Abstract
The antibiogram is an essential resource for institutions to track changes in antimicrobial resistance and to guide empirical antimicrobial therapy. In this Viewpoint, data and examples from literature are presented that suggest institutions have not completely adopted the standardized approach in developing antibiograms, as variations in the development methodologies of antibiograms exist despite consensus guidelines (M39) published by CLSI. We emphasize developing antibiograms in line with the M39 recommendations will help ensure that they are accurate, reliable and valid, and highlight that understanding the limitations of antibiogram data is critical to ensuring appropriate interpretation and application to clinical decision-making. We also stress the importance of easy accessibility and education on antibiogram use, to allow for prescribers to select the most optimal empirical treatment regimens and propose the creation of an abbreviated antibiogram for frontline users. Multidisciplinary antimicrobial stewardship programmes are vital to accomplishing these goals.
Considerations in antibiogram development and reporting
The cumulative antibiogram is a periodic profile of antimicrobial susceptibilities of various organisms isolated from patients within an institution or can be developed to track patterns of resistance in broader geographic areas using data from multiple institutions. It is commonly utilized to monitor recent antimicrobial susceptibility patterns in order to guide empirical antimicrobial therapy selection.1 Components of the cumulative antibiogram include organism names, number of isolates analysed, antimicrobial agents tested and the percentage of each organism interpreted as susceptible to listed antimicrobial agents based on the CLSI recommended breakpoints.2,3 While antibiograms have been commonly utilized by institutions over the past decades, there are variations in the development methodologies of cumulative antibiograms. Additionally, determination of susceptibility rates may be based on flawed calculation methodologies.4 Contributions to flawed susceptibility calculations can include delayed adoption of the most up-to-date CLSI breakpoints and use of FDA or EUCAST breakpoints.5–8 CLSI updates with lowered breakpoints have been shown to increase the resistance rates of specific organisms.9 Breakpoint changes and implementation remained a challenge for all laboratories, as not all automated susceptibility testing systems have FDA clearance for the revised (current) breakpoints. Additionally, the challenge is compounded by the need for laboratories to perform validation studies.10 Delaying or not updating to the most recent CLSI breakpoint recommendations would inflate antimicrobial percentage susceptibility. The inconsistencies among institutions in the preparation of cumulative antibiograms led to the development of CLSI consensus guidelines. Therefore, the M39 guidance document for the development and implementation of cumulative antibiograms in healthcare facilities was first published in 2006.4 This guideline was created to provide standardized guidance to institutional laboratories on antibiogram development to ensure accuracy, reliability and statistical validity. Of note, M39, as with all guidelines, is not mandatory for institutions to adhere to and is only a recommendation, despite being critical during this era of antimicrobial resistance.
The M39 guideline provides specific recommendations for the collection, storage, analysis and presentation of data and includes example templates showcasing the recommendations. In 2014, CLSI published an update to the M39 guideline. The update included further guidance on the preparation and use of cumulative antibiogram reports, and recommendations on the design and support of the clinical laboratory’s susceptibility data management needs.4 Additionally, CLSI identified modalities of antibiogram presentation to enhance its usefulness, including stratification of susceptibility data by body site (e.g. urine and non-urine isolates), hospital unit (e.g. ICU, emergency department [ED]) and/or specific patient populations. Other components added to the antibiogram have been described, such as drug cost, dosing guides and drug-use policies.2 Thus, the preparation, reporting and utilization of the antibiogram can be customized to meet the needs of each institution.
