Abstract
Background
In Tanzania more than 28% of all multi-drug resistant tuberculosis (MDR-TB) cases occur in Dar es Salaam. However, information about management and clinical outcomes of patients with MDR-TB in the region is scarce, and hence the need for this study.
Methods
A 5-year retrospective cohort study was conducted in six centres in Dar es Salaam. Descriptive statistics were used to summarize social demographics and clinical characteristics. Associations between occurrence of adverse events, regimen change and cure were determined using the Chi-square test whereas factors associated with mortality were determined using the Log-ranking test and Cox regression model.
Results
Three-hundred patient files were found and reviewed. The majority were male 199 (66.3%), aged 25–44 years [176 (58.7%)] and 89 (30.1%) were HIV co-infected. 186 (62%) completed their treatment, 68 (22.0%) were on treatment and 9 (3.3%) were lost to follow-up. The majority, 152 (51.0%) were managed using long MDR-TB regimens. The overall mortality rate was 5.7 per 1000 MDR-TB patients. A higher mortality rate was associated with being ≥45 years [adjusted hazard ratio (AHR): 10.82, 95% CI: 1.14–102.74, P = 0.038), female (AHR: 5.92, 95% CI: 1.75–20.08, P = 0.004), on a short anti-TB regimen (AHR: 4.34, 95% CI: 1.41–13.35, P = 0.010), HIV co-infected [crude hazard ratio (CHR): 2.56, 95% CI: 1.01–6.50, P = 0.048), on concomitant long-term medication use (CHR: 2.99, 95% CI: 1.17–7.64, P = 0.022) and having other co-morbidities (CHR: 3.45, 95% CI: 1.32–9.02, P = 0.011).
Conclusions
MDR-TB mortality was associated with short anti-TB regimens, sex, age, concomitant long-term medication use and HIV coinfection. In this population, use of long and individualized regimens is recommended.
Introduction
The WHO and its partners initiated the STOP TB strategy with targets of reducing TB prevalence and deaths by 50% by 2015 and completely eliminating TB as a public health problem by 2050.1 However, the emergence of MDR-TB threatens to derail the progress that has been made so far.2
In 2018, there were approximately half a million (range, 417 000–556 000) new cases of rifampicin-resistant (RR) TB. 390 000 (78%) of these had MDR-TB).3 On average, an estimated 6.2% of people with MDR-TB have XDR-TB.3 Drug resistance surveillance data show that an estimated 240 000 people died from MDR/RR-TB in 2016. In 2016, 8000 patients with XDR-TB were reported worldwide.3 To date, 123 countries have reported at least one XDR-TB case.
Tanzania is one of the 30 countries with high burden of TB.3 TB case notification in the country increased about two-fold from 2017 to 2018,3 with an average annual increase of 10%. In 2018, a total of 449 MDR-TB cases were notified in Tanzania, of which 409 (91%) started treatment, representing an increment of 7.5% compared with 2017. The majority of MDR-TB cases were from Dar es Salaam (28%) followed by Mwanza (11%), Geita (6%), Pwani and Mara (5% each), with 3% each contribution from Morogoro, Mbeya, Arusha, Mtwara and Lindi. The remaining regions contributed 27% of all cases except one region (Katavi) where no cases were reported.4
Treatment success of susceptible TB in Tanzania has been reported to remain at an average of 90% in a cohort of 68 278 patients, with treatment success for MDR/DR-TB cases started on second-line treatment in 2016 was reported to be 80%.3 Recently, the country introduced a newer short MDR-TB treatment regimen consisting of bedaquiline and delamanid given for 9–11 months as per 2016 WHO recommendations,5 and has decentralized MDR-TB services on the Tanzanian mainland.6 Treatment regimens are classified as either standard short (intensified with at least six or seven first- and second-line anti-TB drugs, duration; 9–11 months), long [with relatively fewer drugs (four or five) but given for a long duration of at least 20 months] or individualized, also called long individualized, which contain repurposed (linezolid) and newer anti-TB drugs (bedaquiline and delamanid). The short regimen is the preferred first choice, and patients who cannot tolerate the drugs in the short regimen are put on the individualized regimen. We conducted this study to determine the management and clinical outcomes of MDR-TB patients under different anti-TB regimens in Dar es Salaam, the largest business centre and former capital of Tanzania, with a population of approximately five million people (almost 10% of the country’s population).7
