Abstract
Background
Mycobacterium abscessus subsp. abscessus (M. abscessus) is a rapidly growing mycobacterium that is resistant to most antibiotics. The number of patients with pulmonary disease caused by M. abscessus is increasing in several regions, and therapy involves long-term antibiotic combination treatments, although no standard treatment regimen has been established.
Objectives
To examine candidate regimens for maintenance of antimicrobial treatment against M. abscessus by measuring MIC using the three-drug chequerboard method.
Methods
We evaluated the drug susceptibility of 70 clinical isolates of M. abscessus using the three-drug chequerboard method. We tested the antimycobacterial agents bedaquiline, clofazimine, amikacin, and sitafloxacin (which showed a relatively low MIC range when used as single agents) alone and in combinations.
Results
The three-drug combinations of bedaquiline/clofazimine/amikacin, and bedaquiline/clofazimine/sitafloxacin were studied. Among isolates for which the fractional inhibitory concentration index (FICI) could be calculated, 29/70 isolates (41%) and 11/70 isolates (16%) showed a synergistic response (FICI ≤0.75) with combined use of bedaquiline/clofazimine/amikacin, or with bedaquiline/clofazimine/sitafloxacin, respectively.
Conclusions
The combination of bedaquiline with clofazimine plus either amikacin or sitafloxacin may be useful as maintenance regimens when treating pulmonary disease caused by M. abscessus.
Introduction
The incidence of non-tuberculosis mycobacterial disease is increasing worldwide,1–5 although the frequency of isolation and causative species vary by region. Among them, disease caused by Mycobacterium abscessus is second only to Mycobacterium avium-intracellulare complex (MAC) disease in East Asia, North America, and Australia.1,6–9 In a nationwide survey conducted in Japan in 2014, the incidence of M. abscessus pulmonary disease was reported as 0.5 cases per 100 000 person-years, which was 5-fold higher than that in 2007.10
M. abscessus is a rapidly growing mycobacterium with three subspecies: M. abscessus subsp. abscessus (M. abscessus), M. abscessus subsp. massiliense, and M. abscessus subsp. bolletii (M. bolletii). Wild strains of M. abscessus and M. bolletii have erm(41)-induced macrolide resistance. Pulmonary disease caused by M. abscessus is difficult to treat because of the development of resistance to clarithromycin, a representative macrolide.11 Additionally, M. abscessus strains are highly resistant to most antibiotics such as tetracyclines, carbapenems, fluoroquinolones, linezolid, and sulfamethoxazole/trimethoprim.12
For the initial treatment of M. abscessus pulmonary disease, oral macrolides and intravenous antibiotics such as imipenem and amikacin are recommended, and subsequent maintenance treatment includes a combination of oral antibiotics and inhaled or liposomal amikacin.13,14 However, despite the practice of long-term treatment using multiple agents, the rate of success is low and the disease is refractory.15 According to a meta-analysis of the treatment of M. abscessus pulmonary disease, the culture conversion rate is 35% and recurrence rate is 40%,16 whereas another report indicated a treatment success rate of 33%.17 In a study performed in Korea, the 10 year mortality rate due to M. abscessus pulmonary disease was reported as 29.8%, and the 15 year mortality rate was 50.6% in 129 cases.18 Clofazimine, linezolid, minocycline, moxifloxacin, sulfamethoxazole/trimethoprim, bedaquiline, and inhaled amikacin are the antibiotics available for continuous treatment.13,19 However, there is insufficient evidence that treatment is successful, and the optimal combination of antibiotics is unclear, necessitating the establishment of a treatment regimen that is more effective than those that are currently used.
There are several reports of effective drug combinations in the treatment of infections caused by M. abscessus.20,21 Using currently available antibiotics with low MICs against M. abscessus, we considered several synergistic drug combinations in drug susceptibility tests to identify a candidate treatment regimen. Among these antibiotics,12 we hypothesized that bedaquiline [a drug for multidrug-resistant tuberculosis (MDR-TB)], clofazimine (used for MDR-TB and leprosy), amikacin (used in treating refractory MAC pulmonary disease), and sitafloxacin (a fluoroquinolone that reduces bacterial load in mice infected with M. abscessus22 and is used to treat refractory MAC pulmonary disease with no serious adverse effects)23,24 are candidate drugs for continuous treatment.
The chequerboard and time–kill curve methods are commonly used to evaluate the combined effect of antibiotics. A limitation of the chequerboard method is that it uses a one-dilution range difference, which may cause a reproducibility error and may greatly affect the interpretation of the results.25 However, the advantage of using the chequerboard method is that it is easier to evaluate large numbers of isolates compared with the time–kill curve method. There have been reports of a synergistic effect of drugs identified against M. abscessus by using the two-drug chequerboard method20,25–28 and against MDR M. tuberculosis29Aspergillus spp.,30 and M. abscessus with a parenteral agent31 using the three-drug chequerboard method.
In this study, we evaluated the drug susceptibility interactions against M. abscessus in vitro using the chequerboard method by the broth microdilution technique that combined the drugs bedaquiline, clofazimine, and amikacin or sitafloxacin. These drugs can be used orally or inhaled without intravenous administration during continuous treatment.
