburger T, Hübner NO, Braun M. et al. Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J Antimicrob Chemother 2010; 65: 1712–9. [DOI] [PubMed] [Google Scholar]
- 44. Goroncy-Bermes P, Brill FHH, Brill H.. Antimicrobial activity of wound antiseptics against extended-spectrum β-lactamase-producing bacteria. Wound Med 2013; 1: 41–3. [Google Scholar]
- 45. Thomas B, Sykes L, Stickler DJ.. Sensitivity of urine-grown cells of Providencia stuartii to antiseptics. J Clin Pathol 1978; 3: 929–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ekizoglu M, Sagiroglu M, Kilic E. et al. An investigation of the bactericidal activity of chlorhexidine digluconate against multidrug-resistant hospital isolates. Turkish J Med Sci 2016; 46: 903–9. [DOI] [PubMed] [Google Scholar]
- 47. Cowley NL, Forbes S, Amezquita A. et al. Effects of formulation on microbicide potency and mitigation of the development of bacterial insusceptibility. Appl Environ Microbiol 2015; 81: 7330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Forbes S, Dobson CB, Humphreys GJ. et al. Transient and sustained bacterial adaptation following repeated sublethal exposure to microbicides and a novel human antimicrobial peptide. Antimicrob Agents Chemother 2014; 58: 5809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thomas L, Maillard JY, Lambert RJ. et al. Development of resistance to chlorhexidine diacetate in Pseudomonas aeruginosa and the effect of a "residual" concentration. J Hosp Infect 2000; 46: 297–303. [DOI] [PubMed] [Google Scholar]
- 50. Bock LJ, Wand ME, Sutton JM.. Varying activity of chlorhexidine-based disinfectants against Klebsiella pneumoniae clinical isolates and adapted strains. J Hosp Infect 2016; 93: 42–8. [DOI] [PubMed] [Google Scholar]
- 51. Wesgate R, Grasha P, Maillard JY.. Use of a predictive protocol to measure the antimicrobial resistance risks associated with biocidal product usage. Am J Infect Control 2016; 44: 458–64. [DOI] [PubMed] [Google Scholar]
- 52. Braoudaki M, Hilton AC.. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. J Clin Microbiol 2004; 42: 73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nicoletti G, Boghossian V, Gurevitch F. et al. The antimicrobial activity in vitro of chlorhexidine, a mixture of isothiazolinones ('Kathon' CG) and cetyl trimethyl ammonium bromide (CTAB). J Hosp Infect 1993; 23: 87–111. [DOI] [PubMed] [Google Scholar]
- 54. Marrie TJ, Costerton JW.. Prolonged survival of Serratia marcescens in chlorhexidine. Appl Environ Microbiol 1981; 42: 1093–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Riazi S, Matthews KR.. Failure of foodborne pathogens to develop resistance to sanitizers following repeated exposure to common sanitizers. Int Biodeter Biodegr 2011; 65: 374–8. [Google Scholar]
- 56. Mengistu Y, Erge W, Bellete B.. In vitro susceptibility of gram-negative bacteria to isolates of chlorhexidine gluconate. East Afr Med J 1999; 76: 243–6. [PubMed] [Google Scholar]
- 57. Ulusoy AT, Kalyoncuoglu E, Reis A. et al. Antibacterial effect of N-acetylcysteine and taurolidine on planktonic and biofilm forms of Enterococcus faecalis. Dent Traumatol 2016; 32: 212–8. [DOI] [PubMed] [Google Scholar]
- 58. Witney AA, Gould KA, Pope CF. et al. Genome sequencing and characterization of an extensively drug-resistant sequence type 111 serotype O12 hospital outbreak strain of Pseudomonas aeruginosa. Clin Microbiol Infect 2014; 20: O609–18. [DOI] [PubMed] [Google Scholar]
- 59. Kampf G (ed.). Chlorhexidine digluconate. In: Antiseptic Stewardship: Biocide Resistance and Clinical Implications. Springer International Publishing, 2018; 429–534. [Google Scholar]
- 60. Reich PJ, Boyle MG, Hogan PG. et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus strains in the neonatal intensive care unit: an infection prevention and patient safety challenge. Clin Microbiol Infect 2016; 22: 645.e1–645.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu Q, Zhao H, Han L. et al. Frequency of biocide-resistant genes and susceptibility to chlorhexidine in high-level mupirocin-resistant, methicillin-resistant Staphylococcus aureus (MuH MRSA). Diagn Microbiol Infect Dis 2015; 82: 278–83. [DOI] [PubMed] [Google Scholar]
- 62. Longtin J, Seah C, Siebert K. et al. Distribution of antiseptic resistance genes qacA, qacB, and smr in methicillin-resistant Staphylococcus aureus isolated in Toronto, Canada, from 2005 to 2009. Antimicrob Agents Chemother 2011; 55: 2999–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vali L, Dashti AA, El-Shazly S. et al. Klebsiella oxytoca with reduced sensitivity to chlorhexidine isolated from a diabetic foot ulcer. Int J Infect Dis 2015; 34: 112–6. [DOI] [PubMed] [Google Scholar]