It is imperative for institutions to ensure M39 guidance recommendations are adopted to ensure accuracy and quality, as antibiograms are frequently used within institutions for formulary decisions, development of hospital treatment guidelines and order sets, monitoring resistance trends and empirical antimicrobial therapy recommendations. Several distinct approaches can be used in generating cumulative antimicrobial susceptibility testing (AST) reports from a microbiology laboratory database. These approaches include manual data collection or the use of analytical surveillance software such as WHONET®, MedMined®, VigiLanz®, TheraDoc® and Pharmacy OneSource®. In addition to third party analytical surveillance software, some electronic health record (EHR) systems such as EPIC® have an application function (Bugsy®) that is capable to producing an antibiogram. Similarly, VigiLanz® and TheraDoc® are capable of generating rolling antibiograms, based on specific dates that are input, thus providing the ability to create an antibiogram based on a specific timeframe. No studies have reported whether any of the above approaches is more advantageous than another, particularly if all analytical surveillance system data have been validated. However, data generation and analysis are less time consuming when utilizing analytical surveillance software. Additionally, it will likely provide a platform for more robust analysis including additional data stratification via location and/or infectious syndromes. M39 also provides guidance on the validation process for data analysis and calculation.3 A checklist is provided in M39 and should be used as a quality assurance check to ensure that the analytical surveillance software utilized calculates the data accurately and selection criteria are met. For example, in order to validate the data analysis output, results generated from one system (e.g. laboratory platform) should be compared with results generated from another system (e.g. the antimicrobial susceptibility instrument), provided both systems use the same calculation algorithms. Finally, M39 recommends that all antimicrobials routinely tested be included in the antibiogram susceptibility analysis even if they are suppressed by cascading rules. Institutions must be vigilant that datasets used for analysis contain all results, including those suppressed, as excluding these results will lead to biased susceptibility for the secondary agents that are released when cascading rules are met.3 Thus, a potential pitfall in producing an EHR-based antibiogram is the skewed susceptibility results unless whole unsuppressed data are released from the automated AST platforms.
Despite the release of M39 over a decade ago, there is considerable variation in the analysis and presentation of cumulative AST data among institutions.2,11 Several nationwide surveys have reported the adoption rate of the M39 recommendations to range from 47% to 60%.2,12,13 Ernst et al.12 examined the adherence of American acute care hospitals, of which 40% were teaching hospitals and most exceeded 200 beds, to the National Committee for Clinical Laboratory Standards (the former name of CLSI) guideline published in 2002. It was found that only 60% of institutions met guideline criteria for the antibiogram compilation, which included annual updating and distribution to infection control and medical staff yearly. A survey in 2006 also found substantial variability in the development and reporting of institutional antibiograms across the United States and found that academic hospitals and hospitals with greater number of personnel were significantly more likely to develop more sophisticated and advanced variations of the antibiogram in addition to the cumulative antibiogram.13 In a later study, Xu et al.2 evaluated hospital policies in regard to the generation, reporting and utilization of antibiograms among 47 hospital laboratories. They found nearly all (98%) of laboratories generated annual antibiograms, but only 47% reported that they had adopted all of the standards recommended by CLSI. The authors also found that, when examining which M39 guideline criteria hospital laboratories were adhering to, only 64% of surveyed laboratories required at least 30 isolates for each reported species, which was the lowest adhered to of the key M39 recommendations. The lack of adherence to this M39 recommendation was also observed in a study conducted by Moehring et al.:14 only 25% of hospital laboratories followed this recommendation, and only 3% of laboratories where less than 30 isolates were reported included footnotes stating the reduced statistical validity with such low isolate numbers. The importance of denoting organisms and susceptibility results without the minimum 30 isolates correlates to the statistical validity of the percentage susceptibility represented in the antibiogram. Using a smaller sample size will result in a wider 95% CI. The 95% CI for 80% susceptibility with a sample size of 30 is 0.623 to 0.909, whereas a sample size of 10 would provide a 95% CI ranging from 0.479 to 0.954, leading to a diminished predictive value of antibiograms when using smaller samples sizes. Subsequently, only 3% of the 37 surveyed laboratories produced antibiograms in complete compliance with CLSI guidelines, while 47% of laboratories in the Xu et al.2 study reported full compliance.14 Based on the studies described above, it is apparent that not all hospital laboratories follow the CLSI M39 guideline in its entirety; though it was found that the majority of institutions did create annual cumulative antibiograms. As CLSI routinely updates the M39 document, with an update currently being developed, the current recommendations for antibiogram development will change and more institutions will need to adopt a new set of standards.
