Abstract
Background
Actionable data on antimicrobial use is important when planning strategic interventions such as antimicrobial stewardship to address the challenge of drug resistance, particularly in resource-constrained settings.
Objectives
To assess the prevalence of antibiotic use, the pattern of commonly used antibiotics and patient factors that may be associated with the increased use of antibiotics in the study hospitals.
Methods
This was a cross-sectional study conducted using the WHO Methodology for Point Prevalence Surveys in hospitals. Chi-squared analysis, Fisher’s exact test and logistic regression were employed to analyse statistically the data obtained.
Results
The overall prevalence of antibiotic use in the hospitals was 60.5%. The commonest indications for antibiotic recommendations were community-acquired infections (36.5%), surgical prophylaxis (26.1%) and hospital-acquired infections (15.7%), among others. Very few (2.7%) of the patients had their samples taken for culture and susceptibility testing to guide therapy. Penicillins (48.7%), cephalosporins (23.5%) and fluoroquinolones (17.4%) were the most commonly prescribed antibiotics. Concurrent malaria infection [adjusted OR (AOR) 0.33, 95% CI 0.11–0.94, P = 0.04] and increasing age (AOR 0.98, 95% CI 0.96–1.00, P = 0.02) were associated with lower risk of antibiotic use.
Conclusions
The prevalence of antibiotic consumption in the hospitals was lower than that reported in similar studies in Ghana, but high relative to some reports from high-income countries. Most antibiotic therapy was empirical and not guided by culture and susceptibility testing. There is the need for application of the WHO AWaRe classification for the selection of antibiotics and increased use of culture and susceptibility data to guide infectious disease therapy.
Introduction
Antimicrobial resistance (AMR) is an important subject in healthcare and public health as it is associated with increased mortality and morbidity among patients. The risk of AMR is high as every individual is susceptible to developing drug-resistant infections.1 Infections due to drug-resistant organisms require more aggressive therapy to be used, including (but not limited to) the use of combinations of different agents and the use of reserved agents. Treatment of resistant infections is associated with higher costs2 for second line drugs, additional investigations, and longer hospitalization.3 Productivity losses due to excess morbidity and premature mortality are related indirect costs posing profound socioeconomic problems and implications for the world.4 WHO has recognized AMR as an important public health threat, requiring immediate intervention to slow its progression around the world.5 The WHO, in a collaborative effort, has already developed the Global Action Plan (GAP) using the One Health approach to fight AMR in veterinary and human medicine.6 With this, countries around the world are expected to tailor interventions and mechanisms to tackle AMR to their local circumstances.
Data and information on the use of antimicrobials in relevant sectors are indispensable in policy-making to tackle AMR. Information-driven solutions make the most sustainable impact with minimal resources and wastage. Real world data is needed to implement locally tailored antimicrobial stewardship programmes (ASPs) to optimize antibiotic use. WHO develops various tools and strategies to support stewardship activities in countries.6 One of these is the 2019 AWaRe Classification database, first developed in 2017, which has 180 antibiotics classified as Access, Watch or Reserve (AWaRe), based on pharmacological class, anatomical therapeutic chemical (ATC) codes and their WHO Essential Medicines List status. This classification provides a useful means to monitor antibiotic use across the classes as an indicator of optimal antibiotic use. Antibiotics in the Access group are those that show a lower potential for resistance than the other groups with a good range of activity against many common pathogens. Watch antibiotics are those with much higher resistance potential and as such require key targets for stewardship interventions to reduce their inappropriate use. The Reserve group are those used for treating infections due to multidrug-resistant pathogens and so should be used as a last line, after all alternatives have been explored, in order to protect them against resistance.7
Several studies have investigated antibiotic use in various settings in Ghana and across the world with varied prevalence of antibiotic use and patient parameters affecting their use.8–16 However, impactful interventions that optimize antimicrobial use require facility-specific data to determine which specific improvements can be made. The current study investigated antibiotic use in three hospitals in the Ashanti region to gather data for implementation of antimicrobial stewardship. We aimed to determine prevalence of antibiotic use, commonly used antibiotics, patient variables associated with increased antibiotic use in the hospitals and to identify targets for interventions by ASPs.
