Abstract
Objectives
Enterobacterales producing ESBL (ESBL-E) have been notable for their rapid expansion in community settings. This systematic review and meta-analysis aimed to summarize evidence investigating the association between ESBL-E infection and adverse clinical outcomes, defined as bacteraemia, sepsis or septic shock, and all-cause mortality in adult patients.
Methods
Database search was conducted in PubMed, Scopus and EMBASE. In general, studies were screened for effect estimates of ESBL-E colonization or infection on clinical outcomes with non-ESBL-producing Enterobacterales as comparator, adult populations and molecular ascertainment of ESBL gene. Meta-analysis was performed using the inverse variance heterogeneity model.
Results
Eighteen studies were identified, including 1399 ESBL-E and 3200 non-ESBL-E infected patients. Sixteen of these studies included only bacteraemic patients. Mortality was studied in 17 studies and ESBL-E infection was significantly associated with higher odds of mortality compared with non-ESBL-producing Enterobacterales infection (OR = 1.70, 95% CI: 1.15–2.49, I2=58.3%). However, statistical significance did not persist when adjusted estimates were pooled (aOR = 1.67, 95% CI: 0.52–5.39, I2=78.1%). Septic shock was studied in seven studies and all included only bacteraemic patients. No association between ESBL-E infection and shock was found (OR = 1.23, 95% CI: 0.75–2.02, I2=14.8%). Only one study investigated the association between ESBL-E infection and bacteraemia.
Conclusions
Infections by ESBL-E appear to be significantly associated with mortality but not septic shock. Available studies investigating bacteraemia and shock as an intermediate outcome of ESBL-E infections are lacking. Future studies investigating the relationship between clinical outcomes and molecular characteristics of resistant strains are further warranted, along with studies investigating this in non-bacteraemic patients.
Introduction
The widespread emergence of MDR Gram-negative bacteria (MDRGNB) has profound effects on the clinical management of infected individuals. Of interest, Enterobacterales producing ESBLs (ESBL-E) have been increasingly detected in both community-onset and community-acquired extra-intestinal infections.1–4 ESBL-E have a reportedly alarming spread in the community, especially with the rapid global dissemination of Escherichia coli ST131 harbouring blaCTX-M in the last decade.5–7 This ST has been investigated for its enhanced capability to transmit from human to human.5–7 A recent review of 62 studies on ESBL-producing E. coli alone reported an 8-fold increase in global carriage rate of healthy individuals in the last two decades.8 With regard to the antimicrobial susceptibility profile, the ESBL-E pathogens are often associated with resistance to fluoroquinolones and expanded-spectrum cephalosporins,6 along with their inherent resistance to β-lactams. With carbapenems commonly prescribed for ESBL-E infections, recent reports have observed co-carriage of carbapenemases in ESBL-E strains.9,10 Infections by ESBL-E have been associated with increased rates of morbidity and mortality, with the pathogen being listed as serious threat in the 2019 Antibiotic Resistance Threats Report.11–13 Several studies have also observed increased virulence potential in ESBL-E strains.14–16
At the time of manuscript preparation, there were three systematic reviews and meta-analyses that quantified the association between ESBL-E infection and mortality.17–19 The majority of included studies only performed phenotypic screening with or without confirmatory test for the detection of ESBL production. The sensitivity and specificity of phenotypic screening and confirmatory tests are dependent on certain conditions, such as the number and type of agents used for screening and the influence of AmpC and other β-lactamase gene co-carriage in ESBL-producing bacteria.2,20,21 Few studies have employed molecular techniques such as PCR to ascertain the presence of ESBL-related genes (blaCTX-M, blaSHV and blaTEM variants), understandably due to availability of resources for routine laboratory testing. Misclassification of exposure arising from differences in sensitivity and specificity of ESBL phenotypic tests may have biased the reported association between ESBL production and clinical outcomes. In addition, the variability of phenotypic tests for ESBL detection employed in existing studies was unexpectedly high and difficult to quantify. Studies are consequently less exchangeable, rendering comparison of results unreliable. To avoid this, an updated and more stringent systematic review and meta-analysis investigating the association between ESBL-E infection and clinical outcomes was warranted by restricting the inclusion to studies that additionally performed molecular testing for ESBL genes.
Moreover, there has been no assessment of summary effect sizes for the associations between ESBL-E and other severe clinical outcomes, such as sepsis, septic shock or bacteraemia. These conditions are well-established predictors of mortality, and therefore, the risk of developing these conditions from ESBL-E infection should be investigated. This systematic review and meta-analysis aimed to summarize the association between ESBL-E infection and adverse clinical outcomes (i.e. bacteraemia, sepsis or septic shock and all-cause mortality) in adult patients, as compared with those with non-ESBL-producing Enterobacterales (non-ESBL-E) infection.
Methods
The protocol was prospectively registered in PROSPERO (CRD42020184483) and the findings of this systematic review and meta-analysis are presented following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Table S1, available as Supplementary data at JAC-AMR Online).22 This review was initially designed to examine the association between MDRGNB (i.e. ESBL-E, carbapenem-resistant Enterobacterales and carbapenem-resistant Acinetobacter) and adverse clinical outcomes. The search strategy was designed according to this objective. During the database search, four systematic reviews focusing on carbapenem-resistant Enterobacterales and mortality were found,23–26 with the most recent searches conducted up to December 2015 and up to August 2016 for carbapenem-resistant Klebsiella pneumoniae specifically. Therefore, a decision was made by the authors to restrict the systematic review to only ESBL-E and carbapenem-resistant Acinetobacter as the MDRGNB of interest, and to perform separate meta-analyses for these distinctly different pathogens. This systematic review and meta-analysis focused on ESBL-E.
Search strategy
A database search was conducted on 6 August 2020 in PubMed, Scopus and EMBASE. The search strategy was constructed by a librarian and included a combination of three search categories. The first category was on antibiotic resistance pattern and included terms relevant to extended-spectrum β-lactamase, ESBL, carbapenem or imipenem resistance. The second category specified the bacteria of interest and included terms such as Enterobacterales, Enterobacteriaceae, Acinetobacter, Escherichia coli, Klebsiella pneumoniae or Proteus mirabilis. The third category specified the outcome of interest and included relevant terms for mortality, sepsis or shock and bacteraemia. The three categories were combined with the appropriate Boolean function and MeSH terms were used for comprehensive scope of search. All search results were included with no restriction on publication year and language of article. The complete search strategies are provided in the Supplementary data.