Materials and methods
Test isolates
A total of 70 M. abscessus isolates was obtained from the sputum of patients at the Japan Anti-tuberculosis Association Fukujuji Hospital and Keio University Hospital between 2004 and 2014. The isolates were cultured from sputum samples only when they met the diagnostic criteria for the American Thoracic Society/IDSA as described in 2007.32 As some patients were referred, it is unknown whether they were treated before the diagnosis. However, for these patients, bedaquiline and clofazimine had not been administered, and the detailed history of past use of aminoglycosides or fluoroquinolones was not known. As this study used only the isolates from the laboratories in the clinical hospitals and did not use any additional samples or personal information of patients, ethics approvals were not required.
Identification of mycobacterial species and subspecies
Species were identified using DNA–DNA hybridization kits (DDH mycobacteria, Kyokuto Pharmaceutical Industrial, Tokyo, Japan). Subspecies were identified by gene homology analysis through direct sequencing of the 16S rRNA, hsp65, and rpoB genes.12
Antimicrobial agents
Bedaquiline (Janssen Pharmaceutical, Beerse, Belgium) and sitafloxacin (Daiichi Sankyo Company, Tokyo, Japan) were kindly provided by pharmaceutical companies. Clofazimine was from Tokyo Chemical Industry (Tokyo, Japan) and amikacin was from Sigma–Aldrich (St Louis MO, USA).
Inoculum preparation
The stored clinical isolates were sub-cultured and grown to the exponential phase in Ogawa medium (Kyokuto Pharmaceutical Industrial). Colonies that developed were transferred to a tube with glass beads and emulsified by bead beating using a vortex. The bacterial suspension was prepared in sterile water, and the concentration was adjusted to a McFarland standard of 0.5. The cells were added to cation-adjusted Mueller-Hinton broth (BD Biosciences, Franklin Lakes, NJ, USA) at a dilution of 1 : 200 to serve as an inoculum of 2 × 106 cfu/mL.
Chequerboard preparation and susceptibility testing
The MIC of each isolate was determined by the broth microdilution method using customized frozen microtitre plates from Eiken Chemical (Tokyo, Japan) as per the CLSI standard (M24 3rd edn).33 The chequerboard was a combination of bedaquiline, clofazimine, and amikacin or sitafloxacin. The final drug concentrations were 0.03–0.25 mg/L for bedaquiline, 0.03–0.25 mg/L for clofazimine, 1–8 mg/L for amikacin, and 1–8 mg/L for sitafloxacin (i.e. four 2-fold dilutions, respectively). The concentrations were rounded to three decimal places.
The suspension was inoculated into a microtitre plate to obtain a final concentration of 5 × 105 cfu/mL (5 × 104 cfu/well). The plates were incubated at 30°C, and the MIC of each drug when used alone and MIC of the chequerboard method when using three drugs was determined on day 5, and the fractional inhibitory concentration index (FICI) was calculated. The FICI of the three-drug chequerboard was defined as follows:
MIC [drug A] combination/MIC [drug A] alone + MIC [drug B] combination/MIC [drug B] alone + MIC [drug C] combination/MIC [drug C] alone. The FICI was used to interpret the test results as per the following criteria: synergism, ≤0.75; additive, >0.75–3; indifference, >3–4; and antagonism, >4.29
Statistical analysis
Statistical analysis was performed using BellCurve for Excel 3.21 (Social Survey Research Information, Tokyo, Japan). Data are expressed as the mean ± standard deviation (SD). The paired t-test was used for data analysis, and a P value of <0.05 was considered as statistically significant.
Results
MICs of bedaquiline, clofazimine, amikacin, and sitafloxacin for M. abscessus
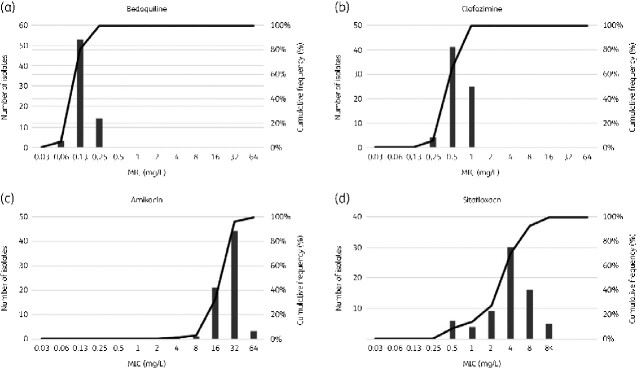
The MICs of bedaquiline, clofazimine, amikacin, and sitafloxacin were determined for 70 isolates of M. abscessus. The MIC range for bedaquiline was 0.06–0.25 mg/L, with an MIC50 (MIC required to inhibit 50% of the isolates) value of 0.13 mg/L and MIC90 value of 0.25 mg/L. The MIC range for clofazimine was 0.25–1 mg/L, with an MIC50 of 0.5 mg/L and MIC90 value of 1 mg/L. The MIC range for amikacin was 4–64 mg/L, with an MIC50 of 32 mg/L and MIC90 value of 32 mg/L, and the MIC range for sitafloxacin was 0.5 to >8 mg/L, with an MIC50 of 4 and MIC90 value of 8 mg/L (Figure 1, Table 1).