Methods
Study design and setting
We employed the WHO Methodology for Point Prevalence survey (PPS) on antibiotic use in hospitals, version 1.1, which has been described elsewhere.17
The facilities were classified according to the criteria set forth in the WHO methodology for PPS17 used for the study. Kwame Nkrumah University of Science and Technology (KNUST) Hospital (UHS) was classified as tertiary, Agogo Presbyterian Hospital (APH) as secondary and Ejisu Government hospital (EGH) as a primary facility.
UHS provides services to more than 200 000 Ghanaians annually. The hospital provides a wide range of services ranging from ambulatory to in-patient care. The hospital has a functioning infection prevention and control programme as well as a drug and therapeutics committee supporting optimization of patient care.18
APH, the secondary hospital, is a 250 bed capacity hospital which caters to patients from all over Ghana and neighbouring countries, especially for ophthalmological care. It serves a municipal catchment area of over 170 822 persons. It is designated as Collaborating Centre for the University of Ghana School of Public Health, Training Centre for Buruli Ulcer Treatment, Ministry of Health (MOH)/WHO designated centre for training in the surgical management of buruli ulcer and one of two sites in Ghana and eight sites in Africa for Malaria Vaccine Trial.19
EGH is a primary hospital facility under the Ghana Health service in a municipality of over 143 762 persons.20 The hospital is in the capital of the municipality which is a bustling market city that sees many people coming in, especially on market days (Thursdays and Sundays).
Point prevalence survey
The PPS was a cross-sectional survey conducted on 26 and 27 November 2019 at a tertiary hospital (UHS) and primary level facility (EGH), respectively and on 10 December 2019 at a secondary level hospital (APH). The different dates were necessary to ensure that all in-patients at each facility were surveyed with the resources available.
Each patient present on the ward at the time of the survey was targeted for inclusion in the study unless the patient did not meet inclusion criteria. Generally, any form with more than 10% missing data would be excluded from the analysis. Table 1 shows the inclusion and exclusion criteria.
Table 1.
Patient inclusion and exclusion criteria for point prevalence survey
| Level | Inclusion | Exclusion |
|---|---|---|
| Ward | All acute care inpatient wards in the hospitals. | Long-term care wards, Emergency departments (except for wards attached to be monitored for more than 24 h), Day surgery and Day care wards. |
| Patients | Hospitalized patient already admitted in the ward as at 08:00 am on day of survey whether receiving antibiotic treatment or not. | Patients admitted after 08:00 am on day of survey, all Day care patients. |
| Antibiotics | Only antibiotics included in ANNEX XI of the WHO protocol administered by oral, parenteral, rectal or inhalation routes were included, antibiotic initiated by 08:00 am on survey day. | Topical antibiotics, antibiotic started after 08:00 am on day of survey. |
Data collection
Anonymized data was collected from the hospital records of in-patients who met the inclusion criteria. The data was collected by the research team with help from select final year Doctor of Pharmacy students from KNUST. A training programme was organized by the research team led by O.K.O.A. and N.K.A.B. on the 25 November 2019 for UHS and EGH as well as on 9 December 2019 for APH, to ensure accuracy and minimize error during data collection. This training involved simulations on obtaining information from patient records, and managing situations and records that strayed from the norm. A mock data collection exercise was conducted to improve accuracy during data collection. A total of 16 persons collected patient data at UHS and EGH and 14 persons at APH. All data was collected on hard copies of forms used in the protocol. No patient sampling was done.
Data management and analyses
Data collected after surveying each hospital was sorted and organized to prevent mix-up during data entry. All the data collected in the study was entered into a REDCap® database and exported into Stata™ 14 for analyses.21,22 No data forms had more than 10% missing data recorded and so all forms were included in the entry and subsequent analyses. Missing data were entered as ‘unknown’ in the database. Descriptive analysis with frequencies and percentages show the patterns within variables. Chi-squared tests and or Fisher’s exact test where appropriate was used to test univariate associations between antibiotics use and patient variables collected in the survey. Finally, forward stepwise logistic regression model with unadjusted and adjusted odds ratios was constructed to establish whether there is a relationship between the antibiotics use and surgery on admission, co-morbidities, nutritional status, use of catheter, tuberculosis and HIV status, peripheral vascular catheter, gender, age and type of infection. A P value of <0.05 was considered statistically significant.