All references from the previous systematic reviews that were not identified in the database search were manually added into the screening list. To ensure that the literature search was comprehensive, the authors also conducted a backward and forward citation search of the articles that were included in this systematic review and meta-analysis. All citations were downloaded and imported into EndNote X9. Duplicates were identified and removed before the citations were exported to Rayyan for the screening process.27
Study selection and screening
The study population was defined as adult patients aged at least 16 years old who presented to healthcare facilities, exposure as ESBL-E colonization or infection, comparison group as patients with non-ESBL-E infections, and the outcomes of interest were bacteraemia, sepsis or septic shock and all-cause mortality. Definitions for these outcomes of interest were accepted as described in each study. Studies were restricted to adults due to underlying differences in types of comorbidities, immune response and available antibiotics between adult and paediatric populations. The inclusion criteria were as follow: (i) studies enrolling patients from healthcare settings; (ii) studies where molecular techniques were employed to detect the presence of ESBL genes (blaCTX-M, blaSHV and blaTEM); and (iii) studies where the effect size for the association between ESBL-E infection and the outcomes of interest was available for extraction or could be estimated from the data provided in the study. The following exclusion criteria were applied: (i) study where there was no comparator group (i.e. only ESBL-E infected patients included in sample), or comparator was not non-ESBL-E; (ii) study where children younger than 16 years old were included; (iii) descriptive epidemiological studies (i.e. case reports and case series); (iv) publication without primary data (e.g. reviews, commentaries, editorials); (v) grey literature and conference abstracts/proceedings; (vi) non-human studies; and (vii) study that ascertained ESBL production with phenotypic tests only. Based on these inclusion and exclusion criteria, abstract and full-text screenings were performed by two reviewers (W.L., Y.E.) independently and any discrepancy was resolved at the end of each screening session. In cases of overlap where multiple studies reported the same group of participants, the study with a larger sample size was selected.
Data extraction
Data extracted from the included full-text articles include authors, year of publication, country, total sample size, study enrolment period, study setting, type of specimen collected, site of infection and characteristics of patients, such as mean or median age, Charlson comorbidity index, APACHE score, gender distribution, proportion of appropriate antibiotic therapy and associated study definition. Additionally, outcomes of interest that were measured, period of follow-up for mortality, bacteria species, adjusted effect sizes, the standard error and adjusted confounders were collected. If the adjusted estimates were not available, unadjusted estimates were extracted or estimated as odds ratio from the reported data in the study. All data were recorded in a pre-designed database from Microsoft Excel. To assess the risk of bias, a modified Newcastle-Ottawa quality assessment scale (NOS)28 for cohort studies similar to that developed by Rottier et al.18 was adapted (Table S2). In general, a study could score 0 to 4 stars for selection, 0 to 2 stars for comparability and 0 to 3 stars for outcome level.
Data analysis
Pooled estimates were calculated using the inverse variance heterogeneity (IVhet) model.29 Estimates for the main outcomes derived from the random effects model were also reported for comparison purposes in the Supplementary data. The heterogeneity of studies was assessed using the I2 index.30 I2 value less than 25% was considered low, between 25% and 50% as moderate and above 50% as high heterogeneity. Subgroup analyses were conducted based on (i) bacteria species to determine differences in pathogenicity among species; (ii) by median year of enrolment (i.e. before 2010 versus after 2010) since prevalence of circulating strains differed in the last two decades; (iii) geographical region where patients were enrolled to examine if adverse outcomes were more likely in regions of high endemicity; and (iv) day of mortality ascertainment (i.e. 14–21 days versus 28–30 days) to determine differences in mortality over days from onset of infection. The analysis for each association of interest was performed only if there were at least three studies available for synthesis.
Sensitivity analyses were performed by restricting the analysis to only bacteraemic patients, cancer patients or studies reporting adjusted risk estimates. Publication bias was visually inspected using the Doi plots and quantitatively assessed using the LFK index.31
All data analysis was performed using the admetan32 and lfk33 modules in Stata/SE 16.1 (College Station, TX, USA).
Results
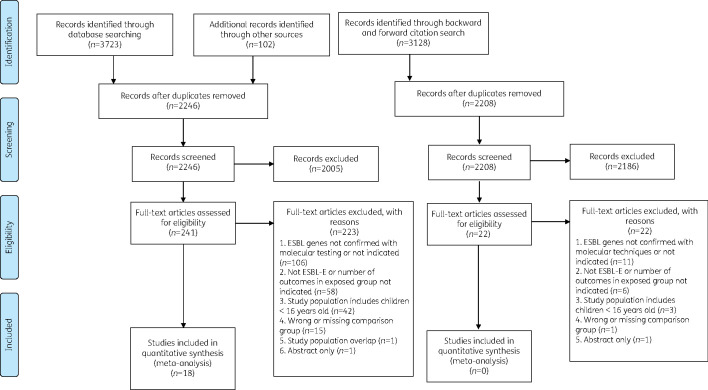
The database search and manual screening of existing systematic reviews identified 2246 unique references. After abstract screening was completed, 241 articles were assessed for eligibility by full text. The predominant reason for study rejection was because the presence of ESBL genes was not ascertained using molecular identification techniques. After full-text screening was completed, 18 studies met the inclusion criteria. The backward-forward citation search of included articles did not yield additional references. The screening process and detailed reasons for exclusion were reported in Figure 1.22 These 18 studies amounted to 1399 ESBL-E and 3200 non-ESBL-E infected patients.34–51
Figure 1.
PRISMA flow diagram for study selection.