Patients and methods
Study design and population
This study was a 5 year (2015–20) retrospective cohort study conducted in six Dar es Salaam Region MDR-TB centres (Muhimbili National Hospital, Mwananyamala Regional Referral Hospital, Temeke Regional Referral Hospital, Amana Regional Referral Hospital, Ukonga MDR-TB and Mbagala MDR- TB centres). Three hundred patient medical files were reviewed in Dar es Salaam MDR-TB centres to establish the clinical characteristics and management of patients with MDR-TB. The confirmation of a resistant TB strain was done using rapid genotypic test (Gene Xpert) or conventional phenotypic culture method on Lowenstein-Jensen media using a proportion method.8 An isolate was regarded as MDR-TB when the isolate was resistant to both isoniazid and rifampicin.9
Data management and analysis
Patient information regarding the management of MDR-TB and social demographic information such as age, marital status, sex and residence, HIV status and information regarding management of MDR-TB and adverse events were recorded as collected from patient files using a checklist/case report form. Patient information was entered manually on a Microsoft Excel Sheet then exported to a statistical package for social science (SPSS version 25, Chicago Inc., USA) for analysis. Descriptive statistics such as social demographics and clinical baseline characteristics were summarized using proportions. The associations between occurrence of adverse events, change in regimens and cure were determined using Chi-square test. Overall mortality rate was calculated using an incidence rate. Furthermore, the Log-ranking test was used to graphically compare the probability of death with time (in months). Factors which had P<0.2 in Log-ranking test qualified for Cox regression analysis where crude (CHR) and adjusted hazard ratios (AHR) were the effect measures for univariate and multivariate Cox regression analysis, respectively. P<0.05 was considered statistically significant and the confidence interval (CI) was 95%.
Ethics
Ethics approval to conduct this study was sought from Muhimbili University of Health and Allied Sciences, Research and Publications Committee (Reference No: MUHAS-REC-2-2020-089). Furthermore, permissions to collect data from patient files were requested from Executive Director for Muhimbili National Hospital, Medical Officers in-charge for Mwananyamala, Temeke and Amana Regional Referral Hospitals, and District Medical Officers in-charge for Mbagala and Ukonga MDR-TB health centres. Confidentiality of patients’ information was ensured through the use of codes (numbers) during data collection, analysis, interpretation and presentation.
Results
Social demographic characteristics of MDR-TB patients
A total of 300 patient records were found and reviewed. The majority of patients were male 199 (66.3%), aged between 25–44 years [176 (58.7%)], married [73 (45.35%)] and from Ilala district [106 (35.3%)] (Table 1). In total, 108 (36.4%) patients had co-morbidities, of whom 89 (30.1%) were HIV positive. The majority of the patients [152 (51.0%)] were treated using long regimens, consisting of relatively fewer drugs (four or five) but given for a long duration of at least 20 months, 62 (20.8%) were on an individualized regimen, which contains repurposed (linezolid) and newer anti-TB drugs (bedaquiline and delamanid) and 84 (28.2%) were on a standard short regimen consisting of at least six or seven first- and second-line anti-TB drugs for a duration of 9–11 months. Switching of regimens was reported in 39 (13.7%) patients and 81 (27.0%) of the studied patients had a documented adverse effect. Concomitant long-term medication use was reported in 100 (33.0%) patients, of whom 85 (85%) were using ART (Table 1).
Table 1.
