Abstract
Background
Cefazolin is the most commonly recommended antimicrobial for surgical antimicrobial prophylaxis (SAP). However, the Australian Surgical National Antimicrobial Prescribing Survey revealed a wide range of antimicrobials prescribed for SAP. Inappropriate use of broad-spectrum antimicrobials is associated with increased patient harm and is a posited driver for antimicrobial resistance.
Objectives
To describe patient, hospital and surgical factors that are associated with appropriateness of the top five prescribed antimicrobials/antimicrobial classes for procedural SAP.
Methods
All procedures audited from 18 April 2016 to 15 April 2019 in the Surgical National Antimicrobial Prescribing Survey were included in the analysis. Estimated marginal means analyses accounted for a range of variables and calculated a rate of adjusted appropriateness (AA). Subanalyses of the top five audited antimicrobials/antimicrobial classes identified associations between variables and appropriateness.
Results
A total of 12 419 surgical episodes with 14 150 prescribed initial procedural doses were included for analysis. When procedural SAP was prescribed, appropriateness was low (57.7%). Allergy status, surgical procedure group and the presence of prosthetic material were positively associated with cefazolin and aminoglycoside appropriateness (P < 0.05). There were no significant positive associations with glycopeptides and third/fourth-generation cephalosporins. The use of broad-spectrum antimicrobials was the most common reason for inappropriate choice (67.9% of metronidazole to 83.3% of third/fourth-generation cephalosporin prescriptions).
Conclusions
Various factors influence appropriateness of procedural SAP choice. Identification of these factors provides targets for antimicrobial stewardship interventions, e.g. procedures where surgeons are regularly prescribing broad-spectrum SAP. These can be tailored to address local hospital prescribing practices.
Introduction
Surgical antimicrobial prophylaxis (SAP) is the most common indication for antimicrobial use in acute hospitals, accounting for 14.1% of 26 714 antimicrobial prescriptions audited in an Australian national point prevalence survey known as the Hospital National Antimicrobial Prescribing Survey (Hospital NAPS) in 2018.1 Of these surgical prophylaxis prescriptions, 39.5% were identified as inappropriate.1 Antimicrobial agent selection for SAP has been identified as an area with poor appropriateness and guideline compliance across national2–4 and international studies.5–11 Inappropriate antimicrobial use may be considered a potential driver for the emergence of antimicrobial resistance (AMR),12 which poses a burden on both the patient and the community.
SAP prescribing is multifaceted and can occur across the pre- and intra-operative (or procedural) and post-procedural settings. The Surgical NAPS was developed to facilitate a more detailed assessment of SAP prescribing including the auditing and reporting of antimicrobial use across these surgical settings.2,13 The Surgical NAPS measures both appropriateness and guideline compliance of SAP prescriptions as these are both clinically meaningful to clinicians to facilitate prescribing behaviour change.
The Therapeutic Guideline: Antibiotic (TG: Antibiotic)14,15 is the recommended guideline for the prescribing of antimicrobials in Australia. Cefazolin is the most commonly recommended antimicrobial for surgical prophylaxis based on multiple factors including its narrow Gram-positive and Gram-negative spectrum coverage for common pathogens, i.e. Staphylococcus aureus, efficacy, safety profile and low cost.14,15
Metronidazole is commonly recommended in addition to cefazolin to provide extended coverage of anaerobic pathogens. Surgical procedure groups where this may be warranted include abdominal, ear, nose and throat, gynaecological, oral/maxillofacial, head and neck and urological.14,15 Vancomycin is only recommended for SAP under defined circumstances. Examples include as an addition to cefazolin if the patient is, or is at risk of being, colonized or infected with MRSA, or as an alternative to cefazolin in patients with immediate or delayed hypersensitivity to penicillins.14,15
Gentamicin is commonly recommended as an alternative SAP agent for patients with immediate severe or delayed severe penicillin hypersensitivity.14,15 Ceftriaxone is a broad-spectrum third-generation cephalosporin antimicrobial covering both Gram-positive and Gram-negative organisms and is not recommended for SAP in current Australian guidelines.14,15
Metronidazole, gentamicin, vancomycin and ceftriaxone represented the second to fifth most commonly prescribed procedural antimicrobials for SAP (4.7%, 3.6%, 2.5% and 1.5%, respectively) as per the Surgical NAPS 2019 report.2,13 These antimicrobials are occasionally recommended; however, the report also demonstrated high rates of inappropriate use when they were prescribed (46.6%, 47.0%, 69.2% and 82.4%, respectively).2
This research aims to determine whether the drivers of appropriateness vary between the different antimicrobials prescribed. It is important, therefore, to try to understand why these antimicrobials are being prescribed and, specifically, which factors (patient, surgical and hospital-related) might be influencing prescribers to choose these broader-spectrum agents. This might include surgical-related factors such as use in emergency and trauma procedures and procedures involving prostheses, or patient-related factors such as allergies and age. Many of these factors are captured by the Surgical NAPS and further analysis may provide insight to inform future guidelines and to inform antimicrobial stewardship (AMS) interventions.
Aims
This study aimed to describe patient, hospital and surgical factors that are associated with appropriateness of antimicrobial choice for procedural SAP, with a focus on the top five antimicrobials/antimicrobial classes prescribed in the Surgical NAPS.
Methods
The Surgical NAPS is an Australian online auditing platform that has been described previously.2,13,16 We provide a summary of these methods here.
Survey design
The Surgical NAPS was co-designed through stakeholder consultation from a range of specialities. It collects data on patient demographics (age and gender) and clinical information [allergy status and American Society of Anesthesiologists (ASA) score].13
Data were collected across a patient’s surgical episode. Three categories of antimicrobial prescriptions were defined for each surgical episode: procedural, post-procedural and existing (Table S1, available as Supplementary data at JAC-AMR Online). Elements of SAP prescribing that were collected include antimicrobial choice, dose, timing, duration and frequency.