A summary of study characteristics can be found in Table 1. A total of 17 studies reported on all causes of mortality, 7 studies septic shock and only one study bacteraemia. The year of study enrolment ranged from 1997 to 2017. In general, 10 studies were conducted in Asia and 8 were in Europe. Sixteen studies included only bacteraemic patients while the other two studies were inclusive of non-bacteraemic patients or individuals with ESBL-E faecal colonization. The most frequent bacterial species isolated was E. coli, followed by three studies on P. mirabilis and two studies each on K. pneumoniae and Enterobacter cloacae. Appropriate initial antibiotic therapy, as defined by individual study, was consistently less frequent in the ESBL-producing group (ranging from 35% to 63%) as compared with the non-ESBL producing group (ranging from 81% to 98%). Conversely, APACHE score was consistently higher in ESBL-E infected patients (ranging from 10 to 19) than the non-ESBL-E infected patients (ranging from 8 to 18). CTX-M was the most commonly detected ESBL type in all but three studies.
Table 1.
Summary detail of 18 ESBL-E studies
| Ref. | First author | Country | Study period | Study population and organism | Outcomes | Infection sitea | ESBL-E group: non-ESBL-E group |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | mean/ median age (years) | % male | mean/ median CCI | mean/ median APACHE | % AIAT | |||||||
| 34 | Tumbarello | Italy | Jan 1999–Dec 2003 | Inpatients with BSI caused by K. pneumoniae | Shock, 21 day mortality | BSI | 48:99 | 64:58 | 64:73 | 3.68:3.07 | 19:16 | 50:98 |
| 35 | Apisarnthanarak | Thailand | Jul 2003– Jun 2004 | Inpatients with community-onset E. coli infection | Mortality | UTI, BSI, pneumonia | 46:46 | 64:63 | 29:28 | — | 10:8 | 63:96 |
| 44 | Gudiol | Spain | Jan 2006–Oct 2008 | Inpatients with cancer and HSCT and BSI caused by E. coli | Shock, 30 day mortality | BSI | 17:118 | 61.5:59.1 | 29:63 | — | — | 35:94 |
| 45 | Tumbarello | Italy | Jan 2006–Dec 2006 | Inpatients with BSI caused by E. coli | Shock, 21 day mortality | BSI | 37:97 | 59:63 | 49:48 | 2.28:2.35 | — | 46:93 |
| 46 | Lee | Taiwan | 2001–08 | Patients with BSI caused by E. cloacae | 28 day mortality | BSI | 121:85 | 63.1:62.9 | 56:64 | — | — | — |
| 47 | Kurihara | Japan | 2001–10 | Patients with BSI caused by P. mirabilis | Shock, 30 day mortality | BSI | 13:51 | 69:78 | 69:47 | — | — | 54:88 |
| 48 | Ha | South Korea | Jan 2010–May 2012 | Patients with cancer and BSI caused by E. coli | Shock, 30 day mortalityb | BSI | 95:255 | 55.9:59.6 | 58:54 | 4:6 | — | 53:95 |
| 49 | Gürntke | Germany | Jan 2008–Dec 2011 | Patients with BSI caused by K pneumoniae | Mortalityc | BSI | 66:286 | — | 71:64 | 6:6 | — | — |
| 50 | Denis | France | Jan 2005–Dec 2008 | Inpatients with BSI caused by E. coli | 30 day mortalityd | BSI | 41:41 | 54:56 | 66:68 | — | 18:18 | 48:85 |
| 51 | Cornejo-Juárez | Mexico | Nov 2012–Jan 2014 | Inpatients with haematological malignancy, without history of chemotherapy, without antimicrobial in last 30 days, with E. coli intestinal colonization | BSI | Colonized | 63:63 | 40.4:43 | 60:64 | — | — | — |
| 36 | Ahn | South Korea | Nov 2005–Dec 2013 | Inpatients with BSI caused by P. mirabilis | Shock, 28 day mortalitye | BSI | 14:48 | 74.5:71 | 64:38 | 2:2 | 13.5:12 | 57:81 |
| 37 | Komatsu | Japan | Jan 2008–May 2013 | Patients with BSI caused by E. coli | 14 day mortality | BSI | 30:85 | 66.5:72.3 | 63:44 | 4.2:2.7 | — | — |
| 38 | Yoon | South Korea | May 2016–Apr 2017 | Patients with BSI caused by E. coli | 30 day mortality | BSI | 463:1029 | — | — | — | — | — |
| 39 | Zhang | China | Jan 2013–Sep 2017 | Inpatients with cancer and BSI caused by E. coli | 30 day mortality | BSI | 160:164 | 61:62 | 46:50 | — | — | — |
| 40 | de Lastours | France | Oct 2016–Jul 2017 | Patients with BSI caused by E. coli | 28 day mortality | BSI | 86:459 | — | — | — | — | — |
| 41 | Nham | South Korea | 2010–12 | Patients with cancer and BSI caused by K. pneumoniae | 30 day mortality | BSI | 50:228 | 55.8:57.7 | 52:61 | 5.16:4.96 | — | 48:97 |
| 42 | Lee | Taiwan | May 2008–Aug 2012 | Patients with BSI caused by E. cloacae and treated with adequate doses of cefepime | 30 day mortalityf | BSI | 40:32 | — | — | — | — | — |
| 43 | Endimiani | Italy | Jan 1997–Jun 2004 | Patients with BSI caused by P. mirabilis | Shock, 60 day mortality | BSI | 9:14 | 70.9:67.3 | 78:50 | 4:4.1 | — | 56:86 |
AIAT, appropriate initial antibiotic therapy; BSI, bloodstream infection; CCI, Charlson comorbidity index; Ref., reference; UTI, urinary tract infection.
Three most prevalent sites of infection in sample.
Adjusted for septic shock, mechanical ventilation, CCI, urinary tract, respiratory tract or intra-abdominal infection as source of bacteraemia.
Adjusted for CCI.
Adjusted for APACHE.
Adjusted for previous antibiotic use, SOFA score.
Adjusted for Pitt bacteraemia score ≥4, rapidly fatal underlying diseases, high-dose cefepime regimen, cefepime susceptible dose dependent isolate.
Using the modified NOS, the risk of bias in 17 studies were assessed with mortality as the outcome and 1 study was assessed with bacteraemia as the outcome. The quality score for selection ranged from 2 to 4. Fifteen of the studies scored the full three stars for outcome ascertainment. However, all but two studies scored zero stars for the comparability component, suggesting very poor comparability among the studies (Table S3). Risk of bias was therefore present in these reported estimates.