Patients’ social demographic and clinical baseline characteristics
| Variable | Categories | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Age group (years) | ≤14 | 3 | 1.0 |
| 15–24 | 51 | 17.0 | |
| 25–44 | 176 | 58.7 | |
| 45–54 | 42 | 14.0 | |
| 55–64 | 16 | 5.3 | |
| ≥65 | 12 | 4.0 | |
| Sex | Male | 199 | 66.3 |
| Female | 101 | 33.7 | |
| Residence | Ilala | 106 | 35.3 |
| Temeke | 113 | 37.7 | |
| Ubungo | 25 | 8.3 | |
| Kinondoni | 50 | 16.7 | |
| Kigamboni | 3 | 1.0 | |
| Outside Dar es salaam | 3 | 1.0 | |
| Marital status | Single | 63 | 39.1 |
| Married | 73 | 45.3 | |
| Divorced | 13 | 8.1 | |
| Cohabiting | 10 | 6.2 | |
| Separated | 1 | 0.6 | |
| Widowed | 1 | 0.6 | |
| HIV | Positive | 89 | 30.1 |
| Co-morbidity | Yes | 108 | 36.4 |
| MDR TB regimena | Short | 84 | 28.2 |
| Long | 152 | 51.0 | |
| Individualized | 62 | 20.8 | |
| Change in regimen | Yes | 39 | 13.7 |
| Adverse effect | Yes | 81 | 27.0 |
| Long-term medication use | Yes | 100 | 33.0 |
| Concomitant long-term drugs | ART | 85 | 85.0 |
| Other drugs | 15 | 15.0 |
Medications used in the short, individualized and long-term regimens (Table S1, available as Supplementary data at JAC-AMR Online).
Of the 300 patients studied, 186 (62%) completed their treatment, 150 (50.0%) were cured and 68 (22.0%) were still on treatment. Out of 300 MDR-TB patients, death was reported in 24 (8.0%) patients; 14 (4.7%) patients were transferred out (referred to other centres) and 9 (3.3%) did not complete their follow-up (loss of follow-up) (Figure 1).
Figure 1.
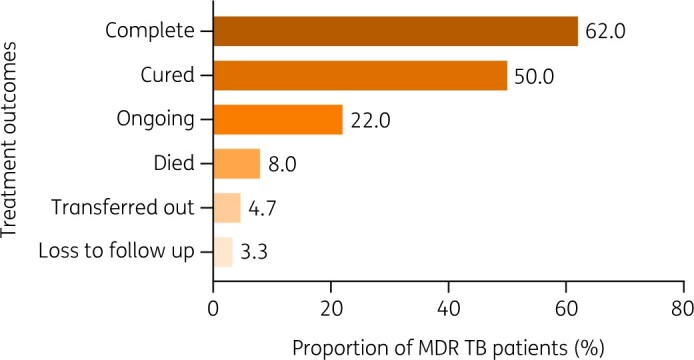
MDR-TB treatment outcomes profile. Of the 300 patients studied, 186 (62%) completed their treatment, 150 (50.0%) were cured, 68 (22.0%) were still on treatment. Out of 300 MDR-TB patients, death was reported in 24 (8.0%) of patients, whereas 14 (4.7%) were transferred out (referred to other centres) and 9 (3.3%) did not complete their follow-up (loss to follow-up).
Mortality rate among MDR TB patients
Mortality rate among MDR-TB patients was calculated as the incidence rate per 1000 patients. The overall mortality rate was found to be 0.0056639 (5.66 per 1000 MDR TB patients). Patients aged ≥45 years had the highest mortality rate (12.97 per 1000 MDR TB patients). Female patients had an incidence rate of 12.82 per 1000 MDR TB patients (higher than male patients); HIV co-infected patients presented a mortality rate of 10.38 per 1000 MDR TB patients. Short anti-TB regimens had the highest mortality rate compared with other regimens (14.41 per 1000 MDR TB patients). Patients with co-morbidities had a higher mortality rate (11.24 per 1000 MDR TB patients), likewise those on concomitant long-term drugs had a higher mortality rate (10.93 per 1000 MDR TB patients). The incidence rate for those with adverse events was 8.18 per 1000 MDR TB patients. Those who changed their regimen had 6.01 deaths per 1000 MDR TB patients and for marital status, the incident rate was 8.78 per 1000 MDR TB patients in those who were married (Table 2).
Table 2.