Ethics
Ethics approval was obtained from the KNUST Committee on Human Research, Publications and Ethics after getting approval from each facility. (CHRPE/AP/654/19).
Results
190 out of 211 inpatients met the inclusion criteria for the study: 44 out of 49 from UHS, 45 of 49 from EGH and 101 of 113 from APH.
The overall prevalence of antibiotic use was 60.5% (Table 2). APH, the secondary hospital, had the highest prevalence at 67.3% while the primary hospital EGH had the lowest prevalence at 51.1%.
Table 2.
Demographics and prevalence of antibiotic use by hospital
| Factor |
n (%) or median (IQR) |
|||
|---|---|---|---|---|
| Overall (N = 190) | UHS (n = 44) | APH (n = 101) | EGH (n = 45) | |
| Gender | ||||
| Female | 120 (63.2%) | 29 (65.9%) | 60 (59.4%) | 31 (68.9%) |
| Male | 63 (33.2%) | 13 (29.6%) | 36 (35.6%) | 14 (31.1%) |
| Age | ||||
| ≥2 years (N = 165) | 30 (21–48) | 27.5 (21.0–42.0) | 35.0 (20.0–57.0) | 29.0 (21.0–44.0) |
| 0–23 months (N = 21) | 0.13 (0.06–0.30) | 0.03 (0.03–0.13) | 0.2 (0.1–0.3) | 0 (0.0–7.0) |
| Patients on antibiotics | 115 (60.5%) | 68 (67.3%) | 24 (54.5%) | 23 (51.1%) |
| Number of antibiotics per patient (N = 115) | ||||
| 1 | 37 (32.2%) | 26 (38.2%) | 4 (16.7%) | 7 (30.4%) |
| 2 | 68 (59.1%) | 34 (50.0%) | 19 (79.2%) | 15 (65.2%) |
| 3 | 10 (8.7%) | 1 (4.2%) | 1 (4.4%) | |
N=Total number of patients; n=number of patients.
Table 3 describes the distribution of the indications for use of antibiotics in the facilities and if such therapy had a culture sample taken or not. Most antibiotics were used for community-acquired infections (36.5%), surgical prophylaxis (26.1%) and for hospital-acquired infections (15.7%). Most prescriptions were empirical as only 2.7% of cases had a sample taken for culture and susceptibility analyses.
Table 3.
Summary of antibiotic indication
| Hospital |
||||
|---|---|---|---|---|
| Factor (n = 115) | Total | UHS | APH | EGH |
| Indication type | ||||
| Community-acquired infection | 42 (36.5%) | 10 (41.7%) | 21 (30.9%) | 11 (47.8%) |
| Surgical prophylaxis | 30 (26.1%) | 9 (37.5%) | 17 (25.0%) | 4 (17.4%) |
| Hospital-acquired infection | 18 (15.7%) | 1 (4.2%) | 15 (22.1%) | 2 (8.7%) |
| Medical prophylaxisa | 16 (13.9%) | 3 (12.5%) | 7 (10.3%) | 6 (26.1%) |
| Other | 9 (7.8%) | 1 (4.2%) | 8 (11.8%) | 0 (0.0%) |
| Reason for antibiotic use in notes? | ||||
| Yes | 89 (88.1%) | 23 (95.8%) | 44 (80.0%) | 22 (100.0%) |
| Culture sample taken? | ||||
| Yes | 3 (2.7%) | 2 (8.3%) | 0 (0.0%) | 1 (4.6%) |
Results shown are n (%).
Antibiotics given to prevent bacterial infections in susceptible groups, such as patients with Crohn’s disease, pregnant women with suspected/susceptible to preterm/premature rupture of membranes to prevent early-onset neonatal Group B streptococcal disease.39
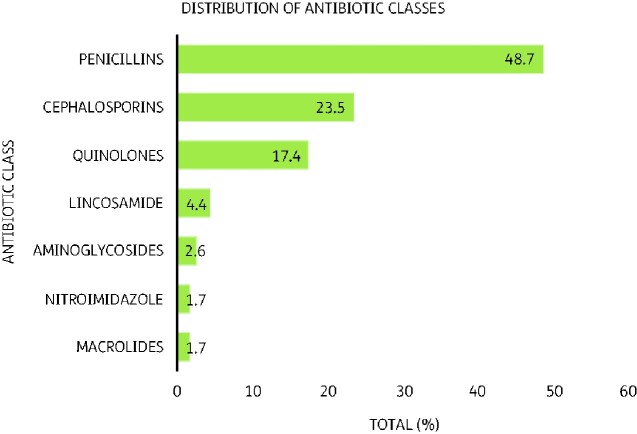
The commonly prescribed antibiotics were penicillins (48.7%) cephalosporins (23.5%) and fluoroquinolones (17.4%) as shown in Figure 1. Table 4 shows the commonly used agents as amoxicillin (36.5%), ciprofloxacin (17.4%), ceftriaxone (11.3%), cefuroxime (9.6%) and ampicillin (7.8%).
