Abstract
Background
This is an update of a previous review. Case reports and case series have described dramatic responses to intravenous immunoglobulin (IVIG) in people with presumed viral myocarditis, and its administration has become commonplace.
Objectives
The primary objective of this review was to compare event‐free (death, requirement for a cardiac transplant, or placement of a left ventricular assist device) or overall (death) survival of adults and children with presumed viral myocarditis treated with IVIG versus those who did not receive IVIG. A secondary objective was to determine if a group of patients with presumed viral myocarditis could be identified (on the basis of age, duration of symptoms, acuity of onset of symptoms, cardiac function at presentation, virological results, or the presence or absence of histological evidence of acute myocarditis on cardiac biopsy in patients in whom a biopsy was performed) who would be the most likely to benefit from IVIG.
Search methods
We searched CENTRAL, MEDLINE, Embase, DARE, CINAHL, Web of Science Core Collection, and LILACS in July 2019, and two trial registries in November 2019. We contacted authors of trials and checked reference lists of relevant papers. We applied no language restrictions.
Selection criteria
We included studies if (1) participants had a clinical diagnosis of acute myocarditis with a left ventricular ejection fraction (LVEF) ≤ 0.45, left ventricular end‐diastolic diameter (LVEDD) > 2 standard deviations (SDs) above the norm, or a left ventricular shortening fraction (LVSF) > 2 SDs below the mean, with duration of cardiac symptoms < 6 months; (2) participants had no evidence of non‐infectious or bacterial cardiac disease; and (3) participants were randomly assigned to receive at least 1 g/kg of IVIG versus no IVIG or placebo.
We excluded studies if (1) participants had received immunosuppression before outcome assessment; or (2) onset of myocarditis was reported to have occurred < 6 months postpartum.
Data collection and analysis
Two review authors independently screened the search results and extracted data. We assessed risk of bias with the Cochrane 'Risk of bias' tool. We conducted meta‐analysis for two outcomes (overall survival and improvement in LVEF) with two adult trials. Other meta‐analyses were not possible because only three relevant trials were included, and researchers analysed markedly different populations and used different outcome measures.
Main results
In this update we added two trials to the two previously included trials. A quasi‐randomised trial was previously included due to a paucity of evidence from randomised trials; however, with the addition of two new randomised trials, it was removed from this update.
For two adult trials, the overall risk of bias was unclear with very low‐certainty evidence for all outcomes. The first trial studied 62 adults with recent‐onset dilated cardiomyopathy randomly assigned to receive IVIG or an equivalent volume of 0.1% albumin in a blinded fashion. The effect on event‐free survival between groups was uncertain (risk ratio (RR) of any event 1.76, 95% confidence interval (CI) 0.48 to 6.40). The second trial studied 41 adults with acute myocarditis randomised to either high‐dose IVIG (1 to 2 g/kg over two days) or no treatment. The IVIG group reported greater survival time after 60 days (no raw data, P < 0.01), but the evidence is uncertain. We pooled the reported number of deaths in both trials, with no evidence of a difference between groups (RR 0.91, 95% CI 0.23 to 3.62, I2 = 31%, very low‐certainty evidence).
The evidence on the effect of IVIG treatment on LVEF (pooled mean difference (MD) −0.01, 95% CI −0.06 to 0.05) after 12 months and an unknown time frame is uncertain. The results for functional capacity, assessed by peak oxygen consumption at 12 months, were uncertain (MD −0.80, 95% CI −4.57 to 2.97). The results for infusion‐related side effects were also uncertain due to a very large CI (RR 20.29, 95% CI 1.25 to 329.93). Lastly, there was uncertain evidence addressing failure to attain complete recovery (RR 0.46, 95% CI 0.19 to 1.14). Evidence for improvement in LVEDD, left ventricular shortening fraction, and hospitalisation status in adults was not reported.
In the single included paediatric trial, the overall risk of bias was low with very low‐certainty evidence for all outcomes. The trial included 86 children in Egypt presenting with acute myocarditis. Children were randomly assigned to 1 g/kg IVIG daily for two consecutive days or placebo followed by echocardiography one and six months post randomisation for recording of LVEDD and LVSF. The evidence for overall survival after six months was uncertain (risk of death RR 0.48, 95% CI 0.20 to 1.15). The evidence was also uncertain for improvement in LVEDD and LVSF after six months (LVEDD MD −4.00, 95% CI −9.52 to 1.52; LVSF no raw data). Evidence for improvement in LVEF, functional capacity, side effects, complete recovery, and hospitalisation status in children was not reported.
Authors' conclusions
Evidence from two trials of very low certainty and with unclear risk of bias provides contradictory evidence on the use of IVIG in the treatment of adults with presumed viral myocarditis. One trial reported that use of IVIG results in longer survival time after 60 days, whilst the other trial found that IVIG does not provide an appreciable benefit. The evidence of a difference in event‐free or overall survival, LVEDD, or LVSF is of very low certainty in a single paediatric trial with a low risk of bias. Until higher‐quality studies with low risk of bias and larger sample sizes have demonstrated benefit in a particular group of patients, the evidence for treatment with IVIG for presumed viral myocarditis is uncertain. Further studies of the pathophysiology of myocarditis would lead to improved diagnostic criteria, which would facilitate future research.
Plain language summary
Intravenous immunoglobulin for presumed viral myocarditis in children and adults
Background
Acute myocarditis is inflammation of the heart and is thought to most commonly begin as a viral infection. The disease affects individuals of all ages. On the basis of multiple case reports and case series, intravenous immunoglobulin (IVIG) has become part of routine practice for treating adults and children with acute myocarditis at many centres.
Results
This is the second update of a previous review, which found one randomised controlled trial (RCT) (a type of study in which participants are assigned to one of two or more treatment groups using a random method) of 62 adults, which suggested that IVIG is not useful in myocarditis. The evidence in this update is current to 2 July 2019, at which point two studies were added: one RCT of 86 children did not find any evidence that IVIG increases survival over placebo, and one RCT of 41 adults did not find evidence of increased survival (less mortality), but did report that patients lived longer for the group treated with IVIG compared to the untreated group. After pooling the available data, there was uncertain evidence of the effect of IVIG in preventing deaths. More RCT evidence is required before IVIG can be routinely recommended for adults or children with myocarditis.
Summary of findings
Background
Description of the condition
Acute myocarditis is a disease that occurs in individuals of all ages. It is presumed to usually start as a viral infection, although autoimmune and idiopathic forms also occur. It remains unclear whether the primary problem is most commonly ongoing damage from a virus, a postinfectious inflammatory reaction, or a combination of the two.
One problem involved in analysing the literature on the treatment of patients with acute myocarditis is that the enrolment criteria for these studies are far from uniform. Reasons for this include the following.
No reference standard has been accepted for the diagnosis of acute myocarditis. Detection of pathogens from cardiac tissue would represent evidence in favour of infectious myocarditis. This seldom occurs, presumably because the concentration of pathogens is typically very low by the time a biopsy is performed. The yield is improved by molecular techniques (Guglin 2012). However, it is not clear whether detection of latent viruses such as cytomegalovirus in cardiac tissue is always indicative of myocarditis. It has been suggested that one way to diagnose acute myocarditis is to perform cardiac biopsies at a minimum of five sites to look for histology fulfilling the Dallas criteria. These criteria require evidence of lymphocytic infiltration and myocyte necrosis with or without degeneration (Towbin 2001). However, studies have shown that biopsies on about half of adults with acute myocarditis at autopsy did not fulfil the Dallas criteria (Towbin 2001). The reason for this is that inflammation can be patchy or transient, and can progress to fibrosis (which would not be interpreted as acute myocarditis) (Levi 2001). Consequently, many studies include patients who do not fulfil the Dallas criteria for acute myocarditis. Alternative World Heart Federation criteria require a diffuse, focal or confluent infiltrate of ≥ 14 leucocytes/mm2 (primarily lymphocytes with up to 4 macrophages/mm2 permitted) (Maisch 2002; Maisch 2013; Meyer 1997). Progress has been reported in the use of diagnostic imaging for diagnosis of acute myocarditis. The 'Lake Louise criteria' can be applied to cardiac magnetic resonance imaging (MRI) for the diagnosis of acute myocarditis (Friedrich 2009). As with an endomyocardial biopsy, the sensitivity of cardiac MRI is greatest if performed early in the course of myocarditis. MRI cannot distinguish viral from other forms of acute myocarditis, but MRI‐guided biopsies have a higher yield than blinded biopsies (Guglin 2012).
The nomenclature of myocarditis is not yet standardised. For example, the term 'acute myocarditis' is used by some study authors to refer to all cases of active myocarditis (Fuse 2000), whereas other study authors use the term only for disease of indistinct onset, using the term 'fulminant' for cases with a distinct onset and clear evidence of a recent viral illness (Hare 2001). Some study authors use the term 'acute myocarditis' for a presumed infectious process, but others include non‐infectious entities. Some experts believe that infectious myocarditis progresses from a phase where viral infection dominates to a phase where autoimmunity dominates (Liu 2001). If viral replication or cytokine production persists, the patient develops a dilated cardiomyopathy (Liu 2001). If viral replication and cytokine production cease, the patient spontaneously recovers. An alternate viewpoint is that the disease begins as rapidly progressive, acute, or chronic myocarditis, and that these three presentations are not part of a continuum (Fenoglio 1983). Because no agreement has been reached on the natural history of acute myocarditis, and no uniform classification scheme has been devised, it is not possible for studies to consistently report results of treatment for different types of myocarditis.
No consensus has been reached regarding which investigations must be done to exclude non‐infectious causes of acute myocardial dysfunction. In previously well paediatric patients, a clinical diagnosis of infectious myocarditis is fairly accurate, although congenital cardiomyopathy can present acutely. In adults, ischaemic heart disease is commonly confused with viral myocarditis. Other causes of acute cardiac dysfunction include drug‐induced dysfunction (from alcohol, organic solvents, cocaine, or chemotherapeutic or cardiac agents), collagen vascular disease, and postpartum cardiomyopathy.