- 64. Cooper R. Iodine revisited. Int Wound J 2007; 4: 124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lepelletier D, Maillard J-Y, Pozzetto B. et al. Povidone iodine: properties, mechanisms of action and role in infection control and Staphylococcus aureus decolonization. Antimicrob Agents Chemother 2020; 64: e00682–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Traoré O, Fayard SF, Laveran H.. An in-vitro evaluation of the activity of povidone-iodine against nosocomial bacterial strains. J Hosp Infect 1996; 34: 217–22. [DOI] [PubMed] [Google Scholar]
- 67. Giacometti A, Cirioni O, Greganti G. et al. Antiseptic compounds still active against bacterial strains isolated from surgical wound infections despite increasing antibiotic resistance. Eur J Clin Microbiol Infect Dis 2002; 21: 553–6. [DOI] [PubMed] [Google Scholar]
- 68. Tremblay YD, Caron V, Blondeau A. et al. Biofilm formation by coagulase-negative staphylococci: impact on the efficacy of antimicrobials and disinfectants commonly used on dairy farms. Vet Microbiol 2014; 172: 511–8. [DOI] [PubMed] [Google Scholar]
- 69. Fuursted K, Hjort A, Knudsen L.. Evaluation of bactericidal activity and lag of regrowth (postantibiotic effect) of five antiseptics on nine bacterial pathogens. J Antimicrob Chemother 1997; 40: 221–6. [DOI] [PubMed] [Google Scholar]
- 70. Anderson RL, Vess RW, Carr JH. et al. Investigations of intrinsic Pseudomonas cepacia contamination in commercially manufactured povidone-iodine. Infect Control Hosp Epidemiol 1991; 12: 297–302. [DOI] [PubMed] [Google Scholar]
- 71. Berkelman RL, Lewin S, Allen JR. et al. Pseudobacteremia attributed to contamination of povidone-iodine with Pseudomonas cepacia. Ann Intern Med 1981; 95: 32–6. [DOI] [PubMed] [Google Scholar]
- 72. Herruzo-Cabrera R, Garcia-Torres V, Rey-Calero J. et al. Evaluation of the penetration strength bactericidal efficacy of a spectrum of action of several antimicrobial creams against isolated microorganisms in a burn centre. Burns 1992; 18: 39–44. [DOI] [PubMed] [Google Scholar]
- 73. Kunisada T, Yamada K, Oda S. et al. Investigation on the efficacy of povidone-iodine against antiseptic-resistant species. Dermatology 1997; 195: 14–8. [DOI] [PubMed] [Google Scholar]
- 74. Lanker Klossner B, Widmer HR. et al. Nondevelopment of resistance by bacteria during hospital use of povidone-iodine. Dermatology 1997; 195 Suppl 2: 10–3. [DOI] [PubMed] [Google Scholar]
- 75. Edwards-Jones V. The benefits of silver in hygiene, personal care and healthcare. Lett Appl Microbiol 2009; 49: 147–52. [DOI] [PubMed] [Google Scholar]
- 76. Unger C, Luck C.. Inhibitory effects of silver ions on Legionella pneumophila grown on agar, intracellular in Acanthamoeba castellanii and in artificial biofilms. J Appl Microbiol 2012; 112: 1212–9. [DOI] [PubMed] [Google Scholar]
- 77. Maillard J-Y, Hartemann P.. Silver as an antimicrobial: facts and gaps in knowledge. Crit Rev Microbiol 2018; 39: 373–83. [DOI] [PubMed] [Google Scholar]
- 78. Morones JR, Elechiguerra JL, Camacho A. et al. The bactericidal effect of silver nanoparticles. Nanotechnol 2005; 16: 2346. [DOI] [PubMed] [Google Scholar]
- 79. Finley PJ, Norton R, Austin C. et al. Unprecedented silver resistance in clinically isolated Enterobacteriaceae: major implications for burn and wound management. Antimicrob Agents Chemother 2015; 59: 4734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hendry AT, Stewart IO.. Silver-resistant Enterobacteriaceae from hospital patients. Can J Microbiol 1979; 25: 915–21. [DOI] [PubMed] [Google Scholar]
- 81. Kuehl R, Brunetto PS, Woischnig AK. et al. Preventing implant-associated infections by silver coating. Antimicrob Agents Chemother 2016; 60: 2467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hosny AE-D, Rasmy SA, Aboul-Magd DS. et al. The increasing threat of silver-resistance in clinical isolates from wounds and burns. Infect Drug Resist 2019; 12: 1985–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li XZ, Nikaido H, Williams KE.. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J Bacteriol 1997; 179: 6127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sütterlin S, Dahlö M, Tellgren-Roth C. et al. High frequency of silver resistance genes in invasive isolates of Enterobacter and Klebsiella species. J Hosp Infect 2017; 96: 256–61. [DOI] [PubMed] [Google Scholar]
- 85. Russell AD, McDonnell G.. Concentration: a major factor in studying biocidal action. J Hosp Infect 2000; 44: 1–3. [DOI] [PubMed] [Google Scholar]
- 86. Jakobsen L, Andersen AS, Friis-Møller A. et al. Silver resistance: an alarming public health concern? Int J Antimicrob Agents 2011; 38: 454–5. [DOI] [PubMed] [Google Scholar]
- 87. Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev 2003; 27: 341–53. [DOI] [PubMed] [Google Scholar]
- 88. Kampf G (ed.). Silver. In: Antiseptic Stewardship: Biocide Resistance and Clinical Implications. Springer International Publishing, 2018; 563–607. [Google Scholar]