Mortality rate stratified by sociodemographic and clinical characteristics of the MDR TB patients in Dar es Salaam
| Characteristic | Category | Total patients (n) | Person time (months) | Deaths, n (%) | Incidence rate/1000 patients |
|---|---|---|---|---|---|
| Age (years) | ≤24 | 39 | 656 | 1 (2.5) | 1.52 |
| 25–44 | 123 | 1905.07 | 9 (7.3) | 4.72 | |
| ≥45 | 39 | 616.97 | 8 (20.5) | 12.97 | |
| Sex | Male | 134 | 2242 | 6 (4.5) | 2.68 |
| Female | 67 | 936.04 | 12 (17.9) | 12.82 | |
| HIV | Positive | 62 | 867.27 | 9 (14.5) | 10.38 |
| Negative | 138 | 2303.77 | 9 (6.5) | 3.91 | |
| MDR TB regimen | Longer | 125 | 2441 | 9 (7.2) | 3.69 |
| Shorter | 60 | 555.04 | 8 (13.3) | 14.41 | |
| Individualized | 16 | 182 | 1 (6.3) | 5.49 | |
| Comorbidity | Yes | 70 | 978.27 | 11 (15.7) | 11.24 |
| No | 131 | 2199.77 | 7 (5.3) | 3.18 | |
| Concomitant long-term medication use | Yes | 66 | 915.27 | 10 (15.2) | 10.93 |
| No | 135 | 2262.77 | 8 (5.9) | 3.54 | |
| Adverse events | Yes | 55 | 855.5 | 7 (12.7) | 8.18 |
| No | 146 | 2322.54 | 11 (7.5) | 4.74 | |
| Change in regimen | Yes | 21 | 333 | 2 (9.5) | 6.01 |
| No | 171 | 2751.04 | 16 (9.4) | 5.82 | |
| Marital status | Married | 58 | 911.47 | 8 (13.8) | 8.78 |
| Not married | 51 | 747.57 | 6 (11.8) | 8.02 |
Adverse events among MDR-TB patients
As shown in Table 3 there was no association between age category, sex, marital status, HIV status, treatment regimen, concomitant long-term medication use or co-morbidity and the occurrence of adverse events (P>0.05) (Table 3).
Table 3.
Factors associated with incidence of adverse events among MDR-TB patients in Dar es Salaam
| Characteristics | Category | Total patients | Adverse events n (%) | P value |
|---|---|---|---|---|
| Age (years) | ≤24 | 54 | 10 (18.5) | 0.055 |
| 25–44 | 176 | 45 (25.6) | ||
| ≥45 | 70 | 26 (37.1) | ||
| Sex | Male | 119 | 49 (24.6) | 0.193 |
| Female | 101 | 32 (31.7) | ||
| Marital status | Married | 83 | 27 (32.5) | 0.810 |
| Not married | 78 | 24 (30.8) | ||
| HIV | Positive | 89 | 30 (33.7) | 0.090 |
| Negative | 207 | 50 (24.2) | ||
| MDR TB regimen | Longer | 152 | 50 (32.9) | 0.077 |
| Shorter | 84 | 18 (21.4) | ||
| Individualized | 62 | 13 (21.0) | ||
| Concomitant long-term medication use | Yes | 100 | 34 (34.0) | 0.053 |
| No | 200 | 47 (23.5) | ||
| Comorbidity | Yes | 108 | 35 (32.4) | 0.108 |
| No | 189 | 45 (23.8) |
Change of regimen among MDR-TB patients
There was no association between age category, sex, marital status, HIV status, treatment regimen, concomitant long-term medication use or co-morbidity and a change of anti-TB regimen (P>0.05) (Table 4).
Table 4.
