Abstract
Background
Little is known about determinants of appropriate antibiotic use in the emergency department (ED). We measured appropriateness of antibiotic use for seven quality indicators (QIs) and studied patient-related factors that determine their variation.
Patients and methods
A retrospective analysis of 948 patients presumptively diagnosed as having an infection needing empirical antibiotic treatment in the ED was performed. Outcomes of seven previously validated QIs were calculated using computerized algorithms. We used logistic regression analysis to identify patient-related factors of QI performance and evaluated whether more appropriate antibiotic use in the ED results in better patient outcomes (length-of-stay, in-hospital mortality, 30 day readmission).
Results
QI performance ranged from 57.3% for guideline-adherent empirical therapy to 97.3% for appropriate route of administration in patients with sepsis. QI performance was positively associated with patients’ disease severity on admission (presence of fever, tachycardia and hypotension). Overall, the clinical diagnosis and thus the guidelines followed influenced QI performance. The difference in complexity between the guidelines was a possible explanation for the variation in QI performance. A QI performance sum score of 100% was associated with reduced in-hospital mortality. QI performance was not associated with readmission rates.
Conclusions
We gained insights into factors that determine quality of antibiotic prescription in the ED. Adherence to the full bundle of QIs was associated with reduced in-hospital mortality. These findings suggest that future stewardship interventions in the ED should focus on the entire process of antibiotic prescribing in the ED and not on a single metric only.
Introduction
Antibiotic stewardship programmes (ASPs) are recommended to increase the quality of antibiotic use and reduce antibiotic resistance.1 A significant part of hospital antibiotic prescribing for infectious diseases is initiated in the emergency department (ED), making the ED a crucial focus of ASPs.2 Studies regarding antibiotic use in the ED have been performed, but little is known about current appropriate antibiotic use in this specific setting. Study results are limited to a specific group of patients [e.g. sepsis, urinary tract infections (UTIs) or pneumonia]3–6 or focus on a single outcome metric (e.g. guideline adherence) while appropriate usage in the ED extends beyond guideline-adherent choice of empirical antibiotic therapy.7 In addition, little is known about the drivers (or determinants) of current antibiotic prescribing in the ED.
To measure the appropriateness of antibiotic use, quality indicators (QIs) can be used. Previous studies have shown that appropriate antibiotic use measured by QIs reduces length of stay (LOS) for hospitalized patients.8–10 Furthermore, compliance with a whole bundle of interventions increases survival among patients with sepsis11 and patients with Staphylococcus aureus bacteremia.12 The sepsis bundles have been the cornerstone of sepsis quality improvement in the ED since 2005.13
It is, however, not known whether appropriate antibiotic use initiated in the ED improves patient outcomes. Most antimicrobial stewardship interventions focus on patients admitted to the hospital ward. However, the first administration of antibiotics is very often administered in the ED, making this an interesting focus for antimicrobial stewardship teams.14 In this study, seven previously validated QIs (single QIs and a QI bundle) were assessed in a large sample of patients admitted to the ED with a (possible) infection who received empirical antibiotic therapy.7,15 We evaluated potential determinants that could explain the variation in QI performance between patients.
Our second goal was to explore whether more appropriate antibiotic use in the ED (better adherence to QIs) results in better patient outcomes (LOS, in-hospital mortality and 30 day readmission rates).
Patients and methods
Ethics
The Medical Ethics Committee of the Radboudumc was notified about the study; formal approval was not required because the project was based on epidemiological data (no. 2016/2938). Patient data were anonymously analysed in a retrospective design. Research involving human subjects, human material or personalized data was not carried out.
Study design and population
We conducted a single-centre, retrospective cohort study in the ED at Radboud University Medical Center, Nijmegen, The Netherlands, an urban, 604 bed tertiary teaching university hospital with approximately 22 500 ED visits per year.
Most patients in the Netherlands visiting an ED for a possible infection are referred by their GP or medical specialist. Only a few patients visit the ED directly without referral. The Dutch Health Care Inspectorate monitors several QIs in the ED, such as the use of an early warning system for the recognition of patients with sepsis, and the availability to healthcare professionals of a biannual training programme for the recognition of patients with sepsis.
All patients aged 18 years or older presenting to the ED in the period September 2015–March 2016 and presumptively diagnosed as having an infection needing empirical antibiotic treatment were included in the study.
Patients were excluded if: (i) antibiotics were used as prophylaxis; or (ii) antibiotics were prescribed before presentation to the ED, e.g. by the family physician, no change to the administered regimen was performed and no new infection was suspected. Patients were selected by extracting details of all antibiotic prescriptions in the ED during the study period from the electronic medical record system, together with clinical and laboratory data. When multiple admissions of a single patient occurred during the study period, only the first admission to the ED was included in our analysis. Because of the retrospective design of our study, the prescribing physicians were unaware that these QIs were monitored.