Once an institution develops an accurate and reliable cumulative antibiogram that adheres to M39 recommendations, institutions should consider exploring the generation of an enhanced antibiogram. Several studies have evaluated different approaches to enhanced antibiogram reporting to improve the functionality of the traditional cumulative antibiogram.15–19 One approach is reporting location-specific susceptibilities since institution-wide cumulative antibiograms may mask important differences in susceptibility rates. Kaufman et al.16 reported that the susceptibilities of Staphylococcus aureus and Pseudomonas aeruginosa isolated in a single surgical ICU unit were significantly lower than those reported in the institution-wide antibiogram. Several studies evaluated alternative antibiogram presentation strategies such as by infection site and patient disposition in addition to location.18,19 Developing a location- or infection site-specific antibiogram will result in diminished sample size and possibly less than the 30 isolate threshold. Potential solutions would be to combine organisms into larger groups (e.g. Enterobacterales) or include multiple years, as this would increase the sample size considerably. Jorgensen et al.18 evaluated their institution’s ED-specific antibiogram susceptibility patterns by patient characteristics including gender, age, residence prior to admission and disposition. Their study revealed distinct differences in urinary Escherichia coli susceptibility as a function of patient characteristics. Lower ampicillin and gentamicin susceptibilities were seen in females aged 18 to 50 years in comparison to females aged 50 years or older. Lower trimethoprim/sulfamethoxazole susceptibilities were seen in patients 65 years old or younger versus those greater than 65 years of age. Hence, antibiograms stratified by patient characteristics may better guide empirical antibiotic selection for urinary tract infections,18,19 highlighting the need to tailor antibiograms to match specific patient populations. In another example, Pogue et al.17 evaluated unit-specific traditional versus combination antibiograms incorporating all ICU Gram-negative isolates recovered from respiratory cultures in order to improve optimal empirical antimicrobial selection in pneumonia. The authors found that the combination of cefepime and tobramycin was the most optimal empirical Gram-negative regimen in ICU patients with a suspected pulmonary source. Generating pathogen susceptibility data stratified by infection syndrome may provide prescribers a streamlined approach to empirical therapy selection for a specific infectious process such as pneumonia.17
Limitations of antibiogram applicability
A traditional cumulative antibiogram has potential limitations that may hinder its general applicability. For example, it does not reveal the timing of isolate collection relative to a patient’s hospital admission. Hence, it cannot reliably distinguish between a community-acquired versus hospital-acquired infection.1 Institutions may consider developing an antibiogram based on timing of culture collection relative to patient admission. A standard definition of 48 h as the pivotal point can be utilized, in which <48 h is classified as community onset and ≥48 h as hospital onset.20,21 This stratification may offer a more accurate analysis of hospital versus community susceptibility rates and potentially lead to better representation of community versus hospital organism ecology, resulting in improved empirical therapy recommendations. Limitations to this approach exist however, such as situations in which cultures are obtained more than 48 h after admission despite the infectious onset being in the community, which can lead to community-onset infections being categorized as hospital-onset infections. Likewise, as a limitation, a colonizing organism already present on a patient, which later causes an infection after admission may be categorized as hospital-acquired. Additionally, the practicality of collecting information needed to determine community versus hospital onset could be labour intensive and individual chart reviews may be required when sophisticated technological means are not available. Another intrinsic limitation is that a traditional antibiogram does not differentiate between an organism that is isolated from a patient as a pathogen versus colonizer. The inclusion of colonizing organisms in the percentage susceptibility calculation may influence susceptibility rates.4
Antibiograms provide binary measures of susceptibility (whether a pathogen is non-susceptible or susceptible) but do not provide quantitative data, such as MIC or further categorization of non-susceptibility into intermediate or resistant.1 Granular data such as MIC distributions that are below the clinical breakpoint are not reported on antibiograms. Thus, information regarding MICs at or near the breakpoint will not be apparent. For example, vancomycin MIC distributions at 2 mg/L have been shown to adversely influence treatment outcomes in invasive MRSA infections despite being within the susceptible range.22 Nevertheless, reporting MIC data or adding further categorization of non-susceptibility on an antibiogram may lead to difficulty in interpretation and use. However, one particularly helpful example of including MIC distribution is with Enterobacter cloacae and cefepime susceptibilities. Here, the organism MIC values dictate susceptibility classification such as susceptible and susceptible dose dependent (SDD). This classification is coupled with an alternative cefepime dosing scheme to maximize drug exposure. For example, if the organism is categorized as cefepime SDD, then maximal doses are recommended to optimize the probability of pharmacodynamic attainment (i.e. MIC = 8 mg/L, cefepime 2 g every 8 h). When cefepime is selected empirically to cover E. cloacae, information on the percentage of isolates that are SDD may prove meaningful.