Hospital demographics captured include: location (state or territory); funding type (public or private); Australian Institute of Health and Welfare (AIHW) peer groups;17 and Australian Bureau of Statistics (ABS) remoteness areas.18
Procedure-related factors include surgical procedure group, elective or emergency surgery status, wound class and presence of prosthesis (including removal or insertion). When multiple surgical ‘procedure groups’ were documented for a patient, only the main procedure system group was included.
Data collection and collation
Surgical NAPS was conducted as a voluntary annual survey during 2016–19 in both public and private hospitals at any timepoint throughout the year. Auditors had the flexibility to audit all procedures at a given timepoint or targeted surgical procedures. Hospitals could participate more than once over the 3 year auditing period. The updated ‘TG: Antibiotics’ version 1615 was made available online on 15 April 2019. All procedures from 18 April 2016 to 15 April 2019 were included in the analysis to focus solely on assessments based on the previous version 15.14
Data were collected by trained auditors (primarily pharmacists, nurses and infectious disease doctors) according to a standardized method and data collection form13 and were entered in the Surgical NAPS online auditing platform.
Appropriateness and guideline compliance are separate measures as they are not mutually exclusive. Appropriateness was a composite measure based on antimicrobial choice, timing of administration, duration and repeat dosing, applying the standardized Appropriateness Assessment Guide (Figure S1). Reasons for inappropriateness were based upon this guide. Guideline compliance of SAP prescribing was assessed against the national guidelines14 or local site-based guidelines available at the time of assessment.
This study focuses on initial antimicrobial choice for SAP, therefore the following exclusions were made: existing antimicrobial therapy and post-procedural antimicrobial prescription (definitions in Table S1); repeat procedural doses or where repeat doses were required but not given; and appropriateness assessments deemed ‘not-assessable’. Data were excluded from univariable and multivariate analysis if prescriptions were categorized as ‘not assessable’ or if categories contained missing or small numbers.
Upon initial data inspection, it was decided a priori to group antimicrobials into their pharmacological classes, with the exception of cefazolin, due to its high volume and first-line recommendation for antimicrobial choice, and metronidazole and chloramphenicol, as no other antimicrobials of their same classes were prescribed. Cefepime was the only fourth-generation cephalosporin and was therefore grouped with third-generation cephalosporins due to similar profiles. Antimicrobials prescribed with a frequency less than 10 were grouped together as ‘others’ and excluded from statistical analysis (Table S2).
The collection and analysis of data from Surgical NAPS has been approved by the Melbourne Health Human Research and Ethics Committee (approval number QA2013066).
Statistical analysis
An initial unadjusted subgroup analysis compared the appropriateness of prescriptions for surgical episodes when antimicrobials were and were not prescribed for both procedural and post-procedural SAP.
Logistic regression models were then used to identify hospital, patient and surgical factors associated with appropriateness. Model selection was performed using a likelihood ratio test and model fit assessed by residual plots. Clinically important variables were selected a priori, based on previous literature for inclusion in univariable and multivariable analysis. Mixed-effects logistic models with unique hospital identifiers as random intercepts provided the best fit to the data and were selected. Two-tailed χ2 tests were conducted and a P value of less than 0.05 was considered statistically significant. ORs were calculated and values <1 were demonstrated to have a negative association of appropriateness i.e. promoted inappropriateness.
Crude estimates of appropriateness were adjusted for factors included in the model by calculating estimated marginal means (EMMs), presented as ‘adjusted appropriateness’ (AA) with 95% confidence intervals. The AA accounts for all factors included in the model by calculating an equal-weighted average across all subgroups. Therefore, the appropriateness is ‘adjusted’ for potential bias that may result from varying subgroup sizes in our sample.
Subgroup analyses were performed for each of the top five prescribed antimicrobials/antimicrobial classes. Percentage of appropriateness was compared for clinically significant categorical variables with χ2 tests.
Statistical analysis was conducted with Stata version 14.1 (StataCorp LP, College Station, TX, USA).
Results
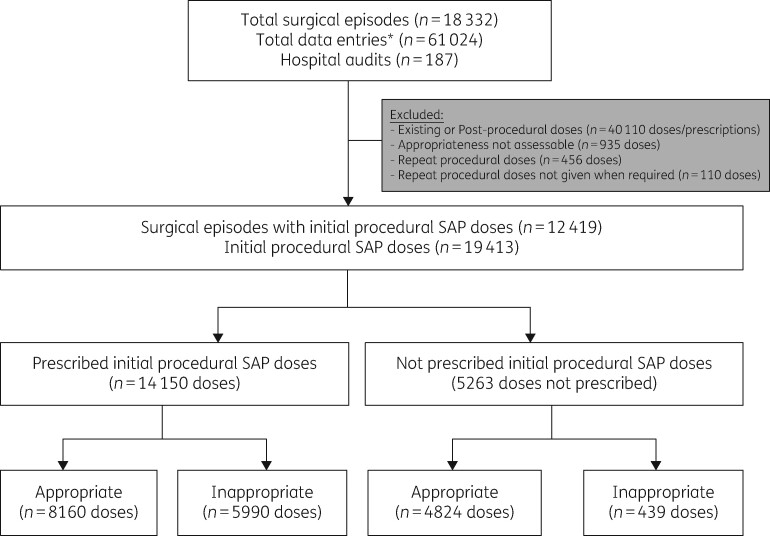
A total of 18 332 surgical episodes across 185 hospital audits were included. Of all surgical episodes with initial procedural SAP prescribed (n = 12 419), 87.8% included at least one cefazolin prescription (n = 10 899). Figure 1 provides a summary of the categories of the included and excluded prescribed antimicrobial doses and prescriptions. Doses with an appropriateness assessment deemed ‘not assessable’ were excluded (n = 935), as were repeat doses (n = 456) and repeat doses not given when required (n = 110). A total of 12 419 surgical episodes with 14 150 prescribed initial procedural doses were included for analysis. The median age of patients was 58 years (range 0–100 years). Female patients accounted for 52.9% of the surgical episodes (n = 7488) and male patients for 46.7% (n = 6609).