We estimated that 933 patients were sufficient to test a maximum of 14 determinants, assuming a maximum indicator performance of 85%.16
QIs and definitions
In the past, specific QIs assessing the appropriateness of antibiotic use for hospitalized patients and for patients with sepsis have been developed and validated using a RAND-modified Delphi procedure.7,15 The clinimetric properties of these indicators have been tested in a large group of hospitalized patients.17 We used seven indicators to assess the appropriateness of antibiotic use in the ED (Table 1).
Table 1.
QIs to measure appropriate antibiotic use in adult patients in the ED
| Number | QI | Numerator description | Denominator description |
|---|---|---|---|
| QIs applicable in the ED | |||
| 1a | antimicrobial therapy in adult patients with sepsis should be started intravenously | number of patients with sepsis who started with empirical systemic antimicrobial therapy intravenously | total number of patients with sepsis who started with empirical systemic antimicrobial therapy |
| 2a | antimicrobial therapy should be started as soon as possible, preferably within 3 h in adult patients with severe sepsis and septic shock | number of patients with severe sepsis or septic shock who started with empirical systemic antimicrobial therapy within the first 3 h after the clinical diagnosis | total number of patients with severe sepsis or septic shock, who started with empirical systemic antimicrobial therapy |
| 3 | before starting antimicrobial therapy, at least two sets of blood cultures should be taken | number of patients from whom at least two blood cultures were taken before empirical systemic antimicrobial therapy was started | total number of patients who started with empirical systemic antimicrobial therapy |
| 4 | specimens for culture from suspected sites of infection should be taken when possibleb | number of patients from whom specimens for culture from suspected sites of infection were taken | total number of patients who started with empirical systemic antimicrobial therapy |
| 5 | an antibiotic plan should be documented in the case notes at the start of systemic antibiotic therapy (antibiotic plan is indication and name) | number of patients who started with systemic antibiotic therapy for whom an antibiotic plan was documented in the case notes | total number of patients who started with systemic antibiotic therapy |
| 6 | empirical systemic antimicrobial therapy (only choice of antimicrobial agent) should be prescribed according to the local (or national) guideline | number of patients who started with empirical systemic antimicrobial therapy according to the guideline | total number of patients who started with empirical systemic antibiotic therapy |
| 7 | first dose of systemic antibiotic therapy should be correctc | number of patients with a correct first dose | total number of patients who started with systemic antibiotic therapy |
QIs 1 and 2 are only applicable to patients with sepsis (≥2 SIRS criteria) or septic shock.
Recommended microbiological testing for each site of infection is presented in Table S1 in Appendix S1.
Adjusted to the setting of the ED; even patients with renal dysfunction can generally be prescribed a one-time dose similar to that for a patient with normal kidney function; reducing the dose in patients with renal failure can even be harmful.33
QI performance
All data needed to compute performance of the QIs were extracted in a uniform way and entered in a database anonymously. The QIs with their numerators and denominators are described in detail in Table 1. We developed computerized algorithms to calculate the scores for each of the QIs for every patient (appropriate = 1 and inappropriate = 0). A QI bundle sum score was calculated by dividing the overall performance of all QIs by the number of QIs that applied to that specific patient. To compute appropriateness scores for the QIs, we used extensive working definitions, which are reported in Appendix S1; the algorithm to compute guideline adherence is reported in Appendix S2 (available as Supplementary data at JAC-AMR Online).
QIs and determinants of appropriate antibiotic use
The following potentially relevant patient determinants were selected: sex, age, Charlson comorbidity index (CCI), allergy to antibiotics, admission time and day, previous antibiotic use within 30 days before admission, ICU admission, recent hospital admission (≤30 days), chronic renal failure, immunocompromised status, an abnormal white blood cell count and the following vital signs: temperature ≥38°C (≥100.4°F), heart rate ≥100/min, systolic blood pressure <100 mm Hg, respiratory rate ≥20/min and Glasgow Coma Scale (GCS) score ≤14 on admission. The clinical diagnosis on admission was the only categorical variable that was used. Extended definitions for the determinants are included in Appendix S1.
QIs and patient outcomes
To evaluate whether more appropriate antibiotic use in the ED results in better patient outcomes, we assessed the association between QI performance (single and bundle sum score) and LOS, in-hospital mortality and 30 day readmission rates. LOS was defined as the number of days between admission and hospital discharge. Readmission was defined as an admission within 30 days after discharge, with an infection in the same organ system as before discharge.
Furthermore, we investigated the relationship between the bundle sum score and mortality by creating 10 subgroups (group 1: bundle sum score 0%–10%, group 2: bundle sum score 10%–20%, etc.). Subgroups with fewer than 30 patients were not analysed.
Statistical analysis
SPSS (version 22.0; SPSS, Inc.) was used for analysing data. Descriptive statistics for continuous variables were represented as mean ± SD for normally distributed variables, otherwise median and IQR were given. Unpaired Student’s t tests were used to compare normally distributed, continuous variables, otherwise non-parametric tests were used. Categorical variables were compared by use of the χ2 test, or Fisher’s exact test when the χ2 test was not appropriate.