While antibiograms are useful, they cannot be relied upon as the sole resource for guiding empirical antimicrobial therapy. Static antibiograms may have limited use in selecting empirical therapy in patients with recurrent or recent infections, as the patient’s microbiological history and prior antibiotic use may provide more useful information during therapy selection. Additionally, pharmacotherapeutic factors such as site of infection, antimicrobial pharmacokinetic/pharmacodynamic properties, contraindications, organism selective pressures and collateral damage, Clostridioides difficile infection risk and efficacy/safety data are all critical considerations in conjunction with antibiogram susceptibility information. More sophisticated antibiograms are on the horizon by way of machine learning to model individual patient data from the EHR to create a personalized antibiogram.23 Modelling personalized antibiograms maybe possible in the near future, but requires validation and inclusion of variables that are not available in the EHR such as antimicrobial history of infectious contacts.
Optimal antibiogram utilization
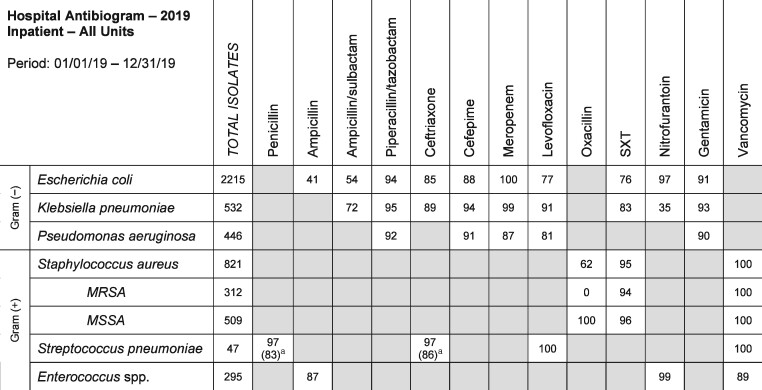
When an antibiogram is used for empirical antimicrobial therapy selection, its application can be more precise when the infecting organism is identified prior to susceptibility results. It is less precise when the infecting organism is not known but is suspected based on the site and/or type of infection. Since antibiograms provide prescribers a resource in the selection of an empirical antimicrobial regimen, it is critical that the antibiogram is a practical and user-friendly resource. Institutions can create a practical abbreviated antibiogram, in addition to the standard cumulative antibiogram and its variations. An example of a practical abbreviated antibiogram is one that lists relevant organisms most commonly encountered in typical infections along with the common antibiotics used to treat these infections (Figure 1). For example, E. coli and Citrobacter freundii likely would have >30 isolate occurrences within a year; however, E. coli would be considered a more relevant organism to list on a practical abbreviated antibiogram while C. freundii can be reserved for the full cumulative antibiogram. Whether the infecting organism is E. coli or C. freundii, knowing or suspecting the causative organism allows for effective utilization of an antibiogram by matching the organism to the most effective and safest therapy. Even without identifying the suspected organism, knowing the Gram-negative susceptibilities to fluoroquinolones and third-generation cephalosporins is very relevant to the front-line prescriber and these should be reported on the abbreviated antibiogram, while susceptibilities to other antibiotics such as cefoxitin and tetracyclines may be less useful and can be reported on the cumulative antibiogram. This would decrease information overload and provide a simpler version for ease of interpretation.
