Abstract
Antibiotics underpin the ‘modern medicine’ that has increased life expectancy, leading to societies with sizeable vulnerable elderly populations who have suffered disproportionately during the current COVID-19 pandemic. Governments have responded by shuttering economies, limiting social interactions and refocusing healthcare. There are implications for antibiotic resistance both during and after these events. During spring 2020, COVID-19-stressed ICUs relaxed stewardship, perhaps promoting resistance. Counterpoised to this, more citizens died at home and total hospital antibiotic use declined, reducing selection pressure. Restricted travel and social distancing potentially reduced community import and transmission of resistant bacteria, though hard data are lacking. The future depends on the vaccines now being deployed. Unequivocal vaccine success should allow a swift return to normality. Vaccine failure followed by extended and successful non-pharmaceutical suppression may lead to the same point, but only after some delay, and with indefinite travel restrictions; sustainability is doubtful. Alternatively, failure of vaccines and control measures may prompt acceptance that we must live with the virus, as in the prolonged 1889–94 ‘influenza’ (or coronavirus OC43) pandemic. Vaccine failure scenarios, particularly those accepting ‘learning to live with the virus’, favour increased outpatient management of non-COVID-19 infections using oral and long t½ antibiotics. Ultimately, all models—except those envisaging societal collapse—suggest that COVID-19 will be controlled and that hospitals will revert to pre-2020 patterns with a large backlog of non-COVID-19 patients awaiting treatment. Clearing this will increase workloads, stresses, nosocomial infections, antibiotic use and resistance. New antibiotics, including cefiderocol, are part of the answer. The prescribing information for cefiderocol is available at: https://shionogi-eu-content.com/gb/fetcroja/pi.
Introduction
The modern medical era began around 1937–42, as systemic sulphonamides and penicillin mitigated the hazard of bacterial infection, opening medical and surgical possibilities that were previously unthinkable.
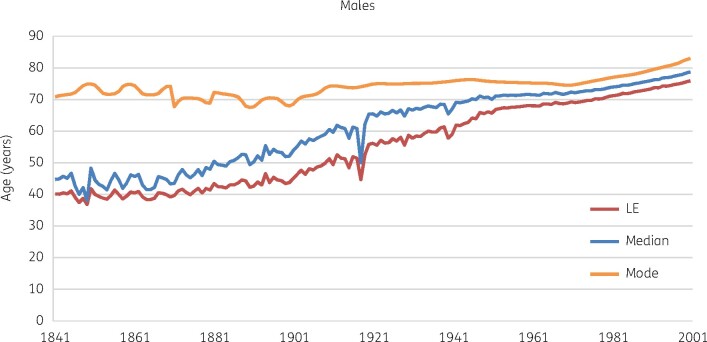
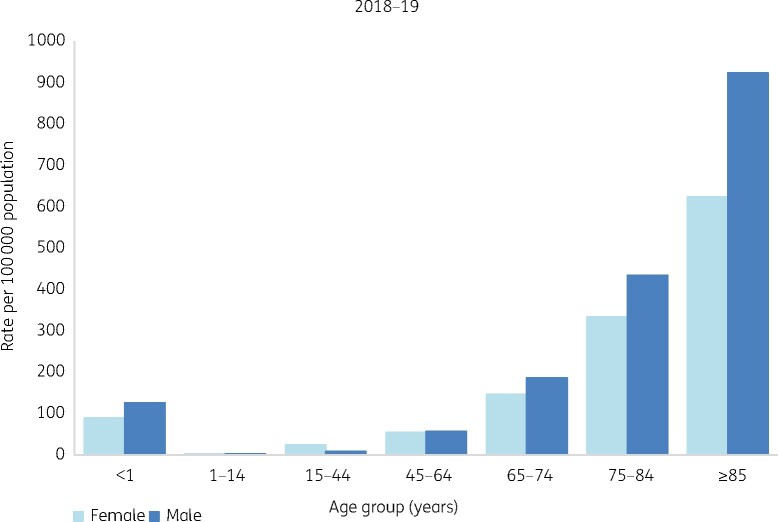
Antibiotics remain the bedrock of what followed. Complex surgery, intensive care, transplants and immunosuppressive treatments all would be impossible if infection could not reliably be controlled. In the community, pneumococcal pneumonia still kills the debilitated, but no longer threatens the likes of Jane Austen’s Marianne Dashwood. Along with earlier improvements in public health, modern medicine has made early non-violent death rare in advanced societies. Mean, median and modal life expectancies have converged (Figure 1) then extended.1 The caveat is that late-life years of ill health have extended too,2 giving a growing frail elderly population with chronic illness and cognitive decline, particularly in Europe, North America and East Asia.3 These citizens are the frequent victims of opportunist Gram-negative bacteria, with accumulating resistance (Figure 2).4
Figure 1.
Three measures of changing lifespan for men in the UK. Data Source: Office for National Statistics.1 LE, life expectancy. Patterns for women are similar though life expectancy is slightly longer.
Figure 2.
Incidence of E. coli bacteraemia in England and Wales, by age. Data source: PHE.4
Until 2020 this medical edifice grew without major viral challenge. Influenza pandemics in 1958–59 and 1968–69 killed many but were terminated by a mixture of strain ‘burnout’ and vaccination.5 HIV took a grim toll but was largely avoidable by personal precautions and now is medically manageable. SARS-CoV-2 has changed the dynamic, whether temporarily or more permanently.
A brief history of COVID-19
First reports of COVID-19 seeped from Wuhan late in 2019, with the causative coronavirus SARS-CoV-2 putatively having jumped from bats in a ‘seafood’ market. Laboratory escape is plausible too, as Wuhan hosts centres for coronavirus research, but is hotly disputed.6
During January to February 2020, outbreaks occurred in China. By February/March infection was spreading in Iran, then Europe. The USA was hit next, with major outbreaks in the northeast, particularly New York and New Jersey. Extensive spread followed in the southern USA, Latin America and India. The pandemic peaked in northern Europe and the north-eastern USA in the early spring, with subsequent declines in infections, hospitalizations and deaths through the late spring and summer before a resurgence in the northern autumn and winter. Argentina, with the seasons reversed, showed the converse pattern, with peak deaths in October, at the end of the southern winter. With some exceptions, including a current (January 2021) upsurge in South Africa, these patterns broadly support the view that SARS-CoV-2 is transitioning from being a ‘new pandemic virus’ to an ‘endemic winter respiratory virus’, joining the four long-established coronaviruses (229E, OC43, NL63 and HKU1) that account for 10%–20% of common colds.7 A few countries, notably Taiwan, Australia and New Zealand, have effectively isolated themselves from the pandemic by a combination of entry restrictions and strict containment efforts whenever small clusters have been detected. Central Africa has been little affected.
Like other single-stranded RNA viruses, SARS-CoV-2 is highly mutable, with over 20 000 sequence variants described. There is current concern about particular variants, including types that first circulated extensively in the UK (VUI202012/01 or B1.1.1.7), South Africa (1.351) and Brazil (P1). These appear to spread more efficiently and, in some cases, may have modifed vaccine-relevant epitopes (see below); there are no substantiated data to indicate that they are more lethal.8
Most COVID-19 infection is mild, inconsequential, and self-limiting. Many only learn that they have been infected when they are found seropositive. Even when virus is detected by RT-PCR, half record no symptoms.9 Among those who do develop symptoms—predominantly fever, cough and shortness of breath along with loss of taste and smell—recovery generally follows after 1 week. But, for a minority, pulmonary symptoms worsen, necessitating hospitalization and, in extremis, supplementary oxygen or ventilation.10,11 Death occurs in 40%–50% of ICU cases,12 increasing with age, male gender, obesity, dementia, diabetes and cardiovascular or pulmonary disease.13
Estimation of fatality rates is fraught, since most mild infections pass unrecorded. In October 2020, the WHO suggested that c. 10% of the world’s population had been infected,14 and that deaths had then reached 1 million. This indicated an infection fatality rate of around 0.13%. Ioannidis,15 using seroprevalence data as the denominator, estimated 0.15%–0.2%. These statistics are reassuring but carry four caveats: (i) the proportion is significantly higher in countries with a large elderly population; (ii) sufficient severe cases can arise to overwhelm local or national ICU capacity, again especially if there is a large vulnerable elderly population;16 (iii) outbreaks in elderly care facilities can kill extensively, as in the UK, Sweden, New York, Italy and Spain;17–19 and (iv) even low mortality rates translate to numerous deaths in large populations. The aspects have dominated political debate, media coverage and policy response. As of this writing (January 2021) the UK NHS has around one-third of its beds occupied by patients infected with SARS-CoV-2, including more than half of its ICU beds, and is clearly showing stresses, emphasized in rolling 24 h news bulletins. Cold review of actual numbers gives a different perspective. From a UK population of 67 million, roughly 1.1 million (2%) were estimated to be infected with SARS-CoV-2 in early January,20 and just 3000—1 citizen in 22 000—were sufficiently sick to need ICU care. In other words, the central issue is a shortage of ICU beds for the minority who become severely ill, and staff to support them, not that COVID-19 has a high fatality rate.