Determinants of QI performance
We created multivariable logistic regression models for each QI. Single relationships between QI adherence and all determinants were studied using univariate analysis. For each QI, a separate multivariate stepwise logistic regression model was constructed. In each model, a single QI was used as the dependent outcome and all the determinants that had bivariate associations with P < 0.20 were investigated as the independent variables. If two independent variables were highly correlated (correlation coefficient >0.6), only one variable was included in the analysis.
For each working diagnosis with at least 30 patients per group, dummy variables were created. For the working diagnosis with less than 30 patients per group, we created an ‘other diagnosis’ dummy variable.
To investigate the discriminative power of each model, receiver operating characteristic (ROC) curves were constructed for the predicted probability of appropriate antibiotic therapy and the actual QI score. These results are presented as the area under the ROC curve (AUC). Each eligible determinant was stepwise added to the model and only remained if the ROC curve improved and the log-likelihood ratio test had a P value <0.20.
QI performance and patient outcomes
To assess the relationship between QI performance, mortality and 30 day readmission rates, we constructed binary logistic regression models with associations assessed by ORs and their Wald confidence intervals (CIs). For LOS, a log transformation was used to satisfy normality assumptions and a linear regression model was used to determine associations between LOS and QI performance. LOS was back-transformed for presentation as geometric mean (95% CI). Possible confounders were selected by bivariate analysis. Each possible confounder that had bivariate associations with P < 0.20 was entered in the multivariate analysis together with each single QI.
When data regarding outcome (6.2%) or determinants of QI performance (5.7%) were missing, patients were excluded from the analysis.
Results
Study population
The patient population consisted of 948 patients with empirical antibiotics prescribed in the ED for a suspected infection. The median patient age was 64.7 years (range 18–95), 56.9% were male, 78.1% of patients had ≥2 Systemic Inflammatory Response Syndrome (SIRS) criteria and 14.3% had a quick SOFA (qSOFA) score of ≥2. A total of 233 patients (24.6%) received a second or a third antibiotic from a different class in the ED. Further baseline characteristics are presented in Table 2.
Table 2.
Baseline characteristics of the 948 participating patients
| Variable | Male | Female | P |
|---|---|---|---|
| Total | 539 | 409 | |
| Median age (range), yearsa | 66.1 (74.6) | 60.0 (77.1) | 0.02 |
| Immunocompromised | 187 (34.7) | 155 (37.9) | 0.31 |
| Allergy to (any) antibiotics | 54 (10.0) | 80 (19.6) | <0.01 |
| Admission at night (7 PM–7 AM) | 192 (35.6) | 147 (35.9) | 0.92 |
| Diagnosis | 0.52 | ||
| LRTI | 156 (28.9) | 118 (28.9) | |
| UTI | 100 (18.6) | 69 (16.9) | |
| skin and soft tissue infection | 28 (5.2) | 27 (6.6) | |
| IA infection | 65 (12.1) | 43 (10.5) | |
| sepsis of undefined origin | 54 (10.0) | 49 (12.0) | |
| other | 74 (13.7) | 45 (11.0) | |
| more than one possible diagnosis | 62 (11.5) | 58 (14.2) | |
| SIRS criteria | |||
| ≥2 (sepsis) | 417 (77.4) | 323 (79.0) | 0.55 |
| qSOFA criteria | |||
| ≥2 (sepsis) | 77 (14.3) | 59 (14.4) | 0.95 |
| CCI, mean (SD) | 5.22 (3.0) | 4.46 (2.6) | <0.01 |
| Antibiotic therapy within past 30 daysb | 200 (37.2) | 149 (36.9) | 0.91 |
| Colonization with ESBL within last year | 15 (2.8) | 7 (1.7) | 0.27 |
| Hospital admission within past 30 days | 91 (16.9) | 58 (14.2) | 0.26 |
| Mean arterial pressure (mm Hg) on admission (SD) | 84.2 (18.8) | 82.3 (19.1) | 0.07 |
| Heart rate (bpm) on admission, mean (SD) | 100.6 (26.3) | 103.8 (21.3) | 0.04 |
| Department of prescribing physician | 0.03 | ||
| internal medicine | 244 (45.3) | 208 (50.9) | |
| emergency medicine | 139 (25.8) | 94 (23.0) | |
| urology | 41 (7.6) | 14 (3.4) | |
| geriatrics | 19 (3.5) | 18 (4.4) | |
| surgery | 31 (5.8) | 15 (3.7) | |
| pulmonology | 28 (5.2) | 32 (7.8) | |
| other | 37 (6.9) | 28 (6.8) | |
| Antibiotics prescribed | >0.05 | ||
| ceftriaxone | 378 (55.1) | 287 (56.3) | |
| metronidazole | 53 (7.7) | 51 (10.0) | |
| ciprofloxacin | 54 (7.9) | 35 (6.9) | |
| piperacillin/tazobactam | 38 (5.5) | 19 (3.7) | |
| ceftazidime | 37 (5.4) | 24 (4.7) | |
| amoxicillin/clavulanic acid | 29 (4.2) | 30 (5.9) | |
| meropenem | 19 (2.8) | 9 (1.8) | |
| other | 78 (11.4) | 55 (10.8) |
Values are n (%) unless otherwise indicated.