Figure 1.
An example of a practical abbreviated antibiogram that lists relevant organisms most commonly encountered in typical infections along with the common antibiotics used for treatment. SXT, trimethoprim/sulfamethoxazole. aSusceptibility rates in parentheses are based on meningitis breakpoints.
With the availability of rapid diagnostics such as MALDI-TOF and nucleic acid amplification tests, a more defined role of the antibiogram with frontline antimicrobial stewardship clinicians is warranted. Improved patient outcomes and shorter time to appropriate antimicrobials have been observed when rapid diagnostics are utilized in conjunction with antimicrobial stewardship programmes (ASP).24,25 In these scenarios, organisms with resistance markers can be identified prior to susceptibility results. It is in this intermediary phase between organism identification and susceptibility result where genus and possibly species of organism(s) likely causing the infection is identified rapidly but susceptibilities are not yet known. Thus, an antibiogram can be a valuable resource in honing empirical antimicrobial selection. For example, if a rapid diagnostic result shows the presence of P. aeruginosa and the patient is not yet on an antipseudomonal agent, part of the antimicrobial selection process should include review of antibiogram susceptibilities to ensure highest probability of susceptibility. ASP personnel can play a critical role in reviewing the antibiogram in conjunction with rapid diagnostic results to ensure appropriate empirical therapy selection. The susceptibility rates from an antibiogram are critical information to consider when determining an appropriate antimicrobial agent for patients with a positive culture.
Susceptibility data derived from an antibiogram should be utilized when generating infectious disease treatment pathways, including order sets. As order sets and clinical pathways are typically institution-specific, the use of the antibiogram to guide antimicrobial selections in each pathway or set will provide the most evidence-based and data-driven empirical therapies. At a minimum, the cumulative antibiogram can be utilized for this purpose; however, if an enhanced antibiogram that is stratified by infectious syndrome type is available, a preference for its use should be considered, as the data obtained can provide more specific information on susceptibility patterns. Thus, when prescribers utilize these treatment pathways or order sets for empirical therapy selection, their selection will have incorporated the institution’s susceptibility patterns. The future antibiogram development will likely coexist with computational models and artificial intelligence to predict antimicrobial susceptibility. Prediction models are available to forecast bacterial resistance and therapy selection based on various factors such as risk factors for site of infection, community/hospital acquisition, microbiology data, diagnosis and antimicrobial consumption.26,27 Unfortunately, antimicrobial resistance models do not take into account social, cultural or behavioural differences associated with resistance development or transmission.28,29
Dissemination of the antibiogram to front-line prescribers in institutions where clinical pathways or order sets are not utilized may prove to be challenging. Studies noted the feasibility of disseminating a pocket antibiogram through electronic means such as a mobile device application.11,14 Posting the antibiogram on the institution’s intranet or in physician work rooms or lounges have also been described.30 Another method to optimize antibiogram utilization is through increased accessibility and visualization such as electronic antibiograms. An electronic antibiogram linked to the EHR can provide a user interface that allows for selection of organism, date ranges, infection source and hospital unit, and provide colour visualization to identify the most susceptible antimicrobial options. Hospitals may also link the static and electronic antibiograms to order sets for easy reference during order entry.31 However, even in the event that antibiograms are readily available and being utilized, their mere availability alone may not lead to appropriate use. It is prudent to educate front-line prescribers on how to appropriately utilize the antibiogram in the context of infectious syndromes, as misinterpretation and/or misutilization of the cumulative antibiogram or its variations when selecting empirical therapy may lead to suboptimal therapy and outcomes. One such example of a situation where use of an antibiogram in isolation may lead to a poor outcome within the context of an infectious syndrome would be the selection of nitrofurantoin based on high susceptibility reported for common urinary pathogens for a patient with pyelonephritis. As nitrofurantoin does not reach adequate concentrations outside of the bladder, this would be a suboptimal therapy. This underscores the importance of utilizing the antibiogram in conjunction with clinical context and patient-specific factors, and not simply selecting an agent based on high susceptibility. Data derived from the antibiogram have limitations and are only one component to consider when selecting optimal empirical therapy.