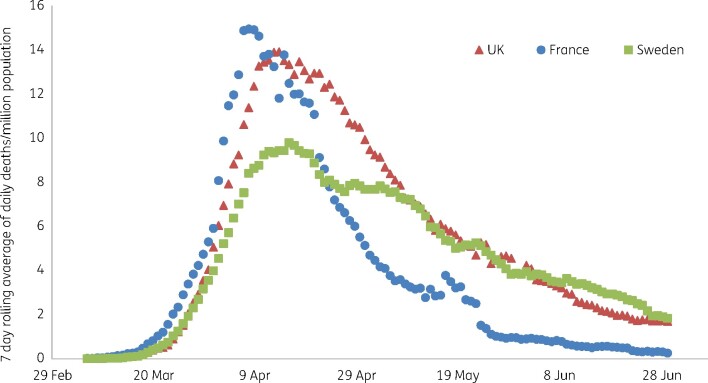
Most governments across Europe, North America and South America have enacted repeated ‘lockdowns’, closing the economy, confining populations and mandating social distancing. Reductions in deaths are attributed to these actions in China (strict lockdown), Europe and New York (varying strictness).21 There is, however, considerable scope for scepticism. In the initial spring wave, UK deaths peaked on 8 April,22 whereas lockdown began on 23 March, suggesting that new infections were already declining, assuming ≥19 days from infection to death (5–6 days incubation, >8 to hospitalization, ≥6 to death). Moreover, there is a remarkable similarity between the spring trajectories of death rates per million population between France, with a strict lockdown, the UK, with a less severe lockdown and Sweden, which had no lockdown beyond general advice on social distancing and restrictions on large events and bar-counter service (Figure 3). The likely explanation is that viral seasonality, not lockdown, underpinned the declines in each country. In an extensive analysis, De Larochelambert et al.23 reviewed deaths against lockdown stringency for 160 countries, finding little relationship and concluding that death rates largely reflected whether a country was in the temperate zone, typically had few deaths due to communicable diseases, and had a large elderly population for whom life expectancy was no longer extending. Strict lockdowns in seven Danish counties, enacted following discovery of a new variant in mink, had no greater effect than milder restrictions in four adjacent counties;24 death and infection trajectories in North and South Dakota are almost superimposable, despite more extensive business closure restrictions (and mask mandates) in the former. Lockdowns have only worked convincingly where they were enforced very strictly against outbreaks that were tiny in global terms, as in Melbourne, or where, as in China, they approximated to classical quarantine, by extracting and confining those found infected.
Figure 3.
First wave deaths from COVID-19 in France (strict lockdown; 13.8% Q2 fall in GDP), UK (moderate lockdown; 20.4% Q2 fall in GDP) and Sweden (no lockdown; 8.6% Q2 fall in GDP).96
Immediate impacts on antibiotic use and resistance
Most non-hospitalized COVID-19 patients receive no antibiotics. On the other hand, antibiotics—typically those used for community-acquired pneumonia (i.e. amoxicillin/clavulanate + macrolide; ceftriaxone + macrolide or levofloxacin)—are prescribed to hospitalized cases, though few have evidence of bacterial infection.25 Thus, Rawson et al.26 estimated that 72% of hospitalized COVID-19 patients received antibiotics but only 8% had bacterial infection and Langford et al.27 published similar figures. This suggests poor stewardship. Others note that bacterial coinfection is rarer than in influenza28 whilst a Swiss study found that ‘early’ antibiotics, before ICU transfer, had little benefit.29 Some hospitals initially administered hydroxychloroquine plus azithromycin against COVID-19 itself, though any benefits, and their mechanism, are disputed and the therapy has fallen into disfavour.30,31
ICU COVID-19 patients are usually intubated and face the risk of ventilator-associated pneumonia, mostly involving the Enterobacterales, Staphylococcus aureus and non-fermenters typical of this infection. Across five UK ICUs we found Klebsiella pneumoniae and Klebsiella aerogenes unusually prevalent in COVID-19 patients,32 whereas a single-hospital French study found an excess of non-fermenters.33
Ventilated COVID-19 patients often receive multiple antibiotic courses. At the height of the pandemic, stewardship policies were overridden,26 with ICU capacity increased. A Spanish hospital reported increased antibiotic use.34 Such data lead to concern that resistance may proliferate in hospitals as a result of COVID-19 pressures, though with scant evidence that it has actually done so. Resistance drivers in the community potentially may increase too. More general practice consultations are remote, and pre-COVID-19 studies suggest that US community physicians are more willing to prescribe antibiotics when consulted online for children35,36 though not for adults.37 Delivery of childhood vaccines has been disrupted,38 favouring resurgence of multiresistant vaccine serotypes of Streptococcus pneumoniae. Disruption of TB treatments will promote recrudescence, resistance and transmission of resistant variants, potentially leading to future treatment difficulties. This is an issue e.g. in India, where TB kills over 420 000 p.a., or around 2.5-fold more than COVID-19 to date (January 2021).39 Dentists—long discouraged from antibiotic use—were reduced to the options of antibiotics, analgesics and extraction, with aerosol-generating procedures forbidden.40,41
However, countervailing forces apply, reducing pressure for resistance. First, much non-COVID-19 hospital activity ceased during peaks of COVID-19 activity.42 In some jurisdictions, particularly the USA, hospital staff were laid off.43 The complex patients who are most vulnerable to multiresistant Gram-negative bacteria were no longer hospitalized. In the UK more people died at home and in care homes rather than in hospitals, where they likely would have received antibiotics.22 IV antibiotic use in English hospitals, measured as DDDs, was 32% lower in April–May 2020 than in April–May 2019 (P. Howard, Leeds Teaching Hospitals NHS Trust, personal communication). Similarly, wholesale IV antibiotic shipments to US hospitals, as DDDs, declined 30.7% in the same comparison (A. Carr, Needham & Company LLC, personal communication) with only 4/36 products showing increases. These data suggest reduced use, though we cannot exclude distortions from stock management inside hospitals, and the decline was only 6.9% if the month of March was added to the comparisons. A more recent report, comparing January to November 2020 with January to November 2019, indicates reduction in unit sales of systemic antibiotics as follows: Spain, 2.1%; France, 3.6%; Germany, 9.3%; Italy, 14%; and the UK, 14.5%.44 Reports of Escherichia coli bacteraemia to England’s mandatory surveillance in the July to September quarter of 2020 were 13.4% below those of the same quarter of 2019, sharply reversing a rising trend.45 The likely explanation is that many septic patients, who ordinarily would present to A&E, are failing to do so and are failing to receive IV antibiotic therapy. They may be represented among the persistently increased numbers of citizens presently dying at home rather than in hospitals.46 Changes in incidence are much less marked for bacteraemias involving pathogens that are mostly healthcare acquired, specifically K. pneumoniae and Pseudomonas aeruginosa.
Second, ICU triage, as applied at the height of the pandemic,47,48 militated against the ‘frequent flyer’ patients likely to be pre-colonized with multiresistant opportunists, favouring hospital-naive patients more likely to retain a susceptible flora.