The range is the difference between the maximum and minimum value.
No data available for seven patients.
The median LOS for admitted patients was 5.4 days (IQR 2.2–8.7 days). Forty-three patients (4.5%) died during admission (Table S2), of whom four died within 24 h after admission to the ED. A total of 55 patients (5.8%) were readmitted for an infection related to the index source of infection.
QI performance and determinants
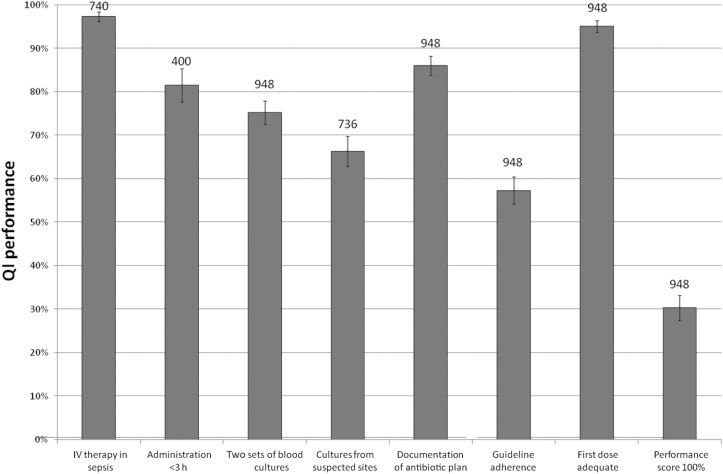
Figure 1 shows the performance of the seven QIs, including the percentage of patients with an overall performance sum score of 100%. Not every indicator applied to all included patients, so the sample size of the QIs varies (Figure 1; numbers above bars). Highest QI adherence was found for ‘antimicrobial therapy in adult patients with sepsis should be started intravenously’ (97.3%). Lowest adherence (57.3%) was found for ‘prescribing empiric antibiotics according to local or national guidelines’.
Figure 1.
Performance levels of QIs for empirical antibiotic therapy for patients admitted to the ED. The numbers above the bars represent the total number of patients.
QI adherence did not differ between patients with and without sepsis, except for the QIs on obtaining blood culture samples (adherence in patients with sepsis 81.1% versus 54.3% in patients without sepsis) and guideline adherence (adherence in patients with sepsis 54.2% versus 68.3% in patients without sepsis).
Table 3 shows the statistically significant results of the logistic regression analysis to explore determinants for each of the QIs and the discriminative power of each model. Due to the strong correlation between the prescriber and the site of infection (for example, a urologist only treats UTIs), we were not able to correct for the prescribing specialty.
Table 3.
Multivariate predictors of performance levels of QIs and associated AUCa
| QI | OR (95% CI) |
|---|---|
| Antimicrobial therapy in adult patients with sepsis should be started intravenously (AUC 0.82) | |
| presence of fever | 5.38 (2.07–14.00) |
| LRTI | 0.16 (0.05–0.59) |
| skin and soft tissue infection | 0.07 (0.02–0.34) |
| Timely initiation of antibiotic therapy (within 3 h) in adult patients with severe sepsis and septic shock (AUC 0.80) | |
| presence of tachycardia | 3.75 (2.08–6.76) |
| renal dysfunction | 3.60 (1.42–9.16) |
| presence of fever | 2.41 (1.33–4.36) |
| presence of hypotension | 2.28 (1.17–4.42) |
| UTI | 2.05 (0.91–4.62) |
| previous antibiotic use | 1.73 (0.90–3.32) |
| IA infection | 0.46 (0.23–0.95) |
| GE | 0.25 (0.08–0.79) |
| Before starting antimicrobial therapy, two sets of blood cultures should be taken (AUC 0.79) | |
| presence of fever | 6.06 (4.23–8.70) |
| GE | 2.29 (0.74–7.08) |
| presence of hypotension | 2.14 (1.32–3.48) |
| immune deficiency | 1.82 (1.22–2.73) |
| renal dysfunction | 1.60 (0.94–2.74) |
| presence of tachycardia | 1.65 (1.15–2.36) |
| CCI total score | 0.91 (0.86–0.98) |
| LRTI | 0.67 (0.46–0.99) |
| IA infection | 0.37 (0.23–0.59) |
| altered mental status (GCS ≤14) | 0.33 (0.16–0.71) |
| Before starting antimicrobial therapy, specimens for culture from suspected sites of infection should be taken (AUC 0.77) | |
| presence of fever | 1.64 (1.12–2.39) |
| antibiotic allergy | 1.64 (0.98–2.75) |
| renal dysfunction | 1.63 (0.98–2.72) |
| UTI | 1.48 (0.95–2.30) |
| previous antibiotic use | 1.35 (0.93–1.97) |
| CCI total score | 0.89 (0.83–0.95) |
| female patients | 0.69 (0.48–0.99) |
| LRTI | 0.18 (0.12–0.28) |
| GE | 0.13 (0.06–0.28) |
| An antibiotic plan should be documented in the case notes at the start of systemic antibiotic therapy (AUC 0.65) | |
| female patients | 1.67 (1.12–2.50) |
| LRTI | 1.66 (0.98–2.82) |
| IA infection | 0.61 (0.34–1.07) |
| other infections | 0.47 (0.24–0.90) |
| sepsis | 0.37 (0.21–0.67) |
| Empirical antibiotics according to local or national guidelines (AUC 0.79) | |
| neutropenic fever | 2.77 (1.01–7.56) |
| UTI | 2.02 (1.37–2.96) |
| antibiotic allergy | 1.81 (1.15–2.84) |
| immune deficiency | 0.68 (0.49–0.95) |
| altered mental status (GCS ≤14) | 0.55 (0.25–1.19) |