A survey study conducted by Tallman et al.30 examined the use of the antibiogram by medical residents for selecting empirical antibiotic therapy in the context of clinical cases. The study authors found that more than 20% of residents could not accurately select therapy with an appropriate spectrum of activity in response to the clinical cases. Even fewer respondents (∼12%) identified the antibiogram as a resource when prescribing empirical antimicrobial therapy. The majority of residents reported using UpToDate or The Sanford Guide as primary resources for their selection of empirical antimicrobial therapy. These resources provide very general spectrum of activity information, but lack institution-specific epidemiological data, which along with patient-specific information may lead to improved empirical therapy selection. Furthermore, 30% of residents did not feel comfortable using an antibiogram for selecting empirical therapy, and only 44% of participants knew where the antibiogram could be accessed. One suggested area of further exploration would be evaluation of antibiogram understanding and utilization among practitioners. Selekman et al.32 evaluated antimicrobial use in paediatric patients with urinary tract infections. It was found that only half of the practitioners utilized the local antibiogram despite having access. The rationale behind the limited antibiogram use was not a study objective and hence not explored but provides a direction of potential future research. Other studies have shown improvement in antimicrobial prescribing after antimicrobial stewardship and/or antibiogram education.33–35 Educating prescribers on antibiogram accessibility and utilization can provide early trainees guidance and confidence in their use as well as improvements in antimicrobial prescribing among practitioners.
It is apparent that various healthcare disciplines such as microbiology, pharmacy, medicine and informatics play critical roles in the design, creation, dissemination, and utilization of antibiograms. Many of these disciplines are part of institutional ASP. The stewardship team should be an integral part of developing valid, accurate, and utilizable antibiograms, whether they be the cumulative antibiogram or a variation of it. ASP need to ensure optimal dissemination of the antibiograms to front-line prescribers, educate on their use and incorporate the data from antibiograms into institutional treatment pathways and electronic medical record order sets for various infectious disease syndromes. Additionally, as institutional ASP are heavily involved in rapid diagnostic device implementation and use, it further underscores the importance of antibiogram use in conjunction with the development of clinical decision support tools and diagnostic platforms.
Conclusions
There exists variability in antibiogram preparation amongst institutions, and the CLSI M39 document should serve as a reference guide to standardize development. M39 has been updated since its creation and will continue to be updated to provide new guidance and recommendations for the preparation of cumulative and enhanced antibiograms. It is imperative that ASP collaborate with prescribers and the microbiology laboratory to ensure proper dissemination, education and utilization of antibiograms. As third-party surveillance software integrated with institutional EMRs become more common and advanced, it may be possible for these programmes to be utilized with machine learning to develop antibiograms that have real-time access to rapid cumulative antibiotic profiling data, MIC trends and patient demographics in order to develop sophisticated ‘smart antibiograms’.36 Further understanding of how to best utilize the cumulative antibiogram and its variations in the clinical setting, and its effects on patient outcomes, is warranted.
Funding
This study was carried out as part of our routine work.
Transparency declarations
Dr Levita Hidayat is a current employee of Merck & Co. Inc., Kenilworth, NJ, USA. At the time of this manuscript, she was employed by Melinta Therapeutics. No funding or support was provided from Merck for this manuscript. All other authors: none to declare.
References
- 1. Pakyz AL. The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2007; 27: 1306–12. [DOI] [PubMed] [Google Scholar]
- 2. Xu R, Polk RE, Stencel L. et al. Antibiogram compliance in University Health System Consortium participating hospitals with Clinical and Laboratory Standards Institute guidelines. Am J Health Syst Pharm 2012; 69: 598–606. [DOI] [PubMed] [Google Scholar]
- 3. CLSI. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data; Approved Guideline—Fourth Edition: M39-A4. 2014.