- 89. Delmar JA, Su CC, Yu EW.. Bacterial multidrug efflux transporters. Annu Rev Biophys 2014; 43: 93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gudipaty SA, Larsen AS, Rensing C. et al. Regulation of Cu(I)/Ag(I) efflux genes in Escherichia coli by the sensor kinase CusS. FEMS Microbiol Lett 2012; 330: 30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Torres-Urquidy O, Bright K.. Efficacy of multiple metals against copper-resistant bacterial strains. J Appl Microbiol 2012; 112: 695–704. [DOI] [PubMed] [Google Scholar]
- 92. Su CC, Long F, Yu EW.. The Cus efflux system removes toxic ions via a methionine shuttle. Protein Sci 2011; 20: 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Solioz M, Odermatt A.. Copper and silver transport by CopB-ATPase in membrane vesicles of Enterococcus hirae. J Biol Chem 1995; 270: 9217–21. [DOI] [PubMed] [Google Scholar]
- 94. Sütterlin S, Tano E, Bergsten A. et al. Effects of silver-based wound dressings on the bacterial flora in chronic leg ulcers and its susceptibility in vitro to silver. Acta Derm Venerol 2012; 92: 34–9. [DOI] [PubMed] [Google Scholar]
- 95. Kremer AN, Hoffmann H.. Subtractive hybridization yields a silver resistance determinant unique to nosocomial pathogens in the Enterobacter cloacae complex. J Clin Microbiol 2012; 50: 3249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Randall CP, Oyama LB, Bostock JM. et al. The silver cation (Ag+): antistaphylococcal activity, mode of action and resistance studies. J Antimicrob Chemother 2013; 68: 131–8. [DOI] [PubMed] [Google Scholar]
- 97. Elkrewi E, Randall CP, Ooi N. et al. Cryptic silver resistance is prevalent and readily activated in certain Gram-negative pathogens. J Antimicrob Chemother 2017; 2: 3043–6. [DOI] [PubMed] [Google Scholar]
- 98. Wu MY, Suryanarayanan K, van Ooij WJ. et al. Using microbial genomics to evaluate the effectiveness of silver to prevent biofilm formation. Water Sci Technol 2007; 55: 413–9. [DOI] [PubMed] [Google Scholar]
- 99. Pal C, Asiani K, Arya S. et al. Metal resistance and its association with antibiotic resistance. Adv Microb Physiol 2017; 70: 261–313. [DOI] [PubMed] [Google Scholar]
- 100. Mashat BH. Polyhexamethylene biguanide hydrochloride: features and applications. Br J Environ Sci 2016; 4: 49–55. [Google Scholar]
- 101. Müller G, Kramer A.. In vitro action of a combination of selected antimicrobial agents and chondroitin sulfate. Chem Biol Interact 2000; 124: 77–85. [DOI] [PubMed] [Google Scholar]
- 102. Koburger T, Müller G, Eisenbeiß W. et al. Microbicidal activity of polihexanide. GMS Krankenhaushyg Interdiszip 2007; 2: Doc44. [Google Scholar]
- 103. Fabry WH, Kock HJ, Vahlensieck W.. Activity of the antiseptic polyhexanide against gram-negative bacteria. Microb Drug Resist 2014; 20: 138–43. [DOI] [PubMed] [Google Scholar]
- 104. Assadian O, Wehse K, Hübner NO. et al. Minimum inhibitory (MIC) and minimum microbicidal concentration (MMC) of polihexanide and triclosan against antibiotic sensitive and resistant Staphylococcus aureus and Escherichia coli strains. GMS Krankenhhyg Interdiszip 2011; 6: Doc06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fabry W, Reimer C, Azem T. et al. Activity of the antiseptic polyhexanide against meticillin-susceptible and meticillin-resistant Staphylococcus aureus. J Global Antimicrob Resist 2013; 1: 195–9. [DOI] [PubMed] [Google Scholar]
- 106. Decker EM, Bartha V, Kopunic A. et al. Antimicrobial efficiency of mouthrinses versus and in combination with different photodynamic therapies on periodontal pathogens in an experimental study. J Periodontal Res 2017; 52: 162–75. [DOI] [PubMed] [Google Scholar]
- 107. Moore LE, Ledder RG, Gilbert P. et al. In vitro study of the effect of cationic biocides on bacterial population dynamics and susceptibility. Appl Environ Microbiol 2008; 74: 4825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Renzoni A, Von Dach E, Landelle C. et al. Impact of exposure of methicillin-resistant Staphylococcus aureus to polyhexanide in vitro and in vivo. Antimicrob Agents Chemother 2017; 61: e00272–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Conceicao T, de Lencastre H, Aires-de-Sousa M.. Efficacy of octenidine against antibiotic-resistant Staphylococcus aureus epidemic clones. J Antimicrob Chemother 2016; 71: 2991–4. [DOI] [PubMed] [Google Scholar]
- 110. Tirali RE, Turan Y, Akal N. et al. In vitro antimicrobial activity of several concentrations of NaOCl and Octenisept in elimination of endodontic pathogens. Oral Surg Oral Med Oral Path Oral Radiol Endod 2009; 108: e117–20. [DOI] [PubMed] [Google Scholar]
- 111. Tylewska-Wierzbanowska S, Rogulska U, Lewandowska G. et al. Bactericidal activity of octenidine to various genospecies of Borrelia burgdorferi, sensu lato spirochetes in vitro and in vivo. Pol J Microbiol 2017; 66: 259–63. [DOI] [PubMed] [Google Scholar]
- 112. Shepherd MJ, Moore G, Wand ME. et al. Pseudomonas aeruginosa adapts to octenidine in the laboratory and a simulated clinical setting, leading to increased tolerance to chlorhexidine and other biocides. J Hosp Infect 2018; 100: e23–9. [DOI] [PubMed] [Google Scholar]