| LRTI | 0.14 (0.10–0.20) |
| other infections | 0.14 (0.07–0.26) |
| GE | 0.10 (0.04–0.25) |
| Initial dose should be adequate (AUC 0.68) | |
| age | 1.02 (1.00–1.04) |
| renal dysfunction | 0.50 (0.25–1.02) |
| previous antibiotic use | 0.48 (0.26–0.91) |
| skin and soft tissue infection | 0.37 (0.14–0.93) |
| Sum score 100% (AUC 0.80) | |
| neutropenic fever | 3.98 (1.82–8.74) |
| presence of fever | 2.37 (1.64–3.43) |
| UTI | 2.19 (1.52–3.16) |
| renal dysfunction | 1.41 (0.91–2.18) |
| female patients | 1.37 (0.98–1.91) |
| other infections | 0.37 (0.17–0.77) |
| LRTI | 0.16 (0.11–0.25) |
| GE | 0.04 (0.01–0.33) |
An OR >1 means a positive association with the QI and an OR <1 means a negative association.
Route of antibiotic administration and adequate dosing
Overall performance of these indicators was high: intravenous (IV) administration was delivered to 97.3% (720/740) of patients with sepsis and the correct dose was administered in 95.0% (901/948) of all patients. Although some determinants for QI performance were found, room for improvement for these indicators was limited.
Time to administration in patients with severe sepsis or septic shock (performance 81.5%, 326/400)
Patients with fever, hypotension or tachycardia were more likely to receive antibiotics within 180 min after admission, as were patients with renal dysfunction. Signs of gastroenteritis (GE) were associated with poor adherence; 11 out of 18 patients (61.1%) received appropriate care.
Blood culture samples collection (performance 75.2%, 713/948)
Collecting blood samples for culture was positively associated with the presence of fever, tachycardia or hypotension. Furthermore, immunocompromised patients and patients with renal dysfunction were more likely to receive appropriate care. A GCS score ≤14, a higher CCI or the presence of an intra-abdominal (IA) infection focus or lower respiratory tract infection (LRTI) were associated with lower adherence.
Additional culture collection (performance 66.3%, 488/736)
Cultures from suspected sites were collected more often in febrile patients, patients with renal insufficiency, patients with an antibiotic allergy and in patients with previous antibiotic use. Highest performance scores were found for UTI (94.0%) and lowest in patients treated for LRTI (46.9%) and GE (41.9%). In patients with LRTI, sputum cultures were ordered in 73.9%. In contrast, Legionella (39.9%) and pneumococcal urinary antigen tests (32.3%) and influenza PCR (53.7%) were obtained less often.
Documentation of an antibiotic plan (performance 86.1%, 816/948)
LRTI as the source of infection and being female were associated with better documentation. A negative association was found for patients with sepsis without a clear infection focus; adherence to this QI was 75% in that group.
Guideline-adherent empirical therapy (performance 57.3%, 543/948)
Guideline adherence was poor for LRTI (33%) and GE (19%). Furthermore, patients presenting to the ED with an uncommon focus of infection had an adherence score of 28% (23/83).
In patients with LRTI, poor guideline adherence was due to the unnecessary administration of broad-spectrum antibiotics, e.g. for providing empirical coverage for atypical pathogens, even if this was not indicated based on the guideline.
Highest guideline adherence was found for patients with UTI (76%) or neutropenic fever (87%). Further, adherence to the local antibiotic guideline was more common in patients with a history of antibiotic allergy. Finally, the presence of an altered mental status or an immunocompromised status were negatively associated with guideline adherence.
QI bundle performance score
Median QI bundle sum score was 83.3% (IQR 67%–99%). Only 287/948 patients (30.3%) had a sum score of 100% (Figure 1).
Patients with fever were more likely to have a score of 100%. The clinical diagnosis was the main determinant for full bundle adherence; the clinical diagnoses neutropenic fever and UTI had a positive association, whereas patients with GE, LRTI or ‘other’ infections had a negative association with a 100% bundle score.