It is not clear how acute myocarditis in children differs from acute myocarditis in adults. However, one study showed that 17 of 18 adults with fulminant myocarditis survived (McCarthy 2000), whereas a paediatric study described survival in only two of nine infants with fulminant myocarditis (Mounts 2001).
Description of the intervention
Intravenous immunoglobulin (IVIG) is a pooled blood product that contains a mix of antibodies taken from the blood of healthy donors. It is a type of immunotherapy.
How the intervention might work
If ongoing infection is the primary problem, IVIG could be efficacious if it contains antibodies to the microbe. IVIG also has anti‐inflammatory properties, so it could be efficacious even if the primary problem is a postinfectious inflammatory reaction or a non‐infectious process.
Why it is important to do this review
Multiple case reports, Nigro 2001; Takeda 1998; Tedeschi 2002, and case series, Alrabate 2013; Drucker 1994; Goland 2008; Haque 2009, have described apparent dramatic responses to IVIG in adults and children with acute myocarditis, with one large case series suggesting no benefit irrespective of severity of illness (Klugman 2009). However, results from a randomised controlled trial showed no advantage in IVIG‐treated adults with recent‐onset dilated cardiomyopathy (or in the subgroup with histological evidence of acute myocarditis) (McNamara 2001).
Objectives
The primary objective of this review was to compare event‐free (death, requirement for a cardiac transplant, or placement of a left ventricular assist device) or overall (death) survival of adults and children with presumed viral myocarditis treated with IVIG versus those who did not receive IVIG. A secondary objective was to determine if a group of patients with presumed viral myocarditis could be identified (on the basis of age, duration of symptoms, acuity of onset of symptoms, cardiac function at presentation, virological results, or the presence or absence of histological evidence of acute myocarditis on cardiac biopsy in patients in whom a biopsy was performed) who would be the most likely to benefit from IVIG.
Methods
Criteria for considering studies for this review
Types of studies
This review included randomised controlled trials (RCTs) that compared study participants treated with IVIG versus participants who did not receive IVIG.
We did not include trials that compared IVIG versus immunosuppressive therapy, as tremendous variability has been noted in the type and dose of immunosuppressive drugs used and in the timing of administration of these drugs. However, if arms of a trial included an IVIG group and a placebo or no‐therapy group, participants from these arms were considered for inclusion in this review if possible.
Types of participants
Inclusion criteria
We included inpatients and outpatients of any age, sex, or race.
Participants had to have a clinical diagnosis of acute myocarditis and at least one of the following:
left ventricular ejection fraction (LVEF) ≤ 0.45;
left ventricular end‐diastolic diameter (LVEDD) > 2 standard deviations (SDs) above the norm as adjusted for body surface area;
left ventricular shortening fraction (LVSF) > 2 SDs less than the mean as adjusted for age, or < 29% in an adult.
The duration of cardiac symptoms before randomisation had to be < 6 months.
As a result of the poor sensitivity of cardiac biopsy as a diagnostic tool for acute myocarditis, and the fact that biopsies are seldom performed in children with suspected myocarditis, a histological diagnosis was not required.
Exclusion criteria
Participants could not have any evidence of non‐infectious or bacterial cardiac disease.
Studies that included participants who had received immunosuppression before the final assessment of outcome following IVIG/no IVIG were excluded, as the benefit of immunosuppression remains controversial.
As the pathogenesis of postpartum cardiomyopathy is likely to differ from that of other cases of acute myocarditis, participants were excluded if onset of myocarditis was reported to be less than six months postpartum.
Types of interventions
Standard therapy for myocarditis is supportive care. In addition to this, participants must have been randomly assigned to receive at least 1 g/kg of any standard formulation of IVIG versus either placebo or no additional therapy.
Types of outcome measures
We planned to analyse outcome measures using the longest follow‐up time reported for each study. We planned to analyse all outcome measures separately in the subgroup of participants who had cardiac biopsies that fulfilled the Dallas or World Heart Federation criteria for acute myocarditis (Aretz 1987; Maisch 1999; Maisch 2000).
Reporting one or more of the outcomes listed here was not an inclusion criterion for the review. Where a published report did not appear to report one of these outcomes, we accessed the trial protocol and attempted to contact the trial authors to ascertain whether the outcomes were measured but not reported. Relevant trials that measured these outcomes but reported no data at all, or data not in a useable format, were included in the review as part of the narrative.
Primary outcomes
Event‐free survival, measured as risk of death or the requirement for cardiac transplant or placement of a left ventricular assist device.
Overall survival, measured as risk of death.
Secondary outcomes
Improvement in LVEF. We planned to examine change from baseline and look for the presence or absence of normalisation.
Improvement in LVEDD. We planned to examine change from baseline and look for the presence or absence of normalisation.
Improvement in LVSF. We planned to examine change from baseline and look for the presence or absence of normalisation.
Improvement in functional capacity (as determined by increased exercise tolerance as measured by any objective test and the New York Heart Association Functional Capacity test).
Occurrence of side effects.
Failure to attain complete recovery.
Hospitalisation status.
Search methods for identification of studies
Electronic searches
We re‐ran search strategies from the previous searches in 2009 (Appendix 1) and 2014 (Appendix 2) on 2 July 2019 (Appendix 3). We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (via CRS Web) and the Database of Abstracts of Reviews of Effects (DARE) (2015, Issue 2 of 4: last issue available), MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily (Ovid, 1946 to 27 June 2019), Embase (Ovid, 1980 to Week 26 2019), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO, 1937 to 2 July 2019), Web of Science Core Collection (Thomson Reuters, 1900 to 2 July 2019), and the Latin American and Caribbean Health Science Information database (LILACS) (BIREME, 1982 to 2 July 2019).
The Cochrane sensitivity‐maximising RCT filter was applied to MEDLINE; for Embase, terms as recommended in the Cochrane Handbook were applied (Lefebvre 2011). An adaptation of the RCT filter was applied to all other databases, except CENTRAL and DARE.
We did not limit the search by language or publication status, and included all years available for each database.
Searching other resources
We reviewed the reference lists of all included studies for further studies.
We searched the following clinical trial registers in January 2014:
National Heart, Lung, and Blood Institute (www.nhlbi.nih.gov/index.htm);
ISRCTN Register (www.controlled-trials.com);
Clinicaltrials.gov (clinicaltrials.gov);
National Research Register Archive and National Institute for Health Research (NIHR) Portfolio Database (portal.nihr.ac.uk);
CenterWatch (www.centerwatch.com/search.asp);
CardioSource (www.cardiosource.com);
www.neri.org/html/research/clinical/pediatric.asp (to 2003).
For this update we searched the following clinical trial registers on 4 November 2019:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/).
Data collection and analysis
Selection of studies
Two review authors (JR, MS) independently examined the titles and abstracts of trials generated by the search to identify those that were potentially relevant. We obtained the full‐texts of those studies deemed potentially relevant, and two review authors (JR, MS) assessed these for inclusion in the review using a standardised form. We planned to resolve any discrepancies through discussion.
Data extraction and management
Two review authors (JR, MS) independently extracted data using a standard data form to capture the following information.
Study characteristics (e.g. design, quality, funding source).
Study participants (e.g. age, severity of illness, duration of symptoms, number of participants randomised, followed up, and analysed).
Intervention (e.g. dose of IVIG).
Outcome measures (e.g. event‐free survival, overall survival, LVEF, LVEDD, LVSF, functional capacity, side effects, complete recovery, hospitalisation status).
Results.
We noted no discrepancies in data extraction. We requested additional unpublished data from the primary author of one included trial (McNamara 1997).
Assessment of risk of bias in included studies
Two review authors (JR, MS) independently assessed all included studies using the Cochrane 'Risk of bias' tool (Higgins 2011). We determined overall risk of bias based on the primary outcome (event‐free survival or overall survival) within each study. We assessed the risk of bias according to the following seven domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table. The domains were all weighted equally, and disagreements were resolved by consensus.
Measures of treatment effect
Dichotomous data (e.g. event‐free survival, overall survival, occurrence of side effects, complete recovery) were expressed as risk ratio (RR) with 95% confidence intervals (CIs). We measured event‐free survival as risk of an event (death, cardiac transplant, placement of a left ventricular assist device) occurring; overall survival as the risk of death; side effects as the risk of side effects occurring; and recovery as failure to attain complete recovery. We derived the number needed to treat for an additional harmful outcome (NNTH) to help clarify the extent of adverse effects. We converted continuous data (e.g. change in LVEF, change in LVEDD, and peak oxygen consumption) to mean differences (MDs) with 95% CIs.
Unit of analysis issues
We planned to analyse cluster‐randomised trials in accordance with the guidance in Section 16.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), but no such studies have as yet been identified. We planned that for trials with multiple arms, we would divide the control group N by the number of arms to avoid double‐counting in meta‐analyses; however, this was not applicable to the studies included in the review.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only).
Assessment of heterogeneity
We inspected forest plots visually to consider the direction and magnitude of effects and the degree of overlap between confidence intervals. We used the I2 statistic to measure heterogeneity amongst the trials in each analysis, but acknowledge that there is substantial uncertainty in the value of I2 when there is only a small number of studies. We also considered the P value from the Chi2 test.
We planned that if we identified substantial heterogeneity (50% to 90%), we would report it and explore possible causes by prespecified subgroup analysis. Where heterogeneity was considerable (75% to 100%), we would not pool studies statistically but present them in forest plots and suppress the summary effect estimate.
Assessment of reporting biases
Due to the small number of included studies, we were only able to conduct one meta‐analysis. As the meta‐analysis only included two studies, it was not possible to examine publication bias using a funnel plot.
Data synthesis
Meta‐analysis was only possible for two outcomes (overall survival and change in LVEF) with data provided from two adult trials. Other meta‐analyses were not possible because only three trials were included in the review, which enrolled completely different populations and measured outcomes in different ways.