- 113. Wand ME, Jamshidi S, Bock LJ. et al. SmvA is an important efflux pump for cationic biocides in Klebsiella pneumoniae and other Enterobacteriaceae. Sci Rep 2019; 9: 1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cooper R, Jenkins L.. A comparison between medical grade honey and table honey. Wounds 2009; 21: 29–36. [Google Scholar]
- 115. Kwakman PHS, te Velde AA, de Boer L. et al. Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS One 2011; 6: e17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lu J, Carter L, Burke N. et al. Manuka-type honeys can eradicate biofilms by Staphylococcus aureus strains with different biofilm-forming abilities. Peer J 2014; 2: e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Alvarez-Suarez J, Gasparrini M, Forbes-Hernandez T. et al. The composition and biological activity of honey: a focus on manuka honey. Foods 2014; 3: 420–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Molan PC. The antibacterial activity of honey: 1. The nature of the antibacterial activity. Bee World 1992; 73: 1–28. [Google Scholar]
- 119. Carter DA, Blair SE, Cokcetin N. et al. Therapeutic manuka honey: no longer so alternative. Front Microbiol 2016; 7: 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Blair S, Cokcetin N, Harry E. et al. The unusual antibacterial activity of medical grade Leptospermum honey: antibacterial spectrum, resistance and transcriptome analysis. Eur J Clin Microbiol Infect Dis 2009; 28: 1199–208. [DOI] [PubMed] [Google Scholar]
- 121. Cooper R, Jenkins L, Henriques AF. et al. Absence of bacterial resistance to medical-grade manuka honey. Eur J Clin Microbiol Infect Dis 2010; 29: 1237–41. [DOI] [PubMed] [Google Scholar]
- 122. Tetz G, Tetz V.. In vitro antimicrobial activity of a novel compound, Mul-1867, against clinically important bacteria. Antimicrob Resist Infect Control 2015; 4: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ueda S, Kuwabara Y.. Susceptibility of biofilm Escherichia coli, Salmonella enteritidis and Staphylococcus aureus to detergents and sanitizers. Biocontrol Sci 2007; 12: 149–53. [DOI] [PubMed] [Google Scholar]
- 124. Azzimonti B, Cochis A, Beyrouthy ME. et al. Essential oil from berries of Lebanese Juniperus excelsa M. Bieb displays similar antibacterial activity to chlorhexidine but higher cytocompatibility with human oral primary cells. Molecules 2015; 20: 9344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tote K, Horemans T, Vanden Berghe D. et al. Inhibitory effect of biocides on the viable masses and matrices of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 2010; 76: 3135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hübner N-O, Matthes R, Koban I. et al. Efficacy of chlorhexidine, polihexanide and tissue-tolerable plasma against Pseudomonas aeruginosa biofilms grown on polystyrene and silicone materials. Skin Pharmacol Physiol 2010; 23 Suppl: 28–34. [DOI] [PubMed] [Google Scholar]
- 127. Choi YS, Kim C, Moon JH. et al. Removal and killing of multispecies endodontic biofilms by N-acetylcysteine. Braz J Microbiol 2018; 49: 184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dostie S, Alkadi LT, Owen G. et al. Chemotherapeutic decontamination of dental implants colonized by mature multispecies oral biofilm. J Clin Periodontol 2017; 44: 403–9. [DOI] [PubMed] [Google Scholar]
- 129. Liaqat I, Sabri AN.. Effect of biocides on biofilm bacteria from dental unit water lines. Curr Microbiol 2008; 56: 619–24. [DOI] [PubMed] [Google Scholar]
- 130. Takenaka S, Trivedi HM, Corbin A. et al. Direct visualization of spatial and temporal patterns of antimicrobial action within model oral biofilms. Appl Environ Microbiol 2008; 74: 1869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Corbin A, Pitts B, Parker A. et al. Antimicrobial penetration and efficacy in an in vitro oral biofilm model. Antimicrob Agents Chemother 2011; 55: 3338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jurczyk K, Nietzsche S, Ender C. et al. In-vitro activity of sodium-hypochlorite gel on bacteria associated with periodontitis. Clin Oral Investig 2016; 20: 2165–73. [DOI] [PubMed] [Google Scholar]
- 133. Peeters E, Nelis HJ, Coenye T.. Evaluation of the efficacy of disinfection procedures against Burkholderia cenocepacia biofilms. J Hosp Infect 2008; 70: 361–8. [DOI] [PubMed] [Google Scholar]
- 134. Shen Y, Stojicic S, Haapasalo M.. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J Endod 2011; 37: 657–61. [DOI] [PubMed] [Google Scholar]