Association between QI adherence and patient outcome
Maximum QI adherence was not associated with reduced LOS. In contrast, documenting an antibiotic plan and performing additional cultures were associated with a longer LOS (Appendix S1, Table S3).
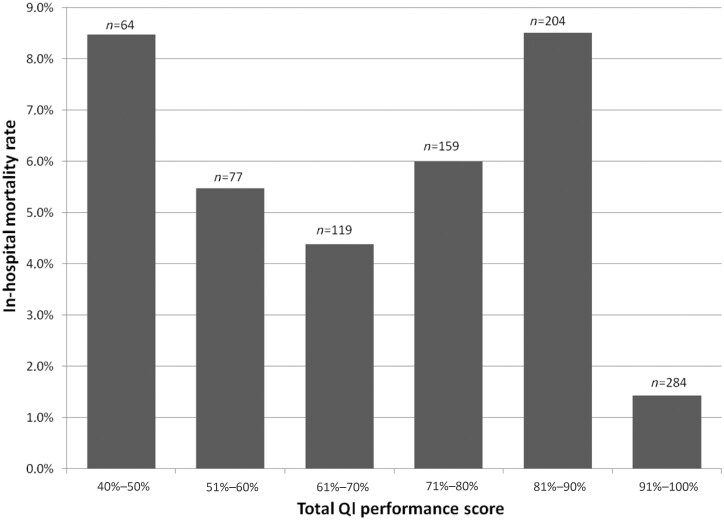
Bundle adherence of 100% was associated with reduced mortality, compared with patients with a lower bundle score (adjusted OR 0.30, 95% CI 0.10–0.92). An increase in bundle score (e.g. from 60% to 70%, or from 70% to 80%) did not result in reduced mortality rates (Appendix S1, Table S4 and Figure 2).
Figure 2.
In-hospital mortality rates. The amount of patients in the 0%–40% group was too low to include in this analysis.
Readmission rates were not associated with any QI performance score (Appendix S1, Table S5).
Discussion
In the present study, we systematically assessed appropriateness of empirical antibiotic use in the ED, using a bundle of validated QIs. Adherence to all seven QIs, thus achieving a 100% bundle performance score, was associated with reduced in-hospital mortality, which suggests that the combination of QIs has a greater effect on the outcome than a single QI.
Bundles are defined by the Institute of Healthcare Improvement as ‘a group of interventions related to a disease process that, when executed together, result in better outcomes than when implemented individually’.18 Bundles aim at converting complex guidelines into meaningful changes in behaviour and clinical outcomes.19 It has been demonstrated before that performing a bundle of recommended care elements in patients treated with antibiotics is more important than performing a single element.20–22 The sepsis bundle has been widely introduced in the ED; in a large study involving almost 30 000 patients with sepsis, increased compliance with sepsis performance bundles was associated with a 25% relative risk reduction in mortality rate.21 However, patients with sepsis only contribute to a small part of antibiotic consumption in the ED.14 Our antibiotic bundle includes both patients with and without sepsis, and can be used by policymakers to assess the quality of antibiotic care and identify factors that need improvement in the ED.
As only 30.3% of all patients had a QI bundle score of 100%, there is ample room for improving antibiotic care in the ED. In order to improve antibiotic behaviour in the ED, potential barriers that hinder implementation should be revealed.23 The success of any implementation depends on the consideration of those barriers and the use of adequate strategies to overcome them. We found several important associations between disease severity, patient characteristics, the clinical diagnosis and measured QI performance. Our findings are in line with other studies, in which parameters that reflect severity of disease were positively associated with culture collection24–26 and early antibiotic treatment.25,27 These results suggest that quality performance is generally better in patients who are more seriously ill.
Overall, guideline adherence for ‘prescribing antibiotic therapy according to the local guideline’ was 57.3% and was importantly influenced by the clinical diagnosis and thus the disease-specific guideline.
Previous studies also found low guideline adherence rates for patients with pneumonia.25,28 First, this may in part be due to the diagnostic uncertainty, as the diagnosis of pneumonia is more complex than that of UTI. Second, the difference in complexity between the guidelines for clinical syndromes may partly explain this finding. In community-acquired pneumonia, antibiotic choice is determined by different patient characteristics (e.g. severity-of-illness score, exposures, seasonal variation), while the guideline for complicated UTI is always straightforward and only recommends checking recent culture results prior to the antibiotic choice. So, in our hospital, ceftriaxone for UTI is practically ‘always’ right.29 This potential negative effect of complex recommendations on guideline adherence has been described before in a qualitative study by Lugtenberg et al.30 and was confirmed in a systematic review.31 Given the fast-paced setting of a busy ED, it can be challenging to correctly apply a complex guideline. Less commonly diagnosed infectious diseases, such as CNS infections, catheter-related infections, obstetric or gynaecological infections, were associated with reduced QI performance, suggesting lack of knowledge as a determinant in these syndromes.