Figure 1.
Workflow summary diagram for data analysis. *Data entries pertains to all data collected within the surgical episode. Each surgical episode collects data on three antimicrobial categories: ‘procedural’, ‘post-procedural’ and ‘existing’, for which an antimicrobial could be ‘prescribed’, ‘not prescribed’ or ‘not assessable’.
Overall appropriateness was low (66.9%) (Table S3). When comparing SAP being prescribed and not being prescribed (regardless of indication), appropriateness was much higher for the latter (57.7% and 91.7%, respectively) (Table S3).
Participating hospitals and audited surgical procedure groups
Of the 187 participating hospitals, 98.4% (n = 184) undertook audits that utilized cefazolin. The remaining three hospitals audited very low numbers of procedures (range: 2–4) with alternative antimicrobials prescribed; cefalotin, chloramphenicol and gentamicin. All Australian states and territories contributed data to the Surgical NAPS (Table S4).
Surgical variables significantly associated with appropriateness of procedural SAP included presence of trauma (OR, 1.41; 95% CI, 1.13–1.77; P = 0.002); presence, removal or insertion of prosthetic material (OR, 1.68; 95% CI, 1.50–1.89; P < 0.001); clean-contaminated wound class (OR, 1.23; 95% CI, 1.08–1.41; P = 0.002); and surgical procedure groups: abdominal (OR, 0.84; 95% CI, 0.70–1.00; P = 0.046), dentoalveolar (OR, 0.25; 95% CI, 0.17–0.37; P < 0.001), gastrointestinal endoscopic (OR, 0.22; 95% CI, 0.09–0.54; P = 0.001), head and neck (OR, 0.46; 95% CI, 0.36–0.60; P < 0.001), neurosurgery (OR, 1.63; 95% CI, 1.23–2.16; P = 0.001), ophthalmology (OR, 2.09; 95% CI, 1.38–3.17; P < 0.001), plastic and reconstructive (OR, 0.59; 95% CI, 0.47–0.73; P < 0.001) and urological (OR, 0.59; 95% CI, 0.47–0.73; P < 0.001) (Table S4).
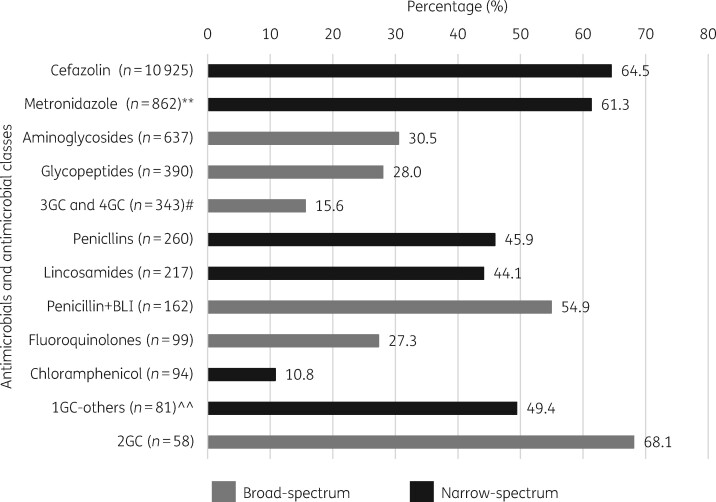
With regard to antimicrobial choice, AA was reported as low for all antimicrobials and classes, ranging from 10.8% for chloramphenicol to 68.1% for second-generation cephalosporins (Figure 2). Broad-spectrum antimicrobials and antimicrobial classes generally had lower rates of appropriateness, i.e. third/fourth-generation cephalosporins (15.5% AA), fluoroquinolones (27.3%), glycopeptides (28.0%) and aminoglycosides (30.5%).
Figure 2.
AA (%) per antimicrobial/antimicrobial class (n = 14 128). **Metronidazole was the only nitroimidazole reported and is therefore reported on its own, as opposed to class. #Only one fourth-generation cephalosporin (n = 5) was reported and grouped with third-generation cephalosporins. ^^Other first-generation cephalosporins refers to all reported, excluding cefazolin. BLI, β-lactamase inhibitor; 1GC, first-generation cephalosporins; 2GC, second-generation cephalosporins; 3GC, third-generation cephalosporins; 4GC, fourth-generation cephalosporins.
Broad-spectrum antimicrobial use
A subgroup analysis of the top five prescribed antimicrobials/antimicrobial classes (n = 13 157) accounted for 93.0% of all procedural SAP doses (n = 14 150). Cefazolin (n = 10 925) and metronidazole (n = 826) are commonly recommended for SAP and accounted for 77.2% and 5.9% of all procedural SAP doses, respectively. The next three most frequently prescribed classes included broad-spectrum antimicrobials (aminoglycosides, glycopeptides and third/fourth-generation cephalosporins).
Table 1 illustrates a range of variables and their associations with appropriateness across the top five antimicrobials/antimicrobial classes. Allergy status (P = 0.015), surgical procedure group (P <0.001) and the presence of prosthetic material (P <0.001) were positively associated with cefazolin appropriateness. There were no significant positive associations between these variables and appropriateness of glycopeptides and third/fourth-generation cephalosporins.
Table 1.