All-cause mortality
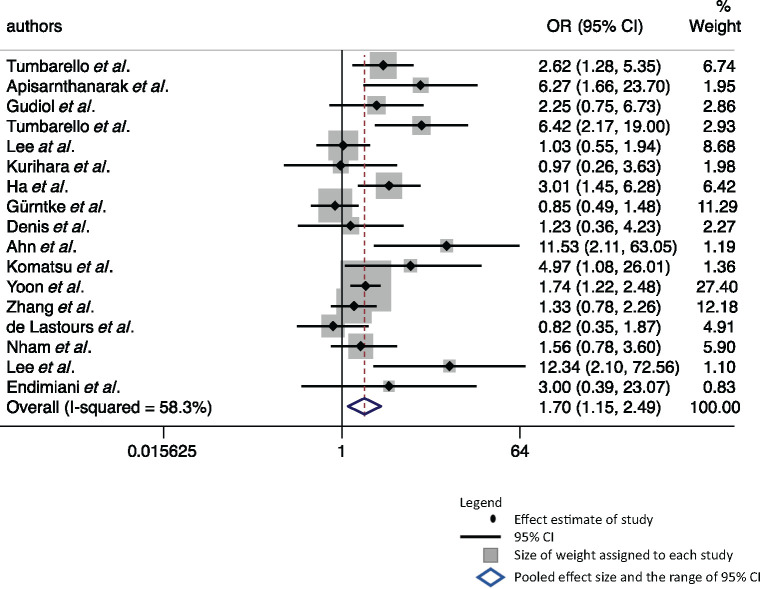
Seventeen studies were pooled to estimate the association between all-cause mortality and ESBL-E infection. The odds of mortality were 70% higher (OR = 1.70, 95% CI: 1.15–2.49) in the group with ESBL producers compared with the non-ESBL-producing counterparts (Figure 2). The heterogeneity of the studies was high (I2=58.3%, 95% CI: 29%–76%). Visualization of the Doi plot (LFK = 3.00) suggests presence of publication bias towards studies that reported ESBL-E infection as a risk factor of mortality (Figure S1).
Figure 2.
Forest plot of 17 studies estimating the association between ESBL-E infection and all-cause mortality. Weights are from Doi’s IVhet model.
Septic shock
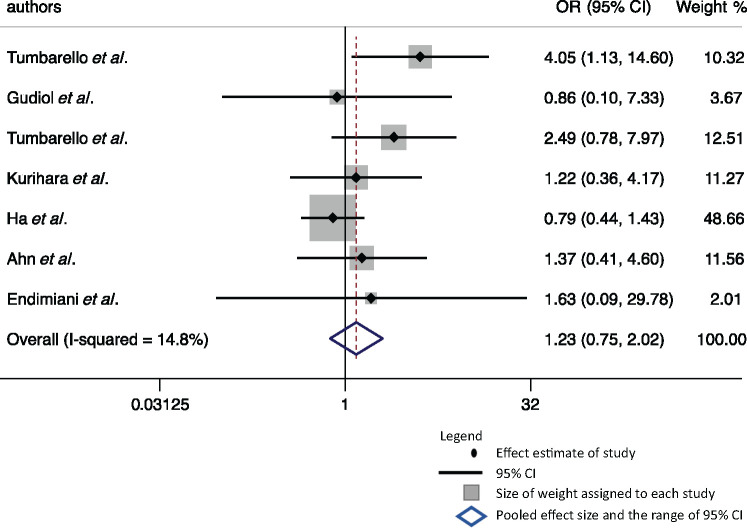
There were seven studies available with data on septic shock, and all included only bacteraemic patients. No significant association was observed between septic shock and ESBL-E infection (OR = 1.23, 95% CI: 0.75–2.02) (Figure 3). The heterogeneity of these studies was low (I2=14.8%, 95% CI: 0%–58%). LFK index was 3.22 and visualization of Doi plot suggests substantial presence of publication bias towards studies that reported ESBL-E infection as a risk factor for septic shock (Figure S2).
Figure 3.
Forest plot of seven studies estimating the association between ESBL-E infection and septic shock. Weights are from Doi’s IVhet model.
Bacteraemia
There was only one study with data available for bacteraemia. The author reported no significant difference in frequency of bacteraemia in ESBL-E and non-ESBL-E colonized patients (OR = 1.88, 95% CI: 0.85–4.13).51
Subgroup analyses
A summary of subgroup analyses can be found in Table 2. Since only two studies each of K. pneumoniae and P. mirabilis were found, these studies were combined with the three studies on E. cloacae for comparison with E. coli infections. There was no statistically significant difference in frequency of septic shock and mortality when comparing between the two bacterial groups. There was also no statistically significant difference in outcomes when comparing between Asia and Europe. Although subgroup analysis for septic shock was not possible by median year of enrolment, there was also no difference in mortality between studies conducted from 2010 to 2017, and studies from 1999 to 2009. Lastly, there was higher mortality in the 14 to 21 days group (OR = 3.60, 95% CI: 2.06–6.30) than in the 28 to 30 days from infection onset group (OR = 1.62, 95% CI: 1.10–2.38); however, the difference was not significantly different.
Table 2.