Figure 1.
Distribution of MIC values for (a) bedaquiline, (b) clofazimine, (c) amikacin, and (d) sitafloxacin in 70 clinical isolates of M. abscessus.
Table 1.
MIC values of bedaquiline, clofazimine, amikacin, and sitafloxacin of 70 clinical isolates of M. abscessus
| MIC (mg/L) |
|||
|---|---|---|---|
| Drug | Range | MIC50 | MIC90 |
| Bedaquiline | 0.06–0.25 | 0.13 | 0.25 |
| Clofazimine | 0.25–1 | 0.5 | 1 |
| Amikacin | 4–64 | 32 | 32 |
| Sitafloxacin | 0.5–8a | 4 | 8 |
Some isolates may have MICs >8 mg/L as this was the highest concentration tested.
MICs and FICI of the combination of bedaquiline, clofazimine and amikacin against M. abscessus
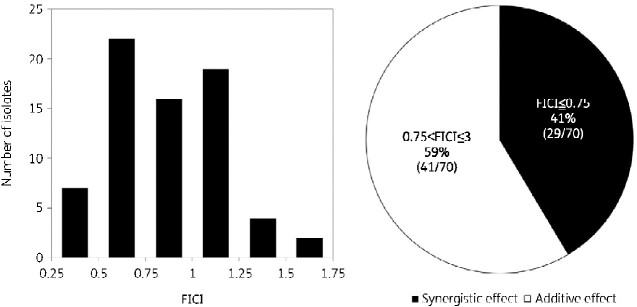
The three-drug chequerboard method using bedaquiline, clofazimine and amikacin was used to measure the MIC within the prepared ranges specified for the drugs in the Methods section for all 70 M. abscessus isolates. The FICI values ranged from 0.30 to 1.7. Synergistic effects with a FICI of 0.75 or less were observed in 41% (29/70) of the tested isolates, and additive effects with a FICI in the range of 0.75–3 were observed in 59% (41/70) of the isolates tested. Moreover, no indifferent or antagonistic isolates were observed (Figure 2).
Figure 2.
Distribution of fractional inhibitory concentration index (FICI) values for 70 clinical isolates of M. abscessus by the chequerboard method using bedaquiline, clofazimine, and amikacin. The pie chart shows the percentage of isolates with synergistic (black shading) and additive (white shading) effects.
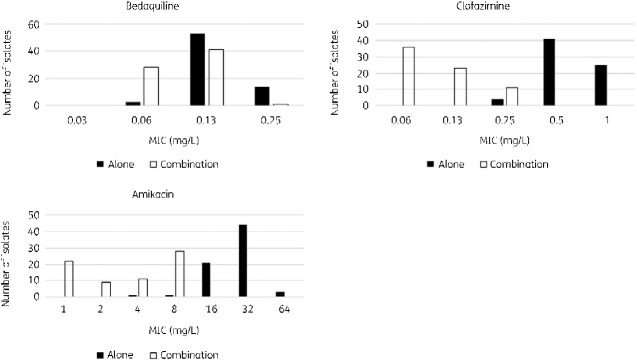
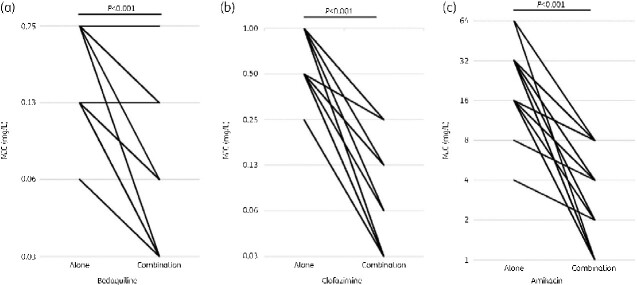
The MIC value for the FICI was defined as the MIC for the three-drug combination and was compared with the MIC for single drug use. The MIC distribution of bedaquiline when used in combination with the other drugs was 0.03–0.25 mg/L, with an MIC50 of 0.06 mg/L and MIC90 of 0.13 mg/L. The MIC distribution of clofazimine when used in combination with other drugs was 0.03–0.25 mg/L, with an MIC50 of 0.03 mg/L and MIC90 of 0.25 mg/L. Similarly, for amikacin, the MIC distribution was 1–8 mg/L, MIC50 was 4 mg/L, and MIC90 was 8 mg/L (Figure 3). The decrease in the observed MIC was 1.04-fold (SD = 0.97, P < 0.001) for bedaquiline, 3.27-fold (SD = 1.17, P < 0.001) for clofazimine, and 3.02-fold (SD = 1.43, P < 0.001) for amikacin, all of which were significant (Figure 4).
Figure 3.
MIC distributions of bedaquiline, clofazimine, and amikacin alone and for the combinatorial use of bedaquiline, clofazimine, and amikacin.
Figure 4.
Change in MIC values of bedaquiline, clofazimine, and amikacin alone and for the combination of (a) bedaquiline, (b) clofazimine, and (c) amikacin.