For peak oxygen consumption, we assumed a correlation of 0.5 and used the methods of Follmann to calculate the SDs of the change from baseline estimates (Follmann 1992). McNamara 2001 reported this variable and calculated the value only on the portion of the sample for which data were available (48/62 participants). The study authors did not provide the breakdown of sample size in each group, only a total sample size. They calculated an estimate of sample size in each group by pro‐rating the original group sample sizes to the new total sample size.
Subgroup analysis and investigation of heterogeneity
We prespecified a subgroup analysis of cardiac biopsies that fulfilled the Dallas, Aretz 1987, or World Heart Federation, Maisch 1999; Maisch 2000, criteria for acute myocarditis to investigate possible heterogeneity; however, due to an insufficient number of included studies we could not undertake this analysis.
Sensitivity analysis
We did not undertake a sensitivity analysis due to the limited number of included studies.
Summary of findings and assessment of the certainty of the evidence
We developed 'Summary of findings' tables (Table 1; Table 2) for both the adult and paediatric populations using the primary outcome of survival (both event‐free survival and overall survival). In order to present a balanced picture of benefits and harms, side effects were also included as an outcome in the adult population; side effects were not reported in children. For each outcome, two review authors (JR, MS) independently applied the GRADE methods to determine the certainty of the evidence as outlined in the GRADE Handbook (Schunemann 2013). We decided whether or not to downgrade the certainty of the evidence one or two levels for each of the five domains (risk of bias, inconsistency, indirectness, imprecision, and publication bias). Any disagreements were resolved through discussion, and all decisions were justified in the footnotes for transparency.
Summary of findings 1. Intravenous immunoglobulin compared with placebo or no treatment for adults with acute myocarditis.
| Intravenous immunoglobulin compared with placebo or no treatment for adults with acute myocarditis | ||||||
|
Patient or population: adults with acute myocarditis Settings: hospital Intervention: IVIG Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | IVIG | |||||
| Event‐free survival (risk of death, cardiac transplant, or left ventricular assist device) ‐ adults with myocarditis, median follow‐up of 23 months | 103 per 1000 | 182 per 1000 (50 to 662) |
RR 1.76 (0.48 to 6.40) | 62 (1) | ⊕⊝⊝⊝ Very low1,2,3 | |
| Overall survival (risk of death) ‐ adults with myocarditis, median follow‐up of 3.5 months | 182 per 1000 | 165 per 1000 (42 to 658) |
RR 0.91 (0.23 to 3.62) | 103 (2) | ⊕⊝⊝⊝ Very low1,3,4 | |
| Side effects ‐ mild infusion effects, 12‐month follow‐up | 0 per 1000 | 2 per 1000 (0 to 33) |
RR 20.29 (1.25 to 329.93) | 62 (1) | ⊕⊝⊝⊝ Very low1,5,6 |
No control participants reported side effects; baseline risk was estimated to be 0.01% based on the limited data available. For the data reported NNTH = 3. |
| *Basis for assumed risk was calculated by the number of events in the control group reported for each survival outcome. For side effects, no events were reported in the control group. To estimate baseline risk we assumed a low baseline risk of 0.01% based on the limited data available for that outcome. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IVIG: intravenous immunoglobulin; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1Downgraded by 1 level due to unclear risk of bias related to lack of clarity around randomisation and blinding. 2Downgraded by 1 level for indirectness as very few participants had proven myocarditis. 3Downgraded by 2 levels for imprecision due to optimal information size not being met and CI including both appreciable benefit and harm. 4Downgraded by 1 level for indirectness as it was it was unclear if biopsies were done to confirm myocarditis and rule out congential cardiomyopathies. 5Downgraded by 1 level for indirectness due to uncertainty of the baseline estimate. 6Downgraded by 1 level for imprecision due to optimal information size not being met and large CI.
Summary of findings 2. Intravenous immunoglobulin compared with placebo or no treatment for children with acute myocarditis.
| Intravenous immunoglobulin compared with placebo or no treatment for children with acute myocarditis | |||||
|
Patient or population: children with acute myocarditis Settings: hospital Intervention: IVIG Comparison: placebo or no treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | IVIG | ||||
| Overall survival (risk of death) ‐ children with myocarditis, 6‐month follow‐up | 295 per 1000 | 142 per 1000 (59 to 340) |
RR 0.48 (0.20 to 1.15) | 86 (1) | ⊕⊝⊝⊝ Very low1,2 |
| *Basis for assumed risk was calculated by the number of events in the control group reported for each survival outcome. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IVIG: intravenous immunoglobulin; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1Downgraded by 2 levels for imprecision due to optimal information size not being met and CI including both appreciable benefit and harm. 2Downgraded by 1 level for indirectness as it was it was unclear if biopsies were done to confirm myocarditis and rule out congential cardiomyopathies.
Results
Description of studies
Results of the search
In the updated search in July 2019, we identified 467 unique references of which 15 references were judged as potentially relevant. We excluded 12 studies that were not randomised or did not evaluate IVIG. One study was identified as ongoing. Two new studies met the inclusion criteria, so in addition to the study included in the previous version of the review, three studies are now included in the review (Figure 1) (El‐Saiedi 2013; Kishimoto 2014; McNamara 2001). Agreement between the two review authors was 100% with respect to study relevance.
1.
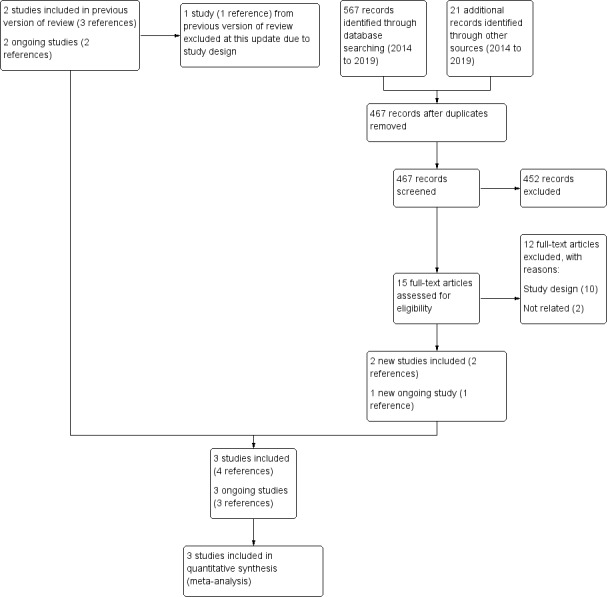
Study flow diagram.
Included studies
See Characteristics of included studies for further details.
Two randomised trials of IVIG in adults and one in children have been reported to date (El‐Saiedi 2013; Kishimoto 2014; McNamara 2001).
McNamara 2001 was conducted in the USA and published in English. This placebo‐controlled trial evaluated the efficacy of IVIG amongst 62 adults (mean age 43.0 years, SD 12.3 years) with new‐onset (within six months) dilated cardiomyopathy, normal coronary angiography, and LVEF ≤ 0.40. All participants had endomyocardial biopsies, but only 10 had cellular inflammation (four fulfilled the Dallas criteria for myocarditis, three had borderline myocarditis, and three had non‐specific inflammation). Participants were randomly assigned to receive 2 g/kg IVIG or an equivalent volume of 0.1% albumin in a blinded fashion.
Kishimoto 2014 was conducted in Japan and published in English. This multicentre randomised trial evaluated the prognosis of 41 adults (range 19 to 80 years of age) with a clinical diagnosis compatible with acute myocarditis. It is not clear how many participants had cardiac biopsies. Participants presented with an LVEF ≤ 0.40, recent onset of symptoms (less than six months), and no evidence of valvular or ischaemic heart disease. Pretreatment catheterisation with coronary angiography and endomyocardial biopsy was completed in 20 participants. Participants were randomised to two groups: IVIG (1 to 2 g/kg over two days) or no treatment.
El‐Saiedi 2013 was conducted in Egypt and published in English. This randomised placebo‐controlled study evaluated the addition of IVIG to conventional therapy in 86 children (ranging from 4 months to 6 years of age) with acute‐onset dilated cardiomyopathy and an LVSF less than 20%. Children were randomly allocated to receive 1 g/kg IVIG or a placebo of 5% glucose intravenous fluids 10 mL/kg daily for two consecutive days. There was no evidence of differences in clinical parameters at baseline between the two groups with the exception of cardiac enzymes (CPK), which were higher in the placebo group (IVIG: 155.6 ± 33.9 versus placebo: 243.2 ± 34.2; P = 0.002).
Funding for the McNamara study was provided by a pharmaceutical company in the form of an educational grant (McNamara 2001). Funding for the Kishimoto study was provided by grants from the Japanese Ministry of Education, Science and Culture, the Shimizu Immunology Foundation, the All Coffee Association, The Universe Foundation, and the Cardiovascular Research Foundation (Kishimoto 2014). The funding source for the El‐Saiedi study was not specified (El‐Saiedi 2013).
Ongoing studies
See Characteristics of ongoing studies for further details.
We identified a total of three ongoing trials. Results have not yet been reported from the European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID) (Hufnagel 2000). This trial involves different therapies depending on the expected pathogenesis of myocarditis, and at least one of the study arms involves immunoglobulin therapy. The Immunoglobulin Therapy for Patients With Idiopathic Cardiomyopathy and Endomyocardial Parvovirus B19 Persistence Trial was completed in June 2018; however, no results have been reported (Heymans 2018). A new trial in India of intravenous IVIG in young patients with recent‐onset dilated cardiomyopathy has been registered, but results are not yet available (Marotrao 2018).
Excluded studies
See Characteristics of excluded studies for further details.
We excluded a total of 26 studies for the following reasons: not an RCT (16 studies), different population (6 studies), different intervention (3 studies), and one study, an abstract, did not report any results.