Relationship between patient’s social demographic and clinical characteristics and changing anti-TB regimen
| Characteristics | Category | Total patients | Changed regimen n (%) | P value |
|---|---|---|---|---|
| Age (years) | ≤24 | 49 | 6 (12.2) | 0.074 |
| 25–44 | 167 | 18 (10.8) | ||
| ≥45 | 69 | 15 (21.7) | ||
| Sex | Male | 188 | 23 (12.2) | 0.321 |
| Female | 97 | 16 (16.5) | ||
| Marital status | Married | 82 | 12 (14.6) | 0.977 |
| Not married | 76 | 11 (14.5) | ||
| HIV | Positive | 82 | 14 (17.1) | 0.297 |
| Negative | 202 | 25 (12.4) | ||
| MDR TB regimen | Longer | 150 | 21 (14.0) | 0.767 |
| Shorter | 78 | 9 (11.5) | ||
| Individualized | 57 | 9 (15.8) | ||
| Concomitant long-term medication use | Yes | 94 | 18 (19.1) | 0.068 |
| No | 191 | 21 (11.0) | ||
| Comorbidity | Yes | 101 | 18 (17.8) | 0.142 |
| No | 182 | 21 (11.5) |
Cure patterns among MDR-TB patients
As shown in Table 5, there was a statistically significant association between sex and cure rate (P = 0.005), HIV status (P = 0.032), concomitant long-term medication use (P = 0.031) and co-morbidity (P = 0.003). There was no association between cure rate and age category, marital status, MDR-TB regimen and adverse events (P>0.05) (Table 5).
Table 5.
Factors associated with cure among MDR-TB patients
| Characteristics | Categories | Total patients | Cured, n (%) | P value |
|---|---|---|---|---|
| Age (years) | ≤24 | 37 | 33 (89.2) | 0.090 |
| 25–44 | 104 | 94 (90.4) | ||
| ≥45 | 33 | 23 (69.7) | ||
| Sex | Male | 116 | 106 (91.4) | 0.005 |
| Female | 58 | 44 (75.9) | ||
| Marital status | Married | 50 | 41 (82.0) | 1.000 |
| Not married | 42 | 35 (83.3) | ||
| HIV | Positive | 50 | 39 (78.0) | 0.032 |
| Negative | 123 | 111 (90.2) | ||
| MDR-TB regimen | Longer | 109 | 97 (89.0) | 0.502 |
| Shorter | 62 | 53 (85.5) | ||
| Concomitant long-term medication use | Yes | 54 | 42 (77.8) | 0.031 |
| No | 120 | 108 (90.0) | ||
| Comorbidity | Yes | 58 | 44 (75.9) | 0.003 |
| No | 115 | 106 (92.2) | ||
| Adverse events | Yes | 50 | 41 (82.0) | 0.307 |
| No | 124 | 109 (87.9) |
P values <0.05 are shown in bold.
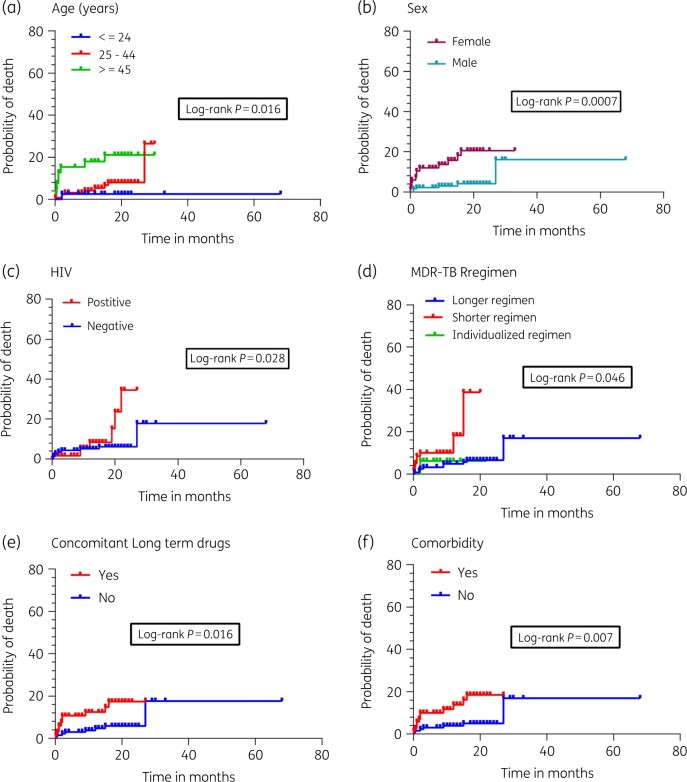
A greater rate of deaths was observed in patients aged 45 years and above (Figure 2a, P = 0.016), females (Figure 2b, P = 0.0007), HIV positive patients (Figure 2c, P = 0.028), those who were on short regimens (Figure 2d, P = 0.046), those on concomitant long-term medication use (Figure 2e, P = 0.016) and those who had co-morbidities (Figure 2f, P = 0.007).