Appropriateness of the top five audited antimicrobials/antimicrobial classes by patient and surgical factors
| Variables | Antimicrobial/antimicrobial class, n (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cefazolin |
metronidazole |
aminoglycosides |
glycopeptides |
3GC/4GCa |
||||||
| total | appropriate | total | appropriate | total | appropriate | total | appropriate | total | appropriate | |
| Total appropriateness, n (%) | 10 925 | 6984 (63.9) | 862 | 472 (54.8) | 637 | 188 (29.5) | 390 | 107 (27.4) | 343 | 34 (9.9) |
| Allergy status χ2 test [P value] | 12.29 [0.015] | 8.67 [0.070] | 10.05 [0.040] | 7.18 [0.127] | 5.51 [0.239] | |||||
| nil known | 9145 | 5889 (64.4) | 721 | 392 (54.4) | 498 | 148 (29.7) | 277 | 69 (24.9) | 303 | 29 (9.6) |
| not documented | 194 | 118 (60.8) | 9 | 6 (66.7) | 13 | 7 (53.9) | 5 | 3 (60.0) | 4 | 0 (0.0) |
| β-lactam allergy | 975 | 578 (59.3) | 83 | 40 (48.2) | 83 | 26 (31.3) | 80 | 29 (36.3) | 22 | 4 (18.2) |
| non-β-lactam allergy | 380 | 253 (66.6) | 39 | 27 (69.2) | 28 | 6 (21.4) | 14 | 3 (21.4) | 11 | 1 (9.1) |
| non-antimicrobial allergy | 221 | 145 (65.6) | 10 | 7 (70.0) | 15 | 1 (6.7) | 14 | 3 (21.4) | 3 | 0 (0.0) |
| Trauma χ2 test [P value] | 2.00 [0.157] | 0.04 [0.851] | 0.06 [0.800] | 0.39 [0.533] | 0.58 [0.447] | |||||
| no | 10 191 | 6497 (63.8) | 843 | 462 (54.8) | 628 | 185 (29.5) | 369 | 100 (27.1) | 338 | 33 (9.8) |
| yes | 734 | 487 (66.4) | 19 | 10 (52.6) | 9 | 3 (33.3) | 21 | 7 (33.3) | 5 | 1 (20.0) |
| Prosthetic material χ2 test [P value] | 292.78 [<0.001] | 2.14 [0.144] | 8.39 [0.004] | 2.12 [0.145] | 0.14 [0.712] | |||||
| no | 6758 | 3903 (57.8) | 791 | 439 (55.5) | 323 | 112 (34.7) | 131 | 42 (32.1) | 261 | 25 (9.6) |
| yes | 4167 | 3081 (73.9) | 71 | 33 (46.5) | 314 | 76 (24.2) | 259 | 65 (25.1) | 82 | 9 (11.0) |
| Surgical procedure group χ2 test [P value] | 661.71 [<0.001] | 33.7 [<0.001] | 153.83 [<0.001] | 6.39 [0.846] | 17.61 [0.128] | |||||
| abdominal | 1741 | 1097 (63.0) | 493 | 297 (60.2) | 32 | 5 (15.6) | 5 | 2 (40.0) | 121 | 17 (14.1) |
| breast | 340 | 218 (64.1) | 2 | 1 (50.0) | 34 | 23 (67.7) | 4 | 1 (25.0) | 1 | 1 (100.0) |
| cardiac | 581 | 385 (66.3) | 0 | — | 57 | 7 (12.3) | 118 | 35 (29.7) | 8 | 0 (0.0) |
| dentoalveolar | 157 | 34 (21.7) | 5 | 4 (80.0) | 0 | — | 0 | — | 0 | — |
| gastrointestinal endoscopic | 23 | 6 (26.1) | 12 | 1 (8.3) | 4 | 3 (75.0) | 0 | — | 9 | 2 (22.2) |
| gynaecological | 527 | 303 (57.5) | 183 | 95 (51.9) | 2 | 2 (100.0) | 0 | — | 7 | 0 (0.0) |
| head and neck | 411 | 186 (45.3) | 7 | 3 (42.9) | 2 | 0 (0.0) | 3 | 0 (0.0) | 3 | 0 (0.0) |
| neurosurgery | 445 | 350 (78.7) | 0 | — | 15 | 0 (0.0) | 20 | 5 (25.0) | 7 | 1 (14.3) |
| obstetrics | 865 | 542 (62.7) | 65 | 23 (35.4) | 3 | 0 (0.0) | 1 | 1 (100.0) | 1 | 0 (0.0) |
| ophthalmology | 426 | 393 (92.3) | 0 | — | 15 | 1 (6.7) | 13 | 3 (23.1) | 2 | 0 (0.0) |
| orthopaedic | 3374 | 2416 (71.6) | 1 | 1 (100.0) | 207 | 18 (8.7) | 180 | 46 (25.6) | 19 | 2 (10.5) |
| plastic and reconstructive | 990 | 452 (45.7) | 13 | 6 (46.2) | 10 | 2 (20.0) | 14 | 5 (35.7) | 4 | 0 (0.0) |
| thoracic | 93 | 70 (75.3) | 2 | 2 (100.0) | 1 | 0 (0.0) | 2 | 0 (0.0) | 1 | 0 (0.0) |
| urological | 677 | 378 (55.8) | 72 | 37 (51.4) | 252 | 127 (50.4) | 9 | 3 (33.3) | 160 | 11 (6.9) |
| vascular | 275 | 154 (56.0) | 7 | 2 (28.6) | 3 | 0 (0.0) | 21 | 6 (28.6) | 0 | — |
Third- and fourth-generation cephalosporins.
Reasons for inappropriateness
Table 2 highlights the multiple reasons for inappropriateness of antimicrobial choice. The most common reason was the antimicrobial spectrum being too broad (55.7%) with the lowest rate of inappropriateness for cefazolin prescriptions (17.3%) and the highest rate for third/fourth-generation cephalosporins (83.3%).
Table 2.