Risk of bias in included studies
Overall risk of bias was unclear in the two adult studies, Kishimoto 2014; McNamara 2001, and low in the paediatric study (Figure 2; Figure 3) (El‐Saiedi 2013).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Selection bias was unclear across the included studies. Methods of randomisation and allocation concealment were not reported in Kishimoto 2014, and there was a lack of detailed explanation regarding the use of block randomisation and double‐blinding in relation to allocation concealment in McNamara 2001. El‐Saiedi 2013 used a random table block stratified by a clinical centre where allocation was managed by a third party and used a placebo, and was therefore judged as having a low risk of selection bias.
Blinding
Performance bias and detection bias were unclear across the included studies. No blinding was reported in Kishimoto 2014, resulting in insufficient information to determine risk of bias. In McNamara 2001, double‐blinding is mentioned but not explained, therefore this study was judged as at unclear risk of bias. El‐Saiedi 2013 used a placebo, and both groups received the same follow‐up, resulting in a determination of low risk of bias for this domain.
Incomplete outcome data
Attrition bias was unclear across the included studies. El‐Saiedi 2013 did not report the number of participants lost to follow‐up, and whilst the outcome data for the primary outcome were complete in McNamara 2001, measurements of functional capacity were incomplete with no explanation provided. We assessed both studies as at unclear risk of bias for this domain. Kishimoto 2014 reported that "all patients were followed up for a median of 3.5 months after therapy", and Table 1 appears to be complete, resulting in a determination of low risk of attrition bias.
Selective reporting
Reporting bias was unclear across the included studies, as insufficient information was reported in El‐Saiedi 2013 and Kishimoto 2014. McNamara 2001 had a low risk of reporting bias as all prespecified outcomes were reported.
Other potential sources of bias
No other potential sources of bias were noted.
Effects of interventions
Survival
The evidence regarding the effect of IVIG on event‐free survival and overall survival in adults (event‐free survival: risk ratio (RR) 1.76, 95% confidence interval (CI) 0.48 to 6.40; very low‐certainty evidence, median follow‐up time of 23 months (range 14 to 41 months); overall survival: pooled RR 0.91, 95% CI 0.23 to 3.62; very low‐certainty evidence) is uncertain (Analysis 1.1; Analysis 1.2) (Kishimoto 2014; McNamara 2001). The Kishimoto study did report longer survival time (defined as time to event) in the IVIG group during an average follow‐up time of 60 days; however, no raw data were reported (P < 0.01) (Kishimoto 2014).
1.1. Analysis.

Comparison 1: IVIG versus placebo or no treatment in adults, Outcome 1: Event‐free survival
1.2. Analysis.

Comparison 1: IVIG versus placebo or no treatment in adults, Outcome 2: Overall survival
In the paediatric study, evidence for overall survival was uncertain (RR of death 0.48, 95% CI 0.20 to 1.15; very low‐certainty evidence; Analysis 2.1) with a follow‐up time of six months (El‐Saiedi 2013). Event‐free survival was not reported.
2.1. Analysis.

Comparison 2: IVIG versus placebo or no treatment in children, Outcome 1: Overall survival
Improvement in LVEF
Evidence was uncertain for improvement in LVEF between the IVIG group and the control group for the two adult trials (mean difference (MD) −0.01, 95% CI −0.06 to 0.05) after 12 months, McNamara 2001, and an unknown time frame, Kishimoto 2014 (Analysis 1.3). The overall certainty of the evidence was very low.
1.3. Analysis.

Comparison 1: IVIG versus placebo or no treatment in adults, Outcome 3: Change in LVEF
Improvement in LVEDD
In the paediatric study, improvement in LVEDD was seen in both the IVIG and control groups after six months, with uncertain evidence of a difference between groups (MD −4.00, 95% CI −9.52 to 1.52; very low‐certainty evidence; Analysis 2.2) (El‐Saiedi 2013).
2.2. Analysis.

Comparison 2: IVIG versus placebo or no treatment in children, Outcome 2: Change in LVEDD
Improvement in LVSF
In the paediatric study, both the IVIG and control groups showed improvement in LVSF after six months, with uncertain evidence of a difference between groups (no numerical data; very low‐certainty evidence) (El‐Saiedi 2013).
Improvement in functional capacity
The results of functional capacity as assessed by peak oxygen consumption was uncertain at 12 months (MD −0.80, 95% CI −4.57 to 2.97; very low‐certainty evidence) (Analysis 1.4) (McNamara 2001). No other measures of functional capacity were reported.
1.4. Analysis.

Comparison 1: IVIG versus placebo or no treatment in adults, Outcome 4: Peak oxygen consumption
Side effects
In adults, the evidence for adverse events potentially related to IVIG was uncertain. Adverse events only occurred in the treated group, and all were described as mild infusion reactions (RR 20.29, 95% CI 1.25 to 329.93; NNTH = 3) (Analysis 1.5) (McNamara 2001). In children, a few participants noted flu‐like symptoms, but no major adverse events were reported including hypotension or anaphylaxis (El‐Saiedi 2013). The overall certainty of the evidence was very low.
1.5. Analysis.

Comparison 1: IVIG versus placebo or no treatment in adults, Outcome 5: Side effects
Failure to attain complete recovery
The evidence regarding the effect of IVIG on whether participants fully recovered was uncertain (RR 0.46, 95% CI 0.19 to 1.14; very low‐certainty evidence) (Analysis 1.6) (Kishimoto 2014).
1.6. Analysis.

Comparison 1: IVIG versus placebo or no treatment in adults, Outcome 6: Failure to attain complete recovery
Hospitalisation status
No evidence regarding hospitalisation status was reported in any of the included studies.
Discussion
Summary of main results
Two adult studies with an unclear risk of bias and one paediatric study with a low risk of bias showed no benefit of IVIG for the primary outcome (transplant‐free or overall survival) (Table 1, Table 2). However, one of the adult studies reported longer survival time in the first 60 days in the IVIG group (Kishimoto 2014). With regard to secondary outcomes, one adult study showed no improvement in LVEF (McNamara 2001); the paediatric study showed no improvement in LVEDD or LVSF (El‐Saiedi 2013); and one adult study showed no improvement in functional capacity when last measured (McNamara 2001).
Overall completeness and applicability of evidence
There is residual doubt, particularly in the paediatric study, as to whether all enrolled participants truly have acute myocarditis (El‐Saiedi 2013). Whilst the meta‐analysis of overall survival showed low heterogeneity, not all of the participants may have had confirmed myocarditis (McNamara 2001). This, combined with the small sample size and the variation in the eligibility criteria and reported outcomes, means that the results of the three included studies may not be applicable to all adults and children with suspected acute myocarditis.
As acute myocarditis is a relatively non‐specific entity, it is possible that a subset of patients may respond to IVIG. This group might include patients whose disease was precipitated by a specific virus, or patients treated with IVIG early in the course of their illness, when they have ongoing viral replication in the myocardium. Paediatric patients may be more likely to respond, as the chance that an acute cardiomyopathy is due to viral myocarditis is probably greater in children than in adults.
Quality of the evidence
The overall certainty of the evidence was very low. The quality of the evidence was downgraded most often due to an unclear risk of bias, as randomisation methods were unclear or not reported, and for imprecision due to the confidence interval failing to exclude either a benefit or a harm and for failing to meet the optimal information size.
Potential biases in the review process
Eligible studies should be readily identified by the screening process, as the terminology for 'myocarditis' and 'IVIG' are not ambiguous. Given that there is equipoise on the use of IVIG for myocarditis, studies are of great interest and are likely to be accepted for publication if the quality is acceptable.
Agreements and disagreements with other studies or reviews
A major limitation of McNamara 2001 is that it seems likely that at least some of the trial participants may not have had viral myocarditis, as only 10 of 62 had inflammation on cardiac biopsy. However, the primary purpose of the biopsies in this trial may have been to rule out fibrosis, so fewer biopsies were taken than would have been taken had the purpose been to confirm myocarditis. This lack of benefit contrasts with multiple case reports and case series suggesting potential benefit of IVIG in suspected viral myocarditis (Alrabate 2013; Drucker 1994; Goland 2008; Haque 2009; McNamara 1997; Nigro 2001; Takeda 1998; Tedeschi 2002). Spontaneous improvement is common with acute myocarditis and can be rapid or gradual, so the improvement noted in these case series may have been part of the natural history of the disease. The findings from McNamara and colleagues are also incongruent with the results of the second RCT of adults with recent‐onset myocarditis that was added at this update. Kishimoto and colleagues reported longer survival times in participants treated with IVIG; however, due to an unclear risk of bias and concerns about imprecision, the certainty of the evidence was very low (Kishimoto 2014).
A recent review reported decreased in‐hospital mortality and improved LVEF with IVIG in 13 adult and paediatric studies with a control group (Huang 2019). They reported improved overall survival with IVIG for acute fulminant myocarditis; however, all studies but El‐Saiedi 2013 were observational studies. It is unclear why they did not include the McNamara and Kishimoto RCTs (Kishimoto 2014; McNamara 2001).
One of the excluded studies warrants mention, as it may be of interest to the reader (Maisch 2004). This was a controlled trial of cytomegalovirus hyperimmunoglobulin (CMVhlg), rather than IVIG, amongst 35 participants with CMV‐positive myocarditis. The results showed a significant difference between groups in favour of treatment in terms of elimination of CMV‐DNA and infiltrate, and improvement of 1 New York Heart Association (NYHA) class. In a review article, it was stated that data from the Marburg Registry support the notion that IVIG has efficacy for myocarditis due to adenovirus, but that for myocarditis due to parvovirus, IVIG decreases inflammation but does not eradicate the virus (Maisch 2013). However, an ongoing trial in the Netherlands is examining the use of IVIG in adults with chronic cardiomyopathy and detection of parvovirus B19 on endomyocardial biopsy (Heymans 2018).
Authors' conclusions
Implications for practice.