MICs and FICI for the combination of bedaquiline, clofazimine and sitafloxacin against M. abscessus
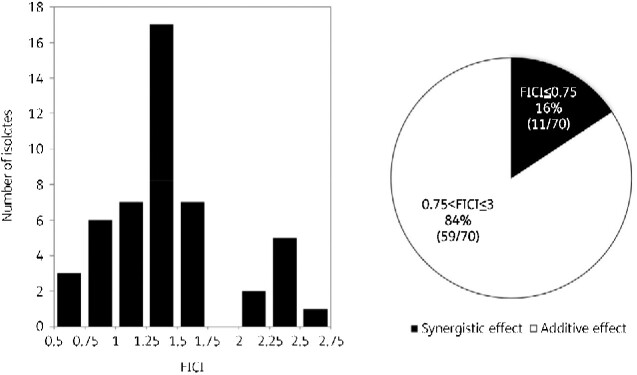
In 53 of the 70 M. abscessus isolates, the three-drug chequerboard method using bedaquiline, clofazimine, and sitafloxacin was able to measure the MIC within the prepared ranges specified for the drugs in the Methods section. For the FICI calculation, an MIC >8 mg/L for sitafloxacin alone was calculated for five isolates at 16 mg/L. However, the true FICI was lower than this value. The remaining 17 of the 70 isolates did not grow within the concentration range of the prepared antibiotics and had an MIC <0.03 mg/L for bedaquiline and clofazimine and <1 mg/L for sitafloxacin. The MIC for bedaquiline and clofazimine was 0.03 mg/L; for sitafloxacin, this value was 1 mg/L. The true FICI was smaller than this value. Among the 70 isolates, the true FICI values could be calculated for 48 isolates; these isolates showed MICs within the prepared range, and the FICI ranged between 0.52 and 2.56 for the combination of bedaquiline, clofazimine and sitafloxacin. When the isolates with a high FICI were included in the calculation, synergistic effects were observed in 16% (11/70) of isolates, with additive effects in 84% (59/70) of isolates (Figure 5). Thus, the FICI values of all 70 isolates were <3, and no indifference or antagonistic phenotypes were observed in the isolates.
Figure 5.
Distribution of fractional inhibitory concentration index (FICI) values for 48 of the 70 clinical isolates of M. abscessus. In the remaining 22 isolates, either the MIC for sitafloxacin alone was greater than 8 mg/L or a lower MIC than the prepared range was observed, and therefore the FICI could not be calculated. The pie chart shows the percentage of synergistic (black shading) or additive (white shading) effects among the 70 isolates for which the FICI was calculated using the highest estimated MIC.
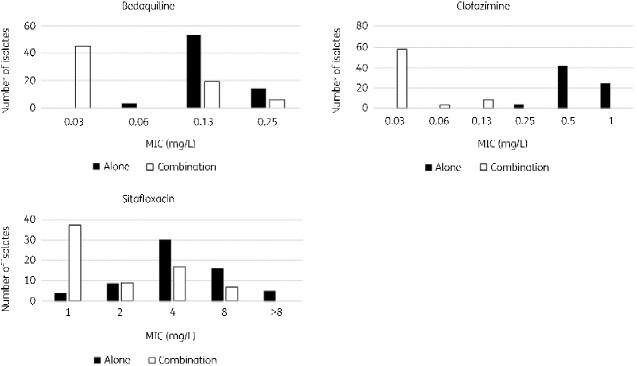
The MIC distribution of bedaquiline when used in combination was 0.03–0.13 mg/L, MIC50 was 0.03 mg/L, and MIC90 was 0.13 mg/L. Similarly, clofazimine, when used in combination, gave an MIC distribution of 0.03–0.13 mg/L, with an MIC50 of 0.03 mg/L and MIC90 of 0.13 mg/L. The MIC distribution for sitafloxacin was 1–8 mg/L, with an MIC50 of 1 mg/L and MIC90 of 4 mg/L (Figure 6).
Figure 6.
MIC distributions of bedaquiline, clofazimine, and sitafloxacin alone and in combination with bedaquiline, clofazimine, and sitafloxacin.
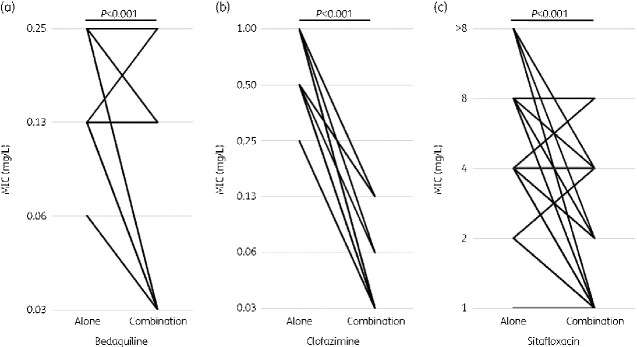
When comparing the MIC of single agents and combinations, the MIC of 0.5 mg/L for sitafloxacin alone was replaced with an MIC of 1.0 mg/L to match the minimum MIC. Under these conditions, the MIC reduction was 1.30-fold (SD = 1.06, P < 0.001) for bedaquiline, 4.0-fold (SD = 0.83, P < 0.001) for clofazimine, and 1.04-fold (SD = 1.31, P < 0.001) for sitafloxacin; these differences were significant (Figure 7).