Figure 1.
Prescribed antibiotics by antibiotic classes.
Table 4.
Prescribed antibiotics by hospital
| Hospital |
||||
|---|---|---|---|---|
| Antibiotica (AWaRe class) | Overall | UHS | APH | EGH |
| Amoxicillin (A) | 42 (36.5%) | 12 (50.0%) | 18 (26.5%) | 12 (52.2%) |
| Ciprofloxacin (W) | 20 (17.4%) | 3 (12.5%) | 15 (22.1%) | 2 (8.7%) |
| Ceftriaxone (W) | 13 (11.3%) | 2 (8.3%) | 11 (16.2%) | 0 (0.0) |
| Cefuroxime (W) | 11 (9.6%) | 3 (12.5%) | 6 (8.8%) | 2 (8.7%) |
| Ampicillin (A) | 9 (7.8%) | 0 (0.0) | 9 (26.5%) | 0 (0.0) |
| Clindamycin (A) | 5 (4.4%) | 0 (0.0) | 3 (4.4%) | 2 (8.7%) |
| Benzylpenicillin (A) | 3 (2.6%) | 0 (0.0) | 1 (1.5%) | 2 (8.7%) |
| Amikacin (A) | 2 (1.7%) | 0 (0.0) | 0 (0.0) | 2 (8.7%) |
| Cefotaxime (W) | 2 (1.7%) | 0 (0.0) | 2 (2.9%) | 0 (0.0) |
| Flucloxacillin (U) | 2 (1.7%) | 0 (0.0) | 2 (2.9%) | 0 (0.0) |
| Metronidazole (A) | 2 (1.7%) | 0(0.0) | 1 (1.5%) | 1 (4.4%) |
| Azithromycin (W) | 1 (0.9%) | 1 (4.2%) | 0 (0.0) | 0 (0.0) |
| Cefpodoxime (U) | 1 (0.9%) | 1 (4.2%) | 0 (0.0) | 0 (0.0) |
| Erythromycin (W) | 1 (0.9%) | 1 (4.2%) | 0 (0.0) | 0 (0.0) |
| Gentamicin (A) | 1 (0.9%) | 1 (4.2%) | 0 (0.0) | 0 (0.0) |
Results shown are n (%).
WHO AWaRe classification: A, Access (46.7%); W, Watch (40%); Re, Reserve (0); U, Unclassified (13.3%).
Antibiotic names are the International Nonproprietary Name (INN).
According to the univariate analysis, surgery, urinary catheter and peripheral vascular catheter use all showed statistically significant association with antibiotic use (Table 5). Results for each individual hospital are shown in Tables S1 to S3 (available as Supplementary data at JAC Online).
Table 5.
Patient variables and association with antibiotic use
| Patients on antibiotics, n (%) |
|||
|---|---|---|---|
| Variable | No | Yes | P value |
| Surgery on admission | |||
| Yes | 5 (13.9%) | 31 (86.1%) | 0.001a |
| Type of surgery | |||
| Minimal | 0 (0.0%) | 4 (100.0%) | |
| NHSN | 1(5.6%) | 17 (94.4%) | 1.000b |
| McCabe score | |||
| Non-Fatal | 62 (37.6%) | 103 (62.4%) | |
| Rapidly Fatal | 10 (52.6%) | 9 (47.4%) | 0.386a |
| Ultimately Fatal | 3 (50.0%) | 3 (50.0%) | |
| Urinary catheter | |||
| Yes | 6 (18.8%) | 26 (81.3%) | |
| Peripheral Vascular Catheter | |||
| Yes | 38 (29.9%) | 89 (70.1%) | <0.001a |
NHSN, National Healthcare Safety Network surgery list defined in Annex IX of WHO protocol.17
Chi-squared test was performed.