Evidence from two trials involving 103 adults provides incongruent evidence regarding the use of intravenous immunoglobulin (IVIG) for the management of presumed viral myocarditis in adults. In both studies, it is unknown whether all participants had viral myocarditis, as this was usually a clinical rather than a biopsy‐proven diagnosis (Kishimoto 2014; McNamara 2001). One of the adult trials did report longer survival time in the first 60 days with a tendency towards longer total survival, but did not detail randomisation methods (Kishimoto 2014). Pooled data showed the risk of death in these two studies to be not significant, with both harm and benefit. Evidence from a single paediatric trial was also inconclusive. The study of 86 children found that IVIG favoured but did not significantly improve survival (El‐Saiedi 2013). Further randomised controlled trials are needed. Until higher‐quality studies demonstrate benefit in a particular group of patients, the evidence for treatment with IVIG for presumed viral myocarditis is uncertain.
Implications for research.
The greatest need is for further studies of the pathophysiology of acute myocarditis, which would provide a better understanding of the aetiology and natural history of the disease. This might lead to improved diagnostic criteria, which would make it much easier for researchers to design studies of treatment options. This might also lead to recognition of subgroups of patients for whom IVIG has greater potential to confer clinical benefit.
What's new
| Date | Event | Description |
|---|---|---|
| 6 January 2020 | New citation required but conclusions have not changed | Two new studies were added; one suggested adults may have improved survival time with intravenous immunoglobulin (IVIG); one reported no therapeutic efficiency for use of IGIV in children. One quasi‐randomised study that was previously included was removed. |
| 2 July 2019 | New search has been performed | Search was updated to July 2019. |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 28 November 2014 | New citation required and conclusions have changed | One new study was added, suggesting that the subgroup of children with encephalitis and myocarditis may benefit from IVIG. |
| 27 November 2014 | New search has been performed | Search was updated to January 2014. |
| 18 June 2008 | Amended | Converted to new review format |
| 18 June 2008 | Amended | Amendment made to authorship. |
| 26 June 2007 | New search has been performed | Searches rerun to June 2007. Two new studies identified as potentially relevant, both of which were excluded. Conclusions of the review unchanged. |
Acknowledgements
Ellen Crumley developed the original search strategy and ran the original search and identified sources of grey literature for the original review. We thank Natasha Wiebe for providing statistical input on the protocol. We thank Kelly Russell for assisting with quality assessment; Andrea Milne for assisting with 'Risk of bias' assessment; and Elizabeth Sumamo for assisting with screening for the 2007 update. For the 2020 update, we thank Diana Keto‐Lambert, who helped with the search, and Sholeh Rahman, who assisted with screening.
Appendices
Appendix 1. Previous search strategies
CINAHL 2007
1 exp immunoglobulins/ 2 (gammaglobulin$ or gamma‐globulin$).tw. 3 ivig$.tw. 4 igg$.tw. 5 immunoglobulin$.tw. 6 ((immune$ or immuno$) adj5 (globulin$ or serum$)).tw. 7 or/1‐6 8 exp Myocardial Diseases/ 9 myocarditis.tw. 10 carditis.tw. 11 myocardiopath$.tw. 12 cardiomyopath$.tw. 13 ((heart$ or myocard$) adj5 (inflammation$ or inflame$)).tw. 14 or/8‐13 15 7 and 14 16 limit 15 to yr="2003 ‐ 2007" 17 Randomized controlled trials/ 18 clinical trial.pt. 19 exp Clinical trials/ 20 (clin$ adj25 trial$).ti,ab. 21 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. 22 placebos.sh. 23 placebo$.ti,ab. 24 random$.ti,ab. 25 exp evaluation studies/ 26 prospective studies.sh. 27 (control$ or prospectiv$ or volunteer$).ti,ab. 28 or/17‐27 29 16 and 28
All Evidence‐Based Medicine Reviews (Cochrane Database of Systematic Reviews, ACP Journal Club, Database of Abstracts of Reviews of Effects and CENTRAL (2003, Issue 2))
1 myocarditis$.mp. 2 carditis$.mp. 3 cardiomyopath$.mp. 4 myocardiopath$.mp. 5 ((heart$ or myocard$) adj5 (inflammation$ or inflame$)).mp. 6 or/1‐5 7 immunoglobulin$.mp. 8 (gammaglobulin$ or gamma‐globulin$).mp. 9 ivig$.mp. 10 igg$.mp. 11 ((immune$ or immuno$) adj5 (globulin$ or serum$)).mp. 12 or/7‐11 13 6 and 12
MEDLINE 2003
1 exp immunoglobulins/ 2 (gammaglobulin$ or gamma‐globulin$).mp. 3 ivig$.mp. 4 exp immunoglobulin g/ 5 igg$.mp. 6 exp immunoglobulins, surface/ 7 immunoglobulin$.mp. 8 ((immune$ or immuno$) adj5 (globulin$ or serum$)).mp. 9 or/1‐8 10 myocarditis$.mp. 11 cardiomyopath$.mp. 12 myocardiopath$.mp. 13 carditis$.mp. 14 ((heart$ or myocard$) adj5 (inflammation$ or inflame$)).mp. 15 or/10‐14 16 9 and 15
EMBASE 2003
1 immunoglobulin$.mp. 2 (gammaglobulin$ or gamma‐globulin$).mp. 3 exp immunoglobulin g/ 4 ivig$.mp. 5 igg$.mp. 6 exp cell surface immunoglobulin/ 7 immunoglobulin/ 8 exp human immunoglobulin/ 9 exp hyperimmune globulin/ 10 ((immune$ or immuno$) adj5 (globulin$ or serum$)).mp. 11 or/1‐10 12 exp myocarditis/ 13 myocarditis$.mp. 14 exp cardiomyopathy/ 15 myocardiopath$.mp. 16 carditis$.mp. 17 ((heart$ or myocard$) adj5 (inflammation$ or inflame$)).mp. 18 cardiomyopath$.mp. 19 exp carditis/ 20 or/12‐19 21 11 and 20
CINAHL 2003
1 immunoglobulin$.mp. 2 (gammaglobulin$ or gamma‐globulin$).mp. 3 ivig$.mp. 4 exp immunoglobulins/ 5 igg$.mp. 6 ((immune$ or immuno$) adj5 (globulin$ or serum$)).mp. 7 or/1‐6 8 myocarditis$.mp. 9 cardiomyopath$.mp. 10 myocardiopath$.mp. 11 carditis$.mp. 12 ((heart$ or myocard$) adj5 (inflammation$ or inflame$)).mp. 13 or/8‐12 14 7 and 13
Appendix 2. Search strategies 2014
CENTRAL
#1 MeSH descriptor Myocarditis explode all trees #2 MYOCARDITIS #3 CARDITIS #4 CARDIOMYOPATH* #5 (HEART near/3 INFLAMMATION ) #6 (MYOCARD* near/3 INFLAMMATION) #7 MYOCARDIOPATH* #8 (#1 or #2 or #3 or #4 or #5 or #6 or #7) #9 MeSH descriptor Immunoglobulins, Intravenous explode all trees #10 IMMUNOGLOBULIN* #11 IMMUNE next GLOBULIN* #12 GAMMAGLOBULIN* #13 GAMMA‐GLOBULIN* #14 IMMUNE next SERUM next GLOBULIN* #15 IVIG* #16 IGG* #17 (#9 or #10 or #11 or #12 or #13 or #14 or #15 or #16) #18 (#8 and #17)
MEDLINE Ovid
1 exp immunoglobulins/ 2 (gammaglobulin$ or gamma‐globulin$).tw. 3 ivig$.tw. 4 exp Immunoglobulin G/ 5 igg$.tw. 6 exp Receptors, Antigen, B‐Cell/ 7 immunoglobulin$.tw. 8 ((immune$ or immuno$) adj5 (globulin$ or serum$)).tw. 9 or/1‐8 10 Myocarditis/ 11 exp Cardiomyopathy, Dilated/ 12 myocarditis.tw. 13 carditis.tw. (1361) 14 myocardiopath$.tw. 15 cardiomyopath$.tw. 16 ((heart$ or myocard$) adj5 (inflammation$ or inflame$)).tw. 17 or/10‐16 18 9 and 17 19 randomized controlled trial.pt. 20 controlled clinical trial.pt. 21 Randomized controlled trials/ 22 random allocation/ 23 double blind method/ 24 single‐blind method/ 25 or/19‐24 26 exp animal/ not humans/ 27 25 not 26 28 clinical trial.pt. 29 exp Clinical Trials as Topic/ 30 (clin$ adj25 trial$).ti,ab. 31 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. 32 placebos/ 33 placebo$.ti,ab. 34 random$.ti,ab. 35 research design/ 36 or/28‐35 37 36 not 26 38 27 or 37 39 18 and 38
EMBASE Ovid
1 exp Immunoglobulin/ 2 exp Myocarditis/ 3 Congestive Cardiomyopathy/ 4 exp immunoglobulins/ 5 (gammaglobulin$ or gamma‐globulin$).tw. 6 ivig$.tw. 7 igg$.tw. 8 immunoglobulin$.tw. 9 ((immune$ or immuno$) adj5 (globulin$ or serum$)).tw. 10 or/4‐9 11 exp Myocarditis/ 12 Congestive Cardiomyopathy/ 13 myocarditis.tw. 14 carditis.tw. 15 myocardiopath$.tw. 16 cardiomyopath$.tw. 17 ((heart$ or myocard$) adj5 (inflammation$ or inflame$)).tw. 18 or/11‐17 19 10 and 18 20 clinical trial/ 21 random$.tw. 22 randomized controlled trial/ 23 trial$.tw. 24 follow‐up.tw. 25 double blind procedure/ 26 placebo$.tw. 27 placebo/ 28 factorial$.ti,ab. 29 (crossover$ or cross‐over$).ti,ab. 30 (double$ adj blind$).ti,ab. 31 (singl$ adj blind$).ti,ab. 32 assign$.ti,ab. 33 allocat$.ti,ab. 34 volunteer$.ti,ab. 35 Crossover Procedure/ 36 Single Blind Procedure/ 37 or/20‐36 38 (exp animal/ or nonhuman/) not exp human/ 39 37 not 38 40 39 and 19
CINAHL EBSCO
( (MH "Myocardial Diseases+") or myocarditis or carditis or cardiomyopath* ) and ( (MH "Immunoglobulins+") or immunoglobulin* or gammaglobulin* or gamma‐globulin* or ivig* or IGG ) and ( ( (MH "Clinical Trials+") or random$ or trial or clinical study or group$ or placebo$ ) )
ISI Web of Science
TS=((myocarditis or carditis or cardiomyopath*) and (IMMUNOGLOBULIN* or GAMMAGLOBULIN* or GAMMA‐GLOBULIN* or ivig* or IGG) and (random* or controlled or trial or RCT or clinical or placebo))
LILACS
myocarditis or carditis or cardiomyopath$ [Palavras] and IMMUNOGLOBULIN$ or GAMMAGLOBULIN$ or GAMMA‐GLOBULIN$ or ivig$ or IGG$ [Palavras] and (random$ or clinical$ or trial$ or RCT) [Palavras]
Appendix 3. Search strategies 2019
CENTRAL and DARE
#1 MeSH descriptor: [Immunoglobulins, Intravenous] explode all trees
#2 immunoglobulin*
#3 immune next globulin*
#4 gammaglobulin*
#5 gamma‐globulin*
#6 immune next serum next globulin*
#7 ivig*
#8 igg*
#9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
#10 MeSH descriptor: [Myocarditis] explode all trees
#11 myocarditis
#12 carditis
#13 cardiomyoath*
#14 heart near/3 inflammation
#15 myocard* near/3 inflammation
#16 myocardiopath*
#17 #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 #9 and #17 (Restrict to date 29/01/2014‐02/07/2019)
MEDLINE Ovid
1. exp Immunoglobulins/
2. (gammaglobulin* or gamma‐globulin*).tw.