- 135. Anand G, Ravinanthan M, Basaviah R. et al. In vitro antimicrobial and cytotoxic effects of Anacardium occidentale and Mangifera indica in oral care. J Pharm Bioallied Sci 2015; 7: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zmantar T, Ben Slama R, Fdhila K. et al. Modulation of drug resistance and biofilm formation of Staphylococcus aureus isolated from the oral cavity of Tunisian children. Braz J Infect Dis 2017; 21: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Houari A, Di Martino P.. Effect of chlorhexidine and benzalkonium chloride on bacterial biofilm formation. Lett Appl Microbiol 2007; 45: 652–6. [DOI] [PubMed] [Google Scholar]
- 138. Takahashi H, Nadres ET, Kuroda K.. Cationic amphiphilic polymers with antimicrobial activity for oral care applications: eradication of S. mutans biofilm. Biomacromolecules 2017; 18: 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rocha GR, Florez SE, de Barros AL. et al. Effect of tt-farnesol and myricetin on in vitro biofilm formed by Streptococcus mutans and Candida albicans. BMC Comp Alter Med 2018; 18: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Stickler D, Hewett P.. Activity of antiseptics against biofilms of mixed bacterial species growing on silicone surfaces. Eur J Clin Microbiol Infect Dis 1991; 10: 416–21. [DOI] [PubMed] [Google Scholar]
- 141. Hill KE, Malic S, McKee R. et al. An in vitro model of chronic wound biofilms to test wound dressings and assess antimicrobial susceptibilities. J Antimicrob Chemother 2010; 65: 1195–206. [DOI] [PubMed] [Google Scholar]
- 142. Bercy P, Lasserre J.. Susceptibility to various oral antiseptics of Porphyromonas gingivalis W83 within a biofilm. Adv Ther 2007; 24: 1181–91. [DOI] [PubMed] [Google Scholar]
- 143. Junka A, Bartoszewicz M, Smutnicka D. et al. Efficacy of antiseptics containing povidone-iodine, octenidine dihydrochloride and ethacridine lactate against biofilm formed by Pseudomonas aeruginosa and Staphylococcus aureus measured with the novel biofilm-oriented antiseptics test. Int Wound J 2014; 11: 730–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pagedar A, Singh J.. Evaluation of antibiofilm effect of benzalkonium chloride, iodophore and sodium hypochlorite against biofilm of Pseudomonas aeruginosa of dairy origin. J Food Sci Technol 2015; 52: 5317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Parsons D, Meredith K, Rowlands VJ. et al. Enhanced performance and mode of action of a novel antibiofilm hydrofiber® wound dressing. Biomed Res Int 2016; 2016: 7616471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Shirdel M, Tajik H, Moradi M.. Combined activity of colloid nanosilver and Zataria multiflora boiss essential oil-mechanism of action and biofilm removal activity. Adv Pharm Bull 2017; 7: 621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Thuptimdang P, Limpiyakorn T, Khan E.. Dependence of toxicity of silver nanoparticles on Pseudomonas putida biofilm structure. Chemosphere 2017; 188: 199–207. [DOI] [PubMed] [Google Scholar]
- 148. Das BC, Dash SK, Mandal D. et al. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab J Chem 2017; 10: 862–76. [Google Scholar]
- 149. Berry JA, Biedlingmaier JF, Whelan PJ.. In vitro resistance to bacterial biofilm formation on coated fluoroplastic tympanostomy tubes. Otolaryngol Head Neck Surg 2000; 123: 246–51. [DOI] [PubMed] [Google Scholar]
- 150. Qin H, Cao H, Zhao Y. et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials 2014; 35: 9114–25. [DOI] [PubMed] [Google Scholar]
- 151. van Hengel IAJ, Riool M, Fratila-Apachitei LE. et al. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials 2017; 140: 1–15. [DOI] [PubMed] [Google Scholar]
- 152. Wirth SM, Bertuccio AJ, Cao F. et al. Inhibition of bacterial surface colonization by immobilized silver nanoparticles depends critically on the planktonic bacterial concentration. J Colloid Interface Sci 2016; 467: 17–27. [DOI] [PubMed] [Google Scholar]
- 153. Wu Y, Quan X, Si X. et al. A small molecule norspermidine in combination with silver ion enhances dispersal and disinfection of multi-species wastewater biofilms. Appl Microbiol Biotechnol 2016; 100: 5619–29. [DOI] [PubMed] [Google Scholar]
- 154. Halstead FD, Rauf M, Bamford A. et al. Antimicrobial dressings: comparison of the ability of a panel of dressings to prevent biofilm formation by key burn wound pathogens. Burns 2015; 41: 1683–94. [DOI] [PubMed] [Google Scholar]
- 155. Bowler PG, Parsons D.. Combatting wound biofilm and recalcitrance with a novel anti-biofilm Hydrofiber® wound dressing. Wound Med 2016; 14: 6–11. [Google Scholar]
- 156. Said J, Walker M, Parsons D. et al. An in vitro test of the efficacy of an anti-biofilm wound dressing. Int J Pharm 2014; 474: 177–81. [DOI] [PubMed] [Google Scholar]
- 157. Davis SC, Harding A, Gil J. et al. Effectiveness of a polyhexanide irrigation solution on methicillin-resistant Staphylococcus aureus biofilms in a porcine wound model. Int Wound J 2017; 14: 937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Bukhary S, Balto H.. Antibacterial efficacy of octenisept, alexidine, chlorhexidine, and sodium hypochlorite against Enterococcus faecalis biofilms. J Endodont 2017; 43: 643–7. [DOI] [PubMed] [Google Scholar]