Fisher’s exact test.
According to the multivariate analysis, the associations between surgery, peripheral vascular catheter and urinary catheter use with antibiotic use were not statistically significant on adjusting for other variables. Increasing age and malaria were associated with decreased adjusted odds of being on antibiotics (Table 6).
Table 6.
Logistic regression to adjust variables for potential confounders (all facilities)
| Variable | UAOR (95% CI) | AOR (95% CI) | P value |
|---|---|---|---|
| Patients on antibiotics | |||
| Age in years | 1.00 (0.98–1.01) | 0.98 (0.96–1.00) | 0.022 |
| Gender | |||
| Female | 1 | 1 | |
| Male | 0.65 (0.35–1.21) | 2.33 (0.88–6.20) | 0.09 |
| Surgery on Admission | |||
| No | 1 | 1 | |
| Yes | 5.1 (1.89–14.00) | 3.2 (0.83–12.56) | 0.092 |
| Comorbidities | |||
| McCabe Score | |||
| Rapidly Fatal | 1 | 1 | |
| Non-Fatal | 1.8 (0.71–4.79) | 2.51 (0.60–10.49) | 0.206 |
| Ultimately Fatal | 1.1 (0.18–6.97) | 0.60 (0.04–9.83) | 0.721 |
| Use of catheter | |||
| No | 1 | 1 | |
| Yes | 3.5 (1.38–9.11) | 4.26 (0.82–22.02) | 0.084 |
| Type of infection | |||
| Malaria | |||
| No | 1 | 1 | |
| Yes | 0.4 (0.19–0.98) | 0.33 (0.11–0.94) | 0.038 |
| Tuberculosis | |||
| No | 1 | ||
| Yes | 0.2 (0.01–2.02) | – | – |
| HIV | |||
| No | 1 | ||
| Yes | 1.5 (0.29–8.12) | – | – |
| Peripheral vascular catheter | |||
| No | 1 | 1 | |
| Yes | 3.5 (1.82–6.80) | 2.31 (0.90–5.87) | 0.08 |
UAOR, unadjusted odds ratio; AOR, adjusted odds ratio. Dashes represent variables that were dropped due to limited numbers and/or collinearity.
Discussion
According to this study, the prevalence of antibiotic use is high relative to other studies, with many patients using two or more antibiotics, and antibiotics in Watch category in the WHO AWaRe classification.7,23 Furthermore, only a small fraction of patients had a sample taken for culture and susceptibility analyses before antibiotic use. The prevalence of patients on at least one antibiotic is comparable to findings from other surveys across Africa and the world. Previous studies in Ghana have recorded 61%–82% prevalence of antibiotic use in hospitals11,13 while other studies from around Africa have reported 50%–70.6% prevalence.8,14,16,24 Studies conducted outside Africa have reported higher prevalence of 77.6% (Pakistan)10 but mainly lower prevalence of 27%–50% in Europe and the United States.14,15,25,26 These studies employed standardized protocols and the differences in antibiotic use may be due to different living conditions, public education27 as well as differences in clinical practice. In our study, the differences in prevalence of antibiotic use may be attributable to the different level of hospital facilities in the study. The prevalence found in the tertiary facility UHS was similar to what was found in a similar facility in Ghana with prevalence of 51.4%.12 This may represent a comparable use of antibiotics across a similar hospital level. This finding supports the use of data from comparable institutions and from the specific facility for policy making. Subsequent interventions for improving antibiotic use will then have maximal impact in individual hospitals versus using generic data and interventions.
The majority of patients on antibiotics were on two antibiotics at the time of the study, which may be a key target for improvement. An example is a case observed in the study where a patient was on ceftriaxone, gentamicin and azithromycin for clinical sepsis. Such a combination is usually aimed at preventing or surmounting possible resistance. The conventional mantra in infectious disease management is to ‘hit hard and to hit early’, which may often include combination of antimicrobial agents for synergy.28 This strategy may be flawed, as, if executed poorly, it could lead to the exponential selection of resistance genes in microbes because competing organisms have been destroyed by the aggressive therapy.29 It is important therefore that therapy is guided by evidence from investigations.