- 4. Hindler JF, Stelling J.. Analysis and presentation of cumulative antibiograms: a new consensus guideline from the Clinical and Laboratory Standards Institute. Clin Infect Dis 2007; 44: 867–73. [DOI] [PubMed] [Google Scholar]
- 5. Yarbrough ML, Wallace MA, Potter RF. et al. Breakpoint beware: reliance on historical breakpoints for Enterobacteriaceae leads to discrepancies in interpretation of susceptibility testing for carbapenems and cephalosporins and gaps in detection of carbapenem-resistant organisms. Eur J Clin Microbiol Infect Dis 2020; 39: 187–95. [DOI] [PubMed] [Google Scholar]
- 6. Bartsch SM, Huang SS, Wong KF. et al. Impact of delays between Clinical and Laboratory Standards Institute and Food and Drug Administration revisions of interpretive criteria for carbapenem-resistant Enterobacteriaceae. J Clin Microbiol 2016; 54: 2757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marchese A, Esposito S, Barbieri R. et al. Does the adoption of EUCAST susceptibility breakpoints affect the selection of antimicrobials to treat acute community-acquired respiratory tract infections? BMC Infect Dis 2012; 12: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Halloran C, Walsh N, O'Grady MC. et al. Assessment of the comparability of CLSI, EUCAST and Stokes antimicrobial susceptibility profiles for Escherichia coli uropathogenic isolates. Br J Biomed Sci 2018; 75: 24–9. [DOI] [PubMed] [Google Scholar]
- 9. Hombach M, Bloemberg GV, Böttger EC.. Effects of clinical breakpoint changes in CLSI guidelines 2010/2011 and EUCAST guidelines 2011 on antibiotic susceptibility test reporting of Gram-negative bacilli. J Antimicrob Chemother 2012; 67: 622–32. [DOI] [PubMed] [Google Scholar]
- 10. Humphries RM, Abbott AN, Hindler JA.. Understanding and addressing CLSI breakpoint revisions: a primer for clinical laboratories. J Clin Microbiol 2019; 57: e00203-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kohlmann R, Gatermann SG.. Analysis and presentation of cumulative antimicrobial susceptibility test data–the influence of different parameters in a routine clinical microbiology laboratory. PLoS One 2016; 11: e0147965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ernst EJ, Diekema DJ, BootsMiller BJ. et al. Are United States hospitals following national guidelines for the analysis and presentation of cumulative antimicrobial susceptibility data? Diagn Microbiol Infect Dis 2004; 49: 141–5. [DOI] [PubMed] [Google Scholar]
- 13. Lautenbach E, Nachamkin I.. Analysis and presentation of cumulative antimicrobial susceptibility data (antibiograms): substantial variability across medical centers in the United States. Infect Control Hosp Epidemiol 2006; 27: 409–12. [DOI] [PubMed] [Google Scholar]
- 14. Moehring RW, Hazen KC, Hawkins MR. et al. Challenges in preparation of cumulative antibiogram reports for community hospitals. J Clin Microbiol 2015; 53: 2977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Binkley S, Fishman NO, LaRosa LA. et al. Comparison of unit-specific and hospital-wide antibiograms: potential implications for selection of empirical antimicrobial therapy. Infect Control Hosp Epidemiol 2006; 27: 682–7. [DOI] [PubMed] [Google Scholar]
- 16. Kaufman D, Haas CE, Edinger R. et al. Antibiotic susceptibility in the surgical intensive care unit compared with the hospital-wide antibiogram. Arch Surg 1998; 133: 1041–5. [DOI] [PubMed] [Google Scholar]
- 17. Pogue JM, Alaniz C, Carver PL. et al. Role of unit-specific combination antibiograms for improving the selection of appropriate empiric therapy for gram-negative pneumonia. Infect Control Hosp Epidemiol 2011; 32: 289–92. [DOI] [PubMed] [Google Scholar]
- 18. Jorgensen S, Zurayk M, Yeung S. et al. Emergency department urinary antibiograms differ by specific patient group. J Clin Microbiol 2017; 55: 2629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grodin L, Conigliaro A, Lee SY. et al. Comparison of UTI antibiograms stratified by ED patient disposition. Am J Emerg Med 2017; 35: 1269–75. [DOI] [PubMed] [Google Scholar]