Figure 7.
Changes in MIC values of bedaquiline, clofazimine, and sitafloxacin when used alone and when (a) bedaquiline, (b) clofazimine, and (c) sitafloxacin are used in combination.
Discussion
In this study, a three-drug chequerboard method using bedaquiline, clofazimine, amikacin, or sitafloxacin was used to test the drug susceptibility of clinical isolates of M. abscessus. Synergism was observed in 41% of isolates when using a combination of bedaquiline, clofazimine, and amikacin and in 16% of isolates when using bedaquiline, clofazimine, and sitafloxacin in the comparison of the three-drug combination versus single drugs, along with a significant reduction in the MIC. Using the three-drug chequerboard method, no indifferent or antagonistic effects were observed.
Bedaquiline is a diarylquinoline drug that inhibits ATP synthases and is used to treat MDR-TB; it is also used as a salvage therapy for patients with MAC and M. abscessus pulmonary disease. The MIC for bedaquiline in most clinical isolates of M. abscessus is <0.25 mg/L,34–37 which is above the average plasma concentration at 400 mg daily for 2 weeks, followed by 200 mg three times per week.38 We also observed a similarly low MIC. However, the MIC distribution for clinical isolates is reportedly higher,39 and studies have described contrasting results showing that bedaquiline is effective40 or ineffective41 in a mouse model. A few patients with refractory pulmonary diseases caused by MAC and M. abscessus have been treated with bedaquiline, which was tolerated.42 It has been reported that combined use of bedaquiline and a β-lactam may inactivate the action of β-lactams.43 However, coadministration with imipenem in vitro does not result in antagonism44 and is effective in a M. abscessus pulmonary disease mouse model.40 Further investigation is needed to determine whether the use of bedaquiline alone is sufficient, and which combinations of drugs are more effective.
The use of clofazimine may also result in improved treatment outcomes for pulmonary disease caused by M. abscessus.45–47 In our study, the MIC distribution for clofazimine used against M. abscessus was low, and a few reports indicate that the MIC90 for clinical isolates was more than 8 mg/L,48,49 suggesting that susceptibility varies among isolates. Further, it has been reported that patients achieved a negative conversion of sputum culture with an MIC value for clofazimine <0.25 mg/L.49 Clofazimine is used at a dose of 50 mg or 100 mg/day in clinical cases of M. abscessus pulmonary disease.46,47,49 It has been reported that bedaquiline and clofazimine, or bedaquiline and amikacin, have no synergistic effect against M. abscessus in vitro according to the chequerboard method.50 However, the combination of bedaquiline and clofazimine exhibited a synergistic effect in the time–kill assay method in vitro50 and in a mouse model.51 Therefore, this combination is a candidate for use in a therapeutic regimen. There are also concerns regarding the emergence of resistant isolates of bedaquiline when treated with the combination of bedaquiline and clofazimine, and thus a combination of three or more drugs should be considered to prevent the emergence of resistant isolates.
In refractory MAC pulmonary disease and M. abscessus pulmonary disease, several studies demonstrated sputum conversion with amikacin inhalation, which may be used as a treatment option.52–55 Amikacin can be used as a maintenance drug for long-term use via an inhaled formulation or by intermittent infusion. The dose of amikacin should be 10–15 mg/kg/day by daily infusion, 15–25 mg/kg/day by three infusions per week, adjusted according to drug level monitoring, 590 mg/day by inhalation of liposomal formulations, and 250–500 mg/day by inhalation of the parenteral formulation.14 Amikacin in combination with clofazimine has been reported to have synergistic effects in vitro,56,57 and may be useful in maintenance regimens.
Among the fluoroquinolones, levofloxacin and moxifloxacin are widely used as second-line treatments for tuberculosis as well as for MAC pulmonary disease.58 However, moxifloxacin was not sufficiently effective in a hollow-fibre59 model or a zebrafish model.60 Although there are no reports of using sitafloxacin in vivo or in patients with M. abscessus pulmonary disease, most isolates have lower MICs of this drug than levofloxacin or moxifloxacin, and it may be used in maintenance regimens. Sitafloxacin is typically administered at a dose of 100 mg/day based on the efficacy of respiratory infections other than mycobacteriosis and can be increased to 200 mg/day. Compared with levofloxacin or moxifloxacin, the daily dose is low, and the resulting blood concentration is lower; thus, the effective dose needed for treating M. abscessus pulmonary disease requires further analysis.