Subgroup analyses of summary effect sizes by type of bacteria, median year of enrolment, geographical region of study and day of mortality ascertainment
| Sepsis/shock |
Mortality |
|||||
|---|---|---|---|---|---|---|
| n studies (n patients) | OR (95% CI) | I2 (%) | n studies (n patients) | OR (95% CI) | I2 (%) | |
| Overall | 7 (915) | 1.23 (0.75–2.02) | 14.8 | 17 (4473) | 1.70 (1.15–2.49) | 58.3 |
| By bacterial species | ||||||
| E. coli | 3 (619) | 0.99 (0.45–2.22) | 32.7 | 10 (3621) | 1.65 (1.03–2.66) | 62.1 |
| K. pneumoniae, P. mirabilis or E. cloacae | 4 (296) | 1.83 (0.92–3.67) | 0 | 7 (852) | 1.83 (0.92–3.61) | 58.4 |
| By median year of enrolment | ||||||
| year 1999–2009 | 6 (565) | 1.87 (1.05–3.32) | 0 | 10 (1297) | 1.66 (0.90-3.05) | 64.9 |
| year 2010–17 | 1 (350) | 0.79 (0.44–1.43) | — | 7 (3176) | 1.72 (1.09–2.74) | 52.8 |
| By geographical region | ||||||
| Asia | 3 (547) | 0.93 (0.57–1.51) | 0 | 10 (3055) | 1.80 (1.11–2.92) | 57.6 |
| Europe | 4 (439) | 2.51 (1.16–5.43) | 0 | 7 (1418) | 1.50 (0.78–2.86) | 68.5 |
| By mortality day | ||||||
| 14 to 21 day | — | — | — | 3 (396) | 3.60 (2.06–6.30) | 0 |
| 28 to 30 day | 11 (3610) | 1.62 (1.10–2.38) | 48.2 | |||
Bold indicates statistical significance.
Sensitivity analyses
When restricting study selection to samples of bacteraemic or cancer patients, 16 and 4 studies were synthesized for mortality outcome, respectively. The odds of mortality remain significantly higher in bacteraemic (OR = 1.65, 95% CI: 1.14–2.41, I2=56.7%) and cancer patients (OR = 1.76, 95% CI: 1.20–2.59, I2=12.3%) with ESBL-E as compared with those with non-ESBL-E infections. On the other hand, sensitivity analyses in bacteraemic and cancer patients for septic shock as outcome was not possible because all seven studies with data on septic shock were conducted in bacteraemic patients and there were insufficient studies (n = 2) for sensitivity analysis in cancer patients.
Five studies with adjusted effect size of ESBL-E infection on mortality were available for synthesis.36,42,48–50 Of interest, only one study controlled for antibiotic therapy and two studies adjusted for underlying comorbidity as confounders.42,48,49 Significantly higher odds of mortality were not observed in patients with ESBL-E infection (aOR = 1.67, 95% CI: 0.52–5.39, I2=78.1%). Sensitivity analyses using adjusted effect estimates for outcomes other than mortality could not be performed, as there was no adjusted estimate available for septic shock and bacteraemia.
When repeating the analyses using the random effects model, the associations between mortality and ESBL-E were consistent, but overestimated the pooled estimates derived from the IVhet model (OR = 1.97, 95% CI: 1.42–2.74, I2=58.3%) (Figure S3). Associations between septic shock and ESBL-E were similarly overestimated but of no significance when using the random effects model (OR = 1.34, 95% CI: 0.83–2.16 I2=14.8%) (Figure S4).
Discussion
In this systematic review and meta-analysis, we observed higher odds of mortality in patients with ESBL-E infection as compared with patients with non-ESBL-E infection. However, when pooling study-adjusted risk estimates only, we observed no significant association between ESBL-E infection and mortality. No significant association between septic shock and ESBL-E infection was found. There was only one study with data on bacteraemia and meta-analysis could not be performed. There appeared to be no significant difference in frequency of mortality and septic shock from ESBL-E infections based on geographical regions of healthcare facilities (Asia versus Europe), bacterial species (E. coli versus K. pneumoniae, P. mirabilis and E. cloacae), year of enrolment (1999–2009 versus 2010–17) or day of mortality ascertainment from infection onset (14 to 21 days versus 28 to 31 days).
This systematic review and meta-analysis could not establish an association between septic shock or bacteraemia and ESBL-E infection due to the lack of existing literature. The seven studies meta-analysed for septic shock were not designed to investigate shock as an outcome or a mediator between ESBL-E infection and mortality. These studies were conducted retrospectively and most had no clear indication if septic shock was documented at presentation or after isolation of ESBL-E. Nonetheless, there appears to be no positive association between septic shock and ESBL-E infection, although this remains to be elucidated by future well-designed longitudinal studies. On the other hand, there was only one study in this review that studied the progression of ESBL-E faecal colonization to development of bacteraemia and no significant association was observed.51 There was 40% concordance rate between the colonizing and infecting ESBL-producing strain,51 comparable to another study that reported 30% concordance rate for MDR organism in general.52 Similarly, no significant difference in bacteraemia occurrences was observed when comparing between those with MDR organism and those with susceptible organism colonisation.52
The results observed in this systematic review and meta-analysis were largely consistent with the other systematic reviews estimating the association between ESBL-E infection and all-cause mortality. The review by Shamsrizi et al.19 reported an increased mortality risk of 70% in bacteraemic patients with ESBL-E infection (RR = 1.70, 95% CI: 1.52–1.90), comparable to an excess of 70% in odds of mortality in this review of bacteraemic patients. However, this review found no significant association between mortality and ESBL-E infection when pooling study-adjusted risk estimates only, in contrast to the previous reviews where significant association was reported after pooling estimates adjusted for inadequate or delayed antibiotic therapy.17–19 This may be because this meta-analysis only included five studies reporting adjusted estimates, one of which adjusted for sepsis as confounder rather than mediator. As sepsis can be regarded as lying on the causal pathway instead, the adjustment for sepsis without any mediation analysis might have underestimated the true association between ESBL-E infection and mortality. This was similarly reported as a limitation of studies in the review by Rottier et al.18
In addition, there could be a potentially bigger role of inappropriate antibiotic therapy and underlying comorbidity in poor prognosis of the infection, rather than pathogenicity associated with ESBL production. A large cohort study of patients with drug-resistant Gram-negative infections observed 20% increase in risk of mortality in those with delayed appropriate antibiotic therapy.53 This was similarly reported in another cohort of patients with sepsis caused by Gram-negative pathogens, where the odds of mortality were four times higher in those without appropriate initial antibiotic therapy.54 More broadly, a systematic review of 122 studies on patients with bacterial infections also reported 56% reduction in odds of mortality for those with appropriate antibiotic therapy.55 This evidence highlights the potential role of inappropriate antibiotic therapy for ESBL infection in poor clinical outcomes. This systematic review and meta-analysis had included only one study that controlled for type of antibiotic therapy and reported no significant association between ESBL-E infection and mortality. Besides, it was observed that all 18 studies reported a lower proportion of appropriate antibiotic therapy in patients with ESBL-E infection as compared with those with non-ESBL-E.