Third, international travel has been dramatically curtailed, and this must reduce the transfer of resistance. London private hospitals ordinarily admit patients from the Middle East, frequently already colonized with resistant Gram-negative opportunists.49 This has stopped. Travellers e.g. to India commonly become colonized by ESBL-producing E. coli.50,51 Again, such travel has essentially ceased. Social distancing and travel restrictions reduce opportunities to catch and import ‘super gonorrhoea’;52,53 though closure of genitourinary medicine clinics54 will facilitate the spread of any already circulating, and a study in Milan indicated no reduction in presentations for acute syphilis and gonorrhoea in early 2020 compared with 2019.55
Social distancing and masks may impact community transmission of respiratory infections, reducing demand for antibiotics. The elderly often acquire pneumococci from grandchildren56 and will not do so if families cannot meet. In Italy, discontinued medical monitoring of otitis media-prone children led to reduced antimicrobial prescriptions in the late winter, without apparent harm.57
A final aspect, of uncertain impact, is the COVID-19-directed use of personal protective equipment. This might be expected to diminish cross-infection, but the inconvenience of changing between patients increased MRSA transmission in the 2003 SARS outbreaks in Canada and Singapore.58,59
What next? Possible scenarios
There are several plausible futures. These are set out below and their implications for resistance, summarized in Table 1, are then considered. There also are extreme possibilities, outlined briefly in the concluding paragraphs of this paper.
Table 1.
Implications of different scenarios for resistance
| Scenario | Central prediction on COVID-19 | Sustainable | Push towards more treatment in the community with oral, OPAT and long t½ agents | Surge of hospital activity to clear backlog | Travel; import of resistance |
|---|---|---|---|---|---|
| Vaccine overwhelmingly successful, and perceived as such | Burden no greater than seasonal influenza with this politically acceptable | Yes | Brief: until population vaccinated
|
Early
|
Briefly reduced, then normalized
|
| Vaccine failure or perceived failure. Prolonged emphasis on track and trace | Control requires eternal vigilance but is achieved and maintained | Doubtful | Brief (if successful): until COVID-19 reduced to low incidence
|
Early (if suppression successful)
|
Reduced for prolonged period
|
| Vaccine failure. Acceptance that virus is established, endemic and that lockdowns are ineffective or cause unacceptable collateral damage | Successive COVID-19 waves, ending in herd immunity; significant further direct mortality | Yes | Extended: until population immunity dominates
|
Delayed
|
Steady reversion to normality
|
Arrows indicate predicted change in selection pressure from the pre-COVID-19 situation: upward, increased selection pressure; horizontal, reversion to status quo ante; downward, reduced selection pressure.
Vaccines directed against SARS-CoV-2 (Table 2) have been developed at impressive speed. Based on interim analyses of ongoing trials, several have been given emergency use authorizations in multiple jurisdictions. Those deployed in Europe and North America are ‘new-technology’ mRNA and adenovirus vector products targeting the SARS-CoV-2 spike protein, which is crucial to viral receptor binding. Classical inactivated virus vaccines have been developed in China and are finding use in South East Asia, Latin America and the Middle East. Deployment is most advanced in Israel, with most (>80%) of the population now vaccinated using the Pfizer BioNTech product, but is progressing rapidly e.g. in the UK, UAE, USA and Chile.
Table 2.
Vaccines against SARS-CoV-2
| Vaccine | Manufacturer | Type | Efficacy | Notes | Reference |
|---|---|---|---|---|---|
| BNT162b2 | Pfizer BioNTech | mRNA | 95% | 122 | |
| mRNA-1273 | Moderna | mRNA | 94.1% | 123 | |
| Sputnik | Gamaleya Institute | adenovirus vector | 91.4% | 124 | |
| ChAdOx1 nCoV-19 | AstraZeneca/Oxford University | adenovirus vector | 53.4%–90.0% | efficacy varied with subgroup, dosage and dosage interval | 125 |
| BBIBP-CorV | Sinopharm | inactivated virus | 79%–86% | 126 | |
| CoronaVac | Sinovac | inactivated virus | 50.4% | 127 |
Although early results are promising, considerable uncertainty remains. First, since use is based on interim trial analyses the duration of protection is unknown. Post-infection immune responses to the classical coronaviruses (229E, HKU1, NL63 and OC43) fade swiftly, restoring vulnerability to infection, though this is generally mild.60 Infection-induced IgG to SARS-CoV-2 declines rapidly too,61,62 especially in asymptomatic cases, suggesting a similar risk, though clinically manifest reinfections seem rare, perhaps owing to persistent T cell-mediated immunity.63 Secondly, there is uncertainty about vaccine responses in the vulnerable elderly with ‘adaptive immunosenescence’.64 Thirdly, it is uncertain whether the vaccines will prevent infection or will reduce severity whilst leaving infected vaccinees as vectors of infection. Last, some emerging virus variants have mutations affecting the spike protein, and it is uncertain whether the present vaccines will reliably cover all present and future variants.8
The optimistic scenario is that vaccines overwhelmingly succeed, reducing the threat of SARS-CoV-2 at least to that of seasonal influenza (which typically has 10 000–30 000 attributed deaths annually in England),65 and that the public accept this situation, allowing a return to normality. At worst, in this scenario, an annual booster shot will be needed, particularly for the elderly and those caring for them, and perhaps with some adaptation to cover prevalent variants, as with influenza vaccines.
The pessimistic scenario is that vaccines provide only modest and brief protection, most probably owing to the proliferation of diverse spike protein variants and/or to general failure to protect the most vulnerable elderly. Failure might also arise if the public, after a year of saturation propaganda, can be satisfied by nothing less than ‘zero COVID’.
Substantial vaccine failure (or unrealistic demands for complete suppression) could be met with indefinite restrictions on social interactions along with extensive track and trace systems. Incoming travellers, including returning nationals, would require testing or quarantine; outgoing travellers would enter a dangerous world unless all countries follow this approach (which they are not doing). The strategy may be sustainable for a remote island, possibly New Zealand, but seems unfeasible in the long term for a trading nation, let alone for a continental union with free movement and varied national approaches to COVID-19.
The alternative response to vaccine failure is to accept that SARS-CoV-2 has become endemic and must circulate, potentially in the form of diversifying spike protein variants that facilitate reinfection. Repeated exposure, together with modestly protective vaccines, should progressively reduce disease severity, especially among the young, who would age with SARS-CoV-2 as we all do with the four long-established coronaviruses. The difficulties with this model are (i) how best to protect the present cohort of most-vulnerable elderly, who lack both prior exposure and the ability to adapt, and (ii) how to re-educate a public that has been ‘trained’ to believe COVID-19 to be far more lethal than is actually the case.66
There is one tantalizing hint of how a future that accepted spread might unfold: the 1889–94 ‘Russian influenza’ pandemic. This is conventionally attributed to H2N2 or H3N8 influenza A,67,68 based on the serology of elderly patients tested decades later. An alternative hypothesis is that coronavirus OC43 was responsible, having evolved apart from a bovine coronavirus shortly beforehand.69 Like COVID-19 and unlike influenza, the 1889–94 infection selectively killed men, spared children70 and caused loss of taste and smell.71 Unlike earlier influenza epidemics it gave repeating similarly sized waves over 5 years, a point thought unusual at the time, and which seems exceptional compared with any influenza epidemic in the preceding 200 years or the subsequent 130.72,73 Such a prolonged pandemic fits a model whereby prior exposure to other coronaviruses gives partial cross-protection, as now postulated for SARS-CoV-2,74,75 but with cohorts regaining vulnerability as immune responses diminished, and perhaps experiencing more than one OC43 infection as immune-escaping mutants were selected. This is speculation, but the parallels are intriguing.
If correct and if predictive (two big ‘ifs’!), it implies that coevolution of man and virus may take half a decade to achieve equilibrium. Even today OC43 can cause lethal care home outbreaks.76
Implications of the scenarios for antibiotic usage and resistance
1. Vaccine success
If vaccines prove overwhelmingly successful there should be a progressive and increasingly exuberant return to the ‘old normal’ in human behaviour and (assuming solvency) travel. Hospitals will face a backlog of elective procedures, along with patients who, fearful of nosocomial COVID-19, had postponed seeking healthcare; one analysis suggests that this backlog may amount to almost 5 million hospital treatment episodes in the UK alone.77 Some will have more severe disease, including more advanced cancers, than would ordinarily be the case. Unless additional hospitals can be commissioned, and (the greater challenge) staffed, there will be considerable workload pressures, which are correlates of increased nosocomial infections,78 antibiotic use, and resistance. In short, once healthcare and travel revert to full capacity, more resistance should be expected.
A partial counterpoise will be the numbers of previously heavy users of healthcare who succumbed to COVID-19 or (because they could not access treatment in the COVID-19-dominated period) to other illnesses. UK excess mortality from March to June 2020 was 30% above normal, with half the deaths falling among care home residents.79 Their demise will reduce hospital demand, but this factor will be small: the great majority of the highly vulnerable population have survived the pandemic.