However, only a small fraction of patients had a sample taken for culture and drug susceptibility analyses before antibiotic therapy was started. This is an important finding as directed therapy is essential in optimizing therapy with antibiotics compared with empirical therapy. Local antibiograms and antimicrobial guidelines are needed as interventions in the hospitals to direct empirical therapy in the face of limited resources. Amoxicillin, ciprofloxacin, ceftriaxone, cefuroxime and ampicillin were the most used antibiotics overall. This is comparable to the top three antibiotics prescribed worldwide.14
None of the facilities at the time of the study had patients on antibiotics classified in the Reserve category of the WHO AWaRe system, similar to another study in Ghana,12 although their availability was not assessed in the current study. However, a good number of antibiotics surveyed belonged to the Watch group with three of them among the most used agents overall, which was higher than findings in Finland (23%) but lower than in Iran (77.3%). Use of Access agents could be increased in Ghana (47%) when compared with that in Singapore (100%) for neonates.23 Interventions could be tailored at using this tool to direct antibiotic use in hospitals in low-resource settings.23,30 Since potentially only a small fraction of the antibiotics prescribed were culture- and susceptibility-directed therapy, using antibiotics in the Watch category as much could be potentially problematic. This can be addressed effectively and inexpensively with hospital-specific formularies with the AWaRe classification taken into consideration. Another intervention could be to increase use of Access agents and restrict the use of Reserve agents to require preauthorization from qualified persons to maintain their low use in the health facilities. Prescriber training and information posters could also be provided to support appropriate prescribing and compliance to rational antibiotic use strategies. Increasing age was associated with slightly decreased adjusted odds of being on an antibiotic. This may be attributed to paediatric patients having immature immune systems and so are at an understandably higher risk of infections requiring antibiotics compared with older individuals. Older persons have more developed immune systems which have evolved to provide protection through exposure to various foreign agents and pathogens from childhood to adulthood.31 A decline in old age is expected but this was not seen in the study as the overall age range for patients did not include geriatrics. Future PPSs could focus on paediatric and geriatric populations in Ghana for evidence to drive policymaking in these vulnerable groups.
A concurrent diagnosis of malaria was protective from receiving antibiotics in patients. Malaria is a febrile illness, endemic to Ghana,32 and with symptoms that could be mistaken for bacterial infections. The availability of cheap rapid diagnostic tests and the high suspicion for malaria in febrile illnesses in Ghana may explain the statistically significant protection. This finding may indicate that prescribers are not irrationally using antibiotics in malaria, which may be commendable.
There were statistically significant associations between having surgery on admission, having a urinary catheter, a peripheral vascular catheter (PVC) in situ and being on an antibiotic in univariate analyses. This finding may be due to their invasive nature, but could be confounded by the severity of illness in these patients requiring such devices or procedures. Catheter-related urinary tract infections are one of the most common nosocomial infections and a common complication of urinary catheters.33–35 Widespread PVC use (estimated to be as high as in up to 80% of inpatients) may contribute to the association with antibiotic use.36 PVC use is associated with severe nosocomial infections and PVC-related nosocomial infections provide a target for reducing infections and antibiotic use.37,38 Adequate infection prevention and control when placing such devices, in addition to their maintenance, may help prevent nosocomial infections related to their use. This may especially be important in APH, where PVC use was significantly associated with antibiotic use while having surgery on admission was not (Table S1). In UHS, surgery and urinary catheter use were significantly associated with antibiotic use, whereas no associations were observed in EGH (Tables S2 and S3).
Antibiotic consumption data are essential in the fight against AMR as they provide an indication of prescribers’ behaviours and are an important metric for improving antibiotic use. This study represents the successful use of the WHO tool17 in surveying antibiotic use in hospitals. It will be especially useful in low-resource settings (as found in low- and middle-income countries) as it is not resource intensive. Our study is limited by the fact that a single PPS may be inadequate to provide information on prescription patterns and trends in antibiotic use in facilities. Subsequent PPS may be conducted during the same period of the initial PPS to account for possible trends in antibiotic use. A strength of our study includes the use of the standardized WHO tool to collect data and the use of multiple centres to collect data.