The effect sizes reported in this review should be of higher precision as compared with previous systematic reviews. Firstly, this review employed the IVhet model instead of the random effects model performed in previous reviews. The IVhet model allows assignment of higher adjusted weight to studies with lower variance or larger sample sizes, and this is maintained even when heterogeneity of included studies is high, as was the case of our studies in this review. The superiority of IVhet model over the use of random effects model has been discussed in detail elsewhere.29,56 Therefore, the estimates reported here were more conservative and likely influenced by larger studies reporting more conservative estimates, as compared with results derived from the random effects model.
Secondly, the inclusion criterion of molecular technique for ascertainment of ESBL gene increased the precision of exposure classification. Sensitivity and specificity of phenotypic tests for ESBL are highly dependent on the type of test and the antibiotic agents used for screening. A study evaluating the use of Vitek 2 with extended cards only reported its highest sensitivity at 79% and sensitivity at 56%.57 Other phenotypic tests, such as double disc diffusion and Etest strip, generally had higher sensitivity only when more than one agent were used in combination.21,57 Of relevance to this systematic review and meta-analysis, one included study by Zhang et al.39 had observed only 92% of their phenotypically screened and confirmed ESBL-producing isolates to harbour an ESBL-related gene. This would have resulted in differential misclassification of 12 patients (out of 324) into the exposed group in their study and biased the study results accordingly. As observational studies are common when investigating the association between ESBL-E and mortality,19 such measurement error is of concern since it introduces bias in these studies that are already prone to confounding, and is especially significant when sample size is small. This emphasizes the potential significance of misclassification in studies that may have employed phenotypic tests with poor sensitivity and specificity for detection of ESBL production. This systematic review and meta-analysis attempted to eliminate this potential misclassification error by restricting inclusion to studies that performed molecular techniques for ESBL gene detection. Despite this, only six studies included here reported ESBL gene detection in the entirety of their exposed group. The reason for lack of molecular ascertainment of ESBL gene in some of the exposed groups was either due to unavailability of isolates for testing, negative PCR results or was unaccounted for (data not shown). This attributable risk of misclassification bias was accordingly reflected in the selection component of our quality assessment.
Lastly, this is the first systematic review and meta-analysis to investigate bacteraemia and sepsis or septic shock as an outcome of ESBL-E infection. These conditions are good predictors for increased risk of mortality. This review has shown gaps in current literature understanding the effect of ESBL-E infection on risk of sepsis, shock or bacteraemia. Research investigating the association between ESBL-E non-bloodstream infections and adverse clinical outcomes is especially lacking as well.
Despite this, this review has several limitations. As compared with the systematic review by Shamsrizi et al.,19 this review included a smaller number of studies largely due to the study criterion requiring molecular detection of ESBL genes in the Enterobacteriaceae strain. The smaller sample size returned a larger degree of uncertainty in the reported pooled effect size. However, the more stringent ascertainment of ESBL production with molecular techniques reduced misclassification error and hence, raised the overall validity of the results. There was also high variability in the type of confounders that were adjusted for in the five studies reporting adjusted risk estimates. Moreover, one study adjusted for septic shock as a potential confounder of the association between ESBL-E infection and mortality. As sepsis or septic shock can be regarded as a mediator, this adjustment would have underestimated the true association between ESBL-E and mortality. As reflected in the poor comparability score from the risk of bias assessment and high heterogeneity among the studies, the pooled estimates here may be highly confounded and should be interpreted with caution. In addition, some degree of publication bias towards studies reporting ESBL-E infection as risk factor for mortality was noted. On the other hand, some studies did not include ESBL infection in the multivariable model since statistical significance was not reached in the univariate model, suggesting also potential publication bias in favour of studies reporting statistically significant results. This review also did not collect effect sizes based on appropriateness of antibiotic therapy and as such, subgroup analysis for this factor was not performed. Lastly, since the majority of studies included only bacteraemic patients, the results from this review have limited generalizability to non-bacteraemic patients. As such, future studies elucidating the prognosis of non-bloodstream ESBL-E infections are warranted to more accurately ascertain the effects of ESBL production on clinical outcomes. This is of clinical significance since there is growing evidence indicating the pathogenic burden of ESBL-E in community-onset and community-acquired urinary tract infections.3,5
Conclusions
In conclusion, this systematic review and meta-analysis reported significantly higher odds of all-cause mortality in patients with ESBL-E infection as compared with those with non-ESBL-E infection. However, this was not observed when pooling study-adjusted risk estimates. No association between ESBL-E infection and septic shock was reported, although most studies were not robustly designed to investigate shock as an outcome or mediator between ESBL-E infection and mortality. There were insufficient data to investigate the association between ESBL-E infection or colonization and bacteraemia. The increase in mortality observed in patients with ESBL-E infection may be attributable to inappropriate antimicrobial therapy or underlying comorbidities, rather than virulence potential that may be associated with ESBL production. This remains to be elucidated by future studies investigating the relationship of drug-resistant and virulent molecular characteristics in ESBL-E strains with clinical outcomes. Appropriate adjustment of confounders will also allow better effect estimation of clinical outcomes attributable to ESBL-E. With ESBL-E being increasingly detected as the infecting pathogen in community-associated urinary tract infections, studies investigating clinical outcomes in non-bacteraemic patients are also lacking and should be highly considered.
Supplementary Material
Acknowledgements
We would like to acknowledge and thank librarian Lars Eriksson for his help in building the search strategy.
Funding
This work was supported by funding to the authors as follows. W.L. and Y.E. receive scholarship support from University of Queensland for Doctor of Philosophy candidature. L.F.-K. was supported by Australian National Health and Medical Research Council Early Career Fellowships (APP1158469). P.N.A.H. receives support from NHMRC Early Career Fellowship (GNT1157530).