3. ivig*.tw.
4. exp Immunoglobulin G/
5. igg*.tw.
6. exp Receptors, Antigen, B‐Cell/
7. immunoglobulin*.tw.
8. ((immune* or immuno*) adj5 (globulin* or serum*)).tw.
9. or/1‐8
10. Myocarditis/
11. exp Cardiomyopathy, Dilated/
12. myocarditis.tw.
13. carditis.tw.
14. myocardiopath*.tw.
15. cardiomyopath*.tw.
16. ((heart* or myocard*) adj5 (inflammation* or inflame*)).tw.
17. or/10‐16
18. 9 and 17
19. randomized controlled trial.pt.
20. controlled clinical trial.pt.
21. randomized.ab.
22. placebo.ab.
23. drug therapy.fs.
24. randomly.ab.
25. trial.ab.
26. groups.ab.
27. 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
28. exp animals/ not humans.sh.
29. 27 not 28
30. 18 and 29
31. limit 30 to ed=20140129‐20190702
EMBASE Ovid
1. exp immunoglobulin/
2. (gammaglobulin* or gamma‐globulin*).tw.
3. ivig*.tw.
4. igg*.tw.
5. immunoglobulin*.tw.
6. ((immune* or immuno*) adj5 (globulin* or serum*)).tw.
7. or/1‐6
8. exp myocarditis/
9. congestive cardiomyopathy/
10. myocarditis.tw.
11. carditis.tw.
12. myocardiopath*.tw.
13. cardiomyopath*.tw.
14. ((heart* or myocard*) adj5 (inflammation* or inflame*)).tw.
15. or/8‐14
16. 7 and 15
17. random$.tw.
18. factorial$.tw.
19. crossover$.tw.
20. cross over$.tw.
21. cross‐over$.tw.
22. placebo$.tw.
23. (doubl$ adj blind$).tw.
24. (singl$ adj blind$).tw.
25. assign$.tw.
26. allocat$.tw.
27. volunteer$.tw.
28. crossover procedure/
29. double blind procedure/
30. randomized controlled trial/
31. single blind procedure/
32. 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31
33. (animal/ or nonhuman/) not human/
34. 32 not 33
35. 16 and 34
36. limit 35 to dd=20140130‐20190702
CINAHL EBSCO
S6 S4 AND S5
S5 EM 20140130‐20190702
S4 S1 AND S2 AND S3
S3 ( ( (MH "Clinical Trials+") or random$ or trial or clinical study or group$ or placebo$ ) )
S2 ( (MH "Immunoglobulins+") or immunoglobulin* or gammaglobulin* or gamma‐globulin* or ivig* or IGG )
S1 ( (MH "Myocardial Diseases+") or myocarditis or carditis or cardiomyopath* )
ISI Web of Science
# 5 #4 Timespan=2014‐2019
# 4 #3 AND #2 AND #1
# 3 TS=(random* or controlled or trial or RCT or clinical or placebo)
# 2 TS=(immunoglobulin* or gammaglobulin* or gamma‐globulin* or ivig* or igg)
# 1 TS=(myocarditis or carditis or cardiomyopath*)
LILACS
myocarditis or carditis or cardiomyopath$ [Words] and immunoglobulin$ or gammaglobulin$ or gamma‐globulin$ or ivig$ or igg$ [Words] and random$ or clinical$ or trial$ or RCT [Words]
Clinicaltrials.gov
IMMUNOGLOBULIN* OR GAMMAGLOBULIN* OR GAMMA‐GLOBULIN* OR ivig* OR immune globulin* OR IGG* | myocarditis OR carditis OR cardiomyopathy
WHO’s ICTRP
Condition: myocarditis OR carditis OR cardiomyopathy
AND Intervention: IMMUNOGLOBULIN* OR GAMMAGLOBULIN* OR GAMMA‐GLOBULIN* OR ivig* OR immune globulin* OR IGG*
Data and analyses
Comparison 1. IVIG versus placebo or no treatment in adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Event‐free survival | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.48, 6.40] |
| 1.2 Overall survival | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.23, 3.62] |
| 1.3 Change in LVEF | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.05] |
| 1.4 Peak oxygen consumption | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐4.57, 2.97] |
| 1.5 Side effects | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 20.29 [1.25, 329.93] |
| 1.6 Failure to attain complete recovery | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.19, 1.14] |
Comparison 2. IVIG versus placebo or no treatment in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Overall survival | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.20, 1.15] |
| 2.2 Change in LVEDD | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐9.52, 1.52] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
El‐Saiedi 2013.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, single‐centre study; table block randomisation stratified by clinical centre. No blinding reported. | |
| Participants | 86 patients (range 4 months to 6 years of age; mean age of 2.1 ± 1.3 years in the IVIG group versus 3.3 ± 1.9 years in the placebo group) with acute‐onset dilated cardiomyopathy, LVSF less than 20%, less than 6 months duration of cardiac symptoms at time of randomisation. Exclusion criteria: neonates, history of cardiac symptoms since birth | |
| Interventions | Treatment of 1 g/kg IVIG (VIGAM‐S. , 2.5 g and 5 g, Bio Products Laboratory Limited) each day for 2 consecutive days versus regular glucose 5% IV fluids 10 mL/kg repeated on 2 consecutive days | |
| Outcomes | Primary endpoint was change in LVSF and LVEDD from baseline to 6 months post randomisation assessed by echocardiography. Secondary endpoint was survival versus death. | |
| Notes | Funding: not reported Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a "random table block stratified by clinical center" |
| Allocation concealment (selection bias) | Low risk | Third party managed group assignment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo used, and both groups received the same follow‐up. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants received the same follow‐up; survival vs death was hard endpoint. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants lost to follow‐up was not reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information reported; data on SF are shown in Figure 2, but numbers are not provided. |
Kishimoto 2014.
| Study characteristics | ||
| Methods | Multicentre trial; randomisation methods not reported | |
| Participants | 41 adults (aged 19 to 80 years; 27 male, 14 female) with an LVEF ≤ 0.40, less than 6 months of cardiac symptoms at randomisation point with myocarditis diagnosed using the Dallas criteria. Patients with acute cardiac failure, arrhythmia, or recent flu‐like illness with electrocardiographic abnormalities were eligible for inclusion. Exclusion criteria: diabetes, thyroid disease, renal disease, uncontrolled hypertension (systolic blood pressure > 140 mmHg and diastolic blood pressure > 90 mmHg), valvular heart disease, previous history of documented ischaemic heart disease, eosinophilic myocarditis | |
| Interventions | 1 to 2 g/kg IVIG (intact, Fc portion: Venilon, Polyglobin‐N, and Venoglobulin‐IH) over 2 days versus no treatment | |
| Outcomes | LVEF measured by echocardiography, presence or absence of a circulatory assist device, degree of recovery | |
| Notes | Funding: grants from the Japanese Ministry of Education, Science and Culture, the Shimizu Immunology Foundation, the All Coffee Association, The Universe Foundation, and the Cardiovascular Research Foundation Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods were not reported. |
| Allocation concealment (selection bias) | Unclear risk | How participants were allocated to groups is not explained. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding was reported; insufficient information to determine risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding was reported; insufficient information to determine risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All patients were followed up for a median of 3.5 months after therapy"; Table 1 appears to be complete. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information reported. |
McNamara 2001.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blind trial; block randomisation was stratified by clinical centre; intention‐to‐treat analysis was not performed | |
| Participants | 62 adults (mean age 43.0, SD 12.3 years; 37 men; 4 participants met the Dallas criteria for acute myocarditis); participants had recent onset (≤ 6 months of symptoms) of dilated cardiomyopathy and LVEF ≤ 0.40. Exclusion criteria: coronary artery disease, significant valvular disease, significant diabetes mellitus, significant hypertension, uncorrected thyroid disease, giant cell myocarditis, sarcoid, haemochromatosis | |
| Interventions | Treatment: 2 g/kg IVIG (Gamimune N, 10%, Bayer Corporation); administered at 1 g/kg IV each day on 2 consecutive days Control: 0.1% albumin in 10% maltose solution given in equivalent volume (10 mL/kg IV) each day on 2 consecutive days | |
| Outcomes | Primary endpoint: change in LVEF from baseline to 6 months and 12 months; secondary endpoints: event‐free survival (events defined as death, cardiac transplantation, or placement of an LVAD) and functional capacity as assessed by metabolic stress testing at 12 months | |
| Notes | Funding: educational grant from the Bayer Corporation Language of publication: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocked randomisation was stratified by clinical centre, but no further details were provided on how the randomisation schedule was created. |
| Allocation concealment (selection bias) | Unclear risk | "Double‐blinding" is mentioned but not explained. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear how those who assessed outcomes were blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blinding" is mentioned but is not explained. It is not clear if the placebo appeared identical to IVIG. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcome data are complete for the primary outcome but incomplete for measurements of functional capacity, with no explanation provided for missing data. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
IV: intravenous
IVIG: intravenous immunoglobulin
LVAD: left ventricular assist device
LVEF: left ventricular ejection fraction
LVEDD: left ventricular end‐diastolic diameter
LVSF: left ventricular shortening fraction
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alrabate 2013 | Not an RCT |
| Anonymous 1997 | Not an RCT |
| Anonymous 2002 | Not an RCT |
| Bauer 2002 | Not an RCT |
| Bhatt 2012 | Quasi‐randomised, not an RCT |
| Bozkurt 1998 | Different population (postpartum cardiomyopathy) |
| Bozkurt 1999 | Not an RCT |
| Drucker 1992 | Not an RCT |
| Drucker 1994 | Not an RCT |
| Felix 2000 | Different intervention (smaller doses) |
| Goland 2008 | Not an RCT |
| Gullestad 2001 | Different population (included participants with symptoms > 6 months) |
| Haque 2009 | Not an RCT |
| Kishimoto 1999 | Not an RCT |
| Klugman 2009 | Not an RCT |
| Levi 2002 | Not an RCT |
| Maisch 1991 | Not an RCT |
| Maisch 1995 | Did not report results |
| Maisch 2004 | Different intervention (CMV hyperimmunoglobulin) |
| Maisch 2007 | Not an RCT |
| McNamara 1997 | Not an RCT |
| Muller 1998 | Different intervention |
| Shioji 2000 | Different population (animals) |
| Staudt 2001 | Different population (included participants with symptoms > 6 months) |
| Takada 1993 | Different population (animals) |
| Takada 1995 | Different population (animals) |
CMV: cytomegalovirus RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Heymans 2018.