Figure 2.
Association between probability of death and time using Log-ranking test. A greater rate of deaths was observed in patients aged 45 years and above (Figure 2a, P = 0.016), females (Figure 2b, P = 0.0007), HIV-positives (Figure 2c, P = 0.028) and those who were on short regimens (Figure 2d, P = 0.046), those on concomitant long-term medication use (Figure 2e, P = 0.016) and those who had co-morbidities (Figure 2f, P = 0.007).
Factors associated with mortality among MDR-TB patients
All factors with P value less than 0.2 in Log-ranking test were subjected to Cox-regression model. Cox-regression analysis found that patients aged ≥45 years had a 10.82-fold increased risk of death with MDR-TB as compared with those aged ≤24 years (AHR: 10.82, 95% CI: 1.14–102.74, P = 0.038). Female patients had 5.92-fold higher chance of dying with MDR-TB as compared with male patients (AHR: 5.92, 95% CI: 1.75–20.08, P = 0.004). Patients who were treated with short regimens were 4.34-fold more likely to die of MDR-TB as compared with those who were on long regimens (AHR: 4.34, 95% CI: 1.41–13.35, P = 0.010). Univariate analysis found an association between mortality and HIV status (CHR: 2.56, 95% CI: 1.01–6.50, P = 0.048), concomitant long-term medication use (CHR: 2.99, 95% CI: 1.17–7.64, P = 0.022) and co-morbidity (CHR: 3.45, 95% CI: 1.32–9.02, P = 0.011). However, there was no statistically significant association between mortality and HIV infection (AHR: 0.22, 95% CI: 0.3–1.60, P = 0.134), concomitant long-term medication use (AHR: 0.85, 95% CI: 0.09–7.76, P = 0.889) or co-morbidity (AHR: 6.81, 95% CI: 0.71–65.66, P = 0.097) (Table 6).
Table 6.
Univariate and multivariate Cox regression analysis for the risk factors for mortality among MDR-TB patients
| Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Categories | CHR | 95% CI | P value | AHR | 95% CI | P value |
| Age (years) | ≥45 | 8.29 | 1.04–66.32 | 0.046 | 10.82 | 1.14–102.74 | 0.038 |
| 25–44 | 2.91 | 0.37–22.93 | 0.312 | 3.48 | 0.39–31.36 | 0.267 | |
| ≤24 | Ref | ||||||
| Sex | Female | 4.76 | 1.77–12.84 | 0.002 | 5.92 | 1.75–20.08 | 0.004 |
| Male | Ref | ||||||
| HIV | Positive | 2.56 | 1.01–6.50 | 0.048 | 0.22 | 0.3–1.60 | 0.134 |
| Negative | Ref | ||||||
| MDR–TB regimen | Short | 3.54 | 1.22–10.32 | 0.021 | 4.34 | 1.41–13.35 | 0.010 |
| Individualized | 1.37 | 0.17–11.08 | 0.771 | 1.48 | 0.17–12.98 | 0.722 | |
| Long | Ref | ||||||
| Concomitant long-term drug | Yes | 2.99 | 1.17–7.64 | 0.022 | 0.85 | 0.09–7.76 | 0.889 |
| No | Ref | ||||||
| Comorbidity | Yes | 3.45 | 1.32–9.02 | 0.011 | 6.81 | 0.71–65.66 | 0.097 |
| No | Ref | ||||||
Ref, reference; CHR, crude hazard ratio; AHR, adjusted hazard ratio; P values <0.05 are shown in bold.