Transparency declarations
D.L.P. has received research grants from Merck, Pfizer and Shionogi outside of the submitted work. He has also received personal fees from Merck, Pfizer, Shionogi, Lysovant, The Medicines Company, Entasis, Venatorx, BioMérieux and Accelerate. P.N.A.H. has received research grants from Merck, Sharp & Dohme (MSD), Sandoz and Shionogi Ltd, outside of the submitted work, as well as personal fees from Pfizer and Sandoz. All other authors: none to declare.
Author contributions
D.L.P., P.N.A.H. and W.L. conceived the aim of this systematic review and meta-analysis. W.L. performed the database search. W.L. and Y.E. were responsible for study screening and selection. W.L. did the data extraction and quality assessment. W.L. and L.F.-K. performed and checked the statistical analysis. W.L. drafted the manuscript. All authors reviewed and provided inputs for the manuscript. All authors approved the final version of the manuscript.
Supplementary data
Tables S1 to S3, Figures S1 to S4 and the search strategies are available as Supplementary data at JAC-AMR Online.
References
- 1.Pitout JD, Laupland KB.. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 2008; 8: 159–66. [DOI] [PubMed] [Google Scholar]
- 2.Paterson DL, Bonomo RA.. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev 2005; 18: 657–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doi Y, Park YS, Rivera JI. et al. Community-associated extended-spectrum -lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis 2013; 56: 641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woerther PL, Burdet C, Chachaty E. et al. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: Toward the globalization of CTX-M. Clin Microbiol Rev 2013; 26: 744–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers BA, Sidjabat HE, Paterson DL.. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J Antimicrob Chemother 2011; 66: 1–14. [DOI] [PubMed] [Google Scholar]
- 6.Price LB, Johnson JR, Aziz M. et al. The epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. mBio 2013; 4: e00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petty NK, Zakour NLB, Stanton-Cook M. et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci USA 2014; 111: 5694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezabih YM, Sabiiti W, Alamneh E. et al. The global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli in the community. J Antimicrob Chemother 2021; 76: 22–9. [DOI] [PubMed] [Google Scholar]
- 9.Gülmez D, Woodford N, Palepou MFI. et al. Carbapenem-resistant Escherichia coli and Klebsiella pneumoniae isolates from Turkey with OXA-48-like carbapenemases and outer membrane protein loss. Int J Antimicrob Agents 2008; 31: 523–6. [DOI] [PubMed] [Google Scholar]
- 10.Baroud M, Dandache I, Araj GF. et al. Underlying mechanisms of carbapenem resistance in extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli isolates at a tertiary care centre in Lebanon: Role of OXA-48 and NDM-1 carbapenemases. Int J Antimicrob Agents 2013; 41: 75–9. [DOI] [PubMed] [Google Scholar]
- 11.CDC 2019. Antibiotic Resistance Threats in the United States, 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf.
- 12.Ramphal R, Ambrose PG.. Extended-spectrum β-lactamases and clinical outcomes: current data. Clin Infect Dis 2006; 42 Suppl 4: S164–72. [DOI] [PubMed] [Google Scholar]
- 13.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 2006; 42: S82–9. [DOI] [PubMed] [Google Scholar]
- 14.Gharrah MM, Mostafa El-Mahdy A, Barwa RF.. Association between virulence factors and extended spectrum β-lactamase producing Klebsiella pneumoniae compared to nonproducing isolates. Interdiscip Perspect Infect Dis 2017; 2017: 7279830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin H-A, Huang Y-L, Yeh K-M. et al. Regulator of the mucoid phenotype A gene increases the virulent ability of extended-spectrum β-lactamase-producing serotype non-K1/K2 Klebsiella pneumonia. J Microbiol Immunol Infect 2016; 49: 494–501. [DOI] [PubMed] [Google Scholar]
- 16.Sahly H, Navon-Venezia S, Roesler L. et al. Extended-spectrum β-lactamase production is associated with an increase in cell invasion and expression of fimbrial adhesins in Klebsiella pneumoniae. Antimicrob Agents Chemother 2008; 52: 3029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwaber MJ, Carmeli Y.. Mortality and delay in effective therapy associated with extended-spectrum β-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 2007; 60: 913–20. [DOI] [PubMed] [Google Scholar]
- 18.Rottier WC, Ammerlaan HSM, Bonten MJM.. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β-lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother 2012; 67: 1311–20. [DOI] [PubMed] [Google Scholar]
- 19.Shamsrizi P, Gladstone BP, Carrara E. et al. Variation of effect estimates in the analysis of mortality and length of hospital stay in patients with infections caused by bacteria-producing extended-spectrum β-lactamases: a systematic review and meta-analysis. BMJ Open 2020; 10: 30266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang K, Guglielmo BJ.. Diagnosis and treatment of extended-spectrum and AmpC β-lactamase- producing organisms. Ann Pharmacother 2007; 41: 1427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willems E, Cartuyvels R, Magerman K. et al. Comparison of different phenotypic assays for the detection of extended-spectrum β-lactamase production by inducible AmpC-producing Gram-negative bacilli. Eur J Clin Microbiol Infect Dis 2013; 32: 549–55. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falagas ME, Tansarli GS, Karageorgopoulos DE. et al. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis 2014; 20: 1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin A, Fahrbach K, Zhao Q. et al. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to Enterobacteriaceae: Results of a systematic literature review and meta-analysis. Open Forum Infect Dis 2018; 5: ofy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu L, Sun X, Ma X.. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob 2017; 16: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohler PP, Volling C, Green K. et al. Carbapenem resistance, initial antibiotic therapy, and mortality in Klebsiella pneumoniae bacteremia: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2017; 38: 1319–28. [DOI] [PubMed] [Google Scholar]
- 27.Ouzzani M, Hammady H, Fedorowicz Z. et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells GA, Shea B, O’Connell D. et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf.