| Study name | Intravenous immunoglobulin (IVIg) for parvovirus B19 (PVB19)‐mediated cardiomyopathy |
| Methods | Randomised controlled trial |
| Participants | Patients with chronic cardiomyopathy and detection of parvovirus B19 in cardiac tissue |
| Interventions | High‐dose intravenous immunoglobulin |
| Outcomes | Primary outcome is change in ejection fraction. |
| Starting date | 2009 |
| Contact information | i.kleinebudde@sanquin.nl |
| Notes |
Hufnagel 2000.
| Study name | European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID) |
| Methods | |
| Participants | |
| Interventions | 1 study arm involves immunoglobulin therapy. |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Recent communication with a co‐investigator uncovered no reported data relevant to the review question. |
Marotrao 2018.
| Study name | Randomized trial on use of intravenous immunoglobulin in young patients with recent onset dilated cardiomyopathy |
| Methods | Randomised, parallel‐group trial |
| Participants | Patients < 25 years of age with dilated cardiomyopathy with left ventricular dysfunction and recent onset of symptoms (duration < 6 months) |
| Interventions | 2 g/kg intravenous immunoglobulin over 3 days |
| Outcomes | Improvement in left ventricular end‐diastolic dimension at 6 months; event‐free survival (i.e. death, hospitalisation, and transplant‐free survival) at 6 months |
| Starting date | 2018 |
| Contact information | patilsuraj762@gmail.com |
| Notes |
Differences between protocol and review
With the 2020 update, differences in how survival was assessed necessitated a change in the primary outcome to also include overall survival (no death) and a name change from 'transplant‐free survival' to 'event‐free survival', to better reflect the definition of the outcome. Additional outcomes of side effects and failure to attain complete recovery were added in the 2015 and 2020 update, respectively. The 'Summary of findings' table, including GRADE assessment, which was added in 2015, was updated in 2020, and additional outcomes were included. With the addition of two new studies, a single meta‐analysis could be conducted in the 2020 update, and the relative effect measured recalculated using risk ratio rather than odds ratio for greater clarity.
The original protocol planned for 'Risk of bias' assessment used the 5‐point Jadad scale. From 2015 update forward, we used the Cochrane 'Risk of bias' tool on all new and previously included studies.
Contributions of authors
JR conceived the review, screened searches initially and for each update, applied inclusion criteria, performed quality assessment and data extraction, participated in writing the review and the updates, and provided clinical expertise.
LH organised retrieval of papers, screened searches, applied inclusion criteria, performed quality assessment and data extraction, edited the review, and provided methodological expertise.
BV conducted the analysis, assisted with interpretation of data, and contributed to writing the review.
MS screened the 2020 update search, applied the inclusion criteria, performed quality assessment, undertook data extraction and analysis, and updated the text.
TK provided clinical and methodological expertise, contributed to interpretation of the results, and edited the review.
Sources of support
Internal sources
Alberta Research Centre for Health Evidence (ARCHE), University of Alberta, Edmonton, Alberta, Canada
Alberta Strategy for Patient‐Oriented Research (SPOR) Knowledge Translation Platform, University of Alberta, Edmonton, Alberta, Canada
External sources
-
NIHR, UK
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care.
Declarations of interest
Joan Robinson's institution has received funding from Pfizer and the US National Institutes of Health for involvement in studies on invasive fungal infection and influenza.
Lisa Hartling, Ben Vandermeer, Meghan Sebastianski, and Terry Klassen have no known conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
El‐Saiedi 2013 {published data only}
- El-Saiedi SA. Randomized controlled trial on the use of intravenous immune globulin in acute pediatric myocarditis. Journal of Clinical Research & Bioethics 2013;5(1):1-5. [Google Scholar]
Kishimoto 2014 {published data only}
- Kishimoto C, Shioji K, Hashimoto T, Nonogi H, Lee JD, Kato S, et al. Therapy with immunoglobulin in patients with acute myocarditis and cardiomyopathy: analysis of leukocyte balance. Heart Vessels 2014;29:336-42. [DOI] [PubMed] [Google Scholar]
McNamara 2001 {published data only}
- McNamara DM, Holubkov R, Starling RC, Dec GW, Loh E, Torre-Amione G, et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation 2001;103(18):2254-9. [DOI] [PubMed] [Google Scholar]
- McNamara DM, Starling RC, Dec GW, Loh E, Torre-Amione G, Gass A, et al. Intervention in myocarditis and acute cardiomyopathy with immune globulin: results from the randomized placebo controlled IMAC trial. Circulation 1999;100(18):104. [Google Scholar]
References to studies excluded from this review
Alrabate 2013 {published data only}
- Alrabte SAH, Bezanti K. Role of intravenous immunoglobulin in the treatment of acute myocarditis. Cardiology in the Young 2013;2013:S96. [Google Scholar]
Anonymous 1997 {published data only}
- Conventional therapy still the mainstay for myocarditis. Drugs & Therapy Perspectives 1997;9(5):5-7. [Google Scholar]
Anonymous 2002 {published data only}
- High-dose intravenous immunoglobulin therapy in acute myocarditis. Respiration & Circulation 2002;50(7):697-703. [Google Scholar]
Bauer 2002 {published data only}
- Bauer S, Gottesman G, Sirota L, Litmanovitz I, Ashkenazi S, Levi I. Severe Coxsackie virus B infection in preterm newborns treated with pleconaril. European Journal of Pediatrics 2002;161(9):491-3. [DOI] [PubMed] [Google Scholar]
Bhatt 2012 {published data only}
- Bhatt GC, Sankar J, Kushwaha KP. Use of intravenous immunoglobulin compared with standard therapy is associated with improved clinical outcomes in children with acute encephalitis syndrome complicated by myocarditis. Pediatric Cardiology 2012;33(8):1370-6. [DOI] [PubMed] [Google Scholar]
Bozkurt 1998 {published data only}
- Bozkurt B, Alvarez RJ, Rosenblum WD, MacGowan GA, Feldman AM, Murali S, et al. Role of immune globulin in the treatment of postpartum cardiomyopathy. Journal of the American College of Cardiology 1998;31(2):330A-1A. [DOI] [PubMed] [Google Scholar]
Bozkurt 1999 {published data only}
- Bozkurt B, Villaneuva FS, Holubkov R, Tokarczyk T, Alvarez RJ, MacGowan GA, et al. Intravenous immune globulin in the therapy of peripartum cardiomyopathy. Journal of the American College of Cardiology 1999;34(1):177-80. [DOI] [PubMed] [Google Scholar]
Drucker 1992 {published data only}
- Drucker NA, Colan SD, Wessel DL, Belser AS, Takahashi M, Lewis AB, et al. Use of intravenous gamma-globulin in acute congestive cardiomyopathy. Circulation 1992;86(4):363. [Google Scholar]
Drucker 1994 {published data only}
- Drucker NA, Colan SD, Lewis AB, Beiser AS, Wessel DL, Takahashi M, et al. Gamma-globulin treatment of acute myocarditis in the pediatric population. Circulation 1994;89(1):252-7. [DOI] [PubMed] [Google Scholar]
Felix 2000 {published data only}
- Felix SB, Staudt A, Dorffel WV, Stangl V, Merkel K, Pohl M, et al. Hemodynamic effects of immunoadsorption and subsequent immunoglobulin substitution in dilated cardiomyopathy - three-month results from a randomized study. Journal of the American College of Cardiology 2000;35(6):1590-8. [DOI] [PubMed] [Google Scholar]
Goland 2008 {published data only}
- Goland S, Czer LS, Siegel RJ, Tabak S, Jordan S, Luthringer D, et al. Intravenous immunoglobulin treatment for acute fulminant cardiomyopathy: series of six patients and review of literature. Canadian Journal of Cardiology 2008;24(7):571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gullestad 2001 {published data only}
- Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, Ihlen H, et al. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation 2001;103(2):220-5. [DOI] [PubMed] [Google Scholar]
Haque 2009 {published data only}
- Haque A, Bhatti S, Siddiqui FJ. Intravenous immune globulin for severe acute myocarditis in children. Indian Pediatrics 2009;46(9):810-1. [PubMed] [Google Scholar]
Kishimoto 1999 {published data only}
- Kishimoto C, Fujita M, Kinoshita M, Iwase T, Fujii B, Murashige A, et al. Immunoglobulin therapy for myocarditis and acute dilated cardiomyopathy. Circulation 1999;100(18):1405. [Google Scholar]