- 159. Cherian B, Gehlot PM, Manjunath MK.. Comparison of the antimicrobial efficacy of octenidine dihydrochloride and chlorhexidine with and without passive ultrasonic irrigation - an invitro study. J Clin Diagn Res 2016; 10: 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ghivari SB, Bhattacharya H, Bhat KG. et al. Antimicrobial activity of root canal irrigants against biofilm forming pathogens- an in vitro study. J Conserv Dent 2017; 20: 147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Guneser MB, Akbulut MB, Eldeniz AU.. Antibacterial effect of chlorhexidine-cetrimide combination, Salvia officinalis plant extract and octenidine in comparison with conventional endodontic irrigants. Dent Mat J 2016; 35: 736–41. [DOI] [PubMed] [Google Scholar]
- 162. Junka AF, Zywicka A, Szymczyk P. et al. A.D.A.M. (antibiofilm dressing’s activity measurement)-simple method for evaluating antibiofilm activity of drug-saturated dressings against wound pathogens. J Microbiol Methods 2017; 143: 6–12. [DOI] [PubMed] [Google Scholar]
- 163. Koban I, Geisel MH, Holtfreter B. et al. Synergistic effects of nonthermal plasma and disinfecting agents against dental biofilms in vitro. ISRN Dent 2013; 2013: 573262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Slee AM, O’Connor JR.. In vitro antiplaque activity of octenidine dihydrochloride (WIN 41464-2) against preformed plaques of selected oral plaque-forming microorganisms. Antimicrob Agents Chemother 1983; 23: 379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Lu J, Cokcetin NN, Burke CM. et al. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Sci Rep 2019; 9: 18160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Maddocks SE, Lopez MS, Rowlands R. et al. Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiol 2012; 158: 781–90. [DOI] [PubMed] [Google Scholar]
- 167. Majtan J, Bohova J, Horniackova M. et al. Anti-biofilm effects of honey against wound pathogens Proteus mirabilis and Enterobacter cloacae. Phytother Res 2014; 28: 69–75. [DOI] [PubMed] [Google Scholar]
- 168. Halstead F, Webber MA, Rauf M. et al. In vitro activity of an engineered honey, medical grade honeys, and antimicrobial wound dressings against biofilm-producing clinical bacterial isolates. J Wound Care 2016; 25: 93–102. [DOI] [PubMed] [Google Scholar]
- 169. Watson D, Berquist S, Nicholson J. et al. Comprehensive in situ killing of six common wound pathogens with manuka honey dressings using a modified AATCC-TM100. Wounds 2017; 29: 262–8. [PubMed] [Google Scholar]
- 170. Brackman G, de Meyer L, Nelis HJ. et al. Biofilm inhibitory and eradicating activity of wound care products against Staphylococcus aureus and Staphylococcus epidermidis biofilms in an in vitro chronic wound model. J Appl Microbiol 2013; 114: 1833–42. [DOI] [PubMed] [Google Scholar]
- 171. Lipsky BA, Senneville E, Abbas ZG. et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diab Metab Res Rev 2020; 36 Suppl 1: e3280. [DOI] [PubMed] [Google Scholar]
- 172. Haesler E, Swanson T, Ousey K. et al. Clinical indicators of wound infection and biofilm: reaching international consensus. J Wound Care 2019; 28 Suppl 3b: S4–S12. [DOI] [PubMed] [Google Scholar]
- 173. Müller G, Kramer A.. biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother 2008; 61: 1281–7. [DOI] [PubMed] [Google Scholar]
- 174. Kramer A, Dissemond J, Kim S. et al. Consensus on wound antisepsis: update 2018. Skin Pharmacol Physiol 2018; 31: 28–58. [DOI] [PubMed] [Google Scholar]
- 175. O’Meara S, Al-Kurdi D, Ologun Y. et al. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2014; issue 1: CD003557. [DOI] [PubMed] [Google Scholar]
- 176. Norman G, Dumville JC, Moore ZEH.. Antibiotics and antiseptics for pressure ulcers. Cochrane Database Syst Rev 2016; issue 4: CD011586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Dumville JC, Lipsky BA, Hoey C. et al. Topical antimicrobial agents for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev 2017; issue 6: CD011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Westby MJ, Dumville JC, Soares MO. et al. Dressings and topical agents for treating pressure ulcers. Cochrane Database Syst Rev 2017; issue 6: CD011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Norman G, Christie J, Liu Z. et al. Antiseptics for burns. Cochrane Database Syst Rev 2017; issue 7: CD011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Norman G, Westerby MJ, Rithalia AM. et al. Dressings and topical agents for treating venous leg ulcers. Cochrane Database Syst Rev 2018; issue 6: CD012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Wolcott RD, Rumbaugh KP, Janes G. et al. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care 2010; 19: 320–8. [DOI] [PubMed] [Google Scholar]
- 182. Wolcott R. Economic aspects of biofilm-based wound care in diabetic foot ulcers. J Wound Care 2015; 24: 189–94. [DOI] [PubMed] [Google Scholar]