2. Perceived vaccine ‘failure’: long-term track and trace seeking ‘zero COVID’
The aim here, following vaccine disappointments, would be to suppress COVID-19 sufficiently that normality of a sort resumes within a closed system, as presently in Taiwan, Australia or New Zealand, all of which achieved early control of viral spread meaning that their hospitals are not under the pressures seen elsewhere. If successful, the medium-term implications for hospital antibiotic utilization would resemble the vaccine case. In the short term, the pressures would be rather different and would continue to resemble those that have pertained in the pandemic itself, both in respect of hospital workload being dominated by COVID-19 and with reduced hospital capacity caused by the needs (i) to socially distance beds, (ii) to cohort patients according to COVID-19 status, and (iii) for numerous staff to self-isolate following track and trace alerts. These factors may drive a shift to outpatient antibiotic therapy and long dosage-interval antibiotics, followed by rise in use, selection pressure and bacterial cross-infection once COVID-19 comes under control and hospitals move to clear their backlog. Such a model must assume drastic long-term reductions in international travel, as it would not be feasible to allow free movement to and from countries lacking similarly stringency. This would impede the transnational flow of resistant bacteria.
The issues with this model are not its implications for antibiotic resistance, which are broadly positive, at least in the short term, but its feasibility and its sustainability. Track and trace systems have, so far, only worked in countries where COVID-19 gained little initial traction, not those, such as the UK, USA and the EU states, where the virus has become endemic and prevalent. In these latter polities, track and trace has been overwhelmed or confounded by undetected cases, spurious late positivity in recovered patients,80 poor concordance between repeat tests81 and poor agreement between different types of test.82 Once infection rates are low, false positives are apt to outnumber true positives, even for a test with e.g. 99% specificity, reducing the positive predictive value.83 The failure of track and trace is illustrated by the extent to which governments have resorted to repeated lockdowns that they had sworn, after Spring 2020, to eschew.
In the view of this author, vaccines would have to come close to being successful, greatly reducing disease prevalence, before the approach becomes practicable. And, if these conditions pertain, it becomes disproportionate to prioritize COVID-19 compared with other infections, notably influenza, that remain significant causes of death in the same demographic. What is more, the economic and social costs will mount as other countries, eschewing this approach, abandon restrictions and their contingent costs. Closed defensive economies rarely prosper. These issues, albeit without the issues of healthcare backlog, will have to be faced also by those countries that have been most successful at suppressing COVID-19 during 2020. Should they deploy a suboptimal vaccine, accepting that they will then have COVID-19 and COVID-19 deaths, or should they remain closed?
3. Vaccine ‘failure’: community control relaxed or abandoned
Given the massive ‘sunk cost,’ control abandonment is now likely only after multiple vaccine disappointments and as the social and economic cost of lockdowns becomes obvious and painful, even to those who presently believe in their efficacy and virtue.
Further viral waves would then be anticipated, largest in countries that initially suppressed COVID-19 most effectively or, more randomly, in those where immunologically distinct variants emerge. If the 1889–94 ‘influenza’ is a model, spikes of infection might extend over years. Vaccines, whilst failing to prevent COVID-19, may mitigate severity and treatments will likely improve. Dexamethasone reduces mortality84 in severely ill patients, and inhaled interferon-β may reduce progression to severe disease.85 Clinical manageability may encourage governments to reduce suppression.
Even so, hospitals will still be hazardous, or be perceived as hazardous, extending pressure to use oral outpatient parenteral antibiotic therapy (OPAT) and long t½ antibiotics. Since this period will be longer than under other scenarios, there will be more impetus to develop such therapies. Single-dose IV oritavancin and dalbavancin give near-universal antistaphylococcal coverage, as do (multidose) oral oxazolidinones, delafloxacin and omadacycline.86 Oral cephalosporin/β-lactamase inhibitor combinations and (carba)penems—sulopenem and tebipenem—are in development,87,88 targeting ESBL producers. Although sulopenem disappointed in complicated urinary tract infections (cUTI),89 it proved effective in uncomplicated urinary tract infections,90 whilst tebipenem was found to be as effective as ertapenem in cUTI.91 Of particular note are combinations of ceftibuten with the oral boronate QPX7728, which inhibits serine and metallo carbapenemase (except IMP types) as well as ESBLs and AmpC enzymes.92
Gradually, normality will return. And maybe sooner than the 1889–94 analogy suggests, given the boost that even partially effective vaccines may provide. Public fear will subside as the huge excess of mild infection is better appreciated. Hospitals, society and travel will revert to pre-pandemic patterns though after a disruption that may persist for several years.
Ultimately all these models predict that COVID-19 will, more or less quickly, decline in importance. As it does so, old concerns will re-emerge, mirroring Churchill’s93 observation after WW1:
‘The position of countries has been violently altered. The modes of thought of men, the whole outlook on affairs, the grouping of parties, all have encountered violent and tremendous change… But as the deluge subsides and the waters fall short, we see the dreary steeples of Fermanagh and Tyrone emerging… The integrity of their quarrel is one of the few institutions unaltered in the cataclysm…’
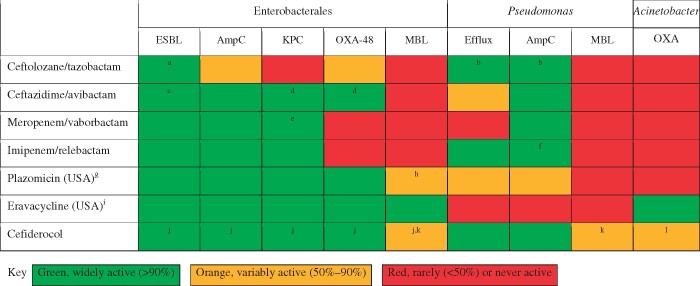
And, in the present context, multiresistant Gram-negatives will renew their challenge. Those seeking a review of prevalent types are directed to the article by Bush and Bradford,94 those wishing to appreciate differing threats of ‘carbapenem resistant’ and ‘carbapenemase-producing’, to our own publication.95 Figure 4 of the present paper summarizes the activity of recently licensed agents against important resistance types, noting where there is demonstrated clinical evidence of efficacy.
Figure 4.
Activity of recently licensed (USA and EU/UK) agents against problem groups of Gram-negative bacteria. Green, widely active (>90%); orange, variably active (50%–90%); red, rarely (<50%) or never active. aTrial evidence of efficacy.128bIn-use evidence of clinical activity against P. aeruginosa likely, based on phenotypes, to have these mechanisms.129cTrial evidence of efficacy.130dIn-use evidence of efficacy and of better outcomes than colistin combinations.131,132eTrial evidence of better outcomes than colistin combinations.133fTrial evidence of activity against imipenem-resistant P. aeruginosa, likely to have owed their phenotypes to combination of loss of porin OprD and expression of AmpC.134gLicensing application withdrawn in EU. hMany isolates with NDM carbapenemases co-produce ArmA or RmtB 16S rRNA methyltransferases, conferring broad aminoglycoside resistance including plazomicin.135iGood in vitro activity against carbapenemase-producing Enterobacterales, but trial failures in cUTI.136jTrial evidence of activity.137kMICs raised for isolates with NDM carbapenemase compared with those for isolates with other carbapenemases; the proportion of these that count as resistant will depend on the breakpoints used.138lIn vitro activity, but excess mortality in CREDIBLE-CR study compared with colistin combinations, associated with Acinetobacter baumannii, suggesting the need for caution.139
Conclusions
COVID-19 is not a great historical pandemic. During 2020 it was reportedly involved in around 1.8 million (3%) of the 60 million deaths that occurred worldwide, and the world population rose by 80 million.96 The 1347–50 Black Death, for comparison, reduced the European population by 33%–60%, with recovery taking 150 years. On 29 September 1918, the troopship SS Leviathan cleared New York with 11 800 aboard. When she docked at Brest 10 days later, 2000 were sick with influenza, 1000 were stretchered ashore and 80 were dead; 15 more died in France.97 For comparison, a COVID-19 outbreak on the USS Theodore Roosevelt infected at least 1200 from a complement of 4000.98 One died. The 1889–94 pandemic killed 125 000 in the UK, 27 000 in its 1889–90 wave. This was from a population of 33 million, or around half of today. Some social scientists blame the influenza for fin de siècle angst,99,100 but life continued. Gilbert and Sullivan’s Gondoliers opened on 7 December 1889, days before the first case, playing continuously until April 1891. Prince Eddy—second in line to the throne—succumbed on 14 January 1892; Lady Windermere’s Fan opened in the February. In October 1918, the Allies’ ‘100 Days Campaign’ crept bloodily eastwards, defeating the German army just as the pandemic peaked.101,102 Across the lines, Berlin alone recorded 1700 influenza deaths on 18 October,103 but retained sufficient energy for street revolution to erupt in November.104 Our forebears, lacking virology, would have mistaken 2020 for a ‘bad flu year’, mourned their dead, but carried on.