Klugman 2009 {published data only}
- Klugman D, Berger JT, Sable CA, He J, Khandelwal SG, Slonim AD. Pediatric patients hospitalized with myocarditis: a multi-institutional analysis. Pediatric Cardiology 2009;31(2):222-8. [DOI] [PubMed] [Google Scholar]
Levi 2002 {published data only}
- Levi D, Alejos J. An approach to the treatment of pediatric myocarditis. Pediatric Drugs 2002;4(10):637-47. [DOI] [PubMed] [Google Scholar]
Maisch 1991 {published data only}
- Maisch B, Outzen H, Roth D, Hiby A, Herzum M, Hengstenberg C, et al. Prognostic determinants in conventionally treated myocarditis and perimyocarditis – focus on antimyolemmal antibodies. European Heart Journal 1991;12(Suppl D):81-7. [DOI] [PubMed] [Google Scholar]
Maisch 1995 {published data only}
- Maisch B, Hufnagel G, Schonian U, Hengstenberg C, for the ESETCID Investigators. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Disease (ESETCID). European Heart Journal 1995;16(Suppl O):173-5. [DOI] [PubMed] [Google Scholar]
Maisch 2004 {unpublished data only}
- Maisch B, Pankuweit S, Funck R, Koelsch. Hyperimmunoglobulin treatment is effective in CMV myocarditis - a controlled treatment trial (abstract P674). European Heart Journal 2004;25(Suppl 1):114. [Google Scholar]
Maisch 2007 {unpublished data only}
- Maisch B, Haake H, Schlotmann N, Pankuweit S. Intermediate dose of pentaglobin eradicates effectively inflammation in parvo B19 and adenovirus positive myocarditis (abstract 1616). Circulation 2007;116:II_338. [Google Scholar]
McNamara 1997 {published data only}
- McNamara DM, Rosenblum WD, Janosko KM, Trost MK, Villaneuva FS, Demetris AJ, et al. Intravenous immune globulin in the therapy of myocarditis and acute cardiomyopathy. Circulation 1997;95(11):2476-8. [DOI] [PubMed] [Google Scholar]
Muller 1998 {published data only}
- Muller J, Wallukat G, Brandes K, Spiegelsberger S, Bieda H, Kupetz W, et al. Successful therapy of idiopathic dilated cardiomyopathy by IgG immunoadsorption, results of a controlled study. Journal of the American College of Cardiology 1998;31(2):67A. [Google Scholar]
Shioji 2000 {published data only}
- Shioji K, Kishimoto C, Sasyama S. Immunoglobulin therapy for acute myocarditis. Respiration & Circulation 2000;48(11):1133-9. [Google Scholar]
Staudt 2001 {published data only}
- Staudt A, Schaper F, Stangl V, Plagemann A, Bohm M, Merkel K, et al. Immunohistological changes in dilated cardiomyopathy induced by immunoadsorption therapy and subsequent immunoglobulin substitution. Circulation 2001;103(22):2681-6. [DOI] [PubMed] [Google Scholar]
Takada 1993 {published data only}
- Takada H, Kishimoto C, Hiraoka Y. Therapy with gamma-globulin suppresses inflammatory lesions in coxsackievirus-B3 myocarditis. Circulation 1993;88(4):600. [Google Scholar]
Takada 1995 {published data only}
- Takada H, Kishimoto C, Hiraoka Y. Therapy with immunoglobulin suppresses myocarditis in a murine coxsackievirus B3 model - antiviral and antiinflammatory effects. Circulation 1995;92(6):1604-11. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Heymans 2018 {unpublished data only}
- Heymans S. Immunoglobulin therapy for patients with idiopathic cardiomyopathy and endomyocardial parvovirus B19 persistence - a prospective, double-blind, randomized, placebo-controlled clinical trial. clinicaltrials.gov/ct2/show/NCT00892112 (first received 4 May 2009).
Hufnagel 2000 {published data only}
- Hufnagel G, Pankuweit S, Richter A, Schonian U, Maisch B, for the ESETCID Investigators. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID). First epidemiological results. Herz 2000;25(3):279-85. [DOI] [PubMed] [Google Scholar]
Marotrao 2018 {unpublished data only}
- Marotrao PS, Verma S. Randomized trial on use of intravenous immunoglobulin in young patients with recent onset dilated cardiomyopathy. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=20913 (registered 18 January 2018).
Additional references
Aretz 1987
- Aretz HT. Myocarditis: the Dallas criteria. Human Pathology 1987;18:619-24. [DOI] [PubMed] [Google Scholar]
Fenoglio 1983
- Fenoglio JJ, Ursell PC, Kellogg CF, Drusin RE, Weiss MB. Diagnosis and classification of myocarditis by endomyocardial biopsy. New England Journal of Medicine 1983;308(1):12-8. [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769-73. [DOI] [PubMed] [Google Scholar]
Friedrich 2009
- Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. Journal of the American College of Cardiology 2009;53(17):1475-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuse 2000
- Fuse K, Kodama M, Okura Y. Predictors of disease course in patients with acute myocarditis. Circulation 2000;102(23):2829-35. [DOI] [PubMed] [Google Scholar]
Guglin 2012
- Guglin M, Nallamshetty L. Myocarditis: diagnosis and treatment. Current Treatment Options in Cardiovascular Medicine 2012;14(6):637-51. [DOI] [PubMed] [Google Scholar]
Hare 2001
- Hare JM, Baughman KL. Fulminant and acute myocarditis: the prognostic value of clinicopathological classification. European Heart Journal 2001;22(4):269-70. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at handbook.cochrane.org.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Available from www.training.cochrane.org/handbook: Cochrane, 2019. [Google Scholar]
Huang 2019
- Huang X, Sun Y, Su G, Li Y, Shuai X. Intravenous immunoglobulin therapy for acute myocarditis in children and adults. International Heart Journal 2019;60(2):359-65. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at handbook.cochrane.org.
Levi 2001
- Levi D, Alejos J. Diagnosis and treatment of pediatric viral myocarditis. Current Opinion in Cardiology 2001;16(2):77-83. [DOI] [PubMed] [Google Scholar]
Liu 2001
- Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation 2001;104(9):1076-82. [DOI] [PubMed] [Google Scholar]
Maisch 1999
- Maisch B, Bültmann B, Factor S, Groene HJ, Hufnagel G, Kawarmura K, et al. World Heart Federation consensus conferences's definition of inflammatory cardiomyopathy (myocarditis): report from two expert committees on histology and viral cardiomyopathy. Heartbeat 1999;4:3-4. [Google Scholar]
Maisch 2000
- Maisch B, Portig I, Ristic A, Hufnagel G, Pankuweit S. Definition of inflammatory cardiomyopathy (myocarditis): on the way to consensus. Herz 2000;25:200-9. [DOI] [PubMed] [Google Scholar]
Maisch 2002
- Maisch B, Ristic AD, Hufnagel G, Funck R, Alter P, Tontsch D, et al. Dilated cardiomyopathies as a cause of congestive heart failure. Herz 2002;27(2):113-34. [DOI] [PubMed] [Google Scholar]
Maisch 2013
- Maisch B, Pankuweit S. Standard and etiology-directed evidence-based therapies in myocarditis: state of the art and future perspectives. Heart Failure Reviews 2013;18(6):761-95. [DOI] [PubMed] [Google Scholar]
McCarthy 2000
- McCarthy RE, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, et al. Long-term outcome of fulminant myocarditis as compared with acute (non-fulminant) myocarditis. New Engand Journal of Medicine 2000;342(10):690-5. [DOI] [PubMed] [Google Scholar]
Meyer 1997
- Meyer T, Grumbach IM, Kreuzer H, Morguet AJ. Giant cell myocarditis due to coxsackie B2 virus infection. Cardiology 1997;88(3):296-9. [DOI] [PubMed] [Google Scholar]
Mounts 2001
- Mounts AW, Amr S, Jamshidi R, Groves C, Dwyer D, Guarner J, et al. A cluster of fulminant myocarditis cases in children, Baltimore, Maryland, 1997. Pediatric Cardiology 2001;22(1):34-9. [DOI] [PubMed] [Google Scholar]
Nigro 2001
- Nigro G, Bastianon V, Colloridi V, Ventriglia F, Gallo P, D'Amati G, et al. Human parvovirus B19 infection in infancy associated with acute and chronic lymphocyte myocarditis and high cytokine levels: report of 3 cases and review. Clinical Infectious Diseases 2001;31(1):65-9. [DOI] [PubMed] [Google Scholar]
Schunemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Takeda 1998
- Takeda Y, Yasuda S, Miyazaki S, Daikoku S, Nakatani S, Nonogi H. High-dose immunoglobulin G therapy for fulminant myocarditis. Japanese Circulation Journal 1998;62(11):871-2. [DOI] [PubMed] [Google Scholar]
Tedeschi 2002
- Tedeschi A, Giannini S, Ciceri L, Massari FM. High-dose intravenous immunoglobulin in the treatment of acute myocarditis. A case report and review of the literature. Journal of Internal Medicine 2002;251(2):169-73. [DOI] [PubMed] [Google Scholar]
Towbin 2001
- Towbin J. Myocarditis and pericarditis in adolescents. Adolescent Medicine 2001;12(1):47-67. [PubMed] [Google Scholar]