Common reasons for inappropriate antimicrobial choice for procedural SAP doses
| Antimicrobial | Inappropriate doses (n) | SAP indicated and inappropriate, n (%) | Total reasons for inappropriate antimicrobial choicea (n) | Types of reasons for inappropriate antimicrobial choice,bn (%) |
|||
|---|---|---|---|---|---|---|---|
| spectrum too broad | spectrum too narrow | allergy mismatch | microbiology mismatch | ||||
| Total | 5372 | 3966 (73.8) | 1007 | 561 (55.7) | 337 (33.5) | 62 (6.2) | 47 (4.7) |
| cefazolin | 3941 | 2842 | 394 | 68 (17.3) | 240 (60.9) | 61 (15.5) | 25 (6.3) |
| aminoglycosides | 449 | 325 | 163 | 133 (81.6) | 28 (17.2) | 1 (0.6) | 1 (0.6) |
| metronidazole | 390 | 290 | 84 | 57 (67.9) | 26 (31.0) | 0 (0.0) | 1 (1.2) |
| 3GC/4GCc | 309 | 260 | 240 | 200 (83.3) | 23 (9.6) | 0 (0.0) | 17 (7.1) |
| glycopeptides | 283 | 249 | 126 | 103 (81.7) | 20 (15.9) | 0 (0.0) | 3 (2.4) |
There was a total of 4826 reasons for inappropriateness of procedural SAP; only 1007 (20.9%) were in relation to the choice of antimicrobial agent.
Rationale for the reasons for inappropriateness are described in Figure S1. Surgical NAPS appropriateness assessment guide.
Third- and fourth-generation cephalosporins.
Surgical procedure groups
Subgroup analysis of appropriate procedural prescriptions of the most common broad-spectrum antimicrobials (aminoglycosides, glycopeptides and third/fourth-generation cephalosporins) (n = 329 doses) identified procedure groups associated with their respective use (Table S5). For aminoglycosides, urological surgery (n = 127) was the most common procedure associated with appropriate use (67.6% of all procedural aminoglycoside doses). Orthopaedic surgery (n = 46, 43.0%) and cardiac surgery (n = 35, 32.7%) accounted for the common procedure groups associated with appropriate glycopeptides. Abdominal surgery accounted for 50% (n = 17) of all appropriate third/fourth-generation cephalosporin doses (Table S5).
Table S5 also illustrates the guideline compliance of these doses deemed appropriate and number of hospitals with local guidelines. For breast surgical procedures, the analysis identified that all appropriate aminoglycoside doses were from one hospital that endorses such use in their local guidelines. Local guidelines informed the appropriateness of broad-spectrum antimicrobial use in 28.3% (n = 93), compared with 52.9% for national guidelines (n = 174).
Discussion
Analysis of the Surgical NAPS data has continued to demonstrate poor rates of appropriateness across procedural SAP. Appropriateness varied across the choice of antimicrobial agent in our surveyed population but was low overall (57.7%). Lower rates of appropriateness were demonstrated where broad-spectrum antimicrobials such as glycopeptides, aminoglycosides and third/fourth-generation cephalosporins were used and these rates differed across a range of surgical procedure groups. Unsurprisingly, the different antimicrobial spectra covered by these antimicrobials reflected their use across different surgical procedures. Additionally, these antimicrobials are not routinely recommended, thus we would expect appropriateness to be low.
Our research contributes to the increasing adoption of appropriateness as a key measure for antimicrobial audits,1,2,16,19–26 as opposed to the sole use of guideline compliance. Standardized appropriateness measures aim to enable assessments to address issues of guideline implementability and recommendation flexibility to account for varying patient characteristics.27
Glycopeptides such as vancomycin provide Gram-positive bacterial coverage and were frequently prescribed by surgeons inappropriately in orthopaedic, cardiac and vascular procedures. In these procedures, vancomycin is indicated as an addition to cefazolin if the patient is, or is at risk of being, colonized or infected with MRSA, or as an alternative to cefazolin in patients with immediate hypersensitivity to penicillins.14 However, neither scenario was reported in the included data. Directed therapy is considered an exception, but was rarely recorded and when it was, the microbiological data to support decision-making were absent. It is unclear whether these data were truly absent or not recorded by the auditor.
Ceftriaxone was the most commonly prescribed third-generation cephalosporin and provides broad Gram-negative bacteria coverage; it was commonly prescribed by surgeons as SAP for abdominal and urological procedures. The use of Gram-negative coverage is warranted in such procedures; however, S. aureus is a common pathogen28,29 and use of broader-spectrum coverage (i.e. ceftriaxone instead of the first-line agent cefazolin) has not been clearly justified in our surveyed population.
The recently updated national guidelines recommend aminoglycosides such as gentamicin for urological procedures due to the emergence of cephalosporin resistance in Enterobacterales.15,29
Whilst this recommendation was not included in the guidelines available for this analysis, we propose that this may have been a driving influence behind our observation of 67.6% of all appropriate gentamicin doses being prescribed for urological procedures.
Our research is novel and has demonstrated associations between risk factors (allergy status, presence of prosthetic material and surgical procedure groups) and appropriateness of antimicrobials for SAP. Surgical procedure groups remain the most common variable negatively associated with appropriateness in this study and in our previously published analysis of procedural and post-procedural SAP prescriptions.16 Surgical procedure groups vary in the terms of risks of infection and as such so do their requirements for SAP, particularly the antimicrobial agent of choice. Thus, surgeons must factor and mitigate these risks when making decisions regarding antimicrobial choice for SAP. Our research has demonstrated that whilst cefazolin is the most commonly recommended and prescribed antimicrobial in our surveyed population, there are still a range of antimicrobials being prescribed for SAP, particularly broad-spectrum antimicrobials that we propose as targets for tailored surgical AMS interventions. These targets include aminoglycoside use in urological, breast and orthopaedic surgery, glycopeptide use in cardiac and orthopaedic surgery and third/fourth-generation cephalosporin use in abdominal and urological surgery.