In general, to increase guideline adherence, stewardship programmes need to focus on barriers related to physicians’ knowledge and attitudes, and barriers connected with guideline-related factors.32 The challenge for hospital antibiotic stewardship teams lies in selecting those interventions that might work best in the ED by addressing the key drivers of current professional antibiotic use in the ED. In this study we show how patient factors drive the appropriateness of antibiotic usage. Given the high patient and staff turnover in the ED, and the need for quick decision-making, (electronic) decision support could be a viable solution for complex algorithms. Furthermore, guidelines should be as short and user-friendly as possible to reduce complexity. Checklists, decision support systems and platforms for the dissemination of guidelines (e.g. tablets, smartphones and mobiles) can be used as suitable strategies to improve accessibility.31 As a further potential successful intervention, regular education—of both physicians in training and senior department leaders in the ED—should focus on (new) guidelines, the importance of culture collection and antibiotic dosing. Such education should also focus on specific patient groups because adherence to complex guidelines, for example in patients with community-acquired pneumonia, was low.
As far as we know, this is the first study that examined the entire process of antibiotic usage in the ED using previously validated QIs. A strength of this study is the rigorous and objective assessment of appropriate antibiotic use, using a systematically developed set of guideline-based QIs. The large sample of 948 patients contributes to the validity of the results. We used accessible variables to identify determinants of appropriate antibiotic usage and to evaluate QI performance, which can be used in future studies to develop and implement new ASPs in the ED. Our study has some limitations. First, this is a single-centre study that was not prospectively conducted. However, the presence of electronic health records provided the possibility of electronic data collection and extraction, which precludes selection bias. As all patients originated from one hospital in this analysis, we were not able to evaluate hospital determinants for appropriate antibiotic use. Second, individual characteristics of prescribing physicians on the ED were not measured. These might also have contributed to the variation in appropriate antibiotic use (e.g. clinical experience, clinical specialism, education in antibiotic prescribing, junior versus attending physician). In teaching and university hospitals in the Netherlands, physicians working in the ED are mainly residents and fellows. Whether the results of our study also apply to EDs only staffed by ED physicians is not known.
Third, we controlled for known confounders, but may not have been able to control for all variables associated with patient outcome. Although we found a significant difference in mortality rate in favour of the 100% bundle score group, the number of patients with that outcome was relatively low. Due to the explorative design that was used to identify possible determinants, associations can be demonstrated, but causal relationships cannot be inferred. The results of this finding should therefore be confirmed in future studies with sufficient power.
Finally, we were not able to assess how QI performance influences downstream care, such as IV–oral switch and streamlining antibiotic therapy based on culture results.
Conclusions
This study generates insight into factors that determine the quality of antibiotic usage in the ED and can be used to develop stewardship interventions to improve antibiotic use in the ED. Adherence to the full bundle of QIs was associated with a reduced mortality rate. This suggests that future interventions should focus on the entire antibiotic process in the ED and not on a single metric.
Supplementary Material
Acknowledgements
We thank Business Intelligence and the student assistants of internal medicine for their help with data management.
Funding
This study was carried out as part of our routine work.
Transparency declarations
None to declare.
Author contributions
M.A.H.B., J.T.O., J.A.S. and M.E.H. conceived the study. M.A.H.B., J.T.O. and J.A.S. supervised the data collection and managed the data, including quality control. F.A. provided statistical advice. J.H. and B.J.K. helped with the study design. M.A.H.B. analysed the data. M.A.H.B. drafted the manuscript, and all authors contributed substantially to its revision. M.A.H.B. takes responsibility for the paper as a whole.
References
- 1. Barlam TF, Cosgrove SE, Abbo LM. et al . Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society For Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pulcini C. Antimicrobial stewardship in emergency departments: a neglected topic. Emerg Med J 2015; 32: 506. [DOI] [PubMed] [Google Scholar]
- 3. Hecker MT, Fox CJ, Son AH. et al . Effect of a stewardship intervention on adherence to uncomplicated cystitis and pyelonephritis guidelines in an emergency department setting. PLoS One 2014; doi:10.1371/journal.pone.0087899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Percival KM, Valenti KM, Schmittling SE. et al . Impact of an antimicrobial stewardship intervention on urinary tract infection treatment in the ED. Am J Emerg Med 2015; 33: 1129–33. [DOI] [PubMed] [Google Scholar]
- 5. Schouten JA, Hulscher ME, Trap-Liefers J. et al . Tailored interventions to improve antibiotic use for lower respiratory tract infections in hospitals: a cluster-randomized, controlled trial. Clin Infect Dis 2007; 44: 931–41. [DOI] [PubMed] [Google Scholar]