We observed synergistic effects only in some isolates; however, the combination of bedaquiline, clofazimine, and amikacin caused a greater decrease in the MIC compared with bedaquiline, clofazimine and sitafloxacin. Twenty-nine isolates showed a synergistic effect with the combination of bedaquiline, clofazimine and amikacin, 11 isolates showed a synergistic effect with the combination of bedaquiline, clofazimine and sitafloxacin, and 8 isolates showed an overlap. The MICs of bedaquiline and clofazimine were within the range of three 2-fold dilutions, and the distribution range of the MICs for amikacin and sitafloxacin was wide, with a few clinical isolates showing synergistic effects. At the CLSI breakpoint, amikacin resistance was ≥64 mg/L and the value for sitafloxacin was undefined, although ciprofloxacin and moxifloxacin showed values of ≥4 mg/L. There were three amikacin-resistant isolates (3/70; 0.04%), and 52 isolates had a sitafloxacin MIC of >4 mg/L (52/70; 74%). As more isolates with synergistic effects were observed in combination with amikacin, this indicates that amikacin is more effective than sitafloxacin against M. abscessus. However, a few isolates had a synergistic effect only with sitafloxacin and not amikacin. In Japan, bedaquiline and clofazimine are only indicated as treatments for MDR-TB and leprosy, respectively, and are not used for any other diseases. Aminoglycosides and fluoroquinolones are used to treat many community-acquired infections, but aminoglycosides are used less frequently, whereas fluoroquinolones are widely used. Although we could not obtain clinical information on exposure to fluoroquinolones and aminoglycosides, the MIC of sitafloxacin in many strains may have been high because the patient had previously been administered fluoroquinolones. Changes in environment and exposure to antibiotics in the human body may alter drug susceptibility through various mechanisms, some of which may remain unknown and widen the range of MICs for antibiotics against M. abscessus.
In this study, we chose inhalation and oral medications, rather than injectable medications, as the regimen for long-term maintenance treatment. Sitafloxacin is an orally administered drug and, in the absence of adverse events, can be administered for longer than amikacin, the longest duration of liposomal inhaled formulation was 84 days,53 which is an advantage. However, in vitro drug susceptibility testing may not reflect the actual clinical efficacy in vivo, and susceptibility to macrolides and amikacin alone has been shown to correlate with the therapeutic effect against M. abscessus. It is not known whether the susceptibility in vitro to the drugs used in this study correlates with their clinical efficacy, but susceptibility is the only way to predict which drugs will be effective in clinical settings. All treatments, including those using bedaquiline, clofazimine, amikacin and sitafloxacin, were well-tolerated and showed promising results, and may be used as an option for continuous treatment of M. abscessus pulmonary disease. Drug susceptibility testing is required for each isolate because the degree of interaction varies between isolates. We demonstrated that the three-drug chequerboard method may be useful when selecting drugs to treat M. abscessus, as well as to determine the combination of drug regimens for the continuous phase depending on susceptibility. Potentially, additional drug–drug interactions other than reduced MICs observed in vitro may be analysed, and future studies can include analyses for drug susceptibility, pharmacokinetics, and pharmacodynamics. In this study, clarithromycin and azithromycin were not chosen for analysis because M. abscessus induces resistance to macrolides. In the guidelines for international respiratory medicine and infectious disease societies, the use of macrolides is recommended even in the presence of macrolide resistance as they have immunomodulatory properties.14 Concomitant use of macrolides necessitates further examination of the interactions between the three drugs and macrolides.
Notably, our study had some limitations. The isolates used in this study were clinically isolated at only two facilities in Tokyo, Japan. The frequency of the species isolated from non-tuberculosis mycobacteria varies by region, and thus the susceptibility of the isolates may also vary. Additionally, we performed the broth microdilution method only once per isolate. Further studies are required to confirm the association between in vitro susceptibility and the clinical course of treatment using this method in multiple settings.
Conclusions
We evaluated the in vitro drug susceptibility of clinical isolates of M. abscessus using the three-drug chequerboard method. Combined treatments with either bedaquiline, clofazimine and amikacin, or bedaquiline, clofazimine and sitafloxacin show potential for treating M. abscessus pulmonary disease.
Funding
This work was supported by the Emerging/Re-emerging Infectious Diseases Project of the Japan Agency for Medical Research and Development (grant no. 18fk0108043h0402).
Transparency declarations
None to declare.