Reasons for inappropriate surgical prophylaxis prescriptions
Inappropriate SAP prescribing has been reported in both Australian3,16,30–32 and international literature.5,6,33–43 Common reasons for inappropriateness identified in this study are consistent with current literature regarding SAP. The timing of administration for procedural SAP remains the most commonly identified reason for inappropriateness in Australia16,31,32 and international research.34,36 Exploring factors influencing antimicrobial choice for SAP is limited and warranted to support targeted AMS interventions. Antimicrobial selection based on broader-spectrum coverage was also a common reason for inappropriateness in our surveyed population. Similarly, a small Iranian audit of 100 SAP cases identified that vancomycin and ceftriaxone accounted for one-fifth of all SAP doses (21%).5 An analysis of Qatar hospital SAP guidelines across 101 patients noted inappropriate antimicrobial selection as the second most common reason for non-compliance (31.5%). Whilst the commonly recommended antimicrobial cefazolin accounted for 44.6% of all antimicrobials, ceftriaxone was the third most common at 16.8%.44
A Turkish point-prevalence study across 16 centres audited 161 SAP doses, with the type of antimicrobial agent most commonly chosen being inappropriate for 40.9%.35 Antimicrobials commonly prescribed were of a broader spectrum and included ceftriaxone, glycopeptides and aminoglycosides.35 Broad-spectrum antimicrobials such as third-generation cephalosporins were also the most commonly prescribed for all surgeries (80%) in an SAP audit of 100 patients in an Indian teaching hospital44 and in a large multicentre prospective Chinese audit including 14 525 surgical procedures (20.5%, 3453 of 16 836 antimicrobial doses).6
Guideline compliance
Higher rates of guideline non-compliance were anticipated, given the second-line nature of aminoglycosides and glycopeptides. This was also anticipated for third/fourth-generation cephalosporins, which are not recommended at all in the Australian guidelines. The lack of guidelines (when assessing guideline compliance) as a means of potential justification for appropriate broad-spectrum SAP use was uncommon (1.5%) (Table S5). Further education of auditors and prescribers to ensure they are aware of what the guidelines cover may help address this issue. Additionally, greater transparency from guideline developers regarding which procedures are and are not covered under each recommendation may provide further decision support to optimize SAP prescriptions.
Compliance with local guidelines was low across the top five antimicrobials (Table S5). However, further analysis of appropriate broad-spectrum agents revealed that local guidelines were potentially endorsing use of broad-spectrum antimicrobials in 28.3% (n = 93) of appropriate doses (n = 329). We were unable to access content of all local hospital guidelines and therefore unable to critically analyse their content for discrepancies between local and national guidelines, and also for potential drivers of broad-spectrum antimicrobial use. This represents a novel area for ongoing research.
Guidelines from the Australian Orthopaedic Association and the American Academy of Orthopaedic Surgeons recommend addition of glycopeptides if the local rate of MRSA is high.45,46 Therefore, we advocate flexibility to adapt the national guidelines based on local epidemiology. We propose that hospitals conduct additional monitoring of antimicrobial adverse effects and surgical site infection microbiology to ensure no additional patient harm is associated with their use. This would support ongoing AMS activities and potentially illustrate further consequences of unwarranted broad-spectrum antimicrobial use.
Limitations
The limitations of the Surgical NAPS data have been described in previous publications.13,16 Key limitations include the voluntary nature of participation; data are not a random sample, which creates potential sampling and selection bias and survey flexibility, i.e. hospitals could audit any patients and/or surgical units. Thus, the data may include targeted audits reflecting higher volume or problematic units, which influences the interpretation of results.
Appropriateness assessments are not entirely objective and one auditor’s interpretation may potentially differ from another. Whilst an assessment rubric (Figure S1) was available and support from the Surgical NAPS team was provided on request, assessment of inter-rater reliability is beyond the scope of this project and has also been discussed elsewhere.2,16
A caveat to all antimicrobial audits is inadequate documentation, which is historically and universally completed poorly for antimicrobial prescriptions.1,19–23 However, we argue that the adoption of appropriateness measurements supports the standardization of these assessments, whilst allowing for some flexibility, as opposed to the measure of guideline compliance.
Our research does not account for a range of factors that the Surgical NAPS does not capture, including surgeon experience and perceptions of risk and their aversion. Qualitative research has highlighted that antimicrobial prescribing is influenced by surgical hierarchy and the fear and risk of causing infections and patient harm.47 Ongoing research is required to explore decision-making of SAP choice and rationale for broad-spectrum use.
Conclusions
There are various factors that influence appropriateness of procedural SAP choice, including patient factors (ASA score and allergies), hospital factors (presence of local guidelines) and surgical factors (presence of trauma, prosthetic material and surgical procedure groups). Abdominal and urological surgery are key targeted areas to improve prescribing of third- and fourth-generation cephalosporins. Urological, breast and orthopaedic surgeries are targeted procedures to improve aminoglycoside prescribing, whilst orthopaedic and cardiac procedures should be targeted to improve the use of glycopeptides. Local guidelines endorsing routine broad-spectrum antimicrobials should consider additional monitoring of the unintentional consequences of their use, i.e. resistance patterns and adverse effects. Identification of these prescribing trends supports an ongoing examination of SAP prescribing practices in Australia and identification of potential targets for interventions across multiple health sectors such as AMS, guideline development and hospital policy.
Supplementary Material
Acknowledgements
We thank Dr Nabeel Imam for initial statistical consultations.
Funding
Surgical NAPS is funded by the Australian Commission on Safety and Quality in Health Care as part of the National Antimicrobial Use and Resistance in Australia Surveillance System, the Australian Government Department of Health, the National Centre for Antimicrobial Stewardship and Melbourne Health.
Transparency declarations
None to declare.
Supplementary data
Tables S1–S5, Figure S1 and the Reviewer report are available as Supplementary data at JAC-AMR Online.
References
- 1.Australian Commission on Safety and Quality in Health Care. Antimicrobial Prescribing Practice in Australian Hospitals: Results of the 2018 Hospital National Antimicrobial Prescribing Survey. 2020. https://irp-cdn.multiscreensite.com/d820f98f/files/uploaded/Hospital%20NAPS%20Public%20Report%20-%202018.pdf.