- 6. Tromp M, Tjan DH, van Zanten AR. et al . The effects of implementation of the Surviving Sepsis Campaign in the Netherlands. Neth J Med 2011; 69: 292–8. [PubMed] [Google Scholar]
- 7. van den Bosch CM, Geerlings SE, Natsch S. et al . Quality indicators to measure appropriate antibiotic use in hospitalized adults. Clin Infect Dis 2015; 60: 281–91. [DOI] [PubMed] [Google Scholar]
- 8. Spoorenberg V, Hulscher ME, Akkermans RP. et al . Appropriate antibiotic use for patients with urinary tract infections reduces length of hospital stay. Clin Infect Dis 2014; 58: 164–9. [DOI] [PubMed] [Google Scholar]
- 9. van den Bosch CM, Hulscher ME, Akkermans RP. et al . Appropriate antibiotic use reduces length of hospital stay. J Antimicrob Chemother 2017; 72: 923–32. [DOI] [PubMed] [Google Scholar]
- 10. Schuts EC, Hulscher M, Mouton JW. et al . Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 847–56. [DOI] [PubMed] [Google Scholar]
- 11. Barochia AV, Cui X, Vitberg D. et al . Bundled care for septic shock: an analysis of clinical trials. Crit Care Med 2010; 38: 668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez-Cortes LE, Del Toro MD, Galvez-Acebal J. et al . Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis 2013; 57: 1225–33. [DOI] [PubMed] [Google Scholar]
- 13. Levy MM, Evans LE, Rhodes A.. The Surviving Sepsis Campaign bundle: 2018 update. Crit Care Med 2018; 46: 997–1000. [DOI] [PubMed] [Google Scholar]
- 14. May L, Cosgrove S, L’Archeveque M. et al . A call to action for antimicrobial stewardship in the emergency department: approaches and strategies. Ann Emerg Med 2013; 62: 69–77.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van den Bosch CM, Hulscher ME, Natsch S. et al . Development of quality indicators for antimicrobial treatment in adults with sepsis. BMC Infect Dis 2014; doi:10.1186/1471-2334-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harrell FE Jr, Lee KL, Mark DB.. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–87. [DOI] [PubMed] [Google Scholar]
- 17. van den Bosch CM, Hulscher ME, Natsch S. et al . Applicability of generic quality indicators for appropriate antibiotic use in daily hospital practice: a cross-sectional point-prevalence multicenter study. Clin Microbiol Infect 2016; doi:10.1016/j.cmi.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 18. Dellinger RP, Vincent JL.. The Surviving Sepsis Campaign sepsis change bundles and clinical practice. Crit Care 2005; 9: 653–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levy MM, Pronovost PJ, Dellinger RP. et al . Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med 2004; 32: S595–7. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen HB, Corbett SW, Steele R. et al . Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med 2007; 35: 1105–12. [DOI] [PubMed] [Google Scholar]
- 21. Levy MM, Rhodes A, Phillips GS. et al . Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015; 43: 3–12. [DOI] [PubMed] [Google Scholar]
- 22. Dellinger RP, Levy MM, Rhodes A. et al . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39: 165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Daalen FV, Geerlings SE, Prins JM, et al. A survey to identify barriers of implementing an antibiotic checklist. Eur J Clin Microbiol Infect Dis 2016; 35: 545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spoorenberg V, Geerlings SE, Geskus RB. et al . Appropriate antibiotic use for patients with complicated urinary tract infections in 38 Dutch Hospital Departments: a retrospective study of variation and determinants. BMC Infect Dis 2015; doi:10.1186/s12879-015-1257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schouten JA, Hulscher ME, Kullberg BJ. et al . Understanding variation in quality of antibiotic use for community-acquired pneumonia: effect of patient, professional and hospital factors. J Antimicrob Chemother 2005; 56: 575–82. [DOI] [PubMed] [Google Scholar]
- 26. van Daalen FV, Kallen MC, van den Bosch CMA. et al . Clinical condition and comorbidity as determinants for blood culture positivity in patients with skin and soft-tissue infections. Eur J Clin Microbiol Infect Dis. 2017; 36: 1853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schuh S, Lindner G, Exadaktylos AK. et al . Determinants of timely management of acute bacterial meningitis in the ED. Am J Emerg Med 2013; 31: 1056–61. [DOI] [PubMed] [Google Scholar]
- 28. McIntosh KA, Maxwell DJ, Pulver LK. et al . A quality improvement initiative to improve adherence to national guidelines for empiric management of community-acquired pneumonia in emergency departments. Int J Qual Health Care 2011; 23: 142–50. [DOI] [PubMed] [Google Scholar]
- 29. Geerlings SE, van Nieuwkoop C, van Haarts E. et al . SWAB Guidelines for Antimicrobial Therapy of Complicated Urinary Tract Infections in Adults. http://www.swab.nl/swab/cms3.nsf/uploads/41949F6BD9ED10EDC1257B7F00212560/$FILE/revised%20uti%20guideline%20FINAL%20010413.pdf.
- 30. Lugtenberg M, Burgers JS, Besters CF. et al . Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract 2011; 12: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fischer F, Lange K, Klose K. et al . Barriers and strategies in guideline implementation-a scoping review. Healthcare (Basel) 2016; doi:10.3390/healthcare4030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baker R, Camosso-Stefinovic J, Gillies C. et al . Tailored interventions to address determinants of practice. Cochrane Database Syst Rev 2015; issue 4: CD005470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Udy AA, Roberts JA, Lipman J.. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013; 39: 2070–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.