References
- 1. Adjemian J, Olivier KN, Seitz AE. et al. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 2012; 185: 881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lai CC, Tan CK, Chou CH. et al. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000-2008. Emerg Infect Dis 2010; 16: 294–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marras TK, Chedore P, Ying AM. et al. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997-2003. Thorax 2007; 62: 661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore JE, Kruijshaar ME, Ormerod LP. et al. Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995–2006. BMC Public Health 2010; 10: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prevots DR, Shaw PA, Strickland D. et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010; 182: 970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang HL, Cheng MH, Lu PL. et al. Epidemiology and predictors of NTM pulmonary infection in Taiwan - a retrospective, five-year multicenter study. Sci Rep 2017; 7: 16300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ko RE, Moon SM, Ahn S. et al. changing epidemiology of nontuberculous mycobacterial lung diseases in a tertiary referral hospital in Korea between 2001 and 2015. J Korean Med Sci 2018; 33: e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim AYH, Chotirmall SH, Fok ETK. et al. Profiling non-tuberculous mycobacteria in an Asian setting: characteristics and clinical outcomes of hospitalized patients in Singapore. BMC Pulm Med 2018; 18: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wassilew N, Hoffmann H, Andrejak C. et al. Pulmonary disease caused by non-tuberculous mycobacteria. Respiration 2016; 91: 386–402. [DOI] [PubMed] [Google Scholar]
- 10. Namkoong H, Kurashima A, Morimoto K. et al. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan. Emerg Infect Dis 2016; 22: 1116–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koh WJ, Jeon K, Lee NY. et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 2011; 183: 405–10. [DOI] [PubMed] [Google Scholar]
- 12. Aono A, Morimoto K, Chikamatsu K. et al. Antimicrobial susceptibility testing of Mycobacteroides (Mycobacterium) abscessus complex, Mycolicibacterium (Mycobacterium) fortuitum, and Mycobacteroides (Mycobacterium) chelonae. J Infect Chemother 2019; 25: 117–23. [DOI] [PubMed] [Google Scholar]
- 13. Haworth CS, Banks J, Capstick T. et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017; 72: ii1–ii64. [DOI] [PubMed] [Google Scholar]
- 14. Daley CL, Iaccarino JM, Lange C. et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline: executive summary. Clin Infect Dis 2020; 71: e1–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morimoto K, Nakagawa T, Asami T. et al. Clinico-microbiological analysis of 121 patients with pulmonary Mycobacteroides abscessus complex disease in Japan - An NTM-JRC study with RIT. Respir Med 2018; 145: 14–20. [DOI] [PubMed] [Google Scholar]
- 16. Pasipanodya JG, Ogbonna D, Ferro BE. et al. Systematic review and meta-analyses of the effect of chemotherapy on pulmonary Mycobacterium abscessus outcomes and disease recurrence. Antimicrob Agents Chemother 2017; 61: e01206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwak N, Dalcolmo MP, Daley CL. et al. Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur Respir J 2019; 54: 1801991. [DOI] [PubMed] [Google Scholar]
- 18. Jhun BW, Moon SM, Jeon K. et al. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: a 15-year follow-up study. Eur Respir J 2020; 55: 1900798. [DOI] [PubMed] [Google Scholar]
- 19. Nathavitharana RR, Strnad L, Lederer PA. et al. Top Questions in the diagnosis and treatment of pulmonary M. abscessus disease. Open Forum Infect Dis 2019; 6: ofz221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aziz DB, Teo JWP, Dartois V. et al. Teicoplanin - tigecycline combination shows synergy against Mycobacterium abscessus. Front Microbiol 2018; 9: 932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu ML, Aziz DB, Dartois V. et al. NTM drug discovery: status, gaps and the way forward. Drug Discov Today 2018; 23: 1502–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saito H, Tomioka H, Sato K. et al. In vitro and in vivo antimycobacterial activities of a new quinolone, DU-6859a. Antimicrob Agents Chemother 1994; 38: 2877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asakura T, Suzuki S, Fukano H. et al. Sitafloxacin-containing regimen for the treatment of refractory Mycobacterium avium complex lung disease. Open Forum Infect Dis 2019; 6: ofz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fujita K, Fujita M, Ito Y. et al. Preliminary evaluation of a sitafloxacin-containing regimen for relapsed or refractory pulmonary Mycobacterium avium complex disease. Open Forum Infect Dis 2016; 3: ofw147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 2003; 52:1. [DOI] [PubMed] [Google Scholar]
- 26. Cheng A, Tsai YT, Chang SY. et al. In Vitro synergism of rifabutin with clarithromycin, imipenem, and tigecycline against the Mycobacterium abscessus complex. Antimicrob Agents Chemother 2019; 63: e02234–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shirata M, Yoshimoto Y, Marumo S. et al. In vitro efficacy of combinations of eight antimicrobial agents against Mycobacteroides abscessus complex. Int J Infect Dis 2020; 97: 270–7. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Z, Lu J, Liu M. et al. In vitro activity of clarithromycin in combination with other antimicrobial agents against Mycobacterium abscessus and Mycobacterium massiliense. Int J Antimicrob Agents 2017; 49: 383–6. [DOI] [PubMed] [Google Scholar]
- 29. Bhusal Y, Shiohira CM, Yamane N.. Determination of in vitro synergy when three antimicrobial agents are combined against Mycobacterium tuberculosis. Int J Antimicrob Agents 2005; 26: 292–7. [DOI] [PubMed] [Google Scholar]