Where COVID-19 is unique is in hitting a modern medicalized population with many elderly and vulnerable, and in humanity’s reaction. Never before was it policy to shutter the economy or to confine the healthy. The WHO’s pandemic influenza plan of 2019 makes no mention of lockdown as a strategy105 and the approach was expressly dismissed in the 1957 influenza pandemic.106,107
It will be for future historians to assess the wisdom or folly of the policies adopted in 2020–21, but it is already arguable that our response generated more harm than the epidemic, leading to impoverishment, delayed treatment and increased mortality for other (e.g. cardiovascular) conditions, disrupted educations and mental illness.108–111 A particularly extensive review of the harms of lockdown is provided by Joffe.112 Many ‘saved’ by lockdowns had little time to live: someone entering a care home in the UK ‘expects’ c. 30 months, and care home residents account for half the UK deaths.113 Those whose prospects are blighted by the response to COVID-19 span the age spectrum. Unless vaccination is successful, or societies are prepared to accept indefinite and stultifying restrictions on liberty, the epidemic must ultimately run its course.
Against this ‘big picture’, effects on antibiotic resistance are a sideshow. Sharp reductions in COVID-19-unrelated medicine, IV antibiotic use and travel are reducing short-term selection pressure nationally, though selection may be locally increased in stressed ICUs. The longer-term effects depend on the success of vaccines or, if they fail, on our response to this failure. If vaccines succeed overwhelmingly, a hectic period will follow as hospitals address a backlog, with some patients sicker than had they been treated earlier. Resulting pressures will promote resistance. If vaccines fail, or if unrealistic hopes lead to a perception of failure, a more atomized society will persist. This will favour oral, OPAT and long t½ antibiotics, reducing hospital-centred selection and cross-infection. Travel will be reduced, limiting import of resistance. But such an approach is unsustainable except in an island choosing indefinite isolation. The dénouement, sooner or later, will be relaxation, further COVID-19 waves, perhaps by vaccine-evading variants, then recovery and normalization.
Some shifts seem set to be maintained, notably more homeworking, which may reduce circulation of other respiratory infections and the contingent, often unwarranted, community demand for antibiotics. In hospitals, all ‘likely’ scenarios favour a short-term reduction in resistance selection, then a bounceback. Ultimately, old challenges will renew, including with carbapenemase producers. Newer antibiotics, including cefiderocol, address these.
Last, there are extreme futures, where economic damage arising from lockdowns or failure of the ‘modern monetary theory’ used to finance COVID-19 responses precipitates civil unrest, loss of confidence and a flight to gold. Lebanon—already in political turmoil in 2019—exemplifies COVID-19 tipping a precarious situation over the edge. During 2020 the lira fell 85% on the dollar, inflation hit 50% monthly and the government was unable to pay healthcare providers. Hospitals suffered blackouts. An early ‘total shutdown’ was followed by an accelerating case tally114,115 and a further shutdown, though it was hard to see how this could be financed, or a good outcome achieved, even without the devastating explosion of 4 August.116 Experience in Libya and Syria shows that carbapenemase-producing bacteria can proliferate in times of chaos.117,118 The inability of a bankrupt Argentina to pay for antibiotics in 2003 was associated, briefly, with reduced use119 though also with worse outcomes for non-infectious conditions,120 and increased mortality in infections.121
If future society is to prosper and to be able to afford modern medicine, it is vital that we avoid such futures, for their human cost will greatly exceed than any toll arising from the virus itself.
Acknowledgements
I am indebted to Phillip Howard, of the Leeds Teaching Hospitals NHS Trust and Alan Carr of Needham & Co., New York for antibiotic prescribing and sales data, and for permission to cite these. I am deeply grateful also to Emily Procter and Milly Gigg of Page Medical for their assistance in formatting figures, and in organizing the referencing; they saved me much time and effort.
Transparency declarations
This article forms part of a Supplement sponsored by Shionogi Europe. The material underwent peer review by the Supplement Editors. Editorial assistance to Shionogi Europe was provided by Page Medical. This paper was commissioned by Shionogi, who have not sought to influence its content; the opinions expressed are those of the author and not necessarily those of Shionogi nor of his employer. D.M.L. has undertaken advisory boards or ad hoc consultancy for Accelerate, Allecra, Antabio, Centauri, Entasis, GlaxoSmithKline, J&J, Meiji, Menarini, Mutabilis, Nordic, Paion, ParaPharm, Pfizer, QPEX, Roche, The Russian Direct Investment Fund, Sandoz, Shionogi, Summit, T.A.Z., VenatoRx, Wockhardt and Zambon. He has presented paid lectures for Astellas, bioMérieux, Beckman Coulter, Cardiome, Cepheid, Hikma, Merck/MSD, Menarini, Nordic, Pfizer and Shionogi. He has direct relevant shareholdings or options in Dechra, GSK, Merck, Perkin Elmer, Pfizer and T.A.Z, amounting to <10% of portfolio value. He also has nominated holdings in Angle, Avacta, Diaceutics, Evgen, Faron, Genedrive, Renalytics, Rua Life Sciences, Synairgen and Verici (all of which have research/products pertinent to medicine) through Enterprise Investment Schemes but has no authority to trade these shares directly.
References
- 1.Office for National Statistics. Mortality in England and Wales: Average Life Span, 2010. 2012. https://webarchive.nationalarchives.gov.uk/20160110132605/http://www.ons.gov.uk/ons/dcp171776_292196.pdf.
- 2.PHE. Chapter 1: Life Expectancy and Healthy Life Expectancy. 2017. https://www.gov.uk/government/publications/health-profile-for-england/chapter-1-life-expectancy-and-healthy-life-expectancy.
- 3.Department of Economic and Social Affairs. World Population Ageing, 2017: Highlights. United Nations. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf.
- 4.PHE, Department of Health and Social Care. Annual Epidemiological Commentary: Gram-Negative Bacteraemia, MRSA Bacteraemia, MSSA Bacteraemia and C. difficile Infections, up to and Including Financial Year April 2018 to March 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/940716/Annual_epidemiology_commentary_April_2019_March_2020.pdf.
- 5.Smadel JE.Influenza vaccine. Public Health Rep 1958; 73: 129–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Cyranoski D. The biggest mystery: what it will take to trace the coronavirus source. Nature, 2020. https://www.nature.com/articles/d41586-020-01541-z. [DOI] [PubMed]
- 7.Su S, Wong G, Shi W. et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 2016; 24: 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. About Variants of the Virus that Causes COVID-19. 2021. https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html.
- 9.Oran D, Topol E.. Prevalence of asymptomatic SARS-CoV-2 infection. Ann Intern Med 2020; 173: 362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). 2019. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html.
- 11.Yang X, Yu Y, Xu J. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Docherty A, Harrison E, Green C. et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369: m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson E, Walker A, Bhaskaran K. et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv2020; doi:10.1101/2020.05.06.20092999.
- 14.Forbes. WHO Estimates Coronavirus Infected 10% of World’s Population. 2020. https://www.forbes.com/sites/jemimamcevoy/2020/10/05/who-estimates-coronavirus-infected-10-of-worlds-population/.
- 15.Ioannidis JPA.Global perspective of COVID-19 epidemiology for a full-cycle pandemic. Eur J Clin Invest 2020; 50: e13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grasselli G, Pesenti A, Cecconi M.. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020; 323: 1545–6. [DOI] [PubMed] [Google Scholar]
- 17.The Economist. Many Covid Deaths in Care Homes are Unrecorded. 2020. https://www.economist.com/europe/2020/05/09/many-covid-deaths-in-care-homes-are-unrecorded.