Conclusions
The prevalence of antibiotic consumption in the hospitals was low relative to reports from some studies in Ghana, but high relative to similar studies from some high-income countries. Most of the antibiotic therapy was empirical and not guided by culture and drug susceptibility testing. The most commonly prescribed antibiotics were penicillins, cephalosporin and fluoroquinolones, with a great proportion being those in the WHO Watch category. Increasing age and patients diagnosed with malaria had lower odds of being prescribed antibiotics. There is the need for pragmatic stewardship initiatives in these hospitals to control the prescribing and consumption of antimicrobials. An example may be the application of the WHO AWaRe classification for the selection of antibiotics, as well as increased use of culture and antimicrobial susceptibility data to guide infectious disease therapy.
Supplementary Material
Acknowledgements
We acknowledge the support of the Ghana Antimicrobial Resistance Policy Platform in the planning and execution of this project. We wish to acknowledge the support of the select members of the class of 2020 Doctor of Pharmacy students led by Maame Ofosuah and Salomey Denkyira who participated in the training and data collection for the study in addition to Drs Judith Foli, Ivan Mozu, Kofi Nyanor, Nana Ofori Adomako and the staff of the Department of Pharmacy Practice, KNUST for their immense support and guidance. We also acknowledge all the staff of the three hospitals that took part in this survey that supported our activities before, during and beyond the data collection.
Funding
This study was supported by internal funding.
Transparency declarations
None to declare.
Author contributions
Conceptualization: O.K.O.A., K.O.B. Methodology: O.K.O.A., K.O.B., A.O.O., N.K.A.B. Validation: all authors. Formal analysis: O.K.O.A. Writing: original draft preparation, all authors; review and editing, all authors. Supervision: K.O.B., A.O.O.
Supplementary data
Tables S1 to S3 are available as Supplementary data at JAC Online
References
- 1. Davies J. Where have all the antibiotics gone? Can J Infect Dis Med Microbiol 2006; 17: 287–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shrestha P, Cooper BS, Coast J et al. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob Resist Infect Control 2018; 7: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One 2017; 12: e0189621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hofer U. The cost of antimicrobial resistance. Nat Rev Microbiol 2019; 17: 3. [DOI] [PubMed] [Google Scholar]
- 5. WHO. Antimicrobial resistance 2019. https://www.who.int/antimicrobial-resistance/en/.
- 6. WHO. Global Action Plan on Antimicrobial Resistance. 2015. https://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/.
- 7. WHO. The 2019 WHO AWaRe classification of antibiotics for evaluation and monitoring of use. 2019. https://apps.who.int/iris/handle/10665/327957.
- 8. Talaat M, Saied T, Kandeel A et al. A point prevalence survey of antibiotic use in 18 hospitals in Egypt. Antibiotics 2014; 3: 450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akhloufi H, Streefkerk RH, Melles DC et al. Point prevalence of appropriate antimicrobial therapy in a Dutch university hospital. Eur J Clin Microbiol Infect Dis 2015; 34: 1631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saleem Z, Hassali MA, Versporten A et al. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: findings and implications. Expert Rev anti Infect Ther 2019; 17: 285–93. [DOI] [PubMed] [Google Scholar]
- 11. Labi AK, Obeng-Nkrumah N, Owusu E et al. Multi-centre point-prevalence survey of hospital-acquired infections in Ghana. J Hosp Infect 2019; 101: 60–8. [DOI] [PubMed] [Google Scholar]
- 12. Labi AK, Obeng-Nkrumah N, Nartey ET et al. Antibiotic use in a tertiary healthcare facility in Ghana: a point prevalence survey. Antimicrob Resist Infect Control 2018; 7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Afriyie DK, Sefah IA, Sneddon J et al. Antimicrobial point prevalence surveys in two Ghanaian hospitals: opportunities for antimicrobial stewardship. JAC-Antimicrob Resist 2020; 2: 10.1093/jacamr/dlaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Versporten A, Zarb P, Caniaux I et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health 2018; 6: e619–e629. [DOI] [PubMed] [Google Scholar]
- 15. Al Matar M, Enani M, Binsaleh G et al. Point prevalence survey of antibiotic use in 26 Saudi hospitals in 2016. J Infect Public Health 2019; 12: 77–82. [DOI] [PubMed] [Google Scholar]
- 16. Okoth C, Opanga S, Okalebo F et al. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: findings and implications. Hosp Pract (1995) 2018; 46: 128–36. [DOI] [PubMed] [Google Scholar]
- 17. WHO. WHO Methodology for Point Prevalence Survey on Antibiotic Use in Hospitals version 1.1. 2018. https://www.who.int/medicines/access/antimicrobial_resistance/WHO-EMP-IAU-2018_01/en/#:∼:text=Point%20Prevalence%20Surveys%20collects%20information,complements%20surveillance%20of%20antimicrobial%20consumption.