- 30. Dannaoui E, Lortholary O, Dromer F.. In vitro evaluation of double and triple combinations of antifungal drugs against Aspergillus fumigatus and Aspergillus terreus. Antimicrob Agents Chemother 2004; 48: 970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pryjma M, Burian J, Thompson CJ.. Rifabutin acts in synergy and is bactericidal with frontline Mycobacterium abscessus antibiotics clarithromycin and tigecycline, suggesting a potent treatment combination. Antimicrob Agents Chemother 2018; 62: e00283–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Griffith DE, Aksamit T, Brown-Elliott BA. et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175: 367–416. [DOI] [PubMed] [Google Scholar]
- 33. CLSI. Susceptibility testing of Mycobacteria, Nocardia spp., and other aerobic Actinomycetes — Third edition : M24. 2018. [PubMed]
- 34. Brown-Elliott BA, Wallace RJ Jr.. In vitro susceptibility testing of bedaquiline against Mycobacterium abscessus complex. Antimicrob Agents Chemother 2019; 63: e01919-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dupont C, Viljoen A, Thomas S. et al. Bedaquiline inhibits the ATP synthase in Mycobacterium abscessus and is effective in infected zebrafish. Antimicrob Agents Chemother 2017; 61: e01225–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huitric E, Verhasselt P, Andries K. et al. In vitro antimycobacterial spectrum of a diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother 2007; 51: 4202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li B, Ye M, Guo Q. et al. Determination of MIC distribution and mechanisms of decreased susceptibility to bedaquiline among clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother 2018; 62: e00175–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diacon AH, Pym A, Grobusch M. et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 2009; 360: 2397–405. [DOI] [PubMed] [Google Scholar]
- 39. Pang Y, Zheng H, Tan Y. et al. In vitro activity of bedaquiline against nontuberculous mycobacteria in China. Antimicrob Agents Chemother 2017; 61: e02627–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Le Moigne V, Raynaud C, Moreau F. et al. Efficacy of bedaquiline, alone or in combination with imipenem, against Mycobacterium abscessus in C3HeB/FeJ mice. Antimicrob Agents Chemother 2020; 64: e00114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lerat I, Cambau E, Bettoni RRD. et al. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis 2014; 209: 905–12. [DOI] [PubMed] [Google Scholar]
- 42. Philley JV, Wallace RJ Jr, Benwill JL. et al. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 2015; 148: 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lindman M, Dick T.. Bedaquiline eliminates bactericidal activity of β-lactams against Mycobacterium abscessus. Antimicrob Agents Chemother 2019; 63: e00827–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarathy JP, Ganapathy US, Zimmerman MD. et al. TBAJ-876, a 3,5-dialkoxypyridine analogue of bedaquiline, is active against Mycobacterium abscessus. Antimicrob Agents Chemother 2020; 64: e02404–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carey GB, Tebas P, Vinnard C. et al. clinical outcomes of clofazimine use for rapidly growing mycobacterial infections. Open Forum Infect Dis 2019; 6: ofz456. [Google Scholar]
- 46. Martiniano SL, Wagner BD, Levin A. et al. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest 2017; 152: 800–9. [DOI] [PubMed] [Google Scholar]
- 47. Yang B, Jhun BW, Moon SM. et al. Clofazimine-containing regimen for the treatment of Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 2017; 61: e02052–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Luo J, Yu X, Jiang G. et al. In vitro activity of clofazimine against nontuberculous mycobacteria isolated in Beijing, China. Antimicrob Agents Chemother 2018; 62: e00072–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kwak N, Whang J, Yang JS et al. Minimal inhibitory concentration of clofazimine among clinical isolates of nontuberculous mycobacteria and its impact on treatment outcome. Chest 2021; 159: 517–23. [DOI] [PubMed] [Google Scholar]
- 50. Ruth MM, Sangen JJN, Remmers K. et al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J Antimicrob Chemother 2019; 74: 935–43. [DOI] [PubMed] [Google Scholar]
- 51. Obregón-Henao A, Arnett KA, Henao-Tamayo M. et al. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob Agents Chemother 2015; 59: 6904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jhun BW, Yang B, Moon SM. et al. Amikacin inhalation as salvage therapy for refractory nontuberculous mycobacterial lung disease. Antimicrob Agents Chemother 2018; 62: e00011-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Olivier KN, Griffith DE, Eagle G. et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med 2017; 195: 814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Olivier KN, Shaw PA, Glaser TS. et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc 2014; 11: 30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yagi K, Ishii M, Namkoong H. et al. The efficacy, safety, and feasibility of inhaled amikacin for the treatment of difficult-to-treat non-tuberculous mycobacterial lung diseases. BMC Infect Dis 2017; 17: 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shen GH, Wu BD, Hu ST. et al. High efficacy of clofazimine and its synergistic effect with amikacin against rapidly growing mycobacteria. Int J Antimicrob Agents 2010; 35: 400–4. [DOI] [PubMed] [Google Scholar]
- 57. van Ingen J, Totten SE, Helstrom NK. et al. In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob Agents Chemother 2012; 56: 6324–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Khadawardi H, Marras TK, Mehrabi M. et al. Clinical efficacy and safety of fluoroquinolone containing regimens in patients with Mycobacterium avium complex (MAC) pulmonary disease. Eur Respir J 2020; 55: 1901240. [DOI] [PubMed] [Google Scholar]
- 59. Ferro BE, Srivastava S, Deshpande D. et al. Moxifloxacin's limited efficacy in the hollow-fiber model of Mycobacterium abscessus disease. Antimicrob Agents Chemother 2016; 60: 3779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nie WJ, Xie ZY, Gao S. et al. Efficacy of moxifloxacin against Mycobacterium abscessus in zebrafish model in vivo. Biomed Environ Sci 2020; 33: 350–8. [DOI] [PubMed] [Google Scholar]