- 2.Melbourne Health. Surgical Prophylaxis Prescribing in Australian Hospitals: Results of the 2017 and 2018 Surgical National Antimicrobial Prescribing Surveys. 2020. https://irp-cdn.multiscreensite.com/d820f98f/files/uploaded/Surgical%20NAPS%20Public%20Report%202017-2018.pdf.
- 3. Bull AL, Russo PL, Friedman ND. et al. Compliance with surgical antibiotic prophylaxis—reporting from a statewide surveillance programme in Victoria, Australia. J Hosp Infect 2006; 63: 140–7. [DOI] [PubMed] [Google Scholar]
- 4. Bull AL, Worth LJ, Spelman T. et al. Antibiotic prescribing practices for prevention of surgical site infections in Australia: increased uptake of national guidelines after surveillance and reporting and impact on infection rates. Surg Infect (Larchmt) 2017; 18: 834–40. [DOI] [PubMed] [Google Scholar]
- 5. Mousavi S, Zamani E, Bahrami F.. An audit of perioperative antimicrobial prophylaxis: compliance with the international guidelines. J Res Pharm Pract 2017; 6: 126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ou Y, Jing BQ, Guo FF. et al. Audits of the quality of perioperative antibiotic prophylaxis in Shandong Province, China, 2006 to 2011. Am J Infect Control 2014; 42: 516–20. [DOI] [PubMed] [Google Scholar]
- 7. Parulekar L, Soman R, Singhal T. et al. How good is compliance with surgical antibiotic prophylaxis guidelines in a tertiary care private hospital in India? A prospective study. Indian J Surg 2009; 71: 15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmitt C, Lacerda RA, Turrini RNT. et al. Improving compliance with surgical antibiotic prophylaxis guidelines: a multicenter evaluation. Am J Infect Control 2017; 45: 1111–5. [DOI] [PubMed] [Google Scholar]
- 9. Nabor MIP, Buckley BS, Lapitan MCM.. Compliance with international guidelines on antibiotic prophylaxis for elective surgeries at a tertiary-level hospital in the Philippines. Healthc Infect 2015; 20: 145–51. [Google Scholar]
- 10. Alemkere G. Antibiotic usage in surgical prophylaxis: a prospective observational study in the surgical ward of Nekemte referral hospital. PLoS One 2018; 13: e0203523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahmoudi L, Ghouchani M, Mahi-Birjand M. et al. Optimizing compliance with surgical antimicrobial prophylaxis guidelines in patients undergoing gastrointestinal surgery at a referral teaching hospital in southern Iran: clinical and economic impact. Infect Drug Resist 2019; 12: 2437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Neill J. Review on antimicrobial resistance. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf.
- 13.Australian Commission on Safety and Quality in Health Care. Surgical National Antimicrobial Prescribing Survey: Results of the 2016 Pilot. 2017. https://www.safetyandquality.gov.au/sites/default/files/migrated/Surgical-National-Antimicrobial-Prescribing-Survey-SNAPS-Report-Results-of-the-2016-Pilot-November-2017.pdf.
- 14.Antibiotic Expert Group. Therapeutic Guidelines: Antibiotic. Version 15. Therapeutic Guidelines Limited, 2014.
- 15.Antibiotic Expert Group. Therapeutic Guidelines: Antibiotic. Version 16. Therapeutic Guidelines Limited, 2019.
- 16. Ierano C, Thursky K, Marshall C. et al. Appropriateness of surgical antimicrobial prophylaxis practices in Australia. JAMA Netw Open 2019; 2: e1915003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Australian Institute of Health and Welfare. Australian Hospital Peer Groups. 2015. https://www.aihw.gov.au/getmedia/79e7d756-7cfe-49bf-b8c0-0bbb0daa2430/14825.pdf.aspx?inline=true.
- 18.Australian Bureau of Statistics. Australian Statistical Geography Standard (ASGS): Volume 5 - Remoteness Structure. 2016. https://www.abs.gov.au/ausstats/abs@.nsf/mf/1270.0.55.005.
- 19.Australian Commission on Safety and Quality in Health Care. Antimicrobial Prescribing Practice in Australia: Results of the 2016 National Antimicrobial Prescribing Survey. 2018. https://www.safetyandquality.gov.au/sites/default/files/migrated/2016-Hospital-NAPS.pdf.
- 20.Australian Commission on Safety and Quality in Health Care. Antimicrobial Prescribing Practice in Australia: Results of the 2017 National Antimicrobial Prescribing Survey. 2018. https://www.safetyandquality.gov.au/sites/default/files/migrated/2017-Hospital-NAPS.pdf.
- 21.Australian Commission on Safety and Quality in Health Care. Antimicrobial Prescribing Practice in Australia: Results of the 2015 National Antimicrobial Prescribing Survey. 2016. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/antimicrobial-prescribing-practice-australian-hospitals-results-2015-hospital-national-antimicrobial-prescribing-survey.
- 22.Australian Commission on Safety and Quality in Health Care. Antimicrobial Prescribing Practice in Australia: Results of the 2014 National Antimicrobial Prescribing Survey. 2015. https://www.safetyandquality.gov.au/sites/default/files/migrated/Antimicrobial-prescribing-practice-in-Aust-hospitals-NAPS-2014-Results.pdf.
- 23.Australian Commission on Safety and Quality in Health Care. Antimicrobial Prescribing Practice in Australia: Results of the 2013 National Antimicrobial Prescribing Survey. 2014. https://www.safetyandquality.gov.au/sites/default/files/migrated/Web-Accessible-2013-NAPS-Report.pdf.