- 183. Malone M, Johani K, Jensen SO. et al. Effect of cadexomer iodine on the microbial load and diversity of chronic non-healing diabetic foot ulcers complicated by biofilm. J Antimicrob Chemother 2017; 72: 2093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Malone M, Schwarzer S, Radzieta M. et al. Effect on total microbial load and community composition with two vs six-week topical cadexomer iodine for treating chronic biofilm infections in diabetic foot ulcers. Int Wound J 2019; 16: 1477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Walker M, Cochrane CA, Bowler PG.. Silver deposition and tissue staining associated with wound dressings containing silver. Ost Wound Manag 2006; 52: 42–50. [PubMed] [Google Scholar]
- 186. Percival SL, Bowler PG, Russell D.. Bacterial resistance to silver in wound care. J Hosp Infect 2005; 60: 1–7. [DOI] [PubMed] [Google Scholar]
- 187. Sheskey PJ, Hancock BC, Moss GP. et al. (eds). Handbook of Pharmaceutical Excipients, 9th edn. Pharmaceutical Press, 2020. [Google Scholar]
- 188. Larson E. Handwashing and skin physiologic and bacteriologic aspects. Infect Control 1985; 6: 14–23. [DOI] [PubMed] [Google Scholar]
- 189. Wesgate R, Robertson A, Barrell M. et al. Impact of test protocols and material binding on the efficacy of antimicrobial wipes. J Hosp Infect 2019; 103: e25–e32. [DOI] [PubMed] [Google Scholar]
- 190. Ampawong S, Aramwit P.. A study of long-term stability and antimicrobial activity of chlorhexidine, polyhexamthethylene biguanide, and silver nanoparticle incorporated in sericin-based wound dressing. J Biomater Sci Polymer Ed 2017; 28: 1286–302. [DOI] [PubMed] [Google Scholar]
- 191. Aramwit P, Muangman P, Namviriyachote N. et al. In vitro evaluation of the antimicrobial effectiveness and moisture binding properties of wound dressings. Int J Mol Sci 2010; 11: 2864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Mohseni M, Shamloo A, Aghababaie Z. et al. A comparative study of wound dressings loaded with silver sulfadiazine and silver nanoparticles: in vitro and in vivo evaluation. Int J Pharmaceut 2019; 564: 350–8. [DOI] [PubMed] [Google Scholar]
- 193. Konop M, Czuwara J, Klodzinska E. et al. Evaluation of keratin biomaterial containing silver nanoparticles as a potential wound dressing in full-thickness skin wound model in diabetic mice. J Tissue Eng Regen Med 2020; 14: 334–46. [DOI] [PubMed] [Google Scholar]
- 194. Szweda P, Gorczyca G, Tylingo R. et al. Comparison of antimicrobial activity of selected, commercially available wound dressing materials. J Wound Care 2018; 27: 320–6. [DOI] [PubMed] [Google Scholar]
- 195. Park J-S, An S-J, Jeong S-I. et al. Chestnut honey impregnated carboxymethyl cellulose hydrogel for diabetic ulcer healing. Polymers 2017; 9: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ferreira MOG, de Lima IS, Morais AIS. et al. Chitosan associated chlorhexidine in gel form: synthesis, characterization and healing wounds application. J Drug Del Sci Technol 2019; 49: 375–82. [Google Scholar]
- 197. Mofidfar M, Kim ES, Larkin E. et al. Antimicrobial activity of silver containing crosslinked poly(acrylic acid) fibers. Micromachines 2019; 10: 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Barbour ME, Maddocks SE, Grady HJ. et al. Chlorhexidine hexametaphosphate as a wound care material coating: antimicrobial efficacy, toxicity and effect on healing. Nanomedicine 2016; 11: 2049–57. [DOI] [PubMed] [Google Scholar]
- 199. Bourdillon KA, Delury CP, Cullen BM.. Biofilms and delayed healing - an in vitro evaluation of silver- and iodine-containing dressings and their effect on bacterial and human cells. Int Wound J 2017; 14: 1066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Stojkovska J, Djurdjevic Z, Janic I. et al. Comparative in vivo evaluation of novel formulations based on alginate and silver nanoparticles for wound treatments. J Biomater Appl 2018; 32: 1197–211. [DOI] [PubMed] [Google Scholar]
- 201. Sarkar R, Ghosh A, Barui A. et al. Repositing honey incorporated electrospun nanofiber membranes to provide anti-oxidant, anti-bacterial and anti-inflammatory microenvironment for wound regeneration. J Mater Sci Mater Med 2018; 29: 31. [DOI] [PubMed] [Google Scholar]
- 202. Moritz S, Wiegand C, Wesarg F. et al. 2014, Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int J Pharm 2014; 471: 45–55. [DOI] [PubMed] [Google Scholar]
- 203. Edwards JV, Prevost NT, Santiago M. et al. Hydrogen peroxide generation of copper/ascorbate formulations on cotton: effect on antibacterial and fibroblast activity for wound healing application. Molecules 2018; 2: 2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Kim H, Izadjoo M.. Antimicrobial activity of a bioelectric dressing using an in vitro wound pathogen colony drip-flow reactor biofilm model. J Wound Care 2016; 25 Suppl 7: S47–S52. [DOI] [PubMed] [Google Scholar]