- 18.ECDC. Risk Factors and Risk Groups. 2020. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/epidemiology.
- 19.NBC New York. NY Nursing Home Reports 98 Deaths Linked to Coronavirus. 2020. https://www.nbcnewyork.com/news/local/ny-nursing-home-reports-98-deaths-linked-coronavirus/2399097/.
- 20.Office for National Statistics. Coronavirus (COVID-19) Infection Survey, UK: 8 January 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/8january2021.
- 21.Flaxman S, Mishra S, Gandy A. et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 2020; 584: 257–61. [DOI] [PubMed] [Google Scholar]
- 22.Office for National Statistics. Deaths Involving COVID-19, UK: Deaths Occurring Between 1 March and 30 April 2020. 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsinvolvingcovid19uk/deathsoccurringbetween1marchand30april2020.
- 23.De Larochelambert Q, Marc A, Antero J. et al. COVID-19 mortality: a matter of vulnerability among nations facing limited margins of adaptation. Front Public Health 2020; 8: 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kepp KP, Bjornskov C. Lockdown effects on Sars-Cov-2 transmission – the evidence from Northern Jutland. medRxiv2021; doi:10.1101/2020.12.28.20248936.
- 25.NICE. COVID-19 Rapid Guideline: Antibiotics for Pneumonia in Adults in Hospital. 2020. https://www.nice.org.uk/guidance/ng173. [PubMed]
- 26.Rawson T, Moore L, Zhu N. et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020; 71: 2459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langford B, So M, Raybardhan S. et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26: 1622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youngs J, Wyncoll D, Hopkins P. et al. Improving antibiotic stewardship in COVID-19: bacterial co-infection is less common than with influenza. J Infect 2020; 81: e55–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buetti N, Mazzuchelli T, Lo Priore E. et al. Early administered antibiotics do not impact mortality in critically ill patients with COVID-19. J Infect 2020; 81: e148–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahase E.Hydroxychloroquine for covid-19: the end of the line? BMJ 2020; 369: m2378. [DOI] [PubMed] [Google Scholar]
- 31.The Centre for Evidence-Based Medicine. What is the Evidence for Using Macrolide Antibiotics to Treat COVID-19? 2020. https://www.cebm.net/covid-19/what-is-the-evidence-for-use-of-macrolide-antobiotics-for-treatmetnof-covid-19/.
- 32.Dhesi Z, Enne V, Brealey D. et al. Organisms causing secondary pneumonias in COVID-19 patients at 5 UK ICUs as detected with the FilmArray test. medRxiv 2020; doi:10.1101/2020.06.22.20131573. [Google Scholar]
- 33.Dudoignon E, Caméléna F, Deniau B. et al. Bacterial pneumonia in COVID-19 critically ill patients: a case series. Clin Infect Dis 2021; 72: 905–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abelenda-Alonso G, Padullés A, Rombauts A. et al. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Control Hosp Epidemiol 2020; 41: 1371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ray K, Shi Z, Gidengil C. et al. Antibiotic prescribing during pediatric direct-to-consumer telemedicine visits. Pediatrics 2019; 143: e20182491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penza K, Murray M, Myers J. et al. Treating pediatric conjunctivitis without an exam: an evaluation of outcomes and antibiotic usage. J Telemed Telecare 2020; 26: 73–8. [DOI] [PubMed] [Google Scholar]
- 37.Shi Z, Mehrotra A, Gidengil C. et al. Quality of care for acute respiratory infections during direct-to-consumer telemedicine visits for adults. Health Affairs 2018; 37: 2014–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.GSK. GSK Delivers Q2 Sales of £7.6 billion −2% AER, −3% CER (Pro-Forma −10% CER). 2020. https://www.gsk.com/en-gb/media/press-releases/gsk-delivers-q2-sales-of-76-billion-2-aer-3-cer-pro-forma-10-cerstar/.
- 39.Udwadia Z, Vora A, Tripathi A. et al. COVID-19 -Tuberculosis interactions: when dark forces collide. Indian J Tuberc 2020; 67 Suppl: S155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prescribing antibiotics for urgent dental care during the pandemic. Br Dent J 2020; 228: 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.HSJ. Dentists Told to Stop Routine Treatment During Covid-19 Crisis. 2020. https://www.hsj.co.uk/coronavirus/dentists-told-to-stop-routine-treatment-during-covid-19-crisis/7027232.article.
- 42.BMA. The hidden impact of COVID-19 on patient care in the NHS in England. 2020. https://www.bma.org.uk/media/2841/the-hidden-impact-of-covid_web-pdf.pdf.
- 43.Beckers Hospital Review. Financial Fallout from COVID-19: 10 Hospitals Laying Off Workers. 2020. https://www.beckershospitalreview.com/finance/financial-fallout-from-covid-19-10-hospitals-laying-off-workers.html.
- 44.IQVIA European Thought Leadership. Impact of COVID-19 on the Pharmaceutical Market – EU4 & UK, Monthly Report: January 29 2021, Data Week Ending January 03, 2021. 2021. https://www.iqvia.com/-/media/iqvia/pdfs/files/iqvia-covid-19-eu4-and-uk-newsletter.pdf?_=1613398280431.
- 45.PHE. Quarterly Epidemiological Commentary: Mandatory MRSA, MSSA, Gram-Negative Bacteraemia and C. difficile Infections Data (up to July to September 2020). 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/942112/Quarterley_Epi_Commentary_December_2020.pdf.
- 46.BBC News. Deaths at Home: More Than 26,000 Extra This Year, ONS Finds. 2020. https://www.bbc.co.uk/news/health-54598728.
- 47.Tambone V, Boudreau D, Ciccozzi M. et al. Ethical criteria for the admission and management of patients in the ICU under conditions of limited medical resources: a shared international proposal in view of the COVID-19 pandemic. Front Public Health 2020; 8: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azoulay É, Beloucif S, Guidet B. et al. Admission decisions to intensive care units in the context of the major COVID-19 outbreak: local guidance from the COVID-19 Paris-region area. Crit Care 2020; 24: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenwood B, Meunier D, Hopkins K. et al. Pseudomonas aeruginosa sequence type 357 with VEB extended-spectrum β-lactamases in the UK: relatedness and resistance. Int J Antimicrob Agents 2018; 52: 301–2. [DOI] [PubMed] [Google Scholar]
- 50.Barreto Miranda I, Ignatius R, Pfüller R. et al. High carriage rate of ESBL-producing Enterobacteriaceae at presentation and follow-up among travellers with gastrointestinal complaints returning from India and Southeast Asia. J Travel Med 2016; 23: tav024. [DOI] [PubMed] [Google Scholar]
- 51.Tängdén T, Cars O, Melhus A. et al. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum β-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother 2010; 54: 3564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eyre D, Sanderson N, Lord E. et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 2018; 23: pii=1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fifer H, Hughes G, Whiley D. et al. Lessons learnt from ceftriaxone-resistant gonorrhoea in the UK and Australia. Lancet Infect Dis 2020; 20: 276–8. [DOI] [PubMed] [Google Scholar]
- 54.British Association for Sexual Health and HIV. BASHH COVID-19 Survey Finds Over Half of Services Have Been Closed. 2020. https://www.bashh.org/news/news/bashh-covid-19-survey-finds-over-half-of-services-have-been-closed.
- 55.Cusini M, Benardon S, Vidoni G. et al. Trend of main STIs during COVID-19 pandemic in Milan, Italy. Sex Transm Infect 2021; 97: 99. [DOI] [PubMed] [Google Scholar]
- 56.Weinberger D, Grant L, Weatherholtz R. et al. Relating pneumococcal carriage among children to disease rates among adults before and after the introduction of conjugate vaccines. Am J Epidemiol 2016; 183: 1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torretta S, Capaccio P, Coro I. et al. Incidental lowering of otitis-media complaints in otitis-prone children during COVID-19 pandemic: not all evil comes to hurt. Eur J Pediatr 2021; 180: 649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poutanen S, Vearncombe M, McGeer A. et al. Nosocomial acquisition of methicillin-resistant Staphylococcus aureus during an outbreak of severe acute respiratory syndrome. Infect Control Hosp Epidemiol 2005; 26: 134–7. [DOI] [PubMed] [Google Scholar]
- 59.Ling M, How K.. Impact of a hospital-wide hand hygiene promotion strategy on healthcare-associated infections. Antimicrob Resist Infect Control 2012; 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Callow K, Parry H, Sergeant M. et al. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect 1990; 105: 435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adams ER, Ainsworth M, Anand R. et al. Antibody testing for COVID-19: a report from the National COVID Scientific Advisory Panel. Wellcome Open Res 2020; 5: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Long Q, Tang X, Shi Q. et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26: 1200–4. [DOI] [PubMed] [Google Scholar]
- 63.Zuo J, Dowell A, Pearce H. et al. Robust SARS-CoV-2-specific T-cell immunity is maintained at 6 months following primary infection. bioRxiv 2020; doi:10.1101/2020.11.01.362319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crooke SN, Ovsyannikova IG, Poland GA. et al. Immunosenescence and human vaccine immune responses. Immun Ageing 2019; 16: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.PHE. Surveillance of Influenza and Other Respiratory Viruses in the UK: Winter 2018 to 2019. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/839350/Surveillance_of_influenza_and_other_respiratory_viruses_in_the_UK_2018_to_2019-FINAL.pdf.