- 18. KNUST. University Health Services. 2020. https://uhs.knust.edu.gh/about-us.
- 19. Agogo Presbyterian Hospital. Presbyterian Health Services – Agogo: Profile. https://agogopresbyhospital.org/main/profile/.
- 20. Ghana Government. Ejisu-Juaben Municipality. http://ejisujuaben.ghanadistricts.gov.gh/.
- 21. Vanderbilt Software. REDCap. REDCap - Research Electronic Data Capture. doi:10.1111/j.1365-2583.2009.00888.x ER.
- 22. StataCorp (2015). Statistical Software: Release 14. College Station, TX: StataCorp LP. [Google Scholar]
- 23. Hsia Y, Lee BR, Versporten A et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Health 2019; 7: e861–e871. [DOI] [PubMed] [Google Scholar]
- 24. Anand Paramadhas BD, Tiroyakgosi C, Mpinda-Joseph P et al. Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert Rev anti Infect Ther 2019; 17: 535–46. [DOI] [PubMed] [Google Scholar]
- 25. Plachouras D, Kärki T, Hansen S et al. Antimicrobial use in European acute care hospitals: Results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use. Euro Surveill 2016; 23: 1800393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magill SS, Edwards JR, Beldavs ZG et al. Prevalence of antimicrobial use in US acute care hospitals, May–September 2011. JAMA 2014; 312: 1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goossens H, Guillemot D, Ferech M et al. National campaigns to improve antibiotic use. Eur J Clin Pharmacol 2006; 62: 373–9. [DOI] [PubMed] [Google Scholar]
- 28. Ehrlich P. Address in pathology, on chemotherapy. Br Med J 1913; 2: 353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pena-Miller R, Laehnemann D, Jansen G et al. When the most potent combination of antibiotics selects for the greatest bacterial load: the Smile-Frown Transition. PLoS Biol 2013; 11: e1001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharland M, Pulcini C, Harbarth S et al. Classifying antibiotics in the WHO essential medicines list for optimal use—be AWaRe. Lancet Infect Dis 2018; 18: 18–20. [DOI] [PubMed] [Google Scholar]
- 31. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B 2015; 282: 20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malaria Global WHO Team. World Malaria Report 2019. 2019. https://apps.who.int/iris/rest/bitstreams/1262394/retrieve.
- 33. Köves B, Magyar A, Tenke P. Spectrum and antibiotic resistance of catheter-associated urinary tract infections. GMS Infect Dis 2017; 5: Doc06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinstein JW, Mazon D, Pantelick E et al. A decade of prevalence surveys in a tertiary-care center: trends in nosocomial infection rates, device utilization, and patient acuity. Infect Control Hosp Epidemiol 1999; 20: 543–8. [DOI] [PubMed] [Google Scholar]
- 35. Kalsi J, Arya M, Wilson P et al. Hospital-acquired urinary tract infection. Int J Clin Pract 2003; 57: 388–91. [PubMed] [Google Scholar]
- 36. Zhang L, Cao S, Marsh N et al. Infection risks associated with peripheral vascular catheters. J Infect Prev 2016; 17: 207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sato A, Nakamura I, Fujita H et al. Peripheral venous catheter-related bloodstream infection is associated with severe complications and potential death: A retrospective observational study. BMC Infect Dis 2017; 17: 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mermel LA. Short-term peripheral venous catheter–related bloodstream infections: a systematic review. Clin Infect Dis 2017; 65: 1757–62. [DOI] [PubMed] [Google Scholar]
- 39. US CDC. Preventing Group B Strep Disease in Newborns. 2018. https://www.cdc.gov/groupbstrep/about/prevention.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.