- 20. Siegman-Igra Y, Fourer B, Orni-Wasserlauf R. et al. Reappraisal of community-acquired bacteremia: a proposal of a new classification for the spectrum of acquisition of bacteremia. Clin Infect Dis 2002; 34: 1431–9. [DOI] [PubMed] [Google Scholar]
- 21. Cardoso T, Almeida M, Friedman ND. et al. Classification of healthcare-associated infection: a systematic review 10 years after the first proposal. BMC Med 2014; 12: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hidayat LK, Hsu DI, Quist R. et al. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166: 2138–44. [DOI] [PubMed] [Google Scholar]
- 23. Corbin CK, Medford RJ, Osei K. et al. Personalized antibiograms: machine learning for precision selection of empiric antibiotics. AMIA Jt Summits Transl Sci Proc 2020; 2020: 108–15. [PMC free article] [PubMed] [Google Scholar]
- 24. Cairns KA, Doyle JS, Trevillyan JM. et al. The impact of a multidisciplinary antimicrobial stewardship team on the timeliness of antimicrobial therapy in patients with positive blood cultures: a randomized controlled trial. J Antimicrob Chemother 2016; 71: 3276–83. [DOI] [PubMed] [Google Scholar]
- 25. MacVane SH, Nolte FS.. Benefits of adding a rapid PCR-based blood culture identification panel to an established antimicrobial stewardship program. J Clin Microbiol 2016; 54: 2455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arepyeva MA, Kolbin AS, Sidorenko SV. et al. A mathematical model for predicting the development of bacterial resistance based on the relationship between the level of antimicrobial resistance and the volume of antibiotic consumption. J Glob Antimicrob Resist 2017; 8: 148–56. [DOI] [PubMed] [Google Scholar]
- 27. Leibovici L, Paul M, Nielsen AD. et al. The TREAT project: decision support and prediction using causal probabilistic networks. Int J Antimicrob Agents 2007; 30Suppl 1: S93–102. [DOI] [PubMed] [Google Scholar]
- 28. Niewiadomska AM, Jayabalasingham B, Seidman JC. et al. Population-level mathematical modeling of antimicrobial resistance: a systematic review. BMC Med 2019; 17: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evans RS, Pestotnik SL, Classen DC. et al. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med 1998; 338: 232–8. [DOI] [PubMed] [Google Scholar]
- 30. Tallman GB, Vilches-Tran RA, Elman MR. et al. Empiric antibiotic prescribing decisions among medical residents: the role of the antibiogram. Infect Control Hosp Epidemiol 2018; 39: 578–83. [DOI] [PubMed] [Google Scholar]
- 31. Simpao AF, Ahumada LM, Larru Martinez B. et al. Design and implementation of a visual analytics electronic antibiogram within an electronic health record system at a tertiary pediatric hospital. Appl Clin Inform 2018; 9: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Selekman RE, Allen IE, Copp HL.. Determinants of practice patterns in pediatric UTI management. J Pediatr Urol 2016; 12: 308.e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang BQ, Wheeler JS, Blanchette LM.. Impact of combination antibiogram and related education on inpatient fluoroquinolone prescribing patterns for patients with health care-associated pneumonia. Ann Pharmacother 2016; 50: 172–9. [DOI] [PubMed] [Google Scholar]
- 34. El-Sokkary RH, Negm EM, Othman HA. et al. Stewardship actions for device associated infections: an intervention study in the emergency intensive care unit. J Infect Public Health 2020; 13: 1927–31. [DOI] [PubMed] [Google Scholar]
- 35. Ahmed SA, Kumar A, Sethi P. et al. Effectiveness of education and antibiotic control programme at All India Institute of Medical Sciences, New Delhi. Natl Med J India 2018; 31: 262–7. [DOI] [PubMed] [Google Scholar]
- 36. van Belkum A, Bachmann TT, Lüdke G. et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat Rev Microbiol 2019; 17: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]