- 66.Geldsetzer P.Use of rapid online surveys to assess people's perceptions during infectious disease outbreaks: a cross-sectional survey on COVID-19. J Med Internet Res 2020; 22: e18790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hilleman MR.Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine 2002; 20: 3068–87. [DOI] [PubMed] [Google Scholar]
- 68.Valleron A, Cori A, Valtat S. et al. Transmissibility and geographic spread of the 1889 influenza pandemic. Proc Natl Acad Sci USA 2010; 107: 8778–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vijgen L, Keyaerts E, Moës E. et al. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J Virol 2005; 79: 1595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valtat S, Cori A, Carrat F. et al. Age distribution of cases and deaths during the 1889 influenza pandemic. Vaccine 2011; 29 Suppl 2: B6–10. [DOI] [PubMed] [Google Scholar]
- 71.Foley C. ‘This revived old plague’1: coping with flu. In: Cox C, Luddy M, eds. Cultures of Care in Irish Medical History, 1750–1970. Palgrave Macmillan, 2010; 141–167. [Google Scholar]
- 72.Creighton C.A History of Epidemics in Britain from A.D. 664 to the Extinction of Plague. Cambridge University Press, 1894. [Google Scholar]
- 73.Patterson KD.Pandemic Influenza, 1700-1900: A Study in Historical Epidemiology. Rowman & Littlefield, 1986. [Google Scholar]
- 74.Sette A, Crotty S.. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat Rev Immunol 2020; 20: 457–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khan T, Rahman M, Al AF. et al. Endemic human coronaviruses induce distinct antibody repertoires in adults and children. bioRxiv2020; doi:10.1101/2020.06.21.163394. [DOI] [PMC free article] [PubMed]
- 76.Patrick D, Petric M, Skowronski D. et al. An outbreak of human coronavirus OC43 infection and serological cross-reactivity with SARS coronavirus. Can J Infect 2006; 17: 330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.The Health Foundation. Elective Care in England: Assessing the Impact of COVID-19 and Where Next. 2020. https://www.health.org.uk/sites/default/files/2020-11/Elective%20care%20in%20England.pdf.
- 78.Hugonnet S, Harbarth S, Sax H. et al. Nursing resources: a major determinant of nosocomial infection? Curr Opin Infect Dis 2004; 17: 329–33. [DOI] [PubMed] [Google Scholar]
- 79.Office for National Statistics. Analysis of Death Registrations Not Involving Coronavirus (COVID-19), England and Wales: 28 December 2019 to 1 May 2020. 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/analysisofdeathregistrationsnotinvolvingcoronaviruscovid19englandandwales28december2019to1may2020/technicalannex.
- 80.PHE. Understanding Cycle Threshold (Ct) in SARS-Cov-2 RT-PCR. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/926410/Understanding_Cycle_Threshold__Ct__in_SARS-CoV-2_RT-PCR_.pdf.
- 81.Healy B, Khan A, Metezai H. et al. The impact of false positive COVID-19 results in an area of low prevalence. Clin Med 2021; 21: e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deeks JJ, Raffle AE.. Lateral flow tests cannot rule out SARS-CoV-2 infection. BMJ 2020; 371: m4787. [DOI] [PubMed] [Google Scholar]
- 83.Watson J, Whiting PF, Brush JE.. Interpreting a covid-19 test result. BMJ 2020; 369: m1808. [DOI] [PubMed] [Google Scholar]
- 84.RECOVERY Collaborative Group; Hornby P, Lim W, Emberson J. et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.University of Southampton. Inhaled Drug Prevents COVID-19 Patients Getting Worse in Southampton Trial. 2020. https://www.uhs.nhs.uk/ClinicalResearchinSouthampton/Research/News-and-updates/Articles/Inhaled-drug-prevents-COVID-19-patients-getting-worse-in-Southampton-trial.aspx.
- 86.Koulenti D, Xu E, Mok YS. et al. Novel antibiotics for multidrug-resistant Gram-positive microorganisms. Microorganisms 2019; 7: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Theuretzbacher U, Bush K, Harbarth S. et al. Critical analysis of antibacterial agents in clinical development. Nat Rev Microbiol 2020; 18: 286–98. [DOI] [PubMed] [Google Scholar]
- 88.Stewart A, Harris P, Henderson A. et al. Oral cephalosporin and β-lactamase inhibitor combinations for ESBL-producing Enterobacteriaceae urinary tract infections. J Antimicrob Chemother 2020; 75: 2384–93. [DOI] [PubMed] [Google Scholar]
- 89.Reuters. BRIEF-Iterum Therapeutics Announces Topline Results from Trial for Urinary Tract Infections Treatment. 2020. https://www.reuters.com/article/brief-iterum-therapeutics-announces-topl/brief-iterum-therapeutics-announces-topline-results-from-trial-for-urinary-tract-infections-treatment-idUKASA00V7L?edition-redirect=uk.
- 90.GlobeNewswire. Iterum Therapeutics Announces Topline Results from its Phase 3 Clinical Trial of Oral Sulopenem for the Treatment of Uncomplicated Urinary Tract Infections. 2020. https://www.globenewswire.com/news-release/2020/06/29/2054608/0/en/Iterum-Therapeutics-Announces-Topline-Results-from-its-Phase-3-Clinical-Trial-of-Oral-Sulopenem-for-the-Treatment-of-Uncomplicated-Urinary-Tract-Infections.html.
- 91.GlobeNewswire. Spero Therapeutics Announces Positive Topline Results from its Phase 3 ADAPT-PO Clinical Trial of Oral Tebipenem HBr in Complicated Urinary Tract Infection and Acute Pyelonephritis. 2020. https://www.globenewswire.com/news-release/2020/09/08/2089966/0/en/Spero-Therapeutics-Announces-Positive-Topline-Results-from-its-Phase-3-ADAPT-PO-Clinical-Trial-of-Oral-Tebipenem-HBr-in-Complicated-Urinary-Tract-Infection-and-Acute-Pyelonephritis.html.
- 92.Hecker S, Reddy K, Lomovskaya O. et al. Discovery of cyclic boronic acid QPX7728, an ultrabroad-spectrum inhibitor of serine and metallo-β-lactamases. J Med Chem 2020; 63: 7491–507. [DOI] [PubMed] [Google Scholar]
- 93.Churchill WS.The World Crisis, 1918-1928: The Aftermath , Vol. 4. Thornton Butterworth Limited, 1929. [Google Scholar]
- 94.Bush K, Bradford PA.. Epidemiology of β-lactamase-producing pathogens. Clin Microbiol Rev 2020; 33: e00047-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Livermore DM, Nicolau DP, Hopkins KL. et al. Carbapenem-resistant Enterobacterales, carbapenem resistant organisms, carbapenemase-producing Enterobacterales, and carbapenemase-producing organisms: terminology past its “sell-by date” in an era of new antibiotics and regional carbapenemase epidemiology. Clin Infect Dis 2020; 71: 1776–82. [DOI] [PubMed] [Google Scholar]
- 96.Worldometer. Real Time World Statistics. 2020. https://www.worldometers.info.
- 97.Explore Magazine. Epidemic at Sea, USS LEVIATHAN, 29 September to October 7, 1918. 2015. https://www.explorermagazin.de/boote/leviahist.pdf.