- 24. Bishop JL, Schulz TR, Kong DC. et al. Similarities and differences in antimicrobial prescribing between major city hospitals and regional and remote hospitals in Australia. Int J Antimicrob Agents 2019; 53: 171–6. [DOI] [PubMed] [Google Scholar]
- 25. Cotta MO, Chen C, Tacey M. et al. What are the similarities and differences in antimicrobial prescribing between Australian public and private hospitals? Intern Med J 2016; 46: 1182–8. [DOI] [PubMed] [Google Scholar]
- 26. Cotta MO, Robertson MS, Upjohn LM. et al. Using periodic point-prevalence surveys to assess appropriateness of antimicrobial prescribing in Australian private hospitals. Intern Med J 2014; 44: 240–6. [DOI] [PubMed] [Google Scholar]
- 27. Ierano C, Ayton D, Peel T. et al. Evaluating the implementability of Antibiotic Surgical Prophylaxis guidelines. Infect Dis Health 2020; 25: 11–21. [DOI] [PubMed] [Google Scholar]
- 28. Pochhammer J, Köhler J, Schäffer M.. Colorectal surgical site infections and their causative pathogens: differences between left-and right-side resections. Surg Infect (Larchmt) 2019; 20: 62–70. [DOI] [PubMed] [Google Scholar]
- 29. Worth LJ, Bull AL, Spelman T. et al. Diminishing surgical site infections in Australia: time trends in infection rates, pathogens and antimicrobial resistance using a comprehensive Victorian surveillance program, 2002–2013. Infect Control Hosp Epidemiol 2015; 36: 409–16. [DOI] [PubMed] [Google Scholar]
- 30. Bull AL, Thursky KA, Richards MJ. et al. Monitoring the appropriateness of surgical antibiotic prophylaxis prescribing in Australia: valid and meaningful indicators provide ‘data for action’. Anaesth Intensive Care 2016; 44: 121–2. [PubMed] [Google Scholar]
- 31. Friedman ND, Styles K, Gray AM. et al. Compliance with surgical antibiotic prophylaxis at an Australian teaching hospital. Am J Infect Control 2013; 41: 71–4. [DOI] [PubMed] [Google Scholar]
- 32. Knox MC, Edye M.. Adherence to surgical antibiotic prophylaxis guidelines in New South Wales, Australia: identifying deficiencies and regression analysis of contributing factors. Surg Infect (Larchmt) 2016; 17: 203–9. [DOI] [PubMed] [Google Scholar]
- 33. Oh AL, Goh LM, Azim NAN. et al. Antibiotic usage in surgical prophylaxis: a prospective surveillance of surgical wards at a tertiary hospital in Malaysia. J Infect Dev Ctries 2014; 8: 193–201. [DOI] [PubMed] [Google Scholar]
- 34. Pittalis S, Ferraro F, Piselli P. et al. Appropriateness of surgical antimicrobial prophylaxis in the Latium region of Italy, 2008: a multicenter study. Surg Infect (Larchmt) 2013; 14: 381–4. [DOI] [PubMed] [Google Scholar]
- 35. Kaya S, Aktas S, Senbayrak S. et al. An evaluation of surgical prophylaxis procedures in Turkey: a multi-center point prevalence study. Eurasian J Med 2016; 48: 24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goede WJ, Lovely JK, Thompson RL. et al. Assessment of prophylactic antibiotic use in patients with surgical site infections. Hosp Pharm 2013; 48: 560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pan A, Ambrosini L, Patroni A. et al. Adherence to surgical site infection guidelines in Italian cardiac surgery units. Infection 2009; 37: 148–52. [DOI] [PubMed] [Google Scholar]
- 38. Hosoglu S, Sunbul M, Erol S. et al. A national survey of surgical antibiotic prophylaxis in Turkey. Infect Control Hosp Epidemiol 2003; 24: 758–61. [DOI] [PubMed] [Google Scholar]
- 39. Abdel-Aziz A, El-Menyar A, Al-Thani H. et al. Adherence of surgeons to antimicrobial prophylaxis guidelines in a tertiary general hospital in a rapidly developing country. Adv Pharmacol Sci 2013; 2013:842593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tourmousoglou CE, Yiannakopoulou EC, Kalapothaki V. et al. Adherence to guidelines for antibiotic prophylaxis in general surgery: a critical appraisal. J Antimicrob Chemother 2008; 61: 214–8. [DOI] [PubMed] [Google Scholar]
- 41. Hohmann C, Eickhoff C, Radziwill R. et al. Adherence to guidelines for antibiotic prophylaxis in surgery patients in German hospitals: a multicentre evaluation involving pharmacy interns. Infection 2012; 40: 131–7. [DOI] [PubMed] [Google Scholar]
- 42. Cai T, Verze P, Brugnolli A. et al. Adherence to European Association of Urology guidelines on prophylactic antibiotics: an important step in antimicrobial stewardship. Eur Urol 2016; 69: 276–83. [DOI] [PubMed] [Google Scholar]
- 43. Thouverez M, Lallemand S, Bailly P. et al. Quelles sont les situations de non-conformité de l’antibioprophylaxie chirurgicale par rapport aux recommandations nationales? Pathol Biol (Paris) 2002; 50: 547–51. [DOI] [PubMed] [Google Scholar]
- 44. Rehan HS, Kakkar AK, Goel S.. Pattern of surgical antibiotic prophylaxis in a tertiary care teaching hospital in India. Int J Infect Control 2010; doi:10.3396/ijic.v6i2.4584. [Google Scholar]
- 45.Arthroplasty Society of Australia. Guidelines for antibiotic prophylaxis at the time of hip and knee arthroplasty. https://www.aoa.org.au/docs/default-source/advocacy/guidelines-for-antibiotic-prophylaxis-at-the-time-of-hip-and-knee-arthroplasty_asa_october-2018.pdf?sfvrsn=aa4ec004_12.
- 46.American Academy of Orthopaedic Surgeons. Diagnosis and Prevention of Periprosthetic Joint Infections. Clinical Practice Guideline. 2019. https://aaos.org/globalassets/quality-and-practice-resources/pji/pji-clinical-practice-guideline-final-9-18-19-.pdf.
- 47. Ierano C, Thursky K, Peel T. et al. Influences on surgical antimicrobial prophylaxis decision making by surgical craft groups, anaesthetists, pharmacists and nurses in public and private hospitals. PLoS One 2019; 14: e0225011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.