- 205. Nejaddehbashi F, Hashemitabar M, Bayati V. et al. Incorporation of silver sulfadiazine into an electrospun composite of caprolactone as an antibacterial scaffold for wound healing in rats. Cell J 2020; 21: 379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Radulescu M, Andronescu E, Dolete G. et al. Silver nanocoatings for reducing the exogenous microbial colonization of wound dressings. Materials (Basel) 2016; 9: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Phillips PL, Yang Q, Davis S. et al. Antimicrobial dressing efficacy against mature Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J 2015; 12: 469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Boekema BKHL, Pool L, Ulrich MMW.. The effect of a honey based gel and silver sulphadiazine on bacterial infections of in vitro burn wounds. Burns 2013; 39: 754–9. [DOI] [PubMed] [Google Scholar]
- 209. Matiasek J, Domig KJ, Djedovic G. et al. The effect of negative pressure wound therapy with antibacterial dressings on an in vitro wound model. J Wound Care 2017; 26: 236–42. [DOI] [PubMed] [Google Scholar]
- 210. Scully R, Hurlow J, Walker M. et al. Clinical and in vitro performance of an antibiofilm hydrofiber wound dressing. J Wound Care 2018; 27: 584–92. [DOI] [PubMed] [Google Scholar]
- 211. Wang Y, Wang C, Xie Y. et al. Highly transparent, highly flexible composite membrane with multiple antimicrobial effects used for promoting wound healing. Carbohydr Polym 2019; 222: 114985. [DOI] [PubMed] [Google Scholar]
- 212. Yabanoglu H, Basaran O, Aydogan C. et al. Assessment of the effectiveness of silver-coated dressing, chlorhexidine acetate (0.5%), citric acid (3%), and silver sulfadiazine (1%), for topical antibacterial effects against the multi-drug resistant Pseudomonas aeruginosa infecting full-skin thickness burn wounds on rats. Int Surg 2013; 98: 416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Lee JW, Song KY.. Evaluation of a polyurethane foam dressing impregnated with 3% povidone-iodine (Betafoam) in a rat wound model. Ann Surg Treat Res 2018; 94: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Paydar S, Ziaeian B, Dehghanian A. et al. A comparison of the effects of topical prolavacid solution (a polyhexamethylene biguanide-based wound cleanser) and medihoney ointment in a rat model of cutaneous wound. Adv Wound Care 2017; 6: 408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Mana TSC, Donskey C, Carty N. et al. Preliminary analysis of the antimicrobial activity of a postoperative wound dressing containing chlorhexidine gluconate against methicillin-resistant Staphylococcus aureus in an in vivo porcine incisional wound model. Am J Infect Cont 2019; 47: 1048–52. [DOI] [PubMed] [Google Scholar]
- 216. Uygur F, Őncül, Evinç R. et al. Effects of three different topical antibacterial dressings on Acinetobacter baumannii-contaminated full-thickness burns in rats. Burns 2009; 35: 270–3. [DOI] [PubMed] [Google Scholar]
- 217. Medeiros VFLP, Azevedo IM, Rego ACM. et al. Antibacterial properties and healing effects of Melipona honey in MRSA-infected wounds of rats. Acta Cir Bras 2016; 31: 327–32. [DOI] [PubMed] [Google Scholar]
- 218. Yeo CK, Vikhe YS, Li P. et al. Hydrogel effects rapid biofilm debridement with ex situ contact-kill to eliminate multidrug resistant bacteria. ACS Appl Mater Interfaces 2018; 10: 20356–67. [DOI] [PubMed] [Google Scholar]
- 219. Touzel RE, Sutton JM, Wand ME.. Establishment of a multi-species biofilm model to evaluate chlorhexidine efficacy. J Hosp Infect 2016; 92: 154–60. [DOI] [PubMed] [Google Scholar]
- 220. Uygur F, Őzyurt M, Evinc R. et al. Comparison of octenidine dihydrochloride (Octenisept®), polihexanide (Prontosan®) and povidone iodine (Betadine®) for topical antibacterial effects in Pseudomonas aeruginosa-contaminated, full skin thickness burn wounds in rats. Cent Eur J Med 2008; 3: 417–21. [Google Scholar]
- 221. Hoekstra MJ, Westgate SJ, Mueller S.. Povidone-iodine ointment demonstrates in vitro efficacy against biofilm formation. Int Wound J 2017; 14: 172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Lipsky B. Diabetic foot infections: current treatment and delaying ‘the post-antibiotic era’. Diabetes Metab Res Rev 2016; 32 Suppl 1: 246–53. [DOI] [PubMed] [Google Scholar]
- 223. Uckay I, Berli M, Sendi P. et al. Principles and practice of antibiotic stewardship in the management of diabetic foot infections. Curr Opin Infect Dis 2019; 32: 95–101. [DOI] [PubMed] [Google Scholar]
- 224. Cooper R, Kirketer-Møller K.. Non-antibiotic antimicrobial interventions and antimicrobial stewardship in wound care. J Wound Care 2018; 27: 355–77. [DOI] [PubMed] [Google Scholar]
- 225. Kampf G (ed.). Antiseptic stewardship for wound and mucous membrane antiseptics. In: Antiseptic Stewardship: Biocide Resistance and Clinical Implications. Springer International Publishing, 2018; 689–694. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.