Discussion
This study found a mortality rate of 5.66 per 1000 MDR TB patients. Death was reported in 24 (8.0%) patients while 9 (3.3%) did not complete their follow-up (lost to follow-up). In this cohort, age and sex were associated with increased mortality. Furthermore, patients co-infected with HIV had a higher rate of dying of MDR-TB as compared with HIV-negative individuals. Patients aged ≥45 years had the highest mortality rate (12.97 per 1000 MDR TB patients). Female patients had an incidence rate of 12.82 per 1000 MDR TB patients, higher than male patients, while HIV co-infected patients presented a mortality rate of 10.38 per 1000 MDR TB patients. A short anti-TB regimen was associated with highest mortality rate compared with other regimens (14.41 per 1000 MDR TB patients). Patients with co-morbidities had a higher mortality rate (11.24 per 1000 MDR TB patients), likewise those on concomitant long-term drugs had a higher rate of dying (10.93 per 1000 MDR TB patients). The incidence rate for those with adverse events was 8.18 per 1000 MDR TB patients. Those who changed their regimen had 6.01 deaths per 1000 MDR TB patients and for marital status, the incident rate was 8.78 per 1000 MDR TB patients in those who were married.
These findings are consistent with other studies and reports. According to the 2018 Tanzania National TB and Leprosy program report, 65% of all MDR TB patients were male, and 30.0% of patients were HIV co-infected, and 54.2% of patients were aged 25–44 years.1 In that report, 15% of all enrolled patients died, while 3.2% were lost to follow up. We speculate that the differences in mortality with our study could be caused by many factors, including time to treatment, as reported elsewhere.10 Our patients were from Dar es Salaam, a city with the highest number of TB diagnostic facilities compared with other regions in the country.10
This study found co-morbidities, HIV/AIDS and age to be associated with increased mortality. A systematic review and meta-analysis of treatment outcomes of MDR patients in sub-Saharan Africa found that HIV-co-infected patients were less likely to be successfully treated than those who were HIV negative.11 Several other studies have reported association between HIV, low initial body weight, co-morbidities/co-infections and age with death from MDR TB.11–15 Advanced age is commonly associated with a weakened immune system and co-morbidities, which impair the body’s ability to fight infections. Both TB and HIV/AIDS are highly debilitating chronic diseases and a patient infected with both is more likely to have a poor prognosis. HIV/AIDS leads to the suppression of the immune system, which in turn becomes less capable of fighting infections such as TB.
This study found MDR-TB patients who were started on a short regimen had a higher mortality rate as compared with those who were started on a long/individualized regimen. Similarly, the STREAM trial reported higher death rates (8.5%) in patients receiving the short regimen, as compared with 6.4% among those who received the long regimen.3 The shorter regimen recorded a higher proportion of deaths (7.6%) than the longer regimen (4.6%) in a recent systematic review and meta-analysis.16 Perhaps this may be attributed to the fact that the short regimen contains many drugs (about at least seven) which have to be used within a short time at their full doses, thus the chances for life-threatening adverse events and non-compliance are high. Although we did not find a higher proportion of adverse events in the short regimen, we believe that this may be caused by lack of documentation. Yet again, this regimen is more likely to be initiated for patients who report in clinically poor condition, and that these patients are more likely to suffer poor prognosis.
Meanwhile, the study found no significant difference in terms of cure rates between the short and long regimens (85.5 versus 89%, P = 0.502). Likewise, a short regimen was similar to a long regimen with respect to the primary efficacy outcome in the STREAM trial.3 However, in the aforementioned systematic review and meta-analysis, treatment success was higher with the shorter regimen than with longer regimens, due to less loss to follow-up with the former. However, the risk of failure or relapse was slightly higher with the shorter regimen.16 The strength of this study lies in the fact that we analysed all available patient files in all the MDR TB treatment centres in Dar es Salaam. We were therefore not limited in terms of sample size. Being a retrospective cohort study, findings from this study are limited by documentation and reporting bias. No statistical test was conducted to measure the effect of missing data on the desirable outcomes, but the analysis was only limited to factors which were correctly reported and documented. Owing to data limitations, the study could not assess the association between MDR TB mortality and initial body weight, smoking, CD4 cell count, ART use or any laboratory parameters such as antibiotic susceptibility. This study was unable to assess the association between a regimen used and time to cure.
Conclusions
The mortality among MDR-TB patients in Dar es Salam was associated with a short anti-TB regimen, concomitant long-term medication use and HIV co-infection. Based on these findings, we suggest that long and individualized regimens should be prioritized for management of MDR-TB in the Dar es Salaam region, while, at the same time, giving special attention to the management of co-infections and co-morbidities.