- 29.Doi SAR, Barendregt JJ, Khan S. et al. Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials 2015; 45: 130–8. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG, Deeks JJ. et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuya-Kanamori L, Barendregt JJ, Doi SAR.. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc 2018; 16: 195–203. [DOI] [PubMed] [Google Scholar]
- 32.Fisher D. admetan: A New, Comprehensive Meta-Analysis Command. Stata Users Group, 2018. [Google Scholar]
- 33.Furuya-Kanamori L, Doi SAR.. LFK: Stata Module to Compute LFK Index and Doi Plot for Detection of Publication Bias in Meta-Analysis. Statistical Software Components S458762. Boston College Department of Economics, 2020. [Google Scholar]
- 34.Tumbarello M, Spanu T, Sanguinetti M. et al. Bloodstream infections caused by extended-spectrum-β-lactamase- producing Klebsiella pneumoniae: Risk factors, molecular epidemiology, and clinical outcome. Antimicrob Agents Chemother 2006; 50: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apisarnthanarak A, Kiratisin P, Saifon P. et al. Clinical and molecular epidemiology of community-onset, extended-spectrum β-lactamase-producing Escherichia coli infections in Thailand: A case-case-control study. Am J Infect Control 2007; 35: 606–12. [DOI] [PubMed] [Google Scholar]
- 36.Ahn JY, Ann HW, Jeon Y. et al. The impact of production of extended-spectrum β-lactamases on the 28-day mortality rate of patients with Proteus mirabilis bacteremia in Korea. BMC Infect Dis 2017; 17: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komatsu Y, Kasahara K, Inoue T. et al. Molecular epidemiology and clinical features of extended-spectrum β-lactamase- or carbapenemase-producing Escherichia coli bacteremia in Japan. PLoS One 2018; 13: e0202276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon EJ, Choi MH, Park YS. et al. Impact of host-pathogen-treatment tripartite components on early mortality of patients with Escherichia coli bloodstream infection: prospective observational study. EBioMedicine 2018; 35: 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Q, Gao HY, Li D. et al. Clinical outcome of Escherichia coli bloodstream infection in cancer patients with/without biofilm formation: a single-center retrospective study. Infect Drug Resist 2019; 12: 359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Lastours V, Laouénan C, Royer G. et al. Mortality in Escherichia coli bloodstream infections: antibiotic resistance still does not make it. J Antimicrob Chemother 2020; 75: 2334–43. [DOI] [PubMed] [Google Scholar]
- 41.Nham E, Huh K, Cho SY. et al. Characteristics and clinical outcomes of extended-spectrum β-lactamase-producing Klebsiella pneumoniae bacteremia in cancer patients. Infect Chemother 2020; 52: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee NY, Lee CC, Li CW. et al. Cefepime therapy for monomicrobial Enterobacter cloacae bacteremia: unfavorable outcomes in patients infected by cefepime-susceptible dose-dependent isolates. Antimicrob Agents Chemother 2015; 59: 7558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endimiani A, Luzzaro F, Brigante G. et al. Proteus mirabilis bloodstream infections: risk factors and treatment outcome related to the expression of extended-spectrum β-lactamases. Antimicrob Agents Chemother 2005; 49: 2598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gudiol C, Calatayud L, Garcia-Vidal C. et al. Bacteraemia due to extended-spectrum β-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother 2010; 65: 333–41. [DOI] [PubMed] [Google Scholar]
- 45.Tumbarello M, Spanu T, Di Bidino R. et al. Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-β-lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother 2010; 54: 4085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee CC, Lee NY, Yan JJ. et al. Bacteremia due to extended-spectrum-β-lactamase-producing Enterobacter cloacae: role of carbapenem therapy. Antimicrob Agents Chemother 2010; 54: 3551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurihara Y, Hitomi S, Oishi T. et al. Characteristics of bacteremia caused by extended-spectrum β-lactamase-producing Proteus mirabilis. J Infect Chemother 2013; 19: 799–805. [DOI] [PubMed] [Google Scholar]
- 48.Ha YE, Kang CI, Cha MK. et al. Epidemiology and clinical outcomes of bloodstream infections caused by extended-spectrum β-lactamase-producing Escherichia coli in patients with cancer. Int J Antimicrob Agents 2013; 42: 403–9. [DOI] [PubMed] [Google Scholar]
- 49.Gürntke S, Kohler C, Steinmetz I. et al. Molecular epidemiology of extended-spectrum β-lactamase (ESBL)-positive Klebsiella pneumoniae from bloodstream infections and risk factors for mortality. J Infect Chemother 2014; 20: 817–9. [DOI] [PubMed] [Google Scholar]
- 50.Denis B, Lafaurie M, Donay JL. et al. Prevalence, risk factors, and impact on clinical outcome of extended-spectrum β-lactamase-producing Escherichia coli bacteraemia: A five-year study. Int J Infect Dis 2015; 39: 1–6. [DOI] [PubMed] [Google Scholar]
- 51.Cornejo-Juárez P, Suárez-Cuenca JA, Volkow-Fernández P. et al. Fecal ESBL Escherichia coli carriage as a risk factor for bacteremia in patients with hematological malignancies. Support Care Cancer 2016; 24: 253–9. [DOI] [PubMed] [Google Scholar]
- 52.Mascitti H, Duran C, Nemo EM. et al. Factors associated with bacteraemia due to multidrug-resistant organisms among bacteraemic patients with multidrug-resistant organism carriage: a case control study. Antimicrob Resist Infect Control 2018; 7: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonine NG, Berger A, Altincatal A. et al. Impact of delayed appropriate antibiotic therapy on patient outcomes by antibiotic resistance status from serious Gram-negative bacterial infections. Am J Med Sci 2019; 357: 103–10. [DOI] [PubMed] [Google Scholar]
- 54.Zilberberg MD, Shorr AF, Micek ST. et al. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care 2014; 18: 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bassetti M, Rello J, Blasi F. et al. A systematic review on the impact of appropriate versus inappropriate initial antibiotic therapy on the outcomes of patients with severe bacterial infections. Int J Antimicrob Agents 2020; 56: 106184. [DOI] [PubMed] [Google Scholar]
- 56.Doi SAR, Thalib L.. A quality-effects model for meta-analysis. Epidemiology 2008; 19: 94–100. [DOI] [PubMed] [Google Scholar]
- 57.Garrec H, Drieux-Rouzet L, Golmard JL. et al. Comparison of nine phenotypic methods for detection of extended-spectrum β-lactamase production by Enterobacteriaceae. J Clin Microbiol 2011; 49: 1048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.