- 98.Payne D, Smith-Jeffcoat S, Nowak G. et al. SARS-CoV-2 infections and serologic responses from a sample of U.S. Navy service members — USS Theodore Roosevelt, April 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith FB.The Russian influenza in the United Kingdom, 1889-1894. Soc Hist Med 1995; 8: 55–73. [DOI] [PubMed] [Google Scholar]
- 100.Honigsbaum M.The Great Dread: cultural and psychological impacts and responses to the ‘Russian’ influenza in the United Kingdom, 1889-1893. Soc Hist Med 2010; 23: 299–319. [Google Scholar]
- 101.Sheffield G.Forgotten Victory. Headline Book Publishing, 2002. [Google Scholar]
- 102.National Museum of Health and Medicine. Influenza Pandemic Chart 1919 (Reeve 003143), OHA 80: Reeve Photograph Collection. 2020. https://www.flickr.com/photos/medicalmuseum/5857153474/in/album-72157614214049255/.
- 103.Schleunes K, Turner H, Barkin K. et al. Germany from 1918 to 1945. In: Encyclopædia Britannica. Encyclopædia Britannica Inc., 2020. [Google Scholar]
- 104.Rürup R.Problems of the German Revolution 1918-19. J Contemp Hist 1968; 3: 109–35. [Google Scholar]
- 105.WHO. Non-Pharmaceutical Public Health Measures for Mitigating the Risk and Impact of Epidemic and Pandemic Influenza. 2019. https://apps.who.int/iris/bitstream/handle/10665/329438/9789241516839-eng.pdf.
- 106.Henderson DA, Courtney B, Inglesby TV. et al. Public health and medical responses to the 1957-58 influenza pandemic. Biosecur Bioterror 2009; 7: 265–73. [DOI] [PubMed] [Google Scholar]
- 107.Inglesby TV, Nuzzo JB, O’Toole T. et al. Disease mitigation measures in the control of pandemic influenza. Biosecur Bioterror 2006; 4: 366–75. [DOI] [PubMed] [Google Scholar]
- 108.Coibion O, Gorodnichenko Y, Weber M.. The cost of the COVID-19 crisis: lockdowns, macroeconomic expectations, and consumer spending. National Bureau of Economic Research Working Paper Series 2020; doi:10.3386/w27141. [Google Scholar]
- 109.Miles D, Stedman M, Heald A.. Living with COVID-19: balancing costs against benefits in the face of the virus. NIER 2020; 253: R60–76. [Google Scholar]
- 110.Wu J, Mamas M, Mohamed M. et al. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart 2021; 107: 113–9. [DOI] [PubMed] [Google Scholar]
- 111.Department of Health and Social Care, Office for National Statistics, Government Actuary’s Department and Home Office. Direct and Indirect Impacts of COVID-19 on Excess Deaths and Morbidity: Executive Summary. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/907616/s0650-direct-indirect-impacts-covid-19-excess-deaths-morbidity-sage-48.pdf.
- 112.Joffe AR.COVID-19: rethinking the lockdown groupthink. Front Public Health 2021; 9: 625778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.BUPA. Length of Stay in Care Homes, Report Commissioned by Bupa Care Services. 2011. https://eprints.lse.ac.uk/33895/1/dp2769.pdf.
- 114.The Telegraph. ‘This Will Get Ugly’: Lebanon's Health System Trapped Between Economic Catastrophe and Coronavirus. 2020. https://www.telegraph.co.uk/global-health/science-and-disease/will-get-ugly-lebanons-health-system-trapped-economic-catastrophe/.
- 115.National Review. Lebanon Hyperinflates. 2020. https://www.nationalreview.com/corner/lebanon-hyperinflates/.
- 116.Devi S.Lebanon faces humanitarian emergency after blast. Lancet 2020; 396: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lerner A, Solter E, Rachi E. et al. Detection and characterization of carbapenemase-producing Enterobacteriaceae in wounded Syrian patients admitted to hospitals in northern Israel. Eur J Clin Microbiol Infect Dis 2016; 35: 149–54. [DOI] [PubMed] [Google Scholar]
- 118.Lafeuille E, Decré D, Mahjoub-Messai F. et al. OXA-48 carbapenemase-producing Klebsiella pneumoniae isolated from Libyan patients. Microb Drug Resist 2013; 19: 491–7. [DOI] [PubMed] [Google Scholar]
- 119.Wirtz V, Dreser A, Gonzales R.. Trends in antibiotic utilization in eight Latin American countries, 1997-2007. Rev Panam Salud Publica 2010; 27: 219–25. [DOI] [PubMed] [Google Scholar]
- 120.Gurfinkel E, Bozovich G, Dabbous O. et al. Socio economic crisis and mortality. Epidemiological testimony of the financial collapse of Argentina. Thromb J 2005; 3: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bantar C, Franco D, Heft C. et al. Does a reduction in antibiotic consumption always represent a favorable outcome from an intervention program on prescribing practice? Int J Infect Dis 2006; 10: 231–5. [DOI] [PubMed] [Google Scholar]
- 122.Polack FP, Thomas SJ, Kitchin N. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baden LR, El Sahly HM, Essink B. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384: 403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sputnik V. The Sputnik V Vaccine’s Efficacy is Confirmed at 91.4% Based on Data Analysis of the Final Control Point of Clinical Trials. 2020. https://sputnikvaccine.com/newsroom/pressreleases/the-sputnik-v-vaccine-s-efficacy-is-confirmed-at-91-4-based-on-data-analysis-of-the-final-control-po/.
- 125.Voysey M, Clemens SAC, Madhi SA. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Medical Xpress. China’s Sinopharm Says Vaccine ‘79% Effective’ Against Covid-19. 2020. https://medicalxpress.com/news/2020-12-china-sinopharm-vaccine-effective-covid-.html.
- 127.Reuters. China’s Sinovac Vaccine Has ‘General Efficacy’ of 50.4% in Brazil Trials, Says Butantan. 2021. https://www.reuters.com/article/healthcoronavirus-brazil-coronavirus/chinas-sinovac-vaccine-has-general-efficacy-of-50-4-in-brazil-trials-says-butantan-idUSE5N2HA01G.
- 128.Popejoy M, Paterson D, Cloutier D. et al. Efficacy of ceftolozane/tazobactam against urinary tract and intra-abdominal infections caused by ESBL-producing Escherichia coli and Klebsiella pneumoniae: a pooled analysis of Phase 3 clinical trials. J Antimicrob Chemother 2017; 72: 268–72. [DOI] [PubMed] [Google Scholar]
- 129.Escolà-Vergé L, Pigrau C, Los-Arcos I. et al. Ceftolozane/tazobactam for the treatment of XDR Pseudomonas aeruginosa infections. Infection 2018; 46: 461–8. [DOI] [PubMed] [Google Scholar]
- 130.Carmeli Y, Armstrong J, Laud P. et al. Ceftazidime/avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 2016; 16: 661–73. [DOI] [PubMed] [Google Scholar]
- 131.van Duin D, Lok J, Earley M. et al. Colistin versus ceftazidime/avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018; 66: 163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sousa A, Pérez-Rodríguez M, Soto A. et al. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 2018; 73: 3170–5. [DOI] [PubMed] [Google Scholar]
- 133.Wunderink R, Giamarellos-Bourboulis E, Rahav G. et al. Effect and safety of meropenem/vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 2018; 7: 439–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Motsch J, Murta de Oliveira C, Stus V. et al. RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis 2020; 70: 1799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Livermore D, Mushtaq S, Warner M. et al. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother 2011; 66: 48–53. [DOI] [PubMed] [Google Scholar]
- 136.Alosaimy S, Abdul‐Mutakabbir J, Kebriaei R. et al. Evaluation of eravacycline: a novel fluorocycline. Pharmacotherapy 2020; 40: 221–38. [DOI] [PubMed] [Google Scholar]
- 137.Bassetti M, Echols R, Matsunga Y. et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21: 226–40. [DOI] [PubMed] [Google Scholar]
- 138.Mushtaq S, Sadouki Z, Vickers A. et al. In-vitro activity of cefiderocol against multidrug-resistant Enterobacterales, Pseudomonas aeruginosa and Acinetobacter baumannii isolates from the UK. Access Microbiol 2020; 2: 50. [Google Scholar]
- 139.FDA, Shionogi. FDA Briefing Document: Meeting of the Antimicrobial Drugs Advisory Committee (AMDAC). 2019. https://www.fda.gov/media/131703/download.