Supplementary Material
Acknowledgements
We thank the officers in charge for granting permission to collect data in their facilities. We acknowledge the support received from medical staff working in MDR-TB centres in Dar es Salaam region.
Funding
This study was funded by the Swedish International Development Cooperation Agency (Sida), Sweden through Muhimbili University of Health and Allied Sciences. The funder did not participate in the design of the study, data collection, analysis, interpretation, and manuscript preparation.
Transparency declarations
None to declare.
References
- 1. World Health Organization (WHO). The Stop TB Strategy. 2010. https://www.who.int/tb/strategy/stop_tb_strategy/en/.
- 2. Zignol M, Hosseini MS, Wright A et al. Global incidence of multidrug‐resistant tuberculosis. J Infect Dis 2006; 194: 479–85. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO). Multidrug-Resistant Tuberculosis (MDR-TB). 2017. https://www.who.int/tb/challenges/mdr/MDR-RR_TB_factsheet_2017.pdf.
- 4. United Republic of Tanzania (URT). The National Tuberculosis and Leprosy Programme. 2018. https://ntlp.go.tz.
- 5. WHO. World Health Organization (WHO) treatment guidelines for drug- resistant tuberculosis. 2016. https://www.who.int/publications/i/item/9789241549639.
- 6. United Republic of Tanzania (URT). The National Tuberculosis and leprosy Programme Annual report for 2017. 2017. https://ntlp.go.tz.
- 7. United Republic of Tanzania (URT). National Bureau of Statistics: 2012 Population and Housing Census Population Distribution by Administrative areas. https://www.nbs.go.tz/nbs/takwimu/census2012/2012_CENSUSVol3DATAsheet.pdf
- 8. National Tuberculosis and Leprosy Program United Republic of Tanzania. Guidelines for Management of Multi Drug Resistant - TB in Tanzania. 2018. https://ntlp.go.tz.
- 9. Baxter R, Hastings N, Law A et al. Standard treatment guidelines and National essential medicines Lists. Anim Genet 2008; 39: 561–3. [DOI] [PubMed] [Google Scholar]
- 10. Mollel E, Lekule I, Lynen L et al. Effect of reliance on Xpert MTB/RIF on time to treatment and multidrug-resistant tuberculosis treatment outcomes in Tanzania : a retrospective cohort study. Int Health 2019; 11: 520–7. [DOI] [PubMed] [Google Scholar]
- 11. Chem ED, Van Hout MC, Hope V. Treatment outcomes and antiretroviral uptake in multidrug-resistant tuberculosis and HIV co-infected patients in Sub Saharan Africa : a systematic review and meta-analysis. BMC Infect Dis 2019; 19: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fantaw D, Feyissa M, Hamid S et al. Assessment of the survival status and risk factors for the mortality among multidrug resistant tuberculosis patients at Adama and Bishoftu general hospitals, Oromia, Ethiopia : a retrospective cohort study. Adv Pharmacoepidemiol Drug Saf 2018; 7: 1–5. [Google Scholar]
- 13. Mollel EW, Chilongola JO. Predictors for mortality among multidrug-resistant tuberculosis patients in Tanzania. J Trop Med 2017;2017: 9241238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chingonzoh R, Manesen MR, Madlavu MJ et al. Risk factors for mortality among adults registered on the routine drug resistant tuberculosis reporting database in the Eastern Cape Province, South Africa, 2011 to 2013. PLoS One 2018; 13: e0202469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Girum T, Tariku Y, Dessu S. Survival status and treatment outcome of Multidrug Resistant Tuberculosis (MDR-TB) among patients treated in Treatment Initiation Centers (TIC) in South Ethiopia: a retrospective cohort study. Ann Med Heal Sci Res 2017; 7: 331–6. [Google Scholar]
- 16. Abidi S, Achar J, Mohamed M et al. Standardised shorter regimens versus individualised longer regimens for rifampin- or multidrug-resistant TB. Eur Respir J 2020; 